Abstract
Objective
Although HIV-infected children are recommended to receive quadrivalent human papillo-mavirus vaccine (QHPV) there is limited information on their response to QHPV. This study in HIV-infected children, evaluated the magnitude and duration of immune responses to QHPV. This report describes type-specific serum antibody responses over a 4-to-5 year period after either 3 or 4 doses of QHPV.
Design/methods
HIV-infected children, ages 7-to-11 years, received 3 doses of QHPV (n = 96) or placebo (n = 30). At 72 weeks QHPV recipients received a fourth dose (n = 84), while placebo recipients began the 3-dose QHPV schedule (n = 27). HPV serotype-specific antibody was determined, by competitive Luminex immunoassay (cLIA) and IgG Luminex immunoassay, at 2, 3.5, and 4-to-5 years after the last dose of QHPV in each treatment arm.
Results
At 4-to-5 years after the last dose of QHPV, antibody titers were significantly higher in 4-dose than in 3-dose group. However, the proportion of vaccinees with a seroresponse in the cLIA assay was not different between the two groups (86–93% for HPV types 6, 11, and 16, and 64% for HPV type 18). These results were very similar to the seroresponse rate in these HIV-infected children at 1 month after completing vaccination.
Conclusions
Children with well-controlled HIV infection who receive 3 doses of the QHPV vaccine maintain seropositivity and antibody levels that are generally similar to children of the same age who are not HIV-infected. Antibody titer correlated strongly with low log HIV RNA, low CD8%, and high CD4%. Additionally, a fourth dose of vaccine in HIV-infected children produces a marked rise in antibody characteristic of an anamnestic response and persistence of high antibody levels.
Conclusions
Study identification: IMPAACT P1085 (V501-021). CLINICALTRIALS.GOV identifier: NCT01206556.
Keywords: Pediatric HIV infection, Human papillomavirus vaccine, Antibody response
1. Introduction
High-risk types of human papillomavirus (HPV) are associated with anogenital precancers and cancers, as well as with specific types of oropharyngeal cancers [1,2]. In addition, low-risk HPV types 6 and11 cause genital warts and are responsible for a significant portion of abnormal cervical cytology [3]. These complications of HPV infection are more common and persistent in patients with human immunodeficiency virus (HIV) infection [4,5]. Partial reconstitution of immune function may mitigate the severe consequences of HPV infection in HPV/HIV dually-infected patients.
Although there is evidence that cervical cancer rates have decreased with combination antiretroviral therapy (cART) in developed countries, worldwide cervical cancer remains elevated in HIV-infected women [6–9]. The data for anal cancer show that rates have leveled off, and in some cases increased in men and women despite the dramatic decrease compared to other AIDS-associated cancers, such as Kaposi Sarcoma [10]. Moreover, despite availability of cART, a large proportion of HIV-infected patients fail to adhere to, or respond to therapy, and fail to adequately reconstitute immune function [11]. The licensed HPV vaccines show effi-cacy against vaccine type HPV infection and their associated morbidities in HIV-uninfected people [12]. This prevents genital warts and cervical cytological abnormalities in women, and prevents penile and anal lesions in men, including genital warts and intraepithelial neoplastic lesions [13]. These vaccines induce persistent high titers of antibody against the vaccine strains of HPV, and several lines of evidence suggest that HPV type-specific antibodies are responsible for efficacy of the vaccine [14,15]. Efficacy after vaccination has been established thus far for 8–10 years [16]. During this interval levels of HPV-specific antibody decline, but remain above levels characteristic of protection after natural infection [17–19]. Quadrivalent HPV vaccine (QHPV) is safe and immunogenic in HIV-infected adults and children, but the duration of antibody persistence is unknown [20–23]. The magnitude of these responses is diminished compared to uninfected individuals, particularly in patients with a low number of CD4+ T cells and high plasma HIV viral load. This is consistent with observations that antibody responses to other vaccine immunogens are decreased in HIV-infected patients [24–26]. There are no efficacy studies of HPV vaccines in HIV-infected patients.
Between 2010 and 2013 we conducted a study in HIV-infected children, ages 7–11 years, to determine the magnitude and duration of immune responses to 3 or 4 doses of the QHPV (NCT01206556) [27]. The outcome measure was primarily type-specific serum antibody, but also included cell-mediated immunity (CMI). The current report describes the persistence of HPV type-specific antibody and CMI in these children during an interval of more than 4 years after either 3 or 4 doses of QHPV.
2. Methods
2.1. Subject population
The original protocol for QHPV administration (P1047) was begun in 2010 [27], at which time children 7 to <12 years of age with HIV infection were enrolled if their baseline CD4% was ≥15 and they had been receiving cART for ≥3 months. Exclusion criteria prior to administration of any dose of study vaccine/placebo included other diseases or medications causing immune suppression; prior diagnosis of sexually transmitted infections, including genital warts; and previous treatment with any blood-derived product within the six months prior to the first injection or any planned during the study. The current study added exclusion of an additional dose of an HPV vaccine other than that administered for the P1047 study. The study was approved by the Ethical Review Committee of each participating site and the parents or guardians of all subjects provided written informed consent; assent was obtained from subjects at sites where this was standard of care.
2.2. Vaccine
Quadrivalent HPV (Types 6, 11, 16, 18) recombinant vaccine (Gardasil) (QHPV) or identical placebo, 0.5 ml, was administered by intramuscular injection as per the package insert.
2.3. Design
Participants were stratified by their CD4% nadir and CD4% at the time of screening into three groups: Group 1: CD4% Nadir < 15 and CD4% ≥ 15 at screening; Group 2: CD4% Nadir ≥ 15 and CD4% ≥ 15 and <25 at screening; and Group 3: CD4% Nadir ≥ 25 and CD4% ≥ 25 at screening. Nadir was defined as the lowest CD4% ever recorded during the subject’s lifetime. The administration of QHPV or identical placebo was assigned randomly, in a double-blinded fashion, in a 3:1 ratio, to 40 subjects in each group at entry (90 to receive QHPV and 30 to receive placebo). Each subject received the same assigned study vaccine 8 and 24 weeks later.
At week 96 subjects who received the QHPV 3-dose standard regimen received a fourth dose of QHPV, while the previous placebo recipients began the QHPV 3-dose standard regimen. All subjects in the current protocol (P1085) were enrolled between 1 and 2 years of their last QHPV vaccination. Interval blood samples for HPV-specific immune responses and HIV status were obtained at approximately 2, 3.5, and 4–5 years after completion of the QHPV schedule for each arm of the P1047 study.
The study was closed early because of funding considerations and the last visit was completed early for some participants. Since Arm B finished vaccination 6 months later than Arm A, Arm B participants were less likely to complete a full 5 years of post-vaccination follow-up. There was a larger decline in sample size over time for the Arm B compared to Arm A [8/22 (36%) vs 15/73 (20%)].
2.4. Clinical laboratory
CD4 number and percent
Analysis of CD4 lymphocyte phenotypes (CD3/4, CD3/8, and CD19) was performed at clinical laboratories at the participating sites that were certified by the Division of AIDS Immunology Quality Assurance Program.
HIV RNA in plasma
The plasma HIV RNA concentration was determined by either the standard or ultrasensitive AMPLICOR HIV-1 MONITOR Test (version 1.5 RNA PCR assay; Roche Diagnostics Corporation).
2.5. HPV-specific antibody assessment
Multiplex Competitive Luminex immunoassay (cLIA)
cLIA was designed to simultaneously quantify antibodies to single neutralizing epitopes on each of four separately manufactured virus like particles (VLPs), one each for HPV 6/11/16/18 [28,29]. Serum anti-HPV 6, 11, 16 and 18 antibody levels were determined in a competitive format [18]. Yeast-derived VLPs identical to those used in the QHPV vaccine were carbodiimide-coupled to a set of four distinct fluorescent Luminex microspheres. Type-specific, phycoerythrin-labeled, neutralizing monoclonal antibodies compete with subject’s serum antibodies for binding to conformationally sensitive, type-specific, neutralizing epitopes on the coupled HPV VLPs. Seropositivity for HPV was defined as having anti-HPV titers ≥20, 16, 20 and 24 mMU/mL, respectively for HPV types 6, 11, 16 and 18. The seropositivity cutoffs were assessed using a panel of sera from subjects highly likely to be HPV-naïve (children) and from subjects who were highly likely to be HPV seropositive [28]. Any sample with a value less than the cutoff was considered serostatus negative. Samples with values equal to or greater than the cutoff were considered serostatus positive.
Total IgG Luminex immunoassay (LIA)
LIA measures a broader set of the antibodies to HPV VLPs types 6, 11, 16, and 18, but does not distinguish between neutralizing and non-neutralizing antibodies [30]. Yeast-derived VLPs are coupled to a set of four distinct fluorescent Luminex microspheres. Antibody concentrations are determined in a 96-well multiplexed, direct binding format by measuring the amount of VLP-specific IgG bound to VLP-microspheres. Following incubation with human serum, fluores-cent signal from an anti-human IgG detection antibody that binds to each serum IgG subclass (1–4) is directly measured on the Bio-Plex (Luminex) instrument [31]. The fluorescent signal from the IgG bound fluorescent detection antibody is proportional to the anti-VLP IgG antibody level. The high, medium, low, and negative controls were collected from humans who were either HPV-seronegative, had low antibody concentrations from natural infection or had medium-to-high antibody concentrations to the HPV types following vaccination. Results are reported as concentration of antibody in mMU/mL.
2.6. Statistics
Baseline characteristics were summarized and compared among the two treatment arms, using Wilcoxon rank sum tests for continuous variables and Fisher’s exact test for categorical variables. To summarize the HPV antibody levels (measured by cLIA and LIA) at each study visit, geometric mean titers (GMTs) and 95% confidence intervals (95% confidence intervals [CIs]) were calculated. The Wilcoxon non-parametric test was used to compare the antibody levels at each study visit between the two treatment arms.
HPV antibody levels from this study (GMTs and 95% CIs) at study visit 4–5 years after completion of 3-dose QHPV (Arm B) were also compared with published levels of QHPV-induced antibody levels from age-similar HIV uninfected children at month 60 after administration of a 3-dose regimen of QHPV vaccine [32].
To compare the change over the study interval in HPV antibody levels between the two treatment arms, longitudinal scatter plots, with estimated mean and 95% CIs from mixed effects longitudinal data analyses were constructed. The HPV antibody titers were log-transformed to better satisfy the assumptions in the mixed effects models.
Spearman’s rank order correlation coefficients were calculated to examine the strength and statistical significance of associations between HPV antibody levels and other continuous variables. Wilcoxon rank sum test or Kruskal-Wallis test were used to assess the associations between HPV antibody levels and categorical variables.
3. Results
In the published QHPV vaccine trial (P1047) 84 of 96 HIV-infected children received a fourth dose of QHPV 96 weeks after receiving the recommended 3 doses of QHPV (Fig. 1; Arm A). At the same time 27 of the 30 original placebo recipients in that trial were begun on the recommended 3-dose QHPV schedule (Fig. 1; Arm B). The current study (P1085) assessed the immune response in these two cohorts at approximately 2, 3.5, and 4–5 years after final vaccinations were completed. The disposition of study participants is outlined in Fig. 1. The two arms were very similar, with respect to their age, CD4%, and viral load at vaccination, but Arm B had significantly fewer participants receiving cART (Table 1), although the HIV clinical status in terms of CD4% and HIV viral load was similar in both arms. CD4 count and HIV viral load did not change significantly over the course of the study follow-up.
Fig. 1.
Consort diagram. 1Brackets [n] = number in indicated category. 2The interval was calculated after the first dose in each arm. Thus, the fourth dose in Arm A was administered 96 weeks after entering the study. 3Three subjects could not be contacted for P1085; 11 subjects refused to continue into P1085.
Table 1.
Demographic characteristics of HPV vaccine recipients.a
| Category | Treatment Arm
|
||
|---|---|---|---|
| A (Nb = 74) | B (N = 23) | P-value | |
| Age at vaccination | |||
| Median (years) | 12 | 12 | 0.426c |
| ≥12 years | 43 (58%) | 13 (57%) | 1.000d |
| Race/ethnicitye | |||
| White (non-Hispanic) | 5 (7%) | 2 (9%) | |
| Black (non-Hispanic) | 37 (50%) | 6 (27%) | 0.097d |
| Hispanic | 31 (42%) | 12 (55%) | |
| Gender (M/F) | 32/42 (57%) | 10/13 (57%) | 1.000d |
| CD4 count (cells/μL) | |||
| Median | 710 | 749 | 0.435c |
| <200 | 1 (1%) | 0 (0%) | 0.835c |
| 200–1000 | 56 (76%) | 17 (74%) | |
| >750 | 17 (23%) | 6 (26%) | |
| CD4% | |||
| Median | 35 | 36 | 0.699c |
| HIV RNA (copies/ml) | |||
| Median | 98 | 85 | 0.472d |
| <400 | 9 (12%) | 4 (17%) | 0.450d |
| 400–5000 | 54 (73%) | 16 (70%) | |
| ≥5000 | 11 (15 %) | 3 (13%) | |
| Antiretroviral (ARV) | |||
| No ARV | 1 (1%) | 2 (9%) | 0.006d |
| 1 class of ARV only | 2 (3%) | 4 (17%) | |
| ≥2 classes of ARVs | 71 (96%) | 17 (74%) | |
Demographics at time of enrollment in the current long-term follow-up study.
N = number of subjects in arms.
Wilcoxon test.
Fisher’s Exact test.
Information unknown for one subject in arm B.
In the current study the recipients of the standard 3-dose schedule (Table 2; Arm B), the seropositivity for HPV 6, 11, or 16 cLIA antibody for the 14 participants tested at 4–5 years after the last vaccine dose was 86–93%. This compares to a 97–100% seropositivity, irrespective of HPV type, that was achieved in HIV-infected children at 1 month after vaccination in the previously published data (from P1047), which described the first 3 doses of vaccine in the participants of Arm A of this study [27]. Geometric mean antibody levels in Arm B at 4–5 years after vaccination were 50–70% lower than the levels for HPV types 6, 11, or 16 at one month after vaccination as previously published [27]. For HPV 18 the seroresponse rate at 4–5 years was 68% lower and the geometric mean titer was reduced by 89% from the one month post-vaccine geometric mean titer. Table 3 compares the 14 HIV-infected subjects at 4–5 years after receiving 3 doses of QHPV (Arm B) in our study with published 5-year data in 240–250 similarly aged uninfected children after QHPV vaccination [32]. Within the limitations of the small sample of HIV-infected children, antibody responses to the HPV genotypes were similar, as assessed by either of the serum assays utilized. The major exception was HPV 11, where LIA was markedly higher for the uninfected vaccinees.
Table 2.
Magnitude and persistence of HPV antibody after 3 or 4 doses of quadrivalent HPV vaccine.
| Interval | Treatment arm
|
P valuea A vs B
|
|||||
|---|---|---|---|---|---|---|---|
| A
|
B
|
cLIA | LIA | ||||
| cLIA | LIA | cLIA | LIA | ||||
| HPV6 | |||||||
| 2.0 years | Nb | 73 | 71 | 22 | 22 | ||
| GM (CI)c | 382 (272;535) | 458 (306;686) | 112 (70;179) | 70 (39;127) | <0.001 | <0.001 | |
| Positive | 71 (97%) | 69 (97%) | 20 (91%) | 19 (86%) | 0.23 | 0.084 | |
| 3.5 years | N | 69 | 67 | 21 | 21 | ||
| GM (CI) | 379 (277;518) | 362 (236;556) | 83 (47;144) | 53 (28;103) | <0.001 | <0.001 | |
| Positive | 68 (99%) | 65 (97%) | 17 (81%) | 17 (81%) | 0.01 | 0.027 | |
| 4–5 years | N | 58 | 58 | 14 | 14 | ||
| GM (CI) | 300 (202;445) | 292 (184;465) | 104 (52;206) | 62 (28;138) | 0.010 | 0.003 | |
| Positive | 55 (95%) | 55 (95%) | 13 (93%) | 12 (86%) | 1.00 | 0.248 | |
| Interval | Treatment arm
|
P value A vs B
|
|||||
| A
|
B
|
cLIA | LIA | ||||
| cLIA | LIA | cLIA | LIA | ||||
|
| |||||||
| HPV11 | |||||||
| 2 years | N | 73 | 71 | 22 | 22 | ||
| GM (CI) | 650 (471;897) | 374 (254;551) | 109 (68;173) | 60 (37;100) | <0.001 | <0.001 | |
| Positive | 71 (97%) | 68 (96%) | 21 (95%) | 19 (86%) | 0.55 | 0.0142 | |
| 3.5 years | N | 69 | 67 | 21 | 21 | ||
| GM (CI) | 545 (389;765) | 300 (196;460) | 78 (42;144) | 50 (28;88) | <0.001 | <0.001 | |
| Positive | 68 (99%) | 64 (96%) | 17 (81%) | 17 (81%) | 0.01 | 0.053 | |
| 4–5 years | N | 58 | 58 | 14 | 14 | ||
| GM (CI) | 408 (282;588) | 242 (155;378) | 82 (42;158) | 48 (22;104) | <0.001 | 0.001 | |
| Positive | 57 (98%) | 56 (97%) | 12 (86%) | 10 (71%) | 0.095 | 0.011 | |
| Interval | Treatment arm
|
P valuea A vs B
|
|||||
| A
|
B
|
cLIA | LIA | ||||
| cLIA | LIA | cLIA | LIA | ||||
|
| |||||||
| HPV16 | |||||||
| 2 years | N | 73 | 71 | 22 | 22 | ||
| GM (CI)b | 1922 (1331;2774) | 1967 (1303;2969) | 376 (165;855) | 357 (161;790) | <0.001 | <0.001 | |
| Positive | 72 (99%) | 71 (100%) | 20 (91%) | 22 (100%) | 0.13 | 1.000 | |
| 3.5 years | N | 69 | 67 | 21 | 21 | ||
| GM (CI) | 1798 (1231;2626) | 1622 (1042;2525) | 277 (109;709) | 306 (139;671) | <0.001 | <0.001 | |
| Positive | 68 (99%) | 67 (100%) | 18 (86%) | 21 (100%) | 0.04 | 1.000 | |
| 4–5 years | N | 58 | 58 | 14 | 14 | ||
| GM (CI) | 1206 (780;1863) | 1117 (699;1786) | 310 (83;1160) | 321 (99;1038) | 0.035 | 0.052 | |
| Positive | 57 (98%) | 58 (100%) | 12 (86%) | 14 (100%) | 0.095 | 1.000 | |
| Interval | Treatment arm
|
P valuea A vs B
|
|||||
| A
|
B
|
cLIA | LIA | ||||
| cLIA | LIA | cLIA | LIA | ||||
|
| |||||||
| HPV18 | |||||||
| 2 years | N | 73 | 70 | 22 | 22 | ||
| GM (CI)b | 198 (121;322) | 230 (143;368) | 39 (18;84) | 45 (22;91) | <0.001 | <0.001 | |
| Positive | 59 (81%) | 64 (91%) | 12 (55%) | 16 (73%) | 0.023 | 0.034 | |
| 3.5 years | N | 69 | 67 | 21 | 21 | ||
| GM (CI) | 159 (98;259) | 189 (114;312) | 35 (17;72) | 44 (23;86) | 0.002 | 0.003 | |
| Positive | 53 (77%) | 60 (90%) | 11 (52%) | 17 (81%) | 0.052 | 0.286 | |
| 4–5 years | N | 58 | 58 | 14 | 14 | ||
| GM (CI) | 108 (64;183) | 130 (78;217) | 42 (15;117) | 49 (19;130) | 0.060 | 0.074 | |
| Positive | 43 (74%) | 51 (88%) | 9 (64%) | 10 (71%) | 0.513 | 0.207 | |
Wilcoxon test for geometric mean comparisons; Fisher’s exact for qualitative (positive) tests.
Two subjects each at 3.5 years and 4–5 years were not tested at 2 years.
Geometric mean (95% confidence interval).
Table 3.
HPV antibody In HIV-infected and uninfected children at 4–5 years after administration of three doses of quadrivalent HPV vaccine.
| HPV Type | HIV-Infected
|
HIV-Uninfecteda
|
P-value (Infected VS Uninfected)
|
||||
|---|---|---|---|---|---|---|---|
| cLIA (n = 14) | LIA (n = 14) | cLIA (n = 239–241) | LIA (n = 252–254) | cLIA | LIA | ||
| 6 | GM (CI) | 104 (52,206) | 62 (28,103) | 126 110,144) | 127 (111,145) | 0.5652 | 0.0765 |
| Positive | 93% | 86% | 94% | 96% | 0.5834 | 0.1247 | |
| 11 | GM (CI) | 82 (42,158) | 48 (22,104) | 141 (122,163) | 144 (125,167)b | 0.1043 | 0.0095 |
| Positive | 86% | 71% | 97% | 98%c | 0.0814 | 0.0005 | |
| 16 | GM (CI) | 310 (83,1160) | 321 (99,1038) | 534 (457,6230) | 548 (471,639) | 0.6977 | 0.3470 |
| Positiveb | 86% | 100% | 99%c | 99% | 0.0159 | 1.0000 | |
| 18 | GM (CI) | 42 (15,117) | 49 (19,130) | 80 (66,98) | 83 (69,101) | 0.2038 | 0.2747 |
| Positive | 64% | 71% | 77% | 87% | 0.3295 | 0.1120 | |
Uninfected comparator group extracted from reference #32. Ferris D, Samakoses R, Block SL; Pediatrics 2014; 134: e657-65.
HPV 16 cLIA seropositive % greater for HIV-uninfected (p = 0.016, Fisher’s exact test).
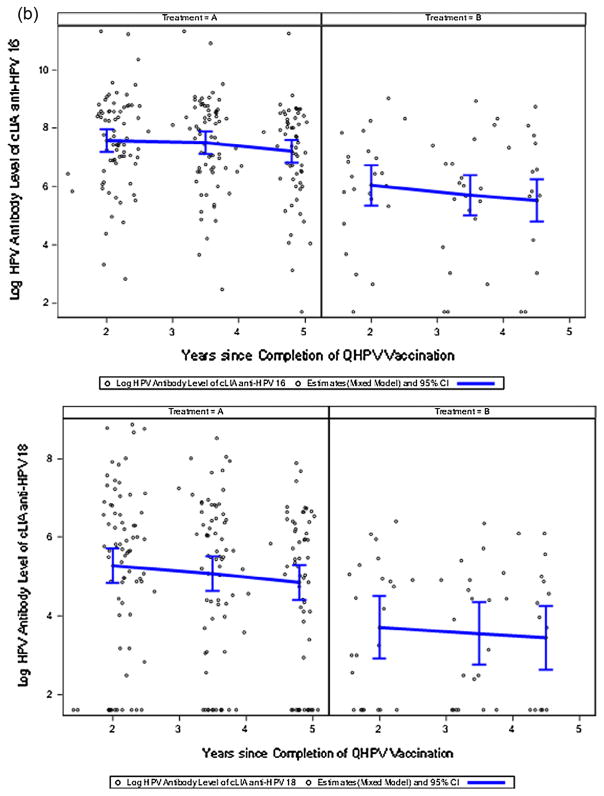
Antibody titers measured in the 4-dose recipients (Table 2; Arm A) were significantly higher (~8–10-fold for cLIA; ~2.5–8.5-fold for LIA) at all time points than those measured in the 3-dose recipients (Table 2; Arm B) throughout the duration of the study. cLIA antibody titers in 4-dose recipients fell ~30% during the course of the study. At the end of the study >95% of vaccinees who received 4 doses of QHPV were seropositive in the cLIA assay for HPV types 6, 11, and 16 antibodies; 75% were seropositive for HPV type 18. Seropositivity rates determined by LIA were similar in direction and non-significantly lower in magnitude. Figs. 2a and 2b show the distribution of log-transformed cLIA antibody titers for the two arms. Similar information on LIA antibody responses are provided in Supplemental Fig. 1. cLIA and LIA antibody titers were highly positively correlated throughout the study (p < 0.001).
Fig. 2.
Fig. 2a. Log10 cLIA anti-HPV 6,11 Antibody Titers (mMU/mL) Over Time.
Fig. 2b. Log10 cLIA anti-HPV 16, 18 Antibody Titers (mMU/mL) Over Time.
A strong negative correlation was demonstrated between antibody titer and high log RNA and high CD8%. Antibody titer was positively correlated with high CD4%, but not with age, gender, or race. This was true for all time points and for each type-specific antibody in Arm A, which had a substantial sample size (Supplemental Tables 1–4).
4. Discussion
In this study of HIV-infected children, antibodies to HPV 6, 11, 16, and 18 remained readily detectable over 4–5 years in children receiving either 3 or 4 vaccine doses. Efficacy trials of HPV vaccines have not been undertaken in HIV-infected patients, but since protection induced by HPV vaccines has been attributed to the presence of HPV-specific antibody - either in serum, the genital tract, or both [14,15], these antibodies have been evaluated in HIV-infected adults after HPV vaccination [33,34]. However, studies of the magnitude and persistence of vaccine-induced HPV antibodies in HIV-infected patients have thus far been limited to young or older adults, and the longest observation period was 48 weeks.
We measured antibody responses to HPV 6, 11, 16 and 18 in HIV-infected children up to 5 years after the administration of the last dose of QHPV. Not surprisingly, we observed that the 4-dose group had significantly higher geometric mean antibody titers at all study time points. However, the proportion of vaccinees with antibody titers indicating a seroresponse in the cLIA assay was not different between the two groups. The proportions of seropositives at 4–5 years after 3 doses of QHPV were 86–93% for HPV types 6, 11, and 16 and 64% for HPV type 18. These results were only marginally lower than the seroresponse rates in these children at 1 month after completing vaccination [27]. Furthermore, the seroresponse rate in the 3-dose group was only modestly reduced compared to results obtained in similar age children without HIV infection who received three doses [32]. It is thought that seroresponse rate is more indicative of protection than antibody titer.
The geometric mean antibody concentrations at 2 years post-vaccination in this report were comparable or higher than the levels observed in an HIV-uninfected adolescent population several years older [32]. These levels declined in the HIV-infected children by 50–70% between 2 and 4–5 years post-vaccination for HPV types 6, 11, or 16, and by 89% for HPV type 18, which was also comparable to the decline observed in uninfected children [32]. As in previous reports, the trends were comparable for either cLIA or LIA antibody determinations, and were closely correlated [35].
The significance of the antibody decline is unclear. Loss of detectable antibody, once achieved, may not necessarily indicate loss of protection. For example, in a previous study of HIV-uninfected women in which declining seropositivity was greater for HPV-18 compared to other types, there were no breakthrough cases of HPV-18–related cervical intraepithelial neoplasia in QHPV recipients among 35 women who were no longer seroresponders [36]. Additionally, an experimental murine model demonstrated protection at picomolar antibody concentrations, which are below the level of detectability with the current serologic assays [37]. While this is reassuring, it is not known if the predictive value of an initial antibody response in an HIV-uninfected host, as stated above, is applicable to HIV-infected children who receive QHPV, considering that in some of these children vaccine responses protective against other pathogens are not maintained [25,38]. The duration of protection against HPV in children vaccinated at an early age will need to persist for an extended period, since protection must last at least for two decades when risk for acquiring HPV is highest because of repeated sexual exposures.
It is because of the uncertainty of the long-term protection in HIV-infected children who receive QHPV vaccines that we determined the magnitude and persistence of HPV-specific antibody after administration of a fourth dose of QHPV. This fourth dose, administered in the parent study (P1047), was generally safe and also stimulated a brisk anamnestic response at 7 days after vaccination [39]. The geometric mean titers measured by cLIA and LIA methods were significantly higher at 4–5 years after the fourth dose of QHPV for all serotypes compared to HIV-infected vaccinees and uninfected historical controls administered 3 doses; >95% of vaccinees were seropositive in the cLIA assay for HPV types 6, 11, and 16 antibodies; 75% were seropositive for HPV type 18. The higher titers against HPV 6, 11, 16 and 18 at 4–5 years after the last dose of vaccine in the 4-dose group compared with the 3-dose groups reflected the higher titers at 1 month after the fourth dose of vaccine [27]. Although these antibody titers were higher in the 4-dose group, the rate of decline was similar to that in the 3-dose group.
Any potential protective value of a fourth dose of QHPV is highly theoretical, given the uncertain significance of loss of previously detectable antibody. This may eventually be determined by evaluating HPV infection or disease in a large clinic population or by long-term effectiveness studies based on managed care databases. Another important consideration in predicting long-term efficacy is the observation that in Arm A, where the sample size was substantial, at all time points and for each HPV-specific antibody there was a strong correlation between antibody titer and low log RNA and CD8%, and high CD4% (Supplemental Table 1). Thus the results presented on persistence of antibody should be viewed in the context of the HIV cohort we studied, which had a median CD4% of 35 and a median viral load of <5000 copies/ml. Untreated patients and those failing therapy may not be as responsive and duration of response could be shorter. On the other hand, it is likely that in an ideal situation where all vaccinees were on cART and had an undetectable viral load, the titer and duration of antibody would have been greater.
The limitations of this study include the relatively small sample size, especially for the 3-dose recipients, relatively high attrition rate in both arms, and the use of historical data for the uninfected comparator cohort. The spectrum of HIV-infected children investigated in this trial may not be representative of typical clinic populations.
In conclusion, children with well-controlled HIV infection who receive 3 doses of the QHPV vaccine maintain seropositivity and antibody levels that are generally similar to children of the same age who are not HIV-infected. A fourth dose of vaccine in these children produces a marked rise in antibody characteristic of an anamnestic response and results in persistence of high antibody levels over 5 years.
Supplementary Material
Acknowledgments
Funding source & sponsors’ role
Funding for this research was provided by Merck & Co., Inc., Kenilworth, NJ, USA, (sponsor) in conjunction with the International Maternal, Pediatric, and Adolescent AIDS Clinical Trials Network (IMPAACT) of the National Institute of Allergy and Infectious Diseases, NIH; National Institute of Mental Health, NIH; and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH.
Although the sponsor formally reviewed a penultimate draft, the opinions expressed are those of the authors and may not necessarily reflect those of the sponsor, IMPAACT, or NIH. All coauthors approved the final version of the manuscript.
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
We gratefully acknowledge the contributions of the site investigators and site staff who conducted the P1085 study:
NJ Med School CRS: Arry Dieudonne, MD, Linda Bettica, RN, BSN, James M. Oleske, MD, MPH; Howard Univ. Washington DC NICHD CRS: Patricia Houston, MD, MS; Strong Memorial Hospital, University of Rochester: Geoffrey A. Weinberg, MD, Barbara Mur-ante, RNC, PNP; Rush University Cook County Hospital Chicago NICHD CRS: James B. McAuley, MD, MPH, Maureen McNichols, RN, CCRC, Kenneth Boyer, MD; University of California San Francisco NICHD CRS: Diane Wara, MD, Ted Ruel, MD, Mica Muscat, PNP; Miller Children’s Hospital Long Beach CA: Audra Deveikis, MD, Jagmohan S. Batra, MD, David Michalik, DO, Tempe Chen, MD.
IMPAACT P1085 Study Team: Myron Levin, MD, Protocol Chair; Edward Handelsman1, MD, NIAID Medical Officer; Jennifer Read, MD, NICHD Medical Officer; Elizabeth Petzold, PhD, Clinical Trials Specialist; Lin-Ye Song, PhD, Protocol Statistician; Barbara Nowak, BA, Protocol Data Manager; Nagamah Deygoo, Protocol Field Representative; William Meyer, PhD, Protocol Virologist; Adriana Weinberg, MD, Protocol Immunologist; Carrie Fry, BS, Laboratory Data Coordinator.
Merck & Co., Inc. provided serology support. In addition, Jon Stek (Merck & Co., Inc.) assisted with the manuscript.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2017.02.021.
Footnotes
Deceased author.
Financial disclosures
No author was paid for their work on this manuscript.
Conflicts of interest
M.J. Levin (chair) and A. Weinberg (immunologist) were members of the core protocol team supported by research grants from the sponsor and M.J. Levin is a consultant to the sponsor.
A Saah is an employee of the sponsor and may hold stock and/or stock options from the sponsor.
References
- 1.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235–42. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch FX, Broker TR, Forman D, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(Suppl 7):H1–H31. doi: 10.1016/j.vaccine.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clifford G, Franceschi S, Diaz M, Munoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24(Suppl 3):S26–34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol, Biomarkers Prevention. 2011;20(12):2551–9. doi: 10.1158/1055-9965.EPI-11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10(12):1152–9. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 6.Del Mistro A, Bertorelle R, Franzetti M, et al. Antiretroviral therapy and the clinical evolution of human papillomavirus-associated genital lesions in HIV-positive women. Clin Infect Dis. 2004;38(5):737–42. doi: 10.1086/381681. [DOI] [PubMed] [Google Scholar]
- 7.Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681–90. doi: 10.1086/650467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler DH, Kakinami L, Modisenyane T, et al. Increased regression and decreased incidence of human papillomavirus-related cervical lesions among HIV-infected women on HAART. Aids. 2012;26(13):1645–52. doi: 10.1097/QAD.0b013e32835536a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hleyhel M, Belot A, Bouvier AM, et al. Risk of AIDS-defining cancers among HIV-1-infected patients in France between 1992 and 2009: results from the FHDH-ANRS CO4 cohort. Clin Infect Dis. 2013;57(11):1638–47. doi: 10.1093/cid/cit497. [DOI] [PubMed] [Google Scholar]
- 10.Simard EP, Pfeiffer RM, Engels EA. Cumulative incidence of cancer among individuals with acquired immunodeficiency syndrome in the United States. Cancer. 2011;117(5):1089–96. doi: 10.1002/cncr.25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. [accessed 11/02/15 2015];Linkage to and Retention in HIV Medical Care. 2015 < http://www.cdc.gov/hiv/prevention/programs/pwp/linkage.html>.
- 12.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63(Rr-05):1–30. [PubMed] [Google Scholar]
- 13.Merck; Corp. MSD, editor. Gardasil Package Insert. 2015. p. 28. [Google Scholar]
- 14.Suzich JA, Ghim SJ, Palmer-Hill FJ, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;92(25):11553–7. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breitburd F, Kirnbauer R, Hubbert NL, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69(6):3959–63. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kjear SK, Nygard M, Dillner J, Munk C, Marshall B, Hansen BT, et al. Long-term effectiveness and safety of Gardasil in the Nordic countries. Sevilla, Spain: European Research Organisation on Genital Infection and Neoplasia;; 2015. Feb 4–7, [Google Scholar]
- 17.Giacomet V, Penagini F, Trabattoni D, et al. Safety and immunogenicity of a quadrivalent human papillomavirus vaccine in HIV-infected and HIV-negative adolescents and young adults. Vaccine. 2014;32(43):5657–61. doi: 10.1016/j.vaccine.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Nygard M, Saah A, Munk C, et al. Evaluation of the long-term anti-human papillomavirus 6 (HPV6), 11, 16, and 18 immune responses generated by the quadrivalent HPV vaccine. Clin Vaccine Immunol. 2015;22(8):943–8. doi: 10.1128/CVI.00133-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz T, Spaczynski M, Kaufmann A, et al. Persistence of immune responses to the HPV-16/18 AS04-adjuvanted vaccine in women aged 15–55 years and first-time modelling of antibody responses in mature women: results from an open-label 6-year follow-up study. BJOG: Int J Obstetrics Gynaecol. 2015;122(1):107–18. doi: 10.1111/1471-0528.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn JA, Xu J, Kapogiannis BG, et al. Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women. Clin Infect Dis. 2013;57(5):735–44. doi: 10.1093/cid/cit319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toft L, Storgaard M, Muller M, et al. Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV-infected adults: a randomized, double-blind clinical trial. J Infect Dis. 2014;209(8):1165–73. doi: 10.1093/infdis/jit657. [DOI] [PubMed] [Google Scholar]
- 22.Toft L, Tolstrup M, Storgaard M, Ostergaard L, Sogaard OS. Vaccination against oncogenic human papillomavirus infection in HIV-infected populations: review of current status and future perspectives. Sexual Health. 2014;11(6):511–23. doi: 10.1071/SH14015. [DOI] [PubMed] [Google Scholar]
- 23.Kojic EM, Kang M, Cespedes MS, et al. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerneis S, Launay O, Turbelin C, Batteux F, Hanslik T, Boelle PY. Long-term immune responses to vaccination in HIV-infected patients: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(8):1130–9. doi: 10.1093/cid/cit937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abzug MJ, Qin M, Levin MJ, et al. Immunogenicity, immunologic memory, and safety following measles revaccination in HIV-infected children receiving highly active antiretroviral therapy. J Infect Dis. 2012;206(4):512–22. doi: 10.1093/infdis/jis386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siberry GK, Patel K, Bellini WJ, et al. Immunity to measles, mumps, and rubella in US children with perinatal HIV infection or perinatal HIV exposure without infection. Clin Infect Dis. 2015;61(6):988–95. doi: 10.1093/cid/civ440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levin MJ, Moscicki AB, Song LY, et al. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr. 2010;55(2):197–204. doi: 10.1097/QAI.0b013e3181de8d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dias D, Van Doren J, Schlottmann S, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol. 2005;12(8):959–69. doi: 10.1128/CDLI.12.8.959-969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opalka D, Lachman CE, MacMullen SA, et al. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol. 2003;10(1):108–15. doi: 10.1128/CDLI.10.1.108-115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opalka D, Matys K, Bojczuk P, et al. Multiplexed serologic assay for nine anogenital human papillomavirus types. Clin Vaccine Immunol. 2010;17(5):818–27. doi: 10.1128/CVI.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown DR, Garland SM, Ferris DG, et al. The humoral response to Gardasil over four years as defined by total IgG and competitive Luminex immunoassay. Hum Vaccin. 2011;7(2):230–8. doi: 10.4161/hv.7.2.13948. [DOI] [PubMed] [Google Scholar]
- 32.Ferris D, Samakoses R, Block SL, et al. Long-term study of a quadrivalent human papillomavirus vaccine. Pediatrics. 2014;134(3):e657–65. doi: 10.1542/peds.2013-4144. [DOI] [PubMed] [Google Scholar]
- 33.Kojic EM, Kang M, Cespedes MS, et al. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin Infect Dis. 2014;59(1):127–35. doi: 10.1093/cid/ciu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkin T, Lee JY, Lensing SY, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010;202(8):1246–53. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown D, Muller M, Sehr P, et al. Concordance assessment between a multiplexed competitive Luminex immunoassay, a multiplexed IgG Luminex immunoassay, and a pseudovirion-based neutralization assay for detection of human papillomaviruse types 16 and 18. Vaccine. 2014;32(44):5880–7. doi: 10.1016/j.vaccine.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–8. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 37.Longet S, Schiller JT, Bobst M, Jichlinski P, Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol. 2011;85(24):13253–9. doi: 10.1128/JVI.06093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutcliffe CG, Moss WJ. Do children infected with HIV receiving HAART need to be revaccinated? Lancet Infect Dis. 2010;10(9):630–42. doi: 10.1016/S1473-3099(10)70116-X. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg A, Song LY, Saah A, et al. Humoral, mucosal, and cell-mediated immunity against vaccine and nonvaccine genotypes after administration of quadrivalent human papillomavirus vaccine to HIV-infected children. J Infect Dis. 2012;206(8):1309–18. doi: 10.1093/infdis/jis489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.