Abstract
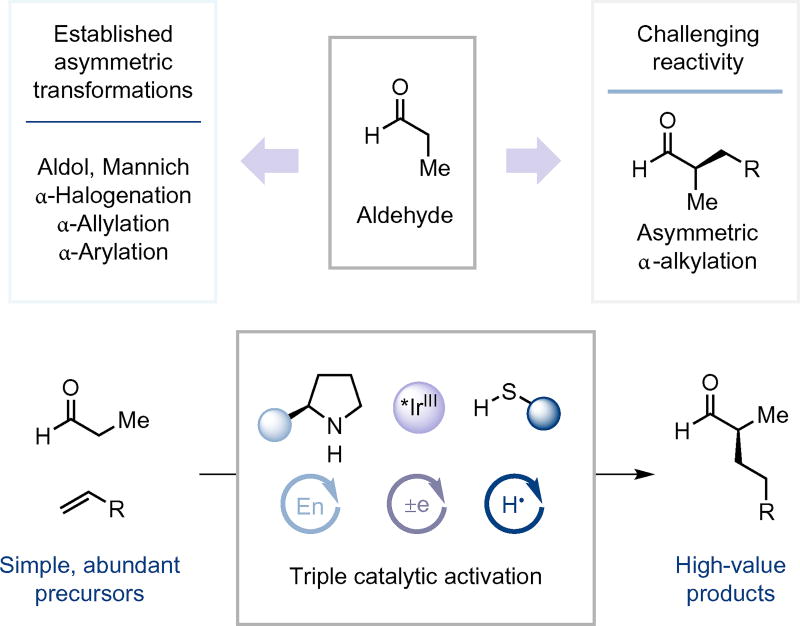
Although the α-alkylation of ketones has already been established, the analogous reaction using aldehyde substrates has proven surprisingly elusive. Despite the structural similarities between the two classes of compounds, the sensitivity and unique reactivity of the aldehyde functionality has typically required activated substrates or specialized additives. Here, we show that the synergistic merger of three catalytic processes—photoredox, enamine and hydrogen-atom transfer (HAT) catalysis—enables an enantioselective α-aldehyde alkylation reaction that employs simple olefins as coupling partners. Chiral imidazolidinones or prolinols, in combination with a thiophenol, iridium photoredox catalyst and visible light, have been successfully used in a triple catalytic process that is temporally sequenced to deliver a new hydrogen and electron-borrowing mechanism. This multicatalytic process enables both intra- and intermolecular aldehyde α-methylene coupling with olefins to construct both cyclic and acyclic products, respectively. With respect to atom and step-economy ideals, this stereoselective process allows the production of high-value molecules from feedstock chemicals in one step while consuming only photons.
The stereoselective α-alkylation of carbonyl compounds has long been a fundamental transformation within the field of organic chemistry. In the 1980s, the seminal research of Evans, Meyers, Oppolzer, Seebach and Myers1 demonstrated that chiral auxiliaries can offer a general approach to the enantioselective construction of α-carbonyl stereocentres2,3, which today remains the method of choice for enolate-based fragment coupling. In recent years a variety of novel catalytic methods have been developed for asymmetric α-carbonyl alkylation, including (1) phase transfer additions of glycine-derived imines, (2) chiral triamine ligation of ketone-derived lithium enolates, and (3) the use of Jacobsen’s Cr (salen) complex with pre-formed stannous enolates4–6. Given these important advances, the direct, enantioselective formation of α-alkyl carbonyls from simple, abundant organic building blocks remains an elusive yet important goal.
Over the last two decades, secondary amine-mediated organocatalysis has enabled the development of mild methods for the catalytic generation of reactive HOMO-raised enamine nucleophiles7–10. This activation platform has found broad utility in a variety of α-functionalization reactions, most notably the enantioselective formation of C–O, C–N, C–X and C–C bonds11. Despite these efforts, the α-alkylation of carbonyl compounds using alkyl halides remains an elusive transformation, mainly due to limitations involving competitive self-aldol reactions and/or catalyst alkylation12.
In 2007 we first demonstrated that open-shell, radical pathways can enable the enantioselective α-functionalization of carbonyl compounds via a SOMO activation pathway13. This process occurs via stoichiometric oxidation of a transiently formed enamine to generate a 3πe− enaminyl radical intermediate. This electrophilic SOMO species can then be intercepted by a variety of prefunctionalized olefins and, following an additional oxidation event, can lead to a number of α-functionalized adducts14,15. While the generality of this 3πe− enaminyl radical activation mode has been demonstrated, widescale adoption has been restricted due to (1) the requirement of two equivalents of a stoichiometric oxidant per bond formation and (2) the need for pre-generated π-nucleophilic olefin partners (for example, silyl ketene acetals, allyl silanes) that can engage the electrophilic 3πe− enaminyl radical.
Recently, we questioned whether photoredox catalysis and organocatalysis might be successfully merged to enable the enantioselective α-alkylation of carbonyl compounds using simple, unactivated olefin coupling partners and without the requirement of stoichiometric oxidants (Fig. 1). We recognized that the combination of both the SOMO and photoredox activation modes might enable hydrogen atom and electron borrowing to achieve enaminyl radical formation before coupling with an olefin substrate16. The generation of simple olefin adducts was deemed feasible, provided the initial enaminyl radical–olefin addition event could be rendered non-reversible. To this end, we rationalized that the use of a third catalytic cycle, involving a reductive hydrogenatom transfer, would capture the radical intermediate arising from the olefin addition step17–19, thereby ensuring that the overall mechanism is redox-neutral (that is, non-oxidative, with only photons being consumed in the process).
Figure 1. Direct enantioselective α-alkylation of aldehydes with olefins via triple catalytic activation.
Aldehydes are known to be efficient substrates for a number of fundamental carbonyl α-functionalization transformations for selective C−O, C−N, C−X and C−C bond formation. Despite this, the catalytic, asymmetric α-alkylation of aldehydes has remained a challenge. This transformation has now been accomplished through the merger of secondary amine organocatalysis, photoredox catalysis and HAT catalysis to produce α-alkyl carbonyl products from simple olefin and aldehyde substrates.
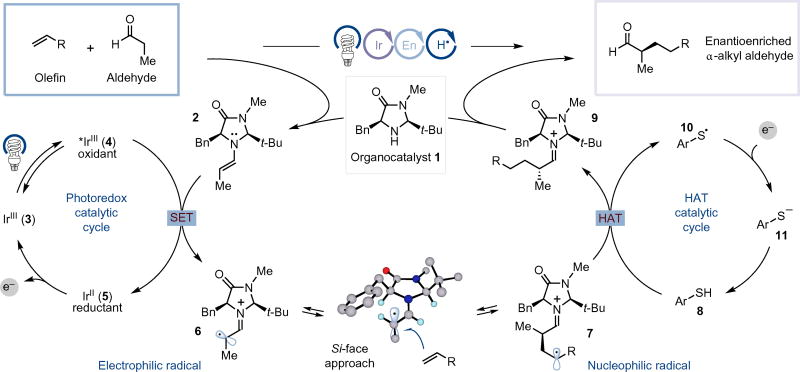
From a conceptual standpoint, we hypothesized that three catalytic cycles could be used in concert to enable this unique alkylation: (1) amine catalysis (= enamine formation), (2) photoredox catalysis (= enamine oxidation to generate an activated 3πe− enaminyl radical and reduction of a thiyl radical), and (3) HAT catalysis (= hydrogenatom transfer to the alkyl radical species after olefin addition) (Fig. 2). As such, a critical feature of this transformation would be the identification of three highly selective yet independent catalysts and their controlled interface. For example, a selective HAT catalyst would be required to successfully discriminate between trapping of the initial electrophilic 3πe− enaminyl radical intermediate and the alkyl radical species generated following olefin coupling. Second, given the reversible nature of the olefin addition event, the kinetic efficiency of the HAT catalyst would dictate the retention or loss of enantiocontrol gained in the SOMO-addition step. Finally, the redox activity of the three catalysts would be interdependent, and efficient regeneration of the ground-state photocatalyst and HAT catalyst would require a multicatalytic process that is temporally synchronized.
Figure 2. Proposed mechanism for aldehyde α-alkylation via photoredox, HAT and organocatalysis.
Condensation of organocatalyst 1 with an aldehyde substrate initiates the catalytic cycle. Concurrently, irradiation of iridium photocatalyst 3 generates an excited state species (4). This intermediate can then oxidize enamine 2 through single electron transfer (SET) and generate the 3πe− enaminyl radical 6. Reversible radical addition across an olefin substrate then produces the carbon radical 7, which is trapped through HAT with thiol HAT catalyst 8. Hydrolysis of iminium 9 provides the enantioenriched α-alkyl aldehyde and regenerates amine catalyst 1. Finally, reduction of thiyl radical 10 by the Ir(II) species (5) subsequently regenerates thiol catalyst 8 as well as the Ir(III) catalyst 3 to complete the remaining redox cycles.
Merging photoredox, organocatalysis and HAT catalysis
A detailed description of our proposed mechanism for the enantioselective α-alkylation of aldehydes is outlined in Fig. 2. We presumed that the process would begin with condensation of amine catalyst 1 and an aldehyde substrate to provide enamine 2. Concurrently, irradiation of the iridium photocatalyst 3 Ir(Fmppy)2(dtbbpy)PF6 (Fmppy = 2-(4-fluorophenyl)-4-(methylpyridine), dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine) with visible light would produce the long-lived (τ = 1.2 µs) excited-state complex 4 (ref. 20). This highly oxidizing charge transfer species ( versus saturated calomel electrode, SCE) would be capable of mediating a single-electron transfer (SET) from the electron-rich enamine 2 to generate reduced IrII complex 5 and the critical 3πe− enaminyl radical species 6. This electrophilic radical should then rapidly undergo addition to an olefin coupling partner to generate a new C–C bond, a stereogenic centre and a secondary alkyl radical. At this stage we hypothesized that this nucleophilic radical 7 would participate in a HAT event with a sufficiently acidic and weak S–H bond (HAT catalyst 8, thiophenol S–H BDE=78 kcalmol−1)21. This HAT process is governed both by the polarity match and relative bond strengths of the donor and acceptor species, and is expected to rapidly trap the alkyl radical species at near diffusion control22. Following HAT, hydrolysis of iminium ion 9 would liberate the organocatalyst while also providing an enantioenriched aldehyde product. Finally, thiyl radical 10 ( versus SCE in dimethylsulfoxide)23 engages in a SET event with the highly reducing IrII complex 5 ( versus SCE) to regenerate both the ground state IrIII complex 3 as well as the HAT thiol catalyst after protonation of thiolate 11.
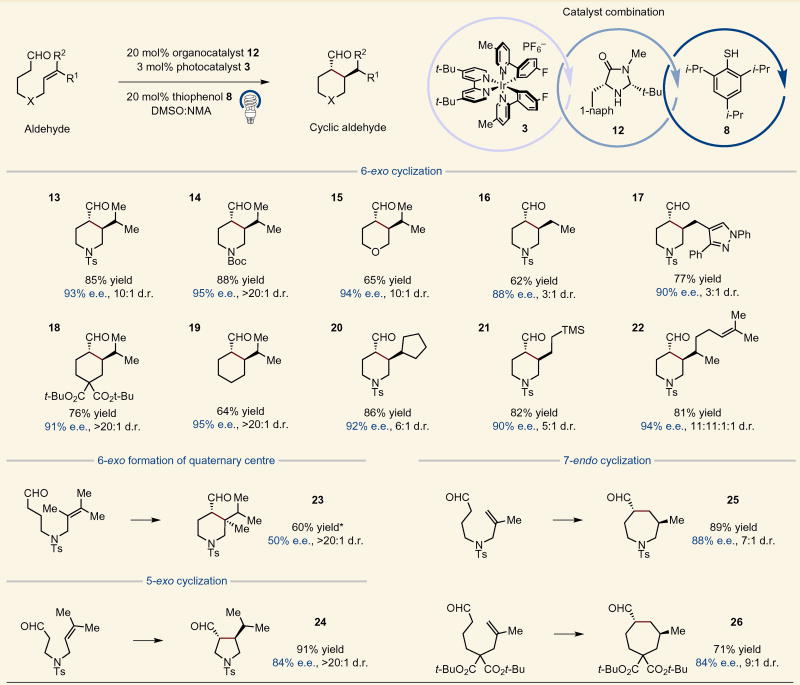
We first tested the proposed aldehyde alkylation on an intramolecular variant to determine if enantioselective ring formation was feasible using this mechanism. As shown in Table 1, heterocyclic or carbocyclic rings were readily generated with high efficiency and enantiocontrol using this tricatalytic method. For example, N-tethered aldehydic olefins with either sulfonamide or carbamate protecting groups are tolerated, giving rise to piperidine adducts in high yield (13 and 14, 85% yield, 93% e.e. and 88% yield, 95% e.e., respectively). Moreover, an ether-linked system provided the desired trans-disubstituted tetrahydropyran with useful efficiency (15, 65% yield, 94% e.e.). Carbocycles were also prepared in good yield via this protocol, as exemplified by the gem-diester-containing substrate 18 (76% yield, 91% e.e.). Notably, the reaction proceeded well using an unsubstituted alkyl tether to generate the disubstituted cyclohexane product (19, 64% yield, 95% e.e., >20:1 d.r.). We also explored the formation of a substituted pyrrolidine product through a 5-exo cyclization and were pleased to obtain the product in excellent yield and moderate stereoselectivity (24, 91% yield, 84% e.e.). The diminished enantioinduction observed in this case is probably due to the enhanced reversibility of the alkylation step as the Baeyer ring strain in five-membered rings exceeds that of six-membered analogues24.
Table 1.
Tether and olefin scope of enantioselective intramolecular α-alkylation of aldehydes.
For each product number (in bold), data are reported as percent isolated yield, and diastereometric ratio (d.r.) was determined by 1H NMR. *(R)-2-tert-butyl-3-methylimidazolidin-4-one (20 mol%) was used. NMA, N-methyl acetamide; Me, methyl; Ts, tosyl; Boc, tert-butyl carbamoyl; TMS, trimethylsilyl; Ph, phenyl. See Supplementary Information for experimental details.
Next, we turned our attention to the alkene component in this intramolecular variant. Trisubstituted olefins proved to be highly efficient (20 and 22, 86% yield, 92% e.e. and 81% yield, 94% e.e., respectively). As might be expected, trisubstituted olefins that incorporate two prochiral carbons achieve high diastereocontrol at the stereocentres involved in ring formation, yet effectively no selectivity in the HAT step (22, 11:11:1:1 d.r.). It should be noted that, in the case of the geraniol-derived example 22, where multiple olefins are present in the tethered aldehyde substrate, only the proximal alkene undergoes radical addition. Notably, 1,2-disubstituted olefins were also effective in the transformation (16, 17 and 21, 62–82% yield, 88–90% e.e.). However, superior efficiency was observed in the cases where radical-stabilization was possible after the cyclization event (17, 21 versus 16). Perhaps most notably, quaternary carbon stereocentres could also be formed via a tethered tetrasubstituted olefin using (R)-2-tert-butyl-3-methylimidazolidin-4-one, albeit with lower enantiocontrol (23, 60% yield, 50% e.e., >20:1 d.r.). Moreover, we also found that seven-membered rings could be formed through a 7-endo cyclization to provide the corresponding azepanes or cycloheptanes in excellent yields (25 and 26, 89% yield, 88% e.e. and 71% yield, 84% e.e., respectively). This process is believed to occur through reversible 6-exo and 7-endo radical cyclizations that ultimately favour the formation of the more thermodynamically stable tertiary radical intermediate before intermolecular HAT.
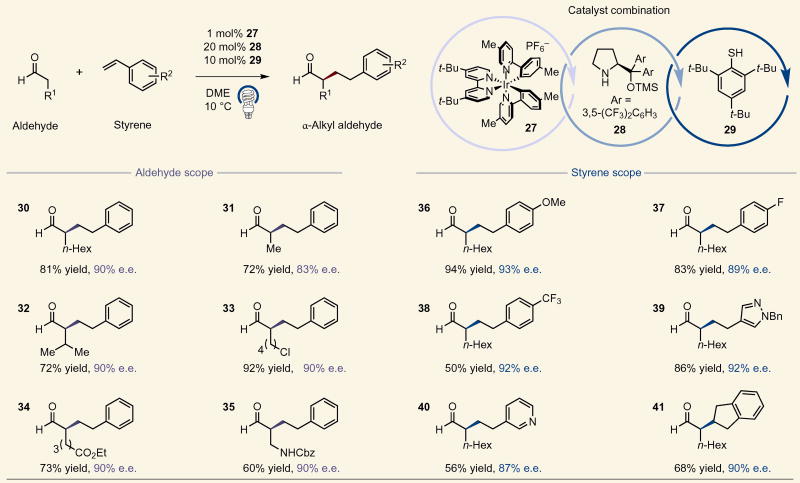
Having successfully demonstrated this enantioselective α-formyl alkylation in a unimolecular sense, we next turned our attention to an intermolecular variant. Initially we focused on widely available styrene coupling partners, given their established propensity as radicalphiles25. Indeed, we observed excellent efficiency and selectivity in this case using a less oxidizing heteroleptic iridium(III) photocatalyst, Ir(dmppy)2(dtbbpy)PF6 (ref. 26) (dmppy = 2-(4-methylphenyl)-4-(methylpyridine)) (27) and the diarylsilylprolinol organocatalyst, α,α-bis[3,5-bis(trifluoromethyl) phenyl]-2-pyrrolidine-methanol trimethylsilyl ether (28). Moreover, the use of a more sterically congested thiophenol catalyst 29 was required in this case to overcome side reactions arising from thiol-ene type processes.
With these conditions in hand, we found that a variety of substituted aldehydes provided the desired alkylated products in good yield and selectivity (Table 2). Interestingly, 6-chlorohexanal provided the desired product (33) in high efficiency rather than the alternative 6-exo-tet intramolecular enamine alkylation adduct. Moreover, β,β-disubstituted and β-amino aldehydes were well-tolerated in the transformation (32 and 35, 72% yield, 90% e.e. and 60% yield, 90% e.e., respectively). Next, we examined the effect of the aromatic group of the styrene component. Notably, we found that a variety of electron-rich and electron-deficient vinyl arenes can be employed while retaining excellent enantioselectivity (36–38, 50–94% yield, 89–93% e.e.). Additionally, heteroaromatics in the form of 3-vinyl pyridine and a 4-vinyl pyrazole were shown to be viable substrates (39 and 40, 86% yield, 92% e.e. and 56% yield, 87% e.e., respectively). Furthermore, 1,2-disubstituted styrenes could also be employed, as exemplified by indene, to generate the corresponding alkylation adduct in excellent yield and enantiocontrol (41, 68% yield, 90% e.e.).
Table 2.
Aldehyde and styrene scope of enantioselective intermolecular α-alkylation.
For each product number (in bold), data are reported as percent isolated yield. Cbz, benzyl carbamoyl; Bn, benzyl. See Supplementary Information for experimental details.
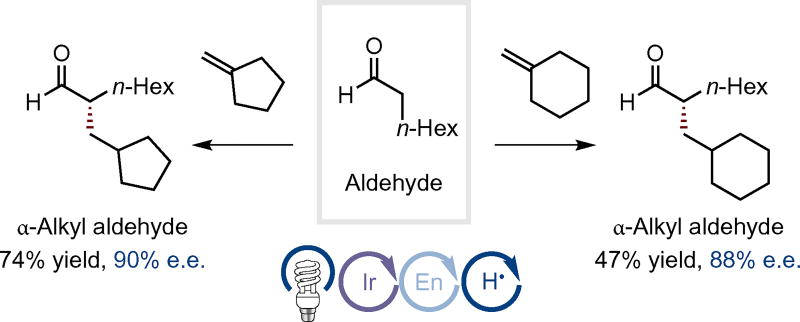
Finally, we examined this α-alkylation manifold with simple olefin coupling partners. Although terminal olefins were found to be less reactive (see Supplementary Information), π-nucleophilic 1,1-disubstituted olefins were found to be suitable, with moderate to useful levels of efficiency. As shown in Fig. 3, we were pleased to find that this triple catalytic activation mode allows methylenecyclopentane and methylenecyclohexane to participate in the desired alkylation event with high levels of enantiocontrol. We attribute the viability of these substrates to be due, in part, to the reversible photocatalytic generation of the key 3πe− enaminyl radical intermediate and subsequent interaction with the HAT catalyst only after addition across the olefin substrate.
Figure 3. Intermolecular α-alkylation of aldehydes with non-functionalized olefins.
Intermolecular coupling between 1,1-disubstituted olefins and octanal. Data are reported as percent isolated yield. Reaction conditions: 1 mol% 27, 20 mol% 28, 10 mol% 29, DME, blue LED light, −65 °C.
In summary, we have developed a novel reaction platform where simple aldehyde substrates undergo coupling with a wide variety of olefin reaction partners to enantioselectively produce α-alkyl carbonyl adducts. This visible light-mediated transformation is accomplished via a hydrogen atom and electron borrowing mechanism that involves three discrete catalytic cycles that are temporally matched to function in concert. This multicatalytic process enables both intra- and intermolecular coupling of aldehydes to construct both cyclic and acyclic products, respectively. We expect the capacity of employing feedstock chemicals in a new enantioselective coupling reaction that employs visible light will find utility across a number of research and industrial applications.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences (NIGMS), the NIH (under award no. R01 GM078201-05) to D.W.C.M., A.G.C., J.T.M., N.J.M. and J.K. and by gifts from Merck, Abbvie, BMS and Janssen. J.T.M. acknowledges the NIH for a postdoctoral fellowship (F32 GM108217-02). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIGMS.
Footnotes
Data availability. The data supporting the findings of this study are available within the paper and its Supplementary Information.
Author contributions
A.G.C., J.T.M., N.J.M. and J.K. performed and analysed experiments. A.G.C., J.T.M., N.J.M., J.K. and D.W.C.M. designed experiments to develop the intramolecular variant of this reaction and probe its utility. A.G.C., N.J.M., and D.W.C.M. designed experiments to develop the intermolecular variant of this reaction and probe its utility. A.G.C. and D.W.C.M. prepared this manuscript.
Supplementary information and chemical compound information are available in the online version of the paper.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Caine D. In: Comprehensive Organic Synthesis Vol. 2. Trost BM, editor. Ch 1.1. Pergamon; 1991. [Google Scholar]
- 2.Corey EJ, Enders D. Applications of N, N-dimethylhydrazones to synthesis. Use in efficient, positionally and stererochemically selective C–C bond formation; oxidative hydrolysis to carbonyl compounds. Tetrahedron Lett. 1976;17:3–6. [Google Scholar]
- 3.Evans DA, Ennis MD, Mathre DJ. Asymmetric alkylation reactions of chiral imide enolates. A practical approach to the enantioselective synthesis of α-substituted carboxylic acid derivatives. J. Am. Chem. Soc. 1982;104:1737–1739. [Google Scholar]
- 4.Dolling U-H, Davis P, Grabowski EJJ. Efficient catalytic asymmetric alkylations. 1. Enantioselective synthesis of (+)-indacrinone via chiral phase-transfer catalysis. J. Am. Chem. Soc. 1984;106:446–447. [Google Scholar]
- 5.Doyle AG, Jacobsen EN. Enantioselective alkylation of acyclic α, α-disubstituted tributyltin enolates catalyzed by a {Cr(salen)} complex. Angew. Chem. Int. Ed. 2007;46:3701–3705. doi: 10.1002/anie.200604901. [DOI] [PubMed] [Google Scholar]
- 6.Mitsuko I, Hagihara A, Kawasaki H, Manabe K, Koga K. Catalytic asymmetric benzylation of achiral lithium enolates using a chiral ligand for lithium in the presence of an achiral ligand. J. Am. Chem. Soc. 1994;116:8829–8830. [Google Scholar]
- 7.Eder U, Sauer G, Wiechert R. New type of asymmetric cyclization to optically active steroid CD partial structures. Angew. Chem. Int. Ed. 1971;10:496–497. [Google Scholar]
- 8.Hajos ZG, Parrish DR. Asymmetric synthesis of bicyclic intermediates of natural product chemistry. J. Org. Chem. 1974;39:1615–1621. [Google Scholar]
- 9.MacMillan DWC. The advent and development of organocatalysis. Nature. 2008;455:304–308. doi: 10.1038/nature07367. [DOI] [PubMed] [Google Scholar]
- 10.Mo F, Dong G. Regioselective ketone α-alkylation with simple olefins via dual activation. Science. 2014;345:68–72. doi: 10.1126/science.1254465. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee S, Yang JW, Hoffmann S, List B. Asymmetric enamine catalysis. Chem. Rev. 2007;107:5471–5569. doi: 10.1021/cr0684016. [DOI] [PubMed] [Google Scholar]
- 12.Vignola N, List B. Catalytic asymmetric intramolecular α-alkylation of aldehydes. J. Am. Chem. Soc. 2004;126:450–451. doi: 10.1021/ja0392566. [DOI] [PubMed] [Google Scholar]
- 13.Beeson TD, Mastracchio A, Hong J, Ashton K, MacMillan DWC. Enantioselective organocatalysis using SOMO activation. Science. 2007;316:582–585. [PubMed] [Google Scholar]
- 14.Jui NT, Garber JAO, Finelli FG, MacMillan DWC. Enantioselective organo-SOMO cycloadditions: a catalytic appraoch to complex pyrrolidines from olefins and aldehydes. J. Am. Chem. Soc. 2012;134:11400–11403. doi: 10.1021/ja305076b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad JC, Kong J, Laforteza BN, MacMillan DWC. Enantioselective arylation of aldehydes via organo-SOMO catalysis. An ortho-selective α-arylation reaction based on an open-shell pathway. J. Am. Chem. Soc. 2009;131:11640–11641. doi: 10.1021/ja9026902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw MH, Twilton J, MacMillan DWC. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016;81:6896–6926. doi: 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts BP. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 1999;28:25–35. [Google Scholar]
- 18.Hamilton DS, Nicewicz DA. Direct catalytic anti-Markovnikov hydroetherification of alkenols. J. Am. Chem. Soc. 2012;134:18577–18580. doi: 10.1021/ja309635w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qvortrup K, Rankic DA, MacMillan DWC. A general strategy for organocatalytic activation of C–H bonds via photoredox catalysis: direct arylation of benzylic ethers. J. Am. Chem. Soc. 2014;136:626–629. doi: 10.1021/ja411596q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowry MS, et al. Single-layer electroluminescent devices and photoinduced hydrogen production from an ionic iridium(III) complex. Chem. Mater. 2005;17:5712–5719. [Google Scholar]
- 21.Venimadhavan S, Amarnath K, Harvey NG, Cheng J-P, Arnett EM. Heterolysis, homolysis, and cleavage energies for the cation radicals of some carbon–sulfur bonds. J. Am. Chem. Soc. 1992;114:221–229. [Google Scholar]
- 22.Franz JA, Bushaw BA, Alnajjar MS. Absolute rate expressions for the abstraction of hydrogen by primary, secondary, and tertiary alkyl radicals from thiophenol. J. Am. Chem. Soc. 1989;111:268–275. [Google Scholar]
- 23.Bordwell FG, Cheng J-P, Harrelson JA., Jr Homolytic bond dissociation energies in solution from equilibrium acidity and electrochemical data. J. Am. Chem. Soc. 1988;110:1229–1231. [Google Scholar]
- 24.Wade LG. Structure and Stereochemistry of Alkanes; Organic Chemistry. 6. Pearson Prentice Hall; 2006. pp. 103–122. [Google Scholar]
- 25.Curran DP. In: Comprehensive Organic Synthesis Vol. 4. Trost BM, editor. Ch 4.1. Pergamon; 1991. [Google Scholar]
- 26.Terrett JA, Clift MD, MacMillan DWC. Direct β-alkylation of aldehydes via photoredox organocatalysis. J. Am. Chem. Soc. 2014;136:6858–6861. doi: 10.1021/ja502639e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.