Abstract
Adult onset cerebellar dysfunction with neuropathy is a commonly encountered condition and is usually due to genetic causes such as spinocerebellar ataxia, gluten ataxia, alcohol related, toxic, degenerative, immune mediated, paraneoplastic causes and so on. Ataxia and neuropathy as presenting features of hepatitis-B related liver disease are very rare and have not been reported so far.
Keywords: hepatitis b, portal hypertension, cirrhosis, movement disorders (other than parkinsons), peripheral nerve disease
Background
Adult onset cerebellar dysfunction with neuropathy is usually due to genetic causes such as spinocerebellar ataxia, gluten ataxia, alcohol related, toxic, degenerative, immune mediated, paraneoplastic causes and so on.1 Acquired hepatocerebellar degeneration is an uncommon clinical entity with a prevalence of 0.8% in patients with liver disease and observed in patients with advanced liver disease (2–33 years after onset of liver disease) and is related to portosystemic shunting.2 On the other hand, cerebellar ataxia and neuropathy have not been reported to precede the diagnosis of liver disease. A review of 1500 patients with cerebellar disease did not report any case due to liver dysfunction.3
This case is being reported because hepatitis-B- related liver disease is amenable to treatment with antivirals and liver transplantation and it is important to recognise cerebellar ataxia and neuropathy as presenting manifestations of cirrhosis.
Case presentation
A 55-year-old man presented with insidious onset gradually progressive neurological signs and symptoms since 49 years of age. He noted weakness of distal lower limbs that was symmetrical and associated with numbness and loss of sensation, which progressed in a glove and stocking pattern. Three years after onset of lower limb symptoms, he developed upper limb symptoms. Around the same time, he developed gradually progressive pancerebellar dysfunction. There was no cognitive decline, no stiffness, dystonia, postural instability or bradykinaesia, sphincter disturbance, seizures or myoclonus. He had not sought medical attention for these symptoms and no specific aetiology had been determined. Around 5 years after onset of these symptoms, he developed abdominal distension and pedal oedema. There were no episodes of altered sensorium, haematemesis, melena, yellowish urine, yellowish discolouration of skin or mucous membranes, pruritus, palmar erythema or spider nevi. There was no breathlessness, cough, chest pain or palpitations. He did not consume alcohol, use illicit drugs, have chronic drug intake or have high-risk sexual behaviour. There was no history suggestive of malabsorption, steatorrhoea or bloating after intake of wheat products. He did not have a history suggestive of thyroid dysfunction. The family history was negative. He was not vaccinated against hepatitis B infection.
On examination, patient had grade IV clubbing and pitting pedal oedema. He was haemodynamically stable and cardiovascular and respiratory systems were normal on clinical examination. He had abdominal free fluid and splenomegaly. There were no signs of hepatic encephalopathy. Patient had signs of pancerebellar involvement, weakness that was predominantly distal in both lower limbs as well as sensory loss in a glove and stocking pattern in both lower limbs up to mid-calf and upper limbs up to the hands. He had an ataxic, wide-based gait with positive Romberg’s sign indicating both cerebellar and sensory ataxic components.
Investigations
Haemogram revealed pancytopenia with normocytic normochromic smear and an erythrocyte sedimentation rate (ESR) of 32 mm in the first hour. Renal functions and electrolytes were normal. Bilirubin and liver enzymes were normal with low albumin of 2.3 g/dL and globulin of 4.8 g/dL with reversed albumin:globulin ratio. His prothrombin time international normalised ratio (PT-INR) was raised at 1.32. His urine did not reveal proteinuria, casts or active sediments. Ultrasound of the abdomen revealed free fluid and splenomegaly with coarse and nodular hepatic echotexture. Upper gastrointestinal endoscopy revealed grade II oesophageal varices. Chest X-ray, echocardiography and ECG were unrevealing. C reactive peptide, lipid profile and thyroid profile were normal. Aetiological workup for liver disease revealed positive hepatitis B surface antigen, negative hepatitis C virus and HIV antibodies. Hepatitis B virus (HBV)DNA level was 200 000 copies/mL. Wilson’s disease and autoimmune hepatitis panel including anti-LKM antibody (Liver kidney microsomal antibody), ANA (Anti nuclear antibody), anti-dsDNA (double stranded DNA), rheumatoid factor and ANCA (Anti neutrophil cytoplasmic antibody) were also negative.
The rest of the workup for ataxia and neuropathy including coeliac serology and SCA (Spinocerebellar ataxia) genetic mutation analysis, serum and urine protein electrophoresis were also negative.
Nerve conduction study showed symmetric axonal polyneuropathy involving all four limbs.
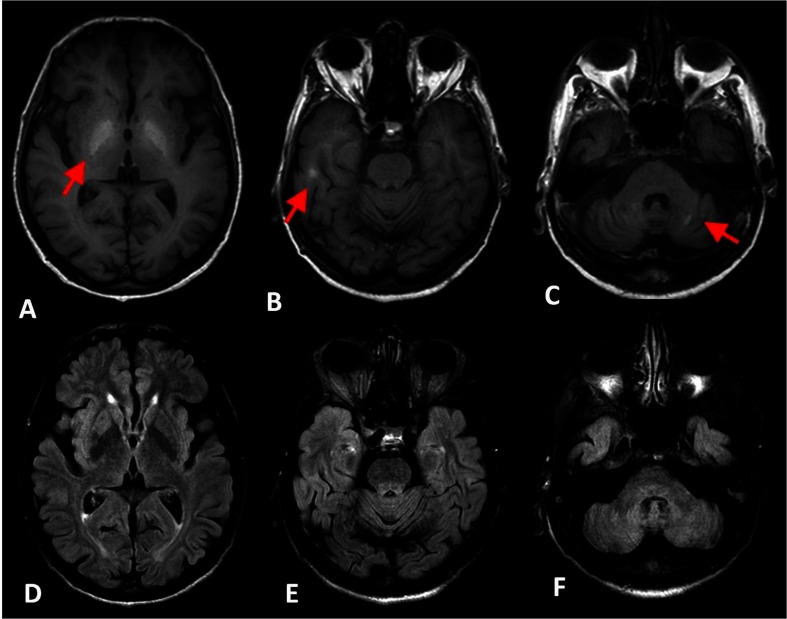
MRI brain (figure 1A–F) demonstrated bilaterally symmetrical T1 hyperintense/T2/FLAIR (Fluid attenuated inversion recovery) hypointense areas in globi pallidi, posterior limbs of internal capsules, ventromedial thalami and dentate nuclei. His MELD (Model of end stage liver disease) score was 9, and Child-Pugh class was class B with 9 points.
Figure 1.
A–C, T1-weighted MRI showing hyperintensity (red arrows) in bilateral globus pallidus (A), cerebral white matter (B) and dentate nuclei (C). D–F, Corresponding FLAIR images with hypointensity.
Differential diagnosis
Progressive cerebellar ataxia with neuropathy has a long list of differentials including toxic such as drug induced and related to heavy metal poisoning, metabolic such as vitamin E deficiency, abetalipoproteinaemia, inflammatory diseases such as CLIPPERS (Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids) syndrome, paraneoplastic causes, anti-GAD (Glutamic acid decarboxylase)associated autoimmune disease, STREAT (Steroid responsive encephalopathy associated with autoimmune thyroiditis) syndrome, degenerative causes such as MSA-C (Multisystem atrophy-cerebellar type), mitochondrial diseases as well as genetic causes such as spinocerebellar ataxia.
In this patient, most of these were ruled out by relevant investigations.
Treatment
Patient was started on tenofovir 300 mg once a day as there was active viral replication and is under follow-up. His ascites resolved, and his neurological symptoms are stable, with no further progression at 8 months of follow-up.
Discussion
The syndrome of hepatocerebral degeneration had been recognised since 1914 by van Woerkem, and since then, a number of neurological manifestations including spasticity, parkinsonism, cerebellar dysfunction and movement disorders have been described. However, the available literature suggests that the liver disease precedes the onset of neurological manifestations by weeks to years.4
Our patient had a long-standing history of neuropathy and cerebellar ataxia before the liver disease and hepatitis B had been diagnosed. Moreover, hepatic encephalopathy never occurred in our patient who had normal cognition. Hepatocerebral degeneration is related to cirrhosis and portosystemic shunting as evidenced by oesophageal varices were present in our patient. This shows that the hepatitis B infection must have been chronic. The inflammatory markers were not raised and the patient had low serum albumin and raised INR reflecting liver dysfunction. Progressive cerebellar dysfunction has not been reported in association with hepatitis B infection, though acute cerebellar ataxia with self-limiting course after hepatitis B vaccination5 has been reported. Though there is association of the liver disease and neurological manifestations, causation could not be definitely proved. However, extensive evaluation for other causes of ataxia, neuropathy and movement disorders failed to reveal any other aetiology for the hepatocerebellar dysfunction. The neurological symptoms may not be reversible with medical management and some may respond to liver transplantation,4 as in our case the patient stabilised, and there was no worsening.
Atypical clinical features
The most common neurological disorders associated with acquired hepatocerebral degeneration (AHCD) are movement disorders. There has been no report of neuropathy associated with AHCD. Five out of eight patients in previously reported series2 had previous episodes of hepatic encephalopathy. Our patient never had encephalopathy.
Atypical imaging features
Acquired hepatocerebral degeneration characterised by T1 hyperintense lesions involving the globus pallidus in all cases and the rest of the regions like middle cerebellar peduncle, cerebellum, putamen, caudate, internal capsule and hemispheric white matter may occur.2 Park et al6 described T2 hyperintensity of the dentate nucleus while this is the first instance T1 hyperintensity of dentate nucleus is being described in this condition.
Learning points.
Neurological manifestations preceding the diagnosis of cirrhosis and portal hypertension are rare in acquired hepatocerebral degeneration.
Association of cerebellar ataxia and neuropathy has so far not been reported in acquired hepatocerebral degeneration.
This is the first case report describing T1 hyperintense lesion of dentate nucleus in acquired hepatocerebral degeneration.
It is important to diagnose this condition early as they offer a window of opportunity to treat to avoid permanent neurological deficits.
Footnotes
Contributors: AE contributed to the diagnosis, management and in writing the manuscript. DD contributed to the diagnosis, management and in writing the manuscript. MT contributed to the diagnosis and management of the case. RB contributed to the diagnosis and management of the case.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Klockgether T. Sporadic ataxia with adult onset: classification and diagnostic criteria. Lancet Neurol 2010;9:94–104. 10.1016/S1474-4422(09)70305-9 [DOI] [PubMed] [Google Scholar]
- 2.Fernández-Rodriguez R, Contreras A, De Villoria JG, et al. Acquired hepatocerebral degeneration: clinical characteristics and MRI findings. Eur J Neurol 2010;17:1463–70. 10.1111/j.1468-1331.2010.03076.x [DOI] [PubMed] [Google Scholar]
- 3.Hadjivassiliou M, Martindale J, Shanmugarajah P, et al. Causes of progressive cerebellar ataxia: prospective evaluation of 1500 patients. J Neurol Neurosurg Psychiatry 2017;88:301–9. 10.1136/jnnp-2016-314863 [DOI] [PubMed] [Google Scholar]
- 4.Ferrara J, Jankovic J. Acquired hepatocerebral degeneration. J Neurol 2009;256:320–32. 10.1007/s00415-009-0144-7 [DOI] [PubMed] [Google Scholar]
- 5.Deisenhammer F, Pohl P, Bösch S, et al. Acute cerebellar ataxia after immunisation with recombinant hepatitis B vaccine. Acta Neurol Scand 1994;89:462–3. 10.1111/j.1600-0404.1994.tb02667.x [DOI] [PubMed] [Google Scholar]
- 6.Park SA, Heo K. Prominent cerebellar symptoms with unusual magnetic resonance imaging findings in acquired hepatocerebral degeneration. Arch Neurol 2004;61:1458–60. 10.1001/archneur.61.9.1458 [DOI] [PubMed] [Google Scholar]