Abstract
Solitary plasmacytoma is a rare disorder comprising 5%–10% of all plasma cell neoplasms. Progression to multiple myeloma is the most common pattern of relapse. Appearance of new lesions without any systemic disease is the most unusual pattern of relapse seen in <2% cases. We present a case of a 46-year-old female who presented with features of third and seventh cranial nerve palsy, diagnosed with solitary plasmacytoma, with no evidence of any systemic disease. As per standard recommendations, the patient received radiotherapy to the local site. The patient developed relapse twice, at three sites, during the follow-up period. Investigations revealed no evidence of any systemic disease. In view of repeat relapses, the patient was started on immune modulatory agent. Two and half years after the last radiotherapy, the patient is symptom free with no evidence of any new lesion.
Keywords: oncology, radiotherapy, haematology (incl blood transfusion)
Background
Solitary plasmacytoma (SP) is a rare disorder comprising about 5%–10% of all plasma cell neoplasms.1–5 SPs are of two types: solitary bone plasmacytoma (SBP) or extramedullary plasmacytoma (EMP).1–5SPs are radio responsive tumours with local failure rates as low as 6% to 31% depending on radiation doses. Progression to multiple myeloma is not uncommon, with risk upto 76% over 10 years for SBP and about 36% for EMP over the same period. Frassica et al reported three patterns of treatment failure: conversion to multiple myeloma (54%, most common), local recurrence (11%) and appearance of new lesions in absence of systemic disease (2%, least common).6 We present a case of a patient with recurrent plasmacytomas without any evidence of multiple myeloma.
Case presentation
A 46-year-old female presented with complaints of diplopia, ptosis of right eyelid, deviation of face and tongue, features of third and seventh cranial nerve palsy and ophthalmoplegia.
Investigations
The patient was investigated—MRI brain revealed large enhancing skull-based tumour involving the clivus and nasopharynx with extensions into the paranasal sinuses, involvement of cavernous sinuses, encasement of Internal carotid arteries, extension into the orbit intracranially (figure 1). Histopathology revealed skull-based tumour with plasmacytoid cells. Immunohistochemistry showed tumour cells positive for epithelial memebrane antigen (EMA) and CD138 and negative for lambda and kappa light chains, CD20 and cytokeratin. Serum protein electrophoresis revealed no M spike; bence jones protein and abnormal bands were not noted in urine protein electrophoresis in 24 hours urine specimen. Bone marrow biopsy revealed 3%–4% plasma cells.
Figure 1.
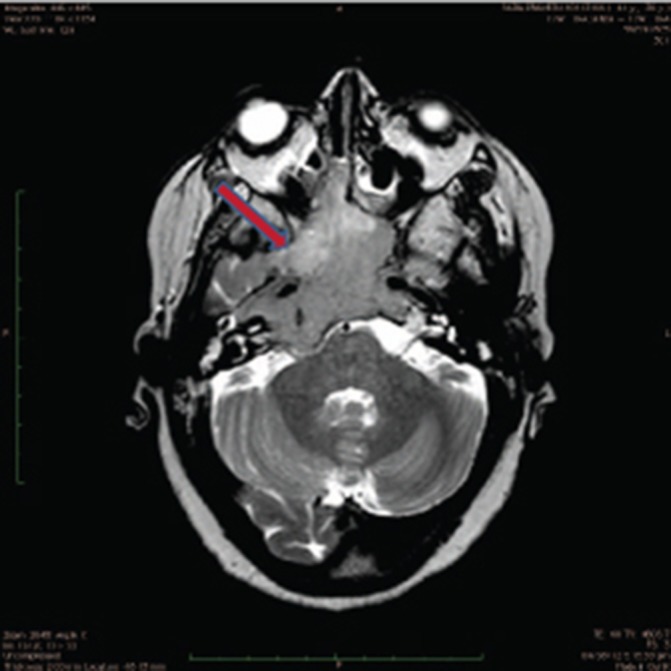
MRI scan of base of skull lesion.
Differential diagnosis
A diagnosis of SP was made.
Treatment
The patient received local radiotherapy 50.4 Gy in 28 fractions in August–September 2012 to the skull base region.
Outcome and follow-up
CT scan three months post-treatment revealed small residual disease; on clinical examination, mild right facial nerve paresis with near normal visual fields on perimetry and normal vision were noted.
The patient remained well for about 16 months when she developed complaints of pain in right femur. A Positron Emission Tomography CT (PET CT) scan was performed, which revealed osteolytic lesions with increased 18 Flurodeoxyglucose (18FDG) uptake in left sacral ala and right femur with extension into the surrounding soft tissue (figures 2 and 3). Minimal residual lesion was seen in base of skull region. Core biopsy from femur was suggestive of plasma cell myeloma. Bone marrow biopsy revealed no evidence of myeloma (5%–6% plasma cells) and no M spike on protein electrophoresis. She underwent intramedullary interlocking femoral nailing of right femur in March 2014 followed by radiotherapy 30 Gy in 10 fractions to sacral ala and right femur lesions. The patient was advised systemic treatment but refused the same.
Figure 2.
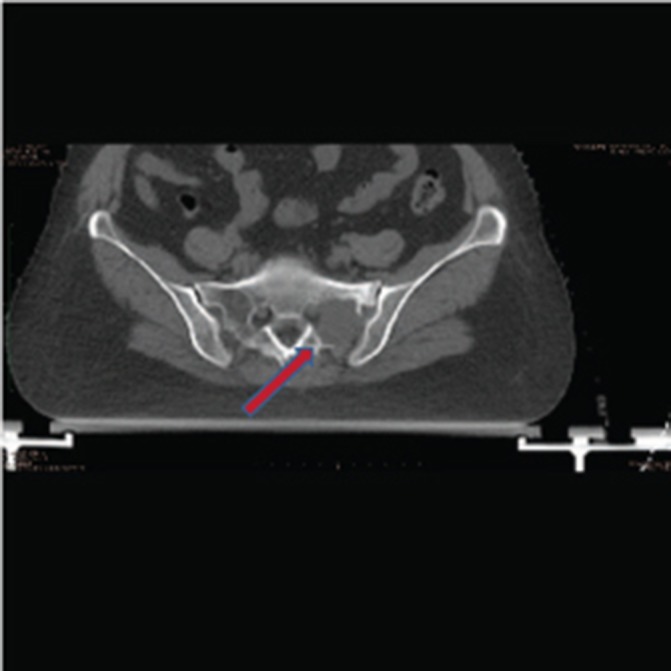
CT scan showing lesion in left sacral ala.
Figure 3.
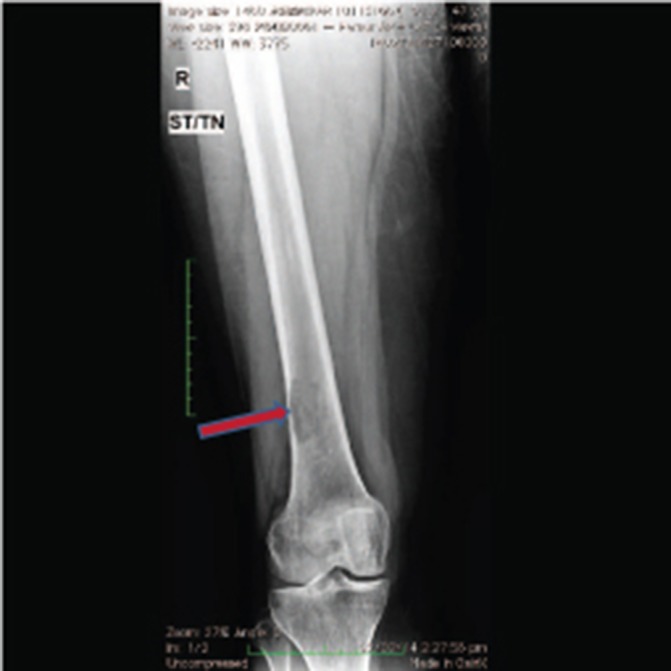
X-ray image showing a solitary lesion in right femur bone.
Six months later, she developed pain in the right forearm. Investigations revealed a lytic lesion in the right radius (figure 4). Myeloma work-up was negative; bone marrow biopsy revealed 5% plasma cells. The patient was again treated as SP, using radiotherapy 20 Gy in five fractions, and then started on cyclophosphamide, thalidomide/dexamethasone-based oral treatment for 6 months and monthly intravenous bisphosphonates, in view of constrained financial status of the patient. This was followed by maintenance Tab Thalidomide 100 mg once daily. The frequency of bisphosphonates was reduced to once every 3 months after the patient had received 6 monthly doses. Two and half years after the last radiotherapy, the patient is on maintenance thalidomide and is now symptom free, with no new lesions.
Figure 4.
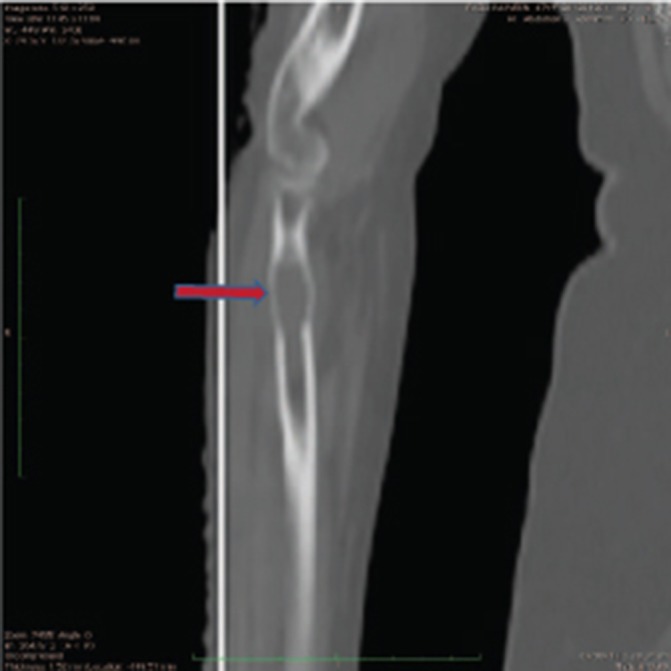
CT scan showing lesion in the upper end of right radius bone.
Discussion
SP is characterised by neoplastic proliferation of monoclonal plasma cells in either soft tissue labelled as EMP or bone labelled as SBP without evidence of any systemic disease. Median age of presentation of SP is 55 to 65 years. Male preponderance is seen with incidence in males twice that of females.7 Diagnostic evaluation includes history, physical examination, complete blood counts, kidney function tests, serum electrolytes, bone marrow biopsy, protein electrophoresis, urine for bence jones protein and skeletal survey. Diagnosis of SBP includes histologically confirmed solitary lesion with normal skeletal survey except at site of lesion, normal bone marrow biopsy (<10% plasma cells) and no myeloma-related organ dysfunction features (CRAB—increased calcium, renal insufficiency, anaemia, or multiple bone lesions).
Imaging modalities including X-ray, CT scan and MRI—both T1 and T2 imaging are required to accurately define extent of lesion. CT scans are more useful in defining the extent of bony destruction, while MRI is more helpful in defining the extent to soft tissue extension and any spinal cord compression.
Treatment of SBP includes radiotherapy, surgery and supportive medication.
SBP is a highly radioresponsive disease with excellent response rates with radiotherapy (RT) alone.7–9 Various dosing schedules have been used to treat SP. Radiotherapy doses of 45–50 Gy in conventional fractionation are standard, though lower doses may be accepted in view of dose constraints to critical structures. Mendenhall et al reported local control rates of 94% with doses>40 Gy vs 64% with doses<40 Gy.10 Common practice in 2D radiotherapy era included one to two vertebrae cranially and caudally. With the advent of CT and MRI, a more accurate delineation of the targets, especially the soft tissue component is possible. There are currently no established guidelines for delineating margins around the gross tumour volume. We used 1.5 to 2 cm margins around the gross tumour volume (GTV) to generate a clinical target volume (CTV) and 0.5 cm expansion for the planning target volume (PTV).
Surgical interventions are restricted to biopsy and orthopaedic stabilisation in case of fracture or impending injury and patients with spinal cord compression where immediate relief is required.11–14 Even if surgical excision is done like in cases of EMP, it should be followed by radiotherapy to local sites to achieve a durable response.7
Prognostic factors have been defined with respect to likelihood of progression to multiple myeloma like age, SBP vs EMP, RT doses, high M protein levels, existence of light chains and persistence of M protein after treatment.15–19 In a Surveillance, Epidemiology, and End Results (SEER) database analysis of 1164 patients of skeletal plasmacytoma, only age (5-year survival of 90% for age 0–29 years, 80% for 30–59 years and 45% for >60 years age group), number of lesions (5-year survival, 59% vs 36% for solitary vs more than two lesions respectively, p=0.032), gender (male—59% vs female—54% 5-year survival, p=0.008) and radiotherapy (5-year survival 60% in patients who recieved radiotherapy vs 46% in patients with no radiotherapy) were factors found to be significant for overall survival. Age was the only factor determining the risk of disease progression to multiple myeloma (age < or >60 years; p=0.027).20 However, no prognostic factors have been separately studied in patients in whom SPs recur at different locations without evidence of multiple myeloma. It has been hypothesised that an indolent form of multiple myeloma exists in most of these patients. Similarly, smouldering myelomas represent a premalignant stage of multiple myeloma and convert to multiple myeloma at the rate of 10% per year. These entities, along with monoclonal gammopathy of unknown significance, represent a spectrum of plasma cell disorders progressing to multiple myeloma. We believe that our patient is at an intermediate stage between asymptomatic plasma cell disorder and symptomatic multiple myeloma.21
The role of systemic therapy is not clear. Conflicting results of disease progression in SP to multiple myeloma have been seen with some studies showing survival advantage and improved progression-free survival rates when RT+chemotherapy was compared with RT alone, while other studies failed to show any beneficial effect of chemotherapy on disease control or progression.15 17 22 23 Tumours with poor prognostic features, such as size more than 5 cm and high-grade histology, may be considered for adjuvant chemotherapy, as many patients have poor response to radiotherapy alone. All these studies have, however, addressed the conversion of SP to multiple myeloma. The role of thalidomide, an anti-angiogenic agent, has been studied in phase II and phase III trials in asymptomatic multiple myelomas, with response rates of close to 35%, with a longer time to progression with the addition of thalidomide.24 25 There is still no clue as to the cause of solitary plamacytoma occurring repeatedly without any systemic features of disease progression. The only possible explanation is an indolent form of multiple myeloma. The role of bisphophonates is also undefined.
Our patient has been on follow-up of 5 years since the first diagnosis and despite recurrent SP, she is doing fine and is disease free on immune modulatory agent.
Learning points.
A comprehensive investigation is important to differentiate between solitary relapse of plasmacytoma and multiple myeloma.
Solitary plasmacytoma relapse at new sites without evidence of systemic disease has a better outcome compared with multiple myeloma.
Local radiotherapy to lesion is associated with minimal toxicity.
Immune modulatory agents are probably effective in decreasing the chances of relapse of solitary plasmcytomas.
Footnotes
Contributors: First author, RK, has been involved in collection and interpretation of data, and writing the manuscript. Second and third authors, SS and SN, have been involved in patient care, including design and delivery of treatment. SN has reviewed and revised the manuscript. All the three authors are involved in follow-up care of the patient.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dimopoulos MA, Moulopoulos LA, Maniatis A, et al. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood 2000;96:2037–44. [PubMed] [Google Scholar]
- 2.Dimopoulos MA, Hamilos G. Solitary bone plasmacytoma and extramedullary plasmacytoma. Curr Treat Options Oncol 2002;3:255–9. 10.1007/s11864-002-0015-2 [DOI] [PubMed] [Google Scholar]
- 3.Ozsahin M, Tsang RW, Poortmans P, et al. Outcomes and patterns of failure in solitary plasmacytoma: a multicenter Rare Cancer Network study of 258 patients. Int J Radiat Oncol Biol Phys 2006;64:210–7. 10.1016/j.ijrobp.2005.06.039 [DOI] [PubMed] [Google Scholar]
- 4.Tong D, Griffin TW, Laramore GE, et al. Solitary plasmacytoma of bone and soft tissues. Radiology 1980;135:195–8. 10.1148/radiology.135.1.7360960 [DOI] [PubMed] [Google Scholar]
- 5.Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine 1976;55:217–38. 10.1097/00005792-197605000-00002 [DOI] [PubMed] [Google Scholar]
- 6.Frassica DA, Frassica FJ, Schray MF, et al. Solitary plasmacytoma of bone: Mayo Clinic experience. Int J Radiat Oncol Biol Phys 1989;16:43–8. 10.1016/0360-3016(89)90008-4 [DOI] [PubMed] [Google Scholar]
- 7.Ozsahin M, Tsang RW, Poortmans P, et al. Outcomes and patterns of failure in solitary plasmacytoma: a multicenter Rare Cancer Network study of 258 patients. Int J Radiat Oncol Biol Phys 2006;64:210–7. 10.1016/j.ijrobp.2005.06.039 [DOI] [PubMed] [Google Scholar]
- 8.Hu K, Yahalom J. Radiotherapy in the management of plasma cell tumors. Oncology 2000;14:101–12. [PubMed] [Google Scholar]
- 9.Dimopoulos MA, Kiamouris C, Moulopoulos LA. Solitary plasmacytoma of bone and extramedullary plasmacytoma. Hematol Oncol Clin North Am 1999;13:1249–57. 10.1016/S0889-8588(05)70124-6 [DOI] [PubMed] [Google Scholar]
- 10.Mendenhall CM, Thar TL, Million RR. Solitary plasmacytoma of bone and soft tissue. Int J Radiat Oncol Biol Phys 1980;6:1497–501. 10.1016/0360-3016(80)90006-1 [DOI] [PubMed] [Google Scholar]
- 11.Soutar R, Lucraft H, Jackson G, et al. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Clin Oncol 2004;16:405–13. 10.1016/j.clon.2004.02.007 [DOI] [PubMed] [Google Scholar]
- 12.Baba H, Maezawa Y, Furusawa N, et al. Solitary plasmacytoma of the spine associated with neurological complications. Spinal Cord 1998;36:470–5. 10.1038/sj.sc.3100572 [DOI] [PubMed] [Google Scholar]
- 13.Huang W, Cao D, Ma J, et al. Solitary plasmacytoma of cervical spine: treatment and prognosis in patients with neurological lesions and spinal instability. Spine 2010;35:278–84. 10.1097/BRS.0b013e3181c9b431 [DOI] [PubMed] [Google Scholar]
- 14.Sinha PK, Paul R, Bandyopadhyay R, et al. Solitary plasmacytoma of the axis vertebra presenting as severe neck pain. J Assoc Physicians India 2011;59:334–5. [PubMed] [Google Scholar]
- 15.Bolek TW, Marcus RB, Mendenhall NP. Solitary plasmacytoma of bone and soft tissue. Int J Radiat Oncol Biol Phys 1996;36:329–33. 10.1016/S0360-3016(96)00334-3 [DOI] [PubMed] [Google Scholar]
- 16.Shih LY, Dunn P, Leung WM, et al. Localised plasmacytomas in Taiwan: comparison between extramedullary plasmacytoma and solitary plasmacytoma of bone. Br J Cancer 1995;71:128–33. 10.1038/bjc.1995.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsang RW, Gospodarowicz MK, Pintilie M, et al. Solitary plasmacytoma treated with radiotherapy: impact of tumor size on outcome. Int J Radiat Oncol Biol Phys 2001;50:113–20. 10.1016/S0360-3016(00)01572-8 [DOI] [PubMed] [Google Scholar]
- 18.Bataille R, Sany J, Serre H. Apparently isolated plasmacytoma of bone. Clinical and prognostic data. 114 cases and review of literature (author’s transl) Nouv Presse Med 1981;10:407–11. [PubMed] [Google Scholar]
- 19.Kilciksiz S, Celik OK, Pak Y, et al. Clinical and prognostic features of plasmacytomas: a multicenter study of Turkish Oncology Group-Sarcoma Working Party. Am J Hematol 2008;83:702–7. 10.1002/ajh.21211 [DOI] [PubMed] [Google Scholar]
- 20.Muhammad Umar Jawad and Sean P Scully. Skeletal plasmacytoma: Progression of disease and impact of local treatment; an analysis of SEER database. J Hematol Oncol 2009;2:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pérez-Persona E, Vidriales MB, Mateo G, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 2007;110:2586–92. 10.1182/blood-2007-05-088443 [DOI] [PubMed] [Google Scholar]
- 22.Avilés A, Huerta-Guzmán J, Delgado S, et al. Improved outcome in solitary bone plasmacytomata with combined therapy. Hematol Oncol 1996;14:111–7. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Fonseca R, Dispenzieri A, et al. Prognostic value of angiogenesis in solitary bone plasmacytoma. Blood 2003;101:1715–7. 10.1182/blood-2002-08-2441 [DOI] [PubMed] [Google Scholar]
- 24.Barlogie B, van Rhee F, Shaughnessy JD, et al. Seven-year median time to progression with thalidomide for smoldering myeloma: partial response identifies subset requiring earlier salvage therapy for symptomatic disease. Blood 2008;112:3122–5. 10.1182/blood-2008-06-164228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witzig TE, Laumann KM, Lacy MQ, et al. A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma. Leukemia 2013;27:220–5. 10.1038/leu.2012.236 [DOI] [PMC free article] [PubMed] [Google Scholar]


