Abstract
Optimal visual recovery following full-thickness traumatic wound dehiscence in a case of operated deep anterior lamellar keratoplasty (DALK) is rarely seen. Here we report a case of 22-year-old male patient presented to our casualty department with complaint of sudden-onset diminution of vision in his right eye following blunt trauma of 1 day duration. DALK had been performed 11 months ago for advanced keratoconus in the same eye. Best-corrected visual acuity (BCVA) in the right eye was hand movement close to face with accurate projection of rays and in the left eye was 20/20. Slit-lamp examination showed the presence of inferior 180° graft dehiscence with broken sutures and shallow anterior chamber with corneal oedema. Repair of the dehiscence with descemetopexy was done under the guidance of intraoperative optical coherence tomography with the successful rescuing of the host Descemet’s membrane. BCVA at 6 months follow-up was 20/40.
Keywords: ophthalmology, anterior chamber
Background
Traumatic wound dehiscence after penetrating keratoplasty (PK) and deep anterior lamellar keratoplasty (DALK) remains a major concern even after many years of surgery. Overall, the incidence of wound dehiscence after PK is 0.6%–5.8% while after DALK is 3.2%. Trauma is the most common cause for the same.1–3 Functional outcomes after graft dehiscence depend on the area of wound dehiscence, duration of presentation, presence of breach in Descemet’s membrane, lens injury, posterior segment damage and others. We report a rare case of good anatomical and functional outcomes in full-thickness traumatic wound dehiscence in operated DALK.
Case presentation
A 22-year-old male patient presented to us with the history of blunt trauma of 1-day duration with complaints of sudden painful diminution of vision, watering and redness in his right eye. DALK surgery had been performed 11 months ago for advanced keratoconus in right eye. Left eye had undergone corneal collagen cross-linking for keratoconus 1 year ago. The patient was using topical prednisolone sodium phosphate 1% once a day along with topical carboxymethyl cellulose sodium 0.5% six times a day in the right eye. Presenting best-corrected visual acuity (BCVA) was hand movement close to face in the right eye and 20/20 in the left eye with accurate projection of rays in both the eyes. There was no relative afferent pathway defect.
Slit-lamp biomicroscopy of the right eye showed the presence of inferior 180° wound dehiscence with broken sutures, a shallow anterior chamber and corneal oedema. Lens was found to be clear (figure 1A,B). Fundus examination was not possible because of corneal oedema. Other eye examination showed prominent corneal nerves, incomplete Fleisher ring with normal fundus examination. Intraocular pressure in the left eye was 14 mm Hg.
Figure 1.

(A,B) Preoperative slit-lamp biomicroscopic images showing inferior 180° graft dehiscence (black arrow), diffuse corneal oedema (red arrow) with the shallow anterior chamber.
Anterior segment optical coherence tomography (ASOCT) (Optovue RTVue, Fremont, California, USA) showed the presence of thick, oedematous donor tissue separated from host Descemet’s membrane, which was torn in the paracentral area with interface fluid, peripheral iridocorneal touch and distorted graft–host junction (figure 2A).
Figure 2.
(A) Preoperative anterior segment optical coherence tomography image showing thick oedematous cornea with interface fluid and distorted graft–host junction. (B) Postoperative anterior segment optical coherence tomography image showing well-apposed graft–host without any interface fluid[white arrows represent the part of the cornea shown in ASOCT ]
Under the guidance of real-time images provided by the intraoperative optical coherence tomography (iOCT) (OPMI LUMERA 700 and RESCAN 700, Carl Zeiss, Meditec, Germany), descemetopexy with air and repair of dehiscence with 10–0 monofilament nylon sutures was done under general anaesthesia (figure 3A and B).
Figure 3.

Intraoperative optical coherence tomography images [ blue arrows represent horizontal scan and pink arrows represent vertical scan ] at the commencement (A) and at the end (B) of surgery showing successful descemetopexy with air.
Investigations
ASOCT revealed thick, oedematous donor tissue separated from the host Descemet’s membrane, which was torn in the paracentral area with interface fluid.
Differential diagnosis
Wound dehiscence with intact Descemet’s membrane.
Treatment
iOCT-guided descemetopexy with air and dehiscence repair was done with interrupted 10–0 monofilament nylon sutures under general anaesthesia (see online supplementary video).
bcr-2017-221495supp001.mp4 (36.1MB, mp4)
Outcome and follow-up
At 6-month follow-up, BCVA in the right eye was 20/40, with well-apposed graft–host junction without interface fluid. (figures 2B and 4A,B). Fundus examination was normal with no evidence of glaucoma.
Figure 4.
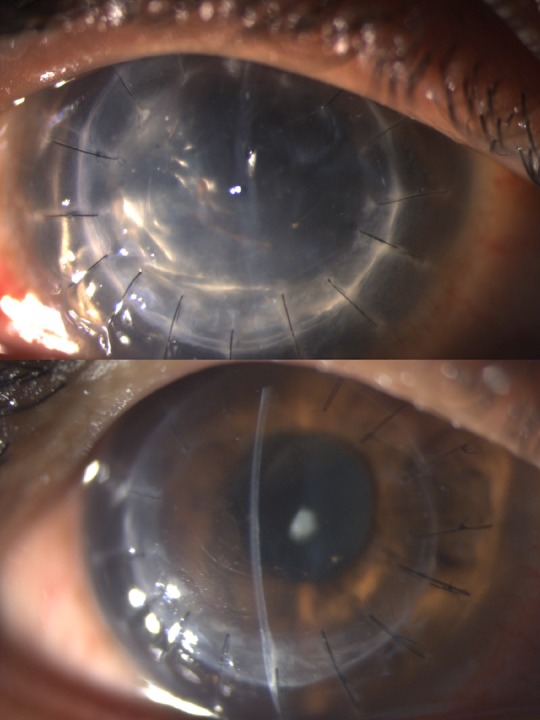
Postoperative slit-lamp biomicroscopic images at day 1 (A) and 6 months follow-up (B) showing well-apposed graft with no interface fluid. At day 1 (A), anterior chamber was almost full with air to provide tamponade.
Discussion
In the recent time, there is a paradigm shift from full thickness to lamellar corneal transplant procedures. The lamellar procedures have benefits of less immunological reaction, use of non-optical grade tissue, minimal postoperative use of steroid, better graft apposition, better healing, avoidance of open sky surgery and others. Even after many years of corneal transplantation, the graft–host junction remains the weakest and the most vulnerable site, which may be jeopardised during blunt trauma.4 5
Even though in DALK surgery, the host Descemet’s membrane is left intact and the patient has a theoretical advantage in graft dehiscence, the overall incidence of graft dehiscence in PK and DALK patients are similar in the various studies.2 3
The corneal wound never gains normal tensile strength after healing. After keratoplasty, wound healing occurs primarily at the endothelial and the epithelial level, while at the stromal level, minimal healing occurs without any collagen fibre anastomosis. A number of factors can affect the wound healing and increase susceptibility for traumatic wound dehiscence. Factors like poor graft apposition, prolonged use of steroids, suture-related complications and avascularity of the graft–host junction could result to a weaker graft–host junction.6 7 Recently, femtosecond laser-assisted keratoplasty is found to be promising in terms of better graft–host apposition and wound healing.8
Causes for wound dehiscence includes trauma, suture-related complications, infection, spontaneous graft dehiscence and others. Among them, trauma is the the most common cause. Common location for wound dehiscence is superonasal or inferotemporal quadrant related to contrecoup or coup injury, respectively.9 iOCT has been used for various anterior segment surgeries like DALK, Descemet stripping automated endothelial keratoplasty, Descemet membrane endothelial keratoplasty, Descemet’s membrane detachment and others. Utility of iOCT for non-traumatic Descemet’s membrane detachment after DALK surgery has been described previously.10 11
Traumatic wound dehiscence in operated DALK is rarely reported in the literature. To the best of our knowledge, there is no report of iOCT-guided management of post-DALK full-thickness traumatic wound dehiscence.12–14
In this case, we made a limbal stab incision at 12 o’clock position adjacent to the intact graft–host junction. Air was then injected into the anterior chamber through the stab incision under the direct guidance of iOCT real-time images. Descemetopexy was done using air under the guidance of iOCT; broken sutures were removed and the area of dehiscence repaired with interrupted 10–0 monofilament nylon sutures. The apposition of the torn Descemet’s membrane to the overlying donor cornea along with the proper alignment of the graft–host junction was confirmed under iOCT.
To summarise, our case illustrates the utility of iOCT in various steps of surgical management in full-thickness wound dehiscence in operated DALK.
Learning points.
Even though Descemet’s membrane left intact in deep anterior lamellar keratoplasty with added benefits of better graft apposition and healing, wound dehiscence can occur with blunt trauma even after many months of surgery.
Timely surgical intervention in a case of graft dehiscence is vital for optimal visual outcomes.
The real-time images of intraoperative optical coherence tomography are proven to be greatly helpful for intraoperative assessment of graft–host interface.
Proper evaluation of the posterior segment, glaucoma and other coexisting complications is necessary for long-term optimal outcomes.
For prevention of graft dehiscence, patient education and encouraging them for usage of protective eyewear are required.
Footnotes
Contributors: MHC, RB, JU and NS have evaluated the case in detail, followed by surgical intervention with optimal outcome.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Farley MK, Pettit TH. Traumatic wound dehiscence after penetrating keratoplasty. Am J Ophthalmol 1987;104:44–9. 10.1016/0002-9394(87)90291-1 [DOI] [PubMed] [Google Scholar]
- 2.Williams MA, Gawley SD, Jackson AJ, et al. Traumatic graft dehiscence after penetrating keratoplasty. Ophthalmology 2008;115:276–8. 10.1016/j.ophtha.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 3.Sari ES, Koytak A, Kubaloglu A, et al. Traumatic wound dehiscence after deep anterior lamellar keratoplasty. Am J Ophthalmol 2013;156:767–72. 10.1016/j.ajo.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 4.Négrel AD, Thylefors B. The global impact of eye injuries. Ophthalmic Epidemiol 1998;5:143–69. 10.1076/opep.5.3.143.8364 [DOI] [PubMed] [Google Scholar]
- 5.Simonsen AH, Andreassen TT, Bendix K. The healing strength of corneal wounds in the human eye. Exp Eye Res 1982;35:287–92. 10.1016/S0014-4835(82)80053-5 [DOI] [PubMed] [Google Scholar]
- 6.Lam FC, Rahman MQ, Ramaesh K. Traumatic wound dehiscence after penetrating keratoplasty-a cause for concern. Eye 2007;21:1146–50. 10.1038/sj.eye.6702407 [DOI] [PubMed] [Google Scholar]
- 7.Calkins JL, Hochheimer BF, Stark WJ. Corneal wound healing: holographic stress-test analysis. Invest Ophthalmol Vis Sci 1981;21:322–34. [PubMed] [Google Scholar]
- 8.Chamberlain WD, Rush SW, Mathers WD, et al. Comparison of femtosecond laser-assisted keratoplasty versus conventional penetrating keratoplasty. Ophthalmology 2011;118:486–91. 10.1016/j.ophtha.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 9.Renucci AM, Marangon FB, Culbertson WW. Wound dehiscence after penetrating keratoplasty: clinical characteristics of 51 cases treated at Bascom Palmer Eye Institute. Cornea 2006;25:524–9. 10.1097/01.ico.0000214232.66979.c4 [DOI] [PubMed] [Google Scholar]
- 10.Titiyal JS, Kaur M, Falera R. Intraoperative optical coherence tomography in anterior segment surgeries. Indian J Ophthalmol 2017;65:116 10.4103/ijo.IJO_868_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma N, Aron N, Kakkar P, et al. Continuous intraoperative OCT guided management of post-deep anterior lamellar keratoplasty descemet’s membrane detachment. Saudi J Ophthalmol 2016;30:133–6. 10.1016/j.sjopt.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee WB, Mathys KC. Traumatic wound dehiscence after deep anterior lamellar keratoplasty. J Cataract Refract Surg 2009;35:1129–31. 10.1016/j.jcrs.2009.01.037 [DOI] [PubMed] [Google Scholar]
- 13.Zarei-Ghanavati S, Zarei-Ghanavati M, Sheibani S. Traumatic wound dehiscence after deep anterior lamellar keratoplasty: protective role of intact descemet membrane after big-bubble technique. Cornea 2010;29:220–1. 10.1097/ICO.0b013e3181a2a7cf [DOI] [PubMed] [Google Scholar]
- 14.Chaurasia S, Ramappa M. Traumatic wound dehiscence after deep anterior lamellar keratoplasty. J Aapos 2011;15:484–5. 10.1016/j.jaapos.2011.06.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bcr-2017-221495supp001.mp4 (36.1MB, mp4)



