Abstract
The authors present a case of a 51-year-old woman with clinical diagnosis of mixed connective tissue disease and overlap systemic lupus erythematosus features, with a 6-month history of progressive painless abdominal distension. On examination, evident signs of ascites were present. Both the abdominal-pelvic ultrasound and CT scan confirmed a large amount of ascites. A diagnostic paracentesis was performed, which revealed typical features of chylous ascites (CA). An extensive diagnostic work-up led by a multidisciplinary team was performed, excluding malignancy, cirrhosis, infectious, as well as cardiac and primary lymphatic causes. The patient was kept under surveillance, with dietary therapy and periodic ascitic drainages. The hypothesis of an autoimmune cause for CA was considered by exclusion. Rituximab therapy was initiated and an excellent response was achieved, with reduction of the rate of accumulation of CA and an increase in quality of life of the patient.
Keywords: connective tissue disease, biological agents, gastrointestinal system
Background
Chylous ascites (CA) is an uncommon form of ascites and its presence under the absence of traumatic causes is even rarer. The diagnosis of the underlying cause of atraumatic CA is extensive, as numerous causes can be linked to its development, starting from the most frequent ones, such as malignancy, cirrhosis and chronic infectious diseases, to the rarer ones, such as fibrosing mesenteritis, retroperitoneal fibrosis, sarcoidosis and systemic lupus erythematosus (SLE). This clinical case underlies the importance of considering autoimmunity diseases, in this case an overlap syndrome between mixed connective tissue disease (MCTD) and SLE, as plausible cause for CA, based on the exclusion of other known causes and a favourable response to rituximab therapy. To the best of our knowledge, this is the first report of rituximab treatment in ascites associated with an overlap syndrome.
Case presentation
A 51-year-old Caucasian woman attended her 6-monthly rheumatology follow-up consultation. The patient was followed in this clinic for overlap syndrome of MCTD with features of SLE. Features of MCTD included lung involvement with severe interstitial lung disease (ILD) and a diffusing capacity for carbon monoxide of 53% predicted, oesophageal dilatation, serositis with mild pericarditis, and high titres of antiribonucleoprotein (anti-RNP) antibodies. According to these, the patient fulfilled the Kasukawa’s criteria for MCTD, but not the Alarcon-Segovia’s criteria, since she did not present history of swollen hands, Raynaud’s phenomenon, myositis, arthritis or acrosclerosis. Features of SLE included history of SLE-subacute-type rash several years before, current serositis with mild pericarditis, sustained lymphopaenia, anti-Sm positivity and hypocomplementaemia. Anti-dsDNA antibodies were negative and there was no history or signs suggestive of renal or neurological involvement. According to these, the patient fulfilled both the 1997 American College of Rheumatology revised classification criteria and the 2012 Systemic Lupus International Collaborating Clinics classification criteria for SLE. The patient did also present skin calcinosis, in this case interpreted as a rare manifestation of MCTD.
She reported a 6-month history of progressive painless abdominal distension, more pronounced in the last 2 months. She denied history of alteration in bowel habits or upper or lower gastrointestinal bleeding. She also reported poor appetite without weight loss. There was ongoing shortness of breath, not worse than usual. No history of fever, jaundice or new skin rashes were present. There was no hair loss, no mouth ulcers, and no pain or swelling of the joints. She denied recent trauma or surgeries.
Other comorbidities included mild chronic kidney disease, upper lobe emphysema (in relation to previous smoking), New York Heart Association (NYHA) class II–III right heart failure, class II obesity and uterine cervical carcinoma in situ treated by colposcopy. For these reasons, she was kept under the care of a multidisciplinary team. There was no history of alcohol consumption or known viral hepatitis.
In the last years, the patient had been clinically stable from the overlap syndrome’s point of view, with no worsening on her ILD with stable functional respiratory tests and high-resolution CT findings in the last 7 years, no new skin rashes, no worsening of calcinosis, controlled reflux and mild lower dysphagia. The only exceptions were the presence of a sustained mild pericardial effusion and serological activity with a mild lymphopaenia of about 0.58×109/L (1.00–4.50×109/L) and C3 hypocomplementaemia of about 0.55 g/L (0.75–1.65 g/L).
She was being treated with azathioprine 100 mg daily and prednisolone 5 mg daily, with other multiple medications addressing her comorbidities including proton pump and ACE inhibitors. She was also under long-term oxygen therapy (2 L/min).
The patient was admitted to the rheumatology ward of our hospital for further investigation.
On admission, on clinical examination she was apyretic, her blood pressure was 107/73 mm Hg and SatO2 was 98% under O2 2 L/min. Cardiopulmonary auscultation was clear, abdomen was tense with clear signs of ascites, and there was a bilateral leg pitting oedema up to the knee. Her left breast was larger than the right one, with one left axillary lymph node palpable. No other palpable lymphadenopathies were present.
Initial investigations
Laboratory tests showed a normocytic anaemia of 92 g/L (115–160 g/dL), mild thrombocytosis of 453×109/L (150−400×109/L), normal coagulation times, hypoalbuminaemia of 22 g/L (33–48 g/L) and elevated C reactive protein of 36 mg/L (<10 mg/L). Interestingly, liver transaminases, bilirubin, creatinine and lactate dehydrogenase (LDH) were within normal values. Urinalysis did not show evidence of proteinuria.
An abdominal-pelvic ultrasound (US) showed a large amount of abdominal and pelvic ascites, a mild enlarged liver with fatty infiltration and no signs of hepatic congestion. An enlarged spleen (14.8 cm) was seen, but the portal and splenic veins were patent with hepatopetal blood flow. The CT scan of the abdomen with contrast confirmed the presence of a large volume of ascites and mild/moderately enlarged retroperitoneal, iliac and inguinal nodes (figures 1 and 2).
Figure 1.
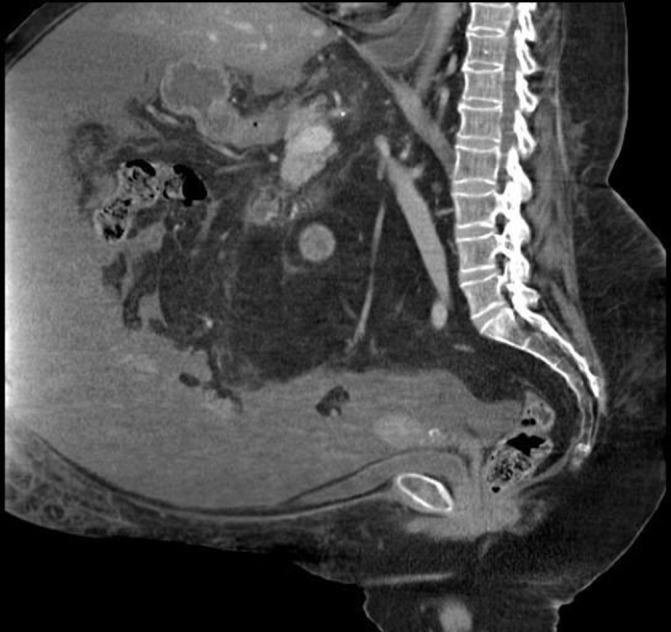
Sagittal abdominal CT scan views showing a widespread large amount of ascites.
Figure 2.
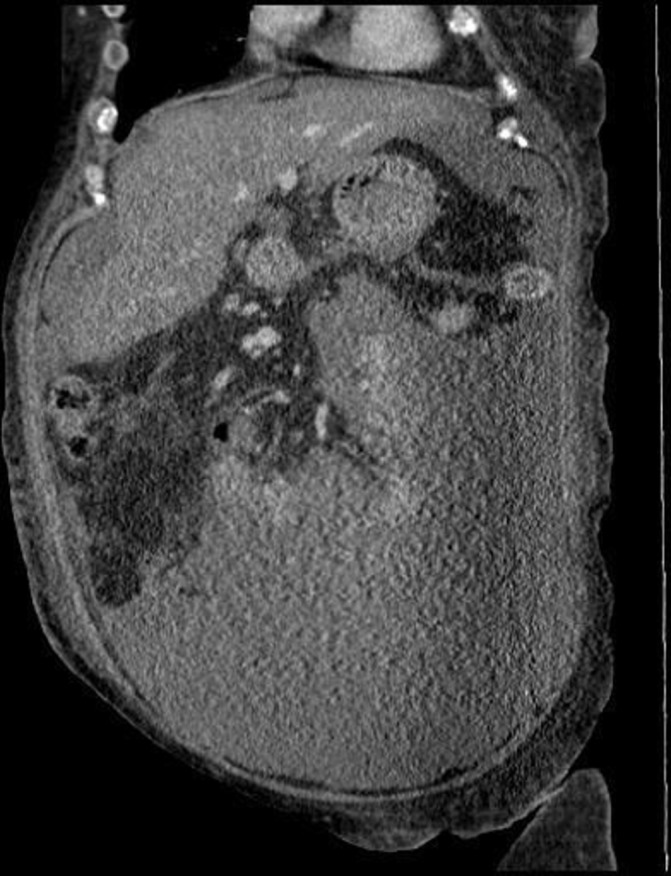
Coronal abdominal CT scan views showing a widespread large amount of ascites.
About 1500 mL of ascitic liquid was extracted by paracentesis. Analysis of the ascitic fluid revealed a milky and cloudy appearance (figure 3) with elevated triglycerides (725 mg/dL), albumin level of 21 g/L with a serum-ascites albumin gradient (SAAG) <1, normal LDH (136 IU/L), normal glucose (85 mg/dL) and amylase (25 UI/L) with cytology revealing moderate cellularity composed of loose aggregates of suboptimally preserved reactive mesothelial cells. These features were compatible with CA.
Figure 3.

Milky and cloudy appearance of the ascitic fluid.
Differential diagnosis and further investigations
An exhaustive investigation was performed, by a multidisciplinary team, to determine the underlying cause of CA. The main differential diagnoses included the following:
Neoplastic cause
The most frequent atraumatic cause of CA in adults is malignancy. In this group, lymphomas account for at least one-third of the cases.1 Other causes are intra-abdominal solid organ malignancies such as in the stomach, oesophagus, pancreas, endometrial, ovaries and prostate, which account for 40% of all malignant causes.2
In this case there were no constitutional symptoms such as weight loss or pronounced asthenia. There were no B-type symptoms suggestive of underlying lymphoma as night sweats or fever. There were no other symptoms suggestive of solid abdominal-pelvic organ malignancy: bowel habits were normal, no signs of upper or lower gastrointestinal bleeding, and no metrorrhagia. Given the history of uterine cervical carcinoma in situ, treated by colposcopy, the patient was kept under regular follow-up in gynaecology consultation, with no abnormal findings reported. Similarly, the patient had a recent endoscopy and colonoscopy, with no signs of gastrointestinal neoplastic lesions. On physical examination, there was an asymmetry between the left and the right breasts, with one left axillary lymph node palpable. No other peripheral lymphadenopathies were present.
The patient has performed a CT of chest, abdomen and pelvis (CT-CAP), which revealed a large amount of ascites, with no masses suspected of malignancy but with mild enlarged nodes in the retroperitoneal iliac and inguinal areas. There were no mediastinal or para-aortic lymph nodes. Chest did not show signs of dilatation on thoracic duct, there was no pleural effusion, but the left breast was abnormal and diffusely oedematous with multiple abnormal left axillary nodes up to 1.8 cm. Biopsy of the left breast and axilla lymph nodes did not show signs of malignancy, with only minor inflammatory features. Biopsy of one of the inguinal lymph nodes was performed and demonstrated features of non-specific inflammatory process. Flow cytometry analysis of the sample did not reveal monoclonal B cell population.
Cytology on ascites fluid did not reveal malignant cells suggestive of occult malignancy. Serum tumour markers were normal, with the exception of CA-125 which was slightly raised.
Serum LDH, electrophoresis and immunoglobulins were normal and not suggestive of other haematological malignancies.
As there was no convincing evidence of primary malignant disease or metastatic disease, the patient was kept under a tight surveillance by the oncology team, with periodic consultations and imaging scans.
Although ascites of unknown aetiology are a common indication for laparoscopy in combination with peritoneal biopsy,3 given the high anaesthetic risk of our patient, the option of a laparoscopy was deferred.
Cirrhotic cause
Despite the fact that ascites are a common manifestation of hepatic cirrhosis, CA presents in only 0.5%–1% of patients with cirrhosis.2 A recent systematic review showed that cirrhosis was responsible for 11% of atraumatic CA.1
In this case, there was no history of alcohol consumption or known viral hepatitis. No jaundice, no fever or other clinical features suggestive of cirrhosis were present. Liver enzymology was normal and there was no hypoprothrombinaemia. Although there was hypoalbuminaemia, SAAG was inferior to 1.1 g/dL, which was not suggestive of cirrhosis. Accordingly, abdominal US did not detect signs of cirrhosis, and portal and splenic veins were patent with hepatopetal blood flow.
Additionally, screening for hepatotropic viruses, including cytomegalovirus, Epstein-Barr and hepatitis B and C, was negative. Liver autoantibodies were also negative.
Based on these findings, the gastroenterology team excluded cirrhosis or any liver disease as the cause of CA.
Infectious cause
Peritoneal tuberculosis is the most common infectious cause of CA and accounts for the majority of the cases in the low-income/middle-income countries.4
Low socioeconomic status, malnutrition, cirrhosis, HIV infection, diabetes mellitus, underlying malignancy, immunosuppressive drugs, cirrhosis and ambulatory peritoneal dialysis are the main risk factors for tuberculous CA.5
In our case, there was no history of risk exposure or symptoms suggestive of active tuberculosis. X-ray did not show features of active tuberculosis, interferon-gamma release assays was negative and the microscopy of ascitic fluid did not reveal acid-fast bacilli. Accordingly, ascitic fluid culture proved to be negative for Mycobacteria, excluding peritoneal tuberculosis.
Cardiac cause
Constrictive pericarditis and severe right heart failure have been reported to cause CA.2 They cause impaired lymph drainage with consequent thoracic duct dilatation and hypertension, leading to an increase in hepatic venous pressure, thereby increasing lymph production.4
Because of the history of right heart failure, the patient has been kept under regular cardiology follow-up. Her diuretic therapy was optimised and a recent echocardiogram revealed stable findings with mild dilatation of the right ventricle with reduced systolic function and mild pericardial effusion, consistent with lupus-like serositis. Also, the leg oedema could be explained by hypoalbuminaemia. N-terminal pro-brain natriuretic peptide (NT pro-BNP) was moderately high (893 ng/dL), lower than what it would be expected in the context of massive ascites of cardiac cause. Additionally, there were no signs of hepatic congestion on US or abnormal liver function tests.
Taking into account the previous findings, the cardiology team excluded cardiac cause of CA in this case.
Lymphatic abnormalities
Congenital lymphatic abnormalities are an important cause of CA in the paediatric population, accounting for 84% of all causes. In adults, the frequency is low, only about 9%.2 Acquired impairments of chyle flow leading to congestion of the lymphatic vessels, as in yellow nail syndrome, can be seen in this age range.1
Although in our clinical case this hypothesis was unlikely, a lymphoscintigraphy was performed to evaluate functional damage on lymphatics and to detect any abnormal lymphatic drainage. No tracer leak was demonstrated within the abdomen or pelvis, leaving this hypothesis less probable.
Despite the fact that lymphangiography use is declining with present non-invasive imaging, this technique still remains as the gold standard in defining cases of lymphatic obstruction.6 In our case, we were unable to perform this exam, given the fact that the major contraindication for its use is cardiovascular or pulmonary disease, especially in patients with pulmonary insufficiency, which was the case of our patient.
Other causes
There are other even rarer causes for CA, like filariasis infection, pancreatitis, sarcoidosis, fibrosing mesenteritis, retroperitoneal fibrosis and nephrotic syndrome, among others.2 Normal findings on CT-CAP, normal serum ACE, normal amylasaemia and absence of proteinuria in the 24-hour protein urine test were some of the reasons why we excluded these hypotheses.
SLE can be a rare cause of CA. The mechanism of SLE causing CA is still not well understood. The cause might be due to SLE-related inflammation of lymphatic system causing damage. Other authors refer that ascites in SLE occur only when complicated by underlying malignancy, nephritic syndrome, congestive heart failure or hepatic cirrhosis.7 A similar type of involvement is theoretically possible in this overlap syndrome, where serositis involvement is an important feature. As this is essentially an exclusion diagnosis, at this moment we could not assume that the overlap syndrome was the underlying cause for CA, but this was kept in the differential diagnosis.
Treatment
The patient was referred to nutritional therapy, with the goal of decreasing the production of chyle, replacing fluid and electrolytes, and maintaining or improving nutrition status. She initiated a high-protein and low-fat diet with short-chain and medium-chain triglycerides.
She was kept under gastroenterology orientation, with regular periodic ascitic drainages, to release pressure from ascitic fluid accumulation in the abdomen. After 1 year of follow-up, there was still no evidence of malignant cause for CA: repeated ascitic fluid cytologies showed no signs of malignant cells, and a second CT of chest and abdomen did not show new lymphadenopathies or progression of the retroperitoneal iliac and inguinal adenopathies.
At this stage, we considered autoimmune serositis related to overlap syndrome involvement as the underlying cause of chronic CA, and for this reason the patient started immunosuppressive therapy with rituximab. This immunosuppressant agent was chosen based on its extrapolated efficacy for treating SLE serositis8 and because this would be a safer option, in a patient where lymphoma has not been completely ruled out. A total of two cycles of rituximab (with two rituximab infusions, 1000 mg each, on day 1 and day 15) separated by a 6-month interval were administrated. The treatment was well tolerated and no adverse outcomes were reported.
Outcome and follow-up
After two cycles of rituximab, her abdominal girth has substantially reduced. The total amount of ascitic fluid, extracted in each drainage, has reduced from 1500 mL to 500–750 mL, and finally to 150 mL/drainage, and the interval between aspirates has increased from weekly to 3 monthly. A control CT scan of the abdomen, with contrast, confirmed the reduction in volume of the ascites (figure 4) and also a decrease in retroperitoneal pelvic lymphadenopathy.
Figure 4.
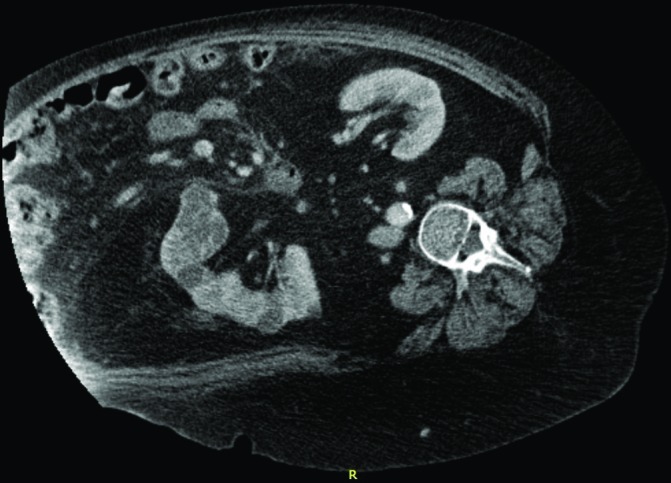
Follow-up abdominal CT scan, performed after rituximab therapy, showing a significant reduction in the ascites volume.
The mild pericarditis and the C3 hypocomplementaemia also resolved after rituximab therapy. A mild lymphopaenia was sustained, as well as the structural damage lesions of the disease, including ILD, oesophageal dilatation and calcinosis. We assumed that this favourable response could only be explained for two reasons: SLE-like serositis in an overlap syndrome as the aetiology of CA or underlying indolent lymphoma not detected after an exhaustive first screening. We intend to keep this patient in a tight clinical and cancer surveillance, but we are satisfied that the response to rituximab has been so positive. So far the hypothesis of overlap syndrome as the cause of CA seems the most probable and sustained diagnosis.
The patient reports feeling much better with rituximab therapy, as CA is less pronounced, causing less physical discomfort, and the need for ascitic drainages is significantly lower. She also refers that her everyday quality of life has gradually increased.
Discussion
CA is a rare form of ascites characterised by a milky-appearing fluid containing high levels of triglycerides. Its incidence ranges from 1 in 20 000 to 1 in 187 000 admissions at large tertiary hospitals.1
CA develops when there is a disruption of the lymphatic system, which occurs due to lymphatic injury or obstruction (from benign or malignant causes).2 Usually CA presents slowly with gradual, painless abdominal distension.9
In clinical practice, trauma and surgery are the most frequent causes of CA, and in these cases CA is secondary to direct injury of lymphatic vessels and subsequent extravasation of chylous into the abdomen. Much less is known about atraumatic causes for CA.9 In a systematic review of atraumatic causes for CA, the most common causes in adults were malignancy (25%), cirrhosis (16%), Mycobacterium infection (15%), and a variety of uncommon causes (23%) that included cardiac causes like constrictive pericarditis and severe right heart failure, fibrosing mesenteritis, nephrotic syndrome, sarcoidosis and SLE, among others.1
In our case, an exhaustive study was performed to uncover the underlying cause, but no conclusive aetiological factor was found. For this reason, we focused on the diagnosis of autoimmune serositis, in an overlap syndrome, as the underlying cause for CA. Although her disease was stable, she kept having an indolent pericardial effusion, revealing that serositis involvement was still active, as well as serological activity. Similar to this case but in the SLE domain, some authors have reported that chronic lupus peritonitis is usually observed in patients with established lupus and that disease activity does not always correlate with its manifestations.10–12 The prompt response to rituximab was one more factor in favour of this hypothesis.
To the best of our knowledge only seven case reports have described this rare association with SLE10–16 but none in the context of an overlap syndrome between MCTD and SLE. In the majority of these case reports, there was a resolution of the CA in response to corticotherapy and/or immunosuppressant agents.
This case provides further evidence of this rare association, provides strong support to the notion that an overlap syndrome can be a possible cause of a CA, and proposes rituximab therapy among the possibly effective therapeutic choices in autoimmune CA.
Learning points.
Atraumatic chylous ascites is a rare clinical condition and when identified should lead to an exhaustive study to determine the underlying aetiology.
Overlap syndrome is an uncommon cause of ascites, even rarer in the case of chylous ascites; thus, the diagnosis can only be suspected after exclusion of other causes.
In our case the prompt response of chylous ascites to an immunosuppressive agent, in this case rituximab, reinforced this diagnosis association.
Footnotes
Contributors: AD did the clinical process review, from the initial symptoms to the final diagnosis, and also elaborated the literature review. GB helped AD in the review process. EV is the assistant physician of oncology who led the differential diagnostic process, providing essential clinical contribution for the exclusion of a malignant cause for chylous ascites. FdG is the assistant physician of rheumatology responsible for the final diagnosis and therapeutic decision of initiating rituximab therapy. He also supervised the report construction from the beginning.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Steinemann DC, Dindo D, Clavien PA, et al. Atraumatic chylous ascites: systematic review on symptoms and causes. J Am Coll Surg 2011;212:899–905. 10.1016/j.jamcollsurg.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 2.Al-Busafi SA, Ghali P, Deschênes M, et al. Chylous Ascites: Evaluation and Management. ISRN Hepatol 2014;2014:1–10. 10.1155/2014/240473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu CM, Lin SM, Peng SM, et al. The role of laparoscopy in the evaluation of ascites of unknown origin. Gastrointest Endosc 1994;40:285–9. 10.1016/S0016-5107(94)70057-5 [DOI] [PubMed] [Google Scholar]
- 4.Cárdenas A, Chopra S. Chylous ascites. Am J Gastroenterol 2002;97:1896–900. 10.1111/j.1572-0241.2002.05911.x [DOI] [PubMed] [Google Scholar]
- 5.Aguado JM, Pons F, Casafont F, et al. Tuberculous peritonitis: a study comparing cirrhotic and noncirrhotic patients. J Clin Gastroenterol 1990;12:550–4. [PubMed] [Google Scholar]
- 6.Campisi C, Bellini C, Eretta C, et al. Diagnosis and management of primary chylous ascites. J Vasc Surg 2006;43:1244–8. 10.1016/j.jvs.2005.11.064 [DOI] [PubMed] [Google Scholar]
- 7.Chen GL, Yang DH, Hsu WH. Chylous ascites and pleural transudate: rare presentations in systemic lupus erythematosus in old age. Case Reports Immunol 2012;2012:1–3. 10.1155/2012/390831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thatayatikom A, White AJ. Rituximab: a promising therapy in systemic lupus erythematosus. Autoimmun Rev 2006;5:18–24. 10.1016/j.autrev.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 9.Browse NL, Wilson NM, Russo F, et al. Aetiology and treatment of chylous ascites. Br J Surg 1992;79:1145–50. 10.1002/bjs.1800791110 [DOI] [PubMed] [Google Scholar]
- 10.Kaklamanis P, Vayopoulos G, Stamatelos G, et al. Chronic lupus peritonitis with ascites. Ann Rheum Dis 1991;50:176–7. 10.1136/ard.50.3.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schousboe JT, Koch AE, Chang RW. Chronic lupus peritonitis with ascites: review of the literature with a case report. Semin Arthritis Rheum 1988;18:121–6. 10.1016/0049-0172(88)90004-2 [DOI] [PubMed] [Google Scholar]
- 12.Prasad S, Abujam B, Lawrence A, et al. Massive ascites as a presenting feature of lupus. Int J Rheum Dis 2012;15:e15–e16. 10.1111/j.1756-185X.2011.01659.x [DOI] [PubMed] [Google Scholar]
- 13.Soysal DE, Hizar Turan S, Ozmen M, et al. A rare case of systemic lupus erythematosus with chylous ascites and chylothorax. Case Rep Rheumatol 2013;2013:1–3. 10.1155/2013/797696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzella DJ, Dettori PN, Hertimian ML, et al. Chylous ascites and chylothorax as presentation of a systemic progression of discoid lupus. J Clin Rheumatol 2013;19:87–9. 10.1097/RHU.0b013e3182847260 [DOI] [PubMed] [Google Scholar]
- 15.B’Chir Hamzaoui S, Abdallah M, Bouslama K, et al. [Chylous ascites revealing a systemic lupus erythematosus (L)]. Gastroenterol Clin Biol 2007;31:100–1. [DOI] [PubMed] [Google Scholar]
- 16.Lee CK, Han JM, Lee KN, et al. Concurrent occurrence of chylothorax, chylous ascites, and protein-losing enteropathy in systemic lupus erythematosus. J Rheumatol 2002;29:1330–3. [PubMed] [Google Scholar]


