Abstract
Melioidosis is endemic in Southeast Asia and tropical Australia with varying clinical features from benign skin lesions to fatal septicaemia. Imaging plays an important role in evaluation of the melioid liver abscesses. A 45-year-old man with underlying diabetes presented with fever and lethargy for 2 weeks and abdominal pain for 2 days. His liver was enlarged on examination. Blood investigations revealed mild leucocytosis and raised liver enzymes. Ultrasound showed multiple multiloculated hypoechoic lesions throughout the liver and spleen. CT of abdomen confirmed that some liver lesions were made up of asymmetric locules of varying sizes (honeycomb sign), while others had hypodense centre with small symmetric peripheral locules in radial fashion (necklace sign). Blood culture was positive for Burkholderia pseudomallei. He was subsequently treated with ceftazidime for a month followed by oral trimethoprim–sulfamethoxazole for 3 months. Follow-up CT of abdomen a month after diagnosis and treatment showed resolving hepatic and splenic lesions.
Keywords: hepatitis and other GI infections, radiology
Background
Melioidosis is endemic in Southeast Asia and tropical Australia. Clinical features of infection can be non-specific and vary from benign skin lesions to fatal septicaemia.1 Imaging specialists may occasionally encounter melioid liver abscesses on ultrasound or CT scan. Familiarity with the imaging characteristics will potentially expedite treatment and avoid unnecessary battery of tests or intervention.
Case presentation
A 45-year-old man with underlying type 2 diabetes mellitus presented to a district hospital (Hospital Tuanku Ampuan Najihah, Kuala Pilah, Malaysia) with fever and lethargy for 2 weeks and abdominal pain for 2 days. He had no history of immunosuppression, chronic alcohol consumption or liver disease. On admission, he was drowsy and hypotensive but afebrile with no tachycardia. Blood pressure normalised after fluid resuscitation. There was hepatomegaly with a liver span of 20 cm on examination. The rest of clinical examinations were unremarkable.
Investigations
Random blood sugar was raised (30.5 mmol/L; normal range <7.8). There were mild leucocytosis (12×109/L; normal range 4–11) and hypoalbuminaemia (29.5 g/L; normal range 35–50 g/L). Other relevant blood results include raised alkaline phosphatase (492 U/L; normal range 30–120), aspartate amino transferase (281 U/L; normal range <40 U/L), alanine amino transferase (190 U/L; normal range <45) and lactate dehydrogenase (488 U/L; normal range <248). Serum sodium level was low (114 mmol/L; normal range 136–145 mmol/L).
Chest radiograph was normal.
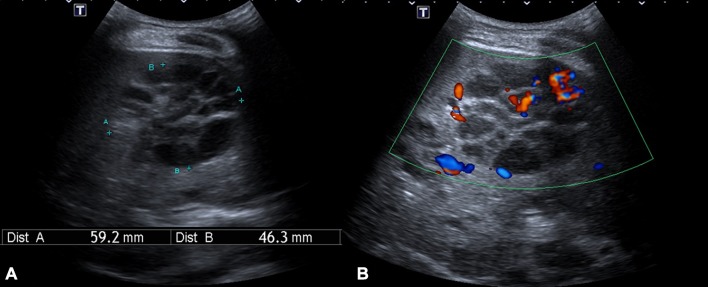
Ultrasound of the abdomen showed multiple multiloculated hypoechoic lesions of varying sizes throughout the liver and spleen with minimal intralesional Doppler signals (figure 1).
Figure 1.
Transabdominal ultrasound at segment V of the liver showed (A) a hypoechoic lesion made up of multiple locules of varying sizes with (B) minimal intralesional Doppler signals.
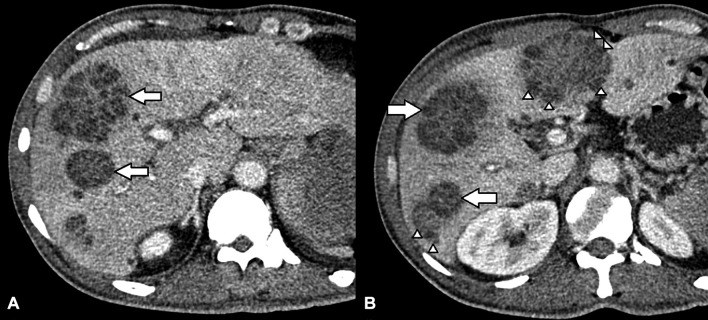
Contrast-enhanced CT of the abdomen performed at a tertiary hospital an hour drive away (Hospital Tuanku Ja’afar, Seremban, Malaysia) confirmed that some liver lesions were made up of asymmetric locules of varying sizes (honeycomb sign), while others had hypodense centre with small symmetric peripheral locules in radial fashion (necklace sign) (figure 2). The splenic lesions were heterogeneously hypodense and irregular. These findings were consistent with multiple liver and splenic abscesses.
Figure 2.
Contrast-enhanced CT scan of the liver above (A) and at the level of (B) the main portal vein showed that some lesions were made up of asymmetric locules of varying sizes (arrows), while others had hypodense centre with small symmetric peripheral locules in radial fashion (arrowheads).
Blood culture at day 3 of admission revealed Burkholderia pseudomallei. No sample was obtained from the liver or splenic lesions for pus culture in view of multiloculations and high suspicion of melioidosis clinically and on imaging.
Differential diagnosis
Tuberculosis, fungal or other Gram-negative bacterial infections such as Escherichia coli and Klebsiella pneumoniae.
Treatment
He was initially suspected to have acute hepatitis and was investigated to identify potential causes. Following ultrasound findings, he was then started on intravenous ceftazidime while waiting for positive blood culture. Intravenous ceftazidime was continued for a month (intensive therapy) followed by oral trimethoprim–sulfamethoxazole (eradication therapy) for 3 months.
Other supportive managements include fluid therapy, sodium correction and blood sugar control.
Outcome and follow-up
His clinical condition improved during intensive therapy with normalisation of liver enzymes, sodium and blood sugar levels.
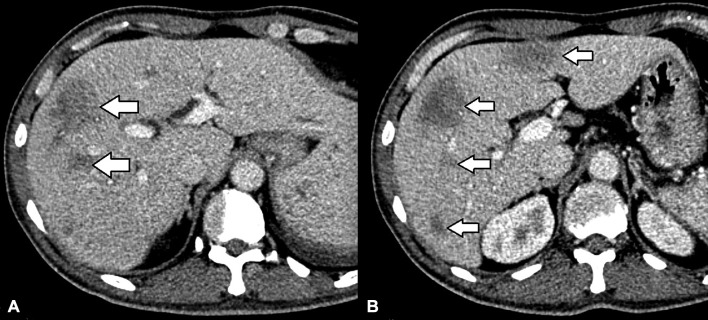
Follow-up CT of abdomen a month after diagnosis and treatment showed resolving hepatic and splenic lesions. The lesions have now become smaller, ill defined and almost isodense to the background parenchyma (figure 3).
Figure 3.
Follow-up CT scan of the liver above (A) and at the level of (B) the main portal vein a month after diagnosis showed that the previously seen lesions in figure 2 have become smaller, ill defined and almost isodense to the liver parenchyma.
Discussion
B. pseudomallei is the causative pathogen of melioidosis. Majority (53%–88%) of patients with clinically apparent melioidosis have underlying predisposing disease, such as diabetes mellitus.1 The three recognised mode of transmission are inhalation, ingestion and inoculation.1 The most common primary clinical presentation is pneumonia (36%) followed by soft tissue abscesses (33%).2 Common sites of abscesses include the subcutaneous tissue (21%), liver (18%), spleen (13%) and lung (13%).2 There have also been sporadic reports of melioid abscesses in prostate, parotid and bones.2 3
It is possible that the pathogen invades the liver via hepatic arteries and portal veins to form hepatic abscesses.3 In general, hepatic abscesses are thought to have up to five descriptive characteristics on CT scan and are not strictly representative of underlying causative organisms.4 The more commonly encountered double target sign or cluster sign, which are indicative of amoebic and bacterial infections, are not usually seen in melioid hepatic abscesses.5 Honeycomb and necklace signs have been reported as characteristic CT findings of the latter.6 Based on a study on 49 Thai participants, the honeycomb pattern could be found in non-melioid lesions, whereas the necklace sign is strongly associated with melioid hepatic abscesses.5 Nevertheless, detection of these lesions along with concurrent splenic abscesses in diabetic patients from endemic regions, suggest high association with melioidosis than other organisms3 It is uncertain how B. pseudomallei forms those characteristic abscesses.
Although ultrasound features of hepatic abscesses have been well described, these were predominantly review articles on differentiating amoebic and pyogenic abscesses.7 8 There is no pathognomonic appearance of liver abscesses on ultrasound and not infrequently the findings can be difficult to differentiate from solid tumours.7 8 Retrospective review of our ultrasound images demonstrates that it is possible to identify honeycomb and necklace patterns on ultrasound. In resource-limited settings, early identification of characteristic features of melioid liver abscesses on ultrasound can potentially obviate the need for more advanced imaging which is not immediately accessible. Identification of these lesions should also alert clinicians from non-endemic regions if travellers from endemic regions present under their care. Nevertheless, imaging should not delay the initiation of antibiotics in patients with suspected sepsis.
The treatment of melioidosis consists of initial intravenous intensive therapy for at least 2 weeks followed by eradication therapy orally for a minimum of 3 months. Duration of intensive therapy can be up to 8 weeks or longer depending on patient’s condition. Preferred antibiotics for intensive therapy are ceftazidime, meropenem and imipenem, while oral eradication therapy of choice is trimethoprim–sulfamethoxazole.9 Image-guided drainage of deep-seated abscesses may be required, but in our case, there were clinical and radiological improvements with medical therapy alone.
In conclusion, liver abscesses with honeycomb and necklace signs can suggest underlying melioidosis. Ultrasound can be a useful tool to aid in the diagnosis of hepatic melioidosis if there is high clinical suspicion.
Learning points.
Liver abscesses with honeycomb and necklace signs can suggest underlying melioidosis.
Ultrasound can be a useful tool to aid in the diagnosis of hepatic melioidosis if there is high clinical suspicion.
Imaging should not delay the initiation of antibiotics in patients with suspected sepsis.
Footnotes
Contributors: The authors SCLO, MMM, NZ and NAAH have contributed sufficiently to the project and have met all criteria to be included as authors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Puthucheary SD. Melioidosis in Malaysia. Med J Malaysia 2009;64:266–74. [PubMed] [Google Scholar]
- 2.Kingsley PV, Leader M, Nagodawithana NS, et al. Melioidosis in Malaysia: a review of case reports. PLoS Negl Trop Dis 2016;10:e0005182 10.1371/journal.pntd.0005182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muttarak M, Peh WC, Euathrongchit J, et al. Spectrum of imaging findings in melioidosis. Br J Radiol 2009;82:514–21. 10.1259/bjr/15785231 [DOI] [PubMed] [Google Scholar]
- 4.Mortelé KJ, Segatto E, Ros PR. The infected liver: radiologic–pathologic correlation. Radiographics 2004;24:937–55. 10.1148/rg.244035719 [DOI] [PubMed] [Google Scholar]
- 5.Apisarnthanarak P, Thairatananon A, Muangsomboon K, et al. Computed tomography characteristics of hepatic and splenic abscesses associated with melioidosis: a 7-year study. J Med Imaging Radiat Oncol 2011;55:176–82. 10.1111/j.1754-9485.2011.02248.x [DOI] [PubMed] [Google Scholar]
- 6.Apisarnthanarak A, Apisarnthanarak P, Mundy LM. Computed tomography characteristics of Burkholderia pseudomallei liver abscess. Clin Infect Dis 2006;42:989–93. 10.1086/501017 [DOI] [PubMed] [Google Scholar]
- 7.Sakijan AS. Ultrasonographic findings of liver abscess. Med J Malaysia 1988;43:332-7. [PubMed] [Google Scholar]
- 8.Ralls PW, Barnes PF, Radin DR, et al. Sonographic features of amebic and pyogenic liver abscesses: a blinded comparison. AJR Am J Roentgenol 1987;149:499–501. 10.2214/ajr.149.3.499 [DOI] [PubMed] [Google Scholar]
- 9.Currie B, Anstey N. Treatment and prognosis of melioidosis. 2017. http://www.uptodate.com/contents/treatment-and-prognosis-of-melioidosis (accessed 14 Aug 2017).