Abstract
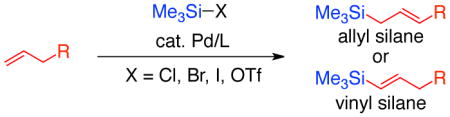
Few methods exist to directly install silyl functionality onto olefins. This Synpacts highlights the state of the art of the silyl-Heck reaction and our recently developed conditions for preparing allyl and vinyl silanes from terminal alkenes using this method.
Keywords: allyl silane, vinyl silane, silyl-Heck reaction, alkene, palladium
Graphical abstact
Unsaturated organosilanes are extraordinarily useful intermediates in synthetic chemistry. Both allyl and vinyl silanes are indispensable nucleophiles in organic synthesis and can be applied in a host of transformations.1 Among many notable applications, Hiyama cross-coupling reactions,2 Fleming-Tamao oxidations, and Hosomi-Sakurai-type crotylations stand out as particularly important examples.3 Organosilanes are also particularly useful reagents due to their low cost, low toxicity, and high stability.1a
Numerous methods exist for the preparation of both allyl and vinyl silanes,4 however, many have limitations that continue to make the preparation of unsaturated organosilanes cumbersome. For example, several methods rely on manipulation of prefunctionalized substrates, wherein the unsaturated silane product does not have increased complexity compared to the starting material (e.g. silyl substitution of allyl ethers or alcohols).5 In some cases, these reactions also require the use of aggressive nucleophiles (such as in silylation of organo-lithium or magnesium reagents), which limits functional group tolerance.6 Hydrosilylation is a simple and mild alternative,7 but requires access to an alkyne starting material, of which there is limited commercial availability. In addition, as these methods are overall reductive in nature, they waste reactive equivalents of the substrate. Finally, a number of methods introduce the silicon center indirectly using C–C bond forming reactions (e.g., alkene metathesis and C–C bond-forming cross-coupling reactions),8 but these strategies require an unsaturated organosilane coupling partner, which ultimately must be synthesized by other means.
In contrast, a method that utilizes alkenes as starting materials for allyl and vinyl silane synthesis would be highly attractive. Alkenes are simple, widely available, inexpensive and stable. However, to date few methods exist to directly attach silyl groups to alkenes to form allyl or vinyl silanes.9
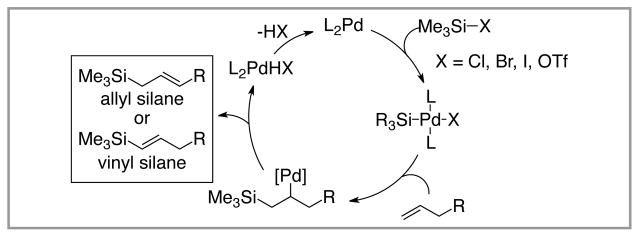
The silyl-Heck reaction is an emerging reaction that allows direct conversion of alkenes into allyl and vinyl silanes. Conceptually, this process can be envisioned as a variant to the widely utilized Heck arylation reaction (Figure 1).10 Oxidative addition of a low-valent late transition metal complex (most often palladium) into a Si-X bond (X = halide or pseudohalide) gives rise to a metal silane complex. Migratory insertion into an alkene followed by β-hydride elimination then delivers either allyl or vinyl silane, depending on the nature of the substrate.
Figure 1.
Mechanistic Outline of the Silyl-Heck Reaction.
Although the potential of this reaction for unsaturated silane synthesis was recognized over 20 years ago (see below), it was not until recently that practical protocols for this reaction have been developed.11 In its current form, silyl-Heck reactions now proceed at mild temperatures (rt to 50 °C), in good to excellent yields, and with excellent functional group tolerance. Both allyl and vinyl silanes can now be prepared in this manner using simple alkene starting materials.
This review highlights the early developments leading to the silyl-Heck reaction and the current state of this area, as well as that of a few related processes. Finally, we discuss future directions of this emerging technology, which we believe will become an increasingly important method for the preparation of organosilanes and the downstream products derived from them.
Early Studies
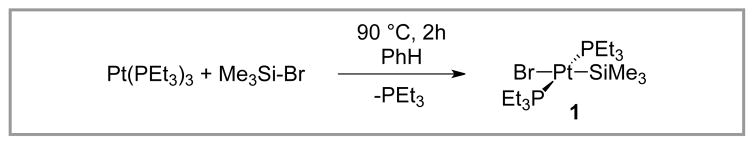
Early studies from the Tanaka group laid the foundation for silyl-Heck reactions. In 1988, they reported the stoichiometric oxidative addition of zero-valent platinum complexes (either Pt(PEt3)3 or Pt(PEt3)4) to Me3Si-I and Me3Si-Br.12 These were the first characterized examples of transition metal insertion into a silyl-halide bond, and the product from the reaction of Me3Si-Br, trans-(PEt3)2Pt(II)(SiMe3)Br (1), was crystallographically characterized. Interestingly, Pt(PPh3)4, Pt(PMe2Ph)4, and Pt(dppe)2 were all reported to be inert in the reaction. Further, unlike the bromo and iodo analogs, insertion into Me3Si-Cl did not occur, even at elevated temperatures. This result was explained by the stronger Si–Cl bond strength (98 kcal/mol), compared to those of Si–Br and Si–I (76 and 57 kcal/mol, respectively).12–13
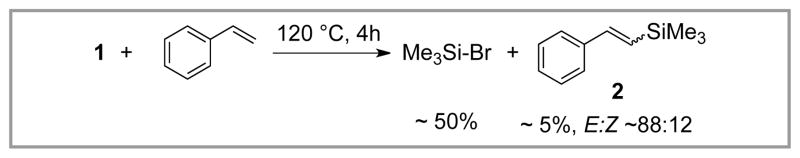
Tanaka recognized the potential of 1 and related intermediates in Heck-type reactions. However, the reaction of 1 with styrene was found to lead primarily to Me3Si-Br (via reductive elimination), and only trace (ca. 5%) of the desired vinyl silane was observed, in this case as an approximate 88:12 E:Z mixture (Scheme 2).14
Scheme 2.
Stoichiometric Reaction of 1 with Styrene.
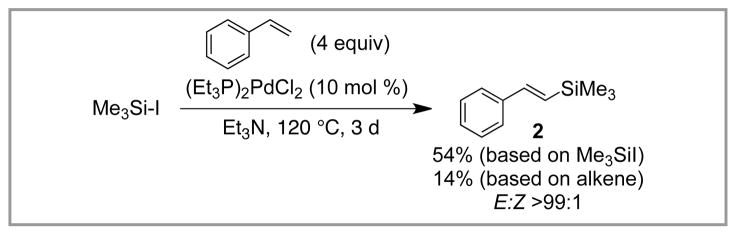
In a parallel study, however, Tanaka had success in observing Heck-type reactivity with Me3Si-I.15 It was found that after prolonged heating at 120 °C in Et3N, PdCl2(PEt3)2 (10 mol %) catalyzes the reaction of Me3Si–I with excess styrene (4 equiv) to give vinyl silane 2 in modest yield (54% based on Me3Si–I, 14% based on styrene, Scheme 3). In this case, 2 was formed as a single regioisomer with >99:1 E:Z selectivity. This seminal report represented the first demonstration of a silyl-Heck reaction and highlighted the challenges associated with making this reaction synthetically practical: low yield, inverted stoichiometry (the more valuable olefin is used in large excess), and limited olefin scope (only three simple styrene derivatives were studied).
Scheme 3.
First Example of A Silyl-Heck Reaction (Tanaka).
Recent Advances
Our group recognized the potential importance of Tanaka’s observations as an entry into unsaturated organosilanes. We postulated that a general protocol for converting alkenes to either allyl or vinyl silanes might emerge if higher yielding, milder reaction conditions were developed that did not require excess alkene.
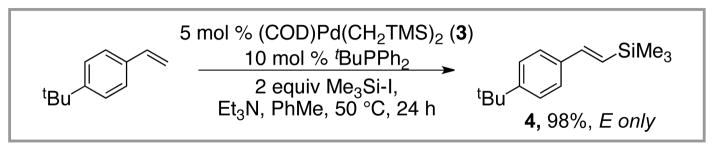
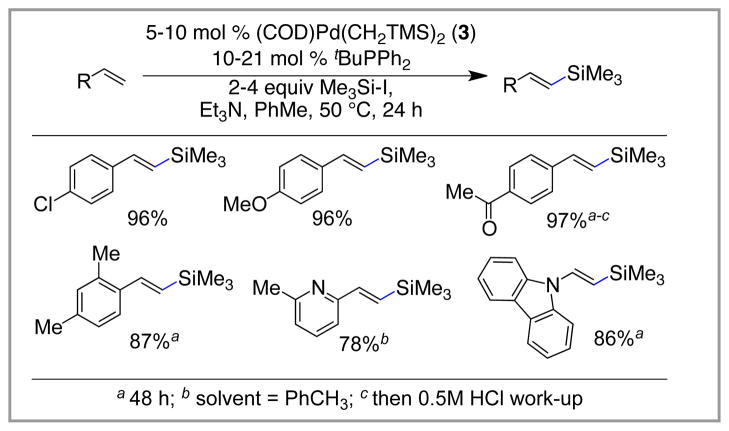
Our initial hypothesis revolved around the identification of a new ligand that would provide a more active palladium catalyst for the reaction. As a first step towards identifying such a catalyst, we elected to study the silylation of 4-tert-butylstyrene using Me3Si-I. Using the conditions outlined in Scheme 4, we rapidly identified tBuPPh2 as a superior ligand for the desired transformation. Under these conditions (5 mol % Pd, 2 equiv Me3Si-I, Et3N, 50 °C, 24 h in PhMe), the vinyl silane was isolated in 98% yield as the E-isomer; the Z-isomer was not detected. These reaction conditions are superior to those previously reported in several ways. First, and most importantly, they are high yielding and use the alkene as the limiting reagent. Second, they operate at much lower temperatures (50 °C) and tolerate the use of solvent (PhMe), which increases the substrate scope of the reaction.
Scheme 4.
Improved Silyl-Heck Reaction (Watson).
In contrast to tBuPPh2, Et3P as well as other common ligands such as Ph3P, Cy3P and tBu3P provide only traces of the desired product under these conditions. We believe that tBuPPh2 is a superior ligand as it meets the dichotomous need for both a large and small ligand. On one hand, the tert-butyl group provides a large, electron-rich phosphine, which leads to a highly active catalyst that avoids coordinative saturation of the metal center. On the other hand, the flat phenyl groups are able to rotate out of the way, which provides sufficient space around the metal center to accommodate the large Me3Si group. This dichotomy of size requirements has been termed the “Goldilocks effect” in an article highlighting the reaction.16
This palladium-catalyzed silylation is effective for preparing a wide range of vinyl silanes from alkenes lacking β-hydrogen atoms. A broad variety of functionalities are tolerated for these substrates, including ethers, halides, ketones, esters, and heterocycles. The method is highly selective for mono-substituted alkenes. In all cases, high yields and complete selectivity for the E-vinyl silane are observed. Scheme 5 highlights a range of the substrates that can be prepared using the silyl-Heck reaction.
Scheme 5.
Preparation of Vinyl Silanes Using the Silyl-Heck Reaction.
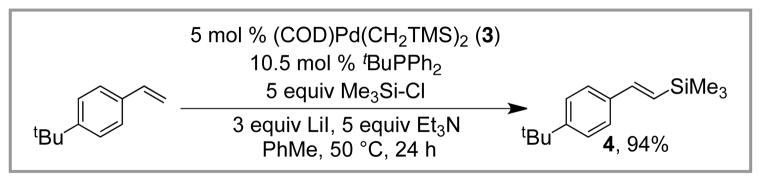
Tanaka’s early studies showed that insertion into the Si-Cl bond is challenging due to its high bond strength. Our group has found that Me3Si-Cl can be utilized in the silyl-Heck reaction if iodide salts are added. For example, 4-tert-butylstyrene can be silylated in high yield using Me3Si-Cl/LiI and the tBuPPh2-derived catalyst (Scheme 6). We assume that in situ halide exchange occurs, leading to the formation of Me3Si-I. In general, similar yields of vinyl silanes are observed with this protocol compared to the direct use of Me3Si-I, and may be advantageous due to the less costly silylating reagent.
Scheme 6.
Conditions for Silyl-Heck Reaction Using TMSCl/LiI.
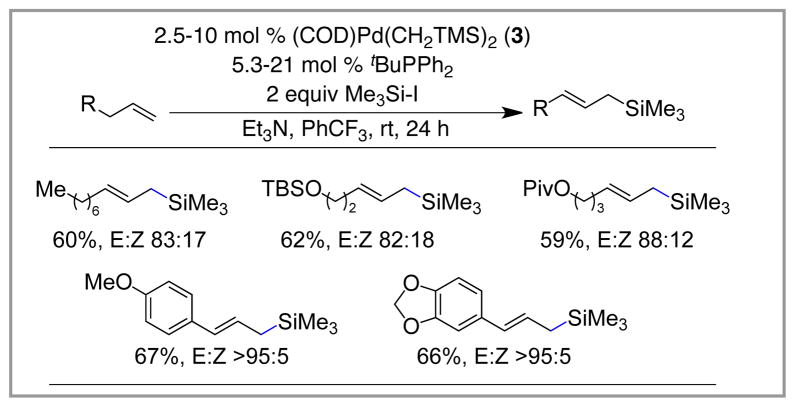
Prior to our work in the area, only styrene substrates had been examined in the silyl-Heck reaction (see above). It was unknown if other types of alkenes could be silylated using this manifold. In particular, α-olefins are an interesting class of substrates, as they are widely available and readily prepared. They also appeared to be a challenging class of substrates, as either allyl or vinyl silanes could emerge as products from the silyl-Heck reaction.
In fact, we found that α-olefins can be used as substrates in the reaction, and that high levels of selectivity for E-allyl silanes are observed. Very little vinyl silane was formed in these reactions, and good to excellent levels of selectivity for the E-allyl over Z-allyl were found. The major competing pathway was isomerization of the substrate to give internal alkenes, which were inert to further reaction. Under the conditions developed for vinyl silane synthesis, this competing reaction greatly decreased the yield of the allyl silane products. However, when the reactions are conducted at room temperature with PhCF3 as the solvent, this competitive process is largely suppressed, and good yields of the allyl silane products can be achieved. The role of the fluorinated solvent in these reactions remains unclear and is the subject of current investigations. Using these conditions, a broad range of terminal alkenes can be transformed into allyl silanes (for examples, Scheme 7). As such, the silyl-Heck reaction provides a very attractive route into this class of high-value nucleophiles.
Scheme 7.
Preparation of Allyl Silanes Using the Silyl-Heck Reaction.
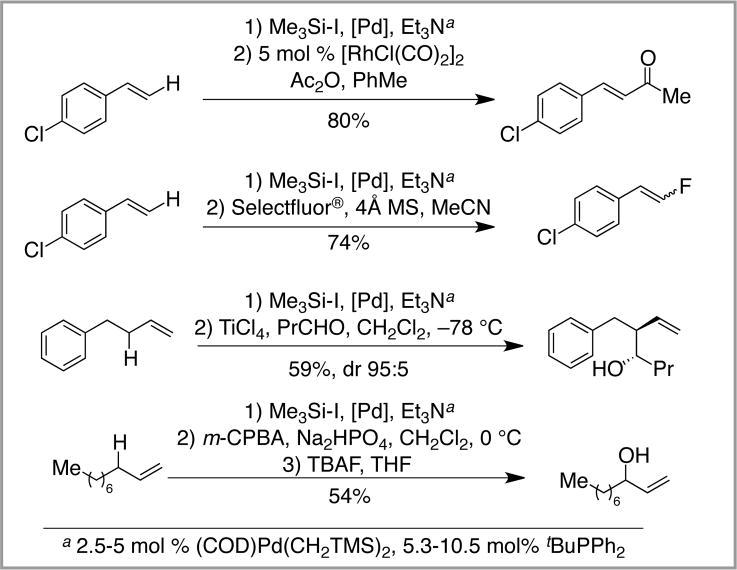
The power of the silyl-Heck reaction lies in the ability to easily access unsaturated organosilanes from widely available starting materials, combined with the vast range of products that can be obtained from the organosilane products. For example, in addition to Hiyama cross-coupling (not shown),17 vinyl silanes can be readily converted into unsaturated ketones18 or vinyl fluorides,19 among many other reactions.1 Likewise, allyl silanes are excellent nucleophiles in a wide range of allylation reactions. They can be used in Hosomi-Sakurai reactions,20 or oxidized to allylic alcohols.21 Moreover, when the silyl-Heck reaction is combined with these other protocols, a highly-flexible method for allylic and vinyl C-H functionalization emerges (Scheme 8).11
Scheme 8.
Formal C-H Functionalization Based Upon Silyl-Heck Reactions and Subsequent Functionalizations.
Related Reactions of Alkynes
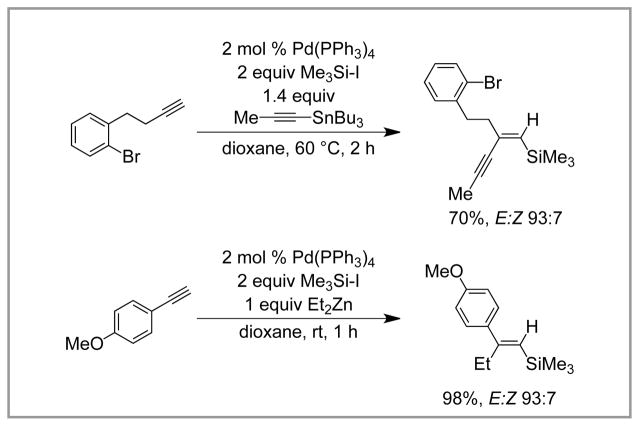
Beginning in 1991, Murai published a series of two papers describing the three-component coupling reaction of terminal alkynes, Me3Si-I, and organometallic nucleophiles (either organostannane or organozinc reagents) catalyzed by [Pd(PPh3)4]. These reactions give rise to trisubstituted vinyl silanes (Scheme 9). Presumably, this transformation is mechanistically related to the silyl-Heck reaction. The reaction proceeds in good to excellent yield (70 – 97%) and good stereoselectivity (E/Z ratios 56:44–98:2). Numerous organometallic groups can be incorporated, including vinyl-, allyl-, and alkynyl organostannanes and alkylzinc reagents, and both Me3Si-I and Me3SiSiMe2-I can be used. A number of terminal aromatic and aliphatic alkynes can be functionalized in this transformation; however, the reaction of alkenes was not reported.
Scheme 9.
Murai’s Three-Component Couplings.
Related Early Metal-Catalyzed Transformations
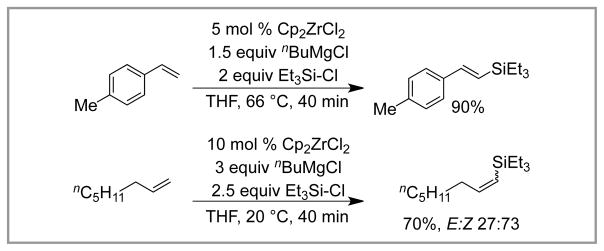
In 1998, Terao and Kambe reported the zirconocene-catalyzed silylation of alkenes with chlorosilanes (Scheme 10).9b, 22 The reaction employs a stoichiometric Grignard reagent to achieve catalyst activation and turnover. The mechanism of the reaction was later shown to be distinct from the silyl-Heck reaction. However, it is included here as the overall transformation is similar, and it utilizes chlorosilanes. The reaction is believed to involve the formation of a Zr(II)-alkene complex, which is subsequently silylated. Notably, this mechanism does not involve insertion into the Si-Cl bond, and likely explains why a large range of chlorosilanes are able to participate in the reaction (including Me3Si-Cl, Et3Si-Cl, and nPr3Si-Cl).
Scheme 10.
Kambe’s Zirconocene-Catalyzed Silylation of Alkenes.
In general, both styrenes and α-olefins give rise to vinyl silanes. In some cases, the E vinyl silane is favored, but in other cases the Z isomer is reported to be major. In most cases, selectivity is moderate. An exception to the formation of vinyl silanes is for reactions involving allyl benzene, which give rise to the allyl silane wherein the alkene is conjugated to the arene. This transformation has the advantage of utilizing inexpensive chlorosilanes, but the need for stoichiometric Grignard reagent limits the substrate scope of the reaction.
Summary and Future Outlook
Although the initial reports of silyl-Heck reactions now date back over 20 years, only recently has the reaction been developed into a practical method for preparing unsaturated organosilanes. The protocol reported by our group allows for the direct conversion of terminal alkenes to allyl and vinyl silanes in an efficient and functional group tolerant way. The products from these reactions are highly useful nucleophiles in a wide variety of reactions; as such, they allow for rapid entry into a broad range of products. Yet, despite the recent progress in this area, challenges remain. First, with the exception of a few disilane examples reported by Murai, to date all methods have been limited to installation of symmetric trialkylsilyl groups. While the vinyl and allyl silanes derived from these products are useful, silyl-Heck reactions that incorporate benzylic, heteroatomic or aromatic silane substituents would be a significant advance. Such products have been shown to be highly versatile reagents in synthesis.2 Second, although the recent advances from our group have demonstrated for the first time that allyl silanes can be prepared from terminal alkenes using silyl-Heck reactions, more remains to be done. In particular, while the yields of the current protocol are synthetically useful, the reactions continue to suffer from competitive isomerization of the starting material. Improved catalysts that avoid this side reaction need to be developed. Third, and finally, within the Heck-type manifold, unassisted activation of Si-Cl bonds has not yet been achieved. This is an important goal, which we expect will further increase functional group tolerance while increasing abundance and reducing cost of the silylating reagent. All these goals, as well as others, are currently under active investigation within the Watson lab.
Scheme 1.
Oxidative Addition of Pt(0) Complexes to Si-X bonds.
Statement of significance of work.
This work reviews the direct preparation of allyl and vinyl silanes using the silyl-Heck reaction. This reaction allows for the preparation of highly useful unsaturated organosilanes from inexpensive, abundant alkenes.
Acknowledgments
The University of Delaware (UD) and the NSF (CAREER CHE-1254360) are gratefully acknowledged for funding and other research support. SESM acknowledges graduate fellowship support by NIH/NIGMS CBI Training Grant 5T32 GM 08550-16.
Biographies
 Sara Martin was born in Pottstown, PA, USA. She received her BSc in Chemistry from Lebanon Valley College in 2009. She is currently a pursuing doctoral studies under the mentorship of Prof. Donald A. Watson at the University of Delaware. Her research projects focus on exploiting transition metal-catalyzed activations of silicon-halogen and -pseudohalogen bonds to form synthetically useful organosilanes.
Sara Martin was born in Pottstown, PA, USA. She received her BSc in Chemistry from Lebanon Valley College in 2009. She is currently a pursuing doctoral studies under the mentorship of Prof. Donald A. Watson at the University of Delaware. Her research projects focus on exploiting transition metal-catalyzed activations of silicon-halogen and -pseudohalogen bonds to form synthetically useful organosilanes.
 Donald A. Watson grew up in Woodland, CA, USA. He earned a BS in Chemistry from University of California, San Diego in 1998, and a PhD in Chemistry in 2004 from the University of California, Irvine under the supervision of Prof. Larry E. Overman. He was an NIH postdoctoral fellow with Prof. Robert G. Bergman at the University of California, Berkeley from 2004–2006, and completed additional postdoctoral studies at the Massachusetts Institute of Technology, working with Prof. Stephen L. Buchwald. In 2009, he joined the faculty at the University of Delaware as an assistant professor.
Donald A. Watson grew up in Woodland, CA, USA. He earned a BS in Chemistry from University of California, San Diego in 1998, and a PhD in Chemistry in 2004 from the University of California, Irvine under the supervision of Prof. Larry E. Overman. He was an NIH postdoctoral fellow with Prof. Robert G. Bergman at the University of California, Berkeley from 2004–2006, and completed additional postdoctoral studies at the Massachusetts Institute of Technology, working with Prof. Stephen L. Buchwald. In 2009, he joined the faculty at the University of Delaware as an assistant professor.
References
- 1.(a) Brook MA. Silicon in Organic, Organometallic, and Polymer Chemistry. Wiley; Chichester: 2000. [Google Scholar]; (b) Fleming I, Barbero A, Walter D. Chem Rev. 1997;97:2063–2192. doi: 10.1021/cr941074u. [DOI] [PubMed] [Google Scholar]
- 2.(a) Nakao Y, Hiyama T. Chem Soc Rev. 2011;40:4893–4901. doi: 10.1039/c1cs15122c. [DOI] [PubMed] [Google Scholar]; (b) Denmark SE, Liu JHC. Angew Chem Int Ed. 2010;49:2978–2986. doi: 10.1002/anie.200905657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Hosomi A, Endo M, Sakurai H. Chem Lett. 1976;5:941–942. [Google Scholar]; (b) Masse CE, Panek JS. Chem Rev. 1995;95:1293–1316. [Google Scholar]; (c) Denmark SE, Fu J. Chem Rev. 2003;103:2763–2793. doi: 10.1021/cr020050h. [DOI] [PubMed] [Google Scholar]
- 4.(a) Oshima K. Vinylsilanes. In: Fleming I, editor. Science of Synthesis. Vol. 4. Thieme; Stuttgart: 2001. pp. 713–754. [Google Scholar]; (b) Sarkar TK. Allylsilanes. In: Fleming I, editor. Science of Synthesis. Vol. 4. Thieme; Stuttgart: 2001. pp. 837–924. [Google Scholar]
- 5.(a) Bourque L, Cleary P, Woerpel KA. J Am Chem Soc. 2007;129:12602–12603. doi: 10.1021/ja073758s. [DOI] [PubMed] [Google Scholar]; (b) Moser R, Nishikata T, Lipshutz BH. Org Lett. 2010;12:28–31. doi: 10.1021/ol9023908. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Selander N, Paasch JR, Szabó KJ. J Am Chem Soc. 2011;133:409–411. doi: 10.1021/ja1096732. [DOI] [PubMed] [Google Scholar]
- 6.Murakami K, Yorimitsu H, Oshima K. J Org Chem. 2009;74:1415–1417. doi: 10.1021/jo802433t. [DOI] [PubMed] [Google Scholar]
- 7.(a) Trost BM, Ball ZT. J Am Chem Soc. 2005;127:17644–17655. doi: 10.1021/ja0528580. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Berthon-Gelloz G, Schumers JM, De Bo G, Markó IE. J Org Chem. 2008;73:4190–4197. doi: 10.1021/jo800411e. [DOI] [PubMed] [Google Scholar]
- 8.(a) Marciniec B, Pietraszuk C. Top Organomet Chem. 2004;11:197–248. [Google Scholar]; (b) Ohmiya H, Yorimitsu H, Oshima K. Org Lett. 2006;8:3093–3096. doi: 10.1021/ol0611144. [DOI] [PubMed] [Google Scholar]
- 9.(a) Marciniec B. Coord Chem Rev. 2005;249:2374–2390. [Google Scholar]; (b) Terao J, Torii K, Saito K, Kambe N, Baba A, Sonoda N. Angew Chem Int Ed. 1998;37:2653–2656. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2653::AID-ANIE2653>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; (c) Jiang Y, Blacque O, Fox T, Frech CM, Berke H. Chem Eur J. 2009;15:2121–2128. doi: 10.1002/chem.200802019. [DOI] [PubMed] [Google Scholar]; (d) Lu B, Falck J. J Org Chem. 2010;75:1701–1705. doi: 10.1021/jo902678p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Cheng C, Simmons EM, Hartwig JF. Angew Chem Int Ed. 2013;52:8984–8989. doi: 10.1002/anie.201304084. [DOI] [PubMed] [Google Scholar]
- 10.Oestreich M. The Mizoroki-Heck Reaction. John Wiley & Sons; Chichester, U.K: 2008. p. 568. [Google Scholar]
- 11.McAtee JR, Martin SES, Ahneman DT, Johnson KA, Watson DA. Angew Chem Int Ed. 2012;51:3663–3667. doi: 10.1002/anie.201200060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita H, Hayashi T, Kobayashi T, Tanaka M, Goto M. J Am Chem Soc. 1988;110:4417–4418. [Google Scholar]
- 13.Armitage DA. In: Comprehensive Organometallic Synthesis. Wilkinson G, Stone FGA, Abel EW, editors. Vol. 2. Pergamon; Oxford: 1982. p. 6. [Google Scholar]
- 14.Yamashita H, Tanaka M, Goto M. Organometallics. 1997;16:4696–4704. [Google Scholar]
- 15.Yamashita H, Kobayashi T, Hayashi T, Tanaka M. Chem Lett. 1991;20:761–762. [Google Scholar]
- 16.Drahl C. Heck Reaction Goes Silyl. Chem Eng News. 2012 Apr 2;90(14):9. [Google Scholar]
- 17.Hiyama T, Shirakawa E. Organosilicon Compounds. In: Miyaura N, editor. Cross-Coupling Reactions. Vol. 219. Springer-Verlag; Berlin/Heidelberg: 2002. pp. 61–85. [Google Scholar]
- 18.Pawlu P, Szudkowska J, Hreczycho G, Marciniec B. J Org Chem. 2011;76:6438–6441. doi: 10.1021/jo201036x. [DOI] [PubMed] [Google Scholar]
- 19.(a) Greedy B, Gouverneur V. Chem Commun. 2001:233–234. [Google Scholar]; (b) Ranjbar-Karimi R. Ultrason Sonochem. 2010;17:768–769. doi: 10.1016/j.ultsonch.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi T, Kabeta K, Hamachi I, Kumada M. Tetrahedron Lett. 1983;24:2865–2868. [Google Scholar]
- 21.Fleming I, Lewis JJ. J Chem Soc, Perkin Trans 1. 1992:3267–3275. [Google Scholar]
- 22.Terao J, Jin Y, Torii K, Kambe N. Tetrahedron. 2004;60:1301–1308. [Google Scholar]