Abstract
The purpose of this research was to determine whether or not routine home visiting (by the Philani Maternal Child Health and Nutrition Project) influences the prevalence of stunted, wasted and underweight children in Cape Town peri-urban settlements. The study was a cross-sectional cohort in which weight and height measurements were collected for all children from 24 matched neighbourhoods; three years earlier 12 of these neighbourhoods were randomized to receive the home visiting intervention and 12 did not. The research took place at all households located within the 24 neighbourhoods in Khayelitsha and Mfuleni peri-urban settlements. Participants included 8715 children aged 0–6 years old (4694 intervention; 4021 control). A total of 41.3% of children were stunted, 3.1% were underweight and 1.4% were wasted. Children in the intervention group were significantly less likely to be underweight or severely underweight for age than children in the control group. While the rates of stunting were also significantly lower in intervention areas, the effect was not clinically significant, and no significant differences were found between the study arms on the prevalence of wasting. The Philani model is effective in the prevention and rehabilitation of underweight children. Philani could strengthen their intervention by focussing specifically on screening for child stunting in addition to underweight children. The results also suggests that efforts to address the long-term adverse effects of undernutrition require structural and economic transformation, in addition to socio-medical intervention.
Keywords: Child nutrition interventions, child undernutrition, stunting, underweight, community-based nutrition
Introduction
Investment in children’s early years is critical for improving lifelong well-being. Delivering interventions in the first 1000 days and in the early years has been shown to be cost effective (Heckman, 2006), to reduce health inequities (Marmot et al., 2008), and there is an increasing evidence base for how early childhood investments can substantially improve adult health (Campbell et al., 2014). However, poverty and both infectious and non-communicable diseases in low and middle income countries (LMIC) often are implicated in creating poor child outcomes.
Child undernutrition is responsible for 3.5 million deaths every year and it is estimated that more than a third of child deaths in low and middle income countries (LMIC) are associated with maternal and child undernutrition (R. E. Black et al., 2008). Undernourished children are more likely to experience lower educational attainment, lower economic productivity and to have shorter height in adulthood (Victora et al., 2008). In addition, depressive symptoms are higher in adolescents who have suffered from undernutrition in early childhood (Galler et al., 2010). Women who have suffered from childhood undernutrition are more likely to give birth to low birth weight children in later life, thus continuing the cycle of undernutrition from one generation to the next (Victora et al., 2008).
Research shows that maternal and child nutritional interventions have the capacity to effect large scale reduction in the burden of associated disease (Bhutta et al., 2008). Cost effective and scalable interventions which address child undernutrition are critical for child survival and wellbeing. There is evidence to suggest that improving nutrition in early childhood in developing countries has long-term economic impacts, in terms of increased adult economic productivity (Hoddinott, Maluccio, Behrman, Flores, & Martorell, 2008). A study of over 1400 Guatemalan adults, for instance, found that those given a nutrition supplement before, but not after, the age of three years earned higher hourly wages, but that this was only the case for men (Hoddinott et al., 2008). Ruel and colleagues have argued that improvements in nutrition require more than bringing nutrition-specific interventions to scale, but would need to include coupling effective nutrition-specific interventions with nutrition-sensitive programmes, the latter being those that address the underlying causes of undernutrition (Ruel, Alderman, Maternal, & Child Nutrition Study, 2013). They go onto argue that, in the context of global and local food systems threatened by many macro- and micro-economic factors, as well as environmental, political, and social factors, nutrition-sensitive programmes – which draw on agriculture, health, social protection, early child development, education, and water and sanitation – sectors, would be imperative to see large-scale undernutrition become a thing of the past in low-income contexts (R. E. Black et al., 2013; Ruel et al., 2013). Nutrition-sensitive programmes have the potential to accelerate progress made by nutrition-specific programmes by improving the household and community environments in which children are raised.
The complexity of the interaction between context and nutrition is reflected in the findings of a systematic review that concluded that child-feeding interventions are underperforming. The authors noted a number of challenges faced by nutrition interventions, pointing to a need for interventions aimed at improving child growth, nutrition, and development outcomes to address multiple determinants of child health, as well as carefully considering the child’s growth context in the delivery of interventions (Kristjansson et al., 2015). In addition, there are intergenerational effects on linear growth (Martorell & Zongrone, 2012). The passing of several generations is necessary to ‘wash out’ the effects of undernutrition (Martorell & Zongrone, 2012) as maternal body mass pre-pregnancy, as well as weight gain during pregnancy, is a strong predictor of birthweight (Martorell & Zongrone, 2012).
In South Africa, approximately 9% of children were underweight, 23.9% were stunted and 4.7% were wasted from 2009–2013 (UNICEF, 2013). The prevalence of child undernutrition is significantly greater in the poor segment of the population (Zere & McIntyre, 2003). South Africa has one of the highest rates of inequality in the world with a Gini of 0.63 in 2009 with the poorest 50% of the population accounting for less than 8% of the country’s income (Lam, Finn, & Leibbrandt, 2015). Unlike Brazil that has seen a significant reduction in inequality since the mid-1990’s, earning inequality has increased in South Africa to the point where the Gini coefficient on earnings in South Africa in 2011 was approximately the same as Brazil in 1976 (Lam et al., 2015). Between 2005 and 2009, approximately 34% of children who died in South African hospitals were severely undernourished, and approximately 29% were underweight for age (Chiba, 2011). An ill child is more likely to be undernourished and this in turn worsens susceptibility to infection. Thus undernutrition is comorbid with conditions such as HIV, TB, diarrhoeal diseases, pneumonia and septicaemia (Chiba, 2011).
The Philani Maternal Child Health and Nutrition Project is a home visiting program that promotes family health by focusing on the support of pregnant mothers, families affected by HIV, prevention of child under-nutrition and the rehabilitation of underweight children. The project employs township mothers to act as Mentor Mothers (MM) from the communities where they work. MM are trained in Philani interventions and conduct home visits to support positive health outcomes (I.M. Le Roux et al., 2013; Rotheram-Borus et al., 2011). All MM are trained in: 1) foundational skills in behaviour change; 2) application of key health information about maternal and child health; and 3) coping with their own life challenges. Among home visiting programs, the competitive advantages of the Philani program are: (1) It addresses multiple health issues concurrently; (2) It is characterized by routine and consistent accountability; and (3) It provides ongoing training to MM over time (Rotheram-Borus et al., 2011). Previous research has shown the Philani intervention to be effective in rehabilitating underweight children from Cape Town peri-urban settlements (I. M. le Roux et al., 2010; I.M. le Roux et al., 2011). All previous findings however have either focussed on community health workers whose sole focus is on the rehabilitation of malnourished children as their sole task (I. M. le Roux et al., 2010; I.M. le Roux et al., 2011) or ‘generalist mentor mothers’ impact on children who were part of a cluster randomized controlled trial (I.M. Le Roux et al., 2013; Rotheram-Borus et al., 2014). In the context of a generalist intervention that focussed on the recruitment of pregnant women (rather than malnourished children), an important question is the extent to which broader benefits of home visiting (not just the pregnant woman and new infant) can be maintained. The purpose of this research was to explore the extent to which the Philani program has a neighbourhood level impact on the prevalence of stunted, wasted and underweight children.
Methods
Context, study sites and participants
A cluster-randomized controlled trial (RCT) has been underway in Khayelitsha and Mfuleni townships in Cape Town since 2008, evaluating the effectiveness of the Philani intervention for improving maternal and child health outcomes related to nutrition, HIV, TB and alcohol use. In this RCT, we identified 26 similarly-sized Cape Town neighbourhoods (450–600 households) that were within five kilometres of health clinics; had five to seven alcohol bars; were non-contiguous or separated by natural barriers; had both formal and informal housing; had similar numbers of child care centres; and had residents of similar duration of residence. Pairs of neighbourhoods were identified in similar areas of each township. In a cluster randomised controlled design, the UCLA team then randomised 26 neighbourhoods within matched pairs to the PIP or the SC condition using simple randomisation. One matched pair was eliminated after 6 months of recruitment due to low numbers of pregnant women (total n=10 pregnant women), leaving 24 study neighbourhoods The RCT collects longitudinal data from a cohort of mother-infant dyads within the 12 intervention and 12 control neighbourhoods.
The control clusters were pure control clusters for which the only reimbursement was for completing an assessment and provided compensation for the time spent on the assessment. We chose the size of the cluster in order to generate a sufficient sample size of at least 20 mothers living with HIV per cluster. This resulted in us sampling neighborhoods with about 40 pregnant mothers per cluster. Buffer neighborhoods and natural barriers were used to provide corridors in order to prevent cross-neighborhood contamination.
Intervention
As part of the RCT, twelve MMs were recruited from the local community to work in each of the 12 intervention neighbourhoods, and trained by Philani to deliver the Philani home visit intervention (manual available at (http://chipts.ucla.edu/resources/?did=899). MM visited all homes in their neighbourhoods frequently for the first 180 days of life of each child (average of 16 visits), and every 6 months thereafter, weighing all children 6 years and under, encouraging clinical care for mothers living with HIV, following up on childhood immunizations and providing ongoing support. Children found to be underweight were followed up repeatedly, according to the Philani protocol until children were no longer classified as under-nourished.
Current study data collection
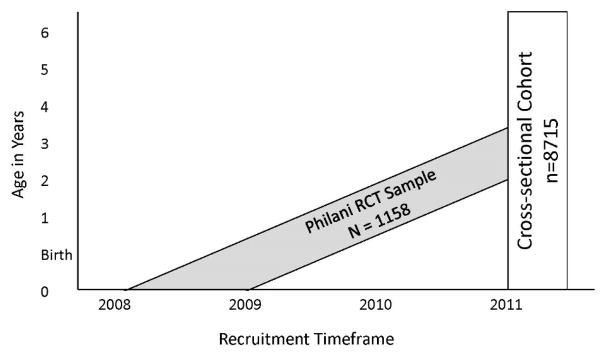
For the current study, a cross sectional survey was conducted in 2011, in which anthropometric data were collected for all children under six years of age in all 24 neighbourhoods. Children in the RCT were 18–36 months old at the time of the survey. Therefore, we assessed not only study children, but all children in the neighbourhoods, some of which were born after the study cohort and some who were born before the RCT started. In 2011, an independent team of data collectors from Stellenbosch University were trained to visit every household within all neighbourhoods, offering to measure the weight and length/height of each child under six years of age (see Figure 1). Refusal rates were not documented in this cross sectional survey, but in the RCT, 98% of households participated with voluntary informed consent.
Figure 1. Children in the cross-sectional cohort.
Graphic representation of the children in the cross-sectional cohort collected in 2011, including the subsample from the Philani randomized controlled trial (RCT).
Weight and length measurement
After verbal consent was granted by a caregiver or guardian in each household, measurements were recorded together with each child’s date of birth and the household address, using a mobile phone. Weight was recorded on calibrated scales while length was measured using a roller meter. Children were weighed without nappies, shoes or clothing. Two weight measurements were made for each child. If the same measurement was repeated, it was recorded. Otherwise a third weight measurement was taken. Length/height was measured supine for children not yet walking, and standing for those who were walking.
The number of children measured in the intervention group was 15% higher than the number in the control condition (n = 4694 versus n = 4021). Data collectors revisited all households periodically for a number of months and on weekend days in an attempt to find as many children as possible in study areas, suggesting that there were in fact fewer children in control areas at the time of data collection, due to reasons such as housing migration.
Statistical analysis
Data were analysed using IBM SPSS Statistics for Windows, version 22.0. The WHO 2006 and WHO 2007 child growth reference data were used to compute z-scores for children aged 0–5 and 5–6 respectively, on height-for-age (ZHAZ), weight-for-age (ZWAZ) and BMI-for-age (ZBMI). Z-scores were calculated for each child using SPSS macros based on WHO 2011 Anthro software. In addition to calculating z-scores, this syntax flags biologically implausible measurements.1 There were 0.4% of weight measurements, 3.1% of height measurements and 5.6% of BMI measurements were flagged as biologically implausible. These measurements were deemed to be errors in data entry, and excluded from the analysis. Each child was subsequently classified as stunted, wasted or being being underweight for age, according to the 2006 WHO standards and cut points included in Table 1. Chi-square tests were performed to compare the frequency of undernutrition on each growth indicator between the intervention and control neighbourhoods. Where significant differences were found, odds ratios were computed for ease of interpretation.
Table 1.
Characteristics of the sample.
| 0 ≤ Age ≤ 6 | Control (N= 4005) | Intervention (N=4668) | Total (N=8673) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| n | % | n | % | n | % | |
| Child Sex | ||||||
| Male | 2016 | 50.3 | 2351 | 50.4 | 4367 | 50.4 |
| Female | 1989 | 49.7 | 2317 | 49.6 | 4306 | 49.6 |
| Child’s age in years | ||||||
| Mean, SD | 2.2 | 1.6 | 2.2 | 1.6 | 2.2 | 1.6 |
| Child’s age group | ||||||
| 0 ≤ Age < 3 | 2295 | 57.3 | 2684 | 57.5 | 4979 | 57.4 |
| 3 ≤ Age < 5 | 1259 | 31.4 | 1503 | 32.2 | 2762 | 31.8 |
| 5 ≤ Age < 6 | 451 | 11.3 | 481 | 10.3 | 932 | 10.7 |
| Weight-for-Age Z Score | ||||||
| Mean (SD) | 0.13 | 1.85 | 0.21 | 1.31 | 0.17 | 1.58 |
| Weight-for-Age < −2 SD | ||||||
| N (%) | 178 | 4.5 | 140 | 3.0 | 318 | 3.7 |
| Height-for-Age Z Score | ||||||
| Mean (SD) | −2.04 | 2.29 | −1.92 | 2.37 | −1.98 | 2.33 |
| Height-for-Age < −2 SD | ||||||
| N (%) | 1992 | 50.1 | 2176 | 47.2 | 4168 | 48.5 |
| Height-for-Length Z Score | ||||||
| Mean (SD) | 1.99 | 2.59 | 2.02 | 2.20 | 2.01 | 2.39 |
| Weight-for-Length < −2 SD | ||||||
| N (%) | 55 | 1.6 | 53 | 1.3 | 108 | 1.4 |
Ethical clearance
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Health Research Ethics Committee of Stellenbosch University (N08-08-218) and the Institutional Review Board at the University of California at Los Angeles (G07-02-033). Verbal consent was obtained from all caregivers and guardians of participating children.
Results
Data were collected for 8673 children aged 0–6 years. There were more male children in the sample (50.3%) compared to female (49.7%). Table 1 illustrates the frequency of stunted, underweight and wasted children across intervention and control neighbourhoods. Across both intervention and control neighbourhoods, a total of 48.5% of children were stunted, 3.7% were underweight and 1.4% were wasted.
Children in the intervention group were 1.5 times less likely to be underweight (X2 = 11.97, df = 1, p = 0.001) and 2.4 times less likely to be severely underweight (X2 = 12.52, df = 1, p = 0.0004). Children in the intervention group were 1.1 times less likely to be stunted (X2 = 5.30, df = 1, p = 0.02). No significant differences were found between the study arms on the prevalence of wasting.
Discussion
A CHW home-based intervention can significantly improve the growth of not only the index child in a maternal and child health intervention delivered in the first 1000 days, but also other children under 6 years of age living in the same household. Findings suggest that children living in areas where the Philani MMs were working were significantly less likely to be underweight and severely underweight than children living in control areas. This is consistent with prior research which showed the effectiveness of the Philani intervention for rehabilitating underweight children (I. M. le Roux et al., 2010; I.M. le Roux et al., 2011). While the rates of stunting were also significantly lower in intervention areas, the effect was not clinically significant.
The Philani intervention focusses on supporting breastfeeding and sustainable nutritional improvements as well as weighing children, which should improve overall growth. These interventions would not only lead to an increase in fat tissue, which should theoretically impact on longitudinal growth as well as weight gain. The lack of a sizeable difference between groups on the prevalence of stunting combined with the very high prevalence of stunting overall in all neighbourhoods may also suggest that the Philani model needs to screen for and address stunting specifically. In recent years, there has been an increased focus of global health policy and intervention on the “first 1000 days” (M. M. Black & Aboud, 2011; Gertler et al., 2014) arising from studies demonstrating the impact of adversity during pregnancy and the first two years. Coupled with this is data showing the cost effectiveness of early investments in improving child development (Campbell et al., 2014; Heckman, 2006). Our finding of a lack of difference in stunting rates from birth to 6 years across conditions may be due to a number of factors. Firstly, the benefits for child growth may require more than simply efficacious interventions in the first 1000 days. A sizeable number of children in the current study did not receive an early intervention but rather were only recruited with acute malnutrition and given an intervention at some point in the years leading up to their sixth birthday. Ensuring sustained benefits across the life-course will require maintaining frequent CHW visits past the first six months. Secondly, the phenomenon of brain-sparing may account for the lack of a finding in stunting. It has been found that psychosocial outcomes are more sensitive to nutritional intervention (Dewey & Adu-Afarwuah, 2008). Brain sparing proposes that when nutritional resources are scarce early in life, they are preferentially directed to the developing brain at the expense of other parts of the body (Auestad, 2000). When a child – previously grossly undernourished – begins to be better nourished, the food resources given to a child may be used for brain development first, and only latterly for growth. Finally, our findings suggest that in order to protect children from undernutrition and stunting, maternal and child nutrition interventions, interventions which provide children with psychosocial stimulation and responsive parenting, and interventions to alleviate poverty, food insecurity, maternal depression, and gender inequality can all be of use (Almond & Currie, 2011). In line with this, efforts to address the long term adverse effects of undernutrition require structural and economic transformation, in addition to socio-medical intervention.
Importantly, what this study shows is that in the context of a successful generalist mentor mother home visiting intervention for targeted children (Rotheram-Borus et al., 2014), the broader neighbourhood benefits on child growth have been maintained. This has important implications for the global shift to the use of generalist community health workers (Singh & Sachs, 2013).
It should also be noted that the ethical protocols and procedures in place as part of the Philani Mentor Mother Project in all study neighbourhoods meant that when a data collector or community recruiter encountered a medical emergency or a child who was severely ill, an emergency referral would have been made to a health facility regardless of which area the child was from. This meant that severely undernourished children from control areas may have received a level of intervention from the project and local health facilities that they may not have normally received.
Strengths and limitations
The strength of the current study is that Philani is a well-established and long standing NGO with relationships to the Department of Health and community leaders. Its well-developed accountability and training systems for both CHW and supervisors are excellent.
Limitations of the study are that some improvements in nutrition might not be reflected in child weight due to concomitant diseases which cause weight loss, such as tuberculosis and HIV (Semba, Darnton-Hill, & de Pee, 2010). The cross sectional design of this study does not allow us to draw conclusion about causality. This is unfortunate, as a longitudinal perspective, from thorough, prospective research, is necessary if we are to understand the determinants of child weight and longitudinal growth. Also, maternal nutrition was not investigated in the present study. Future research could usefully examine the relationship between maternal nutrition before and during pregnancy, and child weight and longitudinal growth, to pinpoint the timeframe during which nutritional investments by the mother have the greatest impacts for the developing child. It should also be noted that ages were calculated based on birth dates, and no data on gestational age at birth was collected to control for premature births, which may somewhat have influenced the results. Finally, research examining the relationship between broader contextual factors, including family education and SES, and child nutrition in South Africa is needed.
Table 2.
Nutritional status of children in the intervention and control township neighbourhoods in Cape Town, South Africa.
| Intervention (n = 4694) | Control (n = 4021) | X2 | OR | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Stunted ZHAZ < −2 | 1821 | 40.1% | 1666 | 42.6% | 5.30 | 1.11 | * |
| Severely stunted ZHAZ < −3 | 862 | 19.0% | 791 | 20.2% | 2.02 | - | |
| Underweight ZWAZ < −2 | 118 | 2.5% | 153 | 3.8% | 11.97 | 1.53 | ** |
| Severely underweight ZWAZ <−3 | 23 | 0.5% | 47 | 1.2% | 12.52 | 2.40 | ** |
| Wasted ZBMI < −2 | 58 | 1.3% | 55 | 1.4% | .273 | - | |
| Severely wasted ZBMI < −3 | 17 | 0.4% | 22 | 0.6% | 1.631 | - | |
p<0.05;
p<0.01
Acknowledgments
Financial support: This study was funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (Grant number 1R01AA017104). ClinicalTrials.gov registration number: NCT00972699. The NIAAA had no role in the design, analysis or writing of this article.
Footnotes
The criteria for biologically implausible data are: Weight-for-age z-score (ZWAZ) ZWAZ<−6 or ZWAZ >5; Length/height-for-age z-score (ZHAZ) ZHAZ<−6 or ZHAZ >6; BMI-for-age z-score (ZBMI) ZBMI <−5 or ZBMI >5
Conflict of interests: Ingrid le Roux is the director of Philani. The other authors have no competing interests.
Authorship: Conception and design, data acquisition, analysis and/or interpretation - MH, MRB, MT. Drafting the article or revising it critically for important intellectual content - MH, MRB, MT, IL. Final approval of the version to be published - MH, MRB, MT, IL.
References
- Almond D, Currie J. Killing me softly: The fetal origins hypothesis. J Econ Perspect. 2011;25(3):153–172. doi: 10.1257/jep.25.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auestad N. Infant nutrition--brain development--disease in later life. An introduction. Dev Neurosci. 2000;22(5–6):472–473. doi: 10.1159/000017477. doi:17477. [DOI] [PubMed] [Google Scholar]
- Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E … Child Undernutrition Study, G. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- Black MM, Aboud FE. Response feeding is embedded in a theoretical framework of responsive parenting. Journal of Nutrition. 2011;141(3):490–494. doi: 10.3945/jn.110.129973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M … Child Undernutrition Study, G. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M … Child Nutrition Study, G. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- Campbell F, Conti G, Heckman JJ, Moon SH, Pinto R, Pungello E, Pan Y. Early childhood investments substantially boost adult health. Science. 2014;343(6178):1478–1485. doi: 10.1126/science.1248429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba AC. Malnutrition. 2011. Retrieved from Pretoria. [Google Scholar]
- Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4(Suppl 1):24–85. doi: 10.1111/j.1740-8709.2007.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler JR, Bryce CP, Waber D, Hock RS, Exner N, Eaglesfield D, … Harrison R. Early childhood malnutrition predicts depressive symptoms at ages 11–17. Journal of Child Psychology and Psychiatry. 2010;51(7):789–798. doi: 10.1111/j.1469-7610.2010.02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler P, Heckman J, Pinto R, Zanolini A, Vermeersch C, Walker S … Grantham-McGregor, S. Labor market returns to an early childhood stimulation intervention in Jamaica. Science. 2014;344(6187):998–1001. doi: 10.1126/science.1251178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman JJ. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312(5782):1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- Hoddinott J, Maluccio JA, Behrman JR, Flores R, Martorell R. Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet. 2008;371(9610):411–416. doi: 10.1016/S0140-6736(08)60205-6. [DOI] [PubMed] [Google Scholar]
- Kristjansson E, Francis DK, Liberato S, Benkhalti Jandu M, Welch V, Batal M, … Petticrew M. Food supplementation for improving the physical and psychosocial health of socio-economically disadvantaged children aged three months to five years. Cochrane Database Syst Rev. 2015;3:CD009924. doi: 10.1002/14651858.CD009924.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D, Finn A, Leibbrandt M. Schooling inequality, returns to schooling, and earnings inequality: Evidence from Brazil and South Africa. 2015. Retrieved from Helsinki. [Google Scholar]
- le Roux IM, le Roux K, Comulada WS, Greco EM, Desmond KA, Mbewu N, Rotheram-Borus MJ. Home visits by neighborhood Mentor Mothers provide timely recovery from childhood malnutrition in South Africa: results from a randomized controlled trial. Nutrition Journal. 2010;9:56. doi: 10.1186/1475-2891-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Roux IM, le Roux K, Mbeutu K, Comulada WS, Desmond KA, Rotheram-Borus MJ. A randomized controlled trial of home visits by neighborhood mentor mothers to improve children’s nutrition in South Africa. Vulnerable Child Youth Stud. 2011;6(2):91–102. doi: 10.1080/17450128.2011.564224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux IM, Tomlinson M, Harwood JM, O’Connor MJ, Worthman CM, Mbewu N, … Rotheram-Borus MJ. Outcomes of home visits for pregnant mothers and their infants: a cluster randomized controlled trial. AIDS. 2013;27(9):1461–1471. doi: 10.1097/QAD.0b013e3283601b53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M, Friel S, Bell R, Houweling TA, Taylor S Commission on Social Determinants of H. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372(9650):1661–1669. doi: 10.1016/S0140-6736(08)61690-6. [DOI] [PubMed] [Google Scholar]
- Martorell R, Zongrone A. Intergenerational influences on child growth and undernutrition. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):302–314. doi: 10.1111/j.1365-3016.2012.01298.x. [DOI] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, le Roux IM, Tomlinson M, Mbewu N, Comulada WS, le Roux K, … Swendeman D. Philani Plus (+): a Mentor Mother community health worker home visiting program to improve maternal and infants’ outcomes. Prev Sci. 2011;12(4):372–388. doi: 10.1007/s11121-011-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Tomlinson M, le Roux IM, Harwood JM, Comulada S, O’Connor MJ, … Worthman CM. A cluster randomised controlled effectiveness trial evaluating perinatal home visiting among South African mothers/infants. PLoS One. 2014;9(10):e105934. doi: 10.1371/journal.pone.0105934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel MT, Alderman H Maternal & Child Nutrition Study G. Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet. 2013;382(9891):536–551. doi: 10.1016/S0140-6736(13)60843-0. [DOI] [PubMed] [Google Scholar]
- Semba RD, Darnton-Hill I, de Pee S. Addressing tuberculosis in the context of malnutrition and HIV coinfection. Food Nutr Bull. 2010;31(4):S345–364. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21214037. [PubMed] [Google Scholar]
- Singh P, Sachs JD. 1 million community health workers in sub-Saharan Africa by 2015. Lancet. 2013;382(9889):363–365. doi: 10.1016/S0140-6736(12)62002-9. [DOI] [PubMed] [Google Scholar]
- UNICEF. Country data: South Africa. 2013 Retrieved from http://www.unicef.org/infobycountry/southafrica_statistics.html.
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L … Child Undernutrition Study G. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zere E, McIntyre D. Inequities in under-five child malnutrition in South Africa. Int J Equity Health. 2003;2(1):7. doi: 10.1186/1475-9276-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]