ABSTRACT
We examined the circulatory mechanisms underlying adaptive increases in thermogenic capacity in deer mice (Peromyscus maniculatus) native to the cold hypoxic environment at high altitudes. Deer mice from high- and low-altitude populations were born and raised in captivity to adulthood, and then acclimated to normoxia or hypobaric hypoxia (simulating hypoxia at ∼4300 m). Thermogenic capacity [maximal O2 consumption (V̇O2,max), during cold exposure] was measured in hypoxia, along with arterial O2 saturation (SaO2) and heart rate (fH). Hypoxia acclimation increased V̇O2,max by a greater magnitude in highlanders than in lowlanders. Highlanders also had higher SaO2 and extracted more O2 from the blood per heartbeat (O2 pulse=V̇O2,max/fH). Hypoxia acclimation increased fH, O2 pulse and capillary density in the left ventricle of the heart. Our results suggest that adaptive increases in thermogenic capacity involve integrated functional changes across the O2 cascade that augment O2 circulation and extraction from the blood.
KEY WORDS: Evolutionary physiology, High-altitude adaptation, Respiration, O2 transport pathway, Aerobic performance
Summary: Adaptive increases in thermogenic capacity in high-altitude deer mice involve integrated functional changes across the O2 cascade that augment O2 circulation and extraction from the blood.
INTRODUCTION
High-altitude natives are valuable model organisms for understanding how physiological systems evolve. The cold and oxygen-depleted (hypoxic) environment at high altitudes requires that endotherms sustain high rates of O2 consumption for thermogenesis and locomotion while facing a diminished O2 supply. Growing evidence suggests that high-altitude natives have overcome this challenge through evolved changes in the physiological systems underlying O2 transport and utilization (Monge and León-Velarde, 1991; Storz et al., 2010b; Scott, 2011; Ivy and Scott, 2015). Studies of high-altitude natives aimed at understanding the evolution of the O2 transport cascade – composed of ventilation, pulmonary diffusion, circulation, tissue diffusion and cellular O2 utilization – are therefore extremely valuable for explaining the physiological mechanisms of evolutionary adaptation.
North American deer mice [Peromyscus maniculatus (Wagner 1845)] are an excellent model species for studies of high-altitude adaptation. Their native altitudinal range extends from below sea level in Death Valley, CA, USA, to over 4300 m above sea level in the Rocky Mountains (Hock, 1964; Snyder et al., 1982; Natarajan et al., 2015). High-altitude populations must sustain high metabolic rates in the wild (Hayes, 1989b), and there appears to be strong directional selection on thermogenic capacity [maximal O2 consumption (V̇O2,max) during cold exposure] to support heat generation in cold alpine environments (Hayes and O'Connor, 1999). In response to this strong selection pressure, high-altitude deer mice have evolved a higher V̇O2,max in hypoxia than low-altitude populations of deer mice and a congeneric lowland species (white-footed mice, P. leucopus) (Cheviron et al., 2012, 2013; Lui et al., 2015). This evidence suggests that highland deer mice have evolved an adaptive increase in thermogenic capacity in hypoxia.
The physiological mechanisms underlying this evolved increase in thermogenic capacity have yet to be fully explained. High-altitude deer mice have evolved a high blood–O2 affinity compared with their lowland counterparts that contributes to increasing V̇O2,max in hypoxia (Snyder et al., 1982; Chappell and Snyder, 1984; Storz et al., 2010a; Natarajan et al., 2013), but it is unclear whether this adaptation improves O2 uptake into the blood in vivo. High-altitude deer mice have also evolved a more oxidative and richly vascularized phenotype of the skeletal muscle (used for shivering and locomotion), in association with differential expression of genes involved in aerobic energy metabolism and angiogenesis (Cheviron et al., 2012, 2014; Lui et al., 2015; Scott et al., 2015; Lau et al., 2017; Mahalingam et al., 2017). Development and acclimatization to cold and/or hypoxia are also known to affect V̇O2,max, cardiopulmonary organ sizes and the capacity for non-shivering thermogenesis in deer mice (Hammond et al., 2001, 2002; Chappell and Hammond, 2004; Shirkey and Hammond, 2014; Velotta et al., 2016). However, we know very little about in vivo cardiorespiratory function at V̇O2,max in this species. This study therefore aims to examine the contribution of differences in arterial O2 saturation and some other aspects of circulatory function to adaptive increases in thermogenic capacity in high-altitude deer mice.
MATERIALS AND METHODS
Animals and acclimation treatments
Captive breeding populations were established from wild deer mouse populations native to high altitude (near the summit of Mount Evans, CO, USA, 39°35′18″N, 105°38′38″W; 4350 m above sea level) (P. m. rufinus) and low altitude (Nine Mile Prairie, Lancaster County, NE, USA, 40°52′12″N, 96°48′20.3″W; 430 m above sea level) (P. m. nebracensis). Wild adults were transported to McMaster University (near sea level) and housed in common-garden conditions, and were bred within each population to produce laboratory-raised progeny. Mice were raised in standard holding conditions (24–25°C, 12 h:12 h light:dark photoperiod) with unlimited access to standard rodent chow and water. All animal protocols followed guidelines established by the Canadian Council on Animal Care and were approved by the McMaster University Animal Research Ethics Board.
Adult mice were raised to ∼6 months of age, and a randomly selected group of individuals (mix of both sexes) from each population were acclimated to either (1) normobaria in standard normoxic conditions or (2) hypobaric hypoxia (barometric pressure of 60 kPa; equivalent to that at an elevation of ∼4300 m) in specially designed hypobaric chambers that have been described previously (Lui et al., 2015). Cages were cleaned twice a week during acclimations, which required that the hypobaric groups be returned to normobaria for a brief period (<30 min). Mice were subjected to subsequent measurements after 6–8 weeks of acclimation.
Respirometry and pulse oximetry
We measured thermogenic capacity in hypoxia in second-generation (F2) mice from high-altitude and low-altitude populations. Maximal rates of O2 consumption (V̇O2,max) were measured during acute cold exposure, using open-flow respirometry in a hypoxic heliox atmosphere (12% O2, 88% He) at −5°C (Rosenmann and Morrison, 1974; Chappell and Hammond, 2004; Cheviron et al., 2012). Respirometry was carried out in a 0.5 l animal chamber that received a constant incurrent flow rate of 1000 ml min−1, regulated using a mass flow controller (MFC-4, Sable Systems, Las Vegas, NV, USA) and a precision flow control valve that was factory calibrated for heliox (Sierra Instruments, Monterey, CA, USA). The chamber was held inside a freezer, in which the ambient temperature was regulated at or slightly below −5°C (measured with a thermocouple; PT-6, Physitemp, Clifton, NJ, USA), and the incurrent gas flowed through copper coils before entering the chamber. Excurrent gas was subsampled at 200 ml min−1, dried with pre-baked Drierite, and analyzed for O2 and CO2 fractions (FoxBox Respirometry System, Sable Systems).
Respirometry experiments were carried out as follows. Baseline O2 and CO2 fractions were first measured without an animal in the chamber. Mice were instrumented with a collar sensor to measure heart rate (fH) and the O2 saturation of arterial blood (SaO2) using a MouseOx Plus pulse oximeter (Starr Life Sciences, Oakmont, PA, USA), and were then transferred to the chamber. The pulse oximetry measurements required that hair be removed from around the neck, which was done 2 days before the experiments using Nair™ hair removal product. Incurrent gas flow rate, chamber temperature, and excurrent O2 and CO2 fractions were measured continuously and were acquired using a PowerLab 8/32 and LabChart 8 Pro software (ADInstruments, Colorado Springs, CO, USA). Pulse oximetry measurements were recorded using Starr Life Sciences acquisition software. Rates of O2 consumption (V̇O2) were calculated using established formulas (Lighton, 2008) and V̇O2,max was defined as the highest V̇O2 achieved over a 30 s period during the trial, which generally occurred after ∼4–6 min in the chamber, when maximal values of SaO2 and fH were also determined. Measurements of core body temperature were made using a rectal probe (RET-3-ISO, Physitemp) immediately after removing the animal from the chamber (after ∼10–12 min in the chamber), and confirmed that all mice were hypothermic at the end of the experiment.
Cardiac histology
Capillarity was measured histologically in the left ventricle of the heart in a separate group of F1 mice from highland and lowland populations. Mice were euthanized with an overdose of isoflurane followed by cervical dislocation. The ventricles were removed, coated in embedding medium, frozen in liquid N2-cooled isopentane and stored at −80°C. Tissue was sectioned (10 μm) perpendicular to the long axis of the heart in a cryostat at −20°C. Capillaries were identified by staining for alkaline phosphatase activity for 1 h at room temperature (assay buffer concentrations in mmol l−1: 1.0 nitroblue tetrazolium, 0.5 5-bromo-4-chloro-3-indoxyl phosphate, 28 NaBO2 and 7 MgSO4; pH 9.3). Images were collected systematically using light microscopy, such that there was equal representation of images from across the left ventricle. A blind observer determined the average value of capillary density for each individual.
Statistics
Two-factor ANOVA was generally used to assess the main effects of population altitude and acclimation environment (interactions were also assessed, but were not generally significant and are not reported). Data for V̇O2,max and the amount of O2 extracted from the blood per heartbeat (the quotient of V̇O2 and fH; also called the O2 pulse) were first corrected for body mass (Mb) before making statistical comparisons. This was accomplished by carrying out least-squares regressions to the equation Y=aMbb (using GraphPad Prism software, La Jolla, CA, USA), including all of the data across all groups, and then calculating the residual from the regression for each individual. These residuals were then used in two-factor ANOVA, and are reported graphically on the right y-axis. The scale of the left y-axis for graphs of our V̇O2,max and O2 pulse data shows the sum of the residual and the expected value for an average-sized 21.6-g mouse (i.e. V̇O2,max or O2 pulse data corrected to a body mass of 21.6 g). We also performed a supplementary statistical analysis of the effects of body mass, population altitude and acclimation environment on V̇O2,max and O2 pulse using linear models (lm) in R (R Core Team, 2016), in which body mass and the variable of interest were log-transformed before making statistical comparisons (the statistical results are extremely similar to those obtained with two-factor ANOVA, and are shown in Table S1). Data are generally reported as means±s.e.m. (except when data points from individual samples are shown). P<0.05 was considered significant.
RESULTS AND DISCUSSION
Thermogenic capacity in hypoxia is enhanced in high-altitude deer mice
Cold-induced V̇O2,max (measured in hypoxia) was greatest in high-altitude deer mice, as reflected by a significant population effect in two-factor ANOVA (Fig. 1A). This was particularly apparent after hypoxia acclimation, when V̇O2,max was 16% higher on average in highlanders than in lowlanders. Because we observed allometric rather than isometric scaling of V̇O2,max to body mass, we used a residual-based approach to correct for body mass before making these comparisons (Fig. 1B), but we obtained very similar statistical results using linear model statistics (Table S1). Body mass was similar between populations (F1,36=1.08, P=0.31) and between acclimation environments (F1,36=0.49, P=0.49) (normoxic highlanders, 22.9±1.1 g; hypoxic highlanders, 21.0±0.6 g; normoxic lowlanders, 20.6±0.8 g; hypoxic lowlanders, 20.9±1.3 g).
Fig. 1.
Thermogenic capacity, measured in hypoxia as the maximal rate of O2 consumption (V̇O2,max) during acute cold exposure, was enhanced in high-altitude deer mice. The effects of population altitude and hypoxia acclimation on hypoxic V̇O2,max were assessed by calculating the residuals (A) for an allometric regression of hypoxic V̇O2,max to body mass (Mb; V̇O2,max=0.46Mb0.65) (B). (A) The left axis shows the hypoxic V̇O2,max for an average-sized 21.6-g mouse, calculated for each group by adding the residual (shown on the right axis) to the V̇O2,max predicted at 21.6 g by the regression (means±s.e.m.). There were significant main effects of population (F1,36=4.42, *P=0.043) and hypoxia acclimation (F1,36=18.25, ‡P<0.001). (B) Grey lines represent 95% confidence intervals of the allometric regression (▾, normoxic highlanders, n=15; ▿, hypoxic highlanders, n=14; ▴, normoxic lowlanders, n=6; ▵, hypoxic lowlanders, n=5).
Our results are consistent with previous findings in deer mice and other high-altitude taxa. The increases in cold- and exercise-induced V̇O2,max in highland deer mice observed by us and others appear to be greatest in hypoxic conditions, and are not as large in normoxic conditions at sea level, suggesting that highlanders are more resistant to the depressing effects of hypoxia on O2 transport (Chappell and Snyder, 1984; Hayes, 1989a; Cheviron et al., 2012, 2013; Lui et al., 2015). Similar differences exist in Andean and Tibetan human populations, in which exercise-induced V̇O2,max is only elevated compared with lowland humans when tested at altitudes above ∼2500 m (Brutsaert, 2016). However, in many of these human studies, it has been hard to distinguish evolved genetic effects from effects of developmental environment and exercise training. Although hypoxia exposure during development also has a strong influence on V̇O2,max in deer mice, directional selection on V̇O2,max at high altitudes appears to have further increased V̇O2,max in high-altitude populations (Fig. 1) (Hayes and O'Connor, 1999; Chappell et al., 2007; Russell et al., 2008; Cheviron et al., 2013; Lui et al., 2015).
High-altitude deer mice maintain higher arterial O2 saturation in hypoxia
Arterial O2 saturation was ∼6–8% higher in highlanders than in lowlanders at V̇O2,max in hypoxia (Fig. 2A). This observation likely results at least in part from the greater blood– and haemoglobin–O2 affinities of highlanders (Snyder et al., 1982; Storz et al., 2010a), which would increase SaO2 at similar conditions of blood O2 and CO2 tensions and pH. This observation could also stem from population differences in arterial O2 tension, arising from differences in pulmonary ventilation or O2 diffusion. Breathing and pulmonary O2 extraction have yet to be examined in deer mice at V̇O2,max, but there appear to be evolved differences in control of breathing by hypoxia under routine conditions in highland deer mice (Ivy and Scott, 2017).
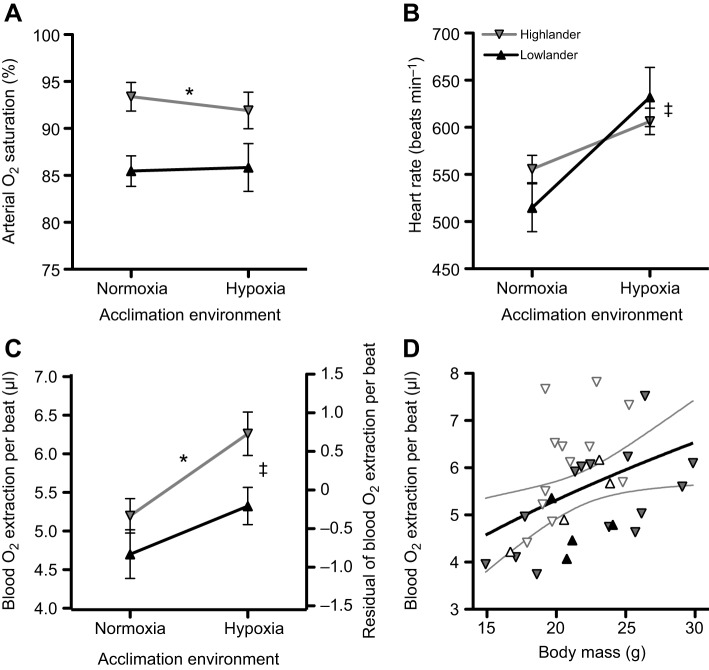
Fig. 2.
Population altitude and hypoxia acclimation affect circulatory O2 delivery at hypoxic V̇O2,max in deer mice. (A) Arterial O2 saturation was higher in highland mice (F1,24=10.46, *P=0.004) but was unaffected by hypoxia acclimation (F1,24=0.063, P=0.803). (B) Heart rate at V̇O2,max increased after hypoxia acclimation (F1,30=15.48, ‡P<0.001) but was similar between populations (F1,30=0.129, P=0.722). The amount of O2 extracted from the blood per heartbeat (‘O2 pulse’, quotient of V̇O2 and heart rate), assessed by calculating the residuals (C) for an allometric regression to body mass (Mb; O2 pulse=1.15Mb0.51; D), was greater in highland mice (F1,30=4.41, *P=0.044) and increased after hypoxia acclimation (F1,30=6.10, ‡P=0.019) (see Fig. 1 and Materials and methods for additional details on this approach). Grey lines in D represent 95% confidence intervals of the allometric regression (▾, normoxic highlanders, n=14; ▿, hypoxic highlanders, n=12; ▴, normoxic lowlanders, n=4; ▵, hypoxic lowlanders, n=4). Data are means±s.e.m., except in D, where data from individuals are shown.
SaO2 was unaffected by hypoxia acclimation (Fig. 2A), and was not always associated with clear population differences in V̇O2,max (Fig. 1). Previous studies using wild-derived strains of deer mice with distinct α-globin haplotypes (on randomized genetic backgrounds) have shown that variation in blood–O2 affinity affects V̇O2,max, such that mice with higher affinity (typical of highland populations) had the highest V̇O2,max when acclimated and tested at high altitude (Chappell and Snyder, 1984; Chappell et al., 1988). This relationship is presumed to arise from a positive association between blood–O2 affinity and SaO2 in hypoxia, but this has not been tested. Here, the higher SaO2 in highlanders compared with lowlanders only appears to be associated with increases in V̇O2,max when mice were acclimated and tested in hypoxia (Fig. 1). However, in normoxia-acclimated mice, highlanders had higher SaO2 without any clear difference in hypoxic V̇O2,max. This suggests that the influence of SaO2 on V̇O2,max may be context dependent, such that the relative benefit of increases in SaO2 may depend upon interactions with other respiratory traits that change after hypoxia acclimation.
Differences in cardiac performance appear to underlie differences in thermogenic capacity
Heart rates (fH) during V̇O2,max in hypoxia were ∼9–23% higher after hypoxia acclimation (Fig. 2B). The amount of O2 extracted from the blood per heartbeat (‘O2 pulse’, quotient of V̇O2 and fH) increased by ∼25–32% after hypoxia acclimation, and was 10–16% greater in the highland population (Fig. 2C, Table S1). The latter observation suggests that cardiac stroke volume (VS) and/or the absolute O2 extraction from the blood (CaO2–CvO2) contributes to the variation in V̇O2,max. This is because all of the above variables are related by the Fick equation, V̇O2=fH×VS×(CaO2–CvO2), such that O2 pulse is equal to the product of stroke volume and blood O2 extraction. This product must therefore be greater in highlanders and increase with hypoxia acclimation.
The observed difference in cardiac performance was likely associated with variation in O2 supply to heart tissue. Hypoxia acclimation increased capillary density – a key determinant of O2 diffusing capacity – by ∼10–12% in the left ventricle (Fig. 3). However, capillary densities were similar between highlanders and lowlanders, so this trait does not underlie population differences in cardiac performance. Nevertheless, it is likely that an interaction between the hypoxia-induced increase in heart capillarity and the population difference in SaO2 resulted in an improved O2 supply to cardiac tissue, and may therefore account for the observed differences in cardiac performance and V̇O2,max. High-altitude adaptation and/or hypoxia acclimation could have also improved the heart's ability to maintain cardiac output during tissue hypoxia. In support of this possibility, some other high-altitude taxa exhibit differences in mitochondrial physiology and metabolic capacity that could improve cardiac function at low O2 tensions (Sheafor, 2003; Scott et al., 2011; Dawson et al., 2016).
Fig. 3.
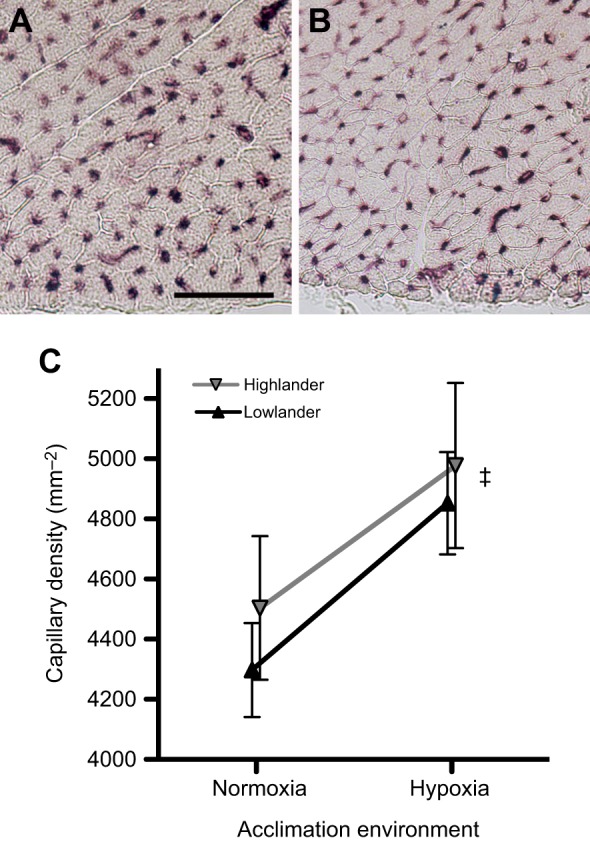
Capillarity of the heart tissue increased after hypoxia acclimation. (A,B) Representative images of left ventricle tissue near the epicardium, stained for alkaline phosphatase activity to identify capillaries, in normoxia-acclimated deer mice from the low-altitude (A) and high-altitude (B) populations (scale bar, 50 μm). (C) There was a significant main effect of hypoxia acclimation on capillary density (F1,30=5.53, ‡P=0.026), but no difference between populations (F1,30=0.57, P=0.455). Data are means± s.e.m., with sample sizes as follows: normoxic highlanders, n=9; hypoxic highlanders, n=8; normoxic lowlanders, n=7; hypoxic lowlanders, n=10.
The functional mechanisms of high-altitude adaptation span the O2 cascade
A key goal of evolutionary physiology is to elucidate the mechanistic basis of adaptive variation in organismal performance (Garland and Carter, 1994; Dalziel et al., 2009). Thermogenesis is a key performance trait that is critical for fitness in endotherms at high altitudes (Hayes and O'Connor, 1999) and can push the respiratory system of many small mammals to its limits (Rosenmann and Morrison, 1974; Chappell and Hammond, 2004). Here, we contribute to the growing evidence suggesting that adaptive increases in thermogenic capacity involve integrated functional changes across the O2 cascade. V̇O2,max in hypoxia appears to be enhanced in high-altitude deer mice (Fig. 1) via increases in pulmonary O2 uptake (Fig. 2A), haemoglobin–O2 affinity (Snyder et al., 1982; Chappell and Snyder, 1984; Storz et al., 2010a; Natarajan et al., 2013), cardiac performance and/or blood O2 extraction (Fig. 2C), and the capacity for O2 diffusion and utilization in skeletal muscle (Cheviron et al., 2012, 2014; Lui et al., 2015; Scott et al., 2015; Mahalingam et al., 2017). Therefore, the concerted evolution of physiological systems underlying O2 transport and utilization appear to be critical to the process of high-altitude adaptation.
Supplementary Material
Acknowledgements
The authors thank Paras Patel and Oliver Wearing for technical assistance with data collection, and two anonymous referees for helpful comments on an earlier version of this manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: G.R.S.; Methodology: K.B.T., G.R.S.; Formal analysis: K.B.T.; Investigation: K.B.T., C.M.I., J.P.V., G.R.S.; Writing - original draft: K.B.T., G.R.S.; Writing - review & editing: K.B.T., C.M.I., J.P.V., J.F.S., G.B.M., Z.A.C., G.R.S.; Supervision: J.F.S., G.B.M., Z.A.C., G.R.S.; Funding acquisition: J.F.S., Z.A.C., G.R.S.
Funding
This research was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to G.R.S., National Science Foundation grants to Z.A.C. (IOS-1354934 and IOS-1634219) and J.F.S. (IOS-1354390), and a National Institutes of Health grant to J.F.S. (HL087216). C.M.I. was supported by an NSERC Postgraduate Scholarship and an Ontario Graduate Scholarship. G.R.S. is supported by the Canada Research Chairs Program. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jeb.biologists.org/lookup/doi/10.1242/jeb.164491.supplemental
References
- Brutsaert T. (2016). Why are high altitude natives so strong at high altitude? Nature vs. nurture: genetic factors vs. growth and development. Adv. Exp. Med. Biol. 903, 101-112. 10.1007/978-1-4899-7678-9_7 [DOI] [PubMed] [Google Scholar]
- Chappell M. A. and Hammond K. A. (2004). Maximal aerobic performance of deer mice in combined cold and exercise challenges. J. Comp. Physiol. B 174, 41-48. 10.1007/s00360-003-0387-z [DOI] [PubMed] [Google Scholar]
- Chappell M. A. and Snyder L. R. G. (1984). Biochemical and physiological correlates of deer mouse α-chain hemoglobin polymorphisms. Proc. Natl. Acad. Sci. USA 81, 5484-5488. 10.1073/pnas.81.17.5484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell M. A., Hayes J. P. and Lee R. G. S. (1988). Hemoglobin polymorphisms in deer mice (Peromyscus maniculatus): physiology of beta-globin variants and alpha-globin recombinants. Evolution 42, 681-688. [DOI] [PubMed] [Google Scholar]
- Chappell M. A., Hammond K. A., Cardullo R. A., Russell G. A., Rezende E. L. and Miller C. (2007). Deer mouse aerobic performance across altitudes: effects of developmental history and temperature acclimation. Physiol. Biochem. Zool. 80, 652-662. 10.1086/521202 [DOI] [PubMed] [Google Scholar]
- Cheviron Z. A., Bachman G. C., Connaty A. D., McClelland G. B. and Storz J. F. (2012). Regulatory changes contribute to the adaptive enhancement of thermogenic capacity in high-altitude deer mice. Proc. Natl. Acad. Sci. USA 109, 8635-8640. 10.1073/pnas.1120523109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron Z. A., Bachman G. C. and Storz J. F. (2013). Contributions of phenotypic plasticity to differences in thermogenic performance between highland and lowland deer mice. J. Exp. Biol. 216, 1160-1166. 10.1242/jeb.075598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron Z. A., Connaty A. D., McClelland G. B. and Storz J. F. (2014). Functional genomics of adaptation to hypoxic cold-stress in high-altitude deer mice: transcriptomic plasticity and thermogenic performance. Evolution 68, 48-62. 10.1111/evo.12257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel A. C., Rogers S. M. and Schulte P. M. (2009). Linking genotypes to phenotypes and fitness: how mechanistic biology can inform molecular ecology. Mol. Ecol. 18, 4997-5017. 10.1111/j.1365-294X.2009.04427.x [DOI] [PubMed] [Google Scholar]
- Dawson N. J., Ivy C. M., Alza L., Cheek R., York J. M., Chua B., Milsom W. K., McCracken K. G. and Scott G. R. (2016). Mitochondrial physiology in the skeletal and cardiac muscles is altered in torrent ducks, Merganetta armata, from high altitudes in the Andes. J. Exp. Biol. 219, 3719-3728. 10.1242/jeb.142711 [DOI] [PubMed] [Google Scholar]
- Garland T. J. and Carter P. A. (1994). Evolutionary physiology. Annu. Rev. Physiol. 56, 579-621. 10.1146/annurev.ph.56.030194.003051 [DOI] [PubMed] [Google Scholar]
- Hammond K. A., Szewczak J. and Krol E. (2001). Effects of altitude and temperature on organ phenotypic plasticity along an altitudinal gradient. J. Exp. Biol. 204, 1991-2000. [DOI] [PubMed] [Google Scholar]
- Hammond K. A., Chappell M. A. and Kristan D. M. (2002). Developmental plasticity in aerobic performance in deer mice (Peromyscus maniculatus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 133, 213-224. 10.1016/S1095-6433(02)00159-9 [DOI] [PubMed] [Google Scholar]
- Hayes J. P. (1989a). Altitudinal and seasonal effects on aerobic metabolism of deer mice. J. Comp. Physiol. B 159, 453-459. 10.1007/BF00692417 [DOI] [PubMed] [Google Scholar]
- Hayes J. P. (1989b). Field and maximal metabolic rates of deer mice (Peromyscus maniculatus) at low and high altitudes. Physiol. Zool. 62, 732-744. 10.1086/physzool.62.3.30157924 [DOI] [Google Scholar]
- Hayes J. P. and O'Connor C. S. (1999). Natural selection on thermogenic capacity of high-altitude deer mice. Evolution 53, 1280-1287. 10.1111/j.1558-5646.1999.tb04540.x [DOI] [PubMed] [Google Scholar]
- Hock R. J. (1964). Physiological responses of deer mice to various native altitudes. In The Physiological Effects of High Altitude (ed. Weihe W. H.), pp. 59-72. New York: Macmillan. [Google Scholar]
- Ivy C. M. and Scott G. R. (2015). Control of breathing and the circulation in high-altitude mammals and birds. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 186, 66-74. 10.1111/apha.12912 [DOI] [PubMed] [Google Scholar]
- Ivy C. M. and Scott G. R. (2017). Control of breathing and ventilatory acclimatization to hypoxia in deer mice native to high altitudes. Acta Physiol. (In press). 10.1111/apha.12912 [DOI] [PubMed] [Google Scholar]
- Lau D. S., Connaty A. D., Mahalingam S., Wall N., Cheviron Z. A., Storz J. F., Scott G. R. and McClelland G. B. (2017). Acclimation to hypoxia increases carbohydrate use during exercise in high-altitude deer mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R400-R411. 10.1152/ajpregu.00365.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighton J. R. B. (2008). Measuring Metabolic Rates: A Manual for Scientists. Oxford University Press. [Google Scholar]
- Lui M. A., Mahalingam S., Patel P., Connaty A. D., Ivy C. M., Cheviron Z. A., Storz J. F., McClelland G. B. and Scott G. R. (2015). High-altitude ancestry and hypoxia acclimation have distinct effects on exercise capacity and muscle phenotype in deer mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R779-R791. 10.1152/ajpregu.00362.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam S., McClelland G. B. and Scott G. R. (2017). Evolved changes in the intracellular distribution and physiology of muscle mitochondria in high-altitude native deer mice. J. Physiol. 595, 4785-4801. 10.1113/JP274130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge C. and León-Velarde F. (1991). Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol. Rev. 71, 1135-1172. [DOI] [PubMed] [Google Scholar]
- Natarajan C., Inoguchi N., Weber R. E., Fago A., Moriyama H. and Storz J. F. (2013). Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340, 1324-1327. 10.1126/science.1236862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C., Hoffmann F. G., Lanier H. C., Wolf C. J., Cheviron Z. A., Spangler M. L., Weber R. E., Fago A. and Storz J. F. (2015). Intraspecific polymorphism, interspecific divergence, and the origins of function-altering mutations in deer mouse hemoglobin. Mol. Biol. Evol. 32, 978-997. 10.1093/molbev/msu403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2016). R: A language and environment for statistical computing. In R Foundation for Statistical Computing Vienna, Austria: (http://www.R-project.org/). [Google Scholar]
- Rosenmann M. and Morrison P. (1974). Maximum oxygen consumption and heat loss facilitation in small homeotherms by He-O2. Am. J. Physiol. 226, 490-495. [DOI] [PubMed] [Google Scholar]
- Russell G. A., Rezende E. L. and Hammond K. A. (2008). Development partly determines the aerobic performance of adult deer mice, Peromyscus maniculatus. J. Exp. Biol. 211, 35-41. 10.1242/jeb.012658 [DOI] [PubMed] [Google Scholar]
- Scott G. R. (2011). Elevated performance: the unique physiology of birds that fly at high altitudes. J. Exp. Biol. 214, 2455-2462. 10.1242/jeb.052548 [DOI] [PubMed] [Google Scholar]
- Scott G. R., Schulte P. M., Egginton S., Scott A. L. M., Richards J. G. and Milsom W. K. (2011). Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Mol. Biol. Evol. 28, 351-363. 10.1093/molbev/msq205 [DOI] [PubMed] [Google Scholar]
- Scott G. R., Elogio T. S., Lui M. A., Storz J. F. and Cheviron Z. A. (2015). Adaptive modifications of muscle phenotype in high-altitude deer mice are associated with evolved changes in gene regulation. Mol. Biol. Evol. 32, 1962-1976. 10.1093/molbev/msv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheafor B. A. (2003). Metabolic enzyme activities across an altitudinal gradient: an examination of pikas (genus Ochotona). J. Exp. Biol. 206, 1241-1249. 10.1242/jeb.00226 [DOI] [PubMed] [Google Scholar]
- Shirkey N. J. and Hammond K. A. (2014). The relationship between cardiopulmonary size and aerobic performance in adult deer mice at high altitude. J. Exp. Biol. 217, 3758-3764. 10.1242/jeb.103713 [DOI] [PubMed] [Google Scholar]
- Snyder L. R. G., Born S. and Lechner A. J. (1982). Blood oxygen affinity in high- and low-altitude populations of the deer mouse. Respir. Physiol. 48, 89-105. 10.1016/0034-5687(82)90052-4 [DOI] [PubMed] [Google Scholar]
- Storz J. F., Runck A. M., Moriyama H., Weber R. E. and Fago A. (2010a). Genetic differences in hemoglobin function between highland and lowland deer mice. J. Exp. Biol. 213, 2565-2574. 10.1242/jeb.042598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Scott G. R. and Cheviron Z. A. (2010b). Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 213, 4125-4136. 10.1242/jeb.048181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velotta J. P., Jones J., Wolf C. J. and Cheviron Z. A. (2016). Transcriptomic plasticity in brown adipose tissue contributes to an enhanced capacity for nonshivering thermogenesis in deer mice. Mol. Ecol. 25, 2870-2886. 10.1111/mec.13661 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.