ABSTRACT
Galectins are a family of lectin binding proteins expressed both intracellularly and extracellularly. Galectin-3 (Gal-3, also known as LGALS3) is expressed at the cell surface; however, Gal-3 lacks a signal sequence, and the mechanism of Gal-3 transport to the cell surface remains poorly understood. Here, using a genome-wide CRISPR/Cas9 forward genetic screen for regulators of Gal-3 cell surface localization, we identified genes encoding glycoproteins, enzymes involved in N-linked glycosylation, regulators of ER-Golgi trafficking and proteins involved in immunity. The results of this screening approach led us to address the controversial role of N-linked glycosylation in the transport of Gal-3 to the cell surface. We find that N-linked glycoprotein maturation is not required for Gal-3 transport from the cytosol to the extracellular space, but is important for cell surface binding. Additionally, secreted Gal-3 is predominantly free and not packaged into extracellular vesicles. These data support a secretion pathway independent of N-linked glycoproteins and extracellular vesicles.
KEY WORDS: Galectin, Glycosylation, Unconventional secretion
Summary: A CRISPR screen identified genes that regulate the cell surface localization of galectins, and data support a secretion pathway for galectin-3 that is independent of N-linked glycoproteins and extracellular vesicles.
INTRODUCTION
Galectins are an evolutionarily conserved family of β-galactose-binding proteins. There are 15 members, all of which contain a carbohydrate-recognition domain (CRD). The family members can be divided into three categories: (1) prototypic single CRD galectins that can form homodimers; (2) galectins that contain two tandem repeat CRDs, and (3) galectin-3, a chimeric protein containing a single CRD and a disordered N-terminal region that facilitates oligomerization (Elola et al., 2015).
Galectins belong to the leaderless class of proteins (defined by the absence of signal peptide and transmembrane domains) that function both in the cytoplasm and outside the cell. Their function in the cytoplasm include roles in cell growth, apoptosis, the cell cycle and cellular immunity (Boyle and Randow, 2013; Liu and Rabinovich, 2005; Nabi et al., 2015; Rabinovich et al., 2002). When galectins are outside the cell, they are known to be retained to the extracellular leaflet of the plasma membrane, typically through their binding to N-linked glycans and core O-linked glycans on glycosylated proteins and lipids. From there, they modulate many cellular processes, including endocytosis, migration and adhesion (Elola et al., 2015; Lakshminarayan et al., 2014; Mazurek et al., 2012; Xin et al., 2015). In addition, galectins are found in the serum, where they regulate the activity of immune cells (Rabinovich et al., 2002).
Interestingly, galectin-3 (Gal-3, also known as LGALS3) is detected at high levels in cardiac patients, and used as a marker for cardiovascular disease and heart failure (Jagodzinski et al., 2015; Medvedeva et al., 2016). Similarly, elevated levels of galectin-1 (Gal-1, also known as LGALS1) are associated with poor prognosis in many cancers, including melanoma, lung, bladder and head cancers (Thijssen et al., 2015).
The mechanism of galectin secretion remains controversial. As mentioned above, galectins lack a signal peptide and do not enter the classical ER/Golgi secretory pathway (Hughes, 1999; Nickel, 2003) and their secretion is not blocked by drugs that inhibit the classical secretory pathway, such as brefeldin A and monensin (Lindstedt et al., 1993; Sato et al., 1993).
Currently, three major mechanisms have been proposed to explain the unconventional secretion of leaderless proteins: (1) direct translocation across the plasma membrane, either through a transporter or by auto-transportation as in the case of FGF2; (2) engulfment into extracellular vesicles (exosomes and microvesicles), and (3) capture into a membrane-bound compartment, such as a secretory autophagosome, late endosome or CUPS (Hughes, 1999; Nickel and Rabouille, 2009; Nickel and Seedorf, 2008).
Evidence is lacking for a mechanism involving direct translocation of galectins across the membrane. In particular, a transporter has yet to be identified and auto-transportation by pore formation is also lacking (Hughes, 1999; Nickel and Rabouille, 2009; Nickel and Seedorf, 2008; Rabouille, 2017). Galectin secretion via microvesicles or exosomes, collectively termed extracellular vesicles (EVs), has been proposed (Cooper and Barondes, 1990; Mehul and Hughes, 1997; Sato et al., 1993; Seelenmeyer et al., 2008). Indeed, Gal-3 and Gal-1 are recruited to the cytoplasmic leaflet of the plasma membrane, where they are secreted in microvesicles generated by plasma membrane budding (Cooper and Barondes, 1990; Mehul and Hughes, 1997). However, contrasting reports show that Gal-1 secretion is not reduced when plasma membrane blebbing is inhibited (Seelenmeyer et al., 2008). Furthermore, secretion in EVs does not explain how galectins are subsequently delivered to the cell surface, although the EVs may be disrupted in the extracellular space to release Gal-3 (Mehul and Hughes, 1997). It has also been proposed that Gal-1 is directly transported across the plasma membrane while coupled to glycoproteins or lipids on the inner leaflet of the membrane. Gal-1 secretion requires a functional CRD for cell surface localization, and binding to glycoproteins, proteins or glycolipids may recruit galectins to the inner leaflet of the plasma membrane and mediate transport across the membrane to the cell surface (Seelenmeyer et al., 2005). However, Chinese hamster ovary (CHO) cells lacking the ability to glycosylate glycoproteins efficiently secrete Gal-1 (Cho and Cummings, 1995), suggesting that glycans do not play a role in secretion. Therefore, not only the mechanism of galectin secretion from the cell remains elusive, but also the role of glycosylation in the secretion process.
What is better established, however, is that moieties of N-linked glycoproteins and lipids that are exposed to the extracellular leaflet of the plasma membrane are important for restricting galectins to the surface of cells after their secretion, and prevent them from diffusing in the extracellular space. For instance, exogenous purified Gal-1, Gal-3 and Gal-8 (also known as LGALS) only bind to the cell surface when N-linked glycosylation pathways are intact and N-linked glycans expressed at the cell surface (Patnaik et al., 2006).
To identify key regulators of Gal-3 cell surface localization (the sum of both its secretion and its retention) and clarify the role of glycosylation, we performed a genome-wide CRISPR/Cas9 forward genetic screen. The most significantly enriched genes identified in the screen encodes glycoproteins, enzymes involved in N-linked glycan maturation and proteins regulating ER-Golgi trafficking.
We focused on the role of two genes identified in the CRISPR screen that encode proteins essential for N-linked glycosylation. When N-linked glycosylation was disrupted, the level of Gal-3 on the cell surface decreased. This was not caused by disruption of Gal-3 secretion from the cytosol to the extracellular space, as free Gal-3 was detected in the medium. This demonstrates that N-linked glycosylation is not required for secretion of Gal-3, but is essential for cell surface binding. These data support a model in which N-linked glycosylation is not required for secretion of Gal-3 to the extracellular space. Furthermore, we tested the role of EVs in Gal-3 secretion and we conclude that they are not involved.
RESULTS
A genome-wide screen identifies genes required for Gal-3 cell surface localization
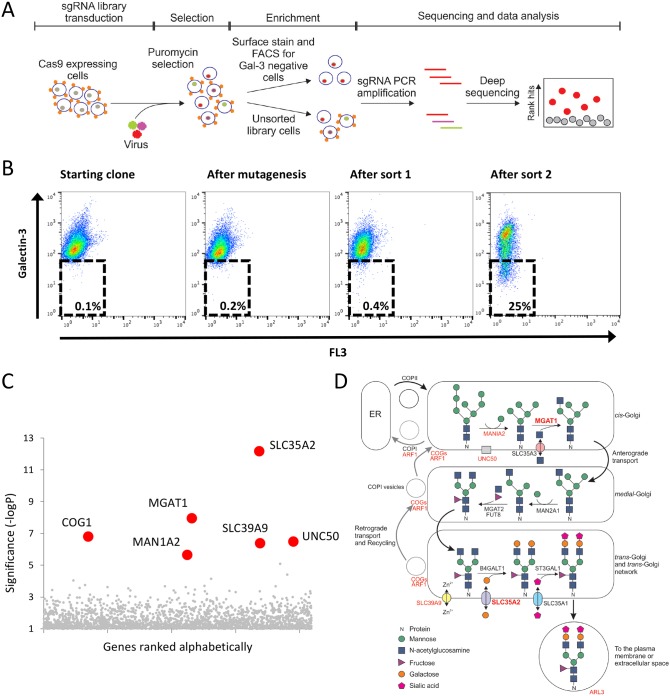
Owing to the limited knowledge about Gal-3 trafficking from the cytosol to the cell surface and its regulation, we set out to identify genes required for cell surface localization of Gal-3. HeLa cells in steady state suspension (sHeLa) express Gal-3 on their surface (Fig. S1A) and there is a small proportion detectable in the medium (Fig. S1B). To be found on the outer leaflet of the cell, Gal-3 must be secreted from the cytosol through an unconventional protein trafficking pathway. Therefore, we performed a genome-wide CRISPR/Cas9 forward genetic screen in sHeLa and enriched for cells with decreased cell surface Gal-3 (Fig. 1A). To ensure optimal screening parameters, sHeLa stably expressing Cas9 nuclease (sHeLa-Cas9) were analysed for Gal-3 surface expression by flow cytometry. Gal-3 surface expression was largely homogenous; however, the small population of Gal-3-negative cells were removed in a pre-clear cell sort to optimize screening parameters. The resulting population (∼1×108 cells) was then transduced with the GeCKO v2 single-guide RNA (sgRNA) library, containing 123,411 guide RNAs targeting 19,050 genes, at a multiplicity of infection of ∼0.3 (Shalem et al., 2014; Timms et al., 2016). Untransduced cells were removed through puromycin selection, and rare cells that had reduced cell surface Gal-3 were enriched by two rounds of fluorescence-activated cell sorting (FACS) (Fig. 1A,B). The sgRNA abundance of the enriched population was quantified by deep sequencing and compared to the control unsorted population (Fig. 1C) (König et al., 2007; Timms et al., 2016). Strikingly the most significantly enriched genes identified in this screen coded for Golgi enzymes involved in N-linked glycosylation or proteins regulating ER-Golgi transport (Fig. 1C,D). These include solute carrier family 35 member A2 (SLC35A2), mannosyl (alpha-1,3-)-glycoprotein beta-1,2-N-acetylglucosaminyltransferase (MGAT1), mannosidase alpha class 1A member 2 (MAN1A2) and component of oligomeric Golgi complex 1 (COG1) (Fig. 1C,D; Table S1). To further analyse the function of the genes identified in this screen, we applied bioinformatic pathway analysis to the 200 most enriched genes (Huang et al., 2009a,b). This analysis showed that, of the genes with known function, many genes coded for glycoproteins such as integrin β3 (ITGB3), laminin subunit beta 2 (LAMB2) and basigin (CD147, also known as BSG), or proteins involved in the transport of glycoproteins within the Golgi and to the cell surface including ADP-ribosylation factor 1 (ARF1) and ADP-ribosylation factor-like protein 3 (ARL3), and proteins with roles in immunity, including NLR family pyrin domain-containing 2 (NLRP2) and tripartite motif-containing proteins 5 and 34 (TRIM5, TRIM34) (Table 1). Interestingly, several proteins identified in this screen are known Gal-3 interactors either on the cell surface, such as the integrins, laminins and CD147, or in the cytosol for the TRIMs (Chauhan et al., 2016; Priglinger et al., 2013).
Fig. 1.
A CRISPR/Cas9-mediated genetic screen identifies genes required for cell surface localization of Gal-3. (A) Schematic of the CRISPR/Cas9 screen in sHeLa to identify genes required for Gal-3 cell surface localization. Cells were transduced with a lentiviral sgRNA library (sgRNA transduction indicated by colours in the nucleus) and cells that were successfully transduced were selected with puromycin. After selection, the population was split into two; one half was sorted by FACS to enrich for cells that have less Gal-3 on the surface (Gal-3 is represented by small orange shapes on the cell surface) and the other was not sorted to represent the entire library. After two rounds of enrichment, the DNA from both the enriched population and the unsorted library was harvested, and enriched sgRNAs were identified by sequencing. Targeted genes were then plotted according to their relative enrichment. (B) CRISPR-mediated mutagenesis was performed on sHeLa using the GeCKO v2 sgRNA library, and rare cells with decreased surface Gal-3 expression were selected by two sequential rounds of FACS. Cell surface Gal-3 was measured on live cells using an anti-Gal-3 antibody conjugated to Alexa Fluor 647. (C) Plot illustrating the hits from the CRISPR screen. The RSA algorithm was used to identify the significantly enriched genes targeted in the selected cells. The most significantly enriched genes are labelled. (D) Schematic of the N-linked glycosylation pathway within the Golgi. Genes identified to be important for Gal-3 surface localization by the CRISPR screen are highlighted in red, and those chosen for further study (MGAT1 and SLC35A2) are shown in bold.
Table 1.
Pathway analysis of the 200 most significantly enriched genes identified in the genome-wide CRISPR screen for Gal-3 cell surface localization

No core ER proteins or enzymes required for N-linked glycosylation upstream of the Golgi were identified in the screen. Furthermore, not all subunits of the COG family were identified. sgRNAs targeting Gal-3 itself were also not enriched in the screen. Analysis of the control unsorted population shows that the screen was not saturating, and that 7% of the sgRNAs in the library were not present and ∼20% showed a coverage of <200 cells/sgRNA (data not shown). Five sgRNAs targeting Gal-3 were efficiently represented, yet these cells were not enriched during the screen. This may indicate that these guides were not effective at targeting Gal-3, or that Gal-3 deletion is lethal or decreased cell growth. Another explanation for the lack of Gal-3 sgRNA enrichment is that Gal-3 secreted by other surrounding cells is able to bind to the surface of Gal-3 null cells, masking the effect of the knockout in our FACS assay. This scenario could also affect other knockout cells, where the sgRNA targets key regulators of Gal-3 secretion but glycosylated binding partners on the cell surface are unaffected. However, some of the hits identified in the screen, such as TRIM34, TRIM5, ARHGAP30 and ARHGAP9, are not known to regulate the glycosylation pathway. Therefore, it remains unclear why Gal-3 was not enriched in this screen.
Additionally, we did not identify genes previously linked to unconventional secretion, such as those coding for the LC3 (also known as MAP1LC3) proteins, GABARAP, GRASP55 (also known as GORASP2) and components of endosomal sorting complexes required for transport (ESCRT) (Table S1) (Nickel and Rabouille, 2009; Rabouille, 2017). To further confirm that autophagy, the GRASP55 and the ESCRT pathways do not regulate cell surface localization of Gal-3, we used LC3 and GABARAP knockout cells (Fig. S2A), GRASP55 esiRNA (Fig. S2B) and a Vps4 (also known as VPS4A) dominant negative mutant (Fig. S2C). In all cases the cell surface expression of Gal-3 remained unaffected, further verifying the absence of these genes in the CRISPR/Cas9 forward genetic screen.
N-linked glycosylation is required for cell surface binding but not galectin secretion
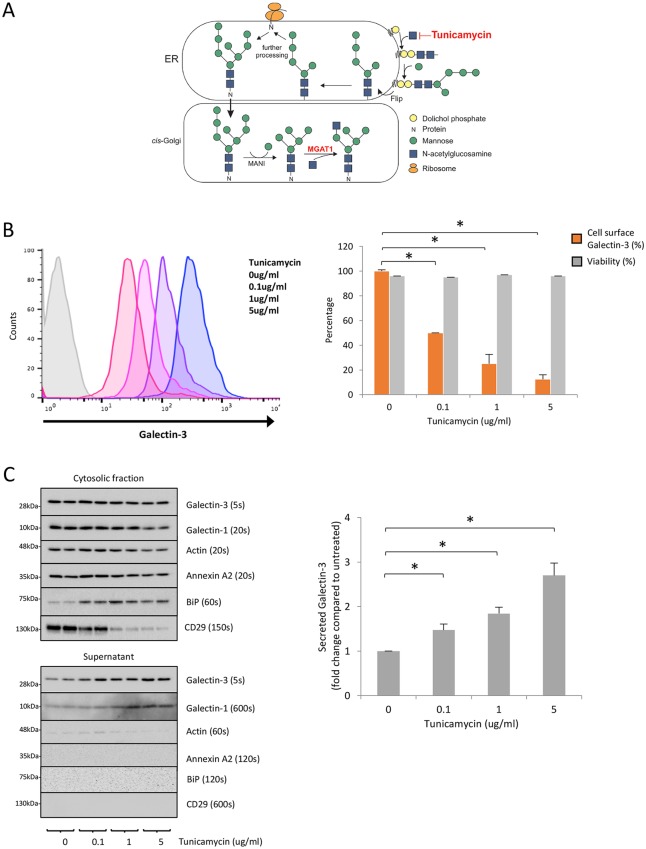
Because it remains controversial whether glycosylation is required for galectin trafficking to the cell surface, we aimed to determine whether defective N-linked glycosylation decreases Gal-3 trafficking to the cell surface, or N-linked glycoproteins are simply required for Gal-3 cell surface binding. Tunicamycin blocks the transfer of N-acetylglucosamine-1-phosphate from UDP-N-acetylglucosamine to dolichol phosphate, the first step in N-linked glycosylation (Fig. 2A) and was used to inhibit glycosylation in sHeLa. Cells were treated with increasing concentrations of tunicamycin to inhibit N-linked glycosylation, and the level of cell surface Gal-3 was assessed by flow cytometry. Cell surface Gal-3 decreased as the concentration of tunicamycin increased (Fig. 2B). Propidium iodide was used to measure cell viability and cells remained viable at all tunicamycin concentrations (Fig. 2B, right). In agreement with the results of the CRISPR screen, this shows that a reduction in N-linked glycosylation (and thus complex glycans at the cell surface) decreases cell surface Gal-3.
Fig. 2.
Tunicamycin decreases cell surface Gal-3 while increasing the level of Gal-3 in the medium. (A) Schematic of tunicamycin inhibition of N-linked glycosylation. Tunicamycin blocks the transfer of N-acetylglucosamine-1-phosphate from UPD-N-acetylglucosamine to dolichol phosphate, the first step in N-linked glycosylation. (B) Tunicamycin reduces cell surface localization of Gal-3. sHeLa were treated with increasing concentrations of tunicamycin diluted in serum-free medium for 24 h. Cell surface Gal-3 was measured on live cells using an anti-Gal-3 antibody conjugated to Alexa Fluor 647; cell viability was also assessed by flow cytometry using propidium iodide. Unstained cells are shown in grey. Quantification is shown on the right. Data are mean±s.e.m. from biological replicates (n=3); *P<0.05 (two-sample Student's t-test comparing cells with treated with the indicated tunicamycin concentrations to untreated cells). (C) Tunicamycin increases the levels of Gal-3 in the culture supernatant. Western blot analysis of cell lysates and supernatants of sHeLa treated with increasing concentrations of tunicamycin (24 h at 37°C in serum-free medium). Note that the tunicamycin treatment was efficient, as shown by the increased level of BiP and decreased level of CD29. Quantification is shown on the right. Data are mean±s.e.m. from biological replicates (n=3); *P<0.05 (two-sample Student's t-test comparing cells treated with the indicated tunicamycin concentrations to untreated cells).
To investigate whether N-linked glycosylation is required for transport of Gal-3 from the cytosol to the extracellular space, the supernatant of sHeLa cells treated with tunicamycin was analysed by western blotting. In this assay, if N-linked glycosylation is indeed required for Gal-3 transport there should be a reduction in the level of Gal-3 in the supernatant compared to untreated cells. Conversely, if N-linked glycosylation is only required for cell surface binding and not for secretion, there should be an increase in free Gal-3 measured in the supernatant. Western blotting showed a concentration-dependent increase in Gal-3 in the supernatant after tunicamycin treatment (Fig. 2C). A similar trend was observed for Gal-1 (Fig. 2C, left). The effectiveness of tunicamycin treatment was confirmed by assessing the relative expression of the ER resident protein BiP (GRP78, also known as HSPA5), where increased expression indicates ER stress (Fig. 2C). Actin and annexin A2 were used as negative controls, with low levels detectable in the supernatant upon tunicamycin treatment (Fig. 2C). These results support the suggestion that Gal-3 and Gal-1 require N-linked glycans to bind to the cell surface (Patnaik et al., 2006). These data also suggest that secretion of galectins from the cytosol to the extracellular space is independent of N-linked glycosylation.
N-linked glycan maturation mediated by MGAT1 and SLC35A2 is required for Gal-3 cell surface binding but not secretion
The use of tunicamycin to block N-linked glycosylation provides proof of principle, but there may be confounding factors due to off-target effects. Therefore, to validate the findings of the CRISPR screen and investigate the role of N-linked glycan maturation, we targeted MGAT1 and SLC35A2; two genes that were highly enriched in the screen and are known to be specifically required for N-linked glycosylation (Fig. 1C,D). In the cis-Golgi, MGAT1 adds N-acetylglucosamine to the sugar backbone of glycoproteins, initiating complex N-linked glycosylation (Fig. 1D). SLC35A2 acts later in the trans-Golgi, transporting UDP-galactose into the trans-Golgi network for addition onto glycoproteins (Fig. 1D).
We generated MGAT1 and SLC35A2 CRISPR knockout cell lines using guide RNAs from an independent CRISPR/Cas9 library (Wang et al., 2015). This provides an additional control for off-target effects as the guide RNAs differed from those used in our original CRISPR screen. Single cell cloning, using FACS, was carried out to obtain knockout clones for MGAT1 and SLC35A2 (Fig. S3). To evaluate the presence of CRISPR-induced mutations in the MGAT1 or SLC35A2 genes, the targeted region of the gene was amplified and sequenced. Alignments and tracking of indels by decomposition (TIDE) analysis confirmed that MGAT1 and SCL35A2 contain CRISPR-induced insertions and deletions (Fig. S3) (Brinkman et al., 2014). MGAT1 and SLC35A2 clones contained a combination of homozygous and compound heterozygous deletions likely to disrupt gene function (Fig. S3). As a positive control, additional MGAT1 and SLC35A2 clones that expressed cell surface Gal-3 to a similar level as untargeted cells (Gal-3 positive) were isolated; these contained no insertions or deletions in the targeted region (Fig. S3).
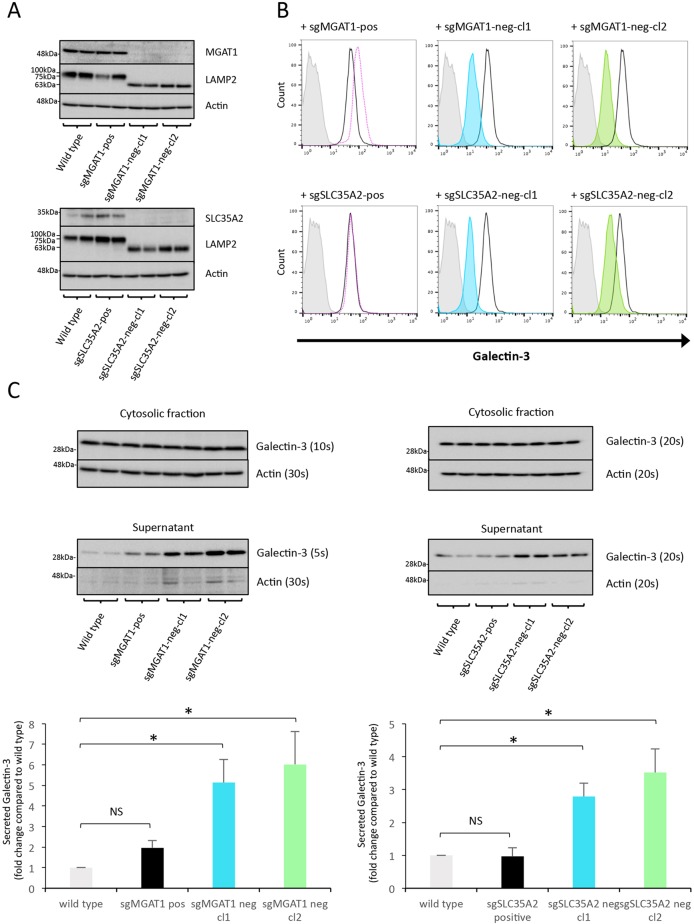
CRISPR-induced deletions led to a loss of target protein expression in both MGAT1 clones and SLC35A2 clones, assessed by western blotting (Fig. 3A). MGAT1 and SLC35A2 protein levels in the Gal-3-positive clones are similar to those in the wild type (Fig. 3A). MGAT1 and SLC35A2 are both essential for N-linked glycosylation, so defective glycosylation would be expected on all N-linked glycoproteins. To assess this, lysosomal-associated membrane protein-2 (LAMP2) glycoforms were analysed by western blotting. MGAT1- and SLC35A2-deficient clones expressed a lower molecular weight form of LAMP2 compared to wild-type and Gal-3-positive sHeLa (Fig. 3A). This indicates that there are fewer mature N-linked glycans added to LAMP2 when MGAT1 or SLC35A2 is absent.
Fig. 3.
MGAT1 and SLC35A2 knockout abrogates Gal-3 cell surface binding but not secretion. (A) Western blot analysis of MGAT1- and SLC35A2-deficient sHeLa. Cell lysates were assessed for either MGAT1 or SLC35A2 protein levels after CRISPR/Cas9 targeting and single cell cloning based on Gal-3 surface expression. LAMP2 was also assessed to analyse defects in glycosylation, and actin was used as a loading control. (B) Cell surface localization of Gal-3 is decreased in MGAT1- and SLC35A2-deficient sHeLa measured by flow cytometry. Cell surface Gal-3 was measured on live cells using an anti-Gal-3 antibody conjugated to Alexa Fluor 647. Grey, no antibody; black line, untransfected; pink dotted line, sgMGAT1-positive clone; blue, sgMGAT1-negative clone 1; green, sgMGAT1-negative clone 2. The same respective colours are used for sgSLC35A2 in the lower panels. (C) Gal-3 is secreted from MGAT1- and SLC35A2-deficient sHeLa. Wild type, positive control and negative clones for MGAT1 (left) and SLC35A2 (right) cells were incubated in serum-free medium for 24 h, and the cells and medium assessed by western blotting. Gal-3 was assessed in the lysate and medium (supernatant); actin was used as a loading control and control for cell lysis. Exposure times are indicated to allow relative comparisons between blots to illustrate the large increase in Gal-3 in the supernatant compared to actin. Quantification of MGAT1 (left) and SLC35A2 (right) is shown in the bottom panels. Data are mean±s.e.m. from biological replicates (n=3); *P<0.05 (two-sample Student's t-test comparing each cell line to wild-type cells). Note that the same actin loading control blots are shown in the top panels of A and C, and also in the top panel of Fig. 5A, as the blots were stripped and reprobed with the indicated antibodies. Similarly, the bottom panel of A and the top panel of Fig. 5B are the same blots.
To confirm that the loss of MGAT1 and SLC35A2 leads to a decrease in the expression of Gal-3 on the cell surface, as identified in the initial CRISPR screen, we assessed cell surface Gal-3 using flow cytometry. Gal-3-positive clones expressing MGAT1 and SLC35A2 were indeed positive for Gal-3 at a comparable level to wild-type sHeLa (Fig. 3B). Likewise, MGAT1- and SLC35A2-deficient clones obtained from the Gal-3-negative population showed a marked reduction in the expression of Gal-3 (Fig. 3B). Therefore, the Gal-3-negative phenotype seen by flow cytometry can be attributed to CRISPR-mediated knockout of MGAT1 and SLC35A2, further validating the original CRISPR screen.
To assess whether loss of MGAT1 or SLC35A2 impacts the transport of Gal-3 from the cytosol to the extracellular space, we analysed Gal-3 secretion by western blotting. Our results show that in both MGAT1- and SLC35A2-deficient cells, Gal-3 is readily detected in the extracellular medium (Fig. 3C). Furthermore, in MGAT1- and SLC35A2-deficient cells there was an increase in the relative amount of Gal-3 in the supernatant compared to the wild-type sHeLa control (Fig. 3C). This was not seen in the Gal-3-positive wild-type clones, which remained similar to wild-type sHeLa (Fig. 3C). Gal-1 also showed a similar trend in SLC35A2 knockout cells (Fig. S4). Therefore, a lack of N-linked glycosylation due to MGAT1 or SLC35A2 deficiency leads to reduced galectin binding to the cell surface and an increase in galectin in the supernatant. This is indicative of a binding defect and not a reduction in secretion.
CHO glycosylation mutants also efficiently secrete galectins
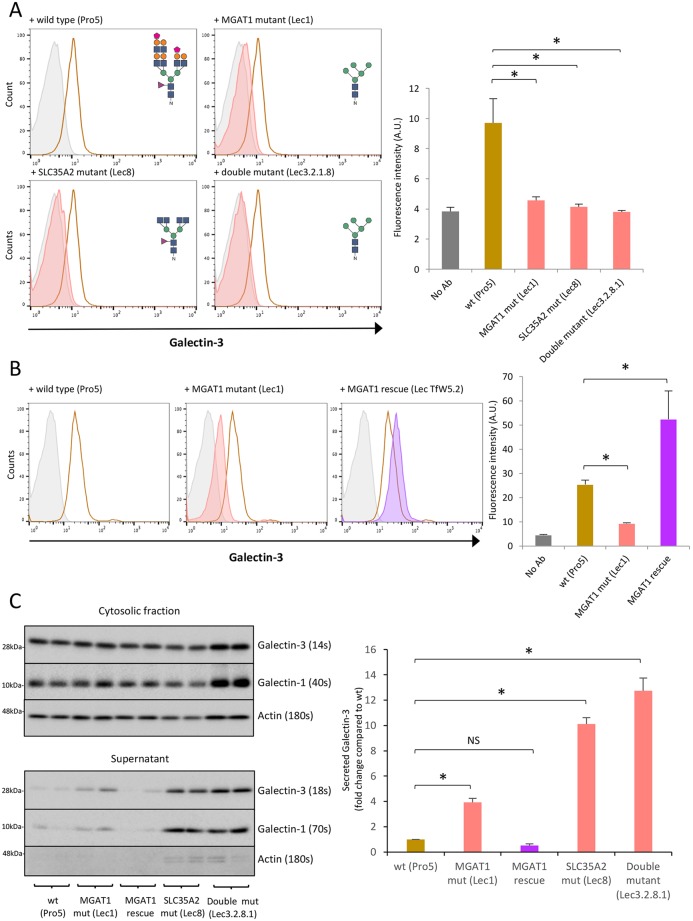
To further assess the role of N-linked glycosylation in the transport of galectins to the cell surface and their secretion, several well-characterized CHO cell lines with glycosylation defects were used to validate our data (Stanley, 1989). These include an MGAT1 loss-of-function mutant (Lec1), an SLC35A2 loss-of-function mutant (Lec8), and an MGAT1 and SLC35A2 loss-of-function double mutant (Lec3.2.8.1) (Stanley, 1989). The aberrations in the N-linked glycans produced from each cell line are depicted in Fig. 4A.
Fig. 4.
MGAT1 and SLC35A2 mutation in CHO Lec cells reduces Gal-3 cell surface binding but does not affect secretion. (A) Gal-3 cell surface localization is decreased in MGAT1 and SLC35A2 mutant CHO lines. Cell surface Gal-3 was measured on live MGAT1 (Lec1), SLC35A2 (Lec8) and double mutant (Lec3.2.1.8) CHO Lec cells compared to wild-type (Pro5) cells by flow cytometry using an anti-Gal-3 antibody conjugated to Alexa Fluor 647. Grey, no antibody; brown, wild type; red, mutants. Predicted N-linked glycans for each cell line are shown on the histograms. Sugar symbols: purple triangle, fructose; green circle, mannose; orange circle, galactose; blue square, N-acetylglucosamine; pink trapezoid, sialic acid. Quantification is shown on the right. Data are mean±s.e.m. from biological replicates (n=3); *P<0.05 [two-sample Student's t-test comparing each mutant CHO line to wild-type (Pro5) cells]. (B) Gal-3 cell surface localization is rescued in MGAT1 rescue CHO Lec cells measured by flow cytometry. Gal-3 was measured as in A. Grey, no antibody; brown, wild type; red, mutants; purple, rescue. Quantification is shown on the right. Data are mean±s.e.m. from biological replicates (n=3); *P<0.05 [two-sample Student's t-test comparing the MGAT1 mutant and rescue lines to wild type (Pro5) cells]. (C) Gal-3 is secreted from MGAT1 and SLC35A2 mutant CHO Lec cells. Wild-type (Pro5), MGAT1 (Lec1), MGAT1 rescue, SLC35A2 mutant (Lec8) and the double mutant (Lec3.2.8.1) were incubated in EX-CELL 325 PF CHO for 48 h, and the cells and medium assessed by western blotting. Gal-3, Gal-1 and actin were analysed in the cell lysates and medium (supernatant). Exposure times are indicated for comparison. Quantification is shown on the right. Data are mean±s.e.m. from biological replicates (n=3); *P<0.05 [two-sample Student's t-test comparing each mutant CHO cell line to wild-type (Pro5) cells].
These cell lines were previously used to analyse galectin-glycan binding specificity, demonstrating that N-linked glycans are the major ligand for Gal-1, -3 and -8 binding at the cell surface (Patnaik and Stanley, 2006). These mutant CHO lines were used here to further assess Gal-3 and Gal-1 cell surface binding and secretion. Flow cytometry analysis of Gal-3 expression on the surface of CHO cells showed that MGAT1 (Lec1) and SLC35A2 (Lec8) single loss-of-function mutant lines, as well as the MGAT1/SLC35A2 (Lec3.2.8.1) double-mutant line, all exhibited a decrease in the level of Gal-3 detectable on the cell surface compared to the wild type (Pro5) (Fig. 4A). This phenotype was reversed in an MGAT1 rescue cell line, confirming that the loss of Gal-3 on the surface is caused by the loss-of-function mutation in the MGAT1 gene (Fig. 4B) (Chen and Stanley, 2003; Kumar and Stanley, 1989). Western blot analysis showed that Gal-3 was secreted by MGAT1 (Lec1) and SLC35A2 (Lec8) loss-of-function cells as expected (Fig. 4C). Furthermore, the level of Gal-3 detectable in the medium was substantially higher than in the wild-type (Pro5) CHO and MGAT1 rescue cell lines (Fig. 4C). This was also evident when Gal-1 secretion was assessed (Fig. 4C). These data are consistent with results obtained in sHeLa lines and further confirm that N-linked glycosylation is not required for Gal-3 secretion.
Secreted Gal-3 is primarily free and not packaged into EVs
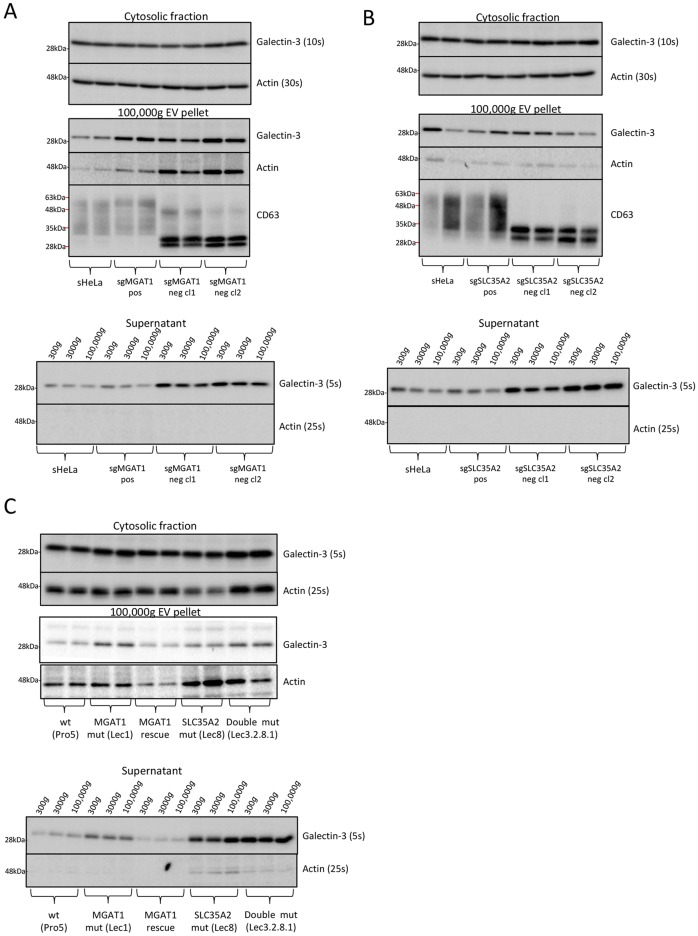
Thus far, we have shown that N-linked glycan maturation is not required for the transport of Gal-3 from the cytosol to the extracellular space and is a regulatory element that retains galectins at the cell surface. Next, we set out to investigate whether secreted Gal-3 is free in the medium or packaged into EVs. There are conflicting data in the literature as to whether galectins are secreted via EVs (Cooper and Barondes, 1990; Mehul and Hughes, 1997; Sato et al., 1993; Seelenmeyer et al., 2008). To investigate this, the medium from wild-type, MGAT1- or SCL35A2-deficient cells was collected and subjected to differential centrifugation. Briefly, cells were removed at 300 g, then the cell debris was removed at 3000 g and EVs pelleted at 100,000 g. The supernatant and EV pellets were separated after centrifugation at 100,000 g and each assessed for Gal-3 by western blotting. The data show similar levels of Gal-3 in the medium after removing EVs at 100,000 g, indicating that the majority of the secreted Gal-3 is free and not packaged in vesicles (Fig. 5A,B). Gal-3 was detectable in the 100,000 g EV pellet of all cell lines, although the levels were somewhat variable, and there was a small increase in the amount of both actin and Gal-3 detected in the EV pellets from MGAT1-deficient clones (Fig. 5A,B). It is important to note that the EV pellets were 50× concentrated compared to the supernatant samples (Fig. 5A,B). To further assess the composition of the 100,000 g pellet, we analysed the tetraspanin CD63, which is known to be enriched in exosomes (Escola et al., 1998). The 100,000 g pellet was CD63 positive and therefore contained some exosomes (Fig. 5A,B). Owing to impaired glycosylation, CD63 runs as a smaller form in the MGAT1- and SLC35A2-deficient EVs (Fig. 5A,B). The lack of glycosylation on CD63 seems to affect antibody detection, and the naked nonglycosylated form was detected better than the glycosylated form. Therefore, it is difficult to comment on the relative levels of CD63 in the EV pellets of the MGAT1- and SLC35A2-deficient cells compared to the wild-type controls. However, we believe that the lack of MGAT1 or SLC35A2 does not affect the formation or level of EVs.
Fig. 5.
Secreted Gal-3 is predominantly soluble and not packaged in EVs. (A) Soluble Gal-3 is secreted from MGAT1-deficient sHeLa. Wild type, positive control and negative clones for MGAT1-deficient cells were incubated in serum-free medium for 24 h. The cells were collected and lysed, whereas the medium was subjected to differential centrifugation at 300, 3000 and 100,000 g. A sample of the medium was collected after each centrifugation step. Gal-3 was assessed in the lysate, the entire 100,000 g EV pellet and medium (supernatant). Actin was used as a loading control and control for cell lysis. Exposure times are indicated for comparison. The 100,000 g EV pellets were also analysed by western blotting for levels of glycosylated and nonglycosylated CD63. (B) Soluble Gal-3 is secreted from SLC35A2-deficient sHeLa cells. Wild type, positive control and negative clones for SLC35A2-deficient cells were incubated in serum-free medium for 24 h. Samples were treated as described in A. (C) Secreted Gal-3 from MGAT1 and SLC35A2 mutant CHO Lec cells is soluble. Wild type (Pro5), MGAT1 (Lec1), MGAT1 rescue, SLC35A2 mutant (Lec8) and the double mutant (Lec3.2.8.1) were incubated in EX-CELL 325 PF CHO for 48 h, and the cells and medium collected. The cells, 100,000 g EV pellet and medium were processed as in A. Gal-3 and actin were analysed by western blotting and exposure times are indicated for comparison. CD63 was not analysed in this experiment as it does not cross-react with hamster CD63. Note that the same actin loading control blots are shown in the top panel of B and the bottom panel of Fig. 3A as the blots were stripped and reprobed with the indicated antibodies. Also, the top panel of A and the top panels of Fig. 3A and C, are the same blots.
We also assessed whether Gal-3 secreted from CHO MGAT1 (Lec1), SLC35A2 (Lec8) and MGAT1/SLC35A2 double (Lec3.2.8.1) mutant cell lines is also free and not packaged into EVs. In agreement with the MGAT1- and SLC35A2-deficient sHeLa, the levels of Gal-3 secreted from the CHO MGAT1 (Lec1), SLC35A2 (Lec8) and MGAT1/SLC35A2 double (Lec3.2.8.1) mutant lines remained unchanged after a 100,000 g centrifugation step (Fig. 5C). There was a small increase in the level of Gal-3 and actin detectable in the EV pellets of MGAT1 (Lec1), SLC35A2 (Lec8) and MAGT1/SLC35A2 double (Lec3.2.8.1) mutants compared to wild-type (Pro5) and rescue lines (Fig. 5C). This may also be reflected in the MGAT1-deficient cells but is not the case for SLC35A2-deficient cells, in which the level was more variable (Fig. 5A,B). Therefore, any difference in the level of EVs secreted is trivial and is unlikely to significantly contribute to the levels of secreted Gal-3. Owing to differences in the species of the cells, we were unable to evaluate CD63 in the EV pellets of CHO cells. Taken together, these results show that Gal-3 associated with EVs is a small proportion of the total secreted Gal-3 and, therefore, cannot be the primary route for trafficking outside the cell.
N-linked glycoproteins are required for the recruitment of intracellular Gal-3 to damaged lysosomal membranes
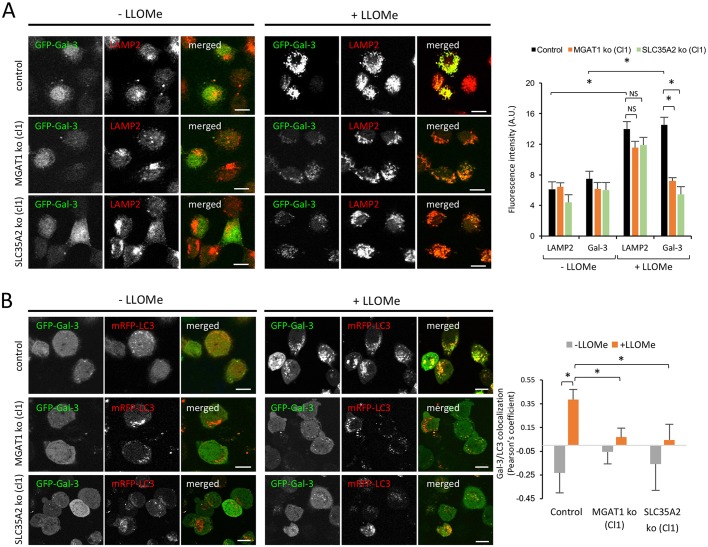
Gal-3 has important roles in regulating cell death and immunity, and is recruited to endolysosomes, lysosomes and phagosomes in response to induced organelle damage and damage caused by bacterial infection (Aits et al., 2015; Feeley et al., 2017; Maejima et al., 2013; Paz et al., 2010). In addition, Gal-3 interacts with TRIM16 to coordinate autophagy to protect against cell damage and bacterial invasion (Chauhan et al., 2016). Recruitment to lysosomes or Shigella-disrupted phagosomes is dependent on Gal-3 binding to N-linked glycans, as shown using a Gal-3 CRD mutant and CHO MGAT1 mutant (Lec1) cells, respectively (Aits et al., 2015; Paz et al., 2010). Therefore, N-linked glycans are not only important for cell surface localization of Gal-3, but are also central for the recruitment of Gal-3 to damaged lysosomes. To further characterize our MGAT1- and SLC35A2-deficient sHeLa lines, we assessed the ability of Gal-3 to redistribute from the cytosol to the membrane of leaky lysosomes (Maejima et al., 2013). To do so, we expressed GFP fused to Gal-3 in wild-type, MGAT1- and SLC35A2-deficient sHeLa lines. All cell lines were then treated with L-Leucyl-L-Leucine methyl ester (LLOMe) to induce lysosomal leakiness, and we assessed the recruitment of GFP-Gal-3 to the site of damage by immunofluorescence (Maejima et al., 2013). In wild-type cells, GFP-Gal-3 was efficiently redistributed from the cytosol to the site of lysosomal damage, colocalizing with LAMP2-positive puncta (Fig. 6A). However, in MGAT1- and SLC35A2-deficient cells the recruitment of GFP-Gal-3 to LAMP2 positive damaged lysosomes was reduced (Fig. 6A). We also assessed recruitment of LC3, as damage to lysosomes should initiate autophagy to degrade the dysfunctional organelle (Maejima et al., 2013). As expected, in wild-type cells treated with LLOMe, GFP-Gal-3-positive puncta were also mRFP-LC3 positive (Fig. 6B). In the MGAT1- and SLC35A2-deficient cells, recruitment of GFP-Gal-3 to mRFP-LC3-positive damaged lysosomes was impaired (Fig. 6B). This further confirms that N-linked glycan maturation is required for the recruitment of Gal-3 to damaged lysosomes and autophagosomes, essential for cellular homeostasis and defence.
Fig. 6.
Recruitment of GFP-Gal3 to damaged lysosomes is reduced in MGAT1- and SLC35A2-deficient cells. (A) Wild-type (control), MGAT1-deficient (clone 1) or SLC35A2-deficient (clone 1) sHeLa transiently expressing GFP-Gal-3 for 24 h were treated with 1 mM LLOMe for 3 h. Cells were fixed with PFA, permeabilized with Triton X100 and subjected to immunocytochemistry using an anti-LAMP2 antibody, then processed for confocal microscopy. The intensity of LAMP2 and Gal-3 signals measured using ImageJ in a minimum of 20 cells per condition is shown on the right. (B) Wild-type (control), MGAT1-deficient (cl1) or SLC35A2-deficient (cl1) sHeLa transiently expressing GFP-Gal-3 and mRFP-LC3 for 24 h were treated with 1 mM LLOMe for 3 h. Cells were fixed with methanol and processed for confocal microscopy. Colocalization (Pearson's coefficient) between Gal-3 and LC3 is shown on the right. Data are mean±s.e.m. from individual cells (n>20); *P<0.05 (two-sample Student's t-test). Scale bars: 10 μm.
DISCUSSION
Cell surface expression of galectins is essential for cellular homeostasis. Despite having important functions in the extracellular space, the mechanism of galectin secretion remains unclear. Galectins do not enter the classical secretory pathway, as they do not contain a signal peptide and their secretion is not affected by drugs that block this pathway (Hughes, 1999). Therefore, they must exit the cell though an unknown unconventional protein trafficking pathway. Currently, there are limited data available to explain the mechanisms of galectin trafficking from the cytosol to the extracellular space, and current theories are controversial. Here, we applied a genome-wide CRISPR screen using the GeCKO v2 library to identify regulators of Gal-3 cell surface localization. Following mutagenesis and enriching for cells with reduced Gal-3 expression at the cell surface, many genes coding for glycoproteins or proteins required for N-linked glycan maturation were identified. While this screen returned many important regulators of Gal-3m it is apparent that the screen was not saturating, as shown by the sequencing data from the control unsorted population. However, five sgRNAs targeting Gal-3 were efficiently represented in the control unsorted population, yet these cells were not enriched during sorting. One explanation for this is that there is free Gal-3 in the medium, secreted by surrounding cells, that binds to the surface of Gal-3-deficient cells masking their Gal-3-negative phenotype. This could also mask other important hits where secretion of Gal-3 is impaired but glycosylation is normal. Unfortunately, we were not able to assess the levels of Gal-3 in the medium and, therefore, do not know if this explains the lack of Gal-3 sgRNA enrichment. Moreover, there are hits identified in this screen that are not known to regulate glycosylation (such as TRIM34, TRIM5, ARHGAP30 and ARHGAP9), which should also be masked in this scenario. Additionally, we did not detect any ER proteins required for glycosylation, upstream of the Golgi, which is somewhat surprising. Loss of these proteins may be lethal or decrease cell proliferation. It is also important to note that the most significantly enriched genes identified by the screen may not be those most important for mediating Gal-3 surface localization; it may simply be that they survive well and are therefore enriched better than others.
Although the screen was not saturating, the results obtained here are consistent with the literature as Gal-3 is known to bind to N-linked glycans present on the cell surface (Patnaik et al., 2006). This is also consistent with the notion that Gal-3 requires N-linked glycans to facilitate trafficking from the cytosol to the cell surface (Seelenmeyer et al., 2005). However, this is controversial, and it was important to establish whether glycoproteins carrying N-linked sugar moieties are required for transport of Gal-3 from the cytosol to the extracellular space. Using tunicamycin and two different MGAT1 and SLC35A2 mutant cell lines, we demonstrate that Gal-3 cell surface binding is dependent on the expression of complex N-linked glycans; however, Gal-3 is efficiently secreted in the absence of N-linked glycans. The secretion of both Gal-3 and Gal-1 was unperturbed in the absence of N-linked glycosylation, clearly demonstrating that their secretion is independent of both the classical secretory pathway and any pathway requiring complex glycoproteins and lipids for transport.
The role of EVs in galectin secretion has been controversial, with conflicting reports in the literature (Cooper and Barondes, 1990; Mehul and Hughes, 1997; Sato et al., 1993; Seelenmeyer et al., 2008). Here, we demonstrate that transport of Gal-3 from the cytosol to the extracellular space is not primarily mediated by EVs in sHeLa and CHO cell lines. Owing to the increased levels of Gal-3 detectable in the medium, MGAT1- and SLC35A2-deficient cells provide an excellent system for assessing whether extracellular Gal-3 is packaged into EVs. Using differential centrifugation, we show that the vast majority of Gal-3 detected in the medium is free and soluble, indicating that Gal-3 is not packaged into EVs. These data support an EV-independent pathway for Gal-3 trafficking to the cell surface and secretion into the extracellular space.
It has previously been shown that Gal-3 is redistributed from the cytosol to glycoproteins on the luminal membrane of damaged endolysosomes/phagosomes (Aits et al., 2015; Feeley et al., 2017; Maejima et al., 2013; Paz et al., 2010). Once associated with the membrane of the damaged organelle, Gal-3 stimulates autophagy to clear the threat (Maejima et al., 2013). Furthermore, it has been shown that Gal-3 is recruited to Shigella-containing phagosomes in wild-type CHO cells but not in MGAT1 (Lec1) mutant CHO cells (Paz et al., 2010). Given these previous data, we tested the MGAT1- and SLC35A2-deficient sHeLa in this context. As expected, Gal-3 recruitment to damaged lysosomes is impaired in the MGAT1- and SLC35A2-deficient cell lines. These data, shown by us and others, may explain why people with congenital disorders of glycosylation (CDG) suffer from recurrent infections (Albahri, 2015; Grünewald et al., 2002; Monticelli et al., 2016). CDG are rare genetic disorders where glycosylation of multiple proteins is deficient or defective due to mutations in the glycosylation pathway; these mutations can occur in COG1, MGAT1 and SLC35A2 genes among many others (Albahri, 2015; Grünewald et al., 2002). CDG cause a range of organ malfunctions; in almost all cases the nervous system is affected and symptoms include developmental disabilities, ataxia hypotonia, hyporeflexia and immunological defects (Albahri, 2015; Grünewald et al., 2002; Monticelli et al., 2016). It is becoming increasingly apparent that patients with immunological defects are more likely to have mutations in resident ER and Golgi enzymes (Monticelli et al., 2016). Consistent with this, our data and previous data from Paz and colleagues suggest that patients with certain forms of CDG could have a reduced ability to sense bacterial or viral entry in the cytosol due to a lack of galectin recruitment to the site of infection (Paz et al., 2010).
Together, these data demonstrate that galectin cell surface binding and secretion are two distinct events. This is consistent with previous studies, which have shown that Gal-3 secretion is unaffected by disruptions in the secretory pathway (Cho and Cummings, 1995; Lindstedt et al., 1993; Sato et al., 1993). Exactly which domains or sequences are essential for mediating galectin secretion are also controversial. It has been shown that the flexible N-terminal domain on Gal-3 is important for secretion; however, this flexible N-terminal domain is absent in other galectins (Menon and Hughes, 1999). Therefore, if there is a common unconventional secretory pathway utilized by the galectin family, it would be somewhat surprising if this was located in the only domain that is not conserved across the galectin family. By contrast, other studies have found that the CRD is essential for the effective secretion of Gal-1 (Seelenmeyer et al., 2005). However, in our analyses, the CRD mutant Gal-3 (R186S), which is unable to bind GlcNAc, did not show any defects in Gal-3 secretion compared to the wild type (Fig. S5) (Salomonsson et al., 2010). It is possible that there are differences in the requirements for secretion between galectin family members, but this would be very surprising as the galectins are highly similar and common transport mechanism would be expected.
Regardless of the exact mechanism, it may be expected that galectins are not secreted via the conventional secretory pathway as their ligand (complex carbohydrates) is a major component of the lumen of the ER and Golgi. If galectins had to move through the ER and Golgi they would come into contact with their ligand, bind, and potentially interrupt the movement of other proteins through the secretory pathway. Therefore, having a separate pathway for trafficking galectins to the cell surface is an excellent way of ensuring that they only meet their ligands where required.
Finally, hundreds of genetic disorders that result from deficiencies in different glycosylation pathways have been described, including several neurological diseases such as autism, epilepsy and CDG (Freeze et al., 2015). Additionally, cancer cells are known to deeply alter the glycosylation pathway inducing hypo- or hyper-glycosylation (Pinho and Reis, 2015). As such, it would be interesting to study whether the alterations in several signalling pathways described in these diseases are associated with a dysregulation of cell surface galectins, given the important role of galectins in signal transduction and cell-to-cell interactions.
MATERIALS AND METHODS
Cell culture
Suspension HeLa cells were cultured in DMEM D6546 (Molecular Probes) plus 10% fetal bovine serum (FBS), 2 mM L-glutamine and 100 Uml−1 penicillin/streptomycin in 5% CO2 at 37°C. LC3 and GABARAP knockout HeLa cells were cultured as described (Nguyen et al., 2016). Lec cells (CHO), obtained from Pamela Stanley (Albert Einstein College of Medicine, New York City, USA), were cultured in MEM alpha, nucleosides (Molecular Probes, 22571038), plus 10% FBS and 100 Uml−1 penicillin/streptomycin in 5% CO2 at 37°C.
Antibodies and reagents
Antibodies used were rat polyclonal anti-Gal-3 [Biolegend; 125408; 1/2000 in western blotting (WB)], rat polyclonal anti-galectin-3 conjugated to Alexa Fluor 647 (Biolegend; 125402; 1/100 in flow cytometry), rabbit polyclonal anti-galectin-1 (a generous gift from Walter Nickel, Heidelberg University, Germany; 1/500 in WB), mouse monoclonal anti-annexin A2 (BD Biosciences; 610071; 1/1000 in WB), rabbit polyclonal anti-actin (Sigma-Aldrich; A2066; 1/2000 in WB), rabbit polyclonal anti-BiP (Abcam; ab21685: 1/1000 in WB), mouse monoclonal anti-LAMP2 (Biolegend; 354302; 1/1000 in WB, 1/100 in immunofluorescence), rabbit polyclonal anti-SLC35A2 (Cambridge Bioscience; HPA036087; 1/500 in WB), rabbit polyclonal anti-MGAT1 (Abcam; ab180578; 1/1000 in WB), mouse monoclonal anti-human CD63 (Thermo Fisher Scientific; 10628D; 1:500 in WB), rabbit polyclonal anti-GFP (Clontech; 632592; 1/2000 in WB), rabbit polyclonal anti-LC3B (Novus Biologicals; NB100-2220; 1/2000 in WB), rabbit polyclonal anti-GABARAP (Abgent; AP1821a; 1/1000 in WB), monoclonal anti-CD29 (BD Biosciences; Clone 18/CD29; 1/2000 in WB) and rabbit polyclonal anti-GRASP55 (Proteintech; 10598-1-AP; 1/1000 in WB).
Reagents included tunicamycin (New England Biolabs; 12819), L-Leucyl-L-leucine methyl ester (Sigma-Aldrich; L7393), propidium iodide solution (Biolegend; 421301), QuickExtract DNA extraction solution (Epicenter; QE0905T), Herculase II fusion DNA polymerase (Agilent; 600675). Oligonucleotides for MGAT1 and SLC35A2 CRISPR targeting and sequencing were obtained from Sigma-Aldrich (Table S2). MISSION esiRNA against GRASP55 was also from Sigma-Aldrich (EHU056901).
Plasmids
pSpCas9(BB)-2A-GFP (PX458) [Addgene plasmid #48138, deposited by Feng Zhang (Ran et al., 2013)], pEGFP-hGal3 [Addgene plasmid #73080, deposited by Tamotsu Yoshimori (Maejima et al., 2013)], pmRFP-LC3 [Addgene plasmid #21075, deposited by Tamotsu Yoshimori (Maejima et al., 2013)] and lentiCas9-Blast [Addgene plasmid #52962, deposited by Feng Zhang (Sanjana et al., 2014)] were used. Vps4 wild type and EQ mutant were a gift from Colin Crump (Crump et al., 2007).
CRISPR screen
The Cas9 nuclease was stably expressed in suspension HeLa cells by lentiviral transduction (Sanjana et al., 2014). Then, ∼1×108 cells were transduced with the GeCKO v2 sgRNA library [Addgene cat #1000000047, deposited by Feng Zhang (Shalem et al., 2014)] at a multiplicity of infection of ∼0.2. Untransduced cells were removed from the library through puromycin selection (1 mg/ml) commencing 48 h after transduction. Rare cells that had lost cell surface Gal-3 were then enriched by sequential rounds of FACS, with the first sort taking place 7 days after transduction with the sgRNA library and the second sort 14 days later. Genomic DNA was extracted (Puregene Core Kit A, Qiagen) from both the sorted cells and an unselected pool of mutagenized cells. sgRNA sequences were amplified by two rounds of PCR, with the second round primers containing the necessary adaptors for Illumina sequencing (Table S2). Sequencing was carried out using a 50 bp single-end read on an Illumina HiSeq2500 instrument, using a custom primer binding immediately upstream of the 20 bp variable segment of the sgRNA. The 3′ ends of the resulting reads were trimmed off the constant portion of the sgRNA, and then mapped to an index of all of the sgRNA sequences in the GeCKO v2 library using Bowtie 2. The resulting sgRNA count tables were then analysed using the RSA algorithm with the default settings (Rivest et al., 1978).
Bioinformatics pathway analysis
The first 200 hits identified in the CRISPR screen were loaded to the analysis wizard of DAVID Bioinformatics Resources 6.8 to perform a pathway analysis (Huang et al., 2009a,b). According to the algorithm, only those genes with known function are included in the pathway analysis and hence not all genes will appear in the tabulated results (Table 1).
CRISPR-mediated gene disruption
For CRISPR/Cas9-mediated gene disruption, oligonucleotides (Sigma-Aldrich) (Table S2) for top and bottom strands of the sgRNA were annealed, and then cloned into the Cas9 expression vector pSpCas9(BB)-2A-GFP (PX458) as previously described (Ran et al., 2013). Transfected cells were sorted for GFP fluorescence and clones were isolated by FACS based on a loss of cell surface Gal-3. Gene disruption was verified by collecting genomic DNA from clonal lines with QuickExtract DNA extraction solution and amplifying the CRISPR/Cas9 targeted region with primers flanking ≥200 base pairs either side of the expected cut site (Table S2). PCR products were sequenced by Sanger sequencing. Insertions and deletions analysed by sequence alignment and TIDE (Brinkman et al., 2014). The TIDE R code, kindly provided by Prof. van Steensel (The Netherlands Cancer Institute, Amsterdam, The Netherlands), was used to analyse clones containing deletions >50 bp.
Flow cytometry
Cells were washed once with serum-free medium, incubated at 4°C for 30 min with an anti-Gal-3 antibody conjugated to Alexa Fluor 647, washed again and analysed on a FACSCalibur (BD Biosciences) equipped with lasers providing 488 nm and 633 nm excitation sources. Alexa Fluor 647 fluorescence was detected with an FL4 detector (661/16 BP). For sorting, cells were immunostained as above and FACS was carried on an Influx (BD Biosciences) or Aria-Fusions (BD Biosciences) cell sorter equipped with lasers providing 488 nm and 640 nm excitation sources. Alexa Fluor 647 fluorescence was detected with a 670/30 BP detector on the Influx and Aria Fusion cell sorters.
Immunofluorescence microscopy
For immunofluorescence microscopy, cells were cultured on coverslips, fixed with 4% paraformaldehyde in PBS for 5 min and permeabilized with 0.1% Triton X100 in PBS for 5 min. Coverslips were incubated with primary antibodies for 2 h, washed three times with PBS, and incubated with secondary antibodies for 30 min. Samples were mounted using ProLong Gold antifade reagent with DAPI (4,6-diamidino-2-phenylindole; Invitrogen) and observed using a Leica SP8 laser confocal microscope.
Immunoblotting
All samples were resolved by 12% SDS-PAGE and transferred to polyvinylidene difluoride membranes for blotting. Membranes were blocked with 5% (w/v) skim milk powder in PBS containing 0.1% Tween 20 (PBST) for 30 min at room temperature. Membranes were then probed with an appropriate dilution of primary antibody overnight at 4°C. Membranes were washed three times in PBST before incubation in diluted secondary antibody for 1 h at room temperature. Membranes were washed as before and developed with Amersham ECL Western Blotting Detection Reagent RPN2106 for the detection of proteins in the cell lysates, or Cyanagen Westar XLS100 for the detection of proteins in the secreted fractions, using a Bio-Rad Chemi Doc XRS system. Membranes were stripped with Restore Plus (Thermo Fisher Scientific, 46430) according to the manufacturer's instructions.
Secretion assay
To measure the secretion of galectins, cells were washed with serum-free medium and incubated for 24 h for sHeLa or 48 h for CHO (Lec). For sHeLa, the serum-free medium was DMEM plus 2 mM L-Glutamine. For CHO (Lec), the serum-free medium was EX-CELL 325 PF CHO (Sigma-Aldrich, C985Z18). Cell supernatants were then collected, centrifuged at 300 g to remove potential remaining cells, either filtered at 0.22 µm or centrifuged at 3000 g to remove cell debris, mixed with sample buffer [50 mM Tris-HCl pH 6.8, 2% SDS (w/v), 0.1% Bromophenol Blue, 10% glycerol and 100 mM DTT] and boiled at 100°C for 5 min. Cell pellets were lysed in lysis buffer (20 mM Tris-HCl pH 6.8, 137 mM NaCl, 1 mM EDTA, 1% Triton X-100 and 10% glycerol) at 4°C for 10 min, and insoluble material was removed by centrifugation at 10,000 g for 10 min at 4°C. Sample buffer was added and the samples boiled (as above). Cell lysates and cell supernatants were then subjected to SDS-PAGE. Densitometry was performed in ImageJ and the differences in the levels of secreted Gal-3 were calculated in each cell line by dividing the amount of Gal-3 in the supernatant by the amount of Gal-3 in the lysate. These values were then used to calculate the fold change relative to the control cells.
Removal of extracellular vesicles
Cells were processed as described for the secretion assay, except after the 3000 g centrifugation step the medium was collected and centrifuged at 100,000 g for 60 min at 4°C. After each centrifugation step a sample of the medium was collected for western blotting. The extracellular vesicle pellet was resuspended in a small volume of nonreducing sample buffer [50 mM Tris-HCl pH 6.8, 2% SDS (w/v), 0.1% Bromophenol Blue, and 10% glycerol]. Half of the EV pellet sample was taken and DTT added to a final concentration of 100 mM. All samples were boiled and resolved by SDS-PAGE. The entire concentrated EV pellet sample was loaded on two gels (reduced and nonreduced) for each sample due to the small scale of the assay. Therefore, the EV pellet was 50× more concentrated than the equivalent supernatant.
Statistical analysis
Significance levels for comparisons between groups were determined with two-sample Student’s t-test.
Supplementary Material
Acknowledgements
We thank Walter Nickel for Gal-1 antibody, Michael D'Angelo for help and expertise with CRISPR/Cas9 INDEL analysis, Bas van Steensel for providing the TIDE R code, Matt Castle for R code expertise, Nuno Rocha for help with the CRISPR cloning method, Pamela Stanley for Lec CHO cell lines, Michael Lazarou for the LC3 and GABARAP knockout cells, and Colin Crump for the Vps4 vectors.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.E.S., S.A.M., P.J.L., K.M.; Methodology: S.E.S., S.A.M., S.J.P., N.S., A.P.H., P.J.L., K.M.; Validation: S.E.S., S.J.P., P.J.L., K.M.; Formal analysis: S.E.S., S.A.M., S.J.P.; Investigation: S.E.S., S.A.M., K.M.; Data curation: K.M.; Writing - original draft: S.E.S., K.M.; Writing - review & editing: S.E.S., S.A.M., S.J.P., N.S., A.P.H., P.J.L., K.M.; Supervision: K.M.; Project administration: K.M.; Funding acquisition: A.P.H., P.J.L., K.M.
Funding
This work was supported by the Wellcome Trust [100574/Z/12/Z; 101835/Z/13/Z to P.J.L.; Wellcome Trust PhD studentship to S.A.M. and S.J.P.], Medical Research Council [MRC_MC_UU_12012/5 to S.E.S. and K.M.] and National Institute for Health Research [to N.S. and A.P.H.]. Deposited in PMC for release after 6 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.206425.supplemental
References
- Aits S., Kricker J., Liu B., Ellegaard A.-M., Hämälistö S., Tvingsholm S., Corcelle-Termeau E., Høgh S., Farkas T., Holm Jonassen A. et al. (2015). Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy 11, 1408-1424. 10.1080/15548627.2015.1063871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albahri Z. (2015). Congenital disorders of glycosylation: a review. Am. J. Pediatr. 1, 6-28. 10.1203/00006450-200211000-00003 [DOI] [Google Scholar]
- Boyle K. B. and Randow F. (2013). The role of ‘eat-me’ signals and autophagy cargo receptors in innate immunity. Curr. Opin. Microbiol. 16, 339-348. 10.1016/j.mib.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Brinkman E. K., Chen T., Amendola M. and van Steensel B. (2014). Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42, e168 10.1093/nar/gku936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan S., Kumar S., Jain A., Ponpuak M., Mudd M. H., Kimura T., Choi S. W., Peters R., Mandell M., Bruun J.-A. et al. (2016). TRIMs and galectins globally cooperate and TRIM16 and galectin-3 co-direct autophagy in endomembrane damage homeostasis. Dev. Cell 39, 13-27. 10.1016/j.devcel.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. and Stanley P. (2003). Five Lec1 CHO cell mutants have distinct Mgat1 gene mutations that encode truncated N-acetylglucosaminyltransferase I. Glycobiology 13, 43-50. 10.1093/glycob/cwg003 [DOI] [PubMed] [Google Scholar]
- Cho M. and Cummings R. D. (1995). Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J. Biol. Chem. 270, 5207-5212. 10.1074/jbc.270.10.5207 [DOI] [PubMed] [Google Scholar]
- Cooper D. N. and Barondes S. H. (1990). Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J. Cell Biol. 110, 1681-1691. 10.1083/jcb.110.5.1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump C. M., Yates C. and Minson T. (2007). Herpes simplex virus type 1 cytoplasmic envelopment requires functional Vps4. J. Virol. 81, 7380-7387. 10.1128/JVI.00222-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elola M. T., Blidner A. G., Ferragut F., Bracalente C. and Rabinovich G. A. (2015). Assembly, organization and regulation of cell-surface receptors by lectin-glycan complexes. Biochem. J. 469, 1-16. 10.1042/BJ20150461 [DOI] [PubMed] [Google Scholar]
- Escola J.-M., Kleijmeer M. J., Stoorvogel W., Griffith J. M., Yoshie O. and Geuze H. J. (1998). Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 273, 20121-20127. 10.1074/jbc.273.32.20121 [DOI] [PubMed] [Google Scholar]
- Feeley E. M., Pilla-Moffett D. M., Zwack E. E., Piro A. S., Finethy R., Kolb J. P., Martinez J., Brodsky I. E. and Coers J. (2017). Galectin-3 directs antimicrobial guanylate binding proteins to vacuoles furnished with bacterial secretion systems. Proc. Natl. Acad. Sci. USA 114, E1698-E1706. 10.1073/pnas.1615771114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze H. H., Eklund E. A., Ng B. G. and Patterson M. C. (2015). Neurological aspects of human glycosylation disorders. Annu. Rev. Neurosci. 38, 105-125. 10.1146/annurev-neuro-071714-034019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünewald S., Matthijs G. and Jaeken J. (2002). Congenital disorders of glycosylation: a review. Pediatr. Res. 52, 618-624. 10.1203/00006450-200211000-00003 [DOI] [PubMed] [Google Scholar]
- Huang W., Sherman B. T. and Lempicki R. A. (2009a). Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1-13. 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Sherman B. T. and Lempicki R. A. (2009b). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44-57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- Hughes R. C. (1999). Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim. Biophys. Acta 1473, 172-185. 10.1016/S0304-4165(99)00177-4 [DOI] [PubMed] [Google Scholar]
- Jagodzinski A., Havulinna A. S., Appelbaum S., Zeller T., Jousilahti P., Skytte-Johanssen S., Hughes M. F., Blankenberg S. and Salomaa V. (2015). Predictive value of galectin-3 for incident cardiovascular disease and heart failure in the population-based FINRISK 1997 cohort. Int. J. Cardiol. 192, 33-39. 10.1016/j.ijcard.2015.05.040 [DOI] [PubMed] [Google Scholar]
- König R., Chiang C.-Y., Tu B. P., Yan S. F., DeJesus P. D., Romero A., Bergauer T., Orth A., Krueger U., Zhou Y. et al. (2007). A probability-based approach for the analysis of large-scale RNAi screens. Nat. Methods 4, 847-849. 10.1038/nmeth1089 [DOI] [PubMed] [Google Scholar]
- Kumar R. and Stanley P. (1989). Transfection of a human gene that corrects the Lec1 glycosylation defect: evidence for transfer of the structural gene for N-acetylglucosaminyltransferase I. Mol. Cell. Biol. 9, 5713-5717. 10.1128/MCB.9.12.5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarayan R., Wunder C., Becken U., Howes M. T., Benzing C., Arumugam S., Sales S., Ariotti N., Chambon V., Lamaze C. et al. (2014). Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrin-independent carriers. Nat. Cell Biol. 16, 595-606. 10.1038/ncb2970 [DOI] [PubMed] [Google Scholar]
- Lindstedt R., Apodaca G., Barondes S. H., Mostov K. E. and Leffler H. (1993). Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J. Biol. Chem. 268, 11750-11757. [PubMed] [Google Scholar]
- Liu F.-T. and Rabinovich G. A. (2005). Galectins as modulators of tumour progression. Nat. Rev. Cancer 5, 29-41. 10.1038/nrc1527 [DOI] [PubMed] [Google Scholar]
- Maejima I., Takahashi A., Omori H., Kimura T., Takabatake Y., Saitoh T., Yamamoto A., Hamasaki M., Noda T., Isaka Y. et al. (2013). Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 32, 2336-2347. 10.1038/emboj.2013.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek N., Byrd J. C., Sun Y., Hafley M., Ramirez K., Burks J. and Bresalier R. S. (2012). Cell-surface galectin-3 confers resistance to TRAIL by impeding trafficking of death receptors in metastatic colon adenocarcinoma cells. Cell Death Differ. 19, 523-533. 10.1038/cdd.2011.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedeva E. A., Berezin I. I., Surkova E. A., Yaranov D. M. and Shchukin Y. V. (2016). Galectin-3 in patients with chronic heart failure: association with oxidative stress, inflammation, renal dysfunction and prognosis. Minerva Cardioangiol. 64, 595-602. [PubMed] [Google Scholar]
- Mehul B. and Hughes R. C. (1997). Plasma membrane targetting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion. J. Cell Sci. 110, 1169-1178. [DOI] [PubMed] [Google Scholar]
- Menon R. P. and Hughes R. C. (1999). Determinants in the N-terminal domains of galectin-3 for secretion by a novel pathway circumventing the endoplasmic reticulum-Golgi complex. Eur. J. Biochem. 264, 569-576. 10.1046/j.1432-1327.1999.00671.x [DOI] [PubMed] [Google Scholar]
- Monticelli M., Ferro T., Jaeken J., Dos Reis Ferreira V. and Videira P. A. (2016). Immunological aspects of congenital disorders of glycosylation (CDG): a review. J. Inherit. Metab. Dis. 39, 765-780. 10.1007/s10545-016-9954-9 [DOI] [PubMed] [Google Scholar]
- Nabi I. R., Shankar J. and Dennis J. W. (2015). The galectin lattice at a glance. J. Cell Sci. 128, 2213-2219. 10.1242/jcs.151159 [DOI] [PubMed] [Google Scholar]
- Nguyen T. N., Padman B. S., Usher J., Oorschot V., Ramm G. and Lazarou M. (2016). Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J. Cell Biol. 215, 857-874. 10.1083/jcb.201607039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W. (2003). The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur. J. Biochem. 270, 2109-2119. 10.1046/j.1432-1033.2003.03577.x [DOI] [PubMed] [Google Scholar]
- Nickel W. and Rabouille C. (2009). Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 10, 148-155. 10.1038/nrm2617 [DOI] [PubMed] [Google Scholar]
- Nickel W. and Seedorf M. (2008). Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu. Rev. Cell Dev. Biol. 24, 287-308. 10.1146/annurev.cellbio.24.110707.175320 [DOI] [PubMed] [Google Scholar]
- Patnaik S. K. and Stanley P. (2006). Lectin-resistant CHO glycosylation mutants. Methods Enzymol. 416, 159-182. 10.1016/S0076-6879(06)16011-5 [DOI] [PubMed] [Google Scholar]
- Patnaik S. K., Potvin B., Carlsson S., Sturm D., Leffler H. and Stanley P. (2006). Complex N-glycans are the major ligands for galectin-1, -3, and -8 on Chinese hamster ovary cells. Glycobiology 16, 305-317. 10.1093/glycob/cwj063 [DOI] [PubMed] [Google Scholar]
- Paz I., Sachse M., Dupont N., Mounier J., Cederfur C., Enninga J., Leffler H., Poirier F., Prevost M.-C., Lafont F. et al. (2010). Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell. Microbiol. 12, 530-544. 10.1111/j.1462-5822.2009.01415.x [DOI] [PubMed] [Google Scholar]
- Pinho S. S. and Reis C. A. (2015). Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540-555. 10.1038/nrc3982 [DOI] [PubMed] [Google Scholar]
- Priglinger C. S., Szober C. M., Priglinger S. G., Merl J., Euler K. N., Kernt M., Gondi G., Behler J., Geerlof A., Kampik A. et al. (2013). Galectin-3 induces clustering of CD147 and integrin-beta1 transmembrane glycoprotein receptors on the RPE cell surface. PLoS ONE 8, e70011 10.1371/journal.pone.0070011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich G. A., Rubinstein N. and Fainboim L. (2002). Unlocking the secrets of galectins: a challenge at the frontier of glyco-immunology. J. Leukoc. Biol. 71, 741-752. [PubMed] [Google Scholar]
- Rabouille C. (2017). Pathways of unconventional protein secretion. Trends Cell Biol. 27, 230-240. 10.1016/j.tcb.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A. and Zhang F. (2013). Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281-2308. 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest R. L., Shamir A. and Adleman L. (1978). A method for obtaining digital signatures and public-key cryptosystems. Commun. ACM 21, 120-126. 10.1145/359340.359342 [DOI] [Google Scholar]
- Salomonsson E., Carlsson M. C., Osla V., Hendus-Altenburger R., Kahl-Knutson B., Öberg C. T., Sundin A., Nilsson R., Nordberg-Karlsson E., Nilsson U. J. et al. (2010). Mutational tuning of galectin-3 specificity and biological function. J. Biol. Chem. 285, 35079-35091. 10.1074/jbc.M109.098160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana N. E., Shalem O. and Zhang F. (2014). Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783-784. 10.1038/nmeth.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Burdett I. and Hughes R. C. (1993). Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex. Exp. Cell Res. 207, 8-18. 10.1006/excr.1993.1157 [DOI] [PubMed] [Google Scholar]
- Seelenmeyer C., Wegehingel S., Tews I., Künzler M., Aebi M. and Nickel W. (2005). Cell surface counter receptors are essential components of the unconventional export machinery of galectin-1. J. Cell Biol. 171, 373-381. 10.1083/jcb.200506026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelenmeyer C., Stegmayer C. and Nickel W. (2008). Unconventional secretion of fibroblast growth factor 2 and galectin-1 does not require shedding of plasma membrane-derived vesicles. FEBS Lett. 582, 1362-1368. 10.1016/j.febslet.2008.03.024 [DOI] [PubMed] [Google Scholar]
- Shalem O., Sanjana N. E., Hartenian E., Shi X., Scott D. A., Mikkelsen T. S., Heckl D., Ebert B. L., Root D. E., Doench J. G. et al. (2014). Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84-87. 10.1126/science.1247005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. (1989). Chinese hamster ovary cell mutants with multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol. Cell. Biol. 9, 377-383. 10.1128/MCB.9.2.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen V. L., Heusschen R., Caers J. and Griffioen A. W. (2015). Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta 1855, 235-247. 10.1016/j.bbcan.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Timms R. T., Menzies S. A., Tchasovnikarova I. A., Christensen L. C., Williamson J. C., Antrobus R., Dougan G., Ellgaard L. and Lehner P. J. (2016). Genetic dissection of mammalian ERAD through comparative haploid and CRISPR forward genetic screens. Nat. Commun. 7, 11786 10.1038/ncomms11786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Birsoy K., Hughes N. W., Krupczak K. M., Post Y., Wei J. J., Lander E. S. and Sabatini D. M. (2015). Identification and characterization of essential genes in the human genome. Science 350, 1096-1101. 10.1126/science.aac7041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M., Dong X.-W. and Guo X.-L. (2015). Role of the interaction between galectin-3 and cell adhesion molecules in cancer metastasis. Biomed. Pharmacother. 69, 179-185. 10.1016/j.biopha.2014.11.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.