ABSTRACT
The integrin αVβ3 is reported to promote angiogenesis in some model systems but not in others. Here, we used optogenetics to study the effects of αVβ3 interaction with the intracellular adapter kindlin-2 (Fermt2) on endothelial cell functions potentially relevant to angiogenesis. Because interaction of kindlin-2 with αVβ3 requires the C-terminal three residues of the β3 cytoplasmic tail (Arg-Gly-Thr; RGT), optogenetic probes LOVpep and ePDZ1 were fused to β3ΔRGT–GFP and mCherry–kindlin-2, respectively, and expressed in β3 integrin-null microvascular endothelial cells. Exposure of the cells to 450 nm (blue) light caused rapid and specific interaction of kindlin-2 with αVβ3 as assessed by immunofluorescence and total internal reflection fluorescence (TIRF) microscopy, and it led to increased endothelial cell migration, podosome formation and angiogenic sprouting. Analyses of kindlin-2 mutants indicated that interaction of kindlin-2 with other kindlin-2 binding partners, including c-Src, actin, integrin-linked kinase and phosphoinositides, were also likely necessary for these endothelial cell responses. Thus, kindlin-2 promotes αVβ3-dependent angiogenic functions of endothelial cells through its simultaneous interactions with β3 integrin and several other binding partners. Optogenetic approaches should find further use in clarifying spatiotemporal aspects of vascular cell biology.
KEY WORDS: Optogenetics, Integrin function, Vascular biology, Kindlin-2
Highlighted Article: Optogenetics show that the interaction of kindlin-2 with β3 integrin and other binding partners supports endothelial cell functions relevant to angiogenesis, including sprouting and podosome formation.
INTRODUCTION
Integrins are heterodimeric, transmembrane adhesion receptors, several classes of which are expressed in vascular endothelial cells, where they influence cellular functions through interactions with components of the extracellular and intracellular milieus. One example is αVβ3, which has been implicated in regulation of endothelial cell adhesion, migration and survival, all key functions during angiogenesis (Avraamides et al., 2008). αVβ3 is highly expressed in proliferative endothelial cells, where it interacts through its extracellular domains with Arg-Gly-Asp-containing matrix proteins, including vitronectin, fibrinogen/fibrin and fibronectin, and through its cytoplasmic tails with intracellular enzymes, adapters and cytoskeletal proteins (Felding-Habermann and Cheresh, 1993; Plow et al., 2014). αVβ3 has been a focus of study for over two decades, but questions remain concerning its precise role in angiogenesis (Demircioglu and Hodivala-Dilke, 2016; Robinson and Hodivala-Dilke, 2011). For example, function-blocking antibodies to αVβ3 inhibit angiogenesis in model systems (Friedlander et al., 1995); however, a small molecule αVβ3 antagonist exhibits variable and dose-dependent effects on angiogenesis (Reynolds et al., 2009). Moreover, global or endothelial cell-specific knockout of αV or β3 integrins fails to blunt tumor angiogenesis in the mouse, and can even enhance it (Murphy et al., 2015; Reynolds et al., 2002).
Recent work has begun to focus on the potential role of kindlin-2 (Fermt2), a ∼78 kDa intracellular adapter protein, in endothelial cell and integrin function (Plow et al., 2014). Kindlin-2 is composed of an N-terminal F0 subdomain and a C-terminal FERM domain made up of F1, F2 and F3 subdomains. The F2 subdomain of kindlin-2, like that of the related kindlin-1 and kindlin-3 proteins, is split by a PH domain (Larjava et al., 2008; Plow et al., 2016; Rognoni et al., 2016; Ussar et al., 2006). Kindlins regulate various aspects of bidirectional signaling mediated by β1, β2 and β3 integrins through direct interactions with integrin β cytoplasmic tails and with intracellular binding partners such as actin, integrin-linked kinase, migfilin, c-Src and phosphoinositides (Malinin et al., 2010; Qu et al., 2014; Rognoni et al., 2016). In the context of endothelial cells, kindlin-2 has been implicated in developmental angiogenesis in zebrafish, and in tumor angiogenesis and αVβ3-dependent intracellular signaling in mice heterozygous for kindlin-2 (Pluskota et al., 2011). However, the mechanisms by which kindlin-2 regulates endothelial cell αVβ3 function remain to be fully explored.
Recently, β3 integrin mutant mice and lung endothelial cells derived from these mice were employed to investigate mechanisms of kindlin-2 interaction with αVβ3 (Liao et al., 2015). Kindlin-2 was found to interact directly with β3 in a manner that requires the C-terminal three residues of the β3 cytoplasmic tail (Arg-Gly-Thr; RGT), potentially similar to the requirement for the C-terminal three residues of β1 for its interaction with kindlin-2 (Li et al., 2017). β3ΔRGT knock-in mice expressing β3 that lacks these three C-terminal residues exhibited reduced physiological (retinal) and pathological (tumor) angiogenesis when compared to wild-type littermates, suggesting a positive role for the αVβ3–kindlin-2 interaction in angiogenesis. Moreover, enforced interaction of kindlin-2 with β3ΔRGT using a chemical dimerizer strategy rescued endothelial cell sprout formation in an in vitro fibrin gel assay (Liao et al., 2015). However, these studies did not examine temporal or spatial details of the αVβ3–kindlin-2 interaction in endothelial cells, nor did they focus on the role of kindlin-2 interactions with other intracellular binding partners.
A potential way to address these remaining issues is to use optogenetic tools. Genetically encoded, light-responsive optogenetic probes are now available for a variety of cell biology applications, enabling rapid (∼s) and potentially reversible manipulation of protein-protein interactions in real time within living cells (Deisseroth, 2015; Karunarathne et al., 2015; Tischer and Weiner, 2014; Weitzman and Hahn, 2014; Zhang et al., 2015). One such optogenetic pair is LOVpep and ePDZb1 (153 and 194 amino acids, respectively). When exposed to 450 nm ‘blue’ light, the Jα helix of LOVpep rapidly undocks from the LOV core and unfolds, enabling heterodimeric interaction with ePDZb1 (Strickland et al., 2012, 2010). Therefore, in the present study, we fused LOVpep to the C-terminus of β3ΔRGT–GFP and ePDZb1 to the N-terminus of mCherry–kindlin-2 and expressed these recombinant proteins in β3-null endothelial cells. This enabled us to study details of the β3ΔRGT/kindlin-2 interaction in response to blue light (Fig. 1). The results demonstrate that kindlin-2 interactions with αVβ3 and its other binding partners promote endothelial cell functions potentially relevant to angiogenesis, including migration and the formation of podosomes and angiogenic sprouts.
Fig. 1.
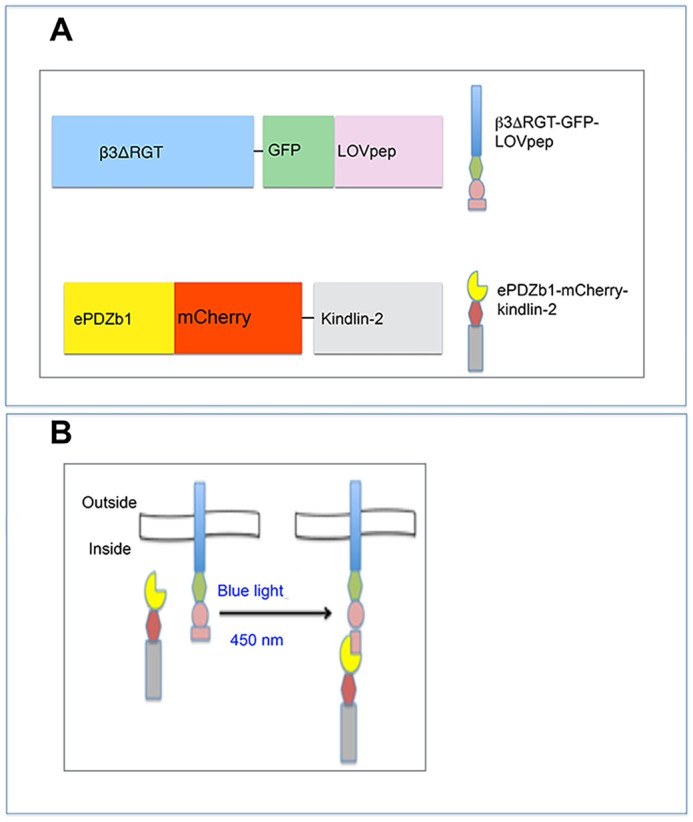
Optogenetic tools to control integrin β3–kindlin-2 interaction. (A) Depictions of the β3ΔRGT–GFP–LOVpep and ePDZb1–mCherry–kindlin-2 fusion proteins. (B) Schematic display of blue light-induced intracellular interaction between β3ΔRGT–GFP–LOVpep and ePDZb1–mCherry–kindlin-2.
RESULTS
Optogenetic control of kindlin-2 interaction with αVβ3 in endothelial cells
The inability of the integrin β3 C-terminal deletion mutant, β3ΔRGT, to interact with kindlin-2 in vitro and in endothelial cells (Liao et al., 2015) provided us an unparalleled opportunity to conditionally induce and study the functional outcome of this interaction using optogenetics. β3ΔRGT–GFP was fused at its C-terminus to LOVpep (β3ΔRGT–GFP–LOVpep), kindlin-2–mCherry was fused at its N-terminus to ePDZb1 (ePDZb1–mCherry–kindlin-2) and these recombinant proteins were stably co-expressed in β3-null, immortalized murine lung endothelial cells (Liao et al., 2015) (Fig. 1A; Fig. S1). Human β3 can pair with murine αV, leading in this case to cell surface expression of αVβ3ΔRGT–GFP–LOVpep and intracellular expression of ePDZb1–mCherry–kindlin-2 (Fig. 1B). Thus, like the fusion of GFP to β3 in the context of the platelet integrin αIIbβ3 (Plançon et al., 2001), we found no deleterious effect of the GFP–LOVpep fusion on surface expression of αVβ3ΔRGT–GFP–LOVpep, nor was there any effect of this fusion on the basal affinity state of αVβ3 as assessed by the ligand-mimetic antibody, WOW-1 Fab (not shown).
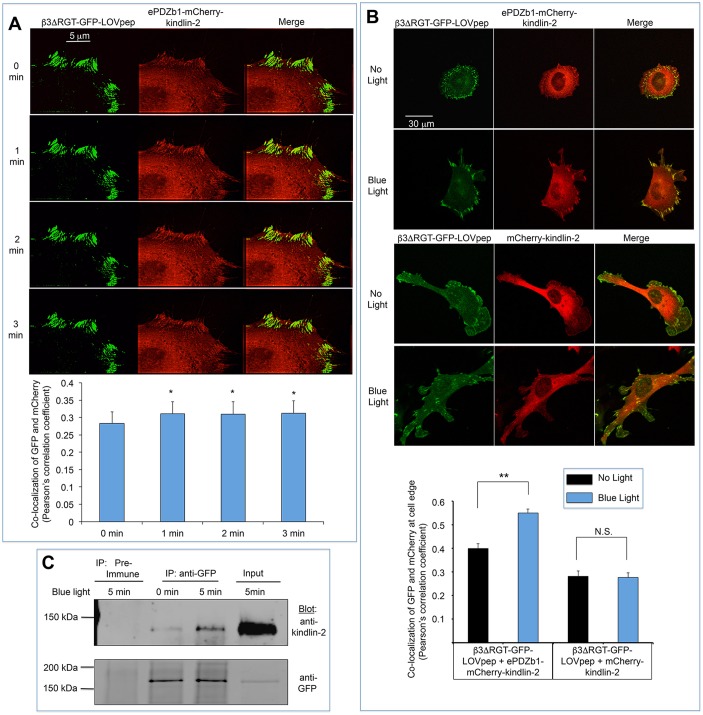
When endothelial cells were plated and allowed to spread on the αVβ3 ligand, fibrinogen, and then exposed to 450 nm blue light, increased colocalization of β3ΔRGT–GFP–LOVpep and ePDZb1–mCherry-kindlin-2 was observed at the cell peripheries and in focal adhesions (Fig. 2A,B). By contrast, no such increased colocalization was observed if mCherry–kindlin-2 lacking ePDZb1 was employed (Fig. 2B), illustrating the specificity of this optogenetic approach. Increased colocalization of β3ΔRGT–GFP–LOVpep and ePDZb1–mCherry–kindlin-2 could be observed as early as 1 min after the introduction of blue light, and could even be observed by co-immunoprecipitation (Fig. 2C). Because β1 integrin in endothelial cells can interact with fibrinogen (Suehiro et al., 1997), we used a function-blocking anti-β1 antibody (HMβ1-1) (Wang et al., 2010) to assess the potential involvement of β1 in this experiment. β1 blockade had no effect on the increase in colocalization of β3ΔRGT–GFP–LOVpep and ePDZb1–mCherry–kindlin-2 induced by blue light (Fig. S2A). Thus, optogenetics can be used to induce a rapid and specific interaction of αVβ3 with kindlin-2, enabling further investigation of the functional consequences of this interaction.
Fig. 2.
Increased association between ePDZb1–mCherry–kindlin-2 and β3ΔRGT–GFP–LOVpep in response to 450 nm blue light. (A) β3−/−-immortalized lung endothelial cells expressing β3ΔRGT–GFP–LOVpep and ePDZb1–mCherry–kindlin-2 were plated on fibrinogen before imaging with time-lapse TIRF microscopy. Blue laser light was used to stimulate the interaction at 100–150 ms illumination every 1 min. A segment of the cell edge is cropped and displayed as a movie montage at 1 min intervals. Scale bar: 5 μm. Colocalization between GFP and mCherry channels was quantified and shown as Pearson's correlation coefficient. Data represent mean±s.e.m. of nine cells from two experiments. *P<0.05 (paired Student’s t-test). (B) Colocalization of β3ΔRGT–GFP–LOVpep and ePDZb1–mCherry–kindlin-2 by confocal imaging. Cells were plated onto coverslips coated with fibrinogen and allowed to spread for 30 min with or without exposure to pulsed blue light for 0.5 s each minute. Cells expressing β3ΔRGT–GFP–LOVpep and mCherry–kindlin-2 (no ePDZb1) were used as a negative control. Scale bar: 30 μm. Colocalization between GFP and mCherry channels was assessed at the edges of the cells and shown as Pearson's correlation coefficient. Data represent mean±s.e.m. of 20–40 cells from two experiments. **P<0.01; N.S., not significant (unpaired Student's t-test). (C) Co-immunoprecipitation of ePDZb1–mCherry–kindlin-2 and β3ΔRGT–GFP–LOVpep. Endothelial cells expressing β3ΔRGT–GFP–LOVpep and ePDZb1–mCherry–kindlin-2 were maintained in the dark or illuminated with blue light for 5 min. Lysates were subjected to immunoprecipitation with pre-immune serum or anti-GFP serum followed by immunoblotting with anti-kindlin-2 or anti-GFP antibody. Blots are representative of three independent experiments.
Optogenetic control of the adhesive and migratory functions of endothelial cell αVβ3 mediated by kindlin-2
Kindlin-2 is known to regulate adhesion and migration of several cell types, including podocytes and tumor cell lines (Guo et al., 2015; Qu et al., 2011; Shi et al., 2007). Because αVβ3 may mediate these same responses in endothelial cells (Mahabeleshwar et al., 2008), the influence of conditional interaction of kindlin-2 with αVβ3 was examined in these cells. When interaction of αVβ3ΔRGT-GFP-LOVpep with ePDZb1–mCherry–kindlin-2 was triggered by blue light, endothelial cell adhesion to fibrinogen was hardly increased, and only at the higher coating concentrations of the ligand (Fig. 3A,B). However, blue light illumination clearly increased cell migration across fibrinogen-coated Transwells (Fig. 3C), and similar results were obtained using an independent endothelial cell line isolated from β3ΔRGT mice (Fig. S3). Because optogenetic interaction of kindlin-2 with β3 did not promote affinity modulation of αVβ3 in endothelial cells as assessed by binding of the WOW-1 Fab antibody (Fig. S4) (Liao et al., 2015), these results suggest that kindlin-2 influences endothelial migration primarily through effects on αVβ3 avidity or on outside-in αVβ3 signaling, similar to the effects of kindlin-3 on platelet αIIbβ3 (Kahner et al., 2012; Mahabeleshwar et al., 2008; Ye et al., 2013).
Fig. 3.
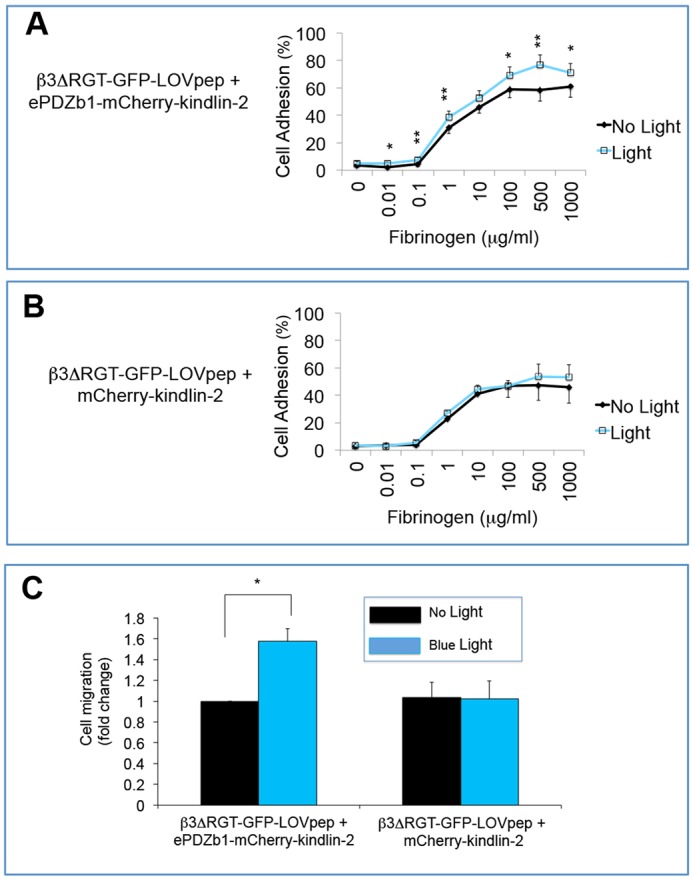
Optogenetic kindlin-2 interaction with integrin β3 promotes endothelial cell adhesion and migration. (A,B) Cells expressing the indicated β3 and kindlin-2 constructs were seeded onto fibrinogen-coated wells and incubated with or without pulsed blue light illumination for 1 h. After washing and fixation, attached cells were stained with Crystal Violet and cell adhesion was quantified as described in the Materials and Methods. Total cell input was assigned a value of 100%. (C) Cell migration across fibrinogen-coated Transwells. Migration of cells in the dark was arbitrarily assigned a fold change as 1. Data represent mean±s.e.m. from at least three experiments. *P<0.05; **P<0.01 (paired Student's t-test).
Kindlin-2 interaction with αVβ3 promotes angiogenic sprouting and podosome formation in endothelial cells
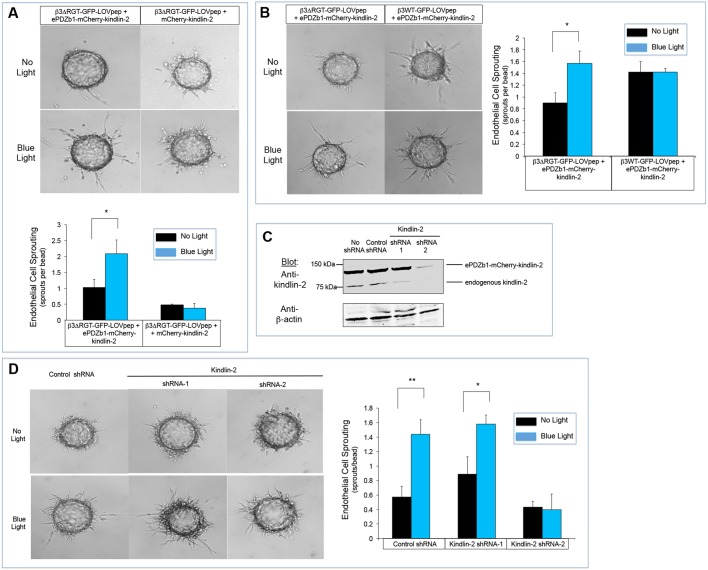
Angiogenesis involves the coordinated actions of many biological processes in response to stimuli such as VEGF, including the sprouting of endothelial tip cells and the formation in these cells of podosomes, structures containing an F-actin core and surrounding proteins, including integrins (Potente et al., 2011; Seano et al., 2014). In a three-dimensional fibrin gel culture, endothelial cells form sprouts, similar to angiogenic tip cells in vivo (Nakatsu et al., 2007). However, consistent with previous observations with αVβ3ΔRGT (Liao et al., 2015), endothelial cells expressing β3ΔRGT–GFP–LOVpep failed to form sprouts in fibrin gels after 24 h of culture, even when kindlin-2 was overexpressed (Fig. 4A,B). In sharp contrast, when the cells co-expressed β3ΔRGT–GFP–LOVpep and ePDZb1–mCherry–kindlin-2, increased sprout formation was observed in response to blue light to a level observed with cells expressing kindlin-2 and wild-type β3, the latter even in the absence of blue light (Fig. 4A,B). As a control, mCherry–kindlin-2 lacking ePDZb1 was unable to induce sprouting in response to blue light (Fig. 4A). Light-induced angiogenic sprouting did not require endogenous kindlin-2 since selective shRNA-mediated knockdown of the endogenous protein failed to prevent sprout formation (Fig. 4C,D). Moreover, the anti-β1 antibody HMβ1-1, failed to prevent blue light-induced sprout formation in endothelial cells expressing β3ΔRGT–GFP–LOVpep and ePDZb1–mCherry–kindlin-2 (Fig. S2C), despite the fact that somewhat fewer angiogenic sprouts were observed in cells treated with the antibody compared to cells treated with isotype control IgG. Thus, angiogenic sprout formation by endothelial cells is promoted by the specific interaction of kindlin-2 with αVβ3, at least in vitro.
Fig. 4.
Optogenetic kindlin-2 interaction with integrin β3 induces angiogenic sprout formation by endothelial cells. (A) Representative images and quantification of sprout formation in endothelial cells expressing the indicated optogenetic integrin β3 and kindlin-2 constructs. After overnight incubation of endothelial cells in the dark or in the presence of blue light (0.5 s illumination every 60 s), angiogenic sprouts were quantified. (B) Comparison of sprout formation by endothelial cells expressing either β3ΔRGT–GFP–LOVpep and/or wild-type β3–GFP–LOVpep in fibrin gel bead assay. Note that blue light-induced interaction with ePDZb1–mCherry–kindlin-2 with β3ΔRGT restores endothelial cell sprout formation to a level seen with wild-type type β3, the latter independent of the presence of blue light. Data represent mean±s.e.m. from four experiments. (C) Lentivirus encoding kindlin-2 shRNA (shRNA-1 and shRNA-2) or a control shRNA was used to transduce endothelial cells expressing β3ΔRGT–GFP–LOVpep and ePDZb1–mCherry–kindlin-2. Note on these western blots that shRNA-1 knocked down the expression of endogenous kindlin-2, but not ePDZb1–mCherry–kindlin-2. By contrast, shRNA-2 knocked down both the endogenous and the recombinant kindlin-2. (D) Representative images and quantification of the effects of kindlin-2 shRNA on endothelial cell sprouting. Data represent mean±s.e.m. from three experiments. **P<0.01; *P<0.05 (paired Student's t-test).
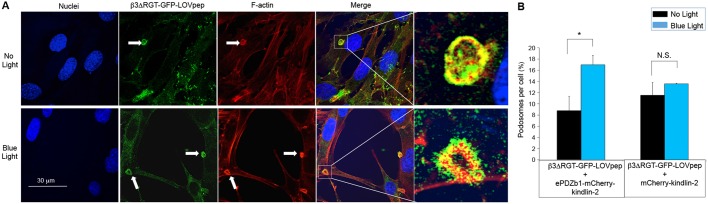
Podosomes can form in many cell types, particularly macrophages and cancer cells, where they may invade into and break down the matrix substratum to affect cell motility (Linder and Kopp, 2005). Podosomes and podosome clusters forming rosettes have been found in endothelial cells (Moreau et al., 2003), where they have been implicated in angiogenesis (Seano et al., 2014; Spuul et al., 2016). We observed the formation of individual podosomes in endothelial cells expressing αVβ3ΔRGT–GFP–LOVpep and ePDZb1–mCherry–kindlin-2 when they were plated on vitronectin, and these structures displayed a distinctive F-actin core surrounded by αVβ3ΔRGT–GFP–LOVpep (Fig. 5A), cortactin (Fig. S5) and vinculin (data not shown). The percentage of cells displaying podosomes increased from 8.8% in the absence of blue light to 17.0% in the presence of such light (P<0.05) (Fig. 5B). This light-induced increase in podosome numbers was not observed in endothelial cells expressing β3ΔRGT–GFP–LOVpep and mCherry–kindlin-2 lacking ePDZb1 (Fig. 5B). Thus, specific optogenetic interaction of kindlin-2 with αVβ3ΔRGT promotes endothelial cell podosome formation. We could not detect proteolytic activities of endothelial podosomes on fibronectin in matrix degradation assays.
Fig. 5.
Interaction between kindlin-2 and β3 increases podosome formation. Endothelial cells expressing the indicated β3ΔRGT and kindlin-2 constructs were seeded onto 10 ng/ml vitronectin-coated coverslips and incubated overnight with or without blue light illumination. (A) Representative image showing β3-GFP (green), F-actin (red) and nuclei (blue). Representative podosomes from the boxed areas in ‘Merge’ are shown in higher magnification on the far right. Scale bar: 30 μm. Arrows indicate the localization of the podosomes. (B) Note that blue light-induced interaction between β3ΔRGT–GFP–LOVpep and ePDZb1–mCherry–kindlin-2 stimulated podosome formation, whereas this did not occur when ePDZb1 was omitted from mCherry–kindlin-2. Data represent mean±s.e.m. from three independent experiments, each analyzing all cells in 10 microscopic fields. *P<0.05; N.S., not significant. (paired Student's t-test).
Kindlin-2 must interact with binding partners in addition to αVβ3 to promote endothelial cell angiogenic functions
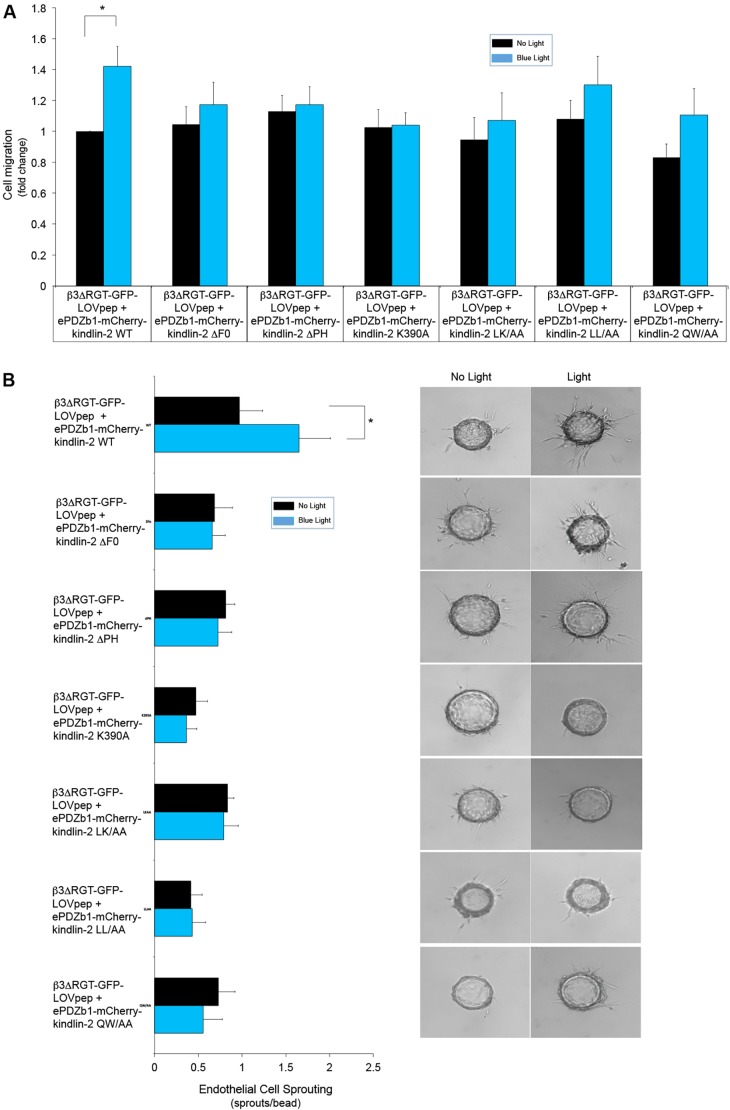
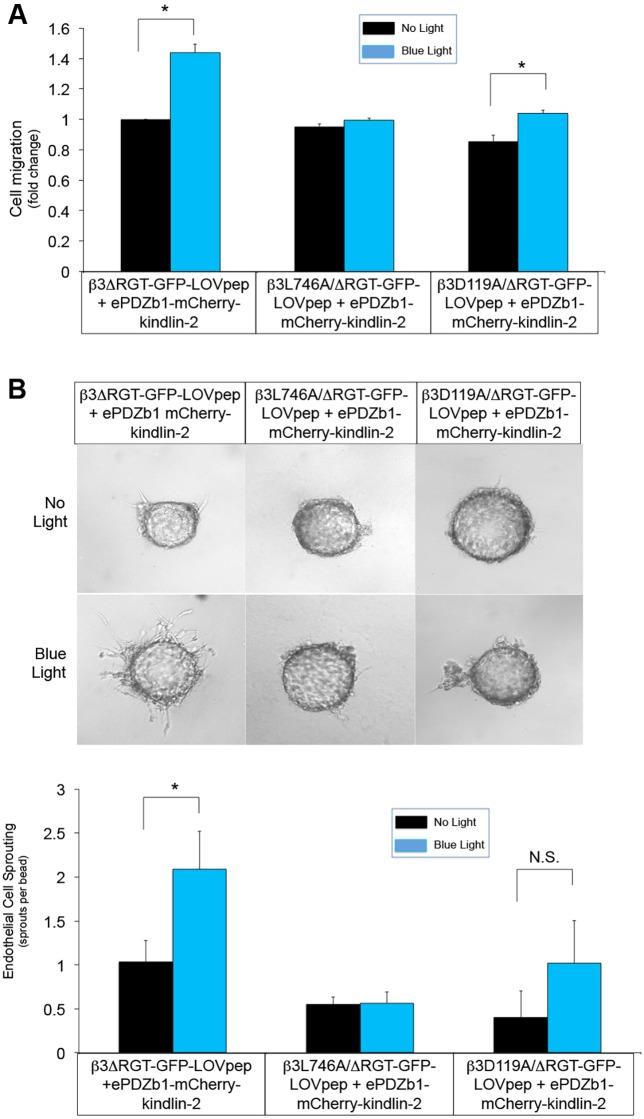
In addition to kindlin-2 F3 subdomain interaction with the β3 integrin tail, other regions in kindlin-2 support interactions with other binding partners. Based on mutational analyses, these regions include F0 subdomain interaction with c-Src (Qu et al., 2014) and actin (Bledzka et al., 2016), PH domain interaction with phosphoinositides (Qu et al., 2011) and paxillin (Theodosiou et al., 2016), and F2 subdomain interaction with integrin-linked kinase (Fukuda et al., 2014). To study these, relevant mutations were introduced into kindlin-2 (Table 1) to create mCherry–mutant kindlin-2–ePDZ1 fusion proteins. Each of the kindlin-2 mutants were then co-expressed in endothelial cells with αVβ3ΔRGT–GFP–LOVpep. Although each kindlin-2 mutant could be recruited to focal adhesions in adherent cells upon exposure to blue light (Fig. S1B and Fig. S6A), none could promote endothelial cell adhesion, migration or angiogenic sprouting (Fig. 6; Fig. S7A). Thus, kindlin-2 might have to interact with several other binding partners in addition to integrin β3 to promote endothelial cell functions relevant to angiogenesis.
Table 1.
Mutant fusion proteins used in this study
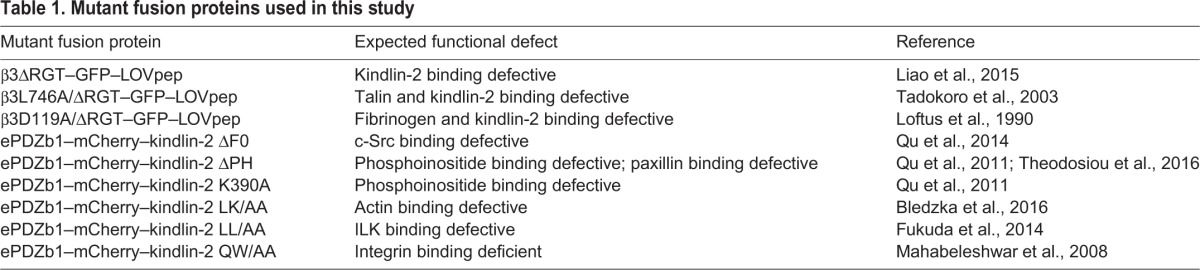
Fig. 6.
The effects of kindlin-2 interaction with β3 integrin on endothelial cells also require kindlin-2 interactions with other binding partners. Mutations were introduced into kindlin-2 as shown in Table 1 and expressed along with β3ΔRGT–GFP–LOVpep. (A) Cell migration across fibrinogen-coated Transwells. Migration of cells expressing β3ΔRGT–GFP–LOVpep and ePDZb1–mCherry–kindlin-2 without illumination was arbitrarily assigned a value of one. (B) Representative images and quantification of endothelial cell sprouting. Data represent mean±s.e.m. from four to five experiments. *P<0.05 (paired Student's t-test).
Kindlin-2 cooperates with talin to enhance activation and/or clustering of platelet integrin αIIbβ3 (Mahabeleshwar et al., 2008; Ye et al., 2013). However, kindlin-2 might also regulate cellular functions in a talin-independent manner (Theodosiou et al., 2016). To differentiate between these two possibilities, a mutation in the cytoplasmic tail of β3 (L746A) was introduced that impairs its interaction with talin but not kindlins (Petrich et al., 2007). In response to blue light, endothelial cells expressing β3L746A/ΔRGT–GFP–LOVpep and kindlin-2–mCherry–ePDZb1 failed to undergo increased αVβ3-dependent cell adhesion (Fig. S7B), migration (Fig. 7A) or angiogenic sprout formation (Fig. 7B). Similar results were obtained with an extracellular β3 mutant (D119A) that impairs extracellular ligand binding. Thus, kindlin-2 interaction with αVβ3 is required, but not sufficient, for several αVβ3 functions potentially relevant to angiogenesis.
Fig. 7.
Optogenetic modulation of endothelial cell functions by kindlin-2 interaction with integrin β3 also requires fibrinogen and talin binding to β3. Point mutations, L746A or D119A, were introduced to create talin binding-defective (L746A) or ligand binding-defective (D119A) β3 mutants. Following expression of β3L746A/ΔRGT–GFP–LOVpep or β3D119A/ΔRGT–GFP–LOVpep along with ePDZb1–mCherry–kindlin-2 in endothelial cells, cell migration (A) and endothelial cell sprouting (B) were quantified. Data represent mean±s.e.m. from three experiments. *P<0.05; N.S., not significant (paired Student's t-test).
DISCUSSION
In the present study, we took advantage of the fact that kindlin-2 interacts directly with the integrin β3 cytoplasmic tail in a manner dependent on the C-terminal three amino acids of β3 (Liao et al., 2015) to study the role of this interaction in endothelial cells. A complementary pair of optogenetic probes, LOV-pep and ePDZb1, were fused to β3ΔRGT and kindlin-2, respectively, to enable spatial and temporal control of the interaction in response to 450 nm light. The major new findings are the following: (1) optogenetic interaction of kindlin-2 with β3ΔRGT promotes several αVβ3-dependent endothelial cell responses in vitro, including adhesion, migration, podosome formation and angiogenic sprouting; (2) these endothelial cell responses also appear to require kindlin-2 interaction with several other known binding partners, including actin, c-Src, integrin-linked kinase and phosphoinositides. Though these results were obtained in vitro, they suggest that simultaneous or near-simultaneous interactions of kindlin-2 with β3 and other binding partners are required for αVβ3-dependent endothelial cell responses relevant to angiogenesis (Avraamides et al., 2008; Pluskota et al., 2011; Potente et al., 2011; Seano et al., 2014). Together with recent reports focused on other aspects of endothelial cell function (Grusch et al., 2014; Kim et al., 2016), the present study illustrates the potential utility of optogenetics for further investigations of multiple facets of endothelial cell biology.
In endothelial cells, both αVβ3 and kindlins have been shown to localize to an actin-rich structure, the podosome (Ussar et al., 2006; Varon et al., 2006), the formation of which precedes vascular branching and angiogenic sprouting, suggesting a potential role of this structure in angiogenesis (Seano et al., 2014). Although the molecular basis of αVβ3 and kindlin-2 function in podosome formation remains to be fully explored, our finding that the αVβ3–kindlin-2 interaction promotes podosome formation as well as angiogenic sprouting provides one plausible explanation for the potential positive contributions of αVβ3 and kindlin-2 to endothelial cell responses that lead to angiogenesis. We note that optogenetic interaction of kindlin-2 with αVβ3 also promoted αVβ3-dependent endothelial cell migration, consistent with the idea that kindlin-2 can stimulate multiple aspects of integrin signaling (Malinin et al., 2010; Qu et al., 2014; Rognoni et al., 2016). Optogenetically controlled kindlin-2 interaction with αVβ3 did not increase αVβ3 affinity in endothelial cells as detected by antibody WOW-1 Fab, suggesting that kindlin-2 rather promotes αVβ3 clustering (Ye et al., 2013) and/or outside-in signaling in endothelial cells.
The physiological functions of kindlin-2 in mice have been challenging to study because genetic deletion leads to embryonic lethality (Montanez et al., 2008). In the present study, we explored the roles of known kindlin-2 binding partners in αVβ3-dependent endothelial functions by introducing specific mutations into the molecule (Table 1). Compared to wild-type ePDZb1–mCherry–kindlin-2, each of the ePDZb1–mCherry–kindlin-2 mutants we studied showed an impaired ability to promote endothelial cell sprout formation. This result could not be explained by failure of the mutants to be expressed in the cells, or by failure of mutant kindlin-2 interaction with αVβ3 in response to blue light. Therefore, we conclude that in order for kindlin-2 to promote angiogenic sprouting, kindlin-2 interactions with actin, c-Src, integrin-linked kinase and phosphoinositides might be required in addition to kindlin-2 interaction with αVβ3. Conceivably, kindlin-2 might nucleate a plasma membrane- and integrin-based macromolecular complex to promote angiogenesis.
β3 integrins are crucial for embryonic development, platelet function and tumor progression. However, there has been controversy as to whether αVβ3 functions as a pro- or anti-angiogenic integrin in endothelial cells (Friedlander et al., 1995; Mahabeleshwar et al., 2006; Reynolds et al., 2002). We reported previously that knock-in mice expressing β3ΔRGT exhibited reduced developmental and tumor angiogenesis (Liao et al., 2015), consistent with a positive role for αVβ3–kindlin-2 in angiogenesis. The results of the current study are consistent with this formulation.
In the past decade, there has been explosive growth in the use of optogenetics to study the mouse nervous system in vivo (Deisseroth, 2015). Optogenetic methodology is finding increasing use in cell biology (Grusch et al., 2014; Kim et al., 2016), and further advances in technology should enable the use of optogenetics in investigation of aspects of the cardiovascular system in model systems in vivo (Reade et al., 2017; Wang et al., 2017).
MATERIALS AND METHODS
DNA constructs
Mid(SS/TM)–GFP–LOVpep (plasmid 34972) and ePDZb1–mCherry (plasmid 34981) were obtained from Addgene [deposited by Michael Glotzer (Strickland et al., 2012)]. To generate β3ΔRGT–GFP–LOVpep, PCR-amplified human β3ΔRGT and GFP–LOVpep, including a nine amino acid linker (GGSGGSGGS), were inserted into the EcoRI and BamHI sites of pLVX-het1 using an In-Fusion cloning kit (Clontech). Similarly, ePDZb1–mCherry and human kindlin-2 were amplified and inserted into the XhoI and BamHI sites of pLVX-het2 to create ePDZb1–mCherry–kindlin-2. Point mutations in β3ΔRGT (β3L746A) or in kindlin-2 (K390A, LK/AA, LL/AA or QW/AA) were introduced by site-directed mutagenesis using a KAPA HiFi kit (Kapa Biosystems). Deletions within kindlin-2 (ΔF0 or ΔPH) were generated by PCR and ligated into the XhoI and BamHI sites of pLVX-het2. Short hairpin RNA (shRNA) constructs targeting kindlin-2 in FG12 were described previously (Kahner et al., 2012). All constructs were verified by DNA sequencing.
Cell culture
Lung endothelial cells isolated from 2-month-old β3−/− mice on a C57/B16 background were transduced and immortalized with retroviruses encoding a temperature-sensitive (tsa58) mutant of the large T antigen (Liao et al., 2015). Cells were maintained in low glucose Dulbecco's modified Eagle's medium with 10% fetal bovine serum, 50 µg/ml heparin (Sigma-Aldrich), 0.5 ng/ml recombinant VEGF and 5 ng/ml basic FGF (BRB Preclinical Repository at National Cancer Institute, Frederick, MD). Lentiviruses encoding β3ΔRGT–GFP–LOVpep or ePDZb1–mCherry–kindlin-2 were transduced into these cells as described (Kahner et al., 2012; Liao et al., 2015). Cells double positive for GFP and mCherry were sorted by flow cytometry on a FACSAria (BD Biosciences). Sorted cells were cultured in the presence of 5 μg/ml puromycin and 100 μg/ml hygromycin at the permissive temperature of 33°C. For experiments, the cells were trypsinized and maintained in short-term culture at 37°C. Endothelial cell identity was confirmed morphologically and by CD31 surface expression in flow cytometry. All animal procedures were performed using protocols approved by the University of California San Diego Institutional Animal Care and Use Committee.
Total internal reflection fluorescence microscopy
Cells were plated on fibrinogen-coated coverslips and mounted in a Chamlide magnetic chamber (Live Cell Instruments), immersed with medium and incubated at 37°C in 5% CO2. Cells were visualized with the Applied Precision OMX DeltaVision Ring TIRF module using a 100× objective (NA 1.40) with two laser lines (488 and 562 nm). Cells were imaged every 1 min for 3–10 min. For activation of the optogenetics system, the 488 nm laser was used to illuminate the sample for 100–150 ms because this is sufficient to expose the cells to 450 nm blue light and activate LOVpep/ePDZb1(van Bergeijk et al., 2015). Data were transferred to a Linux workstation and processed in SoftWoRx software (GE Healthcare Biosciences). Rounds of deconvolution were applied to reduce scattered light captured by the camera. Image processing and analysis were performed with ImageJ and Volocity software (PerkinElmer).
Confocal microscopy and image analysis
Cells on fibrinogen-coated coverslips were illuminated using a blue light-emitting diode (LED) mounted in a customized chamber (500 ms illumination/min; 50 mW/cm2) for 30 min before fixation with 3.7% formaldehyde. Cells were permeabilized with 0.1% Triton X-100 and stained with mouse anti-vinculin antibody (V4505, Sigma-Aldrich, 1:100 dilution). After washing three times with phosphate-buffered saline (PBS), coverslips were mounted on slides using Prolong Diamond antifade reagent (Thermo Fisher Scientific). Images were taken with an Olympus IX81 inverted microscope, using a 100× oil objective (NA 1.4). For colocalization, GFP and mCherry pixels were subjected to background correction. Pixel correlation measurements were made on subareas at cell edges, where focal adhesions were indicated by vinculin staining, and Pearson's correlation coefficient was calculated from 20–40 cells in two independent experiments using Volocity (PerkinElmer). For visualization of podosomes, cells were illuminated for 24 h before being stained with Alexa Fluor 647-conjugated phalloidin (Invitrogen) and Hoechst 33342. Images were captured as described above. Three independent experiments were performed to calculate the percentage of podosomes per cell.
Cell adhesion and migration
96-well plates were coated overnight at 4°C with 100 µl fibrinogen at increasing concentrations, and blocked with 1% bovine serum albumin in PBS for 1 h at room temperature. A cell suspension (100 µl; 106 cells/ml) was seeded to each well and incubated for 1 h at 37°C in 5% CO2 with or without illumination from a blue LED (500 ms illumination/min; ∼50 mW/cm2). After washing three times with PBS, cells were fixed with 3.7% formaldehyde and stained with 0.1% Crystal Violet for 30 min. Crystal Violet was then eluted with 100 µl of 10% acetic acid and absorbance was measured at 590 nm.
For cell migration, Transwells with 8-μm pores were coated on both sides with 100 µg/ml fibrinogen overnight at 4°C. Then 105 endothelial cells in 200 μl endothelial basal medium were added to the upper chamber and 700 μl complete medium containing 2% FBS was added to the lower chamber. Cells were incubated with or without illumination from the top of the Transwells for 16 h at 37°C and 5% CO2. Cells on the upper surface of the chamber were removed with a cotton swab and migrated cells on the lower surface were fixed with methanol at −20°C for 15 min and stained with 0.1% Crystal Violet. Migration was quantified by eluting Crystal Violet in 10% acetic acid and measuring absorbance at 590 nm.
Three-dimensional fibrin gel bead assay
An in vitro model that simulates the process of endothelial cell sprouting was used as described (Nakatsu et al., 2007). Briefly, Cytodex micro-carrier beads (Sigma-Aldrich) coated with endothelial cells were mixed with 2 mg/ml fibrinogen. Thrombin was added to induce a fibrin gel to the top of which were added NIH-3T3 cells. Fibrin gels were incubated at 37°C in 5% CO2 with or without illumination at 450 nm for 24 h. Endothelial cell sprout formation was quantified in a blind manner by scoring sprouts with lengths greater than or equal to the bead diameter.
Western blotting and immunoprecipitation
Cells were lysed in NP-40 buffer [1% Nonidet P-40, 150 mM NaCl, 50 mM Tris-HCl, pH 8.0, and EDTA-free complete protease inhibitor cocktail (Roche Applied Science)]. Proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 5% bovine serum albumin in Tris-buffered saline, membranes were incubated with anti-kindlin-2 antibody (a gift from Dr Cary Wu, University of Pittsburgh, USA) and then secondary antibodies conjugated to IRDye 800CW. Blots were analyzed with the Odyssey imaging system (LI-COR Biosciences). For immunoprecipitation, cell lysates in NP-40 buffer were pre-cleared with protein A-conjugated beads and subjected to overnight incubation with rabbit anti-GFP (a gift from Dr Mark Ginsberg, University of California, San Diego, USA) or pre-immune serum at 4°C. Immune complexes were precipitated by protein-A/G-conjugated beads (Santa Cruz Biotechnology) and analyzed by immunoblotting with anti-kindlin-2 antibody and re-probing with anti-GFP antibody.
Statistical analysis
Two-tailed Student's t-test was used to calculate differences between two groups. P<0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
We thank Drs Cary Wu and Mark Ginsberg for reagents and Drs Sourav Banerjee, Frederic Lagarrigue and Edgar Gutierrez for technical assistance. We also thank the University of California San Diego Neuroscience Microscopy Shared Facility (P30 NS047101) for technical assistance.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Z.L., S.J.S.; Methodology: Z.L., A.K.-F., S.J.S.; Validation: Z.L., S.J.S.; Formal analysis: Z.L.; Investigation: Z.L., S.J.S.; Resources: Z.L., A.K.-F., S.J.S.; Writing - original draft: Z.L., S.J.S.; Writing - review & editing: Z.L., S.J.S.; Visualization: Z.L.; Supervision: S.J.S.; Project administration: S.J.S.; Funding acquisition: S.J.S.
Funding
This work was supported by the National Institutes of Health (HL056595 and HL078784). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.205203.supplemental
References
- Avraamides C. J., Garmy-Susini B. and Varner J. A. (2008). Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 8, 604-617. 10.1038/nrc2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledzka K., Bialkowska K., Sossey-Alaoui K., Vaynberg J., Pluskota E., Qin J. and Plow E. F. (2016). Kindlin-2 directly binds actin and regulates integrin outside-in signaling. J. Cell Biol. 213, 97-108. 10.1083/jcb.201501006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. (2015). Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 18, 1213-1225. 10.1038/nn.4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demircioglu F. and Hodivala-Dilke K. (2016). alphavbeta3 Integrin and tumour blood vessels-learning from the past to shape the future. Curr. Opin. Cell Biol. 42, 121-127. 10.1016/j.ceb.2016.07.008 [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B. and Cheresh D. A. (1993). Vitronectin and its receptors. Curr. Opin. Cell Biol. 5, 864-868. 10.1016/0955-0674(93)90036-P [DOI] [PubMed] [Google Scholar]
- Friedlander M., Brooks P. C., Shaffer R. W., Kincaid C. M., Varner J. A. and Cheresh D. A. (1995). Definition of two angiogenic pathways by distinct av integrins. Science 270, 1500-1502. 10.1126/science.270.5241.1500 [DOI] [PubMed] [Google Scholar]
- Fukuda K., Bledzka K., Yang J., Perera H. D., Plow E. F. and Qin J. (2014). Molecular basis of kindlin-2 binding to integrin-linked kinase pseudokinase for regulating cell adhesion. J. Biol. Chem. 289, 28363-28375. 10.1074/jbc.M114.596692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusch M., Schelch K., Riedler R., Reichhart E., Differ C., Berger W., Ingles-Prieto A. and Janovjak H. (2014). Spatio-temporally precise activation of engineered receptor tyrosine kinases by light. EMBO J. 33, 1713-1726. 10.15252/embj.201387695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Gao J., Zhan J. and Zhang H. (2015). Kindlin-2 interacts with and stabilizes EGFR and is required for EGF-induced breast cancer cell migration. Cancer Lett. 361, 271-281. 10.1016/j.canlet.2015.03.011 [DOI] [PubMed] [Google Scholar]
- Kahner B. N., Kato H., Banno A., Ginsberg M. H., Shattil S. J. and Ye F. (2012). Kindlins, integrin activation and the regulation of talin recruitment to αIIbβ3. PLoS ONE 7, e34056 10.1371/journal.pone.0034056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunarathne W. K. A., O'Neill P. R. and Gautam N. (2015). Subcellular optogenetics - controlling signaling and single-cell behavior. J. Cell Sci. 128, 15-25. 10.1242/jcs.154435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. M., Lee M., Kim N. and Heo W. D. (2016). Optogenetic toolkit reveals the role of Ca2+ sparklets in coordinated cell migration. Proc. Natl. Acad. Sci. USA 113, 5952-5957. 10.1073/pnas.1518412113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H., Plow E. F. and Wu C. (2008). Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 9, 1203-1208. 10.1038/embor.2008.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Deng Y., Sun K., Yang H., Liu J., Wang M., Zhang Z., Lin J., Wu C., Wei Z. et al. (2017). Structural basis of kindlin-mediated integrin recognition and activation. Proc. Natl. Acad. Sci. USA 114, 9349-9354. 10.1073/pnas.1703064114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z., Kato H., Pandey M., Cantor J. M., Ablooglu A. J., Ginsberg M. H. and Shattil S. J. (2015). Interaction of kindlin-2 with integrin β3 promotes outside-in signaling responses by the αVβ3 vitronectin receptor. Blood 125, 1995-2004. 10.1182/blood-2014-09-603035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S. and Kopp P. (2005). Podosomes at a glance. J. Cell Sci. 118, 2079-2082. 10.1242/jcs.02390 [DOI] [PubMed] [Google Scholar]
- Loftus J. C., O'Toole T. E., Plow E. F., Glass A., Frelinger A. L. III and Ginsberg M. H. (1990). A beta 3 integrin mutation abolishes ligand binding and alters divalent cation-dependent conformation. Science 249, 915-918. 10.1126/science.2392682 [DOI] [PubMed] [Google Scholar]
- Mahabeleshwar G. H., Feng W., Phillips D. R. and Byzova T. V. (2006). Integrin signaling is critical for pathological angiogenesis. J. Exp. Med. 203, 2495-2507. 10.1084/jem.20060807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabeleshwar G. H., Chen J., Feng W., Somanath P. R., Razorenova O. V. and Byzova T. V. (2008). Integrin affinity modulation in angiogenesis. Cell Cycle 7, 335-347. 10.4161/cc.7.3.5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinin N. L., Plow E. F. and Byzova T. V. (2010). Kindlins in FERM adhesion. Blood 115, 4011-4017. 10.1182/blood-2009-10-239269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanez E., Ussar S., Schifferer M., Bosl M., Zent R., Moser M. and Fassler R. (2008). Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 22, 1325-1330. 10.1101/gad.469408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau V., Tatin F., Varon C. and Genot E. (2003). Actin can reorganize into podosomes in aortic endothelial cells, a process controlled by Cdc42 and RhoA. Mol. Cell. Biol. 23, 6809-6822. 10.1128/MCB.23.19.6809-6822.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P. A., Begum S. and Hynes R. O. (2015). Tumor angiogenesis in the absence of fibronectin or its cognate integrin receptors. PLoS ONE 10, e0120872 10.1371/journal.pone.0120872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu M. N., Davis J. and Hughes C. C. (2007). Optimized fibrin gel bead assay for the study of angiogenesis. J Vis Exp. e186 10.3791/186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrich B. G., Marchese P., Ruggeri Z. M., Spiess S., Weichert R. A. M., Ye F., Tiedt R., Skoda R. C., Monkley S. J., Critchley D. R. et al. (2007). Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J. Exp. Med. 204, 3103-3111. 10.1084/jem.20071800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plançon S., Morel-Kopp M.-C., Schaffner-Reckinger E., Chen P. and Kieffer N. (2001). Green fluorescent protein (GFP) tagged to the cytoplasmic tail of alphaIIb or beta3 allows the expression of a fully functional integrin alphaIIb(beta3): effect of beta3GFP on alphaIIb(beta3) ligand binding. Biochem. J. 357, 529-536. 10.1042/bj3570529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Meller J. and Byzova T. V. (2014). Integrin function in vascular biology: a view from 2013. Curr. Opin Hematol. 21, 241-247. 10.1097/MOH.0000000000000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Das M., Bialkowska K. and Sossey-Alaoui K. (2016). Of Kindlins and Cancer. Discoveries (Craiova) 4, e59 10.15190/d.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluskota E., Dowling J. J., Gordon N., Golden J. A., Szpak D., West X. Z., Nestor C., Ma Y.-Q., Bialkowska K., Byzova T. et al. (2011). The integrin coactivator kindlin-2 plays a critical role in angiogenesis in mice and zebrafish. Blood 117, 4978-4987. 10.1182/blood-2010-11-321182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M., Gerhardt H. and Carmeliet P. (2011). Basic and therapeutic aspects of angiogenesis. Cell 146, 873-887. 10.1016/j.cell.2011.08.039 [DOI] [PubMed] [Google Scholar]
- Qu H., Tu Y., Shi X., Larjava H., Saleem M. A., Shattil S. J., Fukuda K., Qin J., Kretzler M. and Wu C. (2011). Kindlin-2 regulates podocyte adhesion and fibronectin matrix deposition through interactions with phosphoinositides and integrins. J. Cell Sci. 124, 879-891. 10.1242/jcs.076976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H., Tu Y., Guan J.-L., Xiao G. and Wu C. (2014). Kindlin-2 tyrosine phosphorylation and interaction with Src serve as a regulatable switch in the integrin outside-in signaling circuit. J. Biol. Chem. 289, 31001-31013. 10.1074/jbc.M114.580811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reade A., Motta-Mena L. B., Gardner K. H., Stainier D. Y., Weiner O. D. and Woo S. (2017). TAEL: a zebrafish-optimized optogenetic gene expression system with fine spatial and temporal control. Development 144, 345-355. 10.1242/dev.139238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds L. E., Wyder L., Lively J. C., Taverna D., Robinson S. D., Huang X. Z., Sheppard D., Hynes O. and Hodivala-Dilke K. M. (2002). Enhanced pathological angiogenesis in mice lacking β3 integrin or β3 and β5 integrins. Nat. Med. 8, 27-34. 10.1038/nm0102-27 [DOI] [PubMed] [Google Scholar]
- Reynolds A. R., Hart I. R., Watson A. R., Welti J. C., Silva R. G., Robinson S. D., Da Violante G., Gourlaouen M., Salih M., Jones M. C. et al. (2009). Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat. Med. 15, 392-400. 10.1038/nm.1941 [DOI] [PubMed] [Google Scholar]
- Robinson S. D. and Hodivala-Dilke K. M. (2011). The role of β3-integrins in tumor angiogenesis: context is everything. Curr. Opin. Cell Biol. 23, 630-637. 10.1016/j.ceb.2011.03.014 [DOI] [PubMed] [Google Scholar]
- Rognoni E., Ruppert R. and Fässler R. (2016). The kindlin family: functions, signaling properties and implications for human disease. J. Cell Sci. 129, 17-27. 10.1242/jcs.161190 [DOI] [PubMed] [Google Scholar]
- Seano G., Daubon T., Génot E. and Primo L. (2014). Podosomes as novel players in endothelial biology. Eur. J. Cell Biol. 93, 405-412. 10.1016/j.ejcb.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Shi X., Ma Y.-Q., Tu Y., Chen K., Wu S., Fukuda K., Qin J., Plow E. F. and Wu C. (2007). The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J. Biol. Chem. 282, 20455-20466. 10.1074/jbc.M611680200 [DOI] [PubMed] [Google Scholar]
- Spuul P., Daubon T., Pitter B., Alonso F., Fremaux I., Kramer I., Montanez E. and Génot E. (2016). VEGF-A/notch-induced podosomes proteolyse basement membrane collagen-IV during retinal sprouting angiogenesis. Cell Rep. 17, 484-500. 10.1016/j.celrep.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Strickland D., Yao X., Gawlak G., Rosen M. K., Gardner K. H. and Sosnick T. R. (2010). Rationally improving LOV domain-based photoswitches. Nat. Methods 7, 623-626. 10.1038/nmeth.1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland D., Lin Y., Wagner E., Hope C. M., Zayner J., Antoniou C., Sosnick T. R., Weiss E. L. and Glotzer M. (2012). TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat. Methods 9, 379-384. 10.1038/nmeth.1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suehiro K., Gailit J. and Plow E. F. (1997). Fibrinogen is a ligand for integrin alpha5beta1 on endothelial cells. J. Biol. Chem. 272, 5360-5366. 10.1074/jbc.272.8.5360 [DOI] [PubMed] [Google Scholar]
- Tadokoro S., Shattil S. J., Eto K., Tai V., Liddington R. C., de Pereda J. M., Ginsberg M. H. and Calderwood D. A. (2003). Talin binding to integrin beta tails: a final common step in integrin activation. Science 302, 103-106. 10.1126/science.1086652 [DOI] [PubMed] [Google Scholar]
- Theodosiou M., Widmaier M., Böttcher R. T., Rognoni E., Veelders M., Bharadwaj M., Lambacher A., Austen K., Muller D. J., Zent R. et al. (2016). Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. Elife 5, e10130 10.7554/eLife.10130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer D. and Weiner O. D. (2014). Illuminating cell signalling with optogenetic tools. Nat. Rev. Mol. Cell Biol. 15, 551-558. 10.1038/nrm3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussar S., Wang H.-V., Linder S., Fässler R. and Moser M. (2006). The Kindlins: subcellular localization and expression during murine development. Exp. Cell Res. 312, 3142-3151. 10.1016/j.yexcr.2006.06.030 [DOI] [PubMed] [Google Scholar]
- van Bergeijk P., Adrian M., Hoogenraad C. C. and Kapitein L. C. (2015). Optogenetic control of organelle transport and positioning. Nature 518, 111-114. 10.1038/nature14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon C., Tatin F., Moreau V., Van Obberghen-Schilling E., Fernandez-Sauze S., Reuzeau E., Kramer I. and Genot E. (2006). Transforming growth factor beta induces rosettes of podosomes in primary aortic endothelial cells. Mol. Cell. Biol. 26, 3582-3594. 10.1128/MCB.26.9.3582-3594.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Collighan R. J., Gross S. R., Danen E. H. J., Orend G., Telci D. and Griffin M. (2010). RGD-independent cell adhesion via a tissue transglutaminase-fibronectin matrix promotes fibronectin fibril deposition and requires syndecan-4/2 alpha5beta1 integrin co-signaling. J. Biol. Chem. 285, 40212-40229. 10.1074/jbc.M110.123703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lin W. K., Crawford W., Ni H., Bolton E. L., Khan H., Shanks J., Bub G., Wang X., Paterson D. J. et al. (2017). Optogenetic control of heart rhythm by selective stimulation of cardiomyocytes derived from Pnmt+ cells in murine heart. Sci. Rep. 7, 40687 10.1038/srep40687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman M. and Hahn K. M. (2014). Optogenetic approaches to cell migration and beyond. Curr. Opin. Cell Biol. 30, 112-120. 10.1016/j.ceb.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F., Petrich B. G., Anekal P., Lefort C. T., Kasirer-Friede A., Shattil S. J., Ruppert R., Moser M., Fassler R. and Ginsberg M. H. (2013). The mechanism of kindlin-mediated activation of integrin αIIbβ3. Curr. Biol. 23, 2288-2295. 10.1016/j.cub.2013.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Cui N., Wu Y., Zhong W., Johnson C. M. and Jiang C. (2015). Optogenetic intervention to the vascular endothelium. Vascul. Pharmacol. 74, 122-129. 10.1016/j.vph.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.