Abstract
Synaptogenesis requires orchestrated communication between pre- and postsynaptic cells via coordinated trans-synaptic signaling across the extracellular synaptomatrix. The first Wnt signaling ligand discovered, Drosophila Wingless (Wg; Wnt1 in mammals), plays crucial roles in synaptic development, regulating synapse architecture as well as functional differentiation. Here, we investigate synaptogenic functions of the secreted extracellular deacylase Notum, which restricts Wg signaling by cleaving an essential palmitoleate moiety. At the glutamatergic neuromuscular junction (NMJ) synapse, we find that Notum secreted from the postsynaptic muscle acts to strongly modulate synapse growth, structural architecture, ultrastructural development and functional differentiation. In Notum null flies, we find upregulated extracellular Wg ligand and nuclear trans-synaptic signal transduction, as well as downstream misregulation of both pre- and postsynaptic molecular assembly. Structural, functional and molecular synaptogenic defects are all phenocopied by Wg overexpression, suggesting that Notum acts solely by inhibiting Wg trans-synaptic signaling. Moreover, these synaptic development phenotypes are suppressed by genetically correcting Wg levels in Notum null mutants, indicating that Notum normally functions to coordinate synaptic structural and functional differentiation via negative regulation of Wg trans-synaptic signaling in the extracellular synaptomatrix.
KEY WORDS: Synaptomatrix, Frizzled nuclear import, Neuromuscular junction, Drosophila
Summary: Extracellular enzymatic deacylase regulation of Wnt trans-synaptic signaling in Drosophila is crucial for proper structural, ultrastructural and functional development of the neuromuscular synapses underlying coordinated movement.
INTRODUCTION
In the developing nervous system, Wnt signaling ligands act as potent regulators of multiple stages of neuronal connectivity maturation, stabilization and synaptogenesis, including sculpting structural architecture and determining neurotransmission strength (Ataman et al., 2008; Miech et al., 2008; Packard et al., 2002). The founding Wnt ligand, Drosophila Wingless (Wg), is secreted from presynaptic neurons (Packard et al., 2002) and glia (Kerr et al., 2014) at the developing glutamatergic neuromuscular junction (NMJ) (Jan and Jan, 1976) to bind Frizzled 2 (Fz2) receptors in both anterograde and autocrine signaling (Packard et al., 2002). In the postsynaptic muscle, Wg binding to Fz2 activates the Frizzled Nuclear Import (FNI) signaling pathway, which involves Fz2 endocytosis followed by Fz2 cleavage and Fz2 C-terminus (Fz2-C) nuclear import (Mathew et al., 2005). Fz2-C trafficked in nuclear ribonucleoprotein (RNP) granules regulates translation of synaptic mRNAs, thereby driving expression changes that modulate synapse structural and functional differentiation (Speese et al., 2012). In the presynaptic neuron, Wg binding to Fz2 activates a divergent canonical pathway inhibiting the glycogen synthase kinase 3β (GSK3β) homolog Shaggy (Sgg) to regulate microtubule cytoskeleton dynamics via the microtubule-associated protein 1B (MAP1B) homolog Futsch (Miech et al., 2008). Futsch binding to microtubules regulates architectural changes in synaptic branching and bouton formation. Such multifaceted Wg functions require tight management throughout synaptic development.
A highly conserved extracellular Wg regulator is the secreted deacylase Notum. The Notum gene was discovered in a Drosophila gain-of-function (GOF) mutant screen targeting wing development (Mata et al., 2000). Under scalloped-Gal4 control, Notum GOF causes loss of the wing and duplication of the dorsal thorax (Giráldez et al., 2002). In the developing wing disc, Notum acts as a secreted, extracellular feedback inhibitor of Wg signaling (Gerlitz and Basler, 2002). Notum was recently re-defined as a carboxylesterase that cleaves an essential Wg lipid moiety (palmitoleic acid attached to a conserved serine), leaving it unable to bind to Fz2 and activate downstream signaling (Kakugawa et al., 2015). This Wnt palmitoleate moiety is similarly cleaved by human Notum acting as a highly conserved secreted feedback antagonist in the extracellular space to inactivate Wnt signaling (Langton et al., 2016; Kakugawa et al., 2015). At the Drosophila NMJ, we have found that extracellular regulation of Wg trans-synaptic signaling plays key roles in synaptogenesis (Dani and Broadie, 2012; Parkinson et al., 2013). For example, extracellular matrix metalloproteinase (MMP) enzymes cleave heparan sulfate proteoglycan (HSPG) co-receptors to regulate the Wg trans-synaptic signaling that controls structural and functional synaptic development (Dear et al., 2016). Impairment of this mechanism is causative for fragile X syndrome (FXS) synaptogenic defects (Friedman et al., 2013). Similarly, misregulated extracellular mechanisms impair Wg trans-synaptic signaling in both congenital disorder of glycosylation (CDG) and galactosemia disease states, causing NMJ synaptogenic defects that result in disorders of coordinated movement (Jumbo-Lucioni et al., 2014, 2016; Parkinson et al., 2016). Given these insights, we wished to investigate the putative roles for Notum as a secreted Wg antagonist regulating synaptogenesis.
In the current study, we utilize the well-characterized Drosophila NMJ glutamate synapse model (Harris and Littleton, 2015; Keshishian et al., 1996; Menon et al., 2013) to study Notum requirements in synaptic development. We find that Notum secreted from muscle and glia is resident in the extracellular space surrounding developing synaptic boutons, where it negatively regulates Wg trans-synaptic signaling. In Notum mutants, extracellular Wg ligand levels and downstream Wg signaling are elevated. Null mutants display increased synapse number and strength, altered synaptic vesicle cycling, and synaptic ultrastructural defects including a decrease in subsynaptic reticulum (SSR)/bouton ratio, decreased synaptic vesicle density and an increase in the size of vesicular organelles. Cell-targeted RNAi studies reveal both postsynaptic and perisynaptic requirements, with muscle and glial Notum knockdown resulting in overelaborated NMJ architecture, but neuronal-driven Notum knockdown causing no detectable effects on synaptogenesis. Null Notum defects are all phenocopied by neuronal Wg overexpression, suggesting that synaptogenic phenotypes arise from lack of Wg inhibition. Consistently, genetically correcting Wg levels at the synapse in Notum nulls alleviates synaptogenic phenotypes, demonstrating that Notum functions solely as a negative regulator of Wg signaling. Taken together, these results identify Notum as a secreted Wnt inhibitor resident in the extracellular synaptomatrix with crucial functions regulating trans-synaptic Wnt signaling to coordinate structural and functional synaptogenesis.
RESULTS
Secreted Notum limits Wg levels and downstream trans-synaptic signaling
At the Drosophila NMJ, Wg secreted from neurons and glia regulates structural and functional synaptogenesis (Kerr et al., 2014; Packard et al., 2002), and this Wg signaling is tightly regulated within the extracellular synaptomatrix (Dear et al., 2016; Parkinson et al., 2016). Our goal was to test whether secreted Notum contributes to Wg trans-synaptic signaling control as a palmitoleate deacylase in this extracellular space (Kakugawa et al., 2015). We first tested Notum expression using a CRISPR/Cas9 HA tag in the endogenous Notum locus (Notum-HA; Fig. S1A-C). Introduction of Notum-HA does not detectably perturb Notum function, and the NMJ shows normal architectural development (Fig. S2A,B) and functional differentiation (Fig. S2C-F) compared with the w1118 genetic background control. At the NMJ, we used anti-HA to detect Notum and anti-Discs Large (DLG; Dlg1) to mark postsynaptic scaffolding (Dear et al., 2016; Jumbo-Lucioni et al., 2016). We find that Notum is localized at the NMJ and enriched at synaptic boutons (Fig. 1A). Detergent-free extracellular labeling shows that Notum is secreted into the external synaptomatrix surrounding individual synaptic boutons (Fig. S1D-F). Notum expression is similar to the dynamic Wg pattern at the NMJ (Dear et al., 2016; Jumbo-Lucioni et al., 2016). Extracellular Notum and Wg both surround synaptic boutons, and colocalize around a variable subset of boutons (Fig. S1G). This colocalization shows that Notum and Wg are in close proximity in the extracellular synaptomatrix, allowing Notum the opportunity to deacylate Wg and thus decrease trans-synaptic signaling. In the absence of Notum, Wg signaling is expected to increase at the synapse.
Fig. 1.
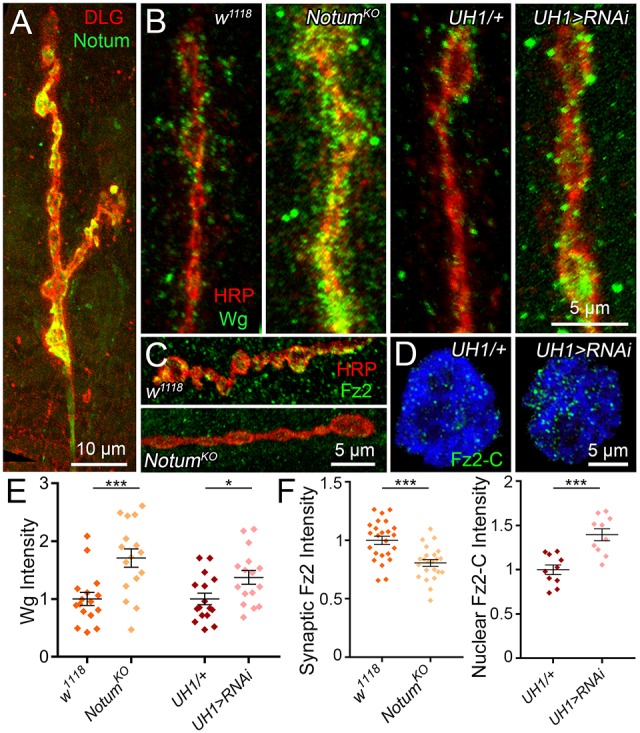
Extracellular Notum reduces Wg ligand levels and trans-synaptic signaling. (A) Representative image of muscle 4 NMJ co-labeled for CRISPR-generated Notum:HA (Notum, green) and synaptic label anti-Discs Large (DLG, red). (B) Representative NMJ bouton images of extracellular anti-Wingless labeling (Wg, green) co-labeled with synaptic marker anti-horseradish peroxidase (HRP, red) in w1118 background control versus NotumKO null mutant and UH1-Gal4/+ transgenic control versus UH1>Notum:RNAi. (C) Representative synaptic bouton images of anti- Fz2-C (green) at the NMJ (HRP, red) in w1118 and NotumKO. (D) Representative images of postsynaptic nuclei co-labeled with anti-Fz2-C (green) and nuclear label DRAQ5 (blue) in UH1/+ control versus UH1>Notum:RNAi. (E) Quantified Wg fluorescent intensities in all four genotypes normalized to control. (F) Quantified Fz2 fluorescent intensities at the NMJ synaptic terminal (left) and postsynaptic nuclei (right) in NotumKO and UH1>Notum:RNAi normalized to controls (w1118 and UH1/+). *P≤0.05, ***P≤0.001.
We used the combination of a Notum knockout null mutation (NotumKO) and two characterized UAS-Notum:RNAi lines to test synaptic Notum roles in the regulation of Wg trans-synaptic signaling (Kakugawa et al., 2015; Perkins et al., 2015). Extracellular Wg ligand levels were assayed using detergent-free immunocytochemical labeling to reveal only secreted Wg (Dear et al., 2016). Anti-horseradish peroxidase (HRP) was used to label the NMJ by binding to extracellular fucosylated N-glycans associated with the presynaptic membrane (Parkinson et al., 2013). We first compared NotumKO with the w1118 genetic background control and found that Wg is strikingly increased in Notum mutants, with elevated expression levels and an expanded spatial domain in the extracellular space surrounding NMJ synaptic boutons (Fig. 1B, left). Comparing Notum RNAi knockdown (UH1-Gal4>UAS-Notum:RNAi) with the transgenic driver alone control (UH1-Gal4/+) reveals a similar, but more modest, increase in extracellular Wg ligand levels at the NMJ synapse (Fig. 1B, right). In quantified measurements, decreasing Notum significantly increases Wg levels in parallel (mean±s.e.m.: normalized UH1-Gal4/+, 1.0±0.10 versus UH1>Notum:RNAi, 1.37±0.12; n=16, P=0.022; Fig. 1E). Completely eliminating Notum in null mutants results in a very strong Wg elevation by >70% compared with controls (w1118, 1.0±0.11 versus NotumKO, 1.71±0.16; n=16, P=0.001; Fig. 1E). These results show that Notum greatly limits Wg expression in the extracellular synaptomatrix.
We next investigated the roles of Notum in Wg trans-synaptic signaling. Presynaptically, Wg binding to Fz2 receptor regulates the MAP1B homolog Futsch to modulate microtubule dynamics (Miech et al., 2008). We therefore assayed Futsch labeling in Notum mutants (Fig. S3A), but observed no discernible difference in quantified comparisons (Fig. S3B). In the muscle, Wg binding drives postsynaptic Fz2 receptor endocytosis, cleavage and Fz2-C fragment transportation into muscle nuclei (FNI signaling pathway) to drive expression changes modifying NMJ structural and functional development (Mathew et al., 2005; Speese et al., 2012). We therefore next tested Fz2 receptor expression at the neuronal membrane in the absence of Notum (Fig. 1C) using the Fz2-C antibody. In NotumKO null mutants, we find a clear decrease in the intensity of Fz2-C punctae surrounding synaptic boutons, consistent with the highly increased Wg ligand levels (Fig. 1B,C). In quantified measurements, Fz2-C receptors are very significantly reduced in Notum mutants compared with controls (w1118, 1.0±0.04 versus NotumKO, 0.81±0.03; n=23, P=0.0001; Fig. 1F, left). We next tested the downstream import of Fz2-C into postsynaptic muscle nuclei (Fig. 1D). Comparing Notum RNAi knockdown (UH1-Gal4>UAS-Notum:RNAi) with transgenic driver alone controls (UH1-Gal4/+), there is a striking increase in the number of Fz2-C punctae in muscle nuclei with loss of Notum function (Fig. 1D). In quantified measurements, nuclear Fz2-C intensity in mutants is increased by 40% compared with controls (UH1-Gal4/+, 1.0±0.06 versus UH1>Notum:RNAi, 1.40±0.09; n=10 nuclei, P=0.001; Fig. 1F, right). These results show that Notum strongly limits Wg trans-synaptic signaling at the developing NMJ.
Notum secreted from muscle and glia regulates presynaptic NMJ architecture
Wg trans-synaptic signaling regulates NMJ growth, synaptic bouton formation and ultrastructural assembly (Packard et al., 2002). We therefore hypothesized that loss of Notum control of Wg trans-synaptic signaling should perturb synaptic architecture. Each NMJ terminal consists of a relatively stereotypical muscle innervation pattern, with consistent axon branching and synaptic bouton formation (Menon et al., 2013). Wg signaling bidirectionally regulates synaptic morphogenesis, with Wg knockdown causing a decrease in synaptic bouton number and Wg overexpression causing an increase in synaptic bouton number (Packard et al., 2002). To test the requirement for Notum in synaptic architectural development, we used immunocytochemistry to co-label the wandering third instar larval NMJ with both presynaptic anti-HRP and postsynaptic anti-DLG markers (Dear et al., 2016; Jumbo-Lucioni et al., 2016). We used characterized Notum RNAi transgenic lines (Perkins et al., 2015) for ubiquitous (UH1-Gal4), neuronal (elav-Gal4), glial (repo-Gal4) and muscle (24B-Gal4) cell-targeted knockdown studies (Fig. 2; Fig. S4A,B). We used the characterized NotumKO null mutant to completely eliminate Notum (Kakugawa et al., 2015), with side-by-side comparisons with presynaptic Wg overexpression (Fig. 3). Transmission electron microscopy (TEM) studies were used in parallel to examine synaptic bouton ultrastructure (Dear et al., 2016; Parkinson et al., 2013) in direct comparison with the confocal analyses (Fig. 3).
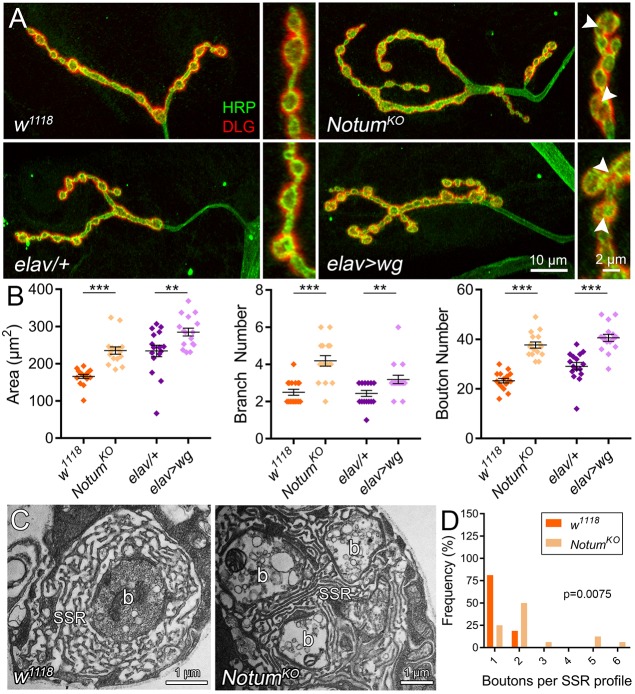
Fig. 2.
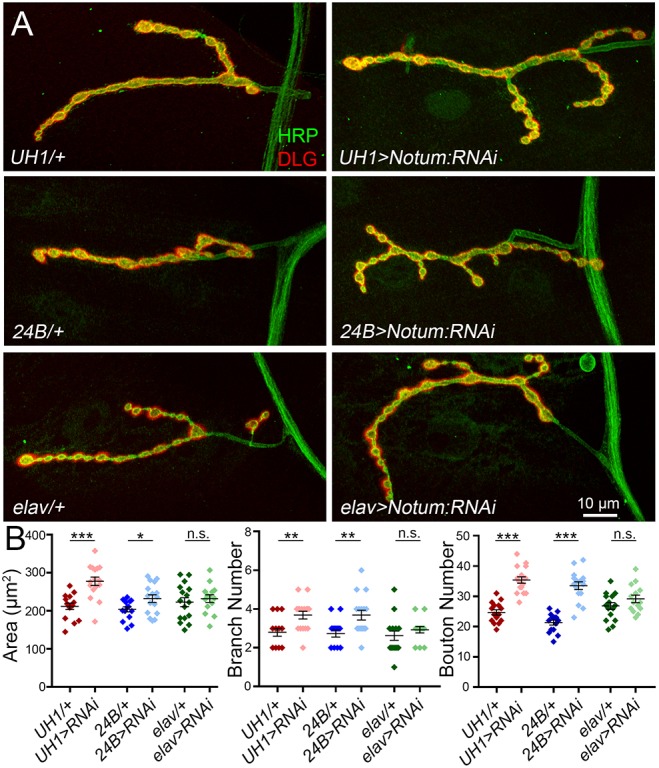
Postsynaptic Notum secretion limits presynaptic structural development. (A) Representative confocal images of muscle 4 NMJs co-labeled for presynaptic HRP (green) and postsynaptic DLG (red) with cell-targeted Notum RNAi knockdown (top, ubiquitous UH1-Gal4/+ versus UH1>Notum:RNAi; middle, postsynaptic muscle 24B-Gal4/+ versus 24B>Notum:RNAi; bottom, presynaptic neuron elav-Gal4/+ versus elav>Notum:RNAi). (B) Quantified NMJ area, branch number and synaptic bouton number for the six genotypes. *P≤0.05, **P≤0.01, ***P≤0.001; n.s., not significant.
Fig. 3.
Elevated Wg signaling phenocopies Notum null mutant synaptic defects. (A) Representative images of muscle 4 NMJs co-labeled for HRP (green) and DLG (red) with Notum null mutant (top row: w1118 background control versus NotumKO) and Wg overexpression (bottom row: elav-Gal4/+ control versus elav>wg). Insets show higher magnification boutons in all four conditions, with clustered boutons in mutants (arrowheads). (B) Quantified NMJ area, branch number and synaptic bouton number for the four genotypes. **P≤0.01 and ***P≤0.001. (C) Representative TEM images of w1118 and NotumKO synaptic boutons. b, bouton; SSR, subsynaptic reticulum. (D) Quantification of bouton number per SSR profile shown in a frequency histogram.
We find that Notum negatively regulates NMJ structural development, with roles limiting growth and synaptic bouton formation (Fig. 2A). When Notum is knocked down ubiquitously (UH1-Gal4>Notum:RNAi), there is a clear increase in NMJ size, branching and bouton number (Fig. 2A). In quantified measurements, global Notum loss causes significant increases in synaptic area (UH1-Gal4/+, 211.6±8.49 µm2 versus UH1>Notum:RNAi, 277.6±11.09; n≥14, P<0.0001), branching (UH1/+, 2.8±0.2 versus UH1>Notum:RNAi, 3.69±0.2; P=0.003) and bouton number (UH1/+, 24.67±0.88 versus UH1>Notum:RNAi, 35.38±1.08; P<0.0001; Fig. 2B). To test cell-specific requirements, Notum was knocked down in neurons (elav-Gal4), muscle (24B-Gal4) or glia (repo-Gal4). Qualitatively, NMJ terminals with muscle-targeted Notum RNAi are expanded indistinguishably from global knockdown, whereas neuron-targeted Notum loss has little discernable effect (Fig. 2A). In quantified measurements, muscle-specific Notum knockdown causes a significant expansion of synaptic area (24B-Gal4/+, 203.7±6.73 µm2 versus 24B>Notum:RNAi, 232.1±10.03; n≥15, P=0.027), branching (24B/+, 2.73±0.18 versus 24B>Notum:RNAi, 3.69±0.25; P=0.005) and bouton number (24B/+, 21.33±0.8 versus 24B>Notum:RNAi, 33.5±1.26; P<0.0001; Fig. 2B). Glial-specific RNAi increases synaptic bouton number more weakly (repo-Gal4/+, 32.19±1.14 versus repo>Notum:RNAi, 39.38±1.88; n=16, P=0.0027; Fig. S4A,B), but does not affect branching. In contrast, neuron-specific Notum knockdown causes no significant change in any synaptic parameter (Fig. 2B). These results show that Notum secreted from muscle and glia both limit presynaptic structure, with muscle-derived Notum having the greater role.
Null NotumKO NMJs show phenotypes similar to ubiquitous Notum knockdown, with striking increases in synapse size, branching and bouton formation (Fig. 3A). In quantified measurements, mutants display highly significant increases in area (w1118, 165.9±5.48 µm2 versus NotumKO, 235.4±9.60; n=16, P<0.0001), branching (w1118, 2.5±0.16 versus NotumKO, 4.19±0.28; P<0.0001) and bouton number (w1118, 23.31±0.90 versus NotumKO, 37.69±1.2; P<0.0001; Fig. 3B). Importantly, Wg overexpression (elav-Gal4>UAS-Wg) causes a very similar synaptic expansion (Fig. 3A), consistent with previous reports (Packard et al., 2002). Neuronal overexpression increasing synaptic Wg ligand levels by 33% (data not shown) causes an obvious expansion of synaptic size, branching and bouton formation (Fig. 3A). In quantified measurements, Wg overexpression increases synaptic area (elav-Gal4/+, 234.3±15.36 µm2 versus elav>wg, 284.9±10.51; n=16, P=0.01), branching (elav/+, 2.44±0.16 versus elav>wg, 3.19±0.23; P=0.01) and bouton number (elav/+, 29.13±1.50 versus elav>wg, 40.56±1.42; P<0.0001; Fig. 3B). Bouton diameter quantification shows a non-significant trend towards small boutons in both comparisons (w1118 versus NotumKO, elav-Gal4/+ versus elav>wg), with boutons closely packed and harder to delineate in both NotumKO and Wg overexpression conditions (Fig. 3A, insets). At the ultrastructural level, control NMJs typically show a single bouton embedded in the SSR, whereas mutants usually have several boutons sharing a single SSR (Fig. 3C). Quantification shows a significant increase in boutons per SSR (w1118, 1.19±0.10 versus NotumKO, 2.44±0.39; P=0.0075; Fig. 3D). These results show that Notum coordinates synapse development by negatively regulating Wg signaling.
Notum limits NMJ synaptic functional differentiation and movement output
Structural and functional development occur simultaneously, but are regulated by distinct molecular mechanisms (Menon et al., 2013). Wg trans-synaptic signaling also regulates synaptic functional differentiation, including both neurotransmission strength and activity-dependent processes that modulate total synaptic output (Ataman et al., 2008; Packard et al., 2002). To test whether secreted Notum contributes to NMJ functional development, spontaneous miniature EJC (mEJC) and nerve stimulation-evoked excitatory junction current (EJC) recordings were made using two-electrode voltage-clamp (TEVC) configuration to obtain linear measurements of synaptic function (Dear et al., 2016; Parkinson et al., 2016). To test consequences on behavioral motor output, coordinated movement was assayed in parallel. We used a well-established roll-over test that measures a precisely orchestrated sequence of bilateral muscle contractions mediated by NMJ function (Bodily et al., 2001; Jumbo-Lucioni et al., 2016). To dissect functional mechanisms, activity-dependent live dye imaging was carried out as a measure of synaptic vesicle (SV) cycling. We used physiological motor nerve stimulation to drive FM1-43 lipophilic dye incorporation, as a measure of both SV endocytosis and pool size, and repeat depolarization in the absence of dye to drive release, as a measure of SV exocytosis within boutons and across the synaptic terminal (Parkinson et al., 2013; Vijayakrishnan et al., 2010). Results of these functional studies are displayed in Figs 4 and 5, and described below.
Fig. 4.
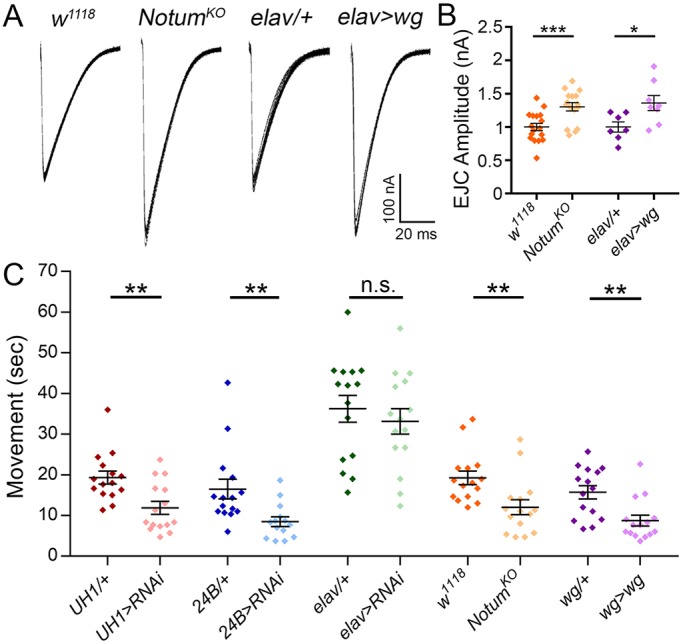
Notum loss strengthens synapse function and improves reaction rate. (A) Representative nerve stimulation-evoked EJC traces (1.0 mM Ca2+) from w1118 background control versus NotumKO null mutant and elav-Gal4/+ transgenic control versus elav>wg overexpression. (B) Quantification of EJC amplitudes in all four genotypes. (C) Coordinated movement rollover reaction time quantification for the denoted ten genotypes. *P≤0.05, **P≤0.01, ***P≤0.001; n.s., not significant.
Fig. 5.
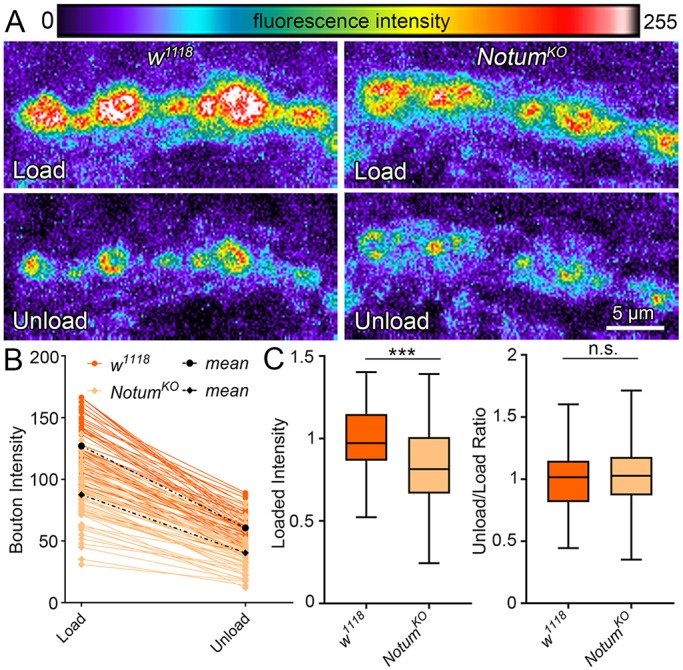
Notum loss alters presynaptic differentiation via vesicle trafficking. (A) Representative synaptic bouton images of FM 1-43 dye imaging with depolarization-induced loading (top) and unloading (bottom) in w1118 control (left) and NotumKO (right). Fluorescent intensity is represented as a heat map. (B) Sample quantification of FM1-43 dye loaded and unloaded bouton fluorescence for all boutons from a single NMJ of each genotype. (C) Box-and-whisker plot quantification of the loaded bouton fluorescence (left) and unload/load ratio (right) for all boutons. ***P≤0.001; n.s. not significant.
Notum negatively regulates NMJ functional differentiation, resulting in elevated neurotransmission strength in NotumKO mutants (Fig. 4A). In quantified measurements, EJC amplitudes are significantly elevated in nulls compared with matched genetic controls (w1118, 1.0±0.06 versus NotumKO, 1.30±0.06; n≥16, P=0.0009; Fig. 4B), with a corresponding increase in mEJC frequency but no change in amplitude (Fig. S5A,B). Elevated function is maintained with high frequency stimulation, with higher quantal content (Fig. S5C,D). Glial repo>Notum:RNAi knockdown causes no changes (Fig. S4C-E), indicating that the requirement is entirely from postsynaptic Notum. As with structure, Notum null functional defects are phenocopied by Wg overexpression (Fig. 4A). In quantified measurements, EJC amplitudes are significantly elevated with Wg overexpression compared with control (elav-Gal4/+, 1.0±0.08 versus elav>wg, 1.36±0.11; n≥7, P=0.024; Fig. 4B). Consequences of elevated NMJ function were tested using the roll-over assay (Movies 1 and 2). Ubiquitous Notum knockdown results in faster movement (UH1-Gal4/+, 19.31±1.57 s versus UH1>Notum:RNAi, 11.87±1.59; n=15, P=0.002), as does muscle-targeted RNAi (24B-Gal4/+, 16.47±2.4 s versus 24B>Notum:RNAi, 8.48±1.17; P=0.007), but no change occurs with neuronal knockdown (P=0.5; Fig. 4C). The glial knockdown is also faster (repo-Gal4/+, 17.83±1.53 s versus repo>Notum:RNAi, 12.93±1.33; P=0.022; Fig. S4F). Notum knockout increases speed (w1118, 19.24±1.63 s versus NotumKO, 12.02±1.82; P=0.006), again phenocopied by Wg overexpression (wg-Gal4/+, 15.69±1.61 s versus wg-Gal4>wg, 8.73±1.32; P=0.002; Fig. 4C). These results show that Notum loss of function (LOF) and Wg GOF similarly augment functional synaptic differentiation and motor output.
Loss of Notum increases both NMJ morphogenesis and functional differentiation (compare Figs 3 and 4), making it difficult to disassociate the structural and functional contributions. Therefore, to independently test functional development on the level of individual synaptic boutons, lipophilic FM1-43 live dye imaging was performed using physiological nerve stimulation to induce SV cycling (Fig. 5). Each synaptic bouton harbors functionally discrete SV pools that participate in active endocytosis-exocytosis turnover cycling. Upon neuronal stimulation, NotumKO mutants clearly and reproducibly load less dye compared with matched controls (Fig. 5A). In quantified measurements, loaded FM1-43 dye intensities per bouton reveal a very highly significant decrease in Notum null boutons relative to matched controls (normalized w1118, 1.0±0.02 versus NotumKO, 0.83±0.06; n≥127 boutons, P=<0.0001; Fig. 5B,C). To study SV release exocytosis, NMJ terminals were depolarized with nerve stimulation a second time in the absence of FM1-43 to drive dye release (Fig. 5A). Both controls and mutants appear comparable in the level of synaptic FM-143 exocytosis. Null Notum boutons load significantly less dye and therefore have less dye to release; however, the unload/load dye ratio in mutants is unchanged compared with matched controls (P=0.55; Fig. 5C, right). These results reveal defects in presynaptic function in the absence of Notum that predict impairments in presynaptic SV organization.
Notum regulates ultrastructural and molecular synaptic assembly
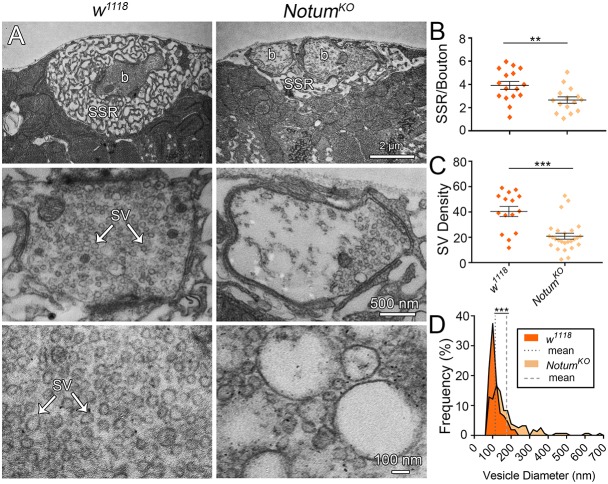
The requirement for Notum for functional synaptogenesis may reflect pre- or postsynaptic roles, or a combination of both. We next tested these mechanistic possibilities with a combination of confocal imaging for synaptic components (Jumbo-Lucioni et al., 2016) and TEM ultrastructure studies (Dear et al., 2016). At the Drosophila NMJ, presynaptic boutons are embedded in an elaborate postsynaptic SSR (Fig. 6A). In Notum mutants, the multiple boutons in a single SSR field are on average reduced in cross-sectional area per bouton (w1118, 5.93±1.23 µm2 versus NotumKO, 3.35±0.51; n≥19 boutons, P=0.028), but if the total bouton areas per SSR are combined, mutants are indistinguishable from controls (w1118, 7.04±1.41 µm2 versus NotumKO, 7.37±1.24; P=0.862). Furthermore, SSR area is obviously reduced in Notum null mutants (Fig. 6A), with the quantified SSR/bouton ratio significantly decreased compared with controls (w1118, 3.91±0.34 versus NotumKO, 2.66±0.28; n≥15 boutons, P=0.009; Fig. 6B). This phenotype is also observed at the confocal level, with a very significant decrease in postsynaptic DLG area (normalized w1118, 1.0±0.08 versus NotumKO, 0.65±0.08; n=16, P=0.005). Within boutons, uniform (40-50 nm) SVs are interspersed with larger vacuoles (>75 nm; Fig. 6A). In Notum null boutons, SVs are very obviously reduced in abundance and more numerous vacuoles are expanded in size (Fig. 6A, right). In quantified measurements, the SV density per synaptic bouton area is greatly decreased in Notum mutants compared with matched controls (w1118, 40.39±3.98 versus NotumKO, 20.87±2.36), a highly significant 50% reduction (n≥15 boutons, P<0.0001; Fig. 6C). Quantification of enlarged vacuole diameter (>75 nm) shows highly significant increases in Notum null mutants (w1118, 113.52±3.15 nm versus NotumKO, 175.10±8.64; P<0.0001; Fig. 6D). These results reveal severely impaired presynaptic and postsynaptic ultrastructural development in the absence of Notum.
Fig. 6.
Ultrastructural synaptic development depends on Notum function. (A) Representative TEM images of w1118 and NotumKO synaptic boutons, with different examples shown at three magnifications; low to include the entire SSR surrounding a bouton (b, top), medium for a presynaptic bouton (middle) and high for better visualization of the synaptic vesicles (SV, bottom). (B) Quantification of SSR/bouton area ratio. (C) Quantification of SV density in NMJ boutons. (D) Frequency distribution of vesicle diameters for both genotypes, with mean diameter indicated by a dotted/dashed line. **P≤0.01, ***P≤0.001.
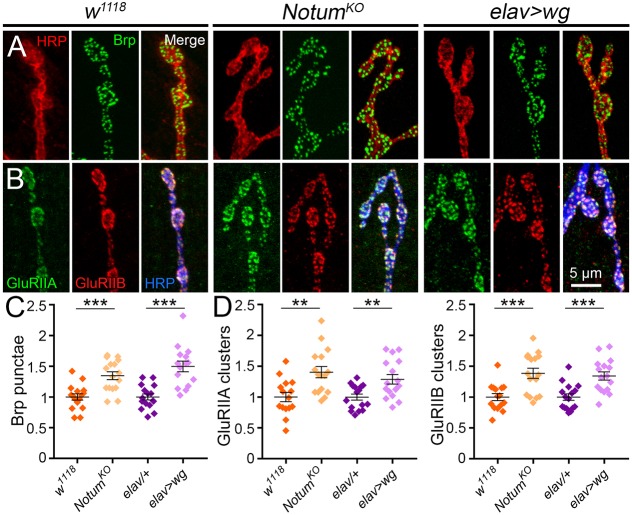
NMJ function depends on the number and composition of postsynaptic glutamate receptors (GluRs) juxtaposing presynaptic Bruchpilot (Brp) active zone release sites (Kittel et al., 2006; Qin et al., 2005; Rasse et al., 2005; Wagh et al., 2006). Brp-positive synapses are elevated in the absence of Notum (Fig. 7A). In quantified measurements, Brp punctae number is very significantly increased in Notum nulls compared with controls (normalized w1118, 1.0±0.05 versus NotumKO, 1.35±0.06; n=16, P=0.0002; Fig. 7C). There are two GluR classes defined by inclusion of either IIA or IIB subunits (Featherstone et al., 2005; Qin et al., 2005). There is a striking increase of both glutamate receptor classes in Notum nulls (Fig. 7B). In quantified measurements, Notum loss results in a highly significant increase in GluRIIA clusters in mutants compared with controls (w1118, 1.0±0.08 versus NotumKO, 1.41±0.09; n≥15, P=0.002; Fig. 7D, left), with a strong increase in overall GluRIIA levels measured by fluorescence intensity (w1118, 1.0±0.05 versus NotumKO, 1.58±0.20; n≥15, P=0.009). Similarly, GluRIIB clusters are increased in Notum nulls (w1118, 1.0±0.06 versus NotumKO, 1.39±0.08; P=0.0006; Fig. 7D, right), although the overall GluRIIB fluorescence intensity is not significantly different (w1118, 1.0±0.06 versus NotumKO, 0.88±0.08; n≥15, P=0.238). Synaptic density (synapse number/bouton) is not changed by loss of Notum (Brp: w1118, 1.0±0.04 versus NotumKO, 0.97±0.05; n≥15, P=0.529; GluRIIA: w1118, 1.0±0.05 versus NotumKO, 0.99±0.04; n≥15, P=0.855; GluRIIB: w1118, 1.0±0.03 versus NotumKO, 0.97±0.04; n≥15, P=0.532). Wg GOF phenocopies pre- and postsynaptic changes (Fig. 7A,B). In quantified measurements, Brp punctae are significantly increased with Wg GOF compared with controls (elav-Gal4/+, 1.0±0.05 versus elav>wg, 1.50±0.09; n≥15, P<0.0001; Fig. 7C). Similarly, both GluR classes are elevated by Wg overexpression, including GluRIIA clusters (elav-Gal4/+, 1.0±0.05 versus elav>wg, 1.29±0.08; n=16, P=0.0041) and GluRIIB clusters (elav-Gal4/+, 1.0±0.05 versus elav>wg, 1.34±0.07; P=0.0004; Fig. 7D). These results indicate Notum restricts synaptic molecular development by limiting Wg trans-synaptic signaling.
Fig. 7.
Notum limits both pre- and postsynaptic molecular assembly. (A) Representative NMJ images co-labeled for HRP (red) and presynaptic anti-Bruchpilot (Brp, green) in w1118, NotumKO and elav>wg. (B) Synaptic boutons co-labeled for HRP (blue), GluRIIA (green) and GluRIIB (red) receptor classes in w1118, NotumKO and elav>wg. (C) Quantification of presynaptic Brp punctae in the genotypes shown, with inclusion of the elav/+ control. (D) Quantification of postsynaptic GluRIIA/GluRIIB in the genotypes shown. **P≤0.01, ***P≤0.001.
Restoring Wg levels in Notum mutants suppresses synaptogenic phenotypes
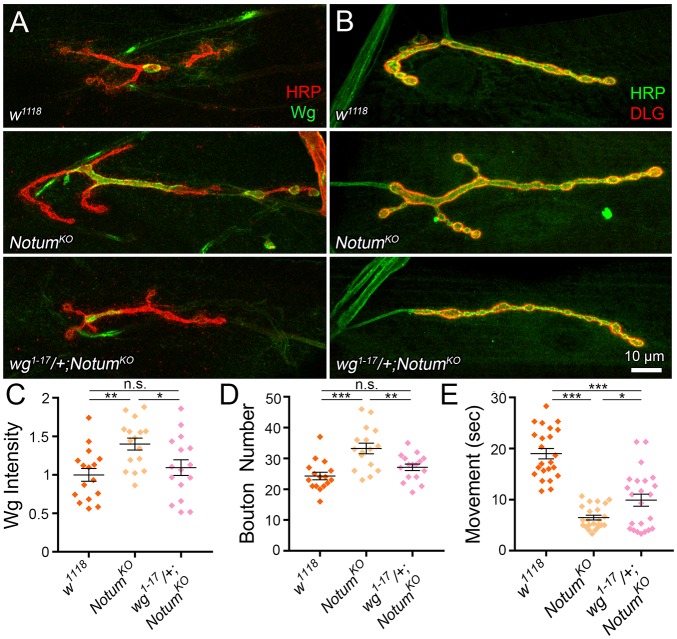
Based on all the studies described above, our working hypothesis is that the Notum mutant synaptogenic phenotypes arise from a lack of extracellular Wg inhibition, allowing for run-away Wg trans-synaptic signaling in the absence of Notum function. The prediction of this hypothesis is that reducing Wg levels towards normal in the Notum background should suppress the synaptogenic phenotypes. To test this prediction, we combined a heterozygous wg null (wg1-17/+; also called wgCX4; Baker, 1987) with the homozygous Notum null to generate the final genotype of wg1-17/+; NotumKO/NotumKO (Fig. 8). We first tested whether this double mutant genetically restores Wg expression. At the NMJ, Wg is normally expressed in a dynamic manner, with highly elevated extracellular ligand levels at a subset of synaptic boutons (Fig. 8A). Null Notum mutants show clearly elevated Wg expression, with a higher intensity and expanded spatial distribution of the secreted signal. In contrast, wg1-17/+; NotumKO/NotumKO synapses exhibit Wg levels that are indistinguishable from controls, with a similar level and pattern of extracellular release (Fig. 8A). In quantified measurements, Notum nulls show significantly elevated synaptic Wg intensity compared with matched controls (normalized w1118, 1.0±0.08 versus NotumKO, 1.4±0.08; n=16, P=0.0069), and removing one copy of wg significantly decreases Wg levels (wg1-17/+; NotumKO/NotumKO, 1.1±0.10; P=0.0474 compared with NotumKO; Fig. 8C). In the double mutants, there is no significant difference remaining in Wg levels at the synapse compared with w1118 (P=0.7325).
Fig. 8.
Restoring normal synaptic Wg levels alleviates Notum null phenotypes. (A) Representative NMJ images co-labeled for HRP (red) and Wg (green) in w1118, NotumKO and the mutant with removal of one copy of wg (wg1-17/+; NotumKO/NotumKO). (B) The same genotypes co-labeled for HRP (green) and DLG (red). (C) Quantification of extracellular Wg ligand level (C) and bouton number (D) in above three genotypes. (E) Coordinated movement rollover reaction time quantification for the three genotypes. *P≤0.05, **P≤0.01, ***P≤0.001; n.s., not significant.
We hypothesized that correcting Wg trans-synaptic signaling in Notum mutants should alleviate defects in synaptic terminal development. To test this hypothesis, we compared NMJ architecture between the genetic controls, Notum nulls and null mutants with restored normal Wg levels (Fig. 8B). Control NMJs display few branches and a highly consistent number of evenly spaced synaptic boutons, whereas Notum mutants are characterized by rampant synaptogenesis with larger terminals, more branching and elevated, more variable synaptic bouton formation (Fig. 8B). In sharp contrast to the null condition, the wg1-17/+; NotumKO/NotumKO synapses exhibit synaptic growth and architecture that is indistinguishable from normal, with a reduction in the terminal area, loss of the excess branching and correction of the supernumerary bouton levels that characterize the Notum mutants (Fig. 8B). In quantified measurements, the Notum nulls show highly significantly elevated synaptic bouton number compared with their matched controls (w1118, 24.31±1.22 versus NotumKO, 33.25±1.74; n=16, P<0.0001), whereas removing one copy of wg significantly decreases bouton number completely back to the control levels (wg1-17/+; NotumKO/NotumKO, 27.13±1.07; P=0.0078 compared with NotumKO; Fig. 8D). In the double mutants with corrected synaptic Wg levels, there is no significant difference remaining in NMJ synaptogenesis compared with genetic controls (P=0.324 compared with w1118 background control). This genetic correction demonstrates that Notum defects in synaptogenesis are due to elevated Wg signaling.
We hypothesized that correcting Wg trans-synaptic signaling in Notum mutants should alleviate the strengthening of neuromuscular function driving faster coordinated movement response times. To test this hypothesis, we tested motor function using the behavioral roll-over assay (Bodily et al., 2001; Jumbo-Lucioni et al., 2016). Control animals exhibit an orchestrated series of bilateral muscle contractions that enable rapid righting behavior, but Notum null mutants are remarkably proficient in this response and show an obvious improvement in coordinated movement time (Fig. 8E). In contrast, deletion of one wg copy from these mutants clearly impairs performance. In quantified measurements, Notum mutants show significantly faster roll-over times compared with matched controls (w1118, 19.0±1.02 s versus NotumKO, 6.49±0.46; n=23, P<0.0001), and correcting Wg levels significantly impairs this response time back towards control levels (wg1-17/+; NotumKO/NotumKO, 9.91±1.18; P=0.032 compared with NotumKO; Fig. 8E). This correction is significant albeit only partial, and a significant difference remains compared with genetic controls (P<0.0001 compared with w1118). This likely reflects the possibility that Notum-Wg interactions happen throughout the nervous system to modulate behavioral output, and may well involve other Wnt signaling interactions in addition to Wg. Taken together, the results of this study strongly support the conclusion that secreted Notum functions to restrict Wg ligand levels in the extracellular synaptomatrix and limit Wg trans-synaptic signaling, and that this function in turn puts a brake on structural and functional synaptogenesis to impede coordinated movement response times.
DISCUSSION
Tightly coordinated trans-synaptic signals are required for proper development of the pre- and postsynaptic apparatus to ensure efficient communication at the synapse. This signaling is both coordinated and controlled in the extracellular space through the actions of secreted and transmembrane glycans, HSPG co-receptors and secreted enzymes, such as matrix metalloproteinase classes (Dear et al., 2016; Jumbo-Lucioni et al., 2016; Parkinson et al., 2016). Wg (Wnt-1) mediates a crucial trans-synaptic signaling pathway regulated by these extracellular synaptic mechanisms, with key roles in both structural and functional synaptogenesis (Ataman et al., 2008; Mathew et al., 2005; Miech et al., 2008; Packard et al., 2002; Speese et al., 2012). Here, we propose Notum as a novel extracellular regulator limiting Wg trans-synaptic signaling to control NMJ synaptogenesis. Wg is post-translationally modified by addition of palmitoleate on a conserved serine (S239) by membrane-bound O-acyltransferase (MBOAT) Porcupine (Kadowaki et al., 1996; Zhai et al., 2004). This lipidation event is required for Fz2 receptor binding and is essential for signaling (Janda et al., 2012). At the synaptic interface, the glycosylphosphatidylinositol (GPI)-anchored glypican Dally-like Protein (Dlp) regulates Wg trans-synaptic signaling (Dani and Broadie, 2012; Kreuger et al., 2004; Lin and Perrimon, 1999; Yan et al., 2009), and Notum was initially described as cleaving such GPI-anchored glypicans from the cell surface (Traister et al., 2008), affecting their ability to interact with the Wg ligand. However, Notum was recently re-defined as a secreted carboxylesterase, not a phospholipase, with structural studies showing a hydrophobic pocket that binds and then cleaves palmitoleate (Kakugawa et al., 2015).
Notum is consistently reported to act primarily as an extracellular Wg feedback inhibitor (Giráldez et al., 2002; Kakugawa et al., 2015). Our studies support this function within the synaptomatrix during synaptogenesis. At the Drosophila NMJ, Wg is secreted from both presynaptic neurons (Packard et al., 2002) and associated peripheral glia (Kerr et al., 2014), with the glial function specifically regulating synaptic transmission strength and postsynaptic glutamate receptor clustering. Our analyses suggest that Notum is secreted from both postsynaptic muscle and peripheral glia, establishing a dynamic, Wg-like expression pattern surrounding synaptic boutons (Dani et al., 2012). In Notum null mutants, Wg signaling is increased at the developing NMJ, revealed by both decreased Fz2 receptor in the synaptic membrane (Wg-driven endocytosis) and an increase in nuclear Fz2-C punctae (FNI pathway). These findings are consistent with Notum function limiting Wg signaling, as established in other developmental contexts (Kakugawa et al., 2015). Notum appears to provide a fascinating directional regulation of Wg trans-synaptic signaling, affecting the anterograde FNI signaling pathway in muscles (Mathew et al., 2005), but not the autocrine divergent canonical pathway in neurons (Miech et al., 2008). Despite the strong elevation in synaptic Wg ligand levels in Notum null mutants, we see no evidence of altered presynaptic Futsch or changes in the microtubule cytoskeleton. However, Notum strongly limits Fz2 C-terminus nuclear import into the postsynaptic nuclei, which is known to drive RNP translational regulation of synaptic mRNAs to control synapse structural and functional differentiation (Speese et al., 2012).
Synaptic morphogenesis and architectural development is strongly perturbed in Notum null mutants, including increased NMJ area, branching and bouton formation, consistent with Notum function inhibiting Wg trans-synaptic signaling (Packard et al., 2002). Elevating presynaptic Wg closely phenocopies Notum synaptic defects, including expanded innervation area, more branching and supernumerary synaptic boutons. Our results show that Notum secreted from muscle and peripheral glia controls Wg in the extracellular space, with targeted Notum RNAi resulting in a similar NMJ expansion to Notum nulls, whereas neuronal Notum knockdown produces no effects. Interestingly, the glial-targeted RNAi increases boutons with no change in branching, whereas muscle knockdown has a stronger impact also affecting branching. Presynaptic Futsch/Map1B microtubule loops have been proposed to mediate Wg-dependent branching and bouton formation (Nahm et al., 2013; Roos et al., 2000; Wang et al., 2007). However, neuronal Wg overexpression has no discernable effect on Futsch-positive microtubule loops. Consistently, Notum LOF also does not impact this pathway, with Notum mutants displaying no change in Futsch-labeled looped, bundled, punctate or splayed microtubules (Jumbo-Lucioni et al., 2016; Packard et al., 2002). Wg binding to the presynaptic Fz2 receptor might activate another divergent Wnt pathway that does not involve Futsch (Menon et al., 2013). Alternatively, Wg signaling via muscle Fz2 may produce a retrograde signal back to the neuron to alter presynaptic development. To test these two possibilities, future studies will employ cell-targeted Fz2 knockdown in Notum nulls to assay for suppression of the synaptic overgrowth phenotypes.
Measures of functional synaptic differentiation reveal elevated neurotransmission and faster motor output function with both Notum knockout and Wg overexpression. These results are consistent with Notum function inhibiting Wg trans-synaptic signaling, and consistent with previously characterized roles of Wg in NMJ functional development (Packard et al., 2002). Notum LOF increases presynaptic function selectively with an elevated mEJC frequency, greater EJC quantal content and heightened synaptic vesicle release during maintained high-frequency stimulation. Some of these effects might be related to to the increased synaptic bouton numbers. Both Notum LOF and Wg GOF also cause NMJ boutons to clump together, with ultrastructural studies showing multiple boutons sharing one SSR profile. These are not satellite boutons (Ashley et al., 2005), but rather aberrantly developing boutons that could result in functional defects. Notum knockdown in glia does not cause detectable mEJC/EJC changes, although Wg from glia regulates NMJ functional properties (Kerr et al., 2014). Interestingly, loss of Notum appears to improve motor performance, and repo-targeted Notum RNAi shows that glial Notum contributes to this function. This is an unusual outcome in a mutant condition, and we assume there must be a counter-balancing cost for the increased neuromuscular function. Live FM dye imaging reveals that Notum mutants load less dye into synaptic boutons upon nerve stimulation, indicating a role in synaptic vesicle endocytosis and/or the developmental regulation of synaptic vesicle pool size (Verstreken et al., 2008). These results show that Notum function limits Wg trans-synaptic signaling to control presynaptic differentiation that is crucial for synapse function and motor output. As with Wg (Kerr et al., 2014), the source of Notum (muscle versus glia) appears to be important for distinct synaptogenic functions. Notum from peripheral glia regulates only bouton formation, whereas Notum from muscle regulates both NMJ growth and function.
Electron microscopy reveals a very strong decrease in synaptic vesicle density in Notum null boutons, providing an explanation for the live FM1-43 dye imaging defects. One of the most striking ultrastructural phenotypes is numerous, enlarged synaptic vesicular bodies. These organelles are highly reminiscent of bulk endosomes, in which a large area of presynaptic membrane is internalized, and will subsequently bud off synaptic vesicles (Clayton and Cousin, 2009). This pathway is usually driven by intense stimulation during activity-dependent bulk endocytosis (ADBE), as first observed at the frog NMJ (Miller and Heuser, 1984). This pathway is induced by high frequency trains of stimulation (Clayton et al., 2008), and several proteins have been identified that affect the formation of bulk endosomes, including Syndapin (Clayton et al., 2009) and Rolling Blackout (RBO; also known as Stambha A) (Vijayakrishnan et al., 2009). At the Drosophila NMJ, conditional rbots mutants block ADBE, reducing the number and size of bulk endosomes (Vijayakrishnan et al., 2009). It will be interesting to test Wg GOF for enlarged endosomal structures, and study their involvement in Wg-dependent synaptic maturation. On the postsynaptic side, Notum also drives proper differentiation. Notum LOF reduces the postsynaptic DLG scaffold and postsynaptic SSR layering. The reduced SSR area in Notum mutants is surprising, given that a reduction in postsynaptic Wg signaling also results in fewer SSR layers (Packard et al., 2002; Kamimura et al., 2013). However, to our knowledge, SSR architecture has not been studied following Wg overexpression. Postsynaptic SSR formation might be sensitive to bidirectional Wg changes, and may be reduced if Wg is tipped in either direction.
Mechanistically, Notum controls both pre- and postsynaptic molecular assembly, with LOF defects phenocopied by Wg overexpression. The results are consistent with Notum function inhibiting Wg trans-synaptic signaling, and consistent with previously characterized roles for Wg in synaptic molecular development (Packard et al., 2002). We analyzed both the presynaptic active zone protein Bruchpilot (Wagh et al., 2006; Menon et al., 2013) and the two postsynaptic GluR classes (Featherstone et al., 2005; Qin et al., 2005). Both presynaptic Brp and postsynaptic GluRs are misregulated in Notum nulls, with an increase in synapse number but not density. Importantly, both Notum LOF and Wg GOF elevate synapse number. Consistently, Wnt7a overexpression in mouse cerebellar cells also increases the number of synaptic sites and causes accumulation of presynaptic proteins required for synaptic vesicle function (Hall et al., 2000). The increased synapse density per NMJ might compensate for reduced neurotransmission per bouton, leading to a net stronger overall NMJ function. In Notum mutants, this could reconcile the elevated synaptic strength measured by electrophysiology compared with compromised single bouton function measured by FM dye imaging and impaired TEM ultrastructure. In any case, synaptic assembly during development is regulated by Notum function limiting Wg trans-synaptic signaling.
Genetically reducing Wg by combining a heterozygous wg null mutation into the homozygous Notum null background reduces extracellular synaptic Wg back to control levels. Wg reduction suppresses synaptogenic defects, restoring increased NMJ area, branching and bouton numbers completely back to normal. Both NotumKO and Wg GOF cause hyperactive movement, with roll-over speeds supporting synaptogenic defects of larger, stronger NMJs in both mutant conditions. However, NotumKO motor function is only partially restored by correcting Wg levels. One explanation for incomplete rescue is that multiple Wnts may contribute to motor behavior. Serine lipidation is conserved for all Wnts, and we know at least two other Wnts act at the Drosophila NMJ (Wnt2, Wnt5; Liebl et al., 2010; Liebl et al., 2008). Wnts are the only secreted ligands suggested to be O-palmitoleated on a serine to function as Notum substrates (Kakugawa et al., 2015). In future studies, we will test contributions of other Wnts. There is growing evidence that Wnts function in activity-dependent mechanisms at both mammalian and Drosophila synapses (Gogolla et al., 2009; Ataman et al., 2008). Our future studies will investigate these mechanisms, exploring both muscle and peripheral glial-derived Notum. We will also study how Notum interacts with other extracellular Wg regulators at the synaptic interface. Secreted and membrane-tethered HSPGs play key roles in Wg regulation at the Drosophila NMJ (Kamimura et al., 2013; Dear et al., 2016), and Notum deacylates Wg in a HSPG-assisted mechanism (Kakugawa et al., 2015). For example, both Wg and Notum bind HSPG Dlp, which could serve as a signaling platform to colocalize them in the synaptic cleft. Our future work will dissect spatial and contextual functions of Notum regulation of Wg signaling at the developing synapse.
MATERIALS AND METHODS
Drosophila stocks
All Drosophila stocks were reared on standard cornmeal/agar/molasses food at 25°C. The genetic background control for all studies was w1118. Null mutant w1118; NotumKO (4)(w+)/TM6b animals, Notum-HA(23)/TM6 and UAS-Notum-V5/Cyo lines were obtained from Jean-Paul Vincent (Francis Crick Institute, London, UK) (Kakugawa et al., 2015). Transgenic studies were performed with neuronal elav-Gal4, glial repo-Gal4, muscle-specific 24B-Gal4 and ubiquitous UH1-Gal4 driver lines (Bloomington Drosophila Stock Center, Indiana University, Bloomington, IN, USA) crossed to characterized UAS-Notum-RNAi lines (#35650 and #55379 from the Harvard Transgenic RNAi Project; TRiP) (Perkins et al., 2015). For Wg studies, overexpression experiments were performed with UAS-wg::GFP (Pfeiffer et al., 2002), and suppression experiments were performed with wg1-17 null mutant (aka wgCX4; Baker, 1987) comparing wg1-17/+ with wg1-17/+; NotumKO/NotumKO.
Generation of Notum-HA line
A C-terminal HA-tagged knock-in version of Notum was generated by CRISPR/Cas9 genome editing as described (Gokcezade et al., 2014). The gRNA target (overlapping with the Notum stop codon) was cloned into the pDCC6 vector. This vector was then co-injected into w1118 embryos with the following ssODN:
CACACGCTCAACAACATGGAGCGCACCGAGTTGGTCAACATGCTCACCCAGCAGGCTAACTACCCATACGACGTCCCTGACTATGCGgggTATCCGTATGATGTGCCAGATTACGCCTAGGCTCACCAAATACCCTGTACCCTTTTGGGGGGATCCGAAAGTGGGCATGGAAATCGT. The two HA epitope tags are indicated in italics and the stop codon is indicated in bold.
Behavioral assays
Coordinated movement assays were conducted using the ‘rollover assay’ as previously reported (Bodily et al., 2001; Jumbo-Lucioni et al., 2016). Using forceps, an individual wandering third instar was placed on a 100×15 mm plate coated with 1% agarose and allowed 30 s to acclimate at 20°C. Using a fine paintbrush, the larva was rolled over so that the ventral midline was exactly upwards (t=0). A stopwatch was used to record righting time, when the dorsal midline was exactly upwards. The assay was repeated three times on the same animal, and the three times were averaged for a single data point.
Immunocytochemistry
Wandering third instars were dissected in physiological solution, fixed and permeabilized with 0.2% Triton X-100 (three times for 10 min each), except for extracellular labeling. Primary antibodies used included: rabbit anti-HRP, mouse anti-DLG, rabbit anti-HA, mouse anti-Wg, rabbit anti-Fz2-C, mouse anti-GluRIIA, rabbit anti-GluRIIB, mouse anti-Brp, mouse anti-Futsch, goat anti-GFP, Cy3-conjugated goat anti-HRP, and Cy5-conjugated goat anti-HRP. Further antibody details are included in Table S1. The fluorescent probe DRAQ5 was used for nuclear staining (1:1000; Invitrogen, 62254). The lectin Vicia Villosa (VVA-TRITC) was used as an NMJ marker (1:200; EY Laboratories, R-4601-2). HA immunoreactivity was visualized using a tyramide signal amplification kit (TSA, Sigma T20911) using HRP-conjugated goat anti-rabbit (1:200; Invitrogen, 31460), biotinylated tyramide and streptavidin-488 (1:200, Invitrogen, S-11223) (Bogdanik et al., 2004). Preparations were processed with primary antibodies overnight at 4°C and secondary antibodies for 2 h at room temperature (RT), washed three times for 10 min each, and mounted in Fluoromount G (Electron Microscopy Sciences).
Confocal imaging
All imaging was performed on a Zeiss LSM 510 META laser-scanning confocal microscope, with images projected in Zen (Zeiss) and analyzed using ImageJ (NIH open source). NMJ area and intensity measurements were made with HRP signal delineated z-stack areas of maximum projection using the threshold and wand-tracing tools within ImageJ. Synaptic boutons were defined as HRP- and DLG-positive varicosities >2 µm. Branches were defined as axonal processes with at least two boutons. Brp and GluR punctae were counted using the multi-point tool within ImageJ. Fz2-C intensity measurements were made with DRAQ5 signal delineated z-stack nuclei with maximum projections.
Western blotting
Western blots from wandering third instars were performed with standard procedures (Gagliardi et al., 2014). Eight larvae were dissected in ice-cold PBS, with the ventral nerve cord (VNC) separated from the body musculature. Both tissues were transferred independently to RIPA buffer [10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl, 1 mM PMSF]. The equivalent of two VNCs and two body musculatures were loaded separately onto the same 4-12% gel and probed with anti-HA.11 (1:2000, Covance).
Electrophysiology
Wandering third instars were dissected longitudinally along the dorsal midline, internal organs removed, and the body walls glued down (Vetbond by 3 M). Peripheral motor nerves were cut at the base of the VNC. Dissections and recordings were carried out at 18°C in physiological saline (in mM): 128 NaCl, 2 KCl, 4 MgCl2, 1.0 CaCl2, 70 sucrose and 5 HEPES {2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid; pH 7.2}. Preparations were imaged with a Zeiss Axioskop microscope using 40× water-immersion objectives. Muscle 6 in abdominal segments 2/3 was impaled with two intracellular microelectrodes (1-mm outer diameter borosilicate capillaries; World Precision Instruments) of ∼15 MΩ resistance filled with 3 M KCl. Muscles were clamped at −60 mV using an Axoclamp-2B amplifier. Spontaneous mEJC recordings were made in 2 min sessions and low-pass filtered. For EJC records, motor nerves were sucked into a fire-polished suction electrode and stimulated using 0.5 ms suprathreshold voltage stimuli at 0.2 Hz from a Grass S88 stimulator. Nerve stimulation-evoked EJC recordings were filtered at 2 kHz. To quantify EJCs, ten consecutive traces were averaged and the average peak value recorded. Clampex 9.0 was used for data acquisition and Clampfit 9 was used for data analysis.
FM imaging
FM1-43 (4 μM; Invitrogen) was added in 1 mM Ca2+ physiological saline (see above). The motor nerve was stimulated with a suction electrode (20 Hz, 1 min), and then the bath saline was replaced several times in quick succession with Ca2+-free saline to halt SV cycling. z-stacks of stimulated (loaded) NMJs were taken with the Zeiss LSM 510 confocal microscope using 40× water immersion objectives. Ca2+-free saline was replaced with 1 mM Ca2+ saline (without FM1-43), and the same motor nerve stimulated (20 Hz, 20 s) for SV exocytosis and dye release. The saline was replaced several times in quick succession with Ca2+-free saline to halt SV cycling. z-stacks of unloaded NMJs were then taken. Images were quantified by outlining individual boutons using the ImageJ elliptical selections tool and measuring fluorescence values in loaded and unloaded conditions. The ratio of unloaded/loaded intensities was calculated in Microsoft Excel (2013). Images for display were exported to Adobe Photoshop.
Electron microscopy
Wandering third instars were dissected and fixed overnight at 4°C in 2.5% glutaraldehyde, followed by secondary fixation in 1% osmium tetroxide for 1 h at RT. Preparations were washed in 0.1 M sodium cacodylate buffer three times (10 min each), followed by ddH2O three times (15 min each). En bloc uranyl acetate staining was carried out using 2% uranyl acetate (2 h at RT, dark). Preparations were rinsed in ddH2O three times (15 min each), followed by an ethanol dehydration series (30, 50, 70, 90, 95, 100, 100%), propylene oxide infiltration and resin embedding (Embed-812). Body wall muscles 6/7 from abdominal segments 2/3 were dissected free and embedded into a semi-hardened resin block. The muscles from four animals were put in each block, with three blocks made per genotype. Blocks were polymerized at 60°C for 48 h. Thick (1 µm) sections were cut using a glass knife, stained with Toluidine Blue for 1 min on a Thermostat slide warmer (45°C) and imaged on a compound microscope at 100× for bouton identification. Once a bouton was found, ultrathin (50 nm) sections were cut using a DiATOME diamond knife on a Leica Ultracut UCT ultramicrotome and then collected on uncoated 200 mesh copper grids. All TEM imaging was performed on a Philips CM10 transmission electron microscope operating at 80 kV, with images collected using a 4 megapixel AMT CCD camera. Bouton area, SSR area, SV number and SV size were measured in ImageJ. Single bouton profiles that were ≤2 µm2 were not considered in the quantification.
Statistical measurements
Statistical comparisons were performed using GraphPad Prism software (Version 7.0 for Windows). Student's t-tests were used for pair-wise comparisons and one-way ANOVAs for data sets of three or more comparisons, followed by a post hoc Tukey's multiple comparisons test. Fisher's exact tests were used with discrete data using R statistical software (Version 3.2.5). Graphs show mean±s.e.m. made with Prism, with significance displayed as *P≤0.05, **P≤0.01, ***P≤0.001 and P>0.05 (not significant, n.s.). Sample sizes reported in the text (n) indicate the number of NMJs, unless otherwise stated.
Acknowledgements
We thank the Bloomington Drosophila Stock Center (Indiana University, Bloomington, IN, USA) and Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA, USA) for genetic lines and antibodies, respectively. We are most especially grateful to Jean-Paul Vincent (The Francis Crick Institute, London, UK) for Notum and Wingless lines, and Andrew Tomlinson (Columbia University, New York, NY, USA) for the Notum-Gal4 (S168). We also thank Vivian Budnik (University of Massachusetts Medical School, Worcester, MA, USA) for the Fz2-C antibody, and Aaron DiAntonio (Washington University, St. Louis, MO, USA) and David Featherstone (University of Illinois, Chicago, IL, USA) for the GluRIIB antibody. We especially thank Emma Rushton for expert assistance with genetics, and other members of the Broadie laboratory for their valuable input on this work.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: D.L.K., K.B.; Methodology: D.L.K., S.C.L., C.A., K.B.; Validation: D.L.K., S.C.L., C.A.; Formal analysis: D.L.K.; Investigation: D.L.K., S.C.L., C.A.; Resources: D.L.K., C.A., K.B.; Writing - original draft: D.L.K.; Writing - review & editing: D.L.K., S.C.L., C.A., K.B.; Visualization: D.L.K., K.B.; Supervision: D.L.K., K.B.; Project administration: D.L.K., K.B.; Funding acquisition: K.B.
Funding
This work was fully supported by a National Institutes of Health grant (MH096832 to K.B.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.148130.supplemental
References
- Ashley J., Packard M., Ataman B. and Budnik V. (2005). Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/mint. J. Neurosci. 25, 5943-5955. 10.1523/JNEUROSCI.1144-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman B., Ashley J., Gorczyca M., Ramachandran P., Fouquet W., Sigrist S. J. and Budnik V. (2008). Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron 57, 705-718. 10.1016/j.neuron.2008.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. (1987). Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 6, 1765-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodily K. D., Morrison C. M., Renden R. B. and Broadie K. (2001). A novel member of the Ig superfamily, turtle, is a CNS-specific protein required for coordinated motor control. J. Neurosci. 21, 3113-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanik L., Mohrmann R., Ramaekers A., Bockaert J., Grau Y., Broadie K. and Parmentier M.-L. (2004). The Drosophila metabotropic glutamate receptor DmGluRA regulates activity-dependent synaptic facilitation and fine synaptic morphology. J. Neurosci. 24, 9105-9116. 10.1523/JNEUROSCI.2724-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E. L. and Cousin M. A. (2009). The molecular physiology of activity-dependent bulk endocytosis of synaptic vesicles. J. Neurochem. 111, 901-914. 10.1111/j.1471-4159.2009.06384.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E. L., Evans G. J. O. and Cousin M. A. (2008). Bulk synaptic vesicle endocytosis is rapidly triggered during strong stimulation. J. Neurosci. 28, 6627-6632. 10.1523/JNEUROSCI.1445-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E. L., Anggono V., Smillie K. J., Chau N., Robinson P. J. and Cousin M. A. (2009). The phospho-dependent dynamin-syndapin interaction triggers activity-dependent bulk endocytosis of synaptic vesicles. J. Neurosci. 29, 7706-7717. 10.1523/JNEUROSCI.1976-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani N. and Broadie K. (2012). Glycosylated synaptomatrix regulation of trans-synaptic signaling. Dev. Neurobiol. 72, 2-21. 10.1002/dneu.20891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani N., Nahm M., Lee S. and Broadie K. (2012). A targeted glycan-related gene screen reveals heparan sulfate proteoglycan sulfation regulates WNT and BMP trans-synaptic signaling. PLoS Genet. 8, e1003031 10.1371/journal.pgen.1003031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dear M. L., Dani N., Parkinson W., Zhou S. and Broadie K. (2016). Two classes of matrix metalloproteinases reciprocally regulate synaptogenesis. Development 143, 75-87. 10.1242/dev.124461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone D. E., Rushton E., Rohrbough J., Liebl F., Karr J., Sheng Q., Rodesch C. K. and Broadie K. (2005). An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction. J. Neurosci. 25, 3199-3208. 10.1523/JNEUROSCI.4201-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. H., Dani N., Rushton E. and Broadie K. (2013). Fragile X mental retardation protein regulates trans-synaptic signaling in Drosophila. Dis. Model Mech. 6, 1400-1413. 10.1242/dmm.012229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi M., Hernandez A., McGough I. J. and Vincent J. P. (2014). Inhibitors of endocytosis prevent Wnt/Wingless signalling by reducing the level of basal β-catenin/Armadillo. J. Cell Sci. 127, 4918-4926. 10.1242/jcs.155424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlitz O. and Basler K. (2002). Wingful, an extracellular feedback inhibitor of Wingless. Genes Dev. 16, 1055-1059. 10.1101/gad.991802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giráldez A. J., Copley R. R. and Cohen S. M. (2002). HSPG modification by the secreted enzyme notum shapes the wingless morphogen gradient. Dev. Cell 2, 667-676. 10.1016/S1534-5807(02)00180-6 [DOI] [PubMed] [Google Scholar]
- Gogolla N., Galimberti I., Deguchi Y. and Caroni P. (2009). Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivites in the adult hippocampus. Neuron 62, 510-525. 10.1016/j.neuron.2009.04.022 [DOI] [PubMed] [Google Scholar]
- Gokcezade J., Sienski G. and Duchek P. (2014). Efficient CRISPR/Cas9 plasmids for rapid and versatile genome editing in Drosophila. G3 (Bethesda) 4, 2279-2282. 10.1534/g3.114.014126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. C., Lucas F. R. and Salinas P. C. (2000). Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100, 525-535. 10.1016/S0092-8674(00)80689-3 [DOI] [PubMed] [Google Scholar]
- Harris K. P. and Littleton J. T. (2015). Transmission, development, and plasticity of synapses. Genetics 201, 345-375. 10.1534/genetics.115.176529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y. and Jan Y. N. (1976). L-Glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J. Physiol. 262, 215-236. 10.1113/jphysiol.1976.sp011593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda C. Y., Waghray D., Levin A. M., Thomas C. and Garcia K. C. (2012). Structural basis of Wnt recognition by frizzled. Science 337, 59-64. 10.1126/science.1222879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumbo-Lucioni P., Parkinson W. and Broadie K. (2014). Overelaborated synaptic architecture and reduced synaptomatrix glycosylation in a Drosophila classic galactosemia disease model. Dis. Model Mech. 7, 1365-1378. 10.1242/dmm.017137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumbo-Lucioni P. P., Parkinson W. M., Kopke D. L. and Broadie K. (2016). Coordinated movement, neuromuscular synaptogenesis and trans-synaptic signaling defects in Drosophila galactosemia models. Hum. Molec. Genet. 25, 3699-3714. 10.1093/hmg/ddw217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T., Wilder E., Klingensmith J., Zachary K. and Perrimon N. (1996). The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 10, 3116-3128. 10.1101/gad.10.24.3116 [DOI] [PubMed] [Google Scholar]
- Kakugawa S., Langton P. F., Zebisch M., Howell S. A., Chang T.-H., Liu Y., Feizl T., Bineva G., O'Reilly N., Snijders A. P. et al. (2015). Notum deacylates Wnt proteins to suppress signalling activity. Nature 519, 187-192. 10.1038/nature14259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura K., Ueno K., Nakagawa J., Hamada R., Saitoe M. and Maeda N. (2013). Perlecan regulates bidirectional Wnt signaling at the Drosophila neuromuscular junction. J. Cell Biol. 200, 219-233. 10.1083/jcb.201207036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr K. S., Fuentes-Medel Y., Brewer C., Barria R., Ashley J., Abruzzi K. C., Sheehan A., Tasdemir-Yilmaz O. E., Freeman M. R. and Budnik V. (2014). Glial Wingless/Wnt regulates glutamate receptor clustering and synaptic physiology at the Drosophila neuromuscular junction. J. Neurosci. 34, 2910-2920. 10.1523/JNEUROSCI.3714-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshishian H., Broadie K., Chiba A. and Bate M. (1996). The Drosophila neuromuscular junction: a model system for studying synaptic development and function. Annu. Rev. Neurosci. 19, 545-575. 10.1146/annurev.ne.19.030196.002553 [DOI] [PubMed] [Google Scholar]
- Kittel R. J., Wichmann C., Rasse T. M., Fouquet W., Schmidt M., Schmid A., Wagh D. A., Pawlu C., Kellner R. R., Willig K. I. et al. (2006). Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science 312, 1051-1054. 10.1126/science.1126308 [DOI] [PubMed] [Google Scholar]
- Kreuger J., Perez L., Giraldez A. J. and Cohen S. M. (2004). Opposing activities of dally-like glypican at high and low levels of wingless morphogen activity. Dev. Cell 7, 503-512. 10.1016/j.devcel.2004.08.005 [DOI] [PubMed] [Google Scholar]
- Langton P. F., Kakugawa S. and Vincent J.-P. (2016). Making, exporting, and modulating Wnts. Trends Cell Biol. 26, 756-765. 10.1016/j.tcb.2016.05.011 [DOI] [PubMed] [Google Scholar]
- Liebl F. L. W., Wu Y., Featherstone D. E., Noordermeer J. N., Fradkin L. and Hing H. (2008). Derailed regulates development of the Drosophila neuromuscular junction. Dev. Neurobiol. 68, 152-165. 10.1002/dneu.20562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl F. L. W., McKeown C., Yao Y. and Hing H. K. (2010). Mutations in Wnt2 alter presynaptic motor neuron morphology and presynaptic protein localization at the Drosophila neuromuscular junction. PLoS ONE 5, e12778 10.1371/journal.pone.0012778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X. and Perrimon N. (1999). Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature 400, 281-284. 10.1038/22343 [DOI] [PubMed] [Google Scholar]
- Mata J., Curado S., Ephrussi A. and Rørth P. (2000). Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell 101, 511-522. 10.1016/S0092-8674(00)80861-2 [DOI] [PubMed] [Google Scholar]
- Mathew D., Ataman B., Chen J., Zhang Y., Cumberledge S. and Budnik V. (2005). Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science 310, 1344-1347. 10.1126/science.1117051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon K. P., Carrillo R. A. and Zinn K. (2013). Development and plasticity of the Drosophila larval neuromuscular junction. Wiley Interdiscip. Rev. Dev. Biol. 2, 647-670. 10.1002/wdev.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech C., Pauer H.-U., He X. and Schwarz T. L. (2008). Presynaptic local signaling by a canonical wingless pathway regulates development of the Drosophila neuromuscular junction. J. Neurosci. 28, 10875-10884. 10.1523/JNEUROSCI.0164-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. M. and Heuser J. E. (1984). Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J. Cell Biol. 98, 685-698. 10.1083/jcb.98.2.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm M., Lee M.-J., Parkinson W., Lee M., Kim H., Kim Y.-J., Kim S., Cho Y. S., Min B.-M., Bae Y. C. et al. (2013). Spartin regulates synaptic growth and neuronal survival by inhibiting BMP-mediated microtubule stabilization. Neuron 77, 680-695. 10.1016/j.neuron.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M., Koo E. S., Gorczyca M., Sharpe J., Cumberledge S. and Budnik V. (2002). The Drosophila Wnt, Wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell 111, 319-330. 10.1016/S0092-8674(02)01047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson W., Dear M. L., Rushton E. and Broadie K. (2013). N-glycosylation requirements in neuromuscular synaptogenesis. Development 140, 4970-4981. 10.1242/dev.099192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson W. M., Dookwah M., Dear M. L., Gatto C. L., Aoki K., Tiemeyer M. and Broadie K. (2016). Synaptic roles for phosphomannomutase type 2 in a new Drosophila congenital disorder of glycosylation disease model. Dis. Model Mech. 9, 513-527. 10.1242/dmm.022939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins L. A., Holderbaum L., Tao R., Hu Y., Sopko R., McCall K., Yang-Zhou D., Flockhard I., Binari R., Flockhard R. et al. (2015). The transgenic RNAi project at Harvard MedicalSchool: resources and validation. Genetics 201, 843-852. 10.1534/genetics.115.180208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer S., Ricardo S., Manneville J.-B., Alexandre C. and Vincent J.-P. (2002). Producing cells retain and recycle Wingless in Drosophila embryos. Curr. Biol. 12, 957-962. 10.1016/S0960-9822(02)00867-9 [DOI] [PubMed] [Google Scholar]
- Qin G., Schwarz T., Kittel R. J., Schmid A., Rasse T. M., Kappei D., Ponimaskin E., Heckmann M. and Sigrist S. J. (2005). Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. J. Neurosci. 25, 3209-3218. 10.1523/JNEUROSCI.4194-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasse T. M., Fouquet W., Schmid A., Kittel R. J., Mertel S., Sigrist C. B., Schmidt M., Guzman A., Merino C., Qin G. et al. (2005). Glutamate receptor dynamics organizing synapse formation in vivo. Nat. Neurosci. 8, 898-905. 10.1038/nn1484 [DOI] [PubMed] [Google Scholar]
- Roos J., Hummel T., Ng N., Klämbt C. and Davis G. W. (2000). Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron 26, 371-382. 10.1016/S0896-6273(00)81170-8 [DOI] [PubMed] [Google Scholar]
- Speese S. D., Ashley J., Jokhi V., Nunnari J., Barria R., Li Y., Ataman B., Koon A., Change Y.-T., Li Q. et al. (2012). Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell 149, 832-846. 10.1016/j.cell.2012.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traister A., Shi W. and Filmus J. (2008). Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochem. J. 410, 503-511. 10.1042/BJ20070511 [DOI] [PubMed] [Google Scholar]
- Verstreken P., Ohyama T. and Bellen H. J. (2008). FM 1-43 labeling of synaptic vesicle pools at the Drosophila neuromuscular junction. Methods Mol. Biol. 440, 349-369. 10.1007/978-1-59745-178-9_26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakrishnan N., Woodruff E. A. III and Broadie K. (2009). Rolling blackout is required for bulk endocytosis in non-neuronal cells and neuronal synapses. J. Cell Sci. 122, 114-125. 10.1242/jcs.036673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakrishnan N., Phillips S. E. and Broadie K. (2010). Drosophila rolling blackout displays lipase domain-dependent and -independent endocytic functions downstream of dynamin. Traffic 11, 1567-1578. 10.1111/j.1600-0854.2010.01117.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh D. A., Rasse T. M., Asan E., Hofbauer A., Schwenkert I., Durrbeck H., Buchner S., Dabauvalle M.-C., Schmidt M., Qin G. et al. (2006). Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 49, 833-844. 10.1016/j.neuron.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Wang X., Shaw W. R., Tsang H. T. H., Reid E. and O'Kane C. J. (2007). Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nat. Neurosci. 10, 177-185. 10.1038/nn1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D., Wu Y., Feng Y., Lin S.-C. and Lin X. (2009). The core protein of glypican Dally-like determines its biphasic activity in wingless morphogen signaling. Dev. Cell 17, 470-481. 10.1016/j.devcel.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L., Chaturvedi D. and Cumberledge S. (2004). Drosophila Wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts; a process that requires Porcupine. J. Biol. Chem. 279, 33220-33227. 10.1074/jbc.M403407200 [DOI] [PubMed] [Google Scholar]