Abstract
The endocannabinoid system (ECS) has been shown to be of great importance in the regulation of numerous physiological and pathological processes. To date, two Class A G-protein-coupled receptors (GPCRs) have been discovered and validated as the main therapeutic targets of this system: the cannabinoid receptor type 1 (CB1), which is the most abundant neuromodulatory receptor in the brain, and the cannabinoid receptor type 2 (CB2), predominantly found in the immune system among other organs and tissues. Endogenous cannabinoid receptor ligands (endocannabinoids) and the enzymes involved in their synthesis, cell uptake, and degradation have also been identified as part of the ECS. However, its complex pharmacology suggests that other GPCRs may also play physiologically relevant roles in this therapeutically promising system. In the last years, GPCRs such as GPR18 and GPR55 have emerged as possible missing members of the cannabinoid family. This categorization still stimulates strong debate due to the lack of pharmacological tools to validate it. Because of their close phylogenetic relationship, the Class A orphan GPCRs, GPR3, GPR6, and GPR12, have also been associated with the cannabinoids. Moreover, certain endo-, phyto-, and synthetic cannabinoid ligands have displayed activity at other well-established GPCRs, including the opioid, adenosine, serotonin, and dopamine receptor families. In addition, the cannabinoid receptors have also been shown to form dimers with other GPCRs triggering cross-talk signaling under specific conditions. In this mini review, we aim to provide insight into the non-CB1, non-CB2 cannabinoid-related GPCRs that have been reported thus far. We consider the physiological relevance of these molecular targets in modulating the ECS.
Keywords: : cannabinoid receptors, endocannabinoid system, GPCRs, orphan receptors
Introduction
The Class A G-protein-coupled receptors (GPCRs), cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2), have been widely confirmed as cannabinoid targets. These receptors have been shown to be involved in numerous physiopathological processes, including pain, inflammation, cancer, metabolic syndromes, hypertension, and neurodegenerative disorders.1 Nonetheless, the complex pharmacology of the endocannabinoid system (ECS) and its wide implication in numerous biological functions suggest the existence of other receptors playing important physiological roles. Consequently, extensive research is currently focused on the identification of potential missing cannabinoid receptors.
Diverse Class A orphans or lately deorphanized GPCRs have been proposed and evaluated as possible ECS members. Nonetheless, the lack of selective ligands for these receptors along with their intricate signaling pathways is delaying a clear elucidation of their relationship with the ECS. Therefore, thus far no other GPCR has been categorized as the cannabinoid receptor type 3 by the International Union of Pharmacology.2
Herein, we intend to provide an overview of the GPCRs that have been postulated as cannabinoid molecular targets and the current available evidence of their relationship with the ECS. Non-GPCR targets of the cannabinoids such as the peroxisome proliferator-activated receptors, ligand-gated ion channels, or transient receptor potential channels have been revised elsewhere and are beyond the scope of this review.3,4
GPR55 and GPR18
Several GPCRs have been postulated to be putative cannabinoid receptors, but so far, only GPR18 and GPR55 have been demonstrated to be targets of a wide variety of endogenous, phytogenic, and synthetic cannabinoid ligands.4 Despite this fact, inconsistencies in pharmacological data in the literature are hampering their categorization.5,6
The cannabinoid-related class A GPCR GPR55 displays low sequence identity with CB1 and CB2 (∼13% and 14%, respectively). GPR55 is widely expressed in the brain, as well as in the peripheral system, co-localizing with the cannabinoid receptors in diverse tissues.7–9 This receptor displays G-protein coupling promiscuity associating with Gα13,8,10 Gαq/11,11 Gα12,11 or Gα12/138,12 depending on the cell line or tissue. GPR55 has been implicated in different physiopathological conditions such as cancer,13–15 pain,11,16,17 metabolic disorders,18,19 vascular functions,20,21 bone physiology,22 and motor coordination.23
The phospholipid lysophosphatidylinositol (LPI) is considered the endogenous GPR55 ligand.8,24,25 In fact, GPR55 has also been named the LPI1 receptor.26 Numerous CB1 and CB2 ligands have also been reported to act as GPR55 modulators.6,27–29 However, significant pharmacological discrepancies have been found depending on the tested functional outcome.6 For instance, the well-known phytocannabinoid Δ9-tetrahydrocannabinol (Δ9-THC) displayed activation of GPR55 according to certain reports,10,11 while it was unable to exert any effect in other functional assays.24,30 Cannabinoid ligands reported to be recognized by GPR55 and their intriguing pharmacology have been recently reviewed elsewhere.31
Although its sequence presents low identity with CB1 and CB2 (∼13% and 8%), GPR18 has also been tightly associated with the ECS.4,32 High expression of GPR18 has been found in the lymphoid tissues, while it is moderately expressed in other organs such as lungs, brain, testis, or ovary.33,34 Initially, GPR18 was found to couple to Gαi/o; however, subsequent findings suggested the participation of the Gαq/11 transduction pathway as well.34–36 Different reports have shown the therapeutic potential of this target in the treatment of pathologies such as intraocular pressure,37 cancer,38 or metabolic disorders39 among others.
N-arachidonoyl glycine (NAGly) has been suggested to be the endogenous GPR18 ligand by several research groups.32,34 However, other researchers were not able to confirm these data.40 Recent investigations point to the existence of another endogenous GPR18 activator: the polyunsaturated fatty acid metabolite, Resolvin D2 (RvD2), which is mainly involved in inflammatory processes.41 In addition, and despite the pharmacological divergences observed among some reports, GPR18 has been shown to recognize an array of CB1 and/or CB2 ligands of endogenous, phytogenic, or synthetic nature (reviewed by others).39,42
The pharmacological discrepancies on the appraisal of cannabinoids in these two receptors, as well as the lack of selective ligands targeting them, are delaying an insightful understanding of the relation of GPR55 and GPR18 with the ECS. These inconsistencies, which may rely on intrinsic properties of these GPCRs, or on the cell type or functional assay, need to be further studied. Intensive efforts are also focused on the structural understanding of these receptors,43 as well as the development of more potent and selective pharmacological tools for the study of these promising therapeutic targets.
GPR3, GPR6, and GPR12
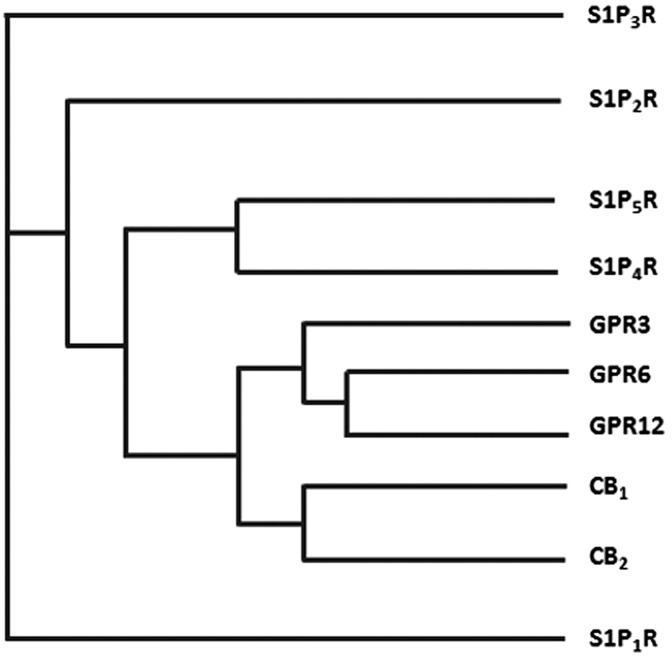
GPR3, GPR6, and GPR12 are three orphan Class A GPCRs that exhibit a very close phylogenetic relationship with the cannabinoid receptors CB1 and CB2 (Fig. 1). Indeed, they belong to the same cluster of receptors, the so-called MECA cluster (which consists of the melanocortin receptors, the endothelial differentiation GPCRs, the cannabinoid receptors, the adenosine binding receptors, and the orphan receptor subset GPR3, −6, and −12).44,45 Because of their phylogenetic proximity, these orphan receptors share common conserved residues and unique sequence motifs with CB1 and CB2.46 According to Fredriksson et al. these orphan receptors may share a common ancestor with the cannabinoid receptors since they share the same chromosomal positions.45
FIG. 1.
Phylogenetic tree of cannabinoid receptors and the closely related Class A GPCRs (S1PR family and the orphan receptors GPR3, GPR6, and GPR12). Data were obtained from GPCRdb.org. CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2; GPCR, G-protein-coupled receptor; S1P, sphingosine-1-phosphate.
GPR3, GPR6, and GPR12, which share over 60% of sequence similarity, were first cloned in 1995.47,48 These receptors constitutively activate adenylate cyclase by coupling to Gαs proteins. In fact, different groups have reported that when expressed in diverse cell lines, they can stimulate adenylate cyclase to levels similar in amplitude to agonist-activated GPCRs.47,49,50 In addition to Gαs, GPR6 and GPR12 have also been suggested to couple to Gαi/o,51,52 but further investigations are required to confirm this G-protein promiscuity.
GPR3, GPR6, and GPR12 are predominantly expressed in the brain and the reproductive system.49 This family of constitutively active GPCRs is involved in neuronal differentiation and growth, as well as in the formation of synaptic contacts.49 Therefore, their role in different neurological processes such as neurite outgrowth,49 Alzheimer's disease,53–57 development of cerebellar granule neurons,58,59 neuropathic pain,60 early phases of cocaine reinforcement,61 emotional-like responses,62 instrumental learning,63 or Parkinson's disease64,65 has been studied. Other pathophysiological conditions such as oocyte maturation,66,67 dyslipidemia,68 and cell proliferation69 may also be impacted by the modulation of some of these receptors.
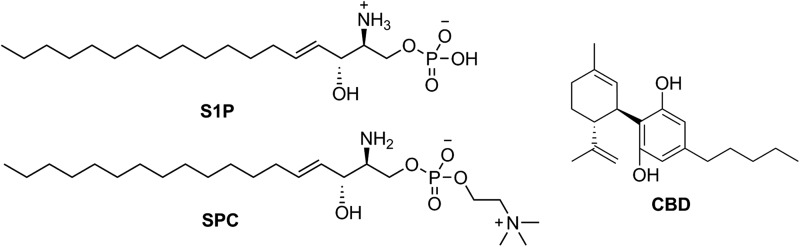
The bioactive lipids, sphingosine-1-phosphate50,52 and/or sphingosylphosphorylcholine,51 have been proposed as endogenous ligands of these receptors (Fig. 2). However, other groups were not able to confirm this claim, and consequently, GPR3, GPR6, and GPR12 are still categorized as orphans.30,70,71 Interestingly, among the very few ligands discovered so far for these receptors, the nonpsychoactive phytocannabinoid cannabidiol (CBD) stands out as being able to target GPR3 and GPR6,72 acting as a β-arrestin2 inverse agonist of both receptors. This functionality is of high interest in the GPR3 field because β-arrestin2 signaling at GPR3 has been directly linked to the manufacture of beta-amyloid plaque (Aβ1–40 and Aβ1–42) in Alzheimer's disease through complex formation with γ-secretase.56,57 Because CBD is an inverse agonist of this signaling pathway at GPR3, it may represent a potential tool for the reduction of amyloid pathology. Other phytocannabinoids and several endocannabinoids were also tested but so far none of them were found to modulate this family of orphan receptors.30,72
FIG. 2.
Structures of the putative GPR3, GPR6, and GPR12 endogenous ligands S1P and SPC and the GPR3 and GPR6 inverse agonist CBD. SPC, sphingosylphosphorylcholine.
So, a relationship between the cannabinoids and the orphan receptors GPR3, GPR6, and GPR12 has been evidenced. Nonetheless, extensive research and more pharmacological tools are needed to extract significant conclusions about the association of these receptors with the ECS and its ligands.
Alkylindole-Sensitive Receptors
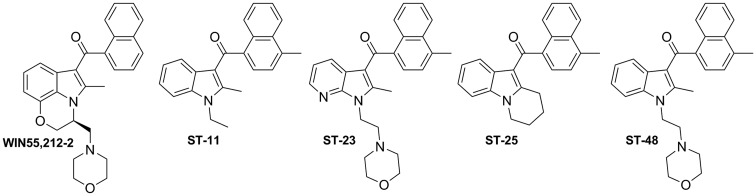
As reported by different research groups, the well-known aminoalkylindole cannabinoid agonist WIN55,212-2 (Fig. 3) displays pharmacological functions independent of the cannabinoid receptors CB1 and CB2.73–75 This fact led to the identification of novel targets commonly referred to as the alkylindole (AI)-sensitive receptors.74,76,77 These cannabinoid-related receptors are modulated by AI derivatives, but not by other classes of cannabinoid ligands.76 Diverse evidence suggests that the AI-sensitive receptors are Gαs-protein coupled receptors that are mainly expressed in microglia and astrocytomas.76–79 However, their biological functions, pharmacology, and therapeutic value remain to be unraveled due to the lack of selective pharmacological tools.
FIG. 3.
Alkylindole derivatives WIN55,212–2, ST-11, ST-23, ST-25, and ST-48.
Recent studies from Stella and coworkers revealed the role of AI-sensitive receptors in the modulation of microglial cell migration and proliferation highlighting their potential in the treatment of gliomas.77,78 Moreover, these authors have identified a series of naphthoyl AI derivatives, ST-11, ST-23, ST-25, and ST-48 (Fig. 3) among them, that bind to the AI-sensitive receptors.78 These compounds display affinities in the nanomolar range when competing with [3H]WIN55,212-2 in DBT (Delayed Brain Tumor) cells which endogenously express AI-sensitive receptors, while lacking CB1 and CB2 receptors.80 Compound ST-11 stands out from this study because of its potency at AI-sensitive receptors, while not interacting with CB1 and CB2 receptors. In addition, in vitro assays revealed that this compound inhibits cell migration and proliferation in the aforementioned mouse glioma cell line, DBT. Further studies revealed that ST-11 can reduce glioblastoma growth in a syngeneic mouse model.81
Even though extensive research is clearly needed to understand the pathophysiological function of these receptors, reported data suggest that AI-sensitive receptor agonists could represent a novel class of potential brain cancer antitumor drugs.
Cannabinoid-Related Oligomers
Numerous studies have shown that GPCRs, cannabinoid receptors among them, can exist and function as dimers or complexes of higher order.82–85 This oligomerization may affect receptor signaling, receptor trafficking, and ligand binding. The physiological relevance of such dimerization has not yet been fully established for the cannabinoid receptors; nonetheless, the presence of cannabinoid homo- and heterodimers in specific tissues has been intensely reported over the last years.
For the CB1 receptor, heteromers have been suggested to exist under certain physiological conditions with serotonin,86 angiotensin,87 opioid,88–90 GPR55,91 somatostatin,92 orexin,93,94 dopamine,95–97 and adenosine98 receptors among others (Table 1). Although CB2 has been less investigated, recent research revealed that it may form heterodimers with CB1,99 with GPR55,100,101 with the serotonin receptor 5HT1A,102 or with the chemokine receptor CXCR4.103 The expression of these heterodimers has been associated with different pathologies. For instance, the CB2−CXCR4 and the CB2−GPR55 dimers have been associated with cancer progression, while the CB1−A2A and the CB1−D2 heteromers have been suggested to have physiological implications in neurodegenerative disorders such as Huntington's or Parkinson's diseases. All these data suggest that the ECS interacts in a significant manner with several other endogenous systems.
Table 1.
Cannabinoid-Related G-Protein-Coupled Receptor Dimers Reported So Far

| Heterodimers | Homodimers | ||
|---|---|---|---|
| CB1−D2 | 95,97 | CB1−CB1 | 104–106 |
| CB1−A2A | 98 | CB2−CB2 | 107–109 |
| CB1−5HT2A | 86 | ||
| CB1−AT1 | 87 | ||
| CB1−GPR55 | 91 | ||
| CB1−SST5 | 92 | ||
| CB1−OX1 | 94 | ||
| CB1−OX2 | 93 | ||
| CB1−μOR | 90 | ||
| CB1−δOR | 88 | ||
| CB1−CB2 | 99 | ||
| CB2−GPR55 | 100,101 | ||
| CB2−5HT1A | 102 | ||
| CB2−CXCR4 | 103 | ||
CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2.
With regard to cannabinoid receptor homodimerization, more data have been published on CB1 homodimers than on their CB2 counterparts. The presence of CB1 receptor homodimers has been reported in different biological tissues,104–106 but their functional role has not been determined. In contrast, CB2 homodimers have been evidenced,107–109 but their pharmacological potential has not been explored yet.
In this field, bivalent ligands have emerged as promising new pharmacological entities and potential tools for the biological study of their respective dimeric receptors.110–113 Despite their poor pharmacokinetic properties,114 bivalent ligands can exhibit enhanced activity and selectivity over their respective corresponding parent ligands offering unique pharmacological strategies. Bivalent ligands have been synthesized and evaluated for several GPCRs. Opioid,115,116 dopamine,117,118 and histamine119 are some of the receptors for which a bivalent compound provided higher activity than their monomer counterparts. CB1 homobivalent120–122 and heterobivalent123–125 ligands have been also reported and explored. However, currently available receptor structural information challenges the fact that bivalent ligands can simultaneously bind to both receptors within the dimer, especially in the case of lipid receptors as the cannabinoids.126 Therefore, novel drug design approaches to target dimers, as well as new techniques to determine bivalent binding, remain to be explored.
Homo- and heterodimerization likely influences the manner in which the ECS responds to ligands. Nevertheless, unambiguous data about their physical association in native tissues, as well as their pharmacology, are needed to clearly identify what biological functions are impacted by cannabinoid dimers.
Well-Established GPCRs Related to the Cannabinoids
Certain endo-, phyto-, and synthetic cannabinoid ligands have been shown to modulate well-known GPCRs. These GPCRs include members of established families such as the opioid, serotonin, muscarinic, dopamine, and adenosine families. For instance, the endocannabinoid anandamide has been shown to act at the adenosine receptor A3,127 the muscarinic acetylcholine receptors M1 and M4,128 and the serotonin receptors 5-HT1A and 5-HT2A129 among others. In addition, phytocannabinoids such as Δ9-THC and CBD have been shown to modulate the μ− and δ−opioid receptors,130 while other plant-derived compounds such as CBG (cannabigerol) and Δ9-THCV (tetrahydrocannabivarin) display activity at the 5-HT1A receptor.131,132 Likewise, synthetic cannabinoids, such as the CB1 inverse agonists taranabant (MK-0364) and rimonabant (SR141716), have also displayed activity in well-established targets. These include the adenosine A3 and the tachykinin NK2 receptors.133
Some of these cannabinoid ligands have been proposed to interact allosterically with the aforementioned targets. It is worth mentioning that the efficacy that most of these cannabinoids exhibit toward these GPCRs is lower than the one displayed at the CB1 and/or CB2 receptors. Therefore, there is no evidence indicating a necessary recategorization of these receptors.
Other GPCRs
Because of their ability to recognize lipids and their relatively close phylogenetic relationship with CB1 and CB2, several other Class A orphan or recently deorphanized GPCRs such as GPR40, GPR43, GPR41, GPR120 (currently classified as free fatty acid receptors FFA1, FFA2, FFA3, and FFA4, respectively), GPR23, GPR92 (recently categorized as lysophosphatidic acid receptors LPA4 and LPA5), GPR84, GPR119, or GPR35 have been postulated as possible cannabinoid receptor candidates.4 However, there is no available evidence since they do not meet some of the criteria established by the International Union of Pharmacology.4,70
Conclusions
Two cannabinoid receptors, CB1 and CB2, have been validated at the molecular level as the main targets of the ECS. These two GPCRs have been widely explored in the development of numerous pathophysiological processes, and their therapeutic potential for the treatment of different diseases has been extensively confirmed.1 Great efforts are being made to structurally understand these receptors; in fact, CB1 in its inactive134,135 and active136 states has been recently crystallized. Despite possible crystallization artifacts, these structures will help shedding light into the complex pharmacology of the cannabinoid receptors.
Growing evidence suggests that other cannabinoid or cannabinoid-like receptors remain to be identified as important players of the ECS. Different endogenous, phytogenic, and/or synthetic cannabinoid ligands have been reported to modulate GPCRs such as GPR18, GPR55, GPR3, GPR6, or the AI-sensitive receptors, among others. Pharmacological discrepancies and the lack of selective ligands for these receptors are delaying the characterization of their relationship with the ECS. Consequently, no CB3 receptor has yet been confirmed.2
Adding more complexity to the ECS scenario, molecular interactions of the cannabinoid receptors with other GPCRs have been reported. Co-localization or co-immunoprecipitation data suggest the presence of cannabinoid homo- and heterodimers in specific native tissues. Cannabinoid receptor dimerization may not only influence the pharmacology of these receptors but also it may provide new signaling pathways through the interacting protomers. However, due to the lack of appropriate tools, there is still limited in vivo information about the expression of cannabinoid dimers. Hence, it remains a challenge to elucidate their therapeutic relevance under specific physiological conditions.
Currently, appropriate characterization of cannabinoid ligands should take into account the activity at the aforementioned GPCRs. Possible biased agonism of ligands, allosterism, or cross-talk signaling could be determining the intricate GPCR pharmacology. In addition, differential coupling and regulation of G-proteins or the formation of oligomers are among GPCR intrinsic properties that might be delaying the validation of novel potential cannabinoid targets. Therefore, further research is needed to fully understand the physiopathological role of these non-CB1, non-CB2 GPCRs in the modulation of the ECS.
Abbreviations Used
- AI
alkylindole
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- CBD
cannabidiol
- ECS
endocannabinoid system
- GPCR
G-protein-coupled receptor
- LPI
lysophosphatidylinositol
- THC
tetrahydrocannabinol
Acknowledgments
The authors acknowledge research support from NIH/NIDA grants R01 DA003934 and K05 DA021358 (P.H.R.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos Trans R Soc B 2012;367:3353–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IUPHAR BPS Guide to PHARMACOLOGY. Cannabinoid receptors. www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=13 (accessed June7, 2017)
- 3.Morales P, Hurst DP, Reggio PH. In: Molecular targets of the phytocannabinoids: a complex picture. In: Kinghorn AD, Gibbons S, eds. Phytocannabinoids: Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa. Cham, Switzerland: Springer International Publishing, 2017; Vol. 103, pp. 103–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pertwee RG, Howlett AC, Abood ME, et al. . International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 2010;62:588–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IUPHAR BPS Guide to PHARMACOLOGY. GPR18, GPR55 and GPR119. www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=114 (accessed June7, 2017)
- 6.Ross RA. The enigmatic pharmacology of GPR55. Trends Pharmacol Sci 2009;30:156–163 [DOI] [PubMed] [Google Scholar]
- 7.Balenga NAB, Henstridge CM, Kargl J, et al. . Pharmacology, signaling and physiological relevance of the G protein-coupled receptor 55. In: Neubig RR, ed. Pharmacology of G Protein Coupled Receptors. San Diego, CA: Advances in Pharmacology, Volume 62, Elsevier Inc., 2011, pp. 251–277. [DOI] [PubMed] [Google Scholar]
- 8.Henstridge CM, Balenga NAB, Ford LA, et al. . The GPR55 ligand L-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J 2009;23:183–193 [DOI] [PubMed] [Google Scholar]
- 9.Sawzdargo M, Nguyen T, Lee DK, et al. . Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain research. Mol Brain Res 1999;64:193–198 [DOI] [PubMed] [Google Scholar]
- 10.Ryberg E, Larsson N, Sjögren S, et al. . The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 2007;152:1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauckner JE, Jensen JB, Chen H-Y, et al. . GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A 2008;105:2699–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henstridge CM, Balenga NA, Schröder R, et al. . GPR55 ligands promote receptor coupling to multiple signalling pathways. Br J Pharmacol 2010;160:604–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andradas C, Blasco-Benito S, Castillo-Lluva S, et al. . Activation of the orphan receptor GPR55 by lysophosphatidylinositol promotes metastasis in triple-negative breast cancer. Oncotarget 2016;7:47565–47575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann NA, Yang J, Trauger SA, et al. . The GPR55 agonist, L-α-lysophosphatidylinositol, mediates ovarian carcinoma cell-induced angiogenesis. Br J Pharmacol 2015;172:4107–4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kargl J, Andersen L, Hasenöhrl C, et al. . GPR55 promotes migration and adhesion of colon cancer cells indicating a role in metastasis. Br J Pharmacol 2016;173:142–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey LM, Gutierrez T, Deng L, et al. . Inflammatory and neuropathic nociception is preserved in GPR55 knockout mice. Sci Rep 2017;7:944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staton PC, Hatcher JP, Walker DJ, et al. . The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain 2008;139:225–236 [DOI] [PubMed] [Google Scholar]
- 18.Tudurí E, Imbernon M, Hernández-Bautista RJ. GPR55: a new promising target for metabolism? J Mol Endocrinol 2017;58:R191–R202 [DOI] [PubMed] [Google Scholar]
- 19.Tudurí E, López M, Diéguez C, et al. . GPR55 and the regulation of glucose homeostasis. Int J Biochem Cell Biol 2017;88:204–207 [DOI] [PubMed] [Google Scholar]
- 20.AlSuleimani YM, Hiley CR. The GPR55 agonist lysophosphatidylinositol relaxes rat mesenteric resistance artery and induces calcium release in rat mesenteric artery endothelial cells. Br J Pharmacol 2015;172:3043–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bondarenko AI, Montecucco F, Panasiuk O, et al. . GPR55 agonist lysophosphatidylinositol and lysophosphatidylcholine inhibit endothelial cell hyperpolarization via GPR-independent suppression of Na+-Ca2+ exchanger and endoplasmic reticulum Ca2+ refilling. Vascul Pharmacol 2017;89:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whyte LS, Ryberg E, Sims NA, et al. . The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc Natl Acad Sci U S A 2009;106:16511–16516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C-S, Chen H, Sun H, et al. . GPR55, a G-protein coupled receptor for lysophosphatidylinositol, plays a role in motor coordination. PLoS One 2013;8:e60314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oka S, Nakajima K, Yamashita A, et al. . Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun 2007;362:928–934 [DOI] [PubMed] [Google Scholar]
- 25.Oka S, Toshida T, Maruyama K, et al. . 2-Arachidonoyl-sn-glycero-3-phosphoinositol: a possible natural ligand for GPR55. J Biochem 2009;145:13–20 [DOI] [PubMed] [Google Scholar]
- 26.Kihara Y, Maceyka M, Spiegel S, et al. . Lysophospholipid receptor nomenclature review: IUPHAR review 8. Br J Pharmacol 2014;171:3575–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriconi A, Cerbara I, Maccarrone M, et al. . GPR55: current knowledge and future perspectives of a purported “Type-3” cannabinoid receptor. Curr Med Chem 2010;17:1411–1429 [DOI] [PubMed] [Google Scholar]
- 28.Nevalainen T, Irving AJ. GPR55, a Lysophosphatidylinositol receptor with cannabinoid sensitivity? Curr Top Med Chem 2010;1:799–813 [DOI] [PubMed] [Google Scholar]
- 29.Sharir H, Abood ME. Pharmacological characterization of GPR55, a putative cannabinoid receptor. Pharmacol Ther 2010;126:301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin H, Chu A, Li W, et al. . Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem 2009;284:12328–12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morales P, Jagerovic N. Advances towards the discovery of GPR55 ligands. Curr Med Chem 2016;23:2087–2100 [DOI] [PubMed] [Google Scholar]
- 32.McHugh D, Hu SSJ, Rimmerman N, et al. . N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci 2010;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gantz I, Muraoka A, Yang YK, et al. . Cloning and chromosomal localization of a gene (GPR18) encoding a novel seven transmembrane receptor highly expressed in spleen and testis. Genomics 1997;42:462–466 [DOI] [PubMed] [Google Scholar]
- 34.Kohno M, Hasegawa H, Inoue A, et al. . Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun 2006;347:827–832 [DOI] [PubMed] [Google Scholar]
- 35.Console-Bram L, Brailoiu E, Brailoiu GC, et al. . Activation of GPR18 by cannabinoid compounds: a tale of biased agonism. Br J Pharmacol 2014;171:3908–3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takenouchi R, Inoue K, Kambe Y, et al. . N-arachidonoyl glycine induces macrophage apoptosis via GPR18. Biochem Biophys Res Commun 2012;418:366–371 [DOI] [PubMed] [Google Scholar]
- 37.Miller S, Leishman E, Oehler O, et al. . Evidence for a GPR18 role in diurnal regulation of intraocular pressure. Invest Ophthalmol Vis Sci 2016;57:6419–6426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin Y, Verdegaal EME, Siderius M, et al. . Quantitative expression profiling of G-protein-coupled receptors (GPCRs) in metastatic melanoma: the constitutively active orphan GPCR GPR18 as novel drug target. Pigment Cell Melanoma Res 2011;24:207–218 [DOI] [PubMed] [Google Scholar]
- 39.Rajaraman G, Simcocks A, Hryciw DH, et al. . G protein coupled receptor 18: a potential role for endocannabinoid signaling in metabolic dysfunction. Mol Nutr Food Res 2016;60:92–102 [DOI] [PubMed] [Google Scholar]
- 40.Lu VB, Puhl HL, Ikeda SR. N-arachidonyl glycine does not activate G protein–coupled receptor 18 signaling via canonical pathways. Mol Pharmacol 2013;83:267–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang N, Dalli J, Colas RA, et al. . Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med 2015;212:1203–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McHugh D. GPR18 in microglia: implications for the CNS and endocannabinoid system signalling. Br J Pharmacol 2012;167:1575–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lingerfelt MA, Zhao P, Sharir HP, et al. . Identification of crucial amino acid residues involved in agonist signaling at the GPR55 receptor. Biochemistry 2017;56:473–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fredriksson R, Schio HB. The repertoire of G-protein–coupled receptors in fully. Mol Pharmacol 2005;67:1414–1425 [DOI] [PubMed] [Google Scholar]
- 45.Fredriksson R, Lagerström MC, Lundin L-G, et al. . The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 2003;63:1256–1272 [DOI] [PubMed] [Google Scholar]
- 46.Morales P, Hurst DP, Reggio PH. Methods for the development of in silico GPCR models. In: Reggio PH, ed. Cannabinoids and Their Receptors. London: Methods in Enzymology, Volume 593, Elsevier Inc., 2017, pp. 405–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eggerickx D, Denef JF, Labbe O, et al. . Molecular cloning of an orphan G-protein-coupled receptor that constitutively activates adenylate cyclase. Biochem J 1995;309:837–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song Z-H, Modi W, Bonner TI. Molecular cloning and chromosomal localization of human genes encoding three closely related G protein-coupled receptors. Genomics 1995;28:347–349 [DOI] [PubMed] [Google Scholar]
- 49.Tanaka S, Ishii K, Kasai K, et al. . Neural expression of G protein-coupled receptors GPR3, GPR6, and GPR12 up-regulates cyclic AMP levels and promotes neurite outgrowth. J Biol Chem 2007;282:10506–10515 [DOI] [PubMed] [Google Scholar]
- 50.Uhlenbrock K, Gassenhuber H, Kostenis E. Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell Signal 2002;14:941–953 [DOI] [PubMed] [Google Scholar]
- 51.Ignatov A, Lintzel J, Hermans-Borgmeyer I, et al. . Role of the G-protein-coupled receptor GPR12 as high-affinity receptor for sphingosylphosphorylcholine and its expression and function in brain development. J Neurosci 2003;23:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ignatov A, Lintzel J, Kreienkamp HJ, et al. . Sphingosine-1-phosphate is a high-affinity ligand for the G protein-coupled receptor GPR6 from mouse and induces intracellular Ca2+ release by activating the sphingosine-kinase pathway. Biochem Biophys Res Commun 2003;311:329–336 [DOI] [PubMed] [Google Scholar]
- 53.Benoit ME, Hernandez MX, Dinh ML, et al. . C1q-induced LRP1B and GPR6 proteins expressed early in Alzheimer disease mouse models, are essential for the C1q-mediated protection against amyloid neurotoxicity. J Biol Chem 2013;288:654–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang Y, Skwarek-Maruszewska A, Horré K, et al. . Loss of GPR3 reduces the amyloid plaque burden and improves memory in Alzheimer's disease mouse models. Sci Transl Med 2015;7:309ra164. [DOI] [PubMed] [Google Scholar]
- 55.Nelson CD, Sheng M. Gpr3 stimulates Aβ production via interactions with APP and β-arrestin2. PLoS One 2013;8:e74680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thathiah A, Horré K, Snellinx A, et al. . β-arrestin 2 regulates Aβ generation and γ-secretase activity in Alzheimer's disease. Nat Med 2013;19:43–49 [DOI] [PubMed] [Google Scholar]
- 57.Thathiah A, Spittaels K, Hoffmann M, et al. . The orphan G protein-coupled receptor 3 modulates amyloid-beta peptide generation in neurons. Science 2009;323:946–951 [DOI] [PubMed] [Google Scholar]
- 58.Tanaka S, Shaikh IM, Chiocca EA, et al. . The Gs-linked receptor GPR3 inhibits the proliferation of cerebellar granule cells during postnatal development. PLoS One 2009;4:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka S, Miyagi T, Dohi E, et al. . Developmental expression of GPR3 in rodent cerebellar granule neurons is associated with cell survival and protects neurons from various apoptotic stimuli. Neurobiol Dis 2014;68:215–227 [DOI] [PubMed] [Google Scholar]
- 60.Ruiz-Medina J, Ledent C, Valverde O. GPR3 orphan receptor is involved in neuropathic pain after peripheral nerve injury and regulates morphine-induced antinociception. Neuropharmacology 2011;61:43–50 [DOI] [PubMed] [Google Scholar]
- 61.Tourino C, Valjent E, Ruiz-Medina J, et al. . The orphan receptor GPR3 modulates the early phases of cocaine reinforcement. Br J Pharmacol 2012;167:892–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valverde O, Celerier E, Baranyi M, et al. . GPR3 receptor, a novel actor in the emotional-like responses. PLoS One 2009;4:e4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lobo MK, Cui Y, Ostlund SB, et al. . Genetic control of instrumental conditioning by striatopallidal neuron-specific S1P receptor Gpr6. Nat Neurosci 2007;10:1395–1397 [DOI] [PubMed] [Google Scholar]
- 64.Oeckl P, Hengerer B, Ferger B. G-protein coupled receptor 6 deficiency alters striatal dopamine and cAMP concentrations and reduces dyskinesia in a mouse model of Parkinson's disease. Exp Neurol 2014;257:1–9 [DOI] [PubMed] [Google Scholar]
- 65.Oeckl P, Ferger B. Increased susceptibility of G-protein coupled receptor 6 deficient mice to MPTP neurotoxicity. Neuroscience 2016;337:218–223 [DOI] [PubMed] [Google Scholar]
- 66.Hinckley M, Vaccari S, Horner K, et al. . The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol 2005;287:249–261 [DOI] [PubMed] [Google Scholar]
- 67.Mehlmann LM, Saeki Y, Tanaka S, et al. . The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 2004;306:1947–1950 [DOI] [PubMed] [Google Scholar]
- 68.Bjursell M, Gerdin AK, Jönsson M, et al. . G protein-coupled receptor 12 deficiency results in dyslipidemia and obesity in mice. Biochem Biophys Res Commun 2006;348:359–366 [DOI] [PubMed] [Google Scholar]
- 69.Lu X, Zhang N, Meng B, et al. . Involvement of GPR12 in the regulation of cell proliferation and survival. Mol Cell Biochem 2012;366:101–110 [DOI] [PubMed] [Google Scholar]
- 70.Davenport AP, Alexander SPH, Sharman JL, et al. . International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol Rev 2013;65:967–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Southern C, Cook JM, Neetoo-Isseljee Z, et al. . Screening beta-arrestin recruitment for the identification of natural ligands for orphan G-protein-coupled receptors. J Biomol Screen 2013;18:599–609 [DOI] [PubMed] [Google Scholar]
- 72.Laun AS, Song Z-H. GPR3 and GPR6, novel molecular targets for cannabidiol. Biochem Biophys Res Commun 2017;490:17–21 [DOI] [PubMed] [Google Scholar]
- 73.Abood ME, Sorensen RG, Stella N. Endocannabinoids: actions at non-CB1/CB2 cannabinoid receptors. Springer: New York, 2013 [Google Scholar]
- 74.Hájos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience 2001;106:1–4 [DOI] [PubMed] [Google Scholar]
- 75.Monory K, Tzavara ET, Lexime J, et al. . Novel, not adenylyl cyclase-coupled cannabinoid binding site in cerebellum of mice. Biochem Biophys Res Commun 2002;292:231–235 [DOI] [PubMed] [Google Scholar]
- 76.Breivogel CS, Griffin G, Di Marzo V, et al. . Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol 2001;60:155–163 [PubMed] [Google Scholar]
- 77.Fung S, Cherry AE, Xu C, et al. . Alkylindole-sensitive receptors modulate microglial cell migration and proliferation. Glia 2015;63:1797–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fung S, Xu C, Hamel E, et al. . Novel indole-based compounds that differentiate alkylindole-sensitive receptors from cannabinoid receptors and microtubules: characterization of their activity on glioma cell migration. Pharmacol Res 2017;115:233–241 [DOI] [PubMed] [Google Scholar]
- 79.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 2010;58:1017–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cudaback E, Marrs W, Moeller T, et al. . The expression level of CB1 and CB2 receptors determines their efficacy at inducing apoptosis in astrocytomas. PLoS One 2010;5:e8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cherry AE, Haas BR, Naydenov AV, et al. . ST-11: a new brain-penetrant microtubule-destabilizing agent with therapeutic potential for glioblastoma multiforme. Mol Cancer Ther 2016;15:2018–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Farran B. An Update on the physiological and therapeutic relevance of GPCR oligomers. Pharmacol Res 2017;117:303–327 [DOI] [PubMed] [Google Scholar]
- 83.Franco R, Martínez-Pinilla E, Lanciego JL, et al. . Basic pharmacological and structural evidence for class A G-protein-coupled receptor heteromerization. Front Pharmacol 2016;7:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scarselli M, Annibale P, McCormick PJ, et al. . Revealing G-protein-coupled receptor oligomerization at the single-molecule level through a nanoscopic lens: methods, dynamics and biological function. FEBS J 2016;283:1197–1217 [DOI] [PubMed] [Google Scholar]
- 85.Vischer HF, Castro M, Pin J-P. G protein-coupled receptor multimers: a question still open despite the use of novel approaches. Mol Pharmacol 2015;88:561–571 [DOI] [PubMed] [Google Scholar]
- 86.Viñals X, Moreno E, Lanfumey L, et al. . Cognitive impairment induced by Delta9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLoS Biol 2015;13:e1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rozenfeld R, Gupta A, Gagnidze K, et al. . AT1R-CB1R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J 2011;30:2350–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bushlin I, Gupta A, Stockton SD, et al. . Dimerization with cannabinoid receptors allosterically modulates delta opioid receptor activity during neuropathic pain. PLoS One 2012;7:e49789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fujita W, Gomes I, Devi LA. Revolution in GPCR signalling: opioid receptor heteromers as novel therapeutic targets: IUPHAR review 10. Br J Pharmacol 2014;171:4155–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hojo M, Sudo Y, Ando Y, et al. . mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: electrophysiological and FRET assay analysis. J Pharmacol Sci 2008;108:308–319 [DOI] [PubMed] [Google Scholar]
- 91.Martínez-Pinilla E, Reyes-Resina I, Oñatibia-Astibia A, et al. . CB1 and GPR55 receptors are co-expressed and form heteromers in rat and monkey striatum. Exp Neurol 2014;261:44–52 [DOI] [PubMed] [Google Scholar]
- 92.Zou S, Somvanshi RK, Kumar U. Somatostatin receptor 5 is a prominent regulator of signaling pathways in cells with coexpression of cannabinoid receptors 1. Neuroscience 2017;340:218–231 [DOI] [PubMed] [Google Scholar]
- 93.Jäntti MH, Mandrika I, Kukkonen JP. Human orexin/hypocretin receptors form constitutive homo- and heteromeric complexes with each other and with human CB1 cannabinoid receptors. Biochem Biophys Res Commun 2014;445:486–490 [DOI] [PubMed] [Google Scholar]
- 94.Ward RJ, Pediani JD, Milligan G. Heteromultimerization of cannabinoid CB(1) receptor and orexin OX(1) receptor generates a unique complex in which both protomers are regulated by orexin A. J Biol Chem 2011;286:37414–37428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci 1997;17:5327–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kearn CS, Blake-Palmer K, Daniel E, et al. . Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol Pharmacol 2005;67:1697–1704 [DOI] [PubMed] [Google Scholar]
- 97.Przybyla JA, Watts VJ. Ligand-induced regulation and localization of cannabinoid CB1 and dopamine D2L receptor heterodimers. J Pharmacol Exp Ther 2010;332:710–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moreno E, Chiarlone A, Medrano M, et al. . Singular location and signaling profile of adenosine A2A-cannabinoid CB1 receptor heteromers in the dorsal striatum. Neuropsychopharmacology 2017. [Epub ahead of print]; DOI: 10.1038/npp.2017 [DOI] [PMC free article] [PubMed]
- 99.Callén L, Moreno E, Barroso-Chinea P, et al. . Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J Biol Chem 2012;287:20851–20865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Balenga NA, Martínez-Pinilla E, Kargl J, et al. . Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signaling. Br J Pharmacol 2014;171:5387–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moreno E, Andradas C, Medrano M, et al. . Targeting CB2-GPR55 receptor heteromers modulates cancer cell signaling. J Biol Chem 2014;289:21960–21972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pazos MR, Mohammed N, Lafuente H, et al. . Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: role of 5HT(1A) and CB2 receptors. Neuropharmacology 2013;71:282–291 [DOI] [PubMed] [Google Scholar]
- 103.Coke CJ, Scarlett KA, Chetram MA, et al. . Simultaneous activation of induced heterodimerization between CXCR4 chemokine receptor and cannabinoid receptor 2 (CB2) reveals a mechanism for regulation of tumor progression. J Biol Chem 2016;291:9991–10005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mackie K. Cannabinoid receptor homo- and heterodimerization. Life Sci 2005;77:1667–1673 [DOI] [PubMed] [Google Scholar]
- 105.Wager-Miller J, Westenbroek R, Mackie K. Dimerization of G protein-coupled receptors: CB1 cannabinoid receptors as an example. Chem Phys Lipids 2002;121:83–89 [DOI] [PubMed] [Google Scholar]
- 106.Xu W, Filppula S, Mercier R, et al. . Purification and mass spectroscopic analysis of human CB1 cannabinoid receptor functionally expressed using the baculovirus system. J Pept Res 2005;66:138–150 [DOI] [PubMed] [Google Scholar]
- 107.Filppula S, Yaddanapudi S, Mercier R, et al. . Purification and mass spectroscopic analysis of human CB2 cannabinoid receptor functionally expressed using the baculovirus system. J Pept Res 2004;64:225–236 [DOI] [PubMed] [Google Scholar]
- 108.Singh J, Song Z-H, Reggio PH. Structure of a cannabinoid receptor subtype 2 homodimer determined by cysteine and homobifunctional crosslinking experiments combined with computational studies. Biophys J 2012;102:244a [Google Scholar]
- 109.Zvonok N, Yaddanapudi S, Williams J, et al. . Comprehensive proteomic mass spectrometric characterization of human cannabinoid CB2 receptor. J Proteome Res 2007;6:2068–2079 [DOI] [PubMed] [Google Scholar]
- 110.Ferré S, Casadó V, Devi La, et al. . G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev 2014;66:413–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Franco R, Martínez-Pinilla E, Ricobaraza A, et al. . Challenges in the development of heteromer-GPCR-based drugs. Prog Mol Biol Transl Sci 2013;117:143–162 [DOI] [PubMed] [Google Scholar]
- 112.Hiller C, Kühhorn J, Gmeiner P. Class A G-protein-coupled receptor (GPCR) dimers and bivalent ligands. J Med Chem 2013;56:6542–6559 [DOI] [PubMed] [Google Scholar]
- 113.Hübner H, Schellhorn T, Gienger M, et al. . Structure-guided development of heterodimer-selective GPCR ligands. Nat Commun 2016;7:12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shonberg J, Scammells PJ, Capuano B. Design strategies for bivalent ligands targeting GPCRs. ChemMedChem 2011;6:963–974 [DOI] [PubMed] [Google Scholar]
- 115.Fulton BS, Knapp BL, Bidlack JM, et al. . Effect of linker substitution on the binding of butorphan univalent and bivalent ligands to opioid receptors. Bioorg Med Chem Lett 2010;20:1507–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang B, Zhang T, Sromek AW, et al. . Synthesis and binding affinity of novel mono- and bivalent morphinan ligands for κ, μ, and δ opioid receptors. Bioorg Med Chem 2011;19:2808–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gogoi S, Biswas S, Modi G, et al. . Novel bivalent ligands for D2/D3 dopamine receptors: significant cooperative gain in D2 affinity and potency. ACS Med Chem Lett 2012;3:991–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huber D, Löber S, Hübner H, et al. . Bivalent molecular probes for dopamine D2-like receptors. Bioorg Med Chem 2012;20:455–466 [DOI] [PubMed] [Google Scholar]
- 119.Birnkammer T, Spickenreither A, Brunskole I, et al. . The bivalent ligand approach leads to highly potent and selective acylguanidine-type histamine H2 receptor agonists. J Med Chem 2012;55:1147–1160 [DOI] [PubMed] [Google Scholar]
- 120.Fernández-Fernández C, Decara J, Bermúdez-Silva FJ, et al. . Description of a bivalent cannabinoid ligand with hypophagic properties. Arch Pharm 2013;346:171–179 [DOI] [PubMed] [Google Scholar]
- 121.Huang G, Pemp D, Stadtmüller P, et al. . Design, synthesis and in vitro evaluation of novel uni- and bivalent ligands for the cannabinoid receptor type 1 with variation of spacer length and structure. Bioorg Med Chem Lett 2014;24:4209–4214 [DOI] [PubMed] [Google Scholar]
- 122.Zhang Y, Gilliam A, Maitra R, et al. . Synthesis and biological evaluation of bivalent ligands for the cannabinoid 1 receptor. J Med Chem 2010;53:7048–7060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fernández-Fernández C, Callado LF, Girón R, et al. . Combining rimonabant and fentanyl in a single entity: preparation and pharmacological results. Drug Design Devel Ther 2014;8:263–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Le Naour M, Akgün E, Yekkirala A, et al. . Bivalent ligands that target μ opioid (MOP) and cannabinoid1 (CB1) receptors are potent analgesics devoid of tolerance. J Med Chem 2013;56:5505–5513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perrey DA, Gilmour BP, Thomas BF, et al. . Toward the development of bivalent ligand probes of cannabinoid CB1 and Orexin OX1 receptor heterodimers. ACS Med Chem Lett 2014;5:634–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Glass M, Govindpani K, Furkert DP, et al. . One for the price of two…are bivalent ligands targeting cannabinoid receptor dimers capable of simultaneously binding to both receptors? Trends Pharmacol Sci 2016;xx:1–11 [DOI] [PubMed] [Google Scholar]
- 127.Lane JR, Beukers MW, Mulder-Krieger T, et al. . The endocannabinoid 2-arachidonylglycerol is a negative allosteric modulator of the human A3 adenosine receptor. Biochem Pharmacol 2010;79:48–56 [DOI] [PubMed] [Google Scholar]
- 128.Christopoulos A, Wilson K. Interaction of anandamide with the M1 and M4 muscarinic acetylcholine receptors. Brain Res 2001;915:70–78 [DOI] [PubMed] [Google Scholar]
- 129.Boger DL, Patterson JE, Jin Q. Structural requirements for 5-HT2A and 5-HT1A serotonin receptor potentiation by the biologically active lipid oleamide. Proc Natl Acad Sci U S A 1998;95:4102–4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kathmann M, Flau K, Redmer A, et al. . Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol 2006;372:354–361 [DOI] [PubMed] [Google Scholar]
- 131.Cascio MG, Gauson LA, Stevenson LA, et al. . Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol 2010;159:129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cascio MG, Zamberletti E, Marini P, et al. . The phytocannabinoid, Δ9-tetrahydrocannabivarin, can act through 5-HT1A receptors to produce antipsychotic effects. Br J Pharmacol 2015;172:1305–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fong TM, Shearman LP, Stribling DS, et al. . Pharmacological efficacy and safety profile of taranabant in preclinical species. Drug Dev Res 2009;70:349–362 [Google Scholar]
- 134.Hua T, Vemuri K, Pu M, et al. . Crystal structure of the human cannabinoid CB1. Cell 2016;167:750–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shao Z, Yin J, Chapman K, et al. . High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 2016;540:602–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hua T, Vemuri K, Nikas SP, et al. . Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature 2017;547:468–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
References
Cite this article as: Morales P, Reggio PH (2017) An update on non-CB1, non-CB2 cannabinoid related G-protein-coupled receptors, Cannabis and Cannabinoid Research 2:1, 265–273, DOI: 10.1089/can.2017.0036.