Abstract
Microsporidiosis are diseases caused by opportunistic intracellular fungi in immunosuppressed individuals, as well as in transplanted patients, the elderly and children, among others. Diabetes mellitus (DM) is a metabolic disease characterized by hyperglycemia and decreased T cell response, neutrophil function, humoral immunity failure, increasing the susceptibility to infections. Here, we investigated the susceptibility of streptozotocin (STZ)-induced type I diabetic and/or immunosuppressed mice to encephalitozoonosis by Encephalitozoon cuniculi. Microscopically, granulomatous hepatitis, interstitial pneumonia and pielonephritis were observed in all infected groups. STZ treatment induced an immunossupressor effect in the populations of B (B-1 and B2) and CD4+ T lymphocytes. Moreover, infection decreased CD4+ and CD8+ T lymphocytes and macrophages of DM mice. Furthermore, infection induced a significant increase of IL-6 and TNF-α cytokine serum levels in DM mice. IFN-γ, the most important cytokine for the resolution of encephalitozoonosis, increased only in infected mice. In addition to the decreased immune response, DM mice were more susceptible to encephalitozoonosis, associated with increased fungal burden, and symptoms. Additionally, cyclophosphamide immunosuppression in DM mice further increased the susceptibility to encephalitozoonosis. Thus, microsporidiosis should be considered in the differential diagnosis of comorbidities in diabetics.
Introduction
The Filum Microsporidia comprises more than 1.200 species belonging to the kingdom Fungi. Microsporidia are intracelular pathogens, causing microsporidiosis, mostly encephalitozoonosis [1]. In fact, these diseases have been mainly described as opportunistic in humans and other mammals, such as those with HIV/AIDS, which may develop diarrhea and weight loss [1–3]. The main clinical syndromes include: keratoconjuntivitis, pneumonia, enteritis, nephritis, myositis, meningoencephalitis and disseminated infection [3].
Immune response against Encephalitozoon sp. is a cooperative result of adaptive and innate immune responses [4]. T cells are critical for protection against E. cuniculi infection, especially CD8+ T cytotoxic lymphocytes [5]. In fact, T CD8-/- knockout mice are more susceptible to encephalitozoonosis, with severe clinical signs (ascitis, lethargy and weight loss). On the other hand, CD4-/- T mice infected with E. cuniculi are resistant as well as in control animals without T cell deficiency [6].
Encephalitozoon sp. is an opportunist pathogen in immunosuppressed individuals, the elderly and children [7]. Microsporidiosis are not limited to the immunodefficient status, since infection by these pathogens have also been described in immunocompetent individuals [4]. A recent study that investigated parasites in stool samples from 100 DM patients found three diagnosed cases of microsporidiosis [8].
DM is a clinical syndrome associated with deficiency in secretion and/or action of insulin. It is considered an emergent health problem of the 21th century, with about 422 million affected people [9]. Besides typical clinical complications, DM decreases the immune response in T cells, neutrophils, and causes humoral immunity changes, increasing the susceptibility to infection and further disease development [10–14].
Some infections are very prevalent in people with diabetes, such as respiratory infections by Streptococcus pneumonie, pneumonia due to Influenza virus and Mycobacterium tuberculosis. Furthermore, urinary infections such as asymptomatic bacteriuria, fungal and emphysematous cystitis, gastritis due to H. pylori, and oral and esophagic candidiasis have also been described. Additionally, people with diabetes may also develop more severe symptoms and metabolic complications, such as hypoglycemia, ketoacidosis and coma [15–17]. Moreover, DM people have an increased risk in developing skin and mucosal infections, including those caused by Candida spp. [18,19].
In this study, we reported that DM mice were more susceptible to encephalitozoonosis with a decrease of B and T lymphocytes associated with lower IFN-γ serum levels. Moreover, the cyclophosphamide (Cy) immunosuppressive treatment in DM mice further increased the susceptibility to this microsporidiosis.
Materials and methods
Animals
Specific pathogen free (SPF), 8 weeks old, C57BL/6 male mice were obtained from the “Centro de Desenvolvimento de Modelos Experimentais para Biologia e Medicina” (in Portuguese) from Federal University of São Paulo, Brazil. During the experimental period of 35 days, animals were housed at Paulista University Animal Facility and kept in controlled temperature and humidity in microisolators in SPF conditions.
Ethics statement
All procedures were approved by the ethics committee of UNIP (protocol number 010/16).
Pathogen cultivation
Spores of Encephalitozoon cuniculi genotype I–obtained from Waterborne® Inc. (New Orleans, LA, USA)–were cultivated in rabbit kidney cells (RK-13) at “Laboratório de Culturas Celulares—UNIP” of UNIP. RK cells were kept in Eagle´s medium (Cultilab, Campinas, Brazil) supplemented with bovine fetal serum (BFS) (Cultilab, Campinas, Brazil), non-essential amino acids and pyruvate at 10% and penicillin-streptomycin (Sigma-Aldrich, St Louis, USA), and incubated with 5% CO2 at 37C. Every seven days, cultures supernatants were collected and centrifuged for 30 minutes at 500 g to obtain spores, that were further kept at 4°C in PBS 1x. Spores of E. cuniculi were counted in a Neubauer chamber.
Induction of DM by STZ
Type 1 DM was induced by intra-peritoneal administration of STZ at 50 mg/kg/day, during 5 consecutive days [20, 21]. Ten days after the end of the treatment, 10 μl of blood was collected from the tail of each DM animal. Subsequently, glycemic values were measured with Accu Chek Active glucometer (Roche, Mannheim, Germany). DM condition was determined when the glycemic level was above 250 mg/dl [20]. This was considered day 0 for experimental infection.
Treatment with Cy
The immunosuppression group was treated with an intra-peritoneal injection of Cy twice per week (75 mg/kg) (Genuxal®, Asta Medica Oncologia, São Paulo, Brazil), starting at day 0 [22,23].
Experimental infection
Animals were divided in the following experimental groups: mice infected with E. cuniculi (Infected); mice treated with Cy and infected with E. cuniculi (Cy-Infected); DM mice and infected with E. cuniculi (DM-Infected), and DM mice treated with Cy and infected with E. cuniculi (DM-Cy-Infected). Infection was done by the I.P. route with 1x107 spores of E. cuniculi at day 0. Uninfected controls were also kept at the same conditions already described: Uninfected, Cy-Uninfected, DM-Uninfected and DM-Cy-Uninfected. During all experimental period (35 days) the blood glucose, temperature and weight were measured, in the mornings without previous fasting.
Necropsy and tissue sampling
At 35 days post infection, all animals were humanely euthanatized with ketamine (50 mg/ml), xylazine (20 mg/ml) and fentanyl (0,05 mg/ml) injected by the I.P. route. About 1 ml of blood was collected by intra-cardiac puncture. Subsequently, cells were obtained from the peritoneal cavity (PerC) by successive washes of the PerC with 2 ml of PBS supplemented with 2% BFS, until the volume of 10 ml. Afterwards, half of the spleen was collected and macerated in a 70 μm cell strainer. Samples of the spleen, liver, kidneys, lungs and duodenum were fixed in 10% buffered formalin solution for 72 hours and routinely processed for histopathology. All tissue samples were colored with HE procedure and evaluated under light microscopy. The hepatic lesions were photographed at x400 magnification using Opticam® photomicroscope. Five images from “hot spots” region were choosing for morphometric analysis. The lesions were manually delineated using the cursor and the area automatically calculated by MetaMorph Software.
Phenotypical analysis of immune cells
Pellets obtained from the spleen and PerC were centrifuged at 550 g for 5 minutes. Supernatants were discarded and red blood cells were removed with 2 ml of hemolytic buffer at room temperature for 5 minutes. Subsequently, 10 ml of PBS-BFS 2% (Cultilab, Campinas, Brazil) was added and centrifugation was performed at 550 g for 5 minutes. Then, aliquots of samples were counted in a Neubauer chamber. To block Fc receptors, anti-CD16/CD32 antibody was diluted in PBS supplemented with bovine serum albumin (BSA) 1%. Afterwards, cells were washed and further incubated with the following monoclonal antibodies: rat anti-mouse CD19 Peridinin Chlorophyll Protein (PerCP)- conjugated or Allophycocyanin (APC)- conjugated, rat anti-mouse CD23 Phycoeritrin (PE)- conjugated, rat anti-mouse F4/80 APC- conjugated, rat anti-mouse CD11b APC-Cyano Dye 7 (APC-Cy7)- conjugated, rat anti-mouse CD4 PerCP- conjugated and rat anti-mouse CD8 Fluorescein isothiocyanate (FITC)- conjugated (BD-Pharmingen, San Diego, USA). Cell phenotypes were determined as follows: macrophages (CD19-CD11b+F4/80+), B-1 cells (CD23-CD19+), B-2 cells (CD23+CD19+), pre B-1 cells-derived phagocyte (Pre B-1 CDP) (CD19+CD11b+F4/80+) [20], CD4+ T lymphocytes (CD19-CD4+) and CD8+ T lymphocytes (CD19-CD8+). After 20 minutes at 4°C, cells were washed and re-suspended in 300 μl of PBS for flow cytometry acquisition. Gates for cell characterization were determined based on patterns of cell size and granularity. Data was acquired with FACSCanto II (BD Biosciences, Mountain View, USA) at UNIFESP (Discipline of Immunology) and the software FlowJo was used for data analysis (FlowJo LLC, Data Analysis Software, Ashland, USA).
Cytokine quantification
The serum from each animal was collected and stored at −20°C. “CBA Mouse Th1/Th2/Th17 Cytokine Kit” (BD Biosciences, Mountain View, CA, USA) was used according to the manufacturer’s instructions. Briefly, 25 μl of serum from each animal was mixed with each capture bead (IL-2, IL-4, IL-6, IL-10, IL-17, IFN-γ and TNF-α) conjugated with APC and the PE secondary antibody for 2 hours at room temperature under light protection. Subsequently, samples were washed, centrifuged and re-suspended with wash buffer for acquisition with the Flow Cytometer FACSCanto II (BD Biosciences, Mountain View, CA, USA). Analysis was done with the software FCAP Array 3.0. Only the detected cytokines and statistically different results were represented in graphics.
Fungal burden
To investigate the level of infection in the experimental groups, E. cuniculi spores were quantified in the PerC wash of infected mice (see PerC processing above). Briefly, 10 μl from PerC suspension were used for spore visualization of counting by using the fluorescent staining Calcofluor (Sigma-Aldrich, St Louis, USA). Fungal burden was determined by randomly selecting and photographing 10 different fields at 400x. Mean of spores counted were represented in graphics.
Statistical analysis
The normality was calculated with the Shapiro-Wilk test, and the homogeneity of the variance between the groups was verified by the Levene test. Variance analysis (ANOVA) of one and two ways was done, with Tukey post-tests. Confidence intervals were 95% of the media, and analysis was done using the bootstrap tool. In all cases, the level of significance was α<0,05. All analyses were done with the software “IBM SPSS Statistics” version 21.0 for Windows® (IBM Corporation, Armonk, EUA). GraphPad Prism software (GraphPad Software Inc, La Jolla, USA), version 5.0 for Windows ® or Microsoft Excel were used to build the graphics.
Results
Diabetic mice showed more symptoms and increased fungal burden
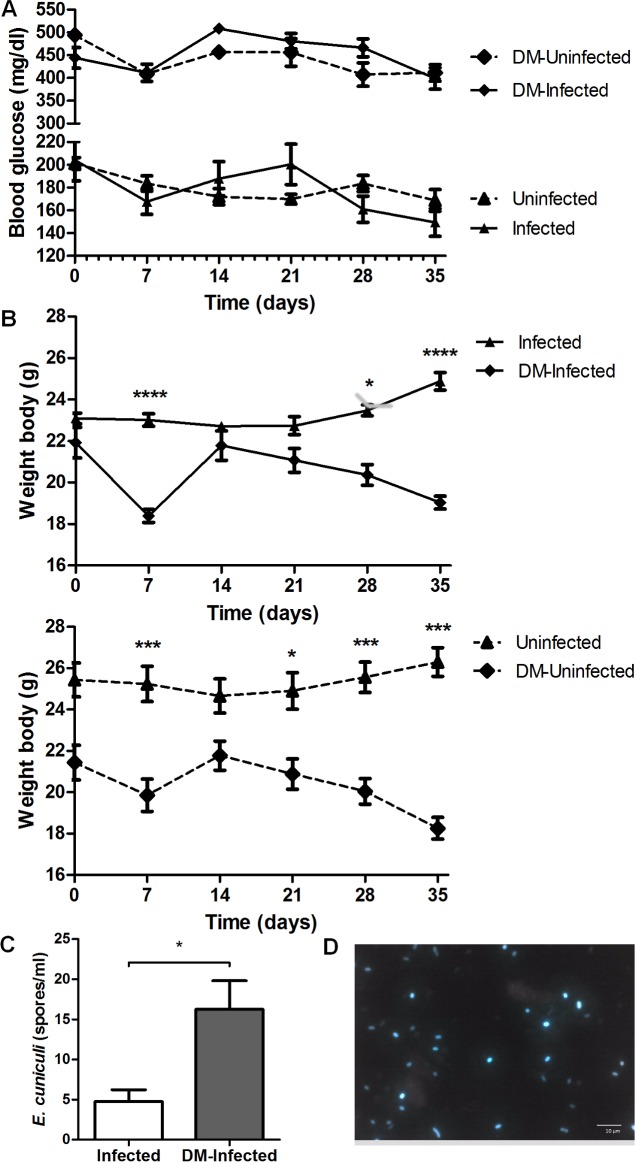
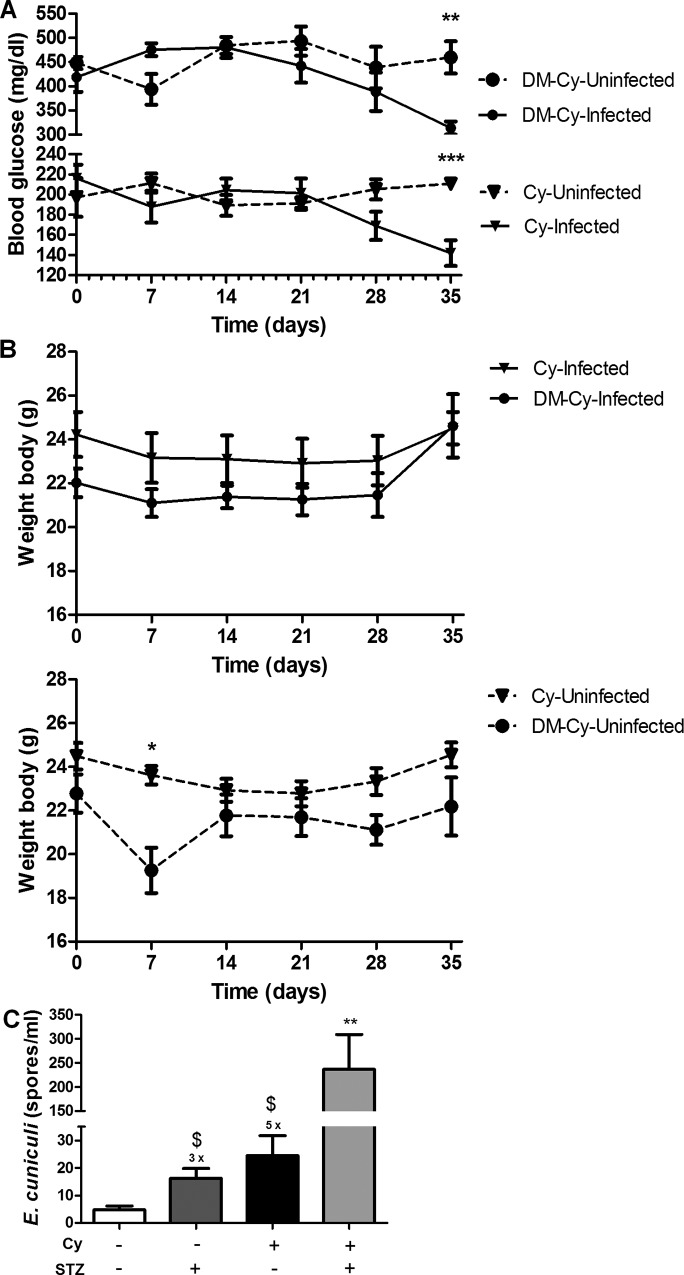
Experimental infection of diabetic mice was performed to investigate the susceptibility to encephalitozoonosis in chronic metabolic diseases such as DM. The animals treated with STZ that developed diabetes after 10 days of the end of treatment, with blood glucose levels higher then 250 mg/dl were included in this study (Fig 1A). As expected non diabetic mice had normal levels (below to 250 mg/dl). Moreover, the median of body weight in all diabetic (infected and non infected) mice was lower than in non-diabetic mice. Weight loss was more severe by the end of the experiment (Fig 1B).
Fig 1. Blood glucose, body weight and fungal burden parameters in mice of experimental groups: Infected, Uninfected, DM-Uninfected and DM-Infected.
A) Blood glucose variation between groups during all experimental period. B) Body weight variation of DM-Infected and Infected mice and its respective controls. One way variance analysis (ANOVA) with Tukey post test showed *** p < 0,01. C) Fungal burden in Infected and DM-Infected mice by E. cuniculi. Non-parametric t-test showing * p<0.01, comparing each group of each period, separately. D) E. cuniculi spores observed in the PerC of DM-Infected mice stained by Calcofluor fluorescent staining.
Serosanguinous exudate was observed only in DM mice and infected with E. cuniculi (DM-Infected). No symptoms were observed in the other groups. Moreover, a higher fungal burden was observed in DM-Infected than infected mice, showing that DM promotes more susceptibility to encephalitozoonosis and disease progression (Fig 1C and 1D).
DM influences the microscopic lesions associated to E. cuniculi infection
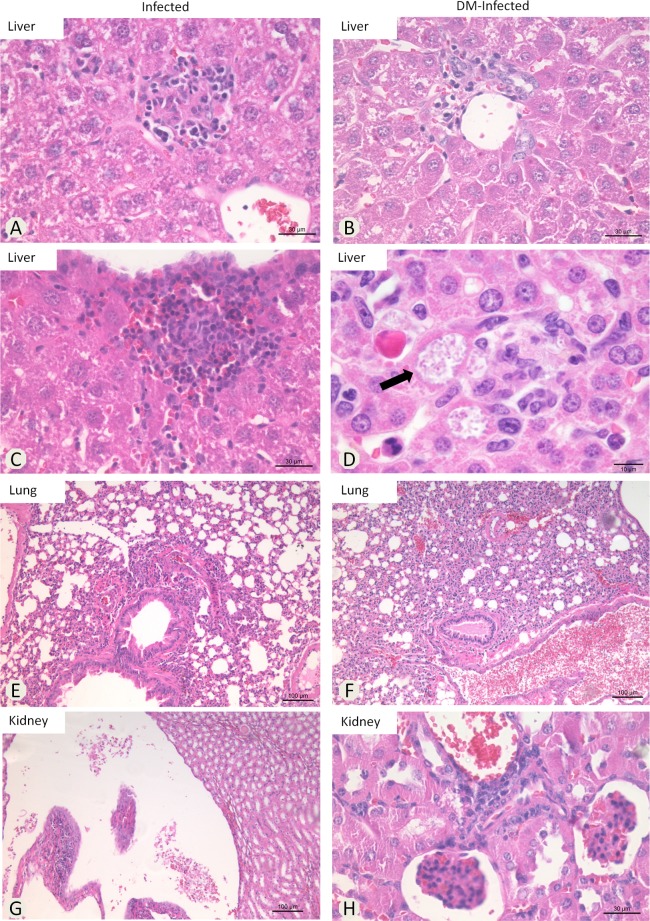
Granulomatous multifocal hepatitis was observed randomly in all E. cuniculi infected groups (DM-Infected and Infected) (Fig 2A, 2B and 2C). Spores clusters were observed associated or not with inflammatory infiltrates (Fig 2D) in DM-Infected mice. Interstitial multifocal pneumonia was observed in all Infected groups (Fig 2E); these lesions were more severe in DM-Infected animals (Fig 2F). Renal lesions included pielonephritis (Fig 2G) and multifocal interstitial nephritis (Fig 2H). Lesions into the liver were measured in all infected groups. The statistical analysis did not demonstrate significant differences between the areas calculated (Table 1). However, we observed more images containing granulomatous infiltrate in Infected mice compared with the DM-Infected group. No lesions were observed in Uninfected mice.
Fig 2. Photomicrographs of histopathological lesions in E. cuniculi Infected and DM-Infected mice.
Liver—mononuclear inflammatory infiltrate located at A) parenchyma, B) portal vein, C) under capsule and D) E. cuniculi clusters into inflammatory infiltrate. Lungs–E and F) interstitial pneumonia. Kidney–G) pyelonephritis and H) nephritis (HE).
Table 1. Morphometric analyses of hepatic lesions from E. cuniculi infected mice.
Lesions´ area of the liver from animals treated or not with cyclophosphamide (Cy) and treated or not with STZ to development of Type 1 Diabetes mellitus (DM).
| group/hepatic lesion | area * | standard deviation* |
|---|---|---|
| Infected | 1.230 | .300 |
| Cy-infected | .746 | .197 |
| DM-infected | 1.017 | .775 |
| Cy-DM-infected | 1.183 | .888 |
*pixels, x106; No statistical difference were observed between the groups (ANOVA/Tukey-Kramer).
DM decreased immune cells population in the peritoneum and spleen
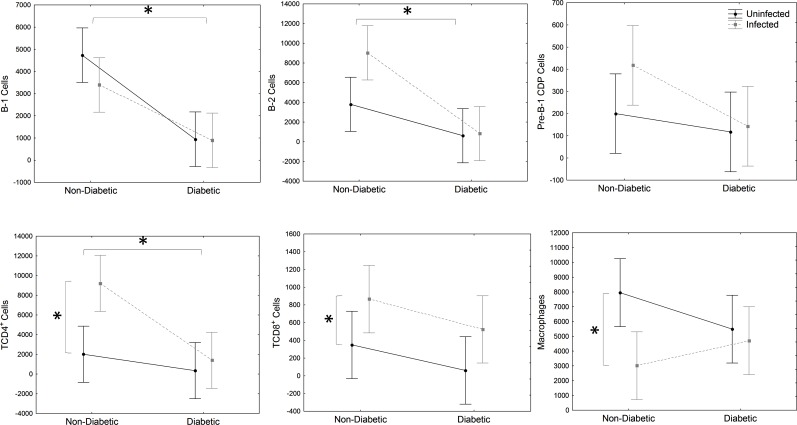
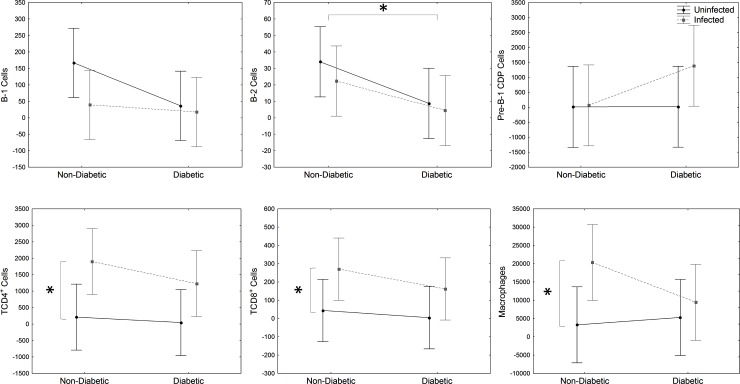
Phenotypical cell analysis included the quantification of macrophages, B and T lymphocytes. Pre-B-1 CDP is a stage of differentiation of B-1 in B-1 CDP, which is described in infection and also diabetes mice model [20,21]. There was a significant decrease of peritoneal B-1, B-2 and CD4+ T lymphocytes of DM and DM-Infected mice than non-diabetic (Infected and Uninfected) mice (Fig 3), showing that DM condition decreased the immune response.
Fig 3. Peritoneal immune cell analysis.
Evaluation of B-1 (CD23-CD19+), B-2 (CD23+CD19+), Pre-B-1CDP (CD19+CD11b+F4/80+) cells, CD4+ (CD19-CD8-CD4+), CD8+ (CD19-CD4-CD8+) T lymphocytes and macrophages (CD19-F4/80+CD11b+) from PerC of STZ-induced DM mice infected with E. cuniculi compared with its controls. Two ways variance analysis (ANOVA) revealed * p<0,05.
Moreover, when comparing Infected and DM-Infected groups with its controls (Uninfected and DM-Uninfected), CD4+ T and CD8+ T cells increased (Fig 3). However, macrophages decreased significantly in Infected and DM-Infected compared to the other groups (Fig 3). There was no significant change regarding B-1, Pre-B-1 CDP and B-2 cells in infection.
These data suggest a possible immunosuppressive effect of diabetic status in B and T lymphocytes in the peritoneal cavity, which may have increased the susceptibility to encephalitozoonosis.
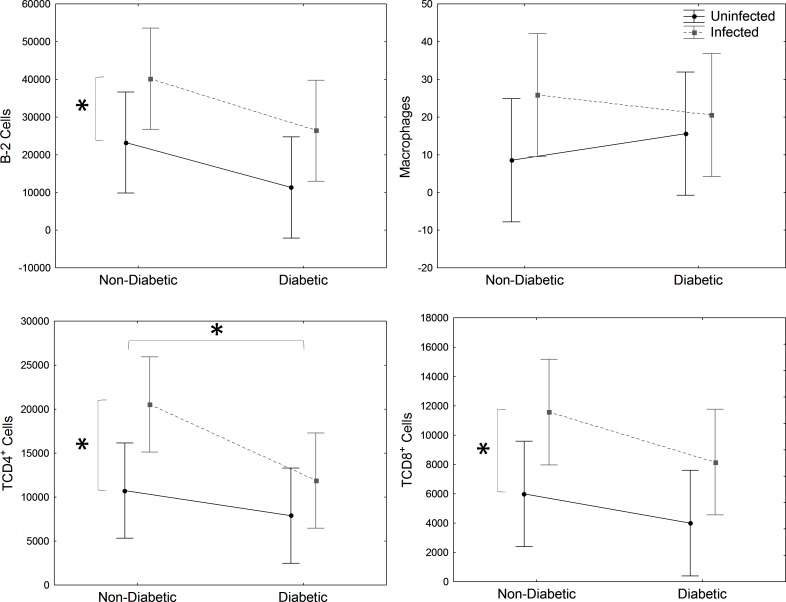
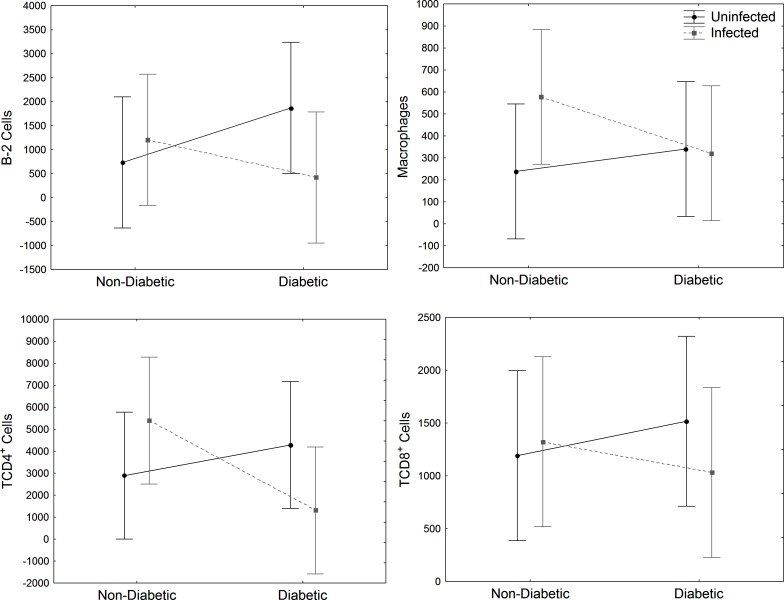
In the spleen, a higher quantity of B-2, CD4+ and CD8+ T cells was observed in infected mice (Infected and DM-infected) than uninfected mice (Uninfected and DM-Uninfected) (Fig 4). However, CD4+ T cells decrease in DM and DM-Uninfected mice (Fig 4). There was no difference in macrophages population.
Fig 4. Spleen immune cell analysis.
Evaluation of B-2 (CD23+CD19+) cells, CD4+ (CD19-CD8-CD4+) and CD8+ T lymphocytes (CD19-CD4-CD8+) and macrophages (CD19-F4/80+CD11b+) from spleen of STZ-induced DM mice infected with E. cuniculi compared with its controls. Two ways variance (ANOVA) analysis revealed p<0,05*.
DM E. cuniculi infected mice showed important decrease in IFN-γ levels
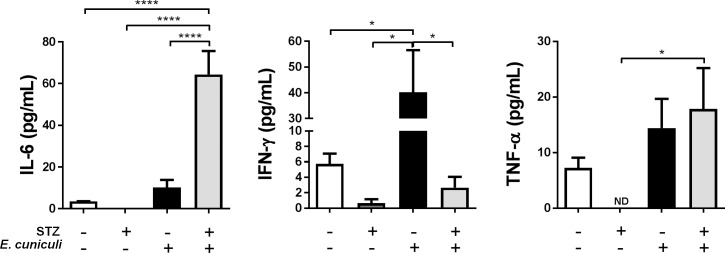
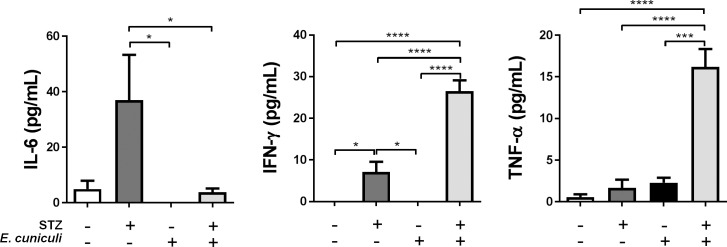
E. cuniculi infection significantly increased pro-inflammatory Th1 cytokines, mainly IFN-γ in Infected group (Fig 5), which is the most important cytokine for the resolution of encephalitozoonosis. In DM-Infected mice IFN- γ levels were decreased and IL-6 and TNF-α were increased (Fig 5).
Fig 5. IL-6, IFN-γ and TNF-α cytokines levels in the serum of STZ-induced DM mice infected with E. cuniculi compared with its controls mice.
+ and–means presence and absence, respectively, of STZ treatment and E. cuniculi infection. Two ways variance analysis (ANOVA) revealed * p<0,05 and **** p<0,0001.
Cy immunosuppression becomes DM-Infected mice more sick
Immunosuppression may increase the susceptibility to encephalitozoonosis in both humans and animals. Thus, we further investigated the effects of immunosuppression by Cy in DM mice infected with E. cuniculi (DM-Cy-Infected). The blood glucose levels were above 400 mg/dl during the experimental period, except at 35 DPI, when it decreased (313 mg/dl), especially compared to DM-Cy-Uninfected mice (459 mg/dl) (Fig 6). The same was observed in non-diabetic mice, since the blood glucose mean levels of Cy-Infected were lower (142 mg/dl) than Cy-Uninfected (210 mg/dl) (Fig 6A). These data suggest that encephalitozoonosis associated with Cy immunosuppression caused a metabolic change decreasing the blood glucose levels of DM mice. On the other hand, DM-Cy-Infected mice were sicker than other groups Cy or non-Cy, as observed in the parameters described below. The body weight not changed in Cy immunosuppressed mice both DM and non-DM (Fig 6B), probably because this mice showed more pronounced ascites.
Fig 6. Blood glucose, body weight and fungal burden parameters in mice immunosuppressed with cyclophosphamide (Cy): Cy-Infected, Cy-Uninfected, DM-Cy-Uninfected and DM-Cy-Infected.
A) Blood glucose variation between immunosuppressed groups during all experimental period. B) Body weight variation of DM-Cy-Infected and Cy-Infected mice and its respective controls. C) Fungal burden in the PerC of E. cuniculi infected mice. + and–means presence or absence, respectively, of Cy treatment and E. cuniculi infection x means time folds compared to Uninfected (-/-) group. Non parametric t-test showed * p<0,05 and ** p<0,01 (A e B) and One way variance analysis (ANOVA) revealed $ p<0,05 comparing with uninfected groups and ** p<0,01 compared to the other groups.
Cy-Infected mice showed a serosanguinous exsudate and splenomegaly. However, a large abdominal distention with abundant serosanguinous exsudate, lethargy and apathy were observed in DM-Cy-Infected mice. Although deaths were not observed, animals were euthanized due to poor clinical conditions in DM-Cy-Infected group at 35 DPI. No clinical symptoms were observed in Uninfected controls. A higher fungal burden (5-folds) was observed in Cy-Infected mice compared to Infected mice (Fig 6C). Moreover, DM-Cy-Infected had a 30-folds higher fungal burden than Infected mice (Fig 6C), showing that immunosuppression plus DM condition increased the susceptibility to encephalitozoonosis development. Histopathological hepatic lesions in DM-Cy-infected mice were similar to DM-infected mice (Table 1).
Cy treatment significantly decreased B-1, B-2, pre-B-1 CDP, CD4+ and CD8+ T cells in the peritoneum and spleen of Cy-Uninfected mice compared to Uninfected control, showing a lymphocytic immunosuppressive effect (p<0,05). Only splenic macrophages increased due to Cy treatment (p<0,05).
Peritoneal B-2 cells decreased significantly in DM-Cy-Infected and DM-Cy-Uninfected compared to non-DM groups (Cy-Infected e Cy-Uninfected) (Fig 7). Although the population of immune cells in the peritoneum decreased by Cy treatment, E. cuniculi infection increased CD4+ and CD8+ T cells and also macrophages in Cy-Infected and DM-Cy-Infected mice compared to uninfected controls (Cy-Uninfected and DM-Cy-Uninfected), showing the importance of these cells against microsporidiosis, even in an immunosuppressive condition (Fig 7). There are no differences pointed in the spleen cells population analyzed (Fig 8).
Fig 7. Peritoneal immune cell analysis.
Evaluation of B-1 (CD23-CD19+), B-2 (CD23+CD19+), Pre-B-1CDP (CD19+F4/80+CD11b+) cells, CD4+ (CD19-CD8-CD4+) and CD8+ (CD19-CD4-CD8+) T lymphocytes and macrophages (CD19-F4/80+CD11b+) from PerC of Cy immunosuppressed and STZ-induced DM mice infected with E. cuniculi compared with its controls. Two ways variance analysis (ANOVA) revealed * p<0,05.
Fig 8. Spleen immune cell analysis.
Evaluation of B-2 (CD23+CD19+) cells, CD4+ (CD19-CD8-CD4+), and CD8+ (CD19-CD4-CD8+) T lymphocytes and macrophages (CD19-F4/80+CD11b+) in spleen Cy immunosuppressed and STZ-induced DM mice infected with E. cuniculi compared with its controls. Two ways variance analysis (ANOVA) revealed * p<0,05.
E. cuniculi infection increased IFN-γ and TNF-α serum levels in Cy-DM-Infected animals compared to the other groups (Fig 9). Additionally, IL-6 and IFN- γ levels increased in DM-Uninfected mice (Fig 9).
Fig 9. IL-6, IFN-γ and TNF-α cytokines levels in the serum of Cy immunosuppressed mice and STZ-induced DM mice infected with E. cuniculi compared with its controls.
+ and–means presence and absence, respectively, of STZ treatment and E. cuniculi infection. One way variance analysis (ANOVA) with Tukey posttest showed * p<0,05, *** p<0,001 and **** p<0,0001.
Discussion
DM is a clinical pathological condition that cause functional abnormalities in patients, such as insufficient immune response against bacterial and mycotic infections [12–15]. Many immunological alterations are related to hipoinsulinemia and hipoglycemia levels in the blood, altering the function of lymphoid organs [10]. For instance, the thymus of DM individuals showed atrophy, loss of cortical-medullar function, increased of extracellular matrix and decreased expression of CCL25 and CXCL12 chemokines [24]. These changes are transient and its detailed clinical importance in DM people has not been fully described. In the present study, DM was induced by STZ in mice that were infected with E. cuniculi. Results showed a higher fungal burden, clinical changes and microscopic lesions in the lung in DM-Infected mice compared to Infected mice. These data demonstrated for the first time that type 1 DM increased the susceptibility to encephalitozoonosis.
HIV-positive, chemotherapy immunosuppressed and diabetes patients, pregnant women, the elderly and children are the mainly risk group to infections by opportunistic pathogens [7,25–27]. So far, the only etiopathological relationship between Microsporidia and Diabetes was a case report of cerebral abscess co-infection due to Encephalitozoon cuniculi genotype I and Streptococcus intermedius in a type II DM patient without immunosuppression [28]. Together, the present results suggest that encephalitozoonosis should be considered an opportunistic disease in DM patients.
The main pathogenic mechanisms associated with the susceptibility of DM individuals to infectious diseases include: the increased virulence due to a hyperglycemia and decreased chemotaxis (interleukins production, leukocyte mobilization and phagocytic activity against infection), glycosuria and intestinal and urinary dysmotility [29].
In DM, innate immune response against infection has been more studied than adaptive immune response. Mice with type 1 DM (STZ-induced) and type 2 DM (due to obesity) infected with Staphylococcus aureus had a more severe disease than its controls, which was related to a decrease in humoral response [30]. Moreover, the increase in disease development in DM individuals has also been associated with a decrease of T cell immune response [18,19]. In our study, B-1, B-2 and CD4+ T cells decreased significantly in the peritoneum of DM-Uninfected and DM-Infected mice compared to non-DM mice (Uninfected and Infected). Previous studies showed a protective effect against encephalitozoonosis mediated by CD8+ T lymphocytes [5,31]. Corroborating these results, we showed increased CD8+ T lymphocytes in the PerC and spleen of DM-Infected and Infected groups. Moreover, previous investigations from our group already showed the importance of B and T lymphocytes in the acquired immunity against encephalitozoonosis [32]. Herein, we suggest that the decrease of B and T populations caused by DM condition may have resulted in increased susceptibility of the encephatozoonosis. Thus, although CD8+ T cells are mainly population against encephalitozoonosis, the decrease of B-1 cells reinforces the importance of this population against this intracellular pathogen.
The effect of glucose against the bacteria Pseudomonas aeruginosa in vivo was already shown in mice with hyperglycemia induced by STZ. Bacterial burden was higher in hyperglycemic animals than its controls and also the metformin treatment reduced both airways glucose and bacterial burden. These data suggest that glucose in the airways is a critical determinant for increased bacterial burden in DM animals [33]. Considering that E. cuniculi is an intracellular pathogen, we speculate that the hyperglycemia could facilitate the microsporidia surveillance in DM mice by in a different way.
In a previous study, we showed that BALB/c mice were more resistant against encephalitozoonosis than BALB/c XID mice, partly due to B-1 cells [32]. Although B-2 cells may not play a crucial role against encephalitozoonosis, antibody levels in infected animals are very high [2], suggesting B-2 cells role in acquired immunity. Therefore, the present results suggested that B-1 and B-2 cells decreased in DM mice could contribute to the increase of encephalitozoonosis susceptibility.
Both in vitro and in vivo studies showed that Th1 inflammatory cytokines (mainly IFN-γ) are crucial against E. cuniculi infection, activating both cytotoxic CD8+ T lymphocytes and macrophages [34,35]. In our study, Infected animals showed a predominant Th1 cytokine response (higher levels of IFN-γ and TNF-α). Th1 cytokines (mainly IFN-γ) develop an important role during microsporidia infection in immunodeficient mice [36], corroborating our results. IFN-γ-/- knockout mice are unable to solving E. cuniculi infection [37] and the adoptively transfer of splenic cells increase their surveillance, suggesting that NK cells producing IFN-γ are important to E. cuniculi destruction [38].
Mononuclear cells from DM people secrete more IL-1 and IL-6 when treated with lipopolysaccharide (LPS) [13]. In the present results, DM-Infected and DM-Cy-Uninfected mice showed a higher level in IL-6, suggesting that DM may also modulate a pro-inflammatory state of the organism. However, other studies reported the decrease in interleukin secretion as a consequence of intrinsic defects in the cells of DM patients [11,39]. The increased glycation in DM individuals may also inhibit IL-10 production by myeloid cells, and IFN-γ /TNF-α by T lymphocytes [40]. The low IL-10 levels may block anti-inflammatory effects on macrophages in DM condition. Also, high levels of IL-6 and IL-12 founded in DM NOD mice address to Th1 pro-inflammatory profile, allowing macrophages precursors in hyperglycemic environment to target to Th1 response even before to entry in infected/inflamed tissue [41]. In our results, DM condition also decreases IFN-γ levels and E. cuniculi infection increases this cytokine in non-DM mice. We speculate that the decrease in IFN-γ in DM mice associated with the decrease in PerC lymphocytes could increase disease susceptibility.
Moreover, Cy treatment in DM-Cy-Infected animals may have caused an immunomodulatory effect, since IFN-γ increased in both DM-Cy-Infected and DM-Cy-Uninfected. Even though, CD8+ T cells decreased significantly in DM-Cy-Infected mice, which was sickest of all infected groups. A higher mortality rate was observed in mice treated with STZ after intra-tracheal Klebsiella pneumonia infection [14]. There was a decrease in granulocyte chemotaxis to the alveolar space associated with a decreased of chemokines (CXCL1, CXCL2) and cytokines (IL-1β and TNF-α). This result suggests a failure in recruiting granulocytes to the infection, increasing the susceptibility to K. pneumoniae pneumonia [14]. The present results showed an increase of TNF-α in DM-Infected mice, however, as described above, this cytokine is not the most effective against encephalitozoonosis.
In previous investigations, we showed that mice immunosuppressed with drugs are important biological models for the study of murine encephalitozoonosis, since immunosuppression favors disease progression, mimicking natural conditions of immunosuppression [22,23,42]. Cy is a cytotoxic alkylating agent, causing cell apoptosis, delaying and suppressing lymphocytic immune response, causing myelosuppression, decreasing neutrophils, T and B lymphocytes, erythrocytes and platelets [43]. In our study, B and T cells decreased significantly by Cy treatment, which was associated with higher fungal burden than infected non-Cy mice, as previously reported. Additionally, Cy treatment may allow depletion of B and T lymphocytes, following extensive mobilization of bone marrow, with cell recovery and proliferation [44,45]. In our study, E. cuniculi infection in Cy-treated mice caused CD4+, CD8+ T cells and macrophage increase, which may be due to this secondary bone marrow stimulatory effect together with infection. However, the number of these cells was lower than Infected mice, which may further explain why Cy increases disease susceptibility.
We observed a metabolic change probably caused by the association of encephalitozoonosis and Cy treatment, carrying on to a blood glucose decrease of both DM and non-DM mice. Microsporidia influences the biochemistry environmental of the host with energetic costs to fungal. Microsporidia uses ATP, since E. cuniculi are frequently observed surrounded by mitochondrias in the cytoplasm of parasitized cells [46]. Even though Microsporidia have reminiscent mitochondria (or mitosome), they need to parasitize a cell to replicate. In fact, there are four ATP transporters in microsporidia. One of them is located in mitosome membrane. The ATP produced by host cell is transported from mitochondria to microsporidia cytoplasm or mitosome [47]. Furthermore, it has been reported that microsporidia infection decrease glycogen of the host and also a rapid glucose absorption [48]. These events could be promoted by parasite’s hexokinase into host cells. The hexokinase catalyzes the first step in glycolysis, through the pentose phosphate via. Therefore, the activity of hexokinase of microsporidia inside host cell could increase the synthesis of nucleotides, amino acids and lipids, which are needed for parasite development [49]. We suggest that the metabolic changes due to E. cuniculi infection could be favored by Cy treatment, promoting susceptibility to disease. This hypothesis is under investigation.
In conclusion, the present results showed that STZ-induced DM mice were more susceptible to encephalitozoonosis, probably due to a decrease in B-1, B-2 and CD4+ T cells and decreased IFN-γ levels, consistent with immunosuppressive condition. Overall, E. cuniculi infection caused more severe disease in DM and Cy-treated mice.
Acknowledgments
We would like to thank Magna Aparecida Maltauro Soares (Butantã Institute) for making the material is light microscope. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, FAPESP, Brazil (Process number 2012/51-727-5), to Dr. Maria Anete Lallo.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP, Brazil, (Process number 2012/51727-5), to Dr Maria Anete Lallo. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Anane S, Attouchi H (2010) Microsporidiosis: epidemiology, clinical data and therapy. Gastroenterol Clin Biol 34(8–9):450–464. doi: 10.1016/j.gcb.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 2.Hinney B, Sak B, Joachim A, Kváč M (2016) More than a rabbit's tale—Encephalitozoon spp. in wild mammals and birds. Int J Parasitol Parasites and Wildlife 5(1):76–87. doi: 10.1016/j.ijppaw.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss LM (2014) Clinical syndromes associated with microsporidiosis In: Weiss LM, Becnel JJ, editors. Microsporidia–pathogens of opportunity. Oxford.Wiley: Blackwell, 2014. pp. 371–401. [Google Scholar]
- 4.Moretto M, Weiss LM, Khan IA (2004) Induction of a rapid and strong antigen-specific intraepithelial lymphocyte response during oral Encephalitozoon cuniculi infection. J Immunol 172 (7): 4402–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan IA, Moretto M, Weiss LM. (2001). Immune response to Encephalitozoon cuniculi infection. Microbes Infec 3 (5):401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valencakova A, Halanova M (2012) Immune response to Encephalitozoon infection review. Comp Immunol Microbiol Infect Dis 35(1):1–7. doi: 10.1016/j.cimid.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 7.Didier ES, Weiss LM (2011) Microsporidiosis: not just in AIDS patients. Curr Opin Infect Dis 24(5):490–495. doi: 10.1097/QCO.0b013e32834aa152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elnadi NA, Hassanien HA, Ahmad AM, Abd Ellah AK (2015) Intestinal parasites in diabetic patients in Sohag University Hospitals, Egypt. J Egypt Soc Parasitol 45(2):443–449. [DOI] [PubMed] [Google Scholar]
- 9.OMS 2016. http://www.who.int/diabetes/en
- 10.Delamaire M, Maugendre D, Moreno M, Le Goff M-C, Allannic H, Genetet B (1997) Impaired leucocyte functions in diabetic patients. Diabet Med 14(1):29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- 11.Geerlings SE, Brouwer EC, Van Kessel KC, Gaastra W, Stolk RP, Hoepelman AI (2000) Cytokine secretion is impaired in women with diabetes mellitus. Eur J Clin Invest 30(11):995–1001. [DOI] [PubMed] [Google Scholar]
- 12.Müller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, et al. (2005) Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis 41(13):281–288. doi: 10.1086/431587 [DOI] [PubMed] [Google Scholar]
- 13.Peleg AY, Weerarathna T, McCarthy JS, Davis TM (2007) Common infections in diabetes: Pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev 23(1):3–13. doi: 10.1002/dmrr.682 [DOI] [PubMed] [Google Scholar]
- 14.Martinez N, Ketheesan N, Martens GW, West K, Lien E, Komfeld H (2016) Defects in early cell recruitment contribute to the increased susceptibility to respiratory Klebsiella pneumoniae infection in diabetic mice. Microbe Intect. 18(10):649–655. doi: 10.1016/j.micinf.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyko EJ, Fihn SD, Scholes D, Chen CL, Normand EH, Yarbro P (2002) Diabetes and the risk of urinary tract infection among postmenopausal women. Diabetes Care 25(10):1778–1783. [DOI] [PubMed] [Google Scholar]
- 16.Hu KK, Boyko EJ, Scholes D, Normand E, Chen CL, Grafton J, et al. (2004) Risk factors for urinary tract infections in postmenopausal women. Arch Intern Med 164(9):989–993. doi: 10.1001/archinte.164.9.989 [DOI] [PubMed] [Google Scholar]
- 17.Kowalewska B, Katarzyna Zorena K, Szmigiero-Kawko M, Wąż P, Myśliwiec M (2016) Higher diversity in fungal species discriminates children with type 1 diabetes mellitus from healthy control. Patient Preference and Adherence 10:591–599. doi: 10.2147/PPA.S97852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calvet HM, Yoshikawa TT (2001) Infections in diabetes. Infect Dis Clin North Am 15(2):407–421. [DOI] [PubMed] [Google Scholar]
- 19.Shah BR, Hux JE (2003) Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 26(2):510–513. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhry ZZ, Morris DL, Moss DR, Sims EK, Chiong Y, Kono T, et al. (2013) Streptozotocin is equally diabetogenic whether administered to fed or fasted mice. Lab Anim. 47(4):57–65. doi: 10.1177/0023677213489548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvares‐Saraiva AM, Novo MC, Oliveira VC, Maricato JT, Lopes JD, Popi AF, et al. (2015) B-1 cells produce insulin and abrogate experimental streptozotocin-induced diabetes. Eur J immunol 45(5):1452–1461. doi: 10.1002/eji.201445409 [DOI] [PubMed] [Google Scholar]
- 22.Lallo MA, Hirschfeld MPM (2012) Encephalitozoonosis in pharmacologically immunosuppressed mice. Expl Parasitol 131(3):339–343. doi: 10.1016/j.exppara.2012.04.019 [DOI] [PubMed] [Google Scholar]
- 23.Lallo MA, Bondan EF (2005) Experimental meningoencephalomyelitis by Encephalitozoon cuniculi in cyclophosphamide-immunosuppressed mice. Arq Neuro-Psiq 63 (2A):246–251. [DOI] [PubMed] [Google Scholar]
- 24.Nagib PR, Gameiro J, Stivanin-Silva LG, de Arruda MS, Villa-Verde DM, Savino W, et al. (2010) Thymic microenvironmental alterations in experimentally induced diabetes. Immunobiology 215 (12):971–979. doi: 10.1016/j.imbio.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 25.Kicia M, Wesolowska M, Jakuszko K, Kopacz Z, Sak B, Květonova D, et al. (2014) Concurrent infection of the urinary tract with Encephalitozoon cuniculi and Enterocytozoon bieneusi in a renal transplant recipient. J Clin Microbiol 52(5):1780–1782. doi: 10.1128/JCM.03328-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller A, Bialek R, Kämper A, Fätkenheuer G, Salzberger B, Franzen C (2001) Detection of microsporidia in travelers with diarrhea. J Clin Microbiol 39(4):1630–1632. doi: 10.1128/JCM.39.4.1630-1632.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lores B, López-Miragaya I, Arias C, Fenoy S, Torres J, del Aguila C (2002) Intestinal microsporidiosis due to Enterocytozoon bieneusi in elderly human immunodeficiency virus—negative patients from Vigo, Spain. Clin Infect Dis 34(7):918–921. doi: 10.1086/339205 [DOI] [PubMed] [Google Scholar]
- 28.Ditrich O, Chrdle A, Sak B, Chmelík V, Kubále J, Dyroková I, et al. (2011) Encephalitozoon cuniculi genotype I as a causative agent of brain abscess in an immunocompetent patient. J Clin Microbiol 49(7):2769–2771. doi: 10.1128/JCM.00620-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casqueiro J, Casqueiro J, Alves C (2012) Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 1:S27–36. doi: 10.4103/2230-8210.94253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farnsworth CW, Shehatou CT, Maynard R, Nishitani K, Kates SL, Zuscik MJ, et al. (2015) A humoral immune defect distinguishes the response to Staphylococcus aureus infections in mice with obesity and type 2 diabetes from that in mice with type 1 diabetes. Infect Immun 83(6): 2264–74. doi: 10.1128/IAI.03074-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braunfuchsová P, Salát J, Kopecký J (2001) CD8+ T-lymphocytes protect SCID mice against Encephalitozoon cuniculi infection. Int. J. Parasitol. 31(7):681–686. [DOI] [PubMed] [Google Scholar]
- 32.da Costa LF, Alvares-Saraiva AM, Dell`Armelina Rocha PR, Spadacci-Morena DD, Perez EC, Mariano M, et al. (2017) B-1 cell decreases susceptibility to encephalitozoonosis in mice. Immunobiology 222(2):218–227. doi: 10.1016/j.imbio.2016.09.018 [DOI] [PubMed] [Google Scholar]
- 33.Gill SK, Hui K, Farne H, Garnett JP, Baines DL, Moore LSP, et al. (2016) Increased airway glucose increases airway bacterial load in hyperglycaemia. Sci Rep 8:27636 doi: 10.1038/srep27636 pmid:27273266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawlor EM, Moretto MM, Khan IA (2010) Optimal CD8 T-cell response against Encephalitozoon cuniculi is mediated by Toll-like receptor 4 upregulation by dendritic cells. Infect Immun 78(7):3097–3102. doi: 10.1128/IAI.00181-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Didier ES, Khan IA (2014) The immunology of microsporidiosis in mammals In: Weiss LM, Becnel JJ, editors. Microsporidia–pathogens of opportunity. Oxford.Wiley: Blackwell, 2014. pp. 307–325. [Google Scholar]
- 36.Salát J, Horká H, Sak B, Kopecký J (2006) Pure CD4+ T-lymphocytes fail to protect perorally infected SCID mice from lethal microsporidiosis caused by Encephalitozoon cuniculi. Parasitol Res 99(6):682–686. doi: 10.1007/s00436-006-0208-x [DOI] [PubMed] [Google Scholar]
- 37.Khan IA, Moretto M (1999) Role of gamma interferon in cellular immune response against murine Encephalitozoon cuniculi infection. Infect Immun 67(4):1887–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salát J, Sak B, Le T, Kopecky J (2004) Susceptibility of IFN- gamma or IL-12 knock-out and SCID mice to infection with two microsporidian species, Encephalitozoon cuniculi and E. intestinalis. Folia Parasitol 51(4): 275–282. [PubMed] [Google Scholar]
- 39.Geerlings SE, Hoepelman AI (1999) Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 26(3–4):256–265. [DOI] [PubMed] [Google Scholar]
- 40.Price CL, Al Hassi HO, English NR, Blakemore AI, Stagg AJ, Knight SC (2010) Methylglyoxal modulates immune responses: relevance to diabetes. J Cell Mol Med 14(6B):1806–1815. doi: 10.1111/j.1582-4934.2009.00803.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohammad MK, Morran M, Slotterbeck B, Leaman DW, Sun Y, Grafenstein HV, et al. (2006) Dysregulated Toll-like receptor expression and signaling in bone marrow-derived macrophages at the onset of diabetes in the non-obese diabetic mouse. Inr Immunol 18(7):1101–1113. doi: 10.1093/intimm/dxl045 [DOI] [PubMed] [Google Scholar]
- 42.Lallo MA, Santos MJ, Bondan EF (2002) Infecção experimental pelo Encephalitozoon cuniculi em camundongos imunossuprimidos com dexametasona. Rev Saúde Pub 36(5):621–626. [DOI] [PubMed] [Google Scholar]
- 43.Brodsky RA (2010) High-dose cyclophosphamide for autoimmunity and alloimmunity. Immunol Res 47(1–3):179–184. doi: 10.1007/s12026-009-8149-y [DOI] [PubMed] [Google Scholar]
- 44.Al Emadi, Jones RJ, Brodsky RA (2009) Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol 6(11):638–647. doi: 10.1038/nrclinonc.2009.146 [DOI] [PubMed] [Google Scholar]
- 45.Brode S, Cooke A (2008) Immune-potentiating effects of the chemotherapeutic drug cyclophosphamide. Crit Rev Immunol 28(2):109–126. [DOI] [PubMed] [Google Scholar]
- 46.Weidner E, Canning EU, Rutledge CR, Meek CL (1999) Mosquito (Diptera: Culicidae) host compatibility and vector competency for the human myositis parasite Trachipleistophora hominis (Phylum Microspora). J. Med Entomol. 36(4):422–525. [DOI] [PubMed] [Google Scholar]
- 47.Tsaousis AD, Kunji ER, Goldberg AV, Lucocq JM, Hirt RP, Embley TM (2008) A novel route for ATP acquisition by the remnant mitochondria of Encephalitozoon cuniculi. Nature 22(7194):553–556. doi: 10.1038/nature06903 pmid:18449191 [DOI] [PubMed] [Google Scholar]
- 48.Metenier G, Vivares CP (2001) Molecular characteristics and physiology of microsporidia. Microbes Infect 3(5): 407–415. [DOI] [PubMed] [Google Scholar]
- 49.Cuomo CA, Desjardins CA, Bakowski MA, Goldberg J, Ma AT, Becnel JJ, et al. (2012) Micosporidian genome analysis reveals evolutionary strategies for obligate intracellular growth. Genome Res 22(12):2478–2488. doi: 10.1101/gr.142802.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.