Abstract
The bovine gastrointestinal tract (GIT) is the main reservoir for enterohaemorrhagic Escherichia coli (EHEC) responsible for food-borne infections. Therefore, it is crucial to develop strategies, such as EHEC suppression by antagonistic microorganisms, to reduce EHEC survival in the GIT of cattle and to limit shedding and food contamination. Most human-derived Lactobacillus reuteri strains produce hydroxypropionaldehyde (HPA), an antimicrobial compound, during anaerobic reduction of glycerol. The capacity of L. reuteri LB1-7, a strain isolated from raw bovine milk, to produce HPA and its antimicrobial activity against an O157:H7 EHEC strain (FCH6) were evaluated in bovine rumen fluid (RF) under strict anaerobiosis. EHEC was totally suppressed when incubated in RF inoculated with L. reuteri LB1-7 and supplemented with 80 mM glycerol (RF-Glyc80). The addition of LB1-7 or glycerol alone did not modify EHEC survival in RF. Glycerol was converted to HPA (up to 14 mM) by LB1-7 during incubation in RF-Glyc80, and HPA production appeared to be responsible for EHEC suppression. The bactericidal activity of L. reuteri LB1-7, the concentration of glycerol required and the level of HPA produced depended on physiological and ecological environments. In vitro experiments also showed that EHEC inoculated in rumen fluid and exposed to L. reuteri and glycerol had a very limited growth in rectal contents. However, L. reuteri exerted an antimicrobial activity against the rumen endogenous microbiota and perturbed feedstuff degradation in the presence of glycerol. The potential administration of L. reuteri and glycerol in view of application to finishing beef cattle at the time of slaughter is discussed. Further in vivo studies will be important to confirm the efficiency of L. reuteri and glycerol supplementation against EHEC shedding in ruminants.
Introduction
Enterohaemorrhagic Escherichia coli (EHEC) are Shiga toxin-producing E. coli (STEC) responsible for severe human diseases such as haemolytic uraemic syndrome [1]. The majority of infections, commonly attributed to the consumption of contaminated food is caused by EHEC with serotype O157:H7 and the gut of ruminants, mainly cattle, is considered as the principal reservoir [2]. Therefore, it is important to develop nutritional or ecological strategies to reduce EHEC survival in the gastrointestinal tract (GIT) of cattle and to limit shedding and further contamination of food products.
Several approaches have been proposed to reduce the prevalence of E. coli O157:H7 in cattle, including feeding of antagonistic microorganisms, vaccination or bacteriophage treatment. Lactobacilli, which are known to exhibit an inhibitory effect against various enteric pathogens, are widely used as probiotics or direct-fed microbials in humans and animals [3, 4]. Lactobacilli display antimicrobial activities as a result of production of metabolites such as lactic acid, bacteriocins or other non-proteinaceous molecules [4].
Lactobacillus reuteri is used commercially as a probiotic microorganism and possesses antimicrobial properties against intestinal pathogens. It is well documented that specific L. reuteri can produce antimicrobial factors, such as hydroxypropionaldehyde (HPA) (also named reuterin), reutericyclin, reutericin, lactate or Mucus Adhesion-Promoting (MAP) protein [5, 6]. HPA has been proven effective against bacteria, fungi and protozoa survival [7, 8]. It has been postulated that HPA inhibits the activity of bacterial ribonucleotide reductase, a key enzyme catalysing the first step in DNA synthesis, which would explain the broad-spectrum activity of HPA [7, 9].
HPA is produced by L. reuteri during a two-step anaerobic fermentation of glycerol: a glycerol dehydratase first catalyses the conversion of glycerol to HPA and HPA is then reduced to 1,3 propanediol (1,3-PD) [7, 8]. In addition, L. reuteri is known to excrete HPA in high amounts when the level of fermentable carbohydrates is low [10]. In germfree mice, L. reuteri administration reduced both colonization and clinical signs due to EHEC infection and resulted in HPA production in the caecum [11].
In cattle, the terminal recto-anal junction has been referred as the major site of E. coli O157:H7 multiplication [12]. However, O157:H7 isolates are found throughout the GIT compartments (including the rumen) of experimentally infected calves and naturally shedding cattle [13, 14]. In addition, O157:H7 isolates in the rectum are clonally similar to isolates found in the rumen and fecal shedding has been shown to be positively associated with the presence of E. coli O157:H7 in the rumen [15]. All these observations demonstrate that the rumen is a reservoir for the contamination of hindgut compartments. Cattle regurgitate digesta during the rumination process and E. coli O157:H7 in the rumen may directly participate to EHEC dissemination in environment and to animal-to-animal transmission. In addition, quorum sensing molecules homoserine lactones (AHLs) promote EHEC colonization of the bovine gut [16]. Interestingly, AHLs are produced by the rumen microbiota but are not found in other GIT compartments highlighting the role of the rumen in the successful EHEC colonization of the bovine gut [16]. For all these reasons, among strategies for decreasing EHEC burden in cattle GIT, it can be proposed to focus on reducing EHEC survival in the rumen.
Because the rumen of cows is under strict anaerobiosis and contains a low level of easily fermentable carbohydrates, we speculated that L. reuteri could produce HPA at this site. In this study, we explored for the first time the potential growth inhibition exerted by L. reuteri towards EHEC in bovine rumen fluid. We tested several L. reuteri strains and demonstrated that L. reuteri LB1-7 isolated from raw bovine milk [17] produced HPA and could achieve a total suppression of EHEC in rumen fluid. In vitro experiments described in this study also showed that the exposure of EHEC to L. reuteri and glycerol in rumen fluid decreased drastically EHEC inoculation of lower bovine digestive segments.
Materials and methods
Bacterial strains and growth conditions
L. reuteri and EHEC strains used in this study are listed in S1 Table. The EHEC strain FCH6 (O157:H7, eae+, stx1-, stx2+) had been isolated from a case of HUS in 2004 due to the consumption of raw milk cheese. EHEC strains were routinely cultured in Luria Bertani (LB) broth (Biokar, Beauvais, France). L. reuteri strains were routinely cultured in De Man, Rogosa and Sharpe (MRS) broth (Biokar, Beauvais, France). Spontaneous rifampicin-resistant mutants of L. reuteri and EHEC strains were isolated on MRS agar (pH 6.8) and LB agar plates containing 100 μg/mL of rifampicin (Sigma-Aldrich, St Quentin Fallavier, France), respectively. Each wild-type strain and its corresponding spontaneous RifR mutant showed identical growth patterns. Sensitivity of the rumen microbiota to rifampicin was confirmed by spotting 100 μL of rumen fluid (RF) samples on MRS agar plate containing rifampicin (50 μg/mL) before incubation at 39°C for 24 hours. Survival of L. reuteri in RF was tested by incubating RF samples (from O2-free, CO2-saturated sterile flasks) inoculated with L. reuteri RifR under anaerobiosis (39°C). The next day, the RF samples were 10-fold serially diluted in sterile phosphate buffered saline (PBS) buffer (pH 7.4), plated on MRS agar plates containing rifampicin and incubated overnight under anaerobiosis at 37°C for CFU counting. The experiments were replicated three times.
Ethics statement
RF and Rec samples were collected from rumen-cannulated cows in the INRA “Herbipôle Experimental Unit”, Auvergne-Rhône-Alpes Research Centre, Saint Genès-Champanelle, France (agreement number: C6334517) for experiments specifically approved by the “Comité d’éthique en matière d’expérimentation animale en Auvergne” (C2EA-02, permit number: 6895-2016091913586944V3). The animals were housed in individual stalls in the INRA “Herbipôle” experimental facilities in accordance with the guidelines of the local ethics committee. Small intestine and caecum contents were collected after the slaughter of animals required in the experimental slaughterhouse of the INRA “Herbipôle Experimental Unit” (permit number: APAFIS#1765–2015091516305 v3). Animals were slaughtered in accordance with the guidelines of the local Ethics Committee and current INRA ethical guidelines for animal welfare by stunning immediately followed by jugular vein bleeding performed by specifically trained abattoir staff (Permit number: 63345001).
Animals and digestive contents sampling
RF samples and rectum contents (Rec) were collected from non-lactating rumen-cannulated Holstein cows (live weight 800 kg on average). They were fed a diet composed of meadow hay and pelleted concentrate (INRA 50183 GV) and 250 g of mineral and vitamin complex. Cows were fed 8 kg of hay, 2 kg of concentrate (DM) in one meal, distributed at 8:00 in the morning.
RF samples were collected manually before feeding through the rumen cannula in O2-free, CO2-saturated sterile and pre-warmed (39°C) flasks as previously described [18]. The samples were immediately filtered through four layers of cheesecloth into CO2-saturated sterile flasks. RF samples (pH 6.2 on average) were confirmed to be negative for O157 E. coli before experiments: i) no colony was obtained after 24 h of incubation of the samples on MacConkey agar containing sorbitol (SMAC) and ii) the genes stx2, rfbE and fliC were not amplified from RF DNA (oligonucleotides used in PCR amplification were described in S2 Table). Bacterial counts from freshly collected RF samples were performed under anaerobiosis using the Most Probable Number (MPN) method and revealed the presence of ≈ 5 x 1010 MPN/mL. At least two cows at three different days were used for each experiment with RF samples. After sampling, rectum contents were rapidly transferred to O2-free, CO2-saturated sterile flasks, diluted (1:1) in sterile reduced potassium phosphate buffer (50 mM potassium phosphate, resazurin 0.1%, 40 mM Na2CO3, 3 mM cysteine, pH 7) and used immediately. Three different cows were used for experiments with Rec samples. Small intestine and caecum contents were collected from three cows at slaughter as previously described [19], rapidly transported to the laboratory, pooled in equal proportions and stored at -20°C until use.
Agar spot test
An agar spot test was performed to detect antimicrobial activity of L. reuteri strains as previously described [20]. The antimicrobial activity was recorded as growth-free inhibition zones (diameter > 1 mm) around the spots.
In vitro HPA production by L. reuteri
HPA was produced under anaerobiosis in PBS buffer (pH 7.4) and RF samples using a two-step fermentation protocol [20]. Briefly, RF samples were first centrifuged twice at 4100 g for 20 min and the supernatants were filter-sterilized through 0.45-μm nylon filter (Merck-Millipore, St Quentin en Yvelines). The filtrate was placed into glass tubes and left without stoppers in an anaerobic chamber (Jacomex, France) (100% CO2) during three days to allow the rumen fluid filtrate to be under anaerobic conditions. The tubes were then recapped with butyl rubber stoppers and filtered again (0.45 μm) under CO2 flux. L. reuteri strains were incubated in MRS broth without shaking under anaerobiosis for 24 hours at 37°C. The cultures were then centrifuged (4100 g, 10 min), washed in sterile PBS buffer and adjusted to ≈ 109 CFU/mL in O2-free, CO2-saturated PBS or filter-sterilized RF samples supplemented with 250 mM glycerol (Fisher-Aldrich). After incubation for 2 hours at 37°C, the bacterial cultures were pelleted and HPA-containing supernatants were collected and filtered (0.22-μm) before storage at 4°C.
HPA quantification
HPA production was quantified by a tryptophan-HCl colorimetric assay as previously described [10]. Briefly, the HPA-containing supernatants were diluted (10-fold) in sterile H2O, mixed with 10 mM tryptophan solution (0.01 M in 0.05 HCl, stabilized with a few drops of toluene) and 12 M HCl before incubation for 30 min at 37°C. Optical density (OD560) was measured with a spectrophotometer Spectronic BioMateTM 3 (Thermo Scientific). Acrolein (Fisher-Aldrich) was used as standard (the method is specific to both reuterin and acrolein quantification [10]). Standard curves were generated using an acrolein solution diluted to a concentration range of 0–20 mM in PBS or filter-sterilized RF. All the samples were filtered (0.45 μm) and diluted in PBS buffer if necessary.
Co-incubation of EHEC and L. reuteri strains
Pre-cultures of EHEC FCH6 RifR or EDL933 RifR inoculated from a single colony were incubated aerobically overnight at 37°C in LB supplemented with rifampicin. Bacterial cultures were then centrifuged (4100 g, 10 min) and the pellets were resuspended in sterile PBS buffer. At the same time, L. reuteri strains were cultured in MRS broth for 24 hours (37°C) without shaking. RF samples or LB broth (5 mL) were introduced into glass tubes equipped with butyl stoppers and screwed caps (Hungate tubes) under a 100% CO2 atmosphere. Ground feed (25 mg) was then added to RF samples (80% meadow hay, 20% concentrate) to mimic a feeding cycle. RF samples or LB broth were inoculated with both L. reuteri (≈ 107 CFU/ml) and the EHEC RifR (≈ 104 CFU/mL) strains. To mimic the physiological conditions encountered in the rumen, the cultures were incubated under strict anaerobiosis at 39°C (bovine temperature) for 24 hours (≈ transit time of forage-rich digesta) with gentle shaking (mixing of digesta). The bacterial cultures were then 10-fold serially diluted in PBS and spotted (10 μL) in triplicate on LB plates containing rifampicin before incubation overnight at 37°C. Each experiment was performed independently at least three times. The values presented are the log10 mean number of CFU/mL.
In vitro feedstuff degradation by the rumen microbiota
The effect of L. reuteri on feedstuff degradation by the rumen microbiota was assessed using the in vitro Daisy II incubation system (ANKOM Technology Corporation, Fairport, NY, USA). This system allows evaluation of feed digestibility during simultaneous incubation of up to 4 glass vessels, which rotate in an insulated chamber maintained at 39.5°C (≈ rumen temperature) [21–23]. RF samples were diluted (1:4, vol:vol) with Goering and Van Soest (GVS) anaerobic buffer [24] and dispensed under O2-free CO2 atmosphere. Feedstuff, under the form of alfalfa hay (AH) or corn silage (CS) (0.25 g of each forage, ground to 1 mm particle size), was bagged (ANKOM filter bags F57, porosity 25 μm, 5.0 cm×5.5 cm) and incubated in the presence of diluted RF for 24 hours. Six glass beads (4 mm diameter) were added to each bag to improve bag immersion in the rumen fluid as previously described [22]. All filter bags were heat-sealed at 0.5 mm from the edge of the bags. One bag with only 6 glass beads was also placed in each jar as blank. Six replicates for each feedstuff were used, and the experiment was repeated three times with RF collected at one-week intervals. For each replicate, one vessel was used as control and contained only RF, buffer, AH and CS bags, and one was inoculated with ≈ 107 CFU/mL of L. reuteri LB1-7 and 80 mM glycerol at the start of incubation. L. reuteri suspension was prepared from an overnight MRS culture at 37°C. The culture was centrifuged and resuspended in GVS buffer. pH was monitored at the start and end of incubation in a 10 mL sample of the incubation mix. Samples were collected and stored (-20°C) for fermentation end products analysis and microbial populations quantification by qPCR (see sections below). After 24 hours of incubation, bags were removed, washed with tap water and dried at 65°C for at least 48 hours for determination of residual DM. Six bags of each feedstuff were not incubated in order to assess passive DM loss by tap water washing. It was then possible to calculate the disappearance of DM (%).
Analysis of fermentation products in the RF samples
Metabolites were quantified by high performance liquid chromatography (HPLC) as previously described [25]. The method was calibrated to detect acetate, citrate, pyruvate, fructose, glucose, lactate, glycerol, 1,3-PD, propionate, isobutyrate, ethanol, butyrate, isovalerate and valerate. The amount of undissociated lactate was calculated using the formula: undissociated acid (mM) = total acid (mM) / (1+10pH-pka) [26]. The pH value of RF was 6.2 and the pKa value of lactate is 3.86.
Incubation of EHEC in rectum contents
Survival of EHEC during its passage in different segments of the bovine GIT was simulated. To this, we performed a first incubation of EHEC in filter-sterilized RF samples (FS-RF) (0.22 μM) followed by a second incubation in Rec samples (RF samples were indeed filtered in order to retrieve only the inoculated bacteria and not the feed particles neither the rumen endogenous microbiota). Briefly, RF samples were centrifuged, filter-sterilized and placed into glass tubes in an anaerobic chamber as described above. The strain FCH6 RifR (≈ 104 CFU/mL) was incubated under strict anaerobiosis with gentle shaking in FS-RF samples (5 mL) inoculated or not with L. reuteri (≈ 107 CFU/mL) and supplemented with 80 mM glycerol. After 24 hours of incubation at 39°C, the samples were centrifuged (4100 g, 10 min) and the bacterial pellet (containing potentially L. reuteri and/or EHEC) was suspended in sterile CO2-satured PBS (0.5 mL). The bacterial suspension was then inoculated into Rec samples freshly collected (containing its endogenous microbiota) (5 mL) and incubated at 39°C under anaerobiosis. The concentration of EHEC RifR was measured after 24h of incubation in FS-RF and after 6 and 24 hours of incubation in Rec samples.
DNA extraction
Genomic DNA from pure culture of L. reuteri was extracted using the Easy-DNATM kit (Fisher Scientific, Illkirch, France). The L. reuteri strains were cultured aerobically for 24 hours in MRS broth at 37°C without shaking. The next day, the bacterial cultures were centrifuged (10,000 g, 15 min) and the pellet was washed twice in sodium phosphate buffer (50 mM, pH 8). Initial cell lysis was performed using proteinase (Easy-DNATM kit) for 5 min at 37°C. Bacterial cells were then disrupted by bead beating for 3 min with 0.2 g of zirconia beads (0.1 mm diameter, Sigma-Aldrich). Subsequent genomic DNA extraction was performed according to the manufacturer’s recommendations.
Total DNA from the rumen and intestinal contents was extracted as previously described [27]. Briefly, bacterial cells were suspended in buffer (50 mM Tris buffer, 0.5 M NaCl, 50 mM EDTA, 4% SDS) (pH 8) and disrupted by bead beating for 3 min with zirconia beads. The mixture was incubated at 70°C for 15 min and centrifuged (16,000 g for 2 min at 15°C). The resulting supernatant was then precipitated, washed and resuspended as previously described [27]. Contaminating RNA was removed using a DNAse-free RNAse (10 mg/mL) and an additional incubation for 15 min at 37°C with 15 μL of Proteinase K (20 mg/mL, Sigma-Aldrich) was also performed. Subsequent DNA purification was performed using the Qiamp Fast DNA Stool mini kit (Qiagen, Courtaboeuf, France).
Oligonucleotide primer design and PCR screening
L. reuteri genes were detected by PCR amplification using the primer pairs described in S2 Table. The primers used to detect Lactobacillus spp. target a 16S rRNA sequence conserved among Lactobacillus spp. but also Leuconostoc spp., Pediococcus spp. and Weissella spp. [28]. Therefore, in this study, the bacteria detected by these primers were designed as Lactobacillus group. DNA sequences from L. reuteri available in databases (JCM 1112 [AP007282.1], DSM 20016 [CP000705.1], FUA3400 [KJ435307.1], SD2112 [CP002844.1], BR11 [GU191838.1] and BPL36 [JQ897939.1]) were aligned (Align Sequences Nucleotide BLAST [http://blast.ncbi.nlm.nih.gov]) to design the primer pairs used to amplify the genes gldC and dhaT (S2 Table). Amplification was performed from genomic DNA extracted from L. reuteri strains as described above. Taq polymerase and enzyme buffer were from MP-Biomedicals (Illkirch, France). The PCR procedure was performed on a Mastercycler Personal apparatus (Eppendorf) using the following programme: initial denaturation at 94°C for 4 min, 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, and a final elongation at 72°C for 10 min.
Bacterial enumeration in RF samples by quantitative-PCR
Total bacteria, Fibrobacter succinogenes, Ruminococus flavefaciens, L. reuteri and the Lactobacillus group were quantified by quantitative PCR (q-PCR). The q-PCR quantification was performed as previously described [27]. The primer pairs targeting the rrs and hsp60 genes are described in S2 Table. The standard curves (108 to 103 rrs or hsp60 copies) targeting Lactobacillus group and L. reuteri, respectively, were established from genomic DNA extracted from L. plantarum and L. reuteri strains. DNA extracted from RF samples inoculated with LB1-7 and L. plantarum (≈ 106 CFU/mL) was used as positive and negative control respectively for L. reuteri quantification. The standard curves (108 to 103 rrs copies) targeting R. flavefaciens and F. succinogenes and total bacteria were established as previously described [27].
Statistical analysis
Values are expressed as mean ± SEM. Statistical analyses were performed with the GraphPad Instat statistical software (La Jolla, CA, USA). Student's t-test was used to compare means and discuss the effect of L. reuteri. All tests were two-tailed paired and the level used to establish significance was P < 0.05.
Results
Survival of EHEC in bovine rumen fluid
The fate of the spontaneous rifampicin mutants of EHEC FCH6 and EDL933 (S1 Table) was assessed in rumen fluid (RF) containing endogenous microbiota under in vitro culture conditions allowing the growth of the endogenous microbiota and mimicking the rumen physiological conditions (see the Materials and Methods section). A concentration of ≈ 104 EHEC/mL was chosen because 3–6 log10 colony-forming unit (CFU)/mL were enumerated from the rumen of steers experimentally infected with E. coli O157:H7 [29]. Results analysis showed that FCH6 RifR survived well after incubation in RF samples (only 0.7 log10 decrease in CFU/mL after 24 hours of incubation) whereas the spontaneous RifR mutant of the reference EHEC strain EDL933 (EDL933 RifR) showed higher cell death after anaerobic incubation in RF (1.9 log10 decrease in CFU/mL) (S1 Fig). The strain FCH6 was thus selected for further studies.
Ability of L. reuteri to produce HPA and to inhibit EHEC growth
Five L. reuteri strains (S1 Table) were tested for their ability to inhibit the growth of EHEC FCH6 on soft BHI agar plates. Results showed that FCH6 was susceptible to L. reuteri strains LB1-7 and F275 under anaerobiosis when 2% glycerol (217 mM) was added whereas it was poorly or not inhibited by the remaining L. reuteri strains, with or without glycerol supplementation (S2 Fig). L. reuteri strains were also tested by PCR to detect the genes gldC and dhaT encoding glycerol dehydratase large subunit (EC 4.2.130) (required to convert glycerol to HPA) and 1,3-propanediol dehydrogenase (EC 1.1.1.202) (required to reduce HPA to 1,3-PD), respectively. Amplified products with the expected size for dhaT were obtained from all the L. reuteri strains tested, whereas the amplicon specific for gldC was only obtained for L. reuteri LB1-7, F275 and 65A (S3 Fig). In accordance with the results of agar spot assay, gldC was not amplified from L. reuteri F70 and 100–23 genomic DNA. The capacity of L. reuteri strains to produce HPA was then tested in PBS buffer supplemented with 250 mM glycerol. Results showed that HPA production at 37°C under anaerobiosis was strain dependent since LB1-7, F275 and 65A produced 114, 84 and 19 mM HPA, respectively. As expected, HPA was not detected in the supernatant of L. reuteri strains 100–23 and F70. The production of HPA by L. reuteri was also tested in filter-sterilized RF samples supplemented with 250 mM glycerol: the strains LB1-7 and F275 produced 189 and 105 mM HPA, respectively, whereas HPA was not detected in the presence of L. reuteri strains 65A, 100–23 and F70. In view of these results, the L. reuteri strain LB1-7 was chosen to perform co-incubation experiments. The L. reuteri strain 100–23 was used as negative control.
Persistence of L. reuteri in bovine intestinal fluids
The quantification of L. reuteri and Lactobacillus group in intestinal contents was performed by qPCR amplification of the genes hsp60 (encoding heat shock protein) and rrs (encoding 16S ribosomal RNA), respectively. Lactobacillus group was detected in RF samples (7.5 log10 rrs copy per μg of DNA ± 0.16), small intestine contents (7.8 log10 rrs copy per μg of DNA ± 0.3), caecum contents (7.4 log10 rrs copy per μg of DNA ± 0.09) and rectum contents (7.6 log10 rrs copy per μg of DNA ± 0.05). In contrast, L. reuteri was undetectable in all GIT compartments tested, including the rumen (detection limit: 2 log10 hsp60 copies). The capacity of L. reuteri LB1-7 to grow in RF samples was then analyzed. After 24 hours of incubation in RF at 39°C under strict anaerobiosis, the spontaneous rifampicin-resistant mutants of LB1-7 (LB1-7 RifR) was enumerated at a concentration similar to the inoculation rate (≈ 107 CFU/mL), demonstrating the capacity of the strain to persist in RF.
Antimicrobial activity of L. reuteri and antimicrobial potency of HPA against EHEC
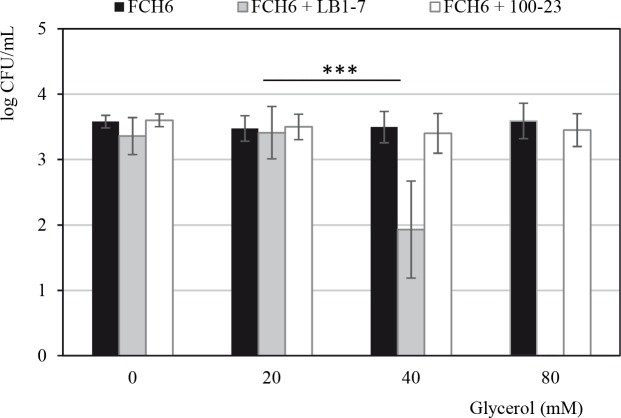
The growth of EHEC FCH6 RifR (≈ 104 CFU/mL) was tested in the presence of different concentrations of L. reuteri LB1-7 (≈ 105, 106 and 107 CFU/mL) and glycerol (20, 40, 80 and 160 mM) in RF samples to determine the optimal conditions to decrease EHEC survival. The strain FCH6 RifR survived well after 24 hours of anaerobic incubation in RF samples in the absence of glycerol or supplemented with 20 or 40 mM glycerol whatever the L. reuteri concentration (S3 Table). In contrast, no EHEC CFU was enumerated when LB1-7 (107 CFU/mL) was inoculated in RF supplemented with 80 or 160 mM glycerol (detection limit: 10 CFU/mL) (Fig 1). The inhibition of higher levels of EHEC (≈ 105 and 106 CFU/mL) were also tested. L. reuteri LB1-7 (≈ 107 CFU/mL) suppressed EHEC FCH6 in RF supplemented with 80 mM glycerol (RF-Glyc80) whatever the EHEC concentration. Similarly, co-incubation of L. reuteri LB1-7 (≈ 107 CFU/mL) and EDL933 RifR (≈ 104 CFU/mL) in RF-Glyc80 also resulted in EHEC suppression after 24 hours of anaerobic incubation. As expected, L. reuteri 100–23 (≈ 107 CFU/mL) had no effect on EHEC survival with or without glycerol (Fig 1). Results also showed that glycerol alone was not responsible for EHEC suppression since L. reuteri LB1-7 RifR showed similar growth curves when incubated in RF and RF-Glyc80. This demonstrated the capacity of L. reuteri to persist in RF in the presence of glycerol. Different dilutions of HPA-containing supernatants were added to RF samples inoculated with FCH6 RifR to test the antimicrobial potency of exogenous HPA. Addition of 25 mM HPA in RF samples (not inoculated with LB1-7) completely suppressed FCH6 RifR after 2 hours of incubation.
Fig 1. Survival of EHEC co-incubated with L. reuteri strains in RF samples supplemented or not with glycerol.
The strain FCH6 RifR was co-inoculated with ≈ 107 CFU/mL of L. reuteri LB1-7 (HPA producer) or 100–23 (negative control) in RF samples under anaerobiosis for 24 hours. The strain FCH6 RifR inoculated alone in RF samples was used as control. RF samples were supplemented or not with glycerol at different concentrations. Bars represent the SEM of three independent experiments. Asterisks indicate statistical significance (***: P<0.001).
Production of HPA by L. reuteri in rumen fluids
Kinetics of EHEC disappearance and HPA accumulation were monitored during co-incubation of FCH6 RifR (≈ 104 CFU/mL) and LB1-7 (≈ 107 CFU/mL) in RF-Glyc80. As shown in Fig 2, a significant decrease in EHEC population was observed from 4 hours of co-incubation (P<0.05) and no EHEC colony could be detected after 6 hours of co-incubation. A total suppression of EHEC was observed when HPA concentration reached ≈ 12.5 mM and a maximal HPA concentration (13.8 mM) was quantified after 8 hours of incubation (Fig 2). As expected, HPA and 1,3-PD accumulation correlated with glycerol disappearance and the glycerol concentration was only 51 mM and 34 mM in RF-Glyc80 after 6 and 24 hours of co-incubation, respectively. The concentrations of HPA and 1,3-PD accumulated in RF-Glyc80 were similar during the first 8 hours of incubation. Noteworthy, the level of 1,3-PD reached 34 mM after 24 hours whereas a decrease in HPA concentration was observed (7.3 mM) (Fig 2 and Table 1). These results suggested that HPA accumulation was transient probably because a part of HPA was reduced to 1,3-PD. As expected, HPA was not detected in the supernatant of RF-Glyc80 inoculated with L. reuteri 100–23 as well as in RF-Glyc80 samples not inoculated with L. reuteri.
Fig 2. EHEC counts and HPA, 1,3-PD and glycerol quantification in RF-Glyc80.
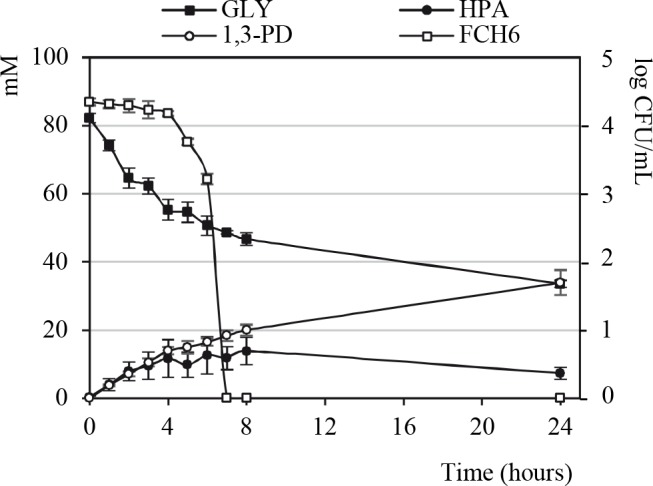
The strain FCH6 RifR (≈ 104 CFU/mL) was co-incubated with L. reuteri LB1-7 (≈ 107 CFU/mL) in RF samples supplemented with 80 mM glycerol under anaerobiosis. At each time point the strain FCH6 RifR was enumerated and accumulation of HPA and 1,3-PD, and disappearance of glycerol were monitored. Bars represent the SEM of three independent experiments. Gly: glycerol.
Table 1. Concentration of glycerol and microbial metabolites.
| Acetate | Propionate | Butyrate | Glycerol | HPA | 1,3-PD | Lactate | |||
|---|---|---|---|---|---|---|---|---|---|
| (mM)a | (mM)a | (mM)a | (mM)b | (mM) | (mM) | (mM) | |||
| RF t = 0 | 61.2 ± 5.5 | 14.1 ± 1.6 | 6.3 ± 0.7 | - | - | - | - | ||
| RF t = 24h | 105.3 ± 2.9 | 33.7 ± 0.8 | 19.0 ± 1.1 | - | - | - | - | ||
| RF + LB1-7 t = 24h | 95.0 ± 2.4NS | 29.9 ±0.9* | 17.5 ± 1.8NS | - | - | - | 1.8 ± 0.09 | ||
| RF-Glyc80 t = 0 | 61.2 ± 5.5 | 14.1 ± 1.6 | 6.3 ± 0.7 | 82.0 ± 3.5 | - | - | - | ||
| RF-Glyc80 t = 24h | 95.8 ± 4.1NS | 40.8 ± 1.6* | 26.1 ± 1.7* | 61.9 ± 5.8 | - | 2.6 ± 0.9 | 0.9 ± 0.35 | ||
| RF-Glyc80 + LB1-7 t = 24h | 79.6 ± 4.8* | 17.8 ± 1.1*** | 7.8 ± 1.2** | 34.2 ± 2.1* | 7.3 ± 1.7 | 34.3 ± 2.8 | 28.7 ± 5.7 | ||
| RF-Glyc80 + 100–23 t = 24h | ND | ND | ND | 46.1 ± 1.9NS | - | 3.5 ± 0.5 | ND | ||
Concentrations of major ruminal fermentation ends products, glycerol, HPA and 1,3-PD were quantified in RF and RF-Glyc80. The samples were inoculated or not with L. reuteri LB1-7. The detection limit of glycerol, 1,3-PD, lactate and HPA were 102 mM, 2 x 102 mM,102 and 5 x 10−2 mM, respectively. Means of three independent replicates are shown with their SEM. ND: not determined; -: undetected.
a: comparisons were made with RF t = 24h
b: comparisons were made with RF-Glyc80 t = 24h
NS: P>0.05 (not significant)
*: P<0.05
**: P<0.01
***: P<0.001.
Values with no superscript were not statistically analyzed.
Because Schaefer et al. show that HPA production by L. reuteri is stimulated by interaction with different bacteria [8], we investigated the potential stimulating role of EHEC in HPA production. A similar maximal concentration of HPA (≈ 14 mM HPA) was quantified at 8 hours of incubation of RF-Glyc80 co-inoculated with LB1-7 and FCH6 RifR or inoculated with LB1-7 alone demonstrating that the presence of EHEC in RF samples did not stimulate the production of HPA by L. reuteri under our experimental conditions.
Production of HPA and antimicrobial activity of L. reuteri LB1-7 in LB broth
HPA production and antimicrobial activity of L. reuteri LB1-7 against EHEC was also investigated during co-incubation in sterile laboratory medium. As shown in Fig 3A, 10 mM glycerol was required to completely suppress FCH6 RifR co-inoculated with LB1-7 in LB broth after 6 hours of co-incubation. Up to 2.1 mM HPA was produced by L. reuteri LB1-7 after 3 hours of co-incubation with FCH6 RifR in LB broth supplemented with 10 mM glycerol (Fig 3B).
Fig 3. Kinetics of EHEC growth or disappearance and HPA production in LB broth.
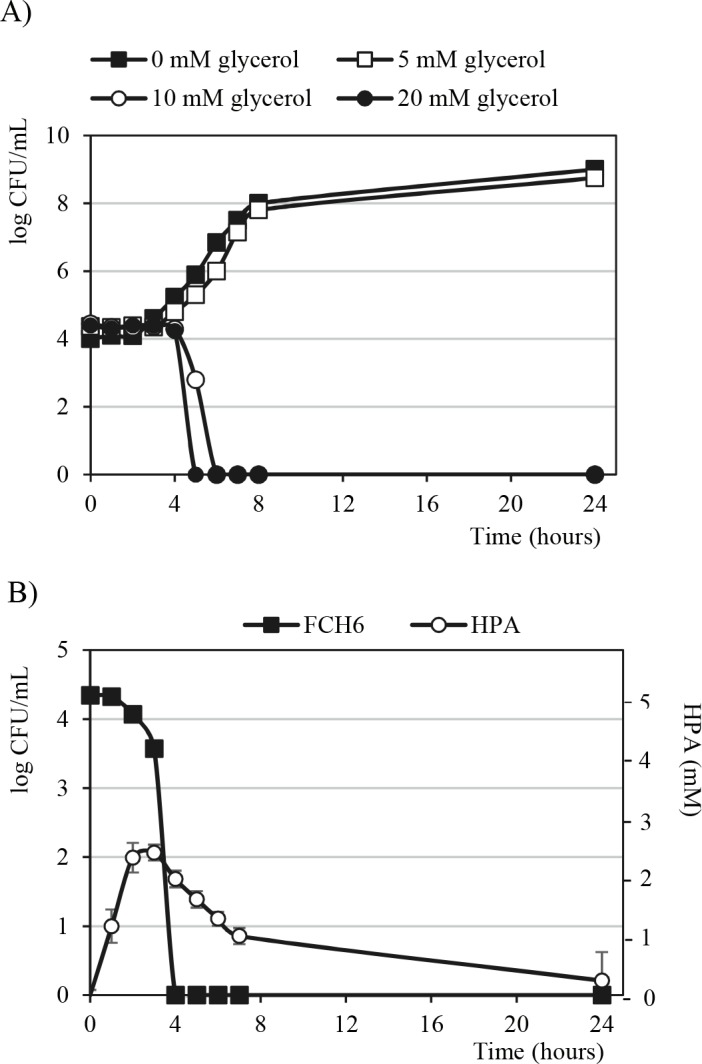
(A) The strain FCH6 RifR (≈ 104 CFU/mL) was co-incubated with L. reuteri LB1-7 (≈ 107 CFU/mL) in LB broth supplemented or not with different concentration of glycerol. The strain FCH6 RifR was then enumerated after 24 hours of incubation under anaerobiosis. Bacterial growth curves are expressed as a single representation of three independent experiments. (B) The strain FCH6 RifR was co-incubated with L. reuteri in LB broth supplemented with 10 mM glycerol under anaerobiosis. At each time point the strain FCH6 RifR was enumerated and accumulation of HPA was quantified. The bacterial growth curve is expressed as a single representation of three independent experiments. Bars represent the SEM of three independent experiments.
Taken together, the results showed that only 10 mM glycerol were required to completely suppress FCH6 RifR in LB medium inoculated with L. reuteri LB1-7 while 80 mM glycerol were necessary in RF. Furthermore, it is noteworthy that the HPA concentration produced by L. reuteri and apparently sufficient to suppress EHEC was 2.1 and 12.5 mM in LB broth and RF samples, respectively.
Glycerol metabolism by endogenous rumen microbiota
Fermentation end products were analysed in RF samples. As expected, the three major short-chain fatty acids present in RF samples freshly collected were acetate, propionate and butyrate (RF t = 0, Table 1). The concentration of additional metabolites was below the detection limit: glucose (< 4 x 10−3 mM), glycerol (< 10−2 mM), 1,3-PD (< 2 x 10−2 mM), isobutyrate (< 10−2 mM), valerate (< 10−2 mM), lactate (< 10−2 mM) and ethanol (< 4 x 10−2 mM). Acetate, propionate and butyrate concentration increased after 24 hours of anaerobic incubation due to fermentation by the endogenous rumen microbiota of simple and complex carbohydrates provided by the ground feed added to RF samples (see the Materials and Methods section) (RF t = 24h, Table 1). The glycerol concentration was still ≈ 62 mM in RF-Glyc80 after 24 hours of incubation, indicating that the rumen microbiota assimilated ≈ 24% of the glycerol. In addition, incubation of RF-Glyc80 resulted in an altered fermentation pattern when compared with RF samples not supplemented with glycerol: disappearance of glycerol was associated with an increase in propionate and butyrate concentration (P<0.05) (Table 1). The 1,3-PD concentration was 2.6 mM in RF-Glyc80 after anaerobic incubation suggesting that the rumen microbiota was able to reduce glycerol to 1,3-PD to some extent. The HPA concentration in RF samples was below the detection limit (< 5 x 10−2 mM).
Impact of L. reuteri on rumen fermentation and glycerol metabolism
The fermentation pattern of RF-Glyc80 samples inoculated with L. reuteri was analyzed (Table 1). After 24 hours of incubation under anaerobiosis, the glycerol concentration was 34.2 mM in RF-Glyc80 inoculated with LB1-7 (Table 1). Comparison with the level of glycerol recovered after incubation of non-inoculated RF-Glyc80 (61.9 mM) suggested that LB1-7 was able to metabolize glycerol, while the change in glycerol concentration in the presence of L. reuteri 100–23 was not significant. As expected, HPA was detected in RF-Glyc80 inoculated with LB1-7 but not with 100–23 (Table 1). Furthermore, as shown above, a relatively high concentration of 1,3-PD (34.3 mM) was quantified in RF-Glyc80 inoculated with L. reuteri LB1-7 whereas the level of 1,3-PD quantified in RF-Glyc80 inoculated with L. reuteri 100–23 was close to that obtained after incubation of RF-Glyc80 alone (Table 1). This suggests that the slight 1,3-PD accumulation observed in RF-Glyc80 inoculated with 100–23 was essentially due to anaerobic reduction of glycerol by the endogenous rumen microbiota. Similar patterns were obtained when RF-Glyc80 was inoculated with L. reuteri alone or co-inoculated with both L. reuteri and EHEC FCH6 RifR.
In the absence of glycerol, incubation of RF with or without L. reuteri led to similar concentrations of acetate and butyrate, and a lower concentration of propionate (P<0.05) (Table 1). In contrast, incubation of L. reuteri LB1-7 in RF-Glyc80 led to lower concentrations of acetate (P<0.05), butyrate (P<0.01), and propionate (P<0.001) (Table 1). Noteworthy, lactate accumulation (28.7 mM; 0.13 mM undisociated from) was observed when LB1-7 was inoculated in RF-Glyc80 but not in RF.
To analyze the role of lactate in EHEC inhibition, the strain FCH6 RifR was incubated in RF-Glyc80 samples supplemented with 30 mM lactate. After 24 hours of incubation under anaerobiosis, a similar EHEC quantification (≈ 104 CFU/mL) was recovered in RF samples supplemented or not with 30 mM lactate suggesting that the LB1-7 bactericidal action was not due to lactate production.
Impact of L. reuteri on the degrading activity of the endogenous rumen microbiota
A potential inhibitory effect of L. reuteri on the feedstuff degradation capacity of the rumen microbiota was investigated. After 24 hours of incubation, 55% and 43% of corn silage and alfalfa hay dry matter (DM) respectively, were degraded by the rumen microbiota (Fig 4A). Rumen microbiota activity was confirmed by the production of SCFAs after 24 hours of incubation (S4 Fig). Inoculation of L. reuteri LB1-7 and glycerol supplementation (80 mM) resulted in a significant decrease of DM degradation: 50.4% and 35.8% of corn silage and alfalfa hay DM were degraded, respectively (P<0.05)(Fig 4A), corresponding to inhibition decrease of 8.3 and 16.7%. A shift in SCFA profile was also measured (S4 Fig), indicating a disruption in the rumen microbiota. All incubation vessels had the same initial pH (7.10). After 24 hours of incubation, the pH was slightly decreased (6.70) in the vessels inoculated with L. reuteri compared with non-inoculated ones (pH 7.05). It is of interest to note that pH 6.70 was still a physiological value for rumen microbiota activity. The decrease in pH value and the fact that lactate and 1,3-PD were recovered in the vessels supplemented with glycerol after 24 hours of LB1-7 incubation (5.2 mM and 7.6 mM, for lactate and 1,3-PD respectively) (S4 Fig) suggested that L. reuteri remained metabolically active during the course of fermentation.
Fig 4. Dry matter degradation (DMD) of forages and fibrolytic ruminal population.
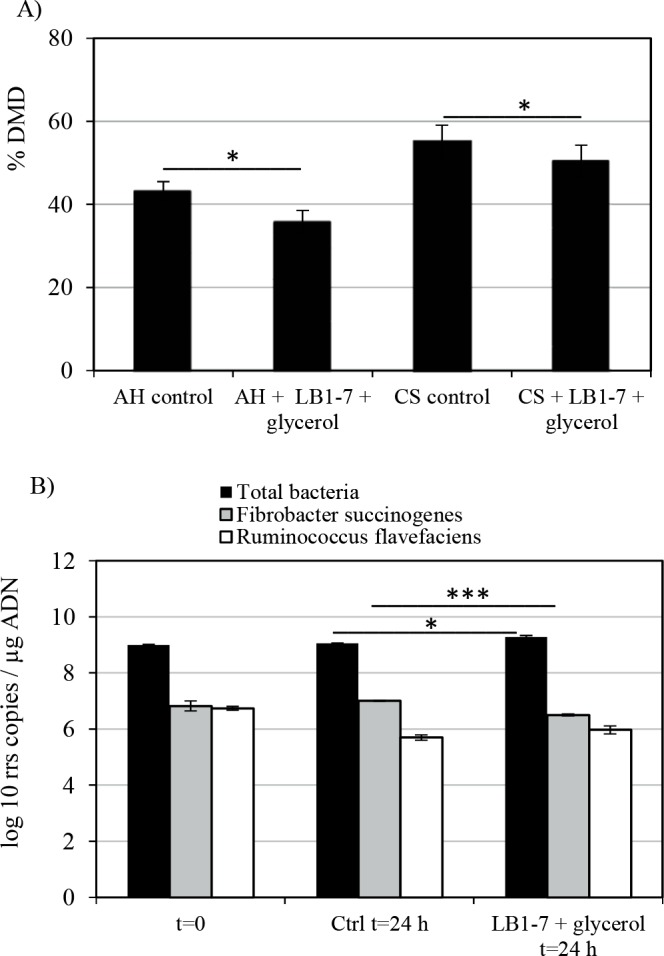
(A) Dry matter degradation of alfalfa hay (AH) and corn silage (CS) by the rumen microbiota after 24 hours of incubation was quantified in the Daisy II incubation system containing RF in the presence or absence of L. reuteri LB1-7 and 80 mM glycerol. Non-incubated bags containing forage were used as control (see the experimental procedures section). The data are expressed as the percentage of dry matter degraded. Bars represent the SEM of three independent experiments. (B) Quantification of the rrs gene copies of the total rumen bacterial population, F. succinogenes and R. flavefaciens in DAISY II vessels. Total DNA template extracted from the Daisy II vessel containing only RF and buffer was used as control (Ctrl). The bacterial populations were quantified before and after 24 hours of incubation. pH was monitored at the start and end of incubation. Bars represent the SEM of three independent experiments. Asterisks indicate statistical significance (*: P<0.05; ***: P<0.001).
In view of these results, a potential antimicrobial activity of L. reuteri against the total rumen bacterial population and two major rumen cellulolytic bacteria, Fibrobacter succinogenes and Ruminococcus flavefaciens, was investigated (Fig 4B). After 24 hours of incubation, the total bacterial population was slightly greater (P<0.05) with L. reuteri inoculation compared with control. R. flavefaciens population decreased during incubation (P<0.05) both in control vessels and in vessels inoculated with L. reuteri and supplemented with glycerol (decrease of 1 and 0.8 log10 rss copy per μg of DNA, respectively) (Fig 4B). The F. succinogenes population remained unchanged in the control vessels during the course of the fermentation whereas a significant decrease (P<0.001) was observed in the vessels inoculated with L. reuteri. This suggested that F. succinogenes was more affected by the presence of L. reuteri than R. flavefaciens, with on average a decrease of 0.5 log10 rrs copy per μg of DNA compared to control vessels.
Survival of EHEC in rectum contents after passage in RF-Glyc80 inoculated with L. reuteri
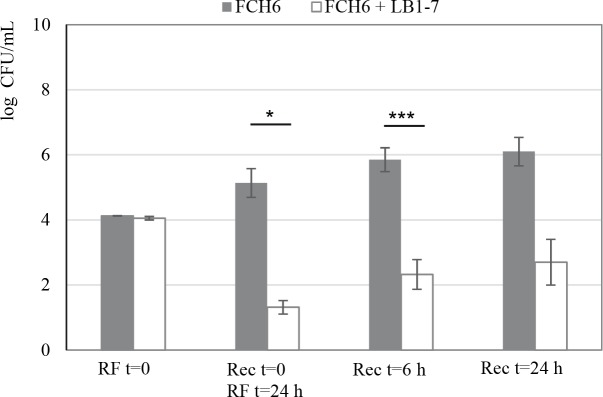
Experiments were performed to mimic the passage of EHEC through different segments of the bovine GIT. To this aim, a first incubation of EHEC was performed in filter-sterilized RF (FS-RF) samples followed by a second incubation in Rec samples. The fate of FCH6 RifR was evaluated after incubation in FS-RF-Glyc80 with L. reuteri LB1-7 and in following Rec samples. The EHEC strain was also incubated in FS-RF without L. reuteri and glycerol as a control. A bacterial concentration of 1.3 log10 FCH6 RifR CFU/mL was recovered after 24 hours of incubation in FS-RF-Glyc80 samples inoculated with L. reuteri LB1-7 (Rec t = 0/RF t = 24h, Fig 5). This concentration was markedly lower than that measured in control RF samples (5.1 log10 CFU/mL, P < 0.05) (Rec t = 0/RF t = 24h, Fig 5). Note that, in contrast to incubation in RF samples containing its endogenous microbiota (Figs 1 and 2), L. reuteri LB1-7 strongly repressed but not totally suppressed the growth of EHEC in FS-RF-Glyc80, suggesting that absence of nutritional competition with the ruminal endogenous microbiota is responsible for the better survival of EHEC. In a second step, bacteria that survived passage through the rumen were inoculated in Rec samples containing its endogenous microbiota. Without prior exposure to L. reuteri and glycerol in RF, EHEC concentration in Rec samples reached 5.9 and 6.1 log10 CFU/mL after 6 and 24 hours of incubation respectively (Fig 5). EHEC concentrations were much lower (2.3 and 2.7 log10 CFU/mL, P < 0.001 and P <0.10 respectively) after 6 and 24 hours of incubation in Rec samples following preliminary exposure to LB1-7 in FS-RF-Glyc80 (Fig 5). The results demonstrated that the passage of EHEC in RF in the presence of L. reuteri and glycerol was efficient to drastically reduce in vitro EHEC survival in rectum content.
Fig 5. Growth or survival of EHEC in rectum contents after incubation in RF.
The strain FCH6 RifR was first incubated in filter-sterilized RF (FS-RF) samples alone or inoculated with ≈ 107 CFU/mL of L. reuteri LB1-7 supplemented with 80 mM glycerol under anaerobiosis for 24 hours. The bacterial pellet was then inoculated into Rec samples and incubated under anaerobiosis. RF = 0 represents inoculation of EHEC in FS-RF samples; Rec t = 0 corresponds to RF t = 24h i.e. number of EHEC surviving the incubation in FS-RF during 24 hours; Rec t = 6h and Rec t = 24h correspond to EHEC survival in Rec samples after 6 and 24 hours of incubation respectively. Bars represent the SEM of three independent experiments. Effect of L. reuteri + Glyc80 is significant *, P<0.05; ***, P<0.001.
Discussion
Strategies can be proposed to reduce the carriage of E. coli O157:H7 in cattle i) during the animal growth period in order to limit pathogen shedding and thus environmental burden and ii) just before slaughter to limit the contamination of carcasses and thus the burden of E. coli O157:H7 entering the processing plant [30]. Many studies have tested the use of direct fed microbials (DFMs) as a pre-harvest strategy to reduce fecal shedding of E. coli O157:H7 in beef cattle [31]. Although results of field studies were variable, a recent meta-analysis showed that distribution of lactic acid bacteria appeared efficient in reducing the prevalence of E. coli O157:H7 fecal shedding [30].
To our knowledge, the use of L. reuteri to reduce EHEC survival in bovine gastrointestinal fluids is investigated for the first time. We have compared several L. reuteri strains and selected L. reuteri LB1-7 able to convert glycerol to HPA and to suppress EHEC in RF containing metabolically active endogenous microbiota. L. reuteri LB1-7 appeared to be of particular interest because of its resistance to simulated gastric and intestinal conditions [17], in addition, it can be potentially endowed with probiotic activities as already demonstrated for other lactobacilli isolated from raw milk [32]. Furthermore, in in vitro simulation of EHEC fate along the bovine digestive tract, we showed that when the EHEC strain are exposed to L. reuteri and glycerol in rumen fluid, its viable load sharply decreased, affecting also EHEC viability in a posterior digestive compartment (i.e. the rectum).
The rumen environment should be appropriate for HPA production by L. reuteri because it is strictly anaerobic and the concentration of available sugars has been reported to be generally low (<1–2 mM post feeding [33]), a required condition for anaerobic glycerol fermentation by L. reuteri (a low glucose concentration favours HPA instead of 1,3-PD accumulation [34]). Indeed, the soluble carbohydrates resulting from polysaccharide breakdown by rumen microbiota are either readily fermented or very rapidly sequestered under the form of oligosaccharides into the cells, preventing soluble sugars to be released in RF [35]. The rumen microbiota is able to produce low levels of 1,3-PD in RF-Glyc80 suggesting transient HPA accumulation. This indicates that some bacteria of the rumen microbiota possess the metabolic pathways to convert glycerol to 1,3-PD via HPA reduction. Among 1,3-PD-producers, Clostridium butyricum and C. perfringens are the most commonly reported mammal intestinal species [36]. Although 1,3-PD production is not described in the rumen of cattle, we speculate that members of the Clostridium genus, which belong to the core ruminal bacterial community [37], could be responsible for HPA production in RF-Glyc80. However, RF-Glyc80 alone was not effective in preventing EHEC multiplication suggesting that transient HPA produced by the endogenous microbiota was insufficient to achieve an antimicrobial effect.
As expected, the acetate:propionate ratio decreased in RF-Glyc80 after 24 hours of incubation indicating that part of the glycerol was fermented by the rumen microbiota. Several ruminal bacterial species such as Anaerovibrio lipolytica and Selenomonas ruminantium are able to ferment glycerol into propionate [38–40]. However, incubation of L. reuteri in RF-Glyc80 resulted in decrease in propionate concentration suggesting that part of the glycerol that could be fermented to propionate by the ruminal microbiota was more efficiently metabolized by L. reuteri. It can also be hypothesized that the HPA produced by L. reuteri in RF-Glyc80 resulted in an inhibition of the propionate producing population.
One of the main functions of the rumen microbiota is the degradation and fermentation of plant biomass into short chain fatty acids providing energy for the animal [41]. Therefore, it is important to analyze the effects of LB1-7 inoculation on the degrading activity of the rumen microbiota. In our study, a significant decrease in corn silage and alfalfa hay degradation in the presence of L. reuteri LB1-7 and glycerol was observed. This decrease is probably due to an inhibitory effect of HPA on the rumen fibrolytic populations, as observed for F. succinogenes. However, qPCR assay targeting bacterial DNA, as well as DM degradation quantification could lead to underestimation of the effect of L. reuteri on the rumen bacteria, due to contribution of DNA and active enzymes released by dead bacterial cells. Fermentation end-products profiles also suggested that L. reuteri exerted an antimicrobial activity against the rumen endogenous microbiota in the presence of glycerol. In addition to the effect on fibrolytic populations, production of HPA by L. reuteri may lead to inhibition of lactate-utilizing and butyrate- and propionate-producing rumen bacteria, resulting in perturbed fermentations. Consequently, a potential supplementation of L. reuteri and glycerol during the animal growing period appears to be unsuitable. However, since EHEC carriage at the time of slaughter represents the potential entry of the pathogen into the meat production process, administration of L. reuteri and glycerol could be considered in view of application to finishing beef cattle, a few days before slaughter, when performance goals (decreased rate of gain—finished weight) are achieved. However, the pre-slaughter glycerol and L. reuteri administration should avoid additional stressing factors that may have a negative impact on meat quality [42]. Therefore, the approach we suggest for reducing E. coli shedding by cattle needs to be carefully evaluated to assess the stress responses in living animal and in post-mortem muscle metabolism, in addition to killing activity against EHEC.
Accumulation of lactate was observed in RF-Glyc80 after incubation of L. reuteri under anaerobiosis. The efficiency of lactate in inhibiting E. coli O157:H7 multiplication in food products and cattle hides is well documented [43, 44]. Furthermore, Ogawa et al. showed that the bactericidal activity of L. casei against E. coli O157:H7 was due to production of undissociated lactate, which permeates the bacterial membrane by diffusion and releases protons into the cell [26]. Our data clearly showed that lactate was not involved in EHEC suppression in rumen contents, probably because the weak level of the corresponding undissociated form was below the minimal inhibitory concentration [26]. Nonetheless, it is possible that the distribution of glycerol and L. reuteri to ruminants leads to EHEC inhibition due to HPA as well as lactate production in the rumen. In further steps, it will be important to analyze the impact of L. reuteri and glycerol association in vivo, in order to assess their effects on bovine gastrointestinal health due to lactate production and microbiota disruption.
The potential use of L. reuteri as a feed supplement to reduce EHEC burden in cattle would necessarily be associated with glycerol administration. It is generally admitted that supplementing feed with a formulation containing freeze-dried micro-organisms is easy to implement, as far as strains stability issues are resolved. Introduction of L. reuteri as direct-fed microbial (DFM) would be of interest because the use of DFMs is widely accepted in ruminant nutrition and is perceived as a natural non-antibiotic way of improving animal performance and health [45]. The use of glycerol as cattle feed supplement has already been proposed to improve animal performance because of its cost-effective energetic value. Because the energy content of glycerol is close to that of corn, the replacement of corn by dry glycerol in cows daily supplementation has been considered [46]. Furthermore, the glycerol concentrations needed for EHEC inhibition by L. reuteri in vitro are close to those used in cattle production [46, 47]. However, in vivo experiments will be needed to determine the glycerol concentration required for production of sufficient HPA to suppress EHEC in the rumen of cows, as part of the glycerol might be absorbed across the rumen wall [48].
In conclusion, L. reuteri LB1-7 appeared to resist the physical and microbiological conditions encountered in the rumen of cows and was effective in suppressing EHEC O157:H7 in RF in vitro. Data presented in this report define valuable information on the optimal antagonistic effect of a selected L. reuteri strain against EHEC (L. reuteri-EHEC concentration ratio, glycerol level, aeration conditions) and provide helpful data to set up more targeted in vivo trials to assess the efficiency of L. reuteri to decrease EHEC shedding by cattle. Future studies will be required to determine i) DFM supplementation parameters, ii) the effect of L. reuteri and glycerol on rumen microbiota and iii) the survival of EHEC strains in lower gastrointestinal tract. In vivo studies will also be necessary to explore the potential of HPA-producing L. reuteri alone or in combination with already commercialized DFMs in order to ultimately validate an efficient pre-harvest strategy in beef cattle with the aim to improve food safety.
Supporting information
The strains FCH6 RifR and EDL933 RifR were incubated in RF samples for 24 hours under anaerobiosis before enumeration. Bars represent the SEM of three independent experiments. Asterisks indicate statistical significance (*: P<0.05; ***: P<0.001).
(TIF)
L. reuteri strains (LB1-7, F275, 65-A, F70 and 100–23) were first spotted onto the surface of Brain Heart Infusion (BHI) agar supplemented with 20 mM glucose and incubated anaerobically. The EHEC strain FCH6 was then inoculated in soft agar with or without glycerol (2%) and poured over the L. reuteri spots (in order to facilitate or not HPA production by spots containing L. reuteri). The plates were then incubated and the antimicrobial activity was recorded as growth-free inhibition zones around the spots as previously described [20].
(TIF)
PCR detection of the genes gldC (A) and dhaT (B) from genomic DNA extracted from L. reuteri strains. Lane 1, molecular size marker (MassRuler DNA Ladder, ThermoFisher Scientific); lane 2, L. reuteri LB1-7; lane 3, L. reuteri 65A; lane 4, L. reuteri F70; lane 5, L. reuteri F275; line 6, L. reuteri 100–23.
The PCR products were subjected to electrophoresis on 1% agarose gel and visualized by ethidium bromide staining.
(TIF)
The Daisy II method was used to analyze the degrading activity of the rumen microbiota. The Daisy II vessels containing RF were supplemented or not with 80 mM glycerol and inoculated or not with L. reuteri LB1-7. The fermentation end-products were quantified in Daisy II vessels before and after 24 hours of anaerobic incubation. Bars represent the SEM of three independent experiments. Asterisks indicate statistical significance (*: P<0.05; ***: P<0.001).
ACE: acetate; PROP: propionate; BUTY: butyrate; GLY: glycerol; LACT: lactate.
(TIF)
The strain FCH6 RifR was incubated under anaerobiosis in Rec samples or co-incubated in Rec samples inoculated with L. reuteri LB1-7 (≈ 107 CFU/mL) and supplemented with 80 mM glycerol. Bars represent the SEM of three independent experiments. Effect of L. reuteri + Glyc80 is significant *, P<0.05.
(TIF)
a: DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany.
b: ISPA, Institute of Sciences of Food Production microbial collection number; National Research Council, Italy.
(DOCX)
DNA sequences from L. reuteri available in DNA databases (JCM 1112 [AP007282.1], DSM 20016 [CP000705.1], FUA3400 [KJ435307.1], SD2112 [CP002844.1], BR11 [GU191838.1] and BPL36 [JQ897939.1]) were aligned (Align Sequences Nucleotide BLAST [http://blast.ncbi.nlm.nih.gov]) to design the primer pairs used to amplify gldC and dhaT.
(DOCX)
(DOCX)
Acknowledgments
We thank Gregory Jubelin and Silvia de Candia for critical reading of the manuscript, and Aurélie Ameilbonne for excellent technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Fonds unique interministériel (OSEO F1010011M) (EF); http://competitivite.gouv.fr/le-lancement-du-10e-appel-a-projets-de-r-d-du-fui/les-resultats-du-10e-appel-a-projets-701.html; European Regional Development Fund (33577) (EF); http://www.europe-en-auvergne.eu/programme-massif-central.html; Conseil Regional d’Auvergne (CPER T2ANSH) (EF); http://m.enseignementsup-recherche.gouv.fr/cid72550/le-contrat-de-projets-etat-region-auvergne-2007-2013.html; LALLEMAND SAS provided support in the form of salaries for authors FCD and LD, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Law D. Virulence factors of Escherichia coli O157 and other Shiga toxin-producing E. coli. Journal of Applied Microbiology. 2000; 88:729–745. [DOI] [PubMed] [Google Scholar]
- 2.Caprioli A, Morabito S, Brugere H, Oswald E. Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Veterinary Research. 2005; 36:289–311. doi: 10.1051/vetres:2005002 [DOI] [PubMed] [Google Scholar]
- 3.Gaggìa F, Mattarelli P, Biavati B. Probiotics and prebiotics in animal feeding for safe food production. International Journal of Food Microbiology. 2010; 141 Suppl 1:S15–S28. [DOI] [PubMed] [Google Scholar]
- 4.Pandey V, Berwal V, Solanki N, Malik NS. Probiotics: Healthy bugs and nourishing elements of diet. Journal of International Society of Preventive & Community Dentistry. 2015; 5:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bøhle LA, Brede DA, Diep DB, Holo H, Nes IF. Specific degradation of the mucus adhesion-promoting protein (MapA) of Lactobacillus reuteri to an antimicrobial peptide. Applied and Environmental Microbiology. 2010; 76:7306–7309. doi: 10.1128/AEM.01423-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talarico TL, Dobrogosz WJ. Chemical characterization of an antimicrobial substance produced by Lactobacillus reuteri. Antimicrobial Agents and Chemotherapy. 1989; 33:674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vollenweider S, Evers S, Zurbriggen K, Lacroix C. Unraveling the hydroxypropionaldehyde (HPA) system: an active antimicrobial agent against human pathogens. Journal of Agricultural and Food Chemistry. 2010; 58:10315–10322. doi: 10.1021/jf1010897 [DOI] [PubMed] [Google Scholar]
- 8.Schaefer L, Auchtung TA, Hermans KE, Whitehead D, Borhan B, Britton RA. The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology; 2010. 156:1589–1599. doi: 10.1099/mic.0.035642-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lüthi-Peng Q, Schärer S, Puhan Z. Production and stability of 3-hydroxypropionaldehyde in Lactobacillus reuteri. Applied Microbiology and Biotechnology. 2002; 60:73–80. doi: 10.1007/s00253-002-1099-0 [DOI] [PubMed] [Google Scholar]
- 10.Vollenweider S, Grassi G, König I, Puhan Z. Purification and structural characterization of 3-hydroxypropionaldehyde and its derivatives. Journal of Agricultural and Food Chemistry. 2003; 51:3287–3293. doi: 10.1021/jf021086d [DOI] [PubMed] [Google Scholar]
- 11.Eaton KA, Honkala A, Auchtung TA, Britton RA. Probiotic Lactobacillus reuteri ameliorates disease due to enterohemorrhagic Escherichia coli in germfree mice. Infection and Immunity. 2011; 79:185–191. doi: 10.1128/IAI.00880-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naylor SW, Low JC, Besser TE, Mahajan A, Gunn GJ, Pearce MC, et al. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infection and Immunity. 2003; 71:1505–1512. doi: 10.1128/IAI.71.3.1505-1512.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown C, Harmon BG, Zhao T, Doyle MP. Experimental Escherichia coli O157:H7 carriage in calves. Applied and Environmental Microbiology. 1997; 63:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keen JE, Laegreid WW, Chitko-McKown CG, Durso LM, Bono JL. Distribution of Shiga-toxigenic Escherichia coli O157 in the gastrointestinal tract of naturally O157-shedding cattle at necropsy. Applied and Environmental Microbiology. 2010; 76:5278–5281. doi: 10.1128/AEM.00400-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker C, Shi X, Sanderson M, Sargeant J, Nagaraja TG. Prevalence of Escherichia coli O157:H7 in gut contents of beef cattle at slaughter. Foodborne Pathogens and Disease. 2010; 7:249–255. doi: 10.1089/fpd.2009.0410 [DOI] [PubMed] [Google Scholar]
- 16.Sperandio V. SdiA sensing of acyl-homoserine lactones by enterohemorrhagic E. coli (EHEC) serotype O157:H7 in the bovine rumen. Gut Microbes. 2010; 1:432–435. doi: 10.4161/gmic.1.6.14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baruzzi F, Poltronieri P, Quero GM, Morea M, Morelli L. An in vitro protocol for direct isolation of potential probiotic lactobacilli from raw bovine milk and traditional fermented milks. Applied Microbiology and Biotechnology. 2011; 90:331–342 doi: 10.1007/s00253-011-3133-6 [DOI] [PubMed] [Google Scholar]
- 18.Chaucheyras-Durand F, Madic J, Doudin F, Martin C. Biotic and abiotic factors influencing in vitro growth of Escherichia coli O157:H7 in ruminant digestive contents. Applied and Environmental Microbiology. 2006; 72:4136–4142. doi: 10.1128/AEM.02600-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertin Y, Chaucheyras-Durand F, Robbe-Masselot C, Durand A, de la Foye A, Harel J, et al. Carbohydrate utilization by enterohaemorrhagic Escherichia coli O157:H7 in bovine intestinal content. Environmental Microbiology. 2013; 15:610–622. doi: 10.1111/1462-2920.12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spinler JK, Taweechotipatr M, Rognerud CL, Ou CN, Tumwasorn S, Versalovic J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe. 2008; 14:166–171. doi: 10.1016/j.anaerobe.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stern MD, Bach A, Calsamiglia S. Alternative techniques for measuring digestion in ruminants. Journal of Animal Science. 1997; 75:2256–2276. [DOI] [PubMed] [Google Scholar]
- 22.Adesogan AT. Effect of bag type on the apparent digestibility of feeds in ANKOM Daisy II incubators. Animal Feed Science and Technology. 2005; 119:333–334. [Google Scholar]
- 23.Damiran D, Del Curto T, Bohnert DW, Findhol SL. Comparison of techniques and grinding size to estimate digestibility of forage based ruminant diets. Animal Feed Science and Technology. 2008; 141:15–35. [Google Scholar]
- 24.Goering HK, Van Soest PJ. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications). Agri Handbook No. 379. ARSUSDA, Washington, DC. 1970.
- 25.Bertin Y, Deval C, de la Foye A, Masson L, Gannon V, Harel J, et al. The gluconeogenesis pathway is involved in maintenance of enterohaemorrhagic Escherichia coli O157:H7 in bovine intestinal content. PLoS One. 2014; 9:e98367 doi: 10.1371/journal.pone.0098367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa M, Shimizu K, Nomoto K, Tanaka R, Hamabata T, Yamasaki S, et al. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157:H7 by probiotic Lactobacillus strains due to production of lactic acid. International Journal of Food Microbiology. 2001; 68:135–140. [DOI] [PubMed] [Google Scholar]
- 27.Mosoni P, Chaucheyras-Durand F, Béra-Maillet C, Forano E. Quantification by real-time PCR of cellulolytic bacteria in the rumen of sheep after supplementation of a forage diet with readily fermentable carbohydrates: effect of a yeast additive. Journal of Applied Microbiology. 2007; 103:2676–2685. doi: 10.1111/j.1365-2672.2007.03517.x [DOI] [PubMed] [Google Scholar]
- 28.Heilig HG, Zoetendal EG, Vaughan EE, Marteau P, Akkermans AD, de Vos WM. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl Environ Microbiol. 2002; 68:114–23. doi: 10.1128/AEM.68.1.114-123.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivas L, Coffey B, McAuliffe O, McDonnell MJ, Burgess CM, Coffey A, et al. In vivo and ex vivo evaluations of bacteriophages e11/2 and e4/1c for use in the control of Escherichia coli O157:H7. Applied and Environmental Microbiology. 2010; 76:7210–7216. doi: 10.1128/AEM.01530-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wisener LV, Sargeant JM, O’Connor AM, Faires MC, Glass-Kaastra SK. The use of direct-fed microbials to reduce shedding of Escherichia coli O157 in beef cattle: a systematic review and meta-analysis. Zoonoses and Public Health. 2015; 62:75–89. doi: 10.1111/zph.12112 [DOI] [PubMed] [Google Scholar]
- 31.Brashears MM, Chaves BD. The diversity of beef safety: A global reason to strengthen our current systems. Meat Sci. 2017; S0309-1740(17)30213-30219. [DOI] [PubMed] [Google Scholar]
- 32.Luongo D, Miyamoto J, Bergamo P, Nazzaro F, Baruzzi F, Sashihara T, et al. Differential modulation of innate immunity in vitro by probiotic strains of Lactobacillus gasseri. BMC Microbiology. 2013; 13:298. doi: doi: 10.1186/1471-2180-13-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McSweeney S, Mackie RI. Micro-organisms and ruminant digestion: state of knowledge, trends and future prospects. FAO Background study paper N° 61; 2012. [Google Scholar]
- 34.Vaidyanathan H, Kandasamy V, Ramakrishnan GG, Ramachandran KB, Jayaraman G, Ramalingam S. Glycerol conversion to 1, 3-Propanediol is enhanced by the expression of a heterologous alcohol dehydrogenase gene in Lactobacillus reuteri. AMB Express. 1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White BA, Lamed R, Bayer EA, Flint HJ. Biomass utilization by gut microbiomes. Annual Review of Microbiology. 2014; 68:279–96. doi: 10.1146/annurev-micro-092412-155618 [DOI] [PubMed] [Google Scholar]
- 36.Biebl H, Menzel K, Zeng AP, Deckwer WD. Microbial production of 1,3-propanediol. Applied Microbiology and Biotechnology. 1999; 52:289–297. [DOI] [PubMed] [Google Scholar]
- 37.Jami E, Mizrahi I. Composition and similarity of bovine rumen microbiota across individual animals. PLoS One. 2012; 7:e33306 doi: 10.1371/journal.pone.0033306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garton GA, Lough AK, Vioque E. Glyceride hydrolysis and glycerol fermentation by sheep rumen contents. Journal of General Microbiology. 1961; 25:215–225. doi: 10.1099/00221287-25-2-215 [DOI] [PubMed] [Google Scholar]
- 39.Danielsson R, Werner-Omazic A, Ramin M, Schnürer A, Griinari M, Dicksved J, et al. Effects on enteric methane production and bacterial and archaeal communities by the addition of cashew nut shell extract or glycerol-an in vitro evaluation. Journal of Dairy Science. 2014; 97:5729–5741. doi: 10.3168/jds.2014-7929 [DOI] [PubMed] [Google Scholar]
- 40.Hobson PN, Mann SO. The isolation of glycerol-fermenting and lipolytic bacteria from the rumen of the sheep. Journal of General Microbiology. 1961; 25:227–240. doi: 10.1099/00221287-25-2-227 [DOI] [PubMed] [Google Scholar]
- 41.Hoover WH, Miller TK. Rumen digestive physiology and microbial ecology. Veterinary Clinics of North America: Food Animal Practice. 1991; 7:311–325. [DOI] [PubMed] [Google Scholar]
- 42.Gruber SL, Tatum JD, Engle TE, Chapman PL, Belk KE, Smith GC. Relationships of behavioral and physiological symptoms of preslaughter stress to beef longissimus muscle tenderness. Journal of Animal Science. 2010; 88:1148–1159. doi: 10.2527/jas.2009-2183 [DOI] [PubMed] [Google Scholar]
- 43.Elramady MG, Aly SS, Rossitto PV, Crook JA, Cullor JS. Synergistic effects of lactic acid and sodium dodecyl sulfate to decontaminate Escherichia coli O157:H7 on cattle hide sections. Foodborne Pathogens and Disease. 2013; 10:661–663. doi: 10.1089/fpd.2012.1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chancey CC, Brooks JC, Martin JN, Echeverry A, Jackson SP, Thompson LD, et al. Survivability of Escherichia coli O157:H7 in mechanically tenderized beef steaks subjected to lactic acid application and cooking under simulated industry conditions. Journal of Food Protection. 2013; 76:1778–1783. doi: 10.4315/0362-028X.JFP-12-566 [DOI] [PubMed] [Google Scholar]
- 45.Chaucheyras-Durand F, Durand H. Probiotics in animal nutrition and health. Beneficial Microbes. 2010; 1:3–9. doi: 10.3920/BM2008.1002 [DOI] [PubMed] [Google Scholar]
- 46.Donkin SS, Koser SL, White M, Doane H, Cecava MJ. Feeding value of glycerol as a replacement for corn grain in rations fed to lactating dairy cows. Journal of Dairy Science. 2009; 92:5111–5119. doi: 10.3168/jds.2009-2201 [DOI] [PubMed] [Google Scholar]
- 47.Lomander H, Frössling J, Ingvartsen KL, Gustafsson H, Svensson C. Supplemental feeding with glycerol or propylene glycol of dairy cows in early lactation—effects on metabolic status, body condition, and milk yield. Journal of Dairy Science. 2012; 95: 2397–2408. doi: 10.3168/jds.2011-4535 [DOI] [PubMed] [Google Scholar]
- 48.Rémond D, Chaise JP, Delval E, Poncet C. Net flux of metabolites across the ruminal wall of sheep fed twice a day with orchardgrass hay. Journal of Animal Sciences. 1993; 71:2529–2538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The strains FCH6 RifR and EDL933 RifR were incubated in RF samples for 24 hours under anaerobiosis before enumeration. Bars represent the SEM of three independent experiments. Asterisks indicate statistical significance (*: P<0.05; ***: P<0.001).
(TIF)
L. reuteri strains (LB1-7, F275, 65-A, F70 and 100–23) were first spotted onto the surface of Brain Heart Infusion (BHI) agar supplemented with 20 mM glucose and incubated anaerobically. The EHEC strain FCH6 was then inoculated in soft agar with or without glycerol (2%) and poured over the L. reuteri spots (in order to facilitate or not HPA production by spots containing L. reuteri). The plates were then incubated and the antimicrobial activity was recorded as growth-free inhibition zones around the spots as previously described [20].
(TIF)
PCR detection of the genes gldC (A) and dhaT (B) from genomic DNA extracted from L. reuteri strains. Lane 1, molecular size marker (MassRuler DNA Ladder, ThermoFisher Scientific); lane 2, L. reuteri LB1-7; lane 3, L. reuteri 65A; lane 4, L. reuteri F70; lane 5, L. reuteri F275; line 6, L. reuteri 100–23.
The PCR products were subjected to electrophoresis on 1% agarose gel and visualized by ethidium bromide staining.
(TIF)
The Daisy II method was used to analyze the degrading activity of the rumen microbiota. The Daisy II vessels containing RF were supplemented or not with 80 mM glycerol and inoculated or not with L. reuteri LB1-7. The fermentation end-products were quantified in Daisy II vessels before and after 24 hours of anaerobic incubation. Bars represent the SEM of three independent experiments. Asterisks indicate statistical significance (*: P<0.05; ***: P<0.001).
ACE: acetate; PROP: propionate; BUTY: butyrate; GLY: glycerol; LACT: lactate.
(TIF)
The strain FCH6 RifR was incubated under anaerobiosis in Rec samples or co-incubated in Rec samples inoculated with L. reuteri LB1-7 (≈ 107 CFU/mL) and supplemented with 80 mM glycerol. Bars represent the SEM of three independent experiments. Effect of L. reuteri + Glyc80 is significant *, P<0.05.
(TIF)
a: DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany.
b: ISPA, Institute of Sciences of Food Production microbial collection number; National Research Council, Italy.
(DOCX)
DNA sequences from L. reuteri available in DNA databases (JCM 1112 [AP007282.1], DSM 20016 [CP000705.1], FUA3400 [KJ435307.1], SD2112 [CP002844.1], BR11 [GU191838.1] and BPL36 [JQ897939.1]) were aligned (Align Sequences Nucleotide BLAST [http://blast.ncbi.nlm.nih.gov]) to design the primer pairs used to amplify gldC and dhaT.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.