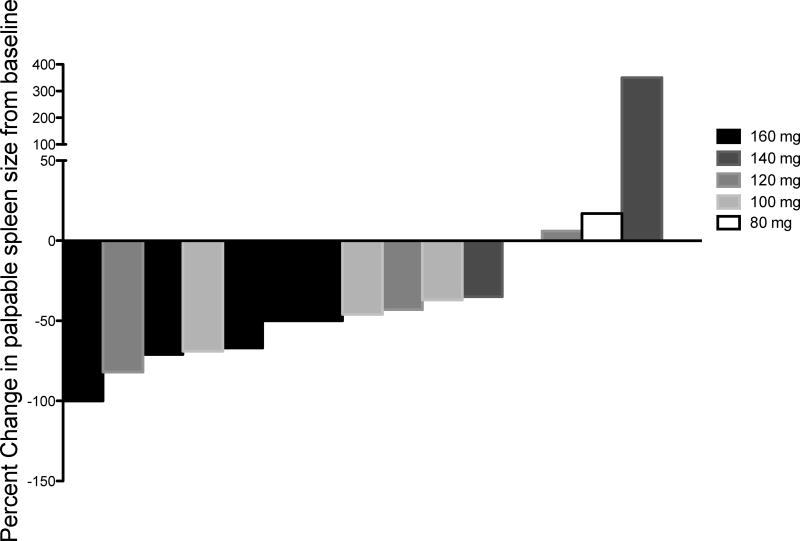Abstract
Despite extensive prior clinical investigation in acute leukemia (60 – 100 mg), dose finding studies have not yet established a true maximum tolerated dose (MTD) for the oral multi-kinase inhibitor lestaurtinib in hematologic malignancies. We performed a multicenter, investigator initiated, phase I (3+3) dose escalation study in patients with JAK2V617F positive myelofibrosis, irrespective of baseline platelet count. A total of 34 patients were enrolled. Dose limiting toxicities were observed in 3 patients overall, at the 100 mg (1) and 160 mg (2) twice daily dose levels. The MTD was 140 mg twice daily. Gastrointestinal toxicity was the most common adverse event, despite a change in drug formulation. Sixteen patients were evaluable for response at 12 weeks; by International Working Group criteria, 7 patients had clinical improvement, 8 had stable disease, and 1 had progressive disease. Eleven patients had a complete or partial response in palpable splenomegaly with a median reduction of 46%. Meaningful reductions in JAK2 V617F allele burden were not observed. To measure JAK2 inhibition in vivo, plasma from treated patients was assayed for its ability to inhibit phosphorylation of STAT5: doses lower than 140 mg had variable and incomplete inhibition. In this phase I study, the MTD for lestaurtinib in patients with myelofibrosis was determined to be 140 mg twice daily. Gastrointestinal adverse events were common, however, significant clinical activity was observed. [NCT00668421]
Keywords: lestuartinib, myelofibrosis, phase I trial, pharmacodynamics, pharmacokinetics
INTRODUCTION
The experience with tyrosine kinase inhibitors (TKIs) in chronic myelogenous leukemia serves as a paradigm for the treatment of other myeloproliferative neoplasms (MPNs) (1). JAK2 mutations identified in the blood cells of patients with the classic Philadelphia-chromosome negative MPNs polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF) initially provided the basis for the hypothesis that JAK2V617F could serve as a target for small molecule therapy of patients with MPNs. Over the last 5 years, several clinical trials of oral JAK1/2 inhibitors investigated whether the clonal evolution and clinical burden of MF could be diminished with JAK2-directed TKI therapy.
Lestaurtinib (CEP701), an orally available staurosporine analog, is a multi-kinase inhibitor that was tested extensively as a FLT3 inhibitor in acute myeloid leukemia (AML) (2–6). Limitations of the drug in AML studies (at dose levels between 60 and 100 mg twice daily) included gastrointestinal toxicity, low free drug levels related to in vivo plasma protein binding and, as a consequence, incomplete target inhibition. Lestaurtinib inhibits JAK2 with a potency comparable to that for FLT3 (in vitro IC50 1 nM). Lestaurtinib was shown to selectively inhibit growth of primary MPN cells in vitro (7). In addition, it was previously evaluated in a single-center phase 2 trial in MF patients (80 mg twice daily, liquid formulation), with limited clinical activity (8), and in a multicenter phase 2 trial in PV and ET patients, with modest clinical activity and only minimal reduction in JAK2V617F allele burden at doses of 80 mg twice daily (capsule formulation) (9). However, a maximum tolerated dose (MTD) for patients with myeloid disorders had not been identified for lestaurtinib. We therefore conducted a phase 1 study to establish the safety of lestaurtinib and identify its MTD in patients with MF. Additional secondary objectives included the evaluation of disease response and of the effect on the granulocyte JAK2V617F allele burden.
METHODS
This was a Myeloproliferative Disorders Research Consortium (MPD-RC)-sponsored multicenter open label, phase I study of lestaurtinib in patients with MF (primary or evolved from PV or ET) carrying the JAK2V617F mutation [NCT00668421]. The primary objective was to identify dose-limiting toxicities (DLT) and define the MTD. Secondary objectives included evaluation of the effect of lestaurtinib treatment on disease response as assessed by the European Myelofibrosis Network (EUMNET) response criteria (10). A post-hoc response assessment using the International Working Group consensus criteria for treatment response in myelofibrosis (IWG) (11) was also performed in an effort to unify this study with other studies of JAK inhibitors.
The design was a standard 3+3 cohort dose escalation study. A DLT was defined as any treatment-emergent grade 3 or greater adverse event (AE) considered possibly, probably or definitely related to the study drug, based on the National Cancer Institute (NCI) common toxicity criteria (CTC) version 3.0. Grade 3 nausea or vomiting was exempt from defining DLT, unless in the setting of maximal antiemetic treatment.
Eligible patients included men and women aged ≥ 18 years with a diagnosis of primary myelofibrosis (PMF), post-polycythemia vera myelofibrosis (PV-MF), or post-essential thrombocythemia myelofibrosis (ET-MF) and carrying the JAK2V617F mutation. Patients were required to have intermediate- or high-risk disease according to the Lille classification system (12), or have symptomatic splenomegaly (≥10 cm below the costal margin). Lestaurtinib was administered orally twice daily on a continuous basis. Five dose levels (80 mg, 100 mg, 120 mg, 140 mg and 160 mg) were evaluated. A liquid formulation (25 mg/ml) was used for the first two dose levels at study initiation, until a capsule formulation (20 mg) (expected to reduce gastrointestinal toxicity) became available. The capsule formulation replaced the liquid, starting again at the 100 mg dose level.
Because frequent expected gastrointestinal AEs were observed early in the treatment of patients at each dose level, the protocol was amended to allow for flexible dosing within the first 28 days at the 160 mg BID starting dose level, with permissive dose reductions for intolerable dyspepsia, diarrhea, or nausea that was grade 1 or 2 but interfered sufficiently with a subject’s quality of life that they were unwilling to continue treatment with the investigational agent at the assigned dose. Patients with manageable grade 1 or 2 dyspepsia, diarrhea, or nausea continued without dose reduction. Five patients were enrolled in this flexible dosing cohort and toxicity was analyzed for the entire cohort.
Patients in this Phase I study who did not experience a DLT could remain on the investigational agent for up to 24 weeks with safety and efficacy assessment being performed at 12 and 24 weeks. Patients who completed 24 weeks of treatment were eligible to continue treatment at the discretion of the site investigator. For a complete description of study design, inclusion/exclusion criteria, dose modifications, schedule of assessments, and JAK2 V617F measurements, see supplemental methods.
Pharmacokinetic assessments
Peripheral blood samples for the measurement of lestaurtinib plasma concentrations were collected at baseline and at weeks 12 and 24. Plasma was harvested on-site within 30 minutes of sampling and frozen. Batched samples were analyzed at Teva Pharmaceuticals, using a validated high-performance liquid chromatography (HPLC) method with fluorescence detection (6).
Pharmacodynamic plasma inhibitory activity of Lestaurtinib
BaF/3 EPOR-GFP JAK2 V617F cells (gift of Dr. Ross Levine, Memorial Sloan Kettering Cancer Center, NY, NY) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum. Cells were re-suspended in fresh medium at a density of 2 ×106 cells/ml immediately prior to the performance of the plasma inhibitory assay (PIA). Patient plasma samples were thawed rapidly and cleared by centrifugation, then stored on ice and protected from light prior to use. Inhibitor-naive plasma from a study subject was used to generate a standard curve for dose response. Lestaurtinib was added to this plasma and diluted serially to concentrations of 5, 10, 20, 30, 40 and 100 µ M, in addition to drug-free plasma. All plasma samples were diluted 1:1 with cells in a total volume of 100 µ l so that the final lestaurtinib concentrations in the standard curve were 2.5, 5, 10, 15, 20 and 50 µ M. Study plasma samples were assayed in duplicate. Cells suspended in plasma were then incubated in a humidified incubator at 37°C and 5% CO2 for 20 minutes. Cells were subsequently fixed in 4% methanol-free formaldehyde. The fixed cells were washed twice with wash buffer (4% BSA in PBS), re-suspended in ice-cold 90% methanol, and stored at −20°C until analysis. Cells were washed twice with wash buffer to remove methanol and stained with phospho-STAT5 AF647 (pY694, BD Biosciences) and total STAT5 (R&D Biosystems) antibodies. Data were collected using a BD FACSCalibur flow cytometer. The mean fluorescence intensity of phospho-STAT5 was calculated for STAT5+ GFP+ dual positive cells using FlowJo (TreeStar) and was displayed with Prism (GraphPad).
RESULTS
Patient Characteristics
The baseline characteristics of the 34 patients enrolled in this phase I study are summarized in Table 1. The median age was 70, and 68% were male. The majority had PMF (62%) and the remainder had ET-/PV-MF. The median disease duration at study entry was 25 months (range, 0–293). The Lille score at study entry was intermediate in 56%; 29% had low -risk disease and 15% high-risk disease. According to the Dynamic International Prognostic Scoring System (DIPSS) (13), 79% of patients had intermediate-2 or high risk disease. Results of baseline cytogenetic analyses were available for 26 patients, with 13 of these patients classified with unfavorable karyotypic abnormalities (14). The median baseline granulocyte JAK2V617F allele burden was 75% (range 9% – 99.4%) for 27 patients. The majority of patients were previously treated, most commonly with hydroxyurea (56%) (see Appendix Table A1). The median baseline hemoglobin was 9.6 g/dL; 53% of patients had a hemoglobin below 10g/dL and 47% had been transfused with at least one unit of red blood cells within the previous six months. The median platelet count at study entry was 146 × 109/L; 12 (35%) patients had a platelet count under 100 × 109/L at study entry, ranging from 12 to 60 × 109/L. 97% of patients had baseline splenomegaly, with a median palpable spleen length of 15 cm.
Table1.
Baseline Patient Demographic and Clinical Characteristics
| Variable | N | % | |
|---|---|---|---|
| Age (years) | Median (Range) | 70 (23,85) | |
|
| |||
| Gender | Male | 23 | 68% |
| Female | 11 | 32% | |
|
| |||
| Disease Subtype | Primary Myelofibrosis | 21 | 62% |
| PV related Myelofibrosis | 10 | 29% | |
| ET related Myelofibrosis | 3 | 9% | |
|
| |||
| Lille Score | 0 | 10 | 29% |
| 1 | 19 | 56% | |
| 2 | 5 | 15% | |
|
| |||
| Baseline Hemoglobin (g/dL) | Median (Range) | 9.6 (6.7, 17.0) | |
|
| |||
| Baseline WBC (×109/L) | Median (Range) | 9.0 (2.6, 92) | |
|
| |||
| Baseline Platelets (×109/L) | Median (Range) | 145.5 (7, 533) | |
|
| |||
| Baseline Weight (kg) | Median (Range) | 70.4 (50.3, 88.5) | |
|
| |||
| Spleen Size at Baseline (cm) | 0 | 1 | 3% |
| 1–10 | 4 | 12% | |
| 11–20 | 21 | 62% | |
| >20 | 8 | 24% | |
| Median (Range) | 15 (2, 30) | ||
|
| |||
| JAK2V617F allele burden (%) | Median (Range) | 75 (9, 99.4) | |
| < 50% | 9 | 26% | |
| >=50% | 18 | 53% | |
| Unknown | 7 | 21% | |
|
| |||
| DIPSS Score* | Low risk (0) | 1 | 3% |
| Intermediate-1 Risk (1) | 6 | 18% | |
| Intermediate-2 Risk (2) | 16 | 47% | |
| High Risk (3+) | 11 | 32% | |
|
| |||
| Red blood cell transfusion | Yes | 16 | 47% |
| in 6 Month Prior to Trial | No | 18 | 53% |
|
| |||
| Platelets <100 × 109/L | Yes | 12 | 35% |
| No | 22 | 65% | |
|
| |||
| Karyotype | Favorable | 13 | 38% |
| Unfavorable | 13 | 38% | |
| Unknown | 6 | 18% | |
|
| |||
| DIPSS Plus Score* | Low risk (0) | 1 | 3% |
| Intermediate-1 Risk (1) | 2 | 6% | |
| Intermediate-2 Risk (2–3) | 14 | 41% | |
| High Risk (4+) | 17 | 50% | |
|
| |||
| Disease duration (months) | Median (Range) | 25(0, 293) | |
|
| |||
| Number of Prior Therapies | Median (Range) | 2(0, 4) | |
Baseline karyotype unknown 6 (18%) patients. These patients were assumed to have favorable karyotype when calculating their DIPSS and DIPSS Plus scores.
Dose Escalation and Toxicity
Patient disposition and distribution of the observed DLTs by dose level are shown in Table 2. Five patients who did not experience a DLT withdrew from the study prior to the completion of 28 days of treatment at the assigned dose. Of these, 4 withdrew due to grade 1 or 2 gastrointestinal events that were possibly or probably drugrelated (diarrhea (2), dyspepsia (1) and one grade 2 pancreatitis in a patient with a history of idiopathic pancreatitis). One patient discontinued the study at the discretion of the treating investigator, for a previously unrecognized pre-existing coagulopathy and concern for occult liver disease. At the original 160 mg fixed dose level, 4 of 7 patients had a protocol-defined treatment interruption prior to 28 days, although only one patient met criteria for a DLT (grade 3 diarrhea). This experience, together with the known pharmacokinetic properties of the drug, precipitated a protocol amendment (April 2010) to allow dose modifications for gastrointestinal toxicity that was mild/moderate in severity (grade ≤2) but intolerable. Five patients were treated under the amended protocol for the phase I portion, with a starting dose of 160 mg and flexible dosing. Of these, 3 required dose reductions (gastrointestinal adverse events; thrombocytopenia) and one experienced a DLT (grade 3 anorexia). The 140 mg twice daily dose was thus identified as the recommended dose for an additional cohort and a subsequent phase II trial. Treatment-emergent hematologic toxicities by dose level, gastrointestinal toxicities by dose level, and all toxicities regardless of attribution are listed in Tables 3, 4 and supplemental Table A2, respectively. There did not appear to be a lower incidence of gastrointestinal toxicity with the capsule versus the liquid formulation, although the capsule formulation was tested at higher dose levels and exposure to treatment was more prolonged.
Table 2.
Patient disposition and distribution of dose limiting toxicity (DLT) by dose level
| Dose Level |
n | Patients evaluable for Phase I |
DLT | Reason withdrawn before 28 days (CAUSE) |
Patients remaining on treatment >12 weeks |
Reasons for withdrawal after 12 weeks |
|---|---|---|---|---|---|---|
| 80* | 4 | 3 | No | 1 (G2 pancreatitis) | 3 | G2 edema; G4 neutropenia; Death1 |
| 100* | 3 | 3 | Yes (Diarrhea) | 0 | 1 | G2 diarrhea |
| 100 | 4 | 3 | No | 1 (Coagulopathy, pre-existing) | 1 | G3 emesis |
| 120 | 3 | 3 | No | 0 | 2 | Lost to follow-up: Patient withdrawal |
| 140 | 8 | 6 | No | 2 (G2 diarrhea; G1 nausea) | 4 | Non-compliance; G2 thrombosis and G3 hyperbilirubinemia; death |
| 160 | 7 | 3 | Yes (Diarrhea) | 3 (G2 dyspepsia; G2 diarrhea; investigator decision) | 3 | G3 muscle weakness |
| 160# | 5 | 5 | Yes (Anorexia) | 0 | 3 | G3 abdominal pain; G4 thrombocytopenia; Death |
| TOTAL | 34 | 26 | 3 | 7 | 17 |
patients treated with liquid formulation; all subsequent was capsule formulation.
flexible dosing (first 28 days) cohort
Death in setting of profound fatigue and weakness
Table 3.
Treatment emergent hematologic toxicities by initial dose level
| Dose Cohort N |
80 n=4 |
100 n=7 |
120 n=3 |
140 n=8 |
160 n=12 |
Total n=34 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| AE | Grade | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 |
|
| |||||||||||||
| Anemia | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 1 | 5 | |
|
| |||||||||||||
| Leukopenia | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 4 | 1 | |
|
| |||||||||||||
| Thrombocytopenia | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 2 | 2 | 5 | |
Table 4.
Gastrointestinal toxicities by initial dose level
| Dose Cohort N |
80 n=4 |
100 n=7 |
120 n=3 |
140 n=8 |
160 n=12 |
Total n=34 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| AE | Grade | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 |
|
| |||||||||||||
| Diarrhea | 2 | 1 | 5 | 2 | 3 | 0 | 6 | 0 | 11 | 1 | 27 | 4 | |
|
| |||||||||||||
| Nausea | 3 | 0 | 5 | 0 | 3 | 0 | 4 | 0 | 9 | 0 | 24 | 0 | |
|
| |||||||||||||
| Dyspepsia | 0 | 0 | 1 | 0 | 1 | 0 | 3 | 0 | 5 | 0 | 10 | 0 | |
|
| |||||||||||||
| Vomiting | 2 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 2 | 0 | 8 | 0 | |
|
| |||||||||||||
| Anorexia | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 3 | 1 | 6 | 3 | |
|
| |||||||||||||
| Dysgeusia | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 3 | 0 | |
|
| |||||||||||||
| Constipation | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | |
|
| |||||||||||||
| Dysphagia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | |
|
| |||||||||||||
| Flatulence | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | |
|
| |||||||||||||
| Gastritis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | |
|
| |||||||||||||
| Hemorrhoids | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | |
|
| |||||||||||||
| Mucositisoral | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
Twelve patients started therapy with a baseline platelet count of <100 × 109/L (range 12, 60 × 109/L); eight of these patients required platelet transfusion support while on therapy as shown in Supplemental Table A3. This subgroup experienced a total of 4 bleeding events (grade 1 epistaxis (2) or gastrointestinal (1); grade 3 gastrointestinal (1). Treatment was discontinued in only one patient due to worsening thrombocytopenia.
Disease Response
Response to therapy could be assessed in the 16 (47%) patients who remained on treatment for at least 12 weeks (Table 5). By IWG response criteria, 7 patients had clinical improvement, 8 had stable disease, and 1 patient had disease progression. Of the 7 patients with clinical improvement, 5 were in the 160 mg dose group. The original study was designed to measure global clinicohematologic response by EUMNET criteria, which reflects composite changes in blood counts, spleen size and constitutional symptoms. Eight patients achieved a global moderate response and 8 had no response; Table 5 lists the responses in spleen size, blood counts, systemic symptoms, and EUMNET and IWG response by dose level for these 16 patients.
Table 5.
Response by initial dose cohort of lestaurtinib for patients on treatment for 3 months or longer
| Response | All Patients on Treatment for ≥3 Months N=16 |
Dose Cohort 80– 100 N=4 |
Dose Cohort 120 N=3 |
Dose Cohort 140 N=3 |
Dose Cohort 160 N=6 |
|
|---|---|---|---|---|---|---|
| Spleen | CR | 1 | 0 | 0 | 0 | 1 |
| PR | 10 | 3 | 2 | 1 | 4 | |
| NR | 5 | 1 | 1 | 2 | 1 | |
|
| ||||||
| Hemoglobin | Stable | 15 | 4 | 3 | 3 | 5 |
| Progression | 1 | 0 | 0 | 0 | 1 | |
|
| ||||||
| Platelet | CR | 3 | 0 | 1 | 0 | 2 |
| count | Stable or Minor Change | 13 | 4 | 2 | 3 | 4 |
|
| ||||||
| White Blood | CR | 3 | 1 | 0 | 0 | 2 |
| Cell count | Stable or Minor Change | 13 | 3 | 3 | 3 | 4 |
|
| ||||||
| Symptoms | Baseline Symptoms & Resolution | 3 | 0 | 1 | 1 | 1 |
| Baseline Symptoms & Incomplete Resolution/Stable | 7 | 4 | 1 | 0 | 2 | |
| No Baseline Symptoms & No Progression | 4 | 0 | 1 | 2 | 1 | |
| No Baseline Symptoms & Progression | 2 | 0 | 0 | 0 | 2 | |
|
| ||||||
| Global | CR | 0 | 0 | 0 | 0 | 0 |
| Response | Major Response | 0 | 0 | 0 | 0 | 0 |
| EUMNET | Moderate Response | 8 | 3 | 2 | 1 | 2 |
| Minor Response | 0 | 0 | 0 | 0 | 0 | |
| No Response | 8 | 1 | 1 | 2 | 4 | |
|
| ||||||
| Global | CR | 0 | 0 | 0 | 0 | 0 |
| Response | Major Response | 10 | 3 | 2 | 1 | 4 |
| Disregarding | Moderate Response | 0 | 0 | 0 | 0 | 0 |
| Symptoms | Minor Response | 0 | 0 | 0 | 0 | 0 |
| EUMNET | No Response | 6 | 1 | 1 | 2 | 2 |
|
| ||||||
| IWG | CR | 0 | 0 | 0 | 0 | 0 |
| Response | PR | 0 | 0 | 0 | 0 | 0 |
| Clinical Improvement | 7 | 1 | 1 | 0 | 5 | |
| Stable Disease | 8 | 3 | 2 | 2 | 1 | |
| Progressive Disease | 1 | 0 | 0 | 1 | 0 | |
One subject achieved a complete response (CR) as assessed by a spleen response at the 160mg dose level, while 10 achieved a partial response (PR). The median percent reduction in palpable splenomegaly for the 15 patients with baseline palpable splenomegaly on treatment for at least 3 months was 46% (range 100% reduction to 350% increase). The percent reduction in splenomegaly for each individual subject is shown in Figure 1. Fifteen patients maintained stability in their hemoglobin levels, distributed evenly across all dose levels. One subject who was transfusion-independent at baseline required red cell transfusions starting on week 6 of treatment (160mg cohort). Patients who were considered transfusion-dependent at baseline remained transfusion-dependent. Three patients (19%) achieved a CR associated with a normalization of the white blood cell count and the platelet count. Although the use of several other JAK2 inhibitors are associated with worsening thrombocytopenia, lestaurtinib use was not associated with dramatic reductions in platelet numbers and was clinically active even in 12 patients entered on trial with a low baseline platelet count (supplemental Table A3). Of 10 patients with constitutional symptoms (sweats, fevers, weight loss, pruritus, abdominal discomfort) reported at baseline, three patients, all of whom received doses of 120 mg or greater, had resolution of these symptoms. None of the patients completing 12 weeks of treatment gained weight during that interval.
Figure 1. Percent change in baseline palpable spleen length at week 12.
Waterfall plot demonstrating percent change in palpable spleen length for each subject at week 12 and colored coded by dose level. A negative percent change indicates a reduction in spleen length. Fifteen patients with baseline palpable splenomegaly were evaluable for this analysis at week 12 of lestaurtinib treatment.
There was no pattern of change in bone marrow histomorphologic abnormalities or change in karyotypic abnormalities noted at baseline in patients treated with lestaurtinib in this trial.
Effect of Lestaurtinib on JAK2 V617F allele burden
Of the 16 patients who received treatment for at least 12 weeks, eight patients had JAK2V617F allele burden determinations at baseline and at 12 weeks. In four of the eight patients, the JAK2V617F allele burden decreased from baseline, with a median decrease of 8.3% (−5.7%, −8.0%, −8.6%, −13.8%), while in the other four, the allele burden increased from baseline, with a median increase of 8.4% (0.1%, 7.6%, 9.1%, 26.7%). As changes of less than 10% in allele burden are not considered significant, lestaurtinib, similar to other JAK2 inhibitors, did not significantly affect JAK2V617F allele burden.
Drug levels and in vitro plasma inhibitory activity
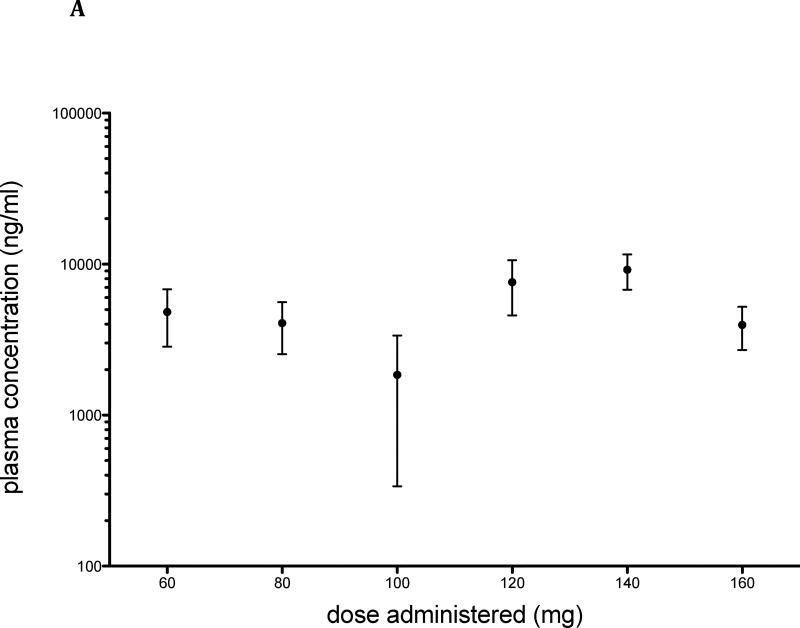
Trough levels of lestaurtinib were measured in treated patients at weeks 12 and 24, and ranged from 197.9 – 18626 ng/mL (Figure 2A). The plasma concentrations of total drug across the dose range evaluated were variable, consistent both with the relatively narrow dose range studied and the variable pharmacokinetic properties of the agent.
Figure 2. Plasma Concentration of lestaurtinib.
Trough levels of lestaurtinib were measured in treated patients by HPLC with fluorescence detection. A: Mean and standard error are presented for each dose administered at time of collection (n = 24: n=2 at 60 and 80 mg; 3 at 100 mg, 5 at 120 mg, 9 at 140 mg and 3 at 160 mg). B: Plasma concentration plotted against spleen response at 3 months (n=12; p = 0.2964).
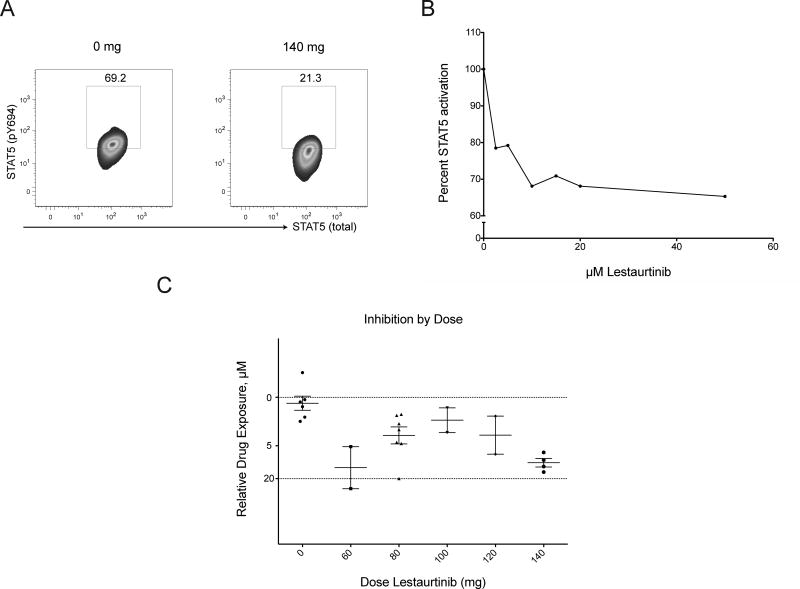
To better define target inhibition using a standardized approach we developed an assay to measure the ability of active drug in serum from treated patients to inhibit phosphorylation of STAT5 in a relevant cell line (Figure 3), a strategy first used to compare relative penicillin activity in the 1950s (15) and more recently adapted to small molecule inhibitor studies in acute leukemia (16). A plasma inhibitory assay is particularly well suited to lestaurtinib, which is highly plasma protein-bound with potential variation in free drug levels across treated patients. In the presence of plasma from a treated patient (140 mg, Figure 3a), phosphorylation of STAT5 in BaF/3 EPOR-GFP JAK2 V617F cells was reduced relative to basal phosphorylation of cells in the presence of treatment-naive plasma (right and left panels, respectively). Most samples from treated patients showed plasma inhibitory activity, although at doses lower than 140 mg, inhibition was variable and incomplete (Figure 3c). There was a significant positive correlation between plasma drug level and inhibitory activity (n=7; p= 0.01; two-tailed spearman rank correlation, Rs=0.89). We noted that patients with the greatest dose reductions (60 mg, Figures 2 and 3c) appeared to have relatively high exposure to drug, suggesting that toxicity may correlate with both drug exposure and effective biologic dose, although given small patient numbers, we interpret these data with caution. Mean plasma drug concentration was higher in patients achieving a partial response in spleen reduction than those without a response, though the difference was non-significant (Figure 2b).
Figure 3. STAT5 Phosphorylation is variably inhibited at lower doses of lestaurtinib.
BaF/3 EPOR-GFP JAK2 V617F cells were incubated in patient plasma and pSTAT5 fluorescence intensity was measured (panel A). A corresponding standard curve was generated using thawed inhibitor-naive plasma from patients with varying concentrations of lestaurtinib added (final concentrations of 2.5 – 50 μM). PhosphoSTAT5 activation is represented as a ratio of treated to untreated cells, as measured by mean fluorescence intensity (MFI, panel B). Plasma samples (baseline and trough levels) collected at designated time points were measured in parallel (panel C): MFI values for study samples (n=20) were converted to a percent of untreated; lines represent mean with SEM at each dose level. Horizontal lines are derived from the standard curve.
DISCUSSION
This phase I study establishes 140 mg twice daily as the MTD of lestaurtinib in patients with JAK2V617F–positive MF. This is the first study of lestaurtinib in adults at these higher dose levels. In contrast to several other JAK inhibitor studies, this study did not exclude patients for thrombocytopenia or neutropenia. As with other JAK inhibitors, treatment-emergent neutropenia and thrombocytopenia were observed, although grade 3/4 anemia or thrombocytopenia was not a major cause of discontinuation from the study. It is hoped that these findings add to the collective experience suggesting that with monitoring and dose modifications, these agents can be more widely used, and that current and future research studies of this class of agents, alone or in combination, can be extended even to the highest-risk MF patients.
Gastrointestinal intolerance was prevalent with lestaurtinib treatment, and represents a major barrier to chronic administration. Although grade 3/4 diarrhea and anorexia were seen in only four and three of the patients, respectively, a high incidence of grade 1/2 diarrhea, nausea, dyspepsia, and vomiting made this drug difficult to administer continuously. Gastrointestinal toxicity to varying degrees has also been associated with most other JAK1/2 inhibitors in clinical development (17). Despite gastrointestinal intolerance, seven patients on this trial received therapy for over 1 year, and four patients have been treated for more than 3 years, suggesting that a subset of patients is able to both tolerate lestaurtinib and obtain clinical benefits.
In general, the development of lestaurtinib across disease types has been hampered by its tolerability profile and by variable steady-state plasma levels; it is highly protein-bound in plasma, binding predominantly to alpha-1-acid glycoprotein, an acute-phase reactant, leading to considerable variation in inhibitory activity across individuals. Consistent with observations in acute myeloid leukemia (6), we found that inhibition of downstream signaling was incomplete in patients with MF, particularly at lower dose levels. For the dose range explored, measurements of phospho-STAT5 inhibition by plasma from treated patients suggest that at doses lower than 140 mg, inhibition was incomplete, underscoring that the therapeutic window for lestaurtinib is narrow. Previously described pharmacokinetic and metabolic factors may contribute to this observation, including decreasing total drug levels over time due to the induction of cytochrome p450 enzymes and increasing levels of alpha-1-acid glycoprotein over time, leading to high-affinity drug binding and even lower available free drug (6).
While this was not an efficacy study, some clinical responses to treatment were observed. By IWG criteria, approximately half of evaluable patients had clinical improvement. By EUMNET criteria (pre-specified secondary endpoint) the best overall responses were moderate global responses in 50% of evaluable patients at three months of therapy, which were largely driven by partial responses in spleen size and symptoms. The EUMNET criteria for response assessment have limitations, including the reliance on measurement of constitutional symptoms and the phase 1 portion of this study did not use a uniform measurement tool or diary for measuring symptoms (18), which may have led to internally inconsistent data with respect to baseline symptoms and/or symptom response. In our dataset however, we found that this would bias our actual response towards no response (considering EUMNET criteria without symptom response, ten patients rather than one achieving a major response; Table 5); this discrepancy was largely driven by an absence of baseline constitutional symptoms captured.
More generally, there may be biologic characteristics of the disease and/or flaws in this targeting approach that would compromise efficacy in MF. Lestaurtinib is a multi-kinase inhibitor, so there may be unintended consequences of non-JAK2 pathway inhibition which could be additive, synergistic, or oppose the control of the clone. In addition, both the on-target and the off-target effects could extend to nonclonal or bystanders cells, and thereby alter response. Only a JAK2V617F mutant-specific inhibitor would be expected to selectively deplete the malignant clone. And while this phase I study was not powered to address efficacy questions, the minimal effect on JAK2V617F allele burden in this study suggests that lestaurtinib did not selectively deplete the malignant clone in the time period observed; this observation is consistent with the findings in larger prior studies of JAK inhibitors in MF (19, 20). In addition, disease-specific features may confer a priori resistance to this treatment approach. Intrinsic resistance to tyrosine kinase inhibitors has recently been elegantly described in CML (21), mechanistically explained by splice variants leading to deficient TKI-induced apoptosis. We have recently shown that cells from patients with MF may be similarly resistant to tyrosine kinase inhibition, and while the mechanism appears to be cell intrinsic, it remains obscure (22).
This dose escalation study establishes a dose of lestaurtinib of 140 mg twice a day as the MTD in patients with MF. Signaling studies in treated patients suggest that reliable downstream target inhibition may overlap with the MTD. Insufficient inhibition of JAK2 may be one mechanism behind the lack of disease remissions in MF. It is hoped that a more effective strategy for successful treatment of MF will evolve from better understanding of the complex disease biology through ongoing collaborative efforts.
METHODS
Inclusion/exclusion criteria (complete)
Eligible patients included men and women aged > 18 years with a diagnosis of primary myelofibrosis (PMF), post-polycythemia vera myelofibrosis (PV-MF), or post-essential thrombocythemia myelofibrosis (ET-MF) and carrying the JAK2V617F mutation. Patients were required to have intermediate- or high-risk disease according to the Lille classification system (12), or have symptomatic splenomegaly (>10 cm below the costal margin). Additional inclusion criteria consisted of a serum bilirubin level ≤2 times the upper limit of normal (ULN), serum glutamic-pyruvic transaminase (SGPT)/alanine aminotransferase [ALT]) levels ≤ 2 × ULN, serum creatinine levels ≤1.5 × ULN, and an ECOG performance status of 0, 1, or 2. Women of childbearing potential were required to have a negative serum pregnancy test at enrollment and women and men of childbearing age were required to practice medically acceptable methods of contraception during the study period and for 30 (women) and 60 (men) days thereafter. Participants considered as candidates to receive an allogeneic hematopoietic stem cell transplant at enrollment were ineligible, and patients were to be off any MF-directed therapy for 4 weeks prior to study entry, with the exception of erythropoietin and hydroxyurea. Nursing and pregnant women were excluded, as were patients with New York Heart Association Grade II or greater congestive heart failure, unstable angina, or a recent history of gastrointestinal bleeding, an ongoing serious non-healing wound, ulcer, or bone fracture, or a major surgical procedure. Patients requiring treatment with a CYP3A4 inhibitor or with known hypersensitivity to any component of lestaurtinib were also ineligible.
Dose modifications
After the first 28 days of treatment, lestaurtinib dosing was interrupted for an individual patient if there was any grade 3–4 non-hematologic toxicity; after resolution or improvement to grade 1 or the baseline level, the dose was reduced to 80 mg twice daily (for patients receiving 120 mg – 160 mg at time of dose reduction) or 60 mg twice daily (for patients receiving 60 mg – 100 mg at the time of reduction). Patients with non-hematologic toxicity who were unable to tolerate the reduced dose level were withdrawn from the treatment phase of the study. Likewise, the drug was interrupted and the dose reduced for significant thrombocytopenia (defined as > 50% reduction from baseline and platelets <100,000/mm3) or neutropenia (defined as a > 50% from baseline and an absolute neutrophil count (ANC) <1000/mm3). Treatment at a reduced dose was resumed when the platelet count or ANC returned to baseline or greater than 100 ×109/L or > 1000/ mm3, respectively. Patients on 80 mg twice daily who experienced recurrent cytopenias that required interruption of therapy were permitted dose reduction to 60 mg twice daily. An amended protocol provided specified dose modifications after the first 28 days for gastrointestinal toxicity that was grade 1 or 2 but interfered sufficiently with a subject’s quality of life that they were unwilling to continue treatment with the investigational agent at the assigned dose. Patients with manageable grade 1 or 2 dyspepsia, diarrhea, or nausea continued without dose reduction.
Schedule of Assessments
In addition to the initial screening visit, there were 12 scheduled treatment visits for evaluation of safety at days 1, 8, 15, 22, 29 and at weeks 6, 9, 12, 15, 18, 21 and 24; for patients on extended drug treatment, visits occurred at 4-week intervals.
JAK2 V617F allele burden measurements
Peripheral blood granulocytes were isolated as previously described (13). Genomic DNA was isolated by acidic phenol extraction (Trizol, Gibco BRL) and quantified for the JAK2 wild type and JAK2V617F alleles using the Ipsogen MutaQuant Kit (gift of the manufacturer) as per the manufacturer’s recommendation. Allele ratios were determined as the percentage of JAK2V617F alleles over the total JAK2 alleles.
Supplementary Material
Acknowledgments
The authors would like to thank Ed Hellriegel (Teva Pharmaceuticals) for performing pharmacokinetic measurements, Ipsogen for generously supplying the Ipsogen MutaQuant Kits, Austin Bailey, PhD and Deborah Kennedy, MD (formerly of Cephalon Oncology) for their facilitation with the study initiation, as well as their expertise and experience with lestaurtinib. To the dedicated research nurses, pharmacists and clinical research associates of the MPD-RC for their hard work and effort in this study.
This study was supported by the Myeloproliferative Disorders Research Consortium (MPD-RC) which is funded by the National Cancer Institute (P01CA108671). A National Institute of Health Research Career Development Award supported EO Hexner (K23HL093366). Lestaurtinib was supplied to the MPD-RC for this trial by Teva pharmaceuticals.
Footnotes
Trail registration: clinicaltrials.gov identifier NCT00668421.
Disclosures of Potential Conflicts of Interest
The authors have no other relevant conflicts of interest to disclose.
Author Contributions
Conception and design: EOH, RW, RM, LP, JDG, LRS, RH
Development and methodology: EOH, HP, RM, LP, JDG
Acquisition of data: EPD, CM, JS, JK, TB
Analysis and interpretation of data: EOH, JM, EPD, LP, RW, GJ, TB, HP, AO, VN, RM, JDG, LRS, RH
Writing and review of the manuscript: EOH, JM, JP, GJR, MRB, DL, EPD, RW, HP, VN, RM, LP, JDG, LRS, RH
Study supervision: EOH, LRS, RH
References
- 1.Goldman JM. A unifying mutation in chronic myeloproliferative disorders. N Engl J Med. 2005;352(17):1744–6. doi: 10.1056/NEJMp058083. [DOI] [PubMed] [Google Scholar]
- 2.Levis M, Tse KF, Smith BD, Garrett E, Small D. A FLT3 tyrosine kinase inhibitor is selectively cytotoxic to acute myeloid leukemia blasts harboring FLT3 internal tandem duplication mutations. Blood. 2001;98(3):885–7. doi: 10.1182/blood.v98.3.885. [DOI] [PubMed] [Google Scholar]
- 3.Levis M, Allebach J, Tse KF, Zheng R, Baldwin BR, Smith BD, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99(11):3885–91. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 4.Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103(10):3669–76. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 5.Knapper S, Burnett AK, Littlewood T, Kell WJ, Agrawal S, Chopra R, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108(10):3262–70. doi: 10.1182/blood-2006-04-015560. Epub 2006/07/22. [DOI] [PubMed] [Google Scholar]
- 6.Levis M, Ravandi F, Wang ES, Baer MR, Perl A, Coutre S, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117(12):3294–301. doi: 10.1182/blood-2010-08-301796. Epub 2011/01/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hexner EO, Serdikoff C, Jan M, Swider CR, Robinson C, Yang S, et al. Lestaurtinib (CEP701)is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2007 doi: 10.1182/blood-2007-04-083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos FP, Kantarjian HM, Jain N, Manshouri T, Thomas DA, Garcia-Manero G, et al. Phase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Blood. 115(6):1131–6. doi: 10.1182/blood-2009-10-246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hexner E, Roboz G, Hoffman R, Luger S, Mascarenhas J, Carroll M, et al. Open-label study of oral CEP-701 (lestaurtinib) in patients with polycythaemia vera or essential thrombocythaemia with JAK2-V617F mutation. Br J Haematol. 2013 doi: 10.1111/bjh.12607. Epub Oct 27. [DOI] [PubMed] [Google Scholar]
- 10.Barosi G, Bordessoule D, Briere J, Cervantes F, Demory JL, Dupriez B, et al. Response criteria for myelofibrosis with myeloid metaplasia: results of an initiative of the European Myelofibrosis Network (EUMNET) Blood. 2005;106(8):2849–53. doi: 10.1182/blood-2005-04-1520. [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A, Barosi G, Mesa RA, Cervantes F, Deeg HJ, Reilly JT, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT) Blood. 2006;108(5):1497–503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- 12.Dupriez B, Morel P, Demory JL, Lai JL, Simon M, Plantier I, et al. Prognostic factors in agnogenic myeloid metaplasia: a report on 195 cases with a new scoring system. Blood. 1996;88(3):1013–8. [PubMed] [Google Scholar]
- 13.Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2010;115(9):1703–8. doi: 10.1182/blood-2009-09-245837. Epub 2009/12/17. [DOI] [PubMed] [Google Scholar]
- 14.Caramazza D, Begna KH, Gangat N, Vaidya R, Siragusa S, Van Dyke DL, et al. Refined cytogenetic-risk categorization for overall and leukemia-free survival in primary myelofibrosis: a single center study of 433 patients. Leukemia. 2011;25(1):82–8. doi: 10.1038/leu.2010.234. Epub 2010/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones WF, Jr, Finland M. Blood levels from orally administered penicillins G and V; relation to food intake. N Engl J Med. 1955;253(18):754–61. doi: 10.1056/NEJM195511032531802. [DOI] [PubMed] [Google Scholar]
- 16.Levis M, Brown P, Smith BD, Stine A, Pham R, Stone R, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108(10):3477–83. doi: 10.1182/blood-2006-04-015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonbol MB, Firwana B, Zarzour A, Morad M, Rana V, Tiu RV. Comprehensive review of JAK inhibitors in myeloproliferative neoplasms. Therapeutic advances in hematology. 2013;4(1):15–35. doi: 10.1177/2040620712461047. Epub 2013/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–8. doi: 10.1182/blood-2011-01-328955. Epub 2011/05/04. [DOI] [PubMed] [Google Scholar]
- 19.Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29(7):789–96. doi: 10.1200/JCO.2010.32.8021. Epub 2011/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. The New England journal of medicine. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. Epub 2012/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng KP, Hillmer AM, Chuah CT, Juan WC, Ko TK, Teo AS, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012;18(4):521–8. doi: 10.1038/nm.2713. Epub 2012/03/20. [DOI] [PubMed] [Google Scholar]
- 22.Kalota A, Jeschke GR, Carroll M, Hexner EO. Intrinsic resistance to JAK2 inhibition in myelofibrosis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(7):1729–39. doi: 10.1158/1078-0432.CCR-12-1907. Epub 2013/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.