Abstract
Background
The aim of this study was to evaluate changes in sex hormone metabolism in patients with threatened miscarriage.
Material/Method
We recruited 73 women in early pregnancy (6–8 weeks of gestation) and divided them into the following 2 groups based on whether they had vaginal bleeding: group A (n=34), the threatened abortion group; and group B (n=39), the normal pregnancy group. Human chorionic gonadotrophin (hCG), estradiol (E2), progesterone (P4), and testosterone (T) serum levels were tested and sex hormone metabolites in the urine were detected using gas chromatography-triple quadrupole mass spectrometry (GC-MS/MS). As the control, data for sex hormones and their metabolites were obtained in normal women of childbearing age without pregnancy (group C: n=23).
Results
E2 and T serum levels were lower in women with threatened miscarriage (group A). Estrone (E1), E2, estriol (E3), 16α-hydroxyestrone (16α-OHE1), 4-methoxyestrone (4-MeOE1), 2-hydroxyestradiol (2-OHE2), and 4-methoxyestradiol (4-MeOE2) levels were significantly lower in group A (P=0.001, 0.003, 0.009, 0.001, 0.012, 0.032, and 0.047, respectively.). Urine levels of dehydroepiandrosterone (DHEA), androstenedione (A2), and the metabolite of (A2) were also significantly lower in group A (P=0.007, 0.009, and 0.011, respectively). The 2-OHE1/E1, 4-OHE1/E1, 2-MeOE1/E1, and 2-MeOE2/E2 ratios were lower in group B, whereas the 2-OHE2/E2, 4-OHE2/E2, and 4-MeOE2/E2 ratios were dramatically lower in all pregnant women (groups A and B) than in group C.
Conclusions
Deficiency in DHEA and abnormal levels of sex hormone metabolites may cause a reduction in the activity of estrogens in women with threatened abortion. These alterations may result in bleeding during the first trimester of pregnancy.
MeSH Keywords: Abortion, Threatened; Gonadal Steroid Hormones; Steroid Metabolism, Inborn Errors
Background
Pregnancy is a process that encompasses embryonic and fetal growth in the maternal uterus and is a complicated physiological process that involves coordinated changes. Threatened abortion refers to a situation in which a small amount of vaginal bleeding occurs before 28 weeks of pregnancy without discharging the pregnancy. The bleeding is usually accompanied by paroxysmal abdominal pain or lower back pain [1]. These symptoms may disappear after resting and treatment, and then the pregnancy may continue. However, if the vaginal bleeding increases or the lower abdominal pain intensifies, it may progress into an inevitable abortion. In all, 25% of all pregnancies involve a threatened pregnancy [1–3], and this rate has increased in recent years. Most cases occur within the first 8–12 weeks of pregnancy, while relatively few occur after 12 weeks. Approximately 14.3–50% of threatened abortion patients will experience a subsequent complete miscarriage [1,4]. Studies have confirmed that experiencing a threatened abortion in the first trimester is associated with an increase in adverse pregnancy outcomes, such as an increased risk of miscarriage in the second trimester and a higher incidence of premature rupture of membranes (PROM), placenta previa, placental abruption, pregnancy-induced hypertension (PIH)/preeclampsia (PE), premature delivery (PTD), low birth weight (LBW), and neonatal intensive care unit (NICU) admission [5–8].
Many factors contribute to threatened abortion, including embryonic or fetal chromosomal abnormalities, environmental and immune factors, and maternal endocrine dysfunction. With the exception of those caused by embryonic or fetal chromosomal abnormalities, many threatened abortions result in a continued pregnancy after appropriate and timely treatment. Among the treatable causes, female endocrine disorders, such as generally luteal insufficiency, are the major culprits [9,10]. However, threatened abortions that are caused by environmental and social factors have become more frequent in recent years.
Communication between the fetus and mother during the first trimester is controlled by sex steroids, including progesterone (P4) and estrogen, as well as prolactin, androgen, and human chorionic gonadotrophin (hCG). There are currently no good indexes that can be used to predict and evaluate pregnancies with bleeding during the first trimester. Blood hCG and P4 levels are the main indexes that are used to diagnose and determine the prognosis in women experiencing a threatened abortion. Estradiol (E2) levels are also used in some cases. The main treatments for threatened abortion are bed rest, luteal support, and combined estrogen and P4 supplements [11]. It is important to note that the use of most hormone remedies is experience-based, and full-scale individual sex hormone analyses are lacking. However, we often find that symptoms (e.g., vaginal bleeding) do not improve and may even become worse after administering standard hormone supplements. In addition, patients complain about the need for repeated blood tests. Sex steroid metabolism depends on 3 factors: a person’s genetic makeup, lifestyle and diet, and the environment. Therefore, increasing our understanding of sex hormone metabolism and interventions with it offers a significant opportunity to reduce the rate of threatened abortions. Hence, developing a non-invasive and comprehensive sex hormone test that can be used to perform a full-scale analysis of sex hormone levels and the levels of their metabolites would benefit patients. In the present study, we used quantitative liquid chromatography-mass spectrometry to compare sex hormone and sex hormone metabolite levels in the urine of women in early pregnancy with and without threatened miscarriage.
Material and Methods
Subjects and samples
The natural pregnancy groups included 73 women in early pregnancy (6–8 weeks of gestation, calculated from the first day of their last menstrual period and after an ultrasound confirmation of a singleton viable pregnancy) who were prospectively recruited from the Outpatient Department of Obstetrics and Gynecology at The First Affiliated Hospital of Anhui Medical University. All pregnant women were divided into 2 groups based on whether they experienced vaginal bleeding in the first trimester. Group A (n=34) was the threatened abortion group, and group B (n=39) was the normal pregnancy group. Women with an irregular menstrual period, a history of recurrent miscarriage, endocrine disease (e.g., thyroid disease), abnormal immunological examination (e.g., ACL or D-D), uterine malformation, or cervical lesions (including cervical incompetence), women who had accepted hormone treatment within 3 the months prior to or during their pregnancy, and women in whom the bleeding originated from an extragenital region were excluded from this study. Data for a set of sex hormones and their metabolites were obtained in normal women of childbearing age who were not pregnant (group C: n=23), and these served as the control group. All the pregnancy outcomes were obtained via chart review or telephone interview. This study was approved by the Institutional Review Board and Ethics Committee of the Affiliated Hospital of Anhui Medical University. All patients enrolled in this study provided informed consent.
All patients underwent blood tests for β-hCG, E2, P4 and testosterone (T) in the morning after fasting following a confirmed diagnosis of an early pregnancy in all included women who satisfied the above criteria.
Urine sample collection: Samples were dried on urine test strips for this study. Morning urine samples were collected on the same day as the blood tests. They were then hung to in a cool, dry, ventilated place at room temperature for 40 to 60 minutes. The completely dried urine test strips were stored at −20°C until they were assayed.
Sex hormones and their metabolites
The following 39 sex hormones and their metabolites were detected in this study: estrogen (E2) and its metabolites, estrone (E1), estriol (E3), 2-hydroxyestrone (2-OHE1), 4-hydroxyestrone (4-OHE1), 16α-hydroxyestrone (16α-OHE1), 2-methoxyestrone (2-MeOE1), 4-methoxyestrone (4-MeOE1), 2-hydroxyestradiol (2-OHE2), 4-hydroxyestradiol (4-OHE2), 2-methoxyestradiol (2-MeOE2), and 4-methoxyestradiol (4-MeOE2);
progesterone and its metabolites, pregnanediol, allopregnanediol, allopregnane, 3α-dihydroprogesterone, 5α-dihydroprogesterone, and 20α-dihydroprogesterone; androgen (A2) and its metabolites, dehydroepiandrosterone (DHEA), androsterone, etiocholanolone, T, 5α-dihydrotestosterone, 5α-androstanediol, 5β-dihydrotestosterone, 5β-androstanediol, and epitestosterone.
The following additional metabolites of steroid hormones were also measured: 11-dexoxycorticosterone, corticosterone, tetrahydro corticosterone, allotetrahydrocorticosterone, cortisol, cortisone, tetrahydrocortisol, and allotetrahydrocortisone.
Laboratory methods
Serum sex hormones were detected using a chemiluminescence method. Detection instrument: Beckman Coulter DXI 800, American; detection agent: Beckman Coulter, USA.
Urinary sex hormones and their metabolites were measured using gas chromatography-triple quadrupole mass spectrometry (GC-MS/MS).
Samples were extracted from dried urine strips using both water and methanol and cleaned on C18-Solid Phase Extraction cartridges. The eluents were dried down and enzymatically hydrolyzed. The liquid extracts of the hydrolysis mixture were dried down and derivatized to trimethylsilyl ethers using MSTFA: NH4I: ethanethiol. A 2-μL volume of final product was injected into an Agilent 7890A gas chromatograph that was coupled to an Agilent 7000B Triple Quadrupole Mass Spectrometer, and all results were analyzed using MassHunter software.
Statistical analysis
The data for all sex hormones and their metabolites are presented as the x±SD or Median (25th–75th%) after they were adjusted for creatinine levels. We used t tests to analyze data with a normal distribution; non-parametric tests were used to analyze data with an abnormal distribution. All statistical analyses were performed using SPSS v. 13.0 (SPSS Inc., USA), and results with a p<0.05 were considered significant.
Results
General patient characteristics and blood test results for the early pregnancy cases and pregnancy outcomes included in this study are shown in Table 1. There were 2 miscarriages in group A and no miscarriages in group B. Except for the 2 miscarriages, all the pregnancies included in the present study proceeded to term birth. However, there were 2 pregnant women with gestational diabetes mellitus (GDM) and 1 with PROM in group A, and only 1 pregnant woman with PROM in group B. There were no differences in age, BMI, and days of gestation between the 2 groups. Serum E2 and T levels were lower in women with threatened miscarriage (group A). However, there were no differences in serum hCG and progesterone (P4) levels in pregnant women with or without threatened miscarriage.
Table 1.
Blood test results in the included cases( x±s or Me(25th–75th%) or n(%)).
| Group | A (n=34) | B (n=39) | P value |
|---|---|---|---|
| Age (years) | 29.18±4.1 | 28.4±4.07 | 0.408 |
| BMI | 22.24±3.05 | 22.24±3.00 | 0.999 |
| days of gestation | 50.98±5.33 | 49.06±6.65 | 0.167 |
| β-HCG (mIU/ml) | 78990.00 (38369.50, 104504.50) | 80489.00 (49821.25, 155945.50) | 0.127 |
| E2 (pmol/L) | 2971.54 (1963.00–4298.00) | 4379.81 (2748.20–7150.50) | 0.009 |
| P (nmol/L) | 77.23±25.47 | 69.94±21.18 | 0.186 |
| T (nmol/L) | 1.71±0.99 | 2.54±1.67 | 0.014 |
| Miscarriage (rate) | 2 (5.9%) | 0 (0%) | 0.213 |
| Pregnancy complication | 3 (9.37%) | 1 (2.56) | 0.333 |
The results for urine levels of sex hormone metabolism in early pregnancy cases are reported in Table 2. Estrogens and their metabolites were present at lower levels in the threatened miscarriage group (group A) than in the normal pregnancy group (group B). Levels of E1, E2, E3, 16α-OHE1, 4-MeOE1, 2-OHE2, and 4-MeOE2 were significantly lower in the threatened miscarriage group (group A) (P=0.001, 0.003, 0.009, 0.001, 0.012, 0.032, and 0.047, respectively.). In group A, urine levels of DHEA, A2, and A2 metabolites were significantly lower (P=0.007, 0.009, and 0.011, respectively). Except for 3α-dihydroprogesterone and tetrahydrocortisol, there was no significant difference in the levels of progesterone or in the metabolites of other steroid hormones (e.g., adrenal cortical hormones) between the 2 groups. From these results, we can infer that a deficiency in DHEA resulted in a decrease in the production of estrogen in the threatened miscarriage group (Figure 1), and a deficiency in estrogen activity may cause bleeding during the first trimester of pregnancy.
Table 2.
Results for sex hormone metabolites in urine (Me(25th–75th%)).
| Group | A | B | P value |
|---|---|---|---|
| E1 | 31.73 (18.610, 49.600) | 59.77 (38.790, 111.690) | 0.001 |
| E2 | 11.605 (7.335, 19.188) | 19.11 (11.400, 32.380) | 0.003 |
| E3 | 15.865 (8.340, 33.480) | 34.86 (14.410, 47.010) | 0.009 |
| 2-OHE1 | 6.12 (3.560, 10.175) | 8.81 (4.980, 16.380) | 0.073 |
| 4-OHE1 | 1.785 (1.043, 2.838) | 2.61 (1.160, 4.350) | 0.200 |
| 16α-OHE1 | 7.4 (3.945, 12.203) | 15.24 (7.550, 35.140) | 0.001 |
| 2-MeOE1 | 3.03 (1.393, 5.048) | 3.85 (1.820, 7.400) | 0.330 |
| 4-MeOE1 | 0.14 (0.095, 0.333) | 0.32 (0.150, 0.540) | 0.012 |
| 2-OHE2 | 1.11 (0.618, 1.833) | 1.67 (0.880, 4.110) | 0.032 |
| 4-OHE2 | 0.815 (0.503, 1.428) | 1.02 (0.650, 1.770) | 0.081 |
| 2-MeOE2 | 0.41 (0.220, 0.673) | 0.49 (0.230, 0.950) | 0.344 |
| 4-MeOE2 | 0.08 (0.050, 0.120) | 0.1 (0.060, 0.220) | 0.047 |
| DHEA | 141.44 (85.215, 776.413) | 600.68 (177.090, 1755.690) | 0.007 |
| Androstenedione | 18.11 (13.773, 37.630) | 39.17 (17.110, 70.050) | 0.009 |
| Androsterone | 1570.69 (1090.595, 2482.478) | 1908.23 (1279.570, 2728.070) | 0.079 |
| Etiocholanolone | 1108.975 (832.088, 1604.67) | 1705.72 (1030.78, 2739.65) | 0.011 |
| Testosterone | 1.605 (0.633, 4.003) | 1.3 (0.460, 3.680) | 0.782 |
| 5α-dihydrotestosterone | 1.1 (0.628, 1.913) | 0.97 (0.530, 1.420) | 0.379 |
| 5α-androstanediol | 13.745 (9.155, 25.885) | 15.24 (10.060, 25.050) | 0.550 |
| 5β-dihydrotestosterone | 0.275 (0.108, 0.623) | 0.1 (0.040, 0.310) | 0.034 |
| 5β-androstanediol | 8.165 (5.313, 13.873) | 12.16 (5.630, 22.170) | 0.162 |
| Epi-testosterone | 17.08 (13.595, 28.205) | 22.41 (13.420, 39.440) | 0.257 |
| Pregnanediol | 5110.825 (3694.588, 8541.313) | 8195.75 (4123.880, 14437.850) | 0.075 |
| Allopregnanediol | 367.04 (211.133, 515.880) | 327.98 (209.050, 610.130) | 0.991 |
| Allopregnanolone | 68.495 (26.798, 106.89) | 72.96 (47.650, 150.050) | 0.486 |
| 3α-dihydroprogesterone | 5.895 (4.200, 9.415) | 9.67 (5.760, 11.630) | 0.017 |
| 20α-dihydroprogesterone | 41 (24.185, 53.210) | 41.01 (25.030, 75.600) | 0.596 |
| 5α-dihydroprogesterone | 7.395 (4.425, 10.543) | 7.64 (5.140, 13.670) | 0.429 |
| Progesterone | 3.22 (2.195, 7.535) | 4.9 (2.970, 10.220) | 0.110 |
| 11-deoxycorticosterone | 8.95 (5.863, 13.523) | 10.36 (4.770, 14.800) | 0.873 |
| Corticosterone | 31.475 (19.143, 45.075) | 38.69 (17.690, 66.290) | 0.183 |
| Tetrahydrocorticosterone | 50.775 (29.515, 79.435) | 55.93 (41.430, 79.990) | 0.181 |
| Allotetrahydrocorticosterone | 82.55 (50.095, 132.535) | 93.57 (61.150, 1520.42) | 0.486 |
| Cortisol | 123.065 (85.180, 177.938) | 132.36 (87.480, 232.870) | 0.299 |
| Cortisone | 152.86 (82.203, 237.113) | 146.83 (96.700, 381.840) | 0.573 |
| Tetrahydrocortisol | 749.615 (538.958, 1331.748) | 987.15 (679.110, 1513.310) | 0.047 |
| Allotetrahydrocortisol | 254.295 (170.398, 415.263) | 381.84 (141.350, 493.360) | 0.401 |
| Tetrahydrocortisone | 2052.6 (1211.518, 3151.913) | 2694.34 (1403.510, 4230.350) | 0.102 |
Figure 1.
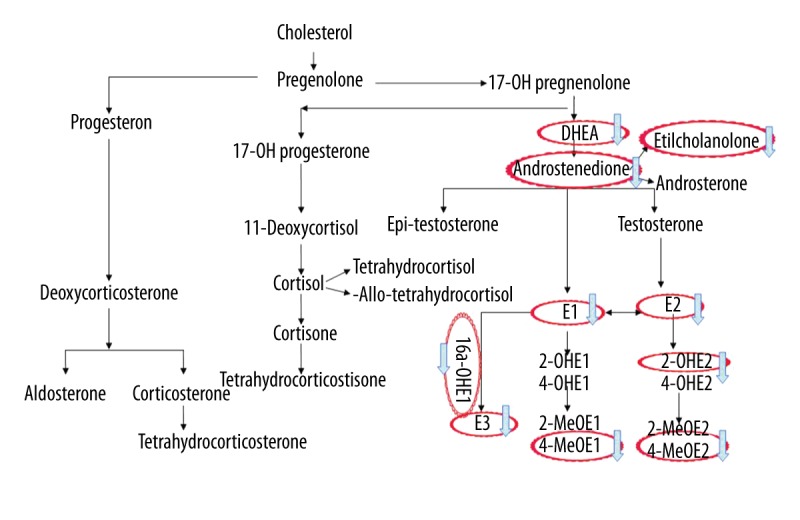
Pathway of sex hormone metabolism and the abnormal in group A. DHEA, A2 and the metabolite of (A2) were also significantly lower in group A. E1, E2, E3, 16α-OHE, 4-MeOE1, and 2-OHE2, and 4-MeOE2 levels were also significantly lower in group A.
Table 3 shows that the activity of the hydroxylation pathway for estrogen was decreased during normal pregnancy (in group B), while there was no significant difference between the threatened abortion group (group A) and the group of normal non-pregnant women (group C). These changes included a decrease in 2OH-E1/E1 and 4OH-E1/E1 ratios. The 2OH-E2/E2 and 4OH-E2/E2 ratios were dramatically lower in the pregnant groups (groups A and B) than in group C, but there was no significant difference between group A and group B. Similar to the hydroxylation pathway, in pregnant women, the methylation pathway for estrogen was also decreased. The 2-MeOE1/E1 and 2-MeOE2/E2 ratios were dramatically lower in pregnant women without threatened abortion (group B) when compared with threatened abortion group (group A). The 4-MeOE1/E1 ratio was lower in group B than in group C, but there was no significant difference between group A and group B. The 4-MeOE2/E2 ratio was lower in pregnant women (group A and group B) than in non-pregnant women (group C). However, there was no significant difference between group A and group B.
Table 3.
Hydroxylation and methylation of estrogen between pregnant women and normal non-pregnant women.
| Group | A | B | C |
|---|---|---|---|
| 2-OHE1/E1 | 0.215 (0.145, 0.313) | 0.140 (0.110, 0.18)** | 0.240 (0.170, 0.350)## |
| 4-OHE1/E1 | 0.060 (0.050, 0.090) | 0.040 (0.030, 0.060)** | 0.080 (0.060, 0.090)## |
| 16a-OHE1/E1 | 0.230 (0.173, 0.453) | 0.290 (0.160, 0.450) | 0.270 (0.170, 0.450) |
| 2-MeOE1/E1 | 0.100 (0.060, 0.143) | 0.050 (0.040, 0.090)** | 0.110 (0.070, 0.150)## |
| 4-MetOE1/E1 | 0.010 (0.001, 0.010) | 0.01 (0.000, 0.010) | 0.01 (0.010, 0.020)# |
| 2-OHE2/E2 | 0.110 (0.060, 0.203) | 0.100 (0.070, 0.140) | 0.200 (0.130, 0.270)*## |
| 4-OHE2/E2 | 0.070 (0.058, 0.090) | 0.060 (0.050, 0.080) | 0.130 (0.070, 0.180)**## |
| 2-MeOE2/E2 | 0.035 (0.030, 0.043) | 0.020 (0.020, 0.030)** | 0.030 (0.020, 0.050) |
| 4-MeOE2/E2 | 0.010 (0.008, 0.010) | 0.010 (0.000, 0.010) | 0.020 (0.010, 0.030)**## |
Compared with group A:
P value <0.05,
P value <0.01;
compared with group B:
P value <0.05,
P value <0.01.
Discussion
A diagnosis of threatened abortion is made when there is vaginal bleeding before 28 weeks of gestation in cases with confirmed fetal cardiac activity by ultrasound and a closed cervix. Bleeding during pregnancy can cause maternal physical and psychological distress, and studies have shown that bleeding may also be associated with adverse maternal and fetal outcomes. The results of the present study are consistent with those of previous studies. Two miscarriages occurred in group A, although there was no significant difference of the miscarriage rates between the 2 groups. There was a trend for an increase in the pregnancy complication rates in the threatened abortion group, but no significant difference was found. These results may be biased because of the small sample size.
Developing convenient and effective methods for the early assessment of this condition is a matter of critical importance. Maternal endocrine levels are a generally accepted etiology, in addition to chromosomal anomalies of the fetus and infectious etiologies, for maternal bleeding. Sex hormone metabolism and balance each play an important role in the maintenance and development of pregnancy. Generally, serum hCG and P4 levels are the main endocrine indexes used to evaluate threatened abortion. However, few reports have examined changes in E2 and T levels in early pregnancy.
In this study, we found that there was no difference in serum hCG levels between the threatened abortion group and the normal pregnancy group. Similar to the result for hCG levels, no significant difference was found in serum P4 values between the 2 groups. However, unlike findings reported in past studies, we found that serum E2 and T levels were significantly lower in the threatened miscarriage group than in the normal pregnancy group. Previous studies suggested that progesterone deficiency is the main reason for bleeding in the first trimester when other reasons have been excluded, and luteal supportive treatment is therefore the main therapy used to treat threatened abortion [12]. An inadequate increase in hCG has been associated with failing pregnancy and miscarriage, and hCG has therefore been used to treat threatened miscarriage, but the results of this treatment regimen have been inconsistent. A previous meta-analysis showed that there was no significant difference in the incidence of subsequent miscarriage in women with threatened abortion who were treated with or without hCG [13]. Studies have also suggested that using a combination of progesterone and estrogen to treat threatened abortion may yield better clinical results, but there is currently no consensus on this issue [14].
The serum hormone levels observed in this study indicated that estrogen deficiency and decreased T levels might be associated with bleeding. The abnormal levels of estrogen and T that induced adverse outcomes during the first trimester of pregnancy may have been caused by a break in the balance between immunization and inflammatory reactions in addition to the maldevelopment of the endometrium. Estrogen deficiency may be caused by changes in sex hormone metabolism in women with threatened abortion. It is therefore clinically important to increase our understanding of sex hormone metabolism during early pregnancy and to provide a basis for research into the etiology of and potential treatments for women with threatened abortion.
Sex hormonal imbalances are associated with miscarriages. The sex hormonal system is a complicated orchestra of hormones that runs on a very specific schedule. If any of these hormones are out of balance, it possibly affects the whole circulation, including affecting early pregnancy. This is the first study to evaluate sex hormone metabonomics in pregnancy during the first trimester. A comprehensive set of sex hormones and their metabolites were detected in urine using GC-MS/MS so that we could explore differences in sex hormone metabolize between pregnant women with and without threatened miscarriage. These data provide a basis for the underlying etiology of and treatments for women with a threatened abortion. A total of 12 estrogen metabolites, 7 progesterone metabolites, and 10 androgen metabolites, in addition to glucocorticoid metabolites, were analyzed in urine samples obtained from the study subjects. Progesterone is a vital hormone produced by the corpus luteum that provides support during early pregnancy. At about 10–12 week’s gestation, placental progesterone production takes over the ovaries. Historically, low levels of circulating progesterone have been associated with vaginal bleeding and impending miscarriage during early pregnancy. Although it has been suggested that lacking progesterone can directly cause miscarriage rather than being a secondary signal of a failing pregnancy, we found no difference in progesterone levels or the levels of its metabolites in urine samples obtained from the threatened miscarriage (group A) and normal pregnancy (group B) groups, consistent with the results of blood tests. However, there was a significant decrease in DHEA and A2 (define A2) in group A. As a precursor of estrogen, DHEA is converted to A2 by 3β-hydroxysteroid dehydrogenase (HSD), and A2 is then converted to E1 and E2 by aromatase. A2 can also be converted to T and epi-testosterone by 17β-HSD and 17α-HSD, respectively (Figure 1). Because they have reduced levels of DHEA, there was a trend toward declines in estrogen and its metabolites, including E1, E2, E3, 16α-OHE1, 4-MeOE1, 2-OHE2, and 4-MeOE2, in the threatened miscarriage group. Estrogen is a highly important hormone in women that plays a critical role in establishing and maintaining pregnancy. Estrogen affects the whole process of pregnancy, including the postpartum recovery period. Estrogen stimulates VEGF production and blood vessel formation to enable maternal-fetal circulation [15]. Estrogen has double-edged functions in immune regulation in that it modulates the immune response by inducing peripheral T cells to secrete the proinflammatory cytokines IFN-g and IL-2 [16], but also promotes tolerance by inducing the secretion of IL-10 [17]. It has therefore been reported that 17β-E2 is better than other indicators for determining the outcome of a pregnancy [18], and our results are consistent with this finding. Furthermore, DHEA is currently used to improve the ovarian environment prior to conception, increase pregnancy rates, and improve pregnancy outcomes for women with diminished ovarian reserve (DOR). Gleicher et al. [19] analyzed the miscarriage rate in 73 women with DOR undergoing DHEA supplementation before pregnancy, and found that miscarriage rates after DHEA treatment not only were lower than in an average national IVF population, but also were comparable to rates reported in normally fertile populations. Supplementation with DHEA may improve egg quality and increase live birth rates for women with a DOR and for infertile women, but not in poor-responder patients undergoing IVF [20]. Our team’s previous study confirmed that in patients with a DOR undergoing IVF treatment, DHEA treatment for 3 months before IVF can increase the level of BMP-15 in follicular fluid samples and improve the quality of embryos [21]. DHEA functions as an immune regulator and play an important role in regulating the immune response in the ovaries. It may be that the physiological mechanism of the immune regulation is the result of the conversion of DHEA to downstream sex steroids. DHEA can also regulate the balance of the Th1/Th2 response and the balance between CD4+/CD8+ T cells [22]. The pregnant women involved in the present study were at the early stage of pregnancy (6–8 weeks), so their placentas were not yet fully formed; therefore, the secretion of sex hormones and immune regulation were mainly performed using ovarian tissue, and once the balance in these processed was disrupted, threatened abortion may have occurred. Our data suggest that a deficiency in DHEA and/or androstenedione is a prognostic indicator of bleeding during early pregnancy.
To the best of our knowledge, the absolute levels of all types of sex hormones (e.g., estrogens, progesterones, androgens, and their metabolites) increase during pregnancy. Hence, this study did not compare the absolute values of these sex hormones and their metabolites in non-pregnant women. However, we found that levels of members of the hydroxylation metabolism pathway (including 2OH-E1/E1 and 4OH-E1/E1) were lower in the normal pregnancy group than in non-pregnant women. However, interestingly, there was no significant difference between the threatened abortion group and the normal non-pregnant women. The ratios of 2OH-E2/E2 and 4OH-E2/E2 were dramatically lower in the pregnant groups. Levels of members of the methylation metabolism pathway were also lower in pregnant women than in non-pregnant women. The ratios of 2-MeOE1/E1 and 2-MeOE2/E2 were dramatically lower in the pregnant women without threatened abortion, but there was no significant difference between the pregnant women with threatened abortion and the normal non-pregnant women. The ratio of 4-MeOE2/E2 was lower in pregnant women than in non-pregnant women. The estrogen metabolites 2-OHE1, 4-OHE1, and 16α-OHE1 are produced via irreversible hydroxylation at the C-2, -4 or -16 26 positions of the steroid ring [23]. The biological effects of different factors depend on their affinity for binding the estrogen receptor (ER). For example, 16-OHE1 is a potent estrogen that can bind covalently to ER, resulting in adverse cell proliferation, whereas 2-OHE1 is weakly estrogenic and exhibits reduced receptor affinity with ER (24). Therefore, 2-OHE1 is considered an antagonist of estrogen that can inhibit cell proliferation [25]. Studies have indicated that the 4-OH metabolic pathway potentially induces genotoxic damage because its products can be further oxidized, resulting in the formation of reactive semi-quinones and quinines [26–28]. Furthermore, 4-OHE can undergo redox circulation to produce reactive oxygen species, which can lead to oxidative damage. However, it is produced at generally lower levels than 2OHE [26,29]. Many studies have investigated the effects of estrogen hydroxylation metabolites on breast cancer. These studies have suggested that a higher ratio of 2/16OHE1 is associated with a lower breast cancer risk and that the 2/16OH ratio is less regulated by metabolic genes. The 2/16OH ratio has therefore been recommended as an independent biomarker of breast cancer risk [28,30,31]. Unlike the hydroxylation of estrogens, the methylation of estrogens can protect DNA from injury by preventing the formation of potentially mutagenic products [32]. Our results suggest that a series of changes occur in estrogen metabolism after pregnancy begins, including a decrease in hydroxylation and methylation metabolism, to provide enough biologically active estrogens to maintain a normal pregnancy. However, in the threatened abortion group, hydroxylation metabolism, especially that involving 2-OHE1 and 4-OHE1, did not decrease in a timely manner, which led to a relatively insufficient level of estrogen. This may be another reason that bleeding can occur in the first trimester of pregnancy.
In this study, dry urine strips and GC-MS/MS were used to detect the levels of sex hormones and their metabolites in urine samples. This method is non-invasive and convenient (patients can collect the sample by themselves without any professional skills), and the samples are easy to store and transport. The results of using urine and this method were consistent with the result of using traditional blood tests. Moreover, this method provides higher repeatability, specificity, and accuracy.
One limitation of this study may be that the sample sizes were not large enough. Further studies that include a larger sample size are ongoing that involve the implementation of clinical interventions based on the results of the study.
Conclusion
We conclude that both blood and urine levels of androgens were lower in the threatened abortion group than in the normal pregnancy group. In the first trimester of pregnancy, the hydroxylation and methylation estrogen pathways exhibited less activity in women with normal pregnancies, but the activity of these pathways was not significantly lower in the threatened miscarriage group. Being deficient in DHEA, a substrate for estrogens, or having abnormal levels of sex hormone metabolites may cause the deficiency in estrogen activity observed in women with threatened abortion. This may in turn generate bleeding during the first trimester of pregnancy. The GC-MS/MS technique offers a method that is more comprehensive, effective, and non-invasive than blood tests for detecting sex hormone metabolites in urine samples.
Acknowledgements
We thank Dr. Guoping Chen at the Centers for Disease Control and Prevention of Anhui Province for his assistance during the power analysis.
Footnotes
Source of support: Departmental sources
Conflict of Interest
There is no conflict of interest to disclose.
References
- 1.Cunningham FG, Gant NF, Leveno KJ, et al., editors. William’s obstetrics. 21st ed. NewYork (NY): McGraw-Hill; 2001. [Google Scholar]
- 2.Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: Prospective study from general practice. BMJ. 1997;315:32–34. doi: 10.1136/bmj.315.7099.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss JL, Malone FD, Vidaver J, et al. Threatened abortion: A risk factor for poor pregnancy outcome, a population-based screening study. Am J Obstet Gynecol. 2004;190:745–50. doi: 10.1016/j.ajog.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Basama FM, Crosfill F. The outcome of pregnancies in 182 women with threatened miscarriage. Arch Gynecol Obstet. 2004;270:86–90. doi: 10.1007/s00404-003-0475-z. [DOI] [PubMed] [Google Scholar]
- 5.Dadkhah F, Kashanian M, Eliasi G. A comparison between the pregnancy outcome in women both with or without threatened abortion. Early Hum Dev. 2010;86:193–96. doi: 10.1016/j.earlhumdev.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Petriglia G, Palaia I, Musella A, et al. Threatened abortion and late-pregnancy complications: A case-control study and review of literature. Minerva Ginecol. 2015;67:491–97. [PubMed] [Google Scholar]
- 7.Ozdemirci S, Karahanoglu E, Esinler D, et al. Influence of threatened miscarriage on pregnancy and early postpartum period: A case-control report. J Matern Fetal Neonatal Med. 2015;28:1186–89. doi: 10.3109/14767058.2014.947577. [DOI] [PubMed] [Google Scholar]
- 8.Evrenos AN, Cakir Gungor AN, Gulerman C, Cosar E. Obstetric outcomes of patients with abortus imminens in the first trimester. Arch Gynecol Obstet. 2014;289:499–504. doi: 10.1007/s00404-013-2979-5. [DOI] [PubMed] [Google Scholar]
- 9.Siklósi G. Luteal insufficiency as the primary cause of habitual abortion – its successful treatment. Acta Biomed Ateneo Parmense. 1992;63:101–11. [PubMed] [Google Scholar]
- 10.Shah D, Nagarajan N. Luteal insufficiency in first trimester. Indian J Endocrinol Metab. 2013;17:44–49. doi: 10.4103/2230-8210.107834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Günzel-Apel A, Urhausen C, Wolf K, et al. Serum progesterone in pregnant bitches supplemented with progestin because of expected or suspected luteal insufficiency. Reprod Domest Anim. 2012;47:55–60. doi: 10.1111/rda.12029. [DOI] [PubMed] [Google Scholar]
- 12.Szabó I, Szilágyi A. Management of threatened abortion. Early Pregnancy. 1996;2:233–40. [PubMed] [Google Scholar]
- 13.Devaseelan P, Fogarty PP, Regan L. Human chorionic gonadotrophin for threatened miscarriage. Cochrane Database Syst Rev. 2010;65:CD007422. doi: 10.1002/14651858.CD007422.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Lim CE, Ho KK, Cheng NC, Wong FW. Combined oestrogen and progesterone for preventing miscarriage. Cochrane Database Syst Rev. 2013;9:CD009278. doi: 10.1002/14651858.CD009278.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildebrandt VA, Babischkin JS, Koos RD, et al. Developmental regulation of vascular endothelial growth/permeability factor messenger ribonucleic acid levels in and vascularization of the villous placenta during baboon pregnancy. Endocrinology. 2001;142:2050–57. doi: 10.1210/endo.142.5.8174. [DOI] [PubMed] [Google Scholar]
- 16.Tanriverdi F, Silveira LF, Maccoll GS, Bouloux PM. The hypothalamic-pituitary-gonadal axis: Immune function and autoimmunity. J Endocrinol. 2003;176:293–304. doi: 10.1677/joe.0.1760293. [DOI] [PubMed] [Google Scholar]
- 17.Cohen-Solal JF, Jeganathan V, Grimaldi CM, et al. Sex hormones and SLE: Influencing the fate of autoreactive B cells. Curr Top Microbiol Immunol. 2006;305:67–88. doi: 10.1007/3-540-29714-6_4. [DOI] [PubMed] [Google Scholar]
- 18.Siimes ASI, Immonen I, Stenman UH, et al. Plasma renin substrate in the prediction of pregnancy outcome in threatened abortion. Br J Obstet Gynaecol. 1983;90:1186–92. doi: 10.1111/j.1471-0528.1983.tb06470.x. [DOI] [PubMed] [Google Scholar]
- 19.Gleicher N, Ryan E, Weghofer A, et al. Miscarriage rates after dehydroepiandrosterone (DHEA) supplementation in women with diminished ovarian reserve: A case control study. Reprod Biol Endocrinol. 2009;7:108. doi: 10.1186/1477-7827-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tartagni M, De Pergola G, Damiani GR, et al. Potential benefit of dehydroepiandrosterone supplementation for infertile but not poor responder patients in a IVF program. Minerva Ginecol. 2015;67:7–12. [PubMed] [Google Scholar]
- 21.Zhang HH, Xu PY, Wu J, et al. Dehydroepiandrosterone improves follicular fluid bone morphogenetic protein-15 and accumulated embryo score of infertility patients with diminished ovarian reserve undergoing in vitro fertilization: a randomized controlled trial. J Ovarian Res. 2014;7:93. doi: 10.1186/s13048-014-0093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Qiu X, Gui Y, et al. Dehydroepiandrosterone improves the ovarian reserve of women with diminished ovarian reserve and is a potential regulator of the immune response in the ovaries. Trends. 2015;9:350–59. doi: 10.5582/bst.2015.01154. [DOI] [PubMed] [Google Scholar]
- 23.Lippert TH, Seeger H, Mueck AO. The impact of endogenous estradiol metabolites on carcinogenesis. Steroids. 2000;65:357–69. doi: 10.1016/s0039-128x(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 24.Liehr JG. Genotoxic effects of estrogens. Mutat Res. 1990;238:269–76. doi: 10.1016/0165-1110(90)90018-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhu BT, Conney AH. Is 2-methoxyestradiol an endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer Res. 1998;58:2269–77. [PubMed] [Google Scholar]
- 26.Cavalieri EL, Stack DE, Devanesan PD, et al. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci USA. 1997;94:10937–42. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavalieri EL, Rogan EG. Depurinating estrogen-DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 2010;6:75–91. doi: 10.2217/fon.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parl FF, Dawling S, Roodi N, Crooke PS. Estrogen metabolism and breast cancer: A risk model. Ann N Y Acad Sci. 2009;1155:68–75. doi: 10.1111/j.1749-6632.2008.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr. 2000;27:67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- 30.Taioli E, Im A, Xu X, et al. Comparison of estrogens and estrogen metabolites in human breast tissue and urine. Reprod Biol Endocrinol. 2010;8:93. doi: 10.1186/1477-7827-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dallal CM, Stone RA, Cauley JA, et al. Urinary estrogen metabolites and breast cancer: A combined analysis of individual level data. Int J Biol Markers. 2013;28:3–16. doi: 10.5301/JBM.2012.9353. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Gaikwad NW, Olson K, et al. Cytochrome P450 isoforms catalyze formation of catechol estrogen quinones that react with DNA. Metabolism. 2007;56:887–94. doi: 10.1016/j.metabol.2007.03.001. [DOI] [PubMed] [Google Scholar]


