Abstract
Patient: Male, 30
Final Diagnosis: Osteosarcoma
Symptoms: Pain
Medication: Rifampin • Oxycodone
Clinical Procedure: Oxycodone usage
Specialty: Oncology
Objective:
Unusual clinical course
Background:
Oxycodone is a semisynthetic opioid receptor agonist, and is frequently used for pain control in patients with cancer. Most oxycodone is metabolized by N-demethylation to noroxycodone by CYP3A. Rifampin is a strong inducer of several drug-metabolizing enzymes, including CYP3A. Hence, rifampin-induced CYP3A activity may decrease the effect of oxycodone.
Case Report:
Osteosarcoma is a highly aggressive primary bone tumor of childhood and adolescence. Here, we report a 30-year-old male with osteosarcoma of the femur with lung metastases in the upper lobe. The lung also contained small, scattered nodular lesions that were identified as tuberculosis. Multi-drug therapy, including rifampin, was administered. The upper-lobe metastatic lesion extended to the brachial plexus and caused severe pain. Over 1000 mg per day of oxycodone was ineffective for pain control. However, morphine was able to control his pain at about one-third the equivalent dose.
Conclusions:
Our patient demonstrated oxycodone resistance due to rifampin. Chemotherapy may have compromised the patient’s immune system, thus theoretically increasing the risk of tuberculosis. Recognition of the interactions between rifampin and oxycodone is important in this and other cancers. Notably, for patients using high doses of oxycodone to manage severe pain, stopping rifampin may lead to oxycodone overdose.
MeSH Keywords: Osteosarcoma, Oxycodone, Rifampin
Background
It is estimated that more than 10 million cases of tuberculosis are diagnosed and about 1.8 million deaths due to tuberculosis occur every year [1,2]. Rifampin is one of the most important drugs used in tuberculosis treatment [2–4]. Rifampin is a strong inducer of several drug-metabolizing enzymes, both in the liver and in the small intestine [2,5].
Oxycodone is a semisynthetic opioid receptor agonist, and is widely used in the treatment of pain [6]. Oxycodone is primarily metabolized in the liver by cytochrome P450 (CYP) enzymes. CYP3A-mediated N-demethylation of oxycodone to noroxycodone accounts for the majority of oxycodone metabolism. CYP3A is inducted by rifampin, which may decrease the analgesic effect of oxycodone [2,7]. CYP3A mediates N-demethylation of oxycodone to noroxycodone, accounting for 80% of oxycodone metabolites. CYP2D6 catalyses the O-demethylation of oxymor-phone, accounting for 10% of metabolites [8,9]. Both noroxycodone and oxymorphone are then metabolized to noroxymorphone by CYP3A and CYP2D6, respectively [2,9,10]. Oxycodone itself is responsible for the analgesic effects on the central opioid receptor [2,10]. Through the induction of CYP3A activity, Rifampin is reported to decrease the area under the oral oxycodone concentration-time curve by 86%. Consequently, the oral bioavailability of oxycodone is decreased from 69% to 21% [2].
Osteosarcoma is an aggressive primary bone tumor of childhood and adolescence with metastatic potential, primarily to the lungs [11]. There is a slight male predominance, with 5.4 and 4.0 diagnoses per million males and females, respectively, made each year in the global incidence [12]. The long bones are more commonly affected than the axial skeleton, including the pelvis (80% and 20%, respectively) [13]. Among the long bones, the femur (40%), tibia (20%), and humerus (10%) are most frequently affected [14]. The current standard treatment protocol for osteosarcoma without distant metastases is surgery plus a multi-drug chemotherapy regimen consisting of cisplatin, doxorubicin, and high-dose methotrexate [15]. Adjuvant chemotherapy is administered with the purpose of eliminating primary tumor cells and micrometastatic cells [12]. The prognosis for osteosarcoma patients without metastasis has improved to about 70% long-term disease-free survival [15,16].
In this case report, we describe oxycodone resistance due to rifampin treatment of tuberculosis in a patient with osteosarcoma, who had intractable pain due to brachial plexus invasion of a metastatic lesion. The increased survival rate of sarcoma patients, as well as the immunosuppressive effects of chemotherapy, may theoretically increase their risk of tuberculosis. It is well known that rifampin interferes with the metabolism of many drugs; however, few case studies have addressed the interaction between rifampin and oxycodone.
Case Report
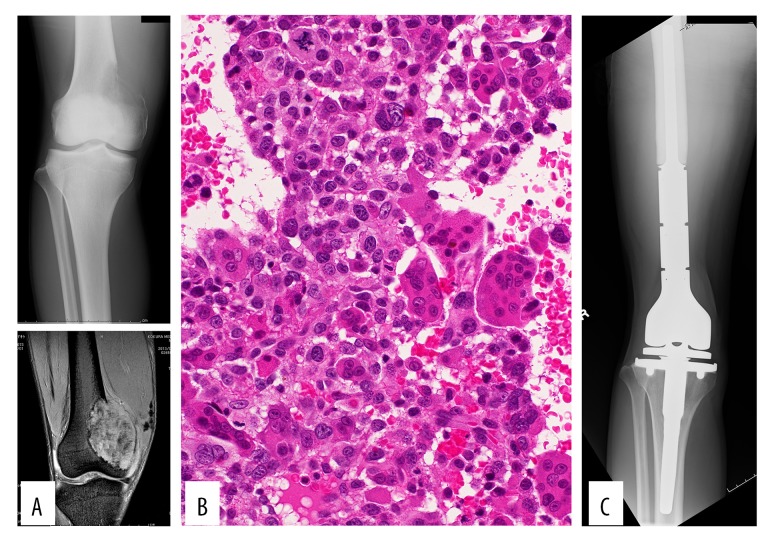
A 30-year-old man noticed right knee pain during the previous 4 months. The pain had increased gradually and worsened during weight-bearing. On physical examination at the initial assessment, the medial distal thigh was swollen, with tenderness. The range of motion in the knee joint was restricted due to the occurrence of pain at deep flexion. The patient’s general condition was good, and no fever was observed. Plain radiographs of the distal femur demonstrated an osteolytic lesion (Figure 1A). MRI revealed a clearly defined tumor expanding to the soft tissue (Figure 1A). A needle biopsy was performed. Histologic analysis indicated proliferation of osteoclast-like multinuclear giant cells with mononuclear short spindle or oval cells. The tumor cells showed some atypical features, including enlarged hyperchromatic nuclei, and were arranged in a sheet-like fashion with foci of lace-like osteoid. Based upon these findings, giant cell-rich osteosarcoma was suggested (Figure 1B). Following the diagnosis of osteosarcoma, the patient received preoperative multi-drug chemotherapy consisting of doxorubicin (60 mg/m2) and cisplatin (120 mg/m2). After 1 cycle of chemotherapy, the patient underwent tumor resection with artificial joint replacement (Figure 1C). The surgical procedure was selected prior to preoperative chemotherapy because the entire lesion was osteolytic (not a typical finding of osteosarcoma), which increased the risk of pathological fracture. The resected material confirmed the diagnosis of osteosarcoma. After surgery, postoperative chemotherapy with doxorubicin and cisplatin was administered. After 3 cycles of this regimen, routine chest computed tomography (CT) (conducted every 3 months) revealed multiple metastases in the lung. On the judgement that the patient was refractory to the current chemotherapy regimen, a regimen including ifosfamide was administered: doxorubicin (30 mg/m2) and ifosfamide (4 g/m2). After 3 cycles of this regimen, the metastatic lesions in the lung increased in size, so another chemotherapy regimen with gemcitabine (1400 mg/m2) and docetaxel (80 mg/m2) was administered every 4 to 5 weeks.
Figure 1.
The plain radiograph demonstrates an ill-defined osteolytic lesion (A-top). MRI STIR (short-tau inversion recovery) shows a lesion with expansion to the soft tissue (A-bottom). The biopsy specimen shows proliferation of osteoclast-like multinuclear giant cells admixed with mononuclear short spindle or oval cells, including atypical cells, suggestive of giant-cell–rich osteosarcoma (B). Resection and replacement was performed (C).
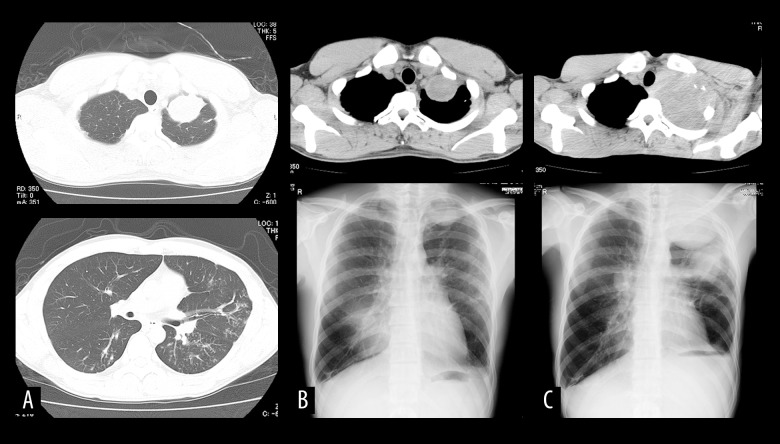
After 9 cycles of the gemcitabine and docetaxel regimen, routine CT revealed that in addition to the metastatic lesions, small, scattered nodular lesions in the lung, which had not been seen on prior CT images, were present (Figure 2A). The small nodular lesions were determined to be tuberculosis, as confirmed by sputum culture. The patient had no fever and no respiratory symptoms, such as coughing, before the tuberculosis diagnosis. When tuberculosis was diagnosed, the patient had received total chemotherapy doses of doxorubicin 330 mg/m2, cisplatin 480 mg/m2, ifosfamide 12 g/m2, gemcitabine 12 600 mg/m2, and docetaxel 720 mg/m2. Tuberculosis treatment was prioritized over treatment of osteosarcoma at an institute that specializes in tuberculosis, because the growth of the metastatic lesions was slow. Tuberculosis treatment was initiated with an oral multi-drug combination of isoniazid 300 mg, rifampin 600 mg, ethambutol 750 mg, and pyrazinamide 1200 g for 2 months. After 2 months, the antibiotic regimen was continued without pyrazinamide.
Figure 2.
CT demonstrates a metastatic lesion in the left upper lung lobe (A-top) and small, scattered nodules (A-bottom). CT and chest plain radiograph show the upper lobe lesion at the time of tuberculosis diagnosis (B), and that it had increased in size 8 months later (C).
After sputum culture was determined to be negative, chemo-therapy for osteosarcoma was restarted at another institution due to the convenience of the location. The patient received chemotherapy with gemcitabine (1800 mg/m2) and docetaxel (80 mg/m2) every 4 weeks. During this period, the metastatic lung lesion increased in size, extending to the brachial plexus and causing worsening pain (Figure 2B, 2C). The pain was partially controlled with oxycodone 1120 mg (equivalent morphine dose 1700 mg). Due to the high dose of oxycodone required, we considered the possibility of oxycodone resistance due to rifampin-induced CYP3A activity. Oxycodone was thus replaced with morphine. We found that 480 mg of morphine, about one-third the equivalent oxycodone dose, was adequate to control the patient’s pain. After 6 cycles of gemcitabine and docetaxel chemotherapy, general fatigue occurred, and the patient could not continue chemotherapy. Four months later, which was 2 years 11 months after the initial visit, the patient died of dyspnea due to spread of the lung lesions.
Discussion
Osteosarcoma is a highly aggressive bone tumor that metastasizes primarily to the lung [11]. In the current case, an upper-lobe metastatic lesion in an osteosarcoma patient extended to the brachial plexus and caused severe pain. Invasion to the brachial plexus in patients with lung cancer or breast cancer is known to cause intractable pain [17,18]. Brachial plexus pain due to cancer invasion can be resistant to treatment with oral non-steroidal anti-inflammatory drugs (NSAIDs), steroids, oral opioids, and even intrathecal opioids [19]. Therefore, high doses of opioids are often necessary to treat severe brachial plexus pain due to cancer invasion.
The increased survival rate of sarcoma patients, as well as the immunosuppressive effects of chemotherapy, may increase their risk of tuberculosis. In the present case, the osteosarcoma patient also suffered from tuberculosis, and tuberculosis treatment was prioritized over treatment of metastatic lesions. This decision was supported by data showing that tuberculosis worsens in lung cancer patients during chemotherapy [20]. In one study, 9 of 904 lung cancer patients developed tuberculosis (1%); tuberculosis occurred within 2 years of lung cancer diagnosis in all 9 patients, and tuberculosis was diagnosed in 3 of 9 cases (33.3%) during chemotherapy [21]. The development of active tuberculosis likely occurred due to the deterioration of both local and systemic immune defenses caused by both the chemotherapeutic agents and the cancer [20,21]. In the current case, the slow growth of the metastatic lesions was also a reason for prioritization of tuberculosis treatment. In cases with rapid growth of neoplastic lesions, chemotherapy for the cancer might be prioritized. In a previous report, simultaneous treatment of both tuberculosis and nasopharyngeal carcinoma was performed [22].
Our patient demonstrated oxycodone resistance due to rifampin, as demonstrated by the ability to control his pain with a lower equivalent dose of morphine. CYP3A is inducted by rifampin, resulting in the increased metabolism of many drugs, including oxycodone [2,7]. Other drugs used to treat tuberculosis, namely isoniazid and ethambutol, have not been reported to induce CYP3A activity. In addition to activating CYP3A, rifampin is also known to induce P-glycoprotein, a membrane efflux transporter found in the intestinal wall and brain capillary endothelium [2,23]. However, the role of P-glycoprotein in the pharmacokinetics of oxycodone seems to be of minor importance [24].
Conclusions
We have presented a case of osteosarcoma with lung metastasis invading the brachial plexus in a patient being treated for tuberculosis. Our patient experienced oxycodone resistance due to rifampin. Immunosuppressive effects of chemo-therapy may increase the risk of tuberculosis in such patients. Recognizing the association between rifampin and oxycodone is also important in other cancers. Notably, in similar patients receiving high doses of oxycodone for treatment of severe pain, stopping rifampin may lead to oxycodone overdose.
Acknowledgments
We appreciate the help of professor of pathology Masanori Hisaoka, who was in charge of the diagnosis and took photographs of histological structures, and the contributions of orthopedic surgeons Jun Fujimori and Shoji Shimose, who performed surgery and administered most of the chemotherapy.
References:
- 1.World Health Organization (WHO) Global Tuberculosis Report. Geneva (CHE): 2016. Available from: URL: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Nieminen TH, Hagelberg NM, Saari TI, et al. Rifampin greatly reduces the plasma concentrations of intravenous and oral oxycodone. Anesthesiology. 2009;110:1371–78. doi: 10.1097/ALN.0b013e31819faa54. [DOI] [PubMed] [Google Scholar]
- 3.Egelund EF, Alsultan A, Peloquin CA. Optimizing the clinical pharmacology of tuberculosis medications. Clin Pharmacol Ther. 2015;98:387–93. doi: 10.1002/cpt.180. [DOI] [PubMed] [Google Scholar]
- 4.Mason PH, Snow K, Asugeni R, et al. Tuberculosis and gender in the Asia-Pacific region. Aust N Z J Public Health. 2017;41(3):227–29. doi: 10.1111/1753-6405.12619. [DOI] [PubMed] [Google Scholar]
- 5.Niemi M, Backman JT, Fromm MF, et al. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819–50. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 6.Kalso E. Oxycodone. J Pain Symptom Manage. 2005;29:S47–56. doi: 10.1016/j.jpainsymman.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Kummer O, Hammann F, Moser C, et al. Effect of the inhibition of CYP3A4 or CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. Eur J Clin Pharmacol. 2011;67:63–71. doi: 10.1007/s00228-010-0893-3. [DOI] [PubMed] [Google Scholar]
- 8.Samer CF, Daali Y, Wagner M, et al. The effects of CYP2D6 and CYP3A activities on the pharmacokinetics of immediate release oxycodone. Br J Pharmacol. 2010;160:907–18. doi: 10.1111/j.1476-5381.2010.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalovic B, Phillips B, Risler LL, et al. Quantitative contribution of CYP2D6 and CYP3A to oxycodone metabolism in human liver and intestinal microsomes. Drug Metab Dispos. 2004;32:447–54. doi: 10.1124/dmd.32.4.447. [DOI] [PubMed] [Google Scholar]
- 10.Lalovic B, Kharasch E, Hoffer C, et al. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: Role of circulating active metabolites. Clin Pharmacol Ther. 2006;79:461–79. doi: 10.1016/j.clpt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Longhi A, Errani C, De Paolis M, et al. Primary bone osteosarcoma in the pediatric age: State of the art. Cancer Treat Rev. 2006;32:423–36. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 13.Lamoureux F, Trichet V, Chipoy C, et al. Recent advances in the management of osteosarcoma and forthcoming therapeutic strategies. Expert Rev Anticancer Ther. 2007;7:169–81. doi: 10.1586/14737140.7.2.169. [DOI] [PubMed] [Google Scholar]
- 14.Klein MJ, Siegal GP. Osteosarcoma: anatomic and histologic variants. Am J Clin Pathol. 2006;125:555–81. doi: 10.1309/UC6K-QHLD-9LV2-KENN. [DOI] [PubMed] [Google Scholar]
- 15.Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: Current challenges and future directions. Expert Rev Anticancer Ther. 2006;6:1075–85. doi: 10.1586/14737140.6.7.1075. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto A, Iwamoto Y. Current status and perspectives regarding the treatment of osteosarcoma: chemotherapy. Rev Recent Clin Trials. 2008;3:228–31. doi: 10.2174/157488708785700267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson PN, Evans RJ. Intractable pain with lung cancer. Pain. 1987;29:163–73. doi: 10.1016/0304-3959(87)91033-5. [DOI] [PubMed] [Google Scholar]
- 18.Wood JJ, Gawler J, Whittle RJ, Staunton MD. Brachial plexopathy in breast carcinoma – an unsolved problem. Eur J Surg Oncol. 1991;17:265–69. [PubMed] [Google Scholar]
- 19.Zinboonyahgoon N, Vlassakov K, Abrecht CR, et al. Brachial plexus block for cancer-related pain: A case series. Pain Physician. 2015;18:E917–24. [PubMed] [Google Scholar]
- 20.Jacobs RE, Gu P, Chachoua A. Reactivation of pulmonary tuberculosis during cancer treatment. Int J Mycobacteriol. 2015;4:337–40. doi: 10.1016/j.ijmyco.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki Y, Imokawa S, Sato J, et al. Cumulative incidence of tuberculosis in lung cancer patients in Japan: A 6-year observational study. Respir Investig. 2016;54:179–83. doi: 10.1016/j.resinv.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Li M, Liu D, et al. Treatment of nasopharyngeal carcinoma with pulmonary tuberculosis and gout: A case report. Oncol Lett. 2014;8:753–57. doi: 10.3892/ol.2014.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greiner B, Eichelbaum M, Fritz P, et al. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest. 1999;104:147–53. doi: 10.1172/JCI6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gimbel J, Ahdieh H. The efficacy and safety of oral immediate-release oxymorphone for postsurgical pain. Anesth Analg. 2004;99:1472–77. doi: 10.1213/01.ANE.0000132548.91622.B3. [DOI] [PubMed] [Google Scholar]