Abstract
Objective:
Systemic lupus erythematosus (SLE) is characterized by chronic inflammation. Plasma atherogenic index (PAI) is a valuable marker for the cardiovascular disease and cardiac risk. The aim of this study was to evaluate the role and clinical use of PAI in atherosclerosis and the cardiac risk in SLE patients.
Materials and Methods:
We included 56 female SLE patients who were selected according to the American College of Rheumatology (1997) diagnosis criteria. Furthermore, we selected age-and body mass index (BMI)-matched 56 female healthy individuals. PAI was measured as a logarithmic value of triglyceride to high-density cholesterol ratio. We used carotid intima media thickness (cIMT) as an inflammatory marker because of its widespread use. The lipid and other biochemical parameters of patient and control groups were examined.
Results:
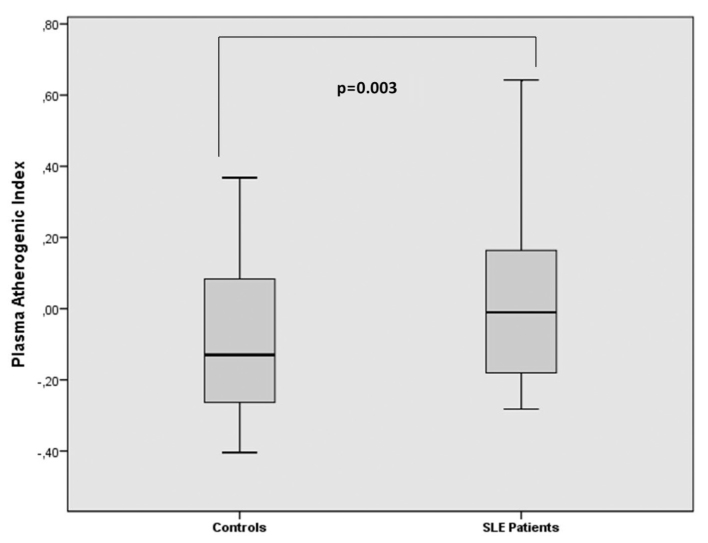
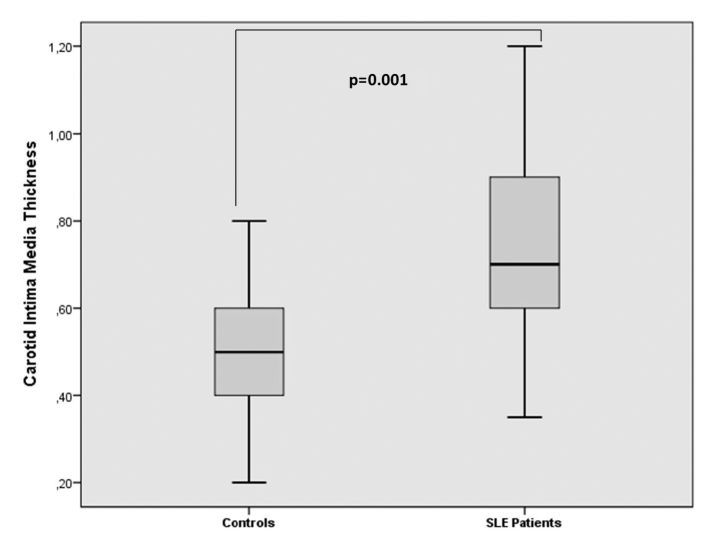
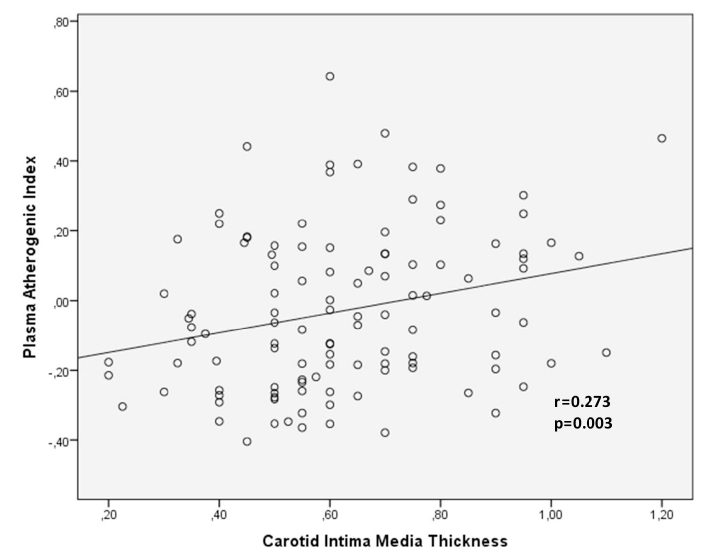
The PAI and cIMT values of SLE patients were 0.04±0.23 and 0.78±0.18 mm, respectively. Besides, for the control group, the PAI value was −0.09±0.20 and cIMT value was 0.50±0.15 mm (p=0.002, p<0.001; respectively). There was a strong correlation between cIMT and PAI (r=0.273, p=0.003). According to the multiple logistic regression analysis, we found that PAI value is an independent factor for cIMT in SLE patients (odds ratio: 2.6, 95 % confidence interval; 1.506–4.374; p=0.029).
Conclusions:
We determined that PAI can be used as an independent indicator for subclinical atherosclerosis in SLE patients.
Keywords: Plasma atherogenic index, carotid intima-media thickness, systemic lupus erythematosus, atherosclerosis
Öz
Amaç:
Sistemik lupus eritematozus (SLE), kronik inflamasyon ile karakterize bir hastalıktır. Plazma aterojenik indeks (PAI), kardiyovasküler hastalık ve kardiyak risk için değerli bir belirteçtir. Bu çalışmanın amacı PAI’in SLE hastalarında aterosklerozu ve kardiyak riski değerlendirmekteki rolü ve klinik kullanılabilirliliğini araştırmaktır.
Gereç ve Yöntem:
Amerikan Romatoloji Koleji (1997) tanı kriterlerine göre değerlendirilen 56 kadın SLE hastası dahil edildi. Ayrıca yaş ve vucüt kitle indeksi uyumlu 56 kadın sağlıklı kişi seçildi. PAI, trigliserit’in yüksek yoğunluklu kolesterole oranı logartimik ölçümüdür. Karotis intima medya kalınlığı (KIMK) inflamatuvar belirteç olarak yaygın olarak kullanıldığından, bu işareti bu çalışmada kullandık. Hasta ve kontrol gruplarının lipid ve diğer biyokimyasal parametreleri incelendi.
Bulgular:
Hasta grubunda PAI 0,04±0,23, KIMK 0,78±0,18 mm, kontrol grubunda PAI −0,09±0,20, KIMK 0,50±0,15 mm olduğu tespit edildi. Hasta grubunda PAI ve KIMK’ın kontrol grubuna kıyasla yüksek olduğu görüldü (sırasıyla p=0,002, p<0,001). SLE hastaları içerisine KIMK ile PAI arasında pozitif korelasyon olduğu görüldü (r=0,273, p=0,003). Multipl logistik regresyon analize göre PAI, KIMK için bağımsız risk faktörü olarak değerlendirildi (Olasılık oranı: 2,6, Güven aralığı %95 1,506–4,374, p=0,029).
Sonuç:
Bu çalışma bize PAI’nın SLE hastalarında subklinik aterosklerozun değerlendirilmesinde kullanılabilecek bağımsız bir belirteç olabileceğini düşündürdü.
Keywords: Plazma aterojenik indeks, karotis intima medya kalınlığı, Sistemik lupus eritematozus, aterosklerozis
Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory and autoimmune connective tissue disease characterized by multiple organ and system involvement. The etiology has not yet been clarified [1]. Fever, skin lesions, joint, renal, hematologic, and central nervous system involvements can be observed during the clinical course of the disease. SLE affects the cardiovascular system, particularly the pericardium. It can also influence the myocardium and endocardium [1–3]. Furthermore, subclinical atherosclerosis and/or related complications in SLE are the most important reasons for the mortality and the morbidity [3, 4].
In SLE patients, systemic inflammation, oxidative stress, and alterations in the lipid metabolism (increment in plasma triglyceride (TG) levels and decrease in the high-density lipoprotein (HDL) levels) primarily possess an important risk for coronary artery disease. In this context, a high atherogenic index (TG-HDL ratio) was demonstrated in SLE patients [5]. However, plasma atherogenic index (PAI) has been measured using a different method that it is obtained using the logarithmic transformation of the TG-HDL ratio and can be efficiently used to determine the cardiac risk and atherosclerosis [6, 7]. Moreover, carotid intima media thickness (cIMT) is a reliable, non-invasive, and easy indicator, which can be applied in ultrasonographic evaluations. It can be safely used to detect subclinical atherosclerosis, which has been shown in many studies [8–10].
In this study, we aim to determine the cardiac risk groups of SLE patients using PAI and cIMT measurements. We also aim to determine whether PAI can be used as a marker for the early detection of the subclinical atherosclerosis in SLE patients.
Materials and Methods
This cross-sectional study was conducted in Necmettin Erbakan University, Medical Faculty, Rheumatology and Cardiology Clinic. We included 56 female SLE patients selected according to the American College of Rheumatology (1997) diagnosis criteria. Furthermore, we selected age-and body mass index (BMI)-matched 56 female and healthy individuals. The basic clinical and laboratory features of the patient and the control groups were examined and recorded. The disease activities of SLE patients were calculated using the SLE disease activity index (SLEDAI). Exclusion criteria were patients with systemic diseases, such as diabetes mellitus, hypertension, known heart disease, thyroid dysfunction, and chronic obstructive pulmonary disease; individuals smoking cigarette and drinking alcohol and who had cancer; patients on lipid-lowering agents, such as statin and fenofibrate; and individual for the control group who were chronically using drugs.The study was approved by the Ethics Committee of Necmettin Erbakan University, Turkey. Written and verbal informed consents of all participants were obtained.
Biochemical analysis
Twelve-hours fasting venous blood samples were collected from patients and control individuals. Total cholesterol (TC), TG, and HDL were analyzed using the Abbot Architect 16000 system with the help of the original reagent analysis. HDL levels were directly analyzed using an enzymatic method without precipitating the HDL. Low-density lipoprotein (LDL) cholesterol levels were calculated using the Friedewald formula (TC=LDL+HDL+TG/5). PAI values were calculated using the log10 TG/HDL formula (6).
Evaluation of carotid intima-media thickness
All examinations were performed by one cardiologist using a Vivid 7 Echocardiography device with an echocardiography device (General Electric, Horten, Norway) with the help of the 10 MHz multi-frequency linear probe. We primarily evaluated the B-mode grayscale imaging and general morphological evaluation of the common carotid artery and internal carotid artery cervical segments after the bifurcation at the axial and longitudinal plane. We conducted measurements from three different points of the right and left main carotid artery. The cIMT measurements were performed by evaluating only the back (away) wall from the CCA. The mean cIMT values were recorded by calculating the mean of triple measurements of carotid artery. Inter-observer variability was less than 6% and intra observer variability is less than 3%.
Statistical analysis
Results were represented as mean ± standard deviation (SD). All statistical analyses were performed using the Statistical Package for Social Sciences program (SPSS) version 18 (IBM, Chicago IL, USA). Data for both groups were homogenously distributed, and the Kolmogorov–Smirnov test was used for the patient and control groups. The Student-t test was used for between-group comparisons. The Pearson correlation test was used for the correlation analysis. The relationship between logistic regression analysis and PAI independent variables was examined. A p<0.05 was considered statistically significant
Results
There was no significant difference between the patient and control groups in terms of their ages and BMI values. The drug use and the disease activities of patients are shown in Table 1. PAI and cIMT values of patients were 0.04±0.23 and 0.78±0.18 mm, respectively. Besides, the control group had PAI value of −0.09±0.20 and cIMT value of 0.50±0.15 mm. The PAI and cIMT values of patient group were higher compared to control individuals (p=0.002, p<0.001, respectively; Figures 1 and 2). The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values of SLE patients were prominently higher compared to control individuals. The clinical and laboratory features of patients and control individuals are shown in Table 1.
Table 1.
The sociodemographic and laboratory parameters in two groups
| SLE patients (n=56) (mean±SD) | Controls (n=56) (mean±SD) | P | |
|---|---|---|---|
| Age (years) | 38.1±10.3 | 36.9±8.8 | 0.486 |
| BMI (kg/m2) | 26.8±5.4 | 26.6±5.1 | 0.849 |
| Glucose (mg/dL) | 93.3±14.9 | 92.3±9.4 | 0.687 |
| Creatinine (mg/dL) | 0.62±0.11 | 0.66±0.07 | 0.034 |
| ALT (IU/L) | 20.9±11.4 | 16.2±5.4 | 0.005 |
| Hb (g/dL) | 12.0±1.3 | 13.2±1.1 | 0.001 |
| WBC (103/L) | 6.9±2.9 | 7.1±1.7 | 0.698 |
| ESR (mm/h) | 31.3±23.2 | 8.3±5.2 | 0.001 |
| CRP (mg/dL) | 8.5±12.1 | 3.0±3.5 | 0.001 |
| TC (mg/dL) | 172.8±37.6 | 181.3±34.4 | 0.200 |
| TG (mg/dL) | 124.3±69.3 | 96.5±38.3 | 0.008 |
| LDL-c (mg/dL) | 100.7±30.9 | 109.2±30.3 | 0.131 |
| HDL-c (mg/dL) | 48.2±12.9 | 52.8±12.4 | 0.054 |
| cIMT (mm) | 0.73±0.18 | 0.50±0.15 | 0.001 |
| PAI | 0.37±0.24 | 0.24±0.21 | 0.003 |
SLE: systemic lupus erythematosus; BMI: body mass index; cIMT: carotid intima media thickness; TC: total cholesterol; TG: triglyceride; LDL-c: low density-lipoprotein cholesterol; HDL-c: high density-lipoprotein cholesterol; PAI: plasma atherogenic index; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; Hb: hemoglobin; WBC: white blood cell counts; ALT: alanine transaminase
Figure 1.
PAI in systemic lupus erythematosus patients and control groups
Figure 2.
Thickness of carodit intima media thickness in systemic lupus erythematosus patients and control groups
In the correlation analysis, a positive correlation was observed between cIMT and PAI (r=0.273, p=0.003; Figure 3). There was a positive correlation between the age and cIMT (r=0.329, p<0.001), BMI (r=0.232, p=0.011), SLEDAI (r=0.454, p<0.001), disease duration (r=0.373, p<0.001), and TG (r=0.253, p=0.005). A positive relationship was observed between SLEDAI and PAI (r=0.232, p=0.011). All correlation analysis results are presentations in Table 2.
Figure 3.
Correlation between atherogenic index and cIMT in SLE patients
Table 2.
cIMT and correlation analysis of other parameters in SLE patients
| cIMT | ||
|---|---|---|
|
| ||
| r value | P | |
| Age | 0.329 | 0.001 |
| BMI | 0.232 | 0.011 |
| Glucose | 0.147 | 0.136 |
| Creatinine | 0.001 | 0.987 |
| ALT | 0.223 | 0.015 |
| HB | −0.223 | 0.011 |
| WBC | 0.096 | 0.300 |
| ESR | 0.253 | 0.006 |
| CRP | 0.222 | 0.015 |
| TC | 0.133 | 0.147 |
| HDL-c | −0.052 | 0.573 |
| TG | 0.253 | 0.005 |
| LDL-c | 0.083 | 0.370 |
| SLEDAI | 0.454 | 0.001 |
| PAI | 0.273 | 0.003 |
SLE: systemic lupus erythematosus; BMI: body mass index; PAI: plasma atherogenic index; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; TC: total cholesterol; HDL-c: high-density lipoprotein cholesterol; TG, triglycerides; LDL-c, low-density lipoprotein cholesterol; ALT: alanine aminotransferase; Hb: hemoglobin; cIMT: carotid intima media thickness; WBC: white blood cell counts; SLEDAI: systemic lupus erythematosus disease activity index
In the multiple logistic regression analysis, the PAI value was determined as an independent factor for subclinical atherosclerosis (odds ratio: 2.6, 95% CI: 1.506–4.374, p=0.029). The multiple logistic regression analysis results are shown in Table 3.
Table 3.
Multiple logistic regression analysis for cIMT in SLE patients
| Independent Variables | Odds ratio | 95% Confidence lnterval | p value |
|---|---|---|---|
| Age | 1.021 | 0.942–1.106 | 0.613 |
| BMI | 1.068 | 0.910–1.254 | 0.419 |
| Glucose | 1.001 | 0.946–1.059 | 0.978 |
| Creatinine | 0.210 | 0.105–0.437 | 0.336 |
| ALT | 1.029 | 0.944–1.121 | 0.518 |
| CRP | 1.022 | 0.970–1.077 | 0.417 |
| ESR | 0.997 | 0.964–1.032 | 0.879 |
| HDL-c | 2.576 | 0.341–2.944 | 0.359 |
| TC | 0.493 | 0.067–3.646 | 0.489 |
| LDL-c | 1.920 | 0.258–2.428 | 0.524 |
| TG | 1.118 | 0.749–1.670 | 0.586 |
| PAI | 2.6 | 1.506–4.374 | 0.029 |
BMI: body mass index; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol; TC: total cholesterol; TG: triglycerides; ALT: alanine aminotransferase; PAI: plasma atherogenic index; cIMT: carotid intima media thickness; SLE: systemic lupus erythematosus
Discussion
In our study, we showed that PAI and cIMT values were higher in female SLE patients compared to controls. According to the correlation analysis, there was a positive correlation between the PAI levels and cIMT in SLE patients. In the regression analysis, PAI was considered an independent predictor of subclinical atherosclerosis.
Although the Atherosclerosis (AS) developmental process has not yet been clarified in SLE patients, the interaction between the AS and SLE is a quite complex process. AS develops due to the accumulation of lipids on the vessel wall. Systemic inflammation plays an important role in the atherosclerotic lesion development and the plaque rupture [11, 12]. The effects of systemic inflammation (primarily cytokines, such as tumor necrosis factor-alpha, interleukin-1, and interleukin-6) lead to alterations in the lipid metabolism and decrease in the synthesis and activity of the lipoprotein lipase synthesis, increase in the hepatic fatty acid synthesis, increase of the lipolysis in the adipose tissue, and decrease in the fatty acid oxidation in the liver [13]. Furthermore, lipoprotein lipase antibodies in SLE patients can contribute to the high TG levels. The alterations in the TG metabolism can cause changes primarily in HDL and also other lipids [14, 15]. The reasons for low HDL levels are the apoprotein A-1 synthesis in the liver and alterations in the lecithin cholesterol acyl transferase activity. Serum amyloid A, which is released from the liver and other tissues in response to cytokines, can lead to the replacement of amyloid A-1 with apoprotein A-1 and increase in the HDL clearance [16]. As a result of these effects, elevated TG levels increase the hepatic very-low-density lipoprotein production, and decrease in the TG rich lipoprotein clearance and decrease in serum HDL levels can be observed [5, 12].
Triglyceride primarily contains LDL and HDL and the alterations in the lipid profile possess a risk for AS development and cardiovascular diseases. TG contains small dense LDL, which a highly atherogenic molecule [13, 17]. Atheroma, which develops due to the accumulation of the cholesterol crystals in the inner layers of the atherosclerotic arteries, causes the narrowing and blockage of the lumen. Systemic inflammation, increased oxidative stress, and alteration in the lipid profile are important in the development and the progression of the process [5, 10, 18]. There are studies in which the TG and HDL and the ratio of TG and HDL can be used to calculate the PAI. Zhan et al. [19] showed that PAI levels were higher in acute coronary syndrome patients compared to controls. Niroumand et al. [7] determined that PAI can be used as an indicator of the cardiovascular diseases. Akbas et al [20] demonstrated that PAI may increase the risk of developing cardiovascular disease.
In various rheumatologic diseases, primarily, SLE and cIMT measurement is used as an indicator for the evaluation of subclinical atherosclerosis, coronary artery disease, the progression of these diseases, and risk determination [21–23]. Eder et al. [22] stated that cIMT was related to the total plaque area and coronary artery disease in SLE patients. Icli et al. [11] and Ammirati et al. [23] showed that cIMT values were higher in SLE patients compared to control individuals. Gheito et al. [24] demonstrated that cIMT levels were higher in SLE patients than in their control group and that it showed a correlation with SLEDAI. Batún Garrido et al. [5] reported the strong correlation between SLE activity and dyslipidemia. They also showed the significant correlation between disease activity and PAI values.
Lipid metabolism alterations in SLE patients that can develop during the course of the disease are important for subclinical atherosclerosis. After this complex process, atherosclerosis development and cardiovascular disease risks increase in SLE patients [5, 12, 25]. In our study, we observed that cIMT and PAI values were higher in SLE patients compared to control individuals; there was a strong and independent relationship between cIMT and PAI, and these findings indicate that PAI can be used in the evaluation of subclinical atherosclerosis in SLE patients.
Our study has a few limitations. These include the small study population, prospective cross-sectional nature of the study, and the fact that the differences between the patients with mild and severe disease activity as per the SLEDAI were not considered.
In conclusions, PAI and cIMT levels of SLE patients are higher compared to control individuals. There is a strong correlation between PAI values and cIMT. PAI values can be used as an independent predictor marker for the early detection or estimation of the subclinical atherosclerosis in SLE patients.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of the Necmettin Erbakan University (Decision Date/No: 04-03-2016/28).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.U.U.; Design - A.U.U., A.K.; Data Collection and/or Processing - D.S., S.A., R.A.B.; Analysis and/or Interpretation - A.U.U., E.C.; Literature Review - A.K., E.C., A.İ.; Writing - A.U.U.; Critical Review - A.U.U., A.İ., E.C.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Kucuk A, Uslu AU, Yilmaz R, Salbas E, Solak Y, Tunc R. Relationship between prevalence and severity of restless legs syndrome and anemia in patients with systemic lupus erythematosus. Int J Rheum Dis. 2015;20:469–73. doi: 10.1111/1756-185X.12793. https://doi.org/10.1111/1756-185X.12793. [DOI] [PubMed] [Google Scholar]
- 2.Barutcu A, Aksu F, Ozcelik F, et al. Evaluation of early cardiac dysfunction in patients with systemic lupus erythematosus with or without anticardiolipin antibodies. Lupus. 2015;24:1019–28. doi: 10.1177/0961203315570164. https://doi.org/10.1177/0961203315570164. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhari D, Madani M Al, Balbissi Md K Al, Paul TK. Lupus Myocarditis Presenting as Life-threatening Overt Heart Failure: A Case Report with Review of Cardiovascular Manifestations of Systemic Lupus Erythematosus. J La State Med Soc. 2015;167:220–2. [PubMed] [Google Scholar]
- 4.Icli A, Cure E, Cumhur Cure M, et al. Novel myokine: irisin may be an independent predictor for subclinic atherosclerosis in Behçet’s disease. J Investig Med. 2016;64:875–81. doi: 10.1136/jim-2015-000044. https://doi.org/10.1136/jim-2015-000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batún Garrido JA de J, Radillo Alba HA, Hernández Nú-ez É, Olán F. Dyslipidaemia and atherogenic risk in patients with systemic lupus erythematosus. Med Clin (Barc) 2016;147:63–6. doi: 10.1016/j.medcli.2016.03.030. https://doi.org/10.1016/j.medcle.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Dobiásová M. AIP--atherogenic index of plasma as a significant predictor of cardiovascular risk: from research to practice. Vnitr Lek. 2006;52:64–71. [PubMed] [Google Scholar]
- 7.Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, et al. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med J Islam Repub Iran. 2015;29:627–35. [PMC free article] [PubMed] [Google Scholar]
- 8.Balta S, Aparci M, Ozturk C, Yildirim AO, Demir M, Celik T. Carotid intima media thickness and subclinical early atherosclerosis. Int J Cardiol. 2016;203:1146. doi: 10.1016/j.ijcard.2015.11.025. https://doi.org/10.1016/j.ijcard.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Kucuk A, Uslu AU, Arslan S, et al. Ischemia-Modified Albumin and Atherosclerosis in Patients With Familial Mediterranean Fever. Angiology. 2016;67:456–60. doi: 10.1177/0003319715595744. https://doi.org/10.1177/0003319715595744. [DOI] [PubMed] [Google Scholar]
- 10.Uslu AU, Kucuk A, Balta S, et al. The relation between ischemia modified albumin levels and carotid intima media thickness in patients with rheumatoid arthritis. Int J Rheum Dis. 2016:12851. doi: 10.1111/1756-185X.12851. https://doi.org/10.1111/1756-185X.12851. [DOI] [PubMed] [Google Scholar]
- 11.Icli A, Cure E, Cure MC, et al. Endocan Levels and Subclinical Atherosclerosis in Patients With Systemic Lupus Erythematosus. Angiology. 2015;67:749–55. doi: 10.1177/0003319715616240. https://doi.org/10.1177/0003319715616240. [DOI] [PubMed] [Google Scholar]
- 12.Feingold KR, Grunfeld C. The Effect of Inflammation and Infection on Lipids and Lipoproteins. In: De Groot LJ, Beck-Peccoz P, Chrousos G, et al., editors. Endotext. South Dartmouth (MA): 2000. [Google Scholar]
- 13.Khovidhunkit W, Kim M-S, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–96. doi: 10.1194/jlr.R300019-JLR200. https://doi.org/10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Reichlin M, Fesmire J, Quintero-Del-Rio AI, Wolfson-Reichlin M. Autoantibodies to lipoprotein lipase and dyslipidemia in systemic lupus erythematosus. Arthritis Rheum. 2002;46:2957–63. doi: 10.1002/art.10624. https://doi.org/10.1002/art.10624. [DOI] [PubMed] [Google Scholar]
- 15.De Carvalho JF, Borba EF, Viana VST, Bueno C, Leon EP, Bonfá E. Anti-lipoprotein lipase antibodies: A new player in the complex atherosclerotic process in systemic lupus erythematosus? Arthritis Rheum. 2004;50:3610–5. doi: 10.1002/art.20630. https://doi.org/10.1002/art.20630. [DOI] [PubMed] [Google Scholar]
- 16.Hosoai H, Webb NR, Glick JM, et al. Expression of serum amyloid A protein in the absence of the acute phase response does not reduce HDL cholesterol or apoA-I levels in human apoA-I transgenic mice. J Lipid Res. 1999;40:648–53. [PubMed] [Google Scholar]
- 17.Olusi SO, George S. Prevalence of LDL atherogenic phenotype in patients with systemic lupus erythematosus. Vasc Heal Risk Manag. 2011;7:75–80. doi: 10.2147/VHRM.S17015. https://doi.org/10.2147/VHRM.S17015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balta S, Aparci M, Ozturk C, Unlu M, Celik T. Carotid intima media thickness can predict coronary artery disease. Int J Cardiol. 2015;201:331. doi: 10.1016/j.ijcard.2014.10.100. https://doi.org/10.1016/j.ijcard.2014.10.100. [DOI] [PubMed] [Google Scholar]
- 19.Zhan Y, Xu T, Tan X. Two parameters reflect lipid-driven inflammatory state in acute coronary syndrome: atherogenic index of plasma, neutrophil-lymphocyte ratio. BMC Cardiovasc Disord. 2016;16:96. doi: 10.1186/s12872-016-0274-7. https://doi.org/10.1186/s12872-016-0274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akbas EM, Timuroglu A, Ozcicek A, et al. Association of uric acid, Atherogenic index of plasma and albuminuria in diabetes mellitus. Int J Clin Exp Med. 2014;7:5737–43. [PMC free article] [PubMed] [Google Scholar]
- 21.Altin C, Sade LE, Gezmis E, et al. Assessment of Subclinical Atherosclerosis by Carotid Intima-Media Thickness and Epicardial Adipose Tissue Thickness in Prediabetes. Angiology. 2016;67:961–9. doi: 10.1177/0003319716643669. https://doi.org/10.1177/0003319716643669. [DOI] [PubMed] [Google Scholar]
- 22.Eder L, Gladman DD, Iba-ez D, Urowitz MB. The correlation between carotid artery atherosclerosis and clinical ischemic heart disease in lupus patients. Lupus. 2014;23:1142–8. doi: 10.1177/0961203314537696. https://doi.org/10.1177/0961203314537696. [DOI] [PubMed] [Google Scholar]
- 23.Ammirati E, Bozzolo EP, Contri R, et al. Cardiometabolic and immune factors associated with increased common carotid artery intimamedia thickness and cardiovascular disease in patients with systemic lupus erythematosus. Nutr Metab Cardiovasc Dis. 2014;24:751–9. doi: 10.1016/j.numecd.2014.01.006. https://doi.org/10.1016/j.numecd.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Gheita TA, Raafat HA, Sayed S, El-Fishawy H, Nasrallah MM, Abdel-Rasheed E. Metabolic syndrome and insulin resistance comorbidity in systemic lupus erythematosus: Effect on carotid intima-media thickness. Z Rheumatol. 2013;72:172–7. doi: 10.1007/s00393-012-1058-9. https://doi.org/10.1007/s00393-012-1058-9. [DOI] [PubMed] [Google Scholar]
- 25.Vásquez-Cede-o DA, Tamariz E, Cevallos MI. Lipid profile in patients with newly diagnosed coronary heart disease: 2012 and 2013 cross-sectional study in Luis Vernaza Hospital, Ecuador. Medwave. 2014;14:e6007. doi: 10.5867/medwave.2014.07.6007. https://doi.org/10.5867/medwave.2014.07.6007. [DOI] [PubMed] [Google Scholar]