Abstract
Summer-weight clothing articles impregnated with permethrin are available as a personal protective measure against human-biting ticks in the United States. However, very few studies have addressed the impact of contact with summer-weight permethrin-treated textiles on tick vigor and behavior. Our aim was to generate new knowledge of how permethrin-treated textiles impact nymphal Ixodes scapularis ticks, the primary vectors in the eastern United States of the causative agents of Lyme disease, human anaplasmosis, and human babesiosis. We developed a series of bioassays designed to: (i) clarify whether permethrin-treated textiles impact ticks through non-contact spatial repellency or contact irritancy; (ii) evaluate the ability of ticks to remain in contact with vertically oriented permethrin-treated textiles, mimicking contact with treated clothing on arms or legs; and (iii) determine the impact of timed exposure to permethrin-treated textiles on the ability of ticks to move and orient toward a human finger stimulus, thus demonstrating normal behavior. Our results indicate that permethrin-treated textiles provide minimal non-contact spatial repellency but strong contact irritancy against ticks, manifesting as a “hot-foot” effect and resulting in ticks actively dislodging from contact with vertically oriented treated textile. Preliminary data suggest that the contact irritancy hot-foot response may be weaker for field-collected nymphs as compared with laboratory-reared nymphs placed upon permethrin-treated textile. We also demonstrate that contact with permethrin-treated textiles negatively impacts the vigor and behavior of nymphal ticks for > 24 h, with outcomes ranging from complete lack of movement to impaired movement and unwillingness of ticks displaying normal movement to ascend onto a human finger. The protective effect of summer-weight permethrin-treated clothing against tick bites merits further study.
Keywords: Ixodes scapularis, Bioassay, Contact irritancy, Permethrin-treated clothing, Toxicity
1. Introduction
Treatment of clothing with the synthetic pyrethroid pesticide permethrin has long been known to protect against exposure to ticks, including human-biters and pathogen vectors such as the Lone star tick (Amblyomma americanum), the American dog tick (Dermacentor variabilis), the Pacific Coast tick (Dermacentor occidentalis), the blacklegged tick (Ixodes scapularis), the western blacklegged tick (Ixodes pacificus), and the common tick (Ixodes ricinus) (Schreck et al., 1978, 1980, 1982a, 1986; Mount and Snoddy, 1983; Lane and Anderson, 1984; Lane, 1989; Evans et al., 1990; Faulde et al., 2003). Field evaluations of the protective effect against crawling ticks or tick bites afforded by wearing permethrin-treated clothing have included: (i) uniforms or summer-weight clothing treated by soaking in a permethrin solution (Schreck et al., 1980, 1982a, 1982b; Evans et al., 1990; Miller et al., 2011); (ii) uniforms or coveralls treated with pressurized permethrin spray (Schreck et al., 1982a, 1986; Mount and Snoddy, 1983; Lane and Anderson, 1984; Lane, 1989; Evans et al., 1990; Jordan et al., 2012); and more recently (iii) factory-impregnated uniforms or summer-weight clothing treated via polymerization of permethrin onto the fiber surface (polymer-coating method) or other proprietary permethrin impregnation methods (Faulde et al., 2008, 2014; Miller et al., 2011; Vaughn and Meshnick, 2011; Vaughn et al., 2014; Richards et al., 2015). These studies collectively demonstrate that wearing permethrin-treated clothing, especially garments that cover most of the body surface, can reduce the risk of tick bites.
Less attention has been given to the behavior and fate of a tick that approaches or makes initial contact with permethrin-treated clothing. Such information is critical to better understand how the level of protection against ticks is influenced by the portion of the body surface covered by permethrin-treated garments. For example, permethrin-treated textiles may protect against ticks through non-contact spatial repellency. In this scenario, ticks approaching a treated textile would avoid making physical contact with it. Alternatively, permethrin-treated textile may impact ticks primarily through contact irritancy (also referred to as contact repellency or tick repellency sensu stricto; Bissinger and Roe, 2010; Halos et al., 2012). In this scenario, ticks would readily make physical contact with a treated textile but then experience discomfort and attempt to escape contact with the irritant surface. The terminology used here to describe how exposure to permethrin-treated textiles impacts tick behavior – resulting in non-contact spatial repellency versus contact irritancy – stems from a previous study on pesticide exposure of mosquitoes (Grieco et al., 2007). We use the term contact irritancy (rather than contact repellency) because it aptly describes the physical response of a tick that makes contact with a permethrin-treated textile: the tick displays an irritant “hot-foot” effect (Halos et al., 2012) and then actively strives to escape contact with the treated textile.
Field observations for adult I. pacificus or D. occidentalis ticks indicated that permethrin-treated clothing acts primarily as a contact irritant rather than a non-contact spatial repellent (Lane and Anderson, 1984; Lane, 1989). A laboratory bioassay providing a choice for D. occidentalis adults to move onto permethrin-treated versus non-treated cloth showed that the ticks readily moved onto the permethrin-treated textile rather than avoiding contact with it (Lane and Anderson, 1984). As long as permethrin-treated clothing is acutely toxic to ticks, a lack of non-contact spatial repellency is not necessarily problematic. A tick that initially contacts human skin and only thereafter approaches a permethrin-treated garment may, if strongly repelled by the treated textile, simply retreat and locate another attachment site. In contrast, a lack of non-contact spatial repellency may lead to the tick walking onto or underneath the permethrin-treated garment. In this scenario, the tick may be exposed to a dose of permethrin sufficient to either cause it to dislodge from the treated textile (and human host) or prevent it from biting before perishing from the toxic effect of the permethrin. This reasoning raises the additional question of whether ticks become incapacitated and unable to bite after realistically brief contact with a permethrin-treated garment.
Initial bioassays with permethrin-treated textiles were performed with ticks being held in continuous contact with the test textile for a pre-determined period of time and then assessed at fixed time points after contact for morbidity/mortality (defined as impaired movement or complete lack of movement even when encouraged to move by prodding or a human breath stimulus; Schreck et al., 1978; Lane and Anderson, 1984; Lane, 1989). In these studies, the vast majority of A. americanum nymphs and D. occidentalis and I. pacificus adults were classified as moribund or dead 1 h after exposure to permethrin-treated textile for no more than 1–2 min. Similarly, Schreck et al. (1986) noted that field-collected I. scapularis (I. dammini) and D. variabilis ticks lost control of normal movement and eventually died after contact with permethrin-treated clothing for ≥15 s. More recent laboratory bioassays have focused on determining the length of time ticks need to be held in continuous contact with a permethrin-treated textile to experience knockdown, defined as inability to move/migrate (Faulde et al., 2003; Faulde and Uedelhoven, 2006). In these studies, 5–7 min of contact with military uniform textiles impregnated with permethrin using the polymer-coating method was required to achieve high levels of knockdown for field-collected I. ricinus nymphs. The main difference between these two types of toxicity bioassays is that the former approach allows for delayed effects of permethrin exposure to manifest before the ticks are scored for vigor, whereas the latter approach is logistically more straightforward, with less manipulation of the ticks as part of the evaluation process, and therefore may generate better standardized data across types of treated textiles, tick species, and life stages. The choice of approach to use therefore should be guided primarily by the specific question to be answered by the toxicity bioassay.
In the United States, summer-weight clothing articles impregnated with permethrin are commercially available under several brand names, with treated garments ranging from socks to shorts, long pants and leggings, and short- and long-sleeved shirts. A single published study has evaluated the protective effect of wearing permethrin-treated summer-weight clothing (shoes, socks, shorts, and t-shirts) against challenges by a vector tick (Miller et al., 2011). This seminal study focused on nymphal I. scapularis ticks, the primary vectors in the eastern United States of the causative agents of Lyme disease, human anaplasmosis, and human babesiosis (Mead, 2015; Eisen et al., 2016). Use of permethrin-treated clothing articles, as compared with similar but non-treated clothing, not only reduced the number of laboratory-reared nymphs that attached after being introduced onto human volunteers but also led to the majority of attached nymphs dying within hours of their attachment (Miller et al., 2011).
Our aim in this study was to generate new knowledge for how I. scapularis nymphs behave when approaching or making initial contact with permethrin-treated textiles, and how their vigor is impacted after brief periods of contact with permethrin-treated textiles. We therefore developed a series of bioassays designed to: (i) clarify whether permethrin-treated textiles impact ticks through non-contact spatial repellency or contact irritancy; (ii) evaluate the ability of ticks to remain in contact with vertically oriented permethrin-treated textiles, mimicking contact with treated clothing on arms or legs; and (iii) determine the impact of timed exposure to permethrin-treated textiles on the ability of ticks to move and orient toward a human finger stimulus, thus demonstrating normal behavior. A final aim that emerged as we developed these bioassays and were able to observe the behavior of nymphal I. scapularis ticks coming into contact with permethrin-treated textiles was to generate a refined classification scheme for the vigor of the ticks after they were exposed to treated textile.
2. Materials and methods
2.1. Source of permethrin-treated textile and nymphal I. scapularis ticks
The permethrin-treated textile used to develop the bioassays came from a single Insect Shield® 100% cotton T-shirt (Insect Shield, LLC, Greensboro, NC, USA). The permethrin-treated test textile was in pristine condition (not washed or worn) for all bioassays. Laboratory-reared I. scapularis nymphs used in the process of developing and evaluating the bioassays originated primarily from tick colonies maintained at the Centers for Disease Control and Prevention, Division of Vector-Borne Diseases, Fort Collins, CO. These ticks included first, second, and third generation nymphs originating from females collected in Fairfield County, CT (CT15, CT14 and CT13, respectively), and first generation nymphs (MN15) originating from females collected in Anoka County and Washington County, MN. Bioassays performed to examine non-contact spatial repellency also included I. scapularis nymphs (OSU) from the Oklahoma State University Tick Rearing Facility (Stillwater, OK, USA). Laboratory-reared nymphal ticks used in the assays were 4–5 mo post-molt. Prior to being used the nymphs were held within desiccators (90–95% RH) in a growth chamber maintained at 21–22 °C with a 16:8 h light:dark cycle. Field-collected I. scapularis nymphs (CT-field) of unknown age used in the assays originated from multiple collection locations within Fairfield County, CT, in 2016. Field-collected nymphs were held in the laboratory at room temperature in desiccators (85–90% RH) for ≥2 wk before being used.
2.2. Classification scheme for tick vigor after contact with permethrin-treated textile
Previous studies to determine the impact of pesticides on the vigor of I. scapularis ticks classified them as either dead or moribund if treated ticks failed to show any signs of life or were incapable of moving, maintaining normal posture, coordinating their legs, or righting themselves, including when breathed upon or physically stimulated (Maupin and Piesman, 1994; Panella et al., 2005; Dolan et al., 2007). Another general scheme to represent stages of tick poisoning after pesticide exposure based on their capacity for movement was presented by Uspensky and Ioffe-Uspensky (2006). Based on our initial observations of I. scapularis nymphs after contact with a permethrin-treated textile, we developed refined methodology and a modified classification scheme to score tick vigor at various post-exposure time points. We avoid using the terms dead (due to the difficulty in distinguishing ticks that are alive but stunned from dead ticks) and moribund. Instead, we use terminology that describes the level of physical activity displayed by ticks following introduction onto a non-treated filter paper surface and stimulation of activity via gentle physical prodding and human breath. Tick vigor following stimulation of activity was scored across four categories of capacity for movement: (i) tick completely motionless; (ii) tick capable of some movement of the legs but unable to right itself or walk; (iii) tick capable of righting itself but not able to move in a coordinated way or readily orient toward a stimulus; and (iv) tick displaying normal movement and response to a stimulus (Fig. 1). Ticks in categories (i–iii) present no more than minimal risk of biting at the point in time they were examined, whereas ticks in category (iv) may present risk of biting. Ticks that were scored as displaying normal movement (iv) were further assessed to determine if they would ascend onto a finger when presented the opportunity. Normal behavior for nymphal ticks not exposed to permethrin-treated textile is to rapidly ascend onto the tip of a finger.
Fig. 1.
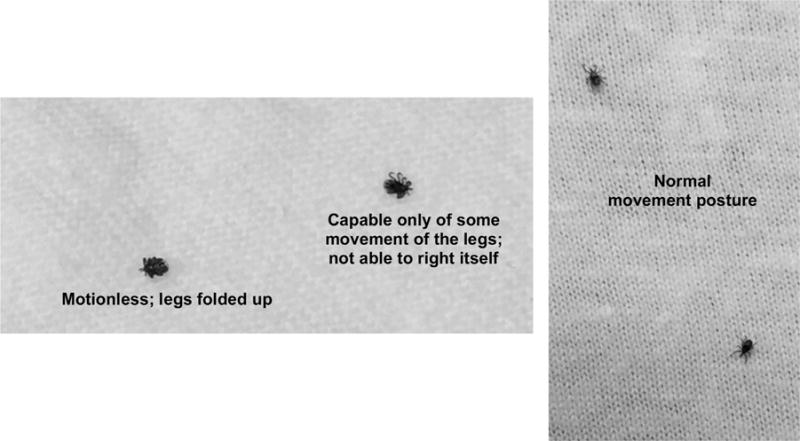
Examples of tick postures after exposure to permethrin-treated textile (left) and normal movement posture (right).
2.3. Bioassays to distinguish non-contact spatial repellency from contact irritancy for ticks approaching a permethrin-treated textile
We explored various bioassays to distinguish non-contact spatial repellency from contact irritancy. One initial option was a “finger assay”, based on earlier bioassays where strong non-contact spatial repellents (e.g., DEET) are applied to skin or cloth wrapped around the center portion of a human finger (Schreck et al., 1995; Carroll et al., 2007). We modified the finger assay to specifically distinguish non-contact spatial repellency from contact irritancy for ticks approaching permethrin-treated textile: ticks were challenged to move upward along the first three phalanxes of a vertically positioned finger, where the first phalanx (tip of the finger) presents a normal finger surface as the tick introduction zone, the second phalanx is tightly wrapped with a 25-mm wide strip of non-treated or treated test textile, and the third phalanx again presents a normal finger surface. In this assay, non-contact spatial repellency would be displayed by ticks approaching but not making physical contact with the treated textile, whereas contact irritancy would be displayed by ticks moving onto the treated textile but then becoming visibly agitated and retreating to the normal finger surface below the treated textile or dislodging from the textile and tumbling off the finger completely. However, initial trials with non-treated cotton textile (JoAnn Fabric #1491315) revealed that, despite their strong natural tendency to move upwards along a vertical surface when seeking a host (Dietrich et al., 2006), ticks were reluctant to leave the skin surface used as the introduction zone and move upward onto the test textile. Out of 50 I. scapularis nymphs (OSU) that climbed onto the tip of the finger in this assay, only 12 (24%) moved upward onto the non-treated test textile within a 5-min observation period and only 1 tick crossed the textile to reach the skin surface above. We therefore concluded that this finger assay did not generate sufficiently strong directed movement to allow us to distinguish non-contact spatial repellency from contact irritancy.
A second vertical assay was designed where ticks were introduced onto textile rather than skin to facilitate immediate and upward directed movement. This assay consisted of a playing card (64 mm wide by 89 mm tall) onto which was sewn: (i) a solid piece of non-treated white cotton textile covering the entire card; and (ii) an additional 13-mm horizontal strip of non-treated or treated test textile, sewn on top of the non-treated background textile, with the upper end of the strip reaching the top of the card (Fig. 2A). The assay was performed with the textile-covered card positioned at a 45° angle. To provide an additional stimulus for upward movement, a finger was held along the top of the card after the ticks were introduced just below the treated strip. The same individual conducted all assay replicates for results presented here. Groups of 5 nymphal ticks (CT-15) per replicate were challenged, over a 5-min period, to approach and attempt to cross horizontal test textile strips in order to reach the finger located at the top of the card. Test strips were made from non-treated textile, permethrin-treated textile, or previously non-treated textile sprayed to saturation with 25% DEET from a pressurized spray can (Off! Deep Woods Insect Repellent, S.C. Johnson & Sons, Inc., Racine, WI) and then allowed to air dry for 15–30 min. DEET was chosen as a positive control for non-contact spatial repellency based on its well documented history as an effective non-contact spatial repellent for ticks (Dautel et al., 1999; Carroll et al., 2005; Bissinger and Roe, 2010; Dolan and Panella, 2011).
Fig. 2.

(A) Vertical (45° angle) bioassay where ticks are challenged to approach and cross a permethrin-treated textile strip to reach a human finger stimulus. (B) Horizontal choice bioassay where ticks are challenged to approach and cross non-treated versus permethrin-treated textile surfaces in order to reach a human finger stimulus.
In this particular assay, non-contact spatial repellency would be displayed by ticks approaching but not making physical contact with the treated textile strip. Strong contact irritancy would be displayed by ticks moving onto the treated textile strip but then becoming agitated (hot-foot effect) and retreating to the non-treated surface below the strip, often by actively flipping over and tumbling downward. Normal behavior or weak contact irritancy would be displayed by ticks moving onto and crossing a non-treated or treated textile strip to reach the finger stimulus above the strip.
Finally, we modified a horizontal choice bioassay described previously by Lane and Anderson (1984) by introducing a human stimulus to induce directed tick movement (Fig. 2B). The assay was conducted within a plastic petri dish (100 mm in diameter) divided into two equally sized halves to test 3 different combinations of textile surfaces: (i) non-treated textile and permethrin-treated textile; (ii) non-treated textile and DEET-treated textile; and (iii) permethrin-treated textile and DEET-treated textile. Ticks were introduced onto a 15 × 15-mm square piece of non-treated textile (introduction zone) located in the middle of the petri dish with the lateral edges extending 7.5 mm onto the two test textiles. To induce directed movement away from the introduction zone and across non-treated or treated textile, human fingers were introduced 10 mm distant from each of the introduction zone’s lateral edges with the tip of each finger resting on the respective test textile. The same individual conducted all assay replicates for results presented here. Groups of 5 nymphal ticks (OSU) per replicate were challenged, over a 5-min period, to move from the introduction zone and cross one of the two textile test surfaces in order to reach the finger stimulus. Locations of ticks within the petri-dish were recorded at 1-min intervals based on whether they remained in the introduction zone, moved onto one of the test textiles, or ascended onto a finger-tip.
2.4. Contact irritancy bioassay with ticks placed upon vertically oriented permethrin-treated textile
We developed a novel contact irritancy bioassay to evaluate the behavior of ticks when they are introduced onto a permethrin-treated textile, such as when a tick transfers from a host-seeking substrate onto a treated sock, pant leg or shirt. This assay employed a playing card (64 mm wide by 89 cm tall) onto which was sewn a solid piece of non-treated textile (same material as described in Section 2.3) or permethrin-treated textile (Fig. 3A). Trials were conducted with the assay card held at different (45 or 90°) vertical angles. Groups of 5 nymphal ticks (laboratory-reared CT-15 or field-collected CT-field) were introduced onto the center of the textile-covered card and the number of ticks remaining on the card were recorded at 1-min intervals over a 5-min period. In this particular assay, strong contact irritancy would manifest as nymphal ticks becoming visibly agitated and actively dislodging from treated textile by flipping over and tumbling downward along the textile-covered card until they fall off the bottom of the card. Ticks placed on non-treated textile should not show signs of agitation, and if they fall off the assay card it should result from walking normally across its edges (bottom, sides, or top) rather than by the distinct tumbling downward motion typical of contact with a permethrin-treated textile. We also scored the vigor of the nymphal ticks (as described in Section 2.2) at 1 and 24 h after contact with non-treated or treated textiles. Field-collected nymphs were held at optimal conditions in the laboratory for ≥2 wk before being used in the assay.
Fig. 3.
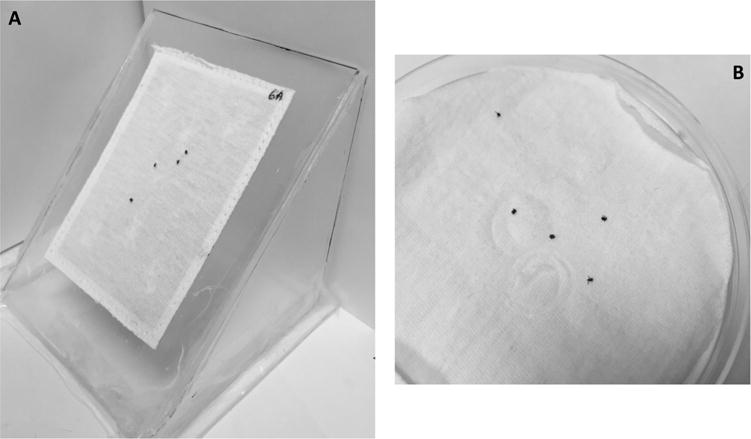
(A) Vertical (45° angle) bioassay to evaluate contact irritancy when ticks are placed upon per-methrin-treated textile. (B) Horizontal toxicity bioassay where ticks are held in continuous contact with permethrin-treated textile.
2.5. Toxicity bioassay with timed exposure of ticks to permethrin-treated textile
Our toxicity bioassay was developed to determine the impact of timed contact with permethrin-treated textile on the ability of nymphal ticks to move and behave normally at selected time points post-exposure. We focused on brief contact times (seconds to a few minutes) to mimic realistic contact with vertically oriented permethrin-treated clothing articles worn during outdoor activities. Preliminary trials indicated that direct contact with a permethrin-treated test textile for 120 s was sufficient to render a majority of laboratory-reared nymphal ticks incapable of normal movement at 1 h after exposure (data not shown). Non-treated filter paper or permethrin-treated textile were cut into circles (100 mm in diameter) and placed within a horizontal plastic petri dish arena (Fig. 3B). Groups of 10 nymphal ticks (laboratory-reared CT-15 or field-collected CT-field) were introduced into the center of the arena and kept in continuous contact with the test surface for a pre-determined period of time, ranging from 10 to 120 s for permethrin-treated textile and 120 s for the non-treated filter paper control. Filter paper was used as the negative control exposure surface for laboratory-reared nymphs because we found it to be much easier to transfer ticks displaying normal movement from filter paper than from non-treated textiles where ticks readily cling to cloth fibers requiring them to be forcibly dislodged. Trials with small numbers of field-collected nymphs used non-treated textile as the negative control exposure surface. After exposure, ticks were held at room temperature in desiccators (90–95% RH) until they were scored for vigor. Field-collected nymphs were held at optimal conditions in the laboratory for ≥2 weeks before being used in the assay. Tick vigor was scored (as described in Section 2.2) at various pre-determined time points after contact with a non-treated surface or treated textile.
2.6. Data analysis
Due to the exploratory nature of bioassay development, a majority of the data presented here are descriptive. Statistical analyses are restricted to specific assay results where sample sizes were sufficient to allow for such data treatment. Statistical tests used, indicated in the text and tables, included Fisher’s Exact Test to compare proportions of ticks displaying specific behaviors or levels of vigor across types of text textiles, exposure times, or time periods elapsed after exposure to test textiles, and likelihood ratio test based on the expectation of equal numbers of ticks moving onto each test textile in the two-choice assay.
3. Results
3.1. Vertical bioassay to distinguish non-contact spatial repellency from contact irritancy
Results for laboratory-reared I. scapularis nymphs (CT15) indicate that the primary action of a permethrin-treated textile against approaching nymphal ticks is contact irritancy rather than non-contact spatial repellency (Table 1). Complete avoidance of physical contact with a test textile (non-contact spatial repellency) was displayed by a higher proportion (100%) of nymphal ticks approaching a DEET-treated textile strip as compared with either a permethrin-treated textile strip (6%) or a non-treated textile strip (4%) (Fisher’s Exact 2-tailed Test; P < 0.001 in both cases). Of the nymphal ticks that made contact with permethrin-treated textile (n = 47) or non-treated textile (n = 48), a higher proportion dislodged from the permethrin-treated textile (74%) as compared with the non-treated textile (0%) (P < 0.001). The majority (88%) of the 48 ticks that walked onto the non-treated textile strip remained on the fabric for the entire 5-min observation period whereas only a small proportion (12%) crossed the strip to reach the finger stimulus above. In contrast, none of the 47 nymphs that walked onto the permethrin-treated textile strip remained on it over the entire 5-min observation period; they either dislodged from the strip (74%) or crossed it to reach the finger stimulus above (26%).
Table 1.
Results of a vertical assay challenging laboratory-reared I. scapularis nymphs (CT15) to approach and cross a 13-mm wide test textile strip (non-treated, permethrin-impregnated, or sprayed with DEET) to reach a human finger stimulus positioned directly above the textile strip.
| Treatment of test textile strip | Total no. nymphs examined | Outcome for 5-min observation period
|
|||
|---|---|---|---|---|---|
| No. (%) nymphs remaining below the test textile strip for the entire 5-min period without making physical contact (non-contact spatial repellency) | No. (%) nymphs walking onto the test textile strip but then becoming visibly agitated and retreating or tumbling off without crossing the test strip (strong contact irritancy) | No. (%) nymphs walking onto the test textile strip and remaining there without signs of agitation (normal behavior) | No. (%) nymphs walking onto and crossing the test textile strip to reach the finger stimulus above (normal behavior or weak contact irritancy) | ||
| DEET | 50 | 50 (100) | 0 (0) | 0 (0) | 0 (0) |
| Permethrin | 50 | 3 (6) | 35 (70) | 0 (0) | 12 (24) |
| Non-treated | 50 | 2 (4) | 0 (0) | 42 (84) | 6 (12) |
3.2. Horizontal choice bioassay to distinguish non-contact spatial repellency from contact irritancy
Laboratory-reared I. scapularis nymphs (OSU) given a two-way choice of moving onto non-treated textile versus permethrin-treated textile or DEET-treated textile in order to reach a human finger overwhelmingly (72–94%) chose to move onto the non-treated textile rather than either type of treated textile (6–28%) (Likelihood ratio test based on the expectation of equal numbers of ticks moving onto either test textile; P < 0.005 in both cases) (Table 2). In the case of nymphal ticks given the choice of moving onto permethrin-treated textile versus DEET-treated textile in order to reach a human finger, the vast majority (88%) of ticks chose to move onto permethrin-treated textile (P < 0.001). Albeit the differences were not statistically significant, the overall probability of a tick having walked onto a test textile to also reach the finger stimulus tended to be higher for non-treated textile (39%, n = 80 ticks) and permethrin-treated textile (35%, n = 55 ticks) than for DEET-treated textile (11%, n = 9 ticks).
Table 2.
Results of a horizontal two-choice assay with laboratory-reared I. scapularis nymphs (OSU) challenged over a 5-min period to cross non-treated, permethrin-treated, and DEET-treated textile surfaces in order to reach a human finger stimulus.
| Two-choice test textile combination | Location of ticks that were outside the Introduction Zone (IZ) at the end of the 5-min test period
|
Likelihood of ticks moving onto a test textile to reach the human finger stimulus
|
|||||
|---|---|---|---|---|---|---|---|
| No. ticks located outside IZ | No. (%) on side with non-treated textilea | No. (%) on side with permethrin-treated textilea | No. (%) on side with DEET-treated textilea | No. (%) on side with non-treated textile that reached fingerb | No. (%) on side with permethrin-treated textile that reached fingerc | No. (%) on side with DEET-treated textile that reached fingerd | |
| Non-treated textile versus permethrin-treated textile | 47 | 34 (72) | 13 (28) | N/A | 13 (38) | 4 (31) | N/A |
| Non-treated textile versus DEET-treated textile | 49 | 46 (94) | N/A | 3 (7) | 18 (39) | N/A | 1 (33) |
| DEET-treated textile versus permethrin-treated textile | 48 | N/A | 42 (88) | 6 (12) | N/A | 15 (36) | 0 (0) |
Number of ticks located outside the IZ was used as the denominator for percentage calculations.
Number of ticks located on the side with non-treated textile was used as the denominator for percentage calculations.
Number of ticks located on the side with permethrin-treated textile was used as the denominator for percentage calculations.
Number of ticks located on the side with DEET-treated textile was used as the denominator for percentage calculations.
3.3. Contact irritancy bioassay with permethrin-treated textile: laboratory-reared ticks
Results for laboratory-reared I. scapularis nymphs (CT15) introduced onto non-treated or permethrin-treated textile in 45 and 90° angle vertical assays showed nymphal ticks to be significantly more likely to remain in contact with a non-treated textile as compared with a permethrin-treated textile at time points ranging from 1 to 5 min post-introduction (Table 3, Fig. 4). Five min after being introduced, 66% of ticks remained on non-treated textile positioned at 45° angle whereas 12% of ticks remained on permethrin-treated textile positioned at the same angle. For textiles positioned at 90° angle, 68% of ticks remained on the non-treated textile after 5 min as compared with 2% of ticks for the permethrin-treated textile. Ticks introduced onto a vertical permethrin-treated textile displayed distinct contact irritancy, resulting in ticks actively flipping over and tumbling downward until they reached the bottom of the assay card and then fell of the test textile. As illustrated in Fig. 4, this evasive downward tumbling action led to more rapid dislodgement of nymphal ticks from the treated textile held at a 90° angle as compared with a 45° angle.
Table 3.
Results of a contact irritancy assay challenging field-collected (CT-field) or laboratory-reared (CT15) I. scapularis nymphs introduced onto vertically oriented non-treated or permethrin-treated textile to remain in contact with the test textile.
| Observation time point and tick vigor | 45 degree angle vertical assay
|
90 degree angle vertical assay
|
||||
|---|---|---|---|---|---|---|
| CT-field
|
CT15
|
CT15
|
||||
| Non-treated textile | Permethrin-treated textile | Non-treated textile | Permethrin-treated textile | Non-treated textile | Permethrin-treated textile | |
| No. (%) ticks remaining on test textile over the 5-min observation perioda | ||||||
| 0 min (introduction) | 10 (100) | 10 (100) | 50 (100) | 50 (100) | 50 (100) | 50 (100) |
| 1 min | 10 (100) | 10 (100)NS | 49 (98) | 46 (92)NS | 40 (80) | 5 (10)*** |
| 2 min | 10 (100) | 10 (100)NS | 48 (96) | 35 (70)*** | 36 (72) | 4 (8)*** |
| 3 min | 10 (100) | 10 (100)NS | 44 (88) | 16 (32)*** | 36 (72) | 1 (2)*** |
| 4 min | 10 (100) | 10 (100)NS | 37 (74) | 8 (16)*** | 36 (72) | 1 (2)*** |
| 5 min | 10 (100) | 10 (100)NS | 33 (66) | 6 (12)*** | 34 (68) | 1 (2)*** |
| Tick vigor 1 h after exposureb | ||||||
| No. (%) completely motionless | 0 (0) | 5 (50) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No. (%) displaying only some movement of the legsc | 0 (0) | 2 (20) | 0 (0) | 46 (92) | 0 (0) | 27 (54) |
| No. (%) displaying only weak/ | 0 (0) | 2 (20) | 0 (0) | 4 (8) | 0 (0) | 7 (14) |
| uncoordinated movementd | ||||||
| No. (%) displaying normal movemente | 10 (100) | 1 (10)*** | 50 (100) | 0 (0)*** | 50 (100) | 16 (32)*** |
| Tick vigor 24 h after exposureb | ||||||
| No. (%) completely motionless | 0 (0) | 8 (80) | 4 (8) | 30 (60) | 3 (6) | 16 (32) |
| No. (%) displaying only some movement of the legsc | 0 (0) | 0 (0) | 0 (0) | 11 (22) | 0 (0) | 7 (14) |
| No. (%) displaying only weak/uncoordinated movementd | 0 (0) | 1 (10) | 0 (0) | 2 (4) | 0 (0) | 3 (6) |
| No. (%) displaying normal movemente | 10 (100) | 1 (10)*** | 46 (92) | 7 (14)*** | 47 (94) | 24 (48)*** |
Statistical comparisons (Fisher’s Exact 1-Tailed Test) of the numbers of nymphs still in contact with permethrin-treated textile versus non-treated textile for the same assay card angle, observation time point, and tick group; adjusted for multiple (n = 5) observation time points using Bonferroni correction: P > 0.01NS; P ≤ 0.01*; P ≤ 0.005**; P ≤ 0.001***.
Statistical comparisons (Fisher’s Exact 1-Tailed Test) of the numbers of nymphs displaying normal movement after exposure to permethrin-treated textile versus non-treated textile for the same assay card angle, time point after exposure, and tick group; adjusted for multiple (n = 2) observation time points using Bonferroni correction: P > 0.02NS; P ≤ 0.01*; P ≤ 0.005**; P ≤ 0.001***.
Ticks capable of some movement of the legs but not able to right themselves or walk.
Ticks capable of righting themselves but not able to move in a coordinated way or orient toward a stimulus.
Ticks displaying normal movement and response to stimulus.
Fig. 4.
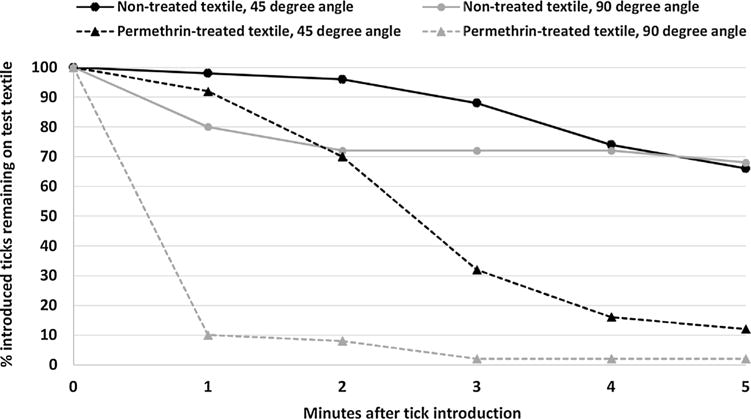
Percentages of laboratory-reared I. scapularis nymphs (CT 15) remaining on test textiles over a 5-min period in vertical contact irritancy assays with non-treated and permethrin-treated textiles positioned at 45 or 90° angle.
We also scored tick vigor at 1 and 24 h after contact with either a non-treated or a permethrin-treated textile. All nymphs introduced onto non-treated textile displayed normal movement 1 h after contact and > 90% displayed normal movement after 24 h (Table 3). Regardless of the angle of the assay card, nymphal ticks contacting a permethrin-treated textile were significantly less likely than those contacting a non-treated textile to display normal movement, with the most pronounced difference seen 1 h after contact. Moreover, ticks exposed to treated textile in the 45° angle assay displayed normal movement less frequently (0%) 1 h after contact than those exposed in the 90° angle treated textile assay (32%) (Fisher’s Exact 2-tailed Test; P < 0.001).
We also noted a significant difference (P < 0.001) between the 45 and 90° angle treated textile assays 24 h after tick contact (14 and 48%, respectively, displaying normal movement), albeit less pronounced due to some previously stunned ticks having regained mobility at this post-exposure time point. These results are most likely related to the longer time nymphal ticks typically spent in contact with permethrin-treated textile in the 45° angle assay compared to the 90° angle assay.
We also document changes in tick vigor at 1 versus 24 h after contact with permethrin-treated textile. Presumably due to delayed toxicity after contact with treated textile, the proportions of ticks scored as being completely motionless increased from 1 to 24 h after contact with permethrin-treated textile held at 45° angle (P < 0.001) and 90° angle (P < 0.001).
3.4. Contact irritancy bioassay with permethrin-treated textile: field-collected ticks
Albeit based on small sample sizes and nymphal ticks of unknown age, Table 3 includes preliminary data for field-collected nymphs in the contact irritancy bioassay. In striking contrast to the laboratory-reared ticks, all field-collected ticks remained on treated textile positioned at 45° angle over the full 5-min observation period. However, similar to the laboratory-reared ticks, the vast majority (90%) of field-collected ticks failed to display normal movement 1 and 24 h after contact with the treated textile.
3.5. Toxicity bioassay: tick vigor in relation to contact time with permethrin-treated textile and time elapsed from end of contact
Several notable descriptive results emerged from the toxicity bioassay where laboratory-reared I. scapularis nymphs (CT15) were held in continuous contact with permethrin-treated textile for 10, 30, 60, or 120 s and then scored for vigor at time points ranging from directly after exposure to 7 d later (Table 4, Fig. 5). All negative control ticks held in contact with filter paper for 120 s displayed normal movement for all examined post-exposure time points, and 29 of 30 nymphs ascended a finger when given the opportunity at the final time point 7 d post-exposure (Table 4).
Table 4.
Results of a toxicity assay where laboratory-reared I. scapularis nymphs (CT15) were held in continuous contact with a permethrin-treated textile for durations of time from 10 to 120 s and assessed for vigor at various time points from 0 min to 7 after exposure.
| Vigor and behavior of ticks | Time of contact with permethrin-treated textile (n = 30 nymphs per contact duration) | Time of contact with non-treated control surface (n = 30 nymphs) | |||
|---|---|---|---|---|---|
| 10 s | 30 s | 60 s | 120 s | 120 s | |
| 0 min after exposure | |||||
| No. (%) completely motionless | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No. (%) displaying only some movement of the legsa | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No. (%) displaying only weak/uncoordinated movementb | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No. (%) displaying normal movementc | 30 (100) | 30 (100) | 30 (100) | 30 (100) | 30 (100) |
| % displaying normal movement that ascended onto a fingerd | 7 | 0 | 0 | 0 | – |
| 15 min after exposure | |||||
| No. (%) completely motionless | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No. (%) displaying only some movement of the legsa | 16 (53) | 22 (73) | 25 (83) | 29 (97) | 0 (0) |
| No. (%) displaying only weak/uncoordinated movementb | 5 (17) | 3 (10) | 1 (3) | 1 (3) | 0 (0) |
| No. (%) displaying normal movementc | 9 (30) | 5 (17) | 4 (13) | 0 (0) | 30 (100) |
| % displaying normal movement that ascended onto a fingerd | 0 | 0 | 0 | – | – |
| 30 min after exposure | |||||
| No. (%) completely motionless | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No. (%) displaying only some movement of the legsa | 19 (63) | 24 (80) | 27 (90) | 30 (100) | 0 (0) |
| No. (%) displaying only weak/uncoordinated movementb | 4 (13) | 2 (7) | 2 (7) | 0 (0) | 0 (0) |
| No. (%) displaying normal movementc | 7 (23) | 4 (13) | 1 (3) | 0 (0) | 30 (100) |
| % displaying normal movement that ascended onto a fingerd | 0 | 0 | 0 | – | – |
| 1 h after exposure | |||||
| No. (%) completely motionless | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No. (%) displaying only some movement of the legsa | 25 (83) | 27 (90) | 27 (90) | 30 (100) | 0 (0) |
| No. (%) displaying only weak/uncoordinated movementb | 4 (13) | 3 (10) | 2 (7) | 0 (0) | 0 (0) |
| No. (%) displaying normal movementc | 1 (3) | 0 (0) | 1 (3) | 0 (0) | 30 (100) |
| % displaying normal movement that ascended onto a fingerd | 100 | – | 100 | – | – |
| 2 h after exposure | |||||
| No. (%) completely motionless | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No. (%) displaying only some movement of the legsa | 26 (87) | 26 (87) | 26 (87) | 29 (97) | 0 (0) |
| No. (%) displaying only weak/uncoordinated movementb | 1 (3) | 2 (7) | 3 (10) | 1 (3) | 0 (0) |
| No. (%) displaying normal movementc | 3 (10) | 2 (7) | 1 (3) | 0 (0) | 30 (100) |
| % displaying normal movement that ascended onto a fingerd | 67 | 50 | 0 | – | – |
| 4 h after exposure | |||||
| No. (%) completely motionless | 0 (0) | 0 (0) | 1 (3) | 5 (17) | 0 (0) |
| No. (%) displaying only some movement of the legsa | 28 (93) | 27 (90) | 28 (93) | 24 (80) | 0 (0) |
| No. (%) displaying only weak/uncoordinated movementb | 2 (7) | 2 (7) | 0 (0) | 1 (3) | 0 (0) |
| No. (%) displaying normal movementc | 0 (0) | 1 (3) | 1 (3) | 0 (0) | 30 (100) |
| % displaying normal movement that ascended onto a fingerd | – | 0 | 0 | – | – |
| 8 h after exposure | |||||
| No. (%) completely motionless | 9 (30) | 5 (17) | 9 (30) | 5 (17) | 0 (0) |
| No. (%) displaying only some movement of the legsa | 13 (43) | 23 (77) | 19 (63) | 25 (83) | 0 (0) |
| No. (%) displaying only weak/uncoordinated movementb | 8 (27) | 1 (3) | 1 (3) | 0 (0) | 0 (0) |
| No. (%) displaying normal movementc | 0 (0) | 1 (3) | 1 (3) | 0 (0) | 30 (100) |
| % displaying normal movement that ascended onto a fingerd | – | 0 | 0 | – | – |
| 1 d after exposure | |||||
| No. (%) completely motionless | 15 (50) | 15 (50) | 20 (67) | 19 (63) | 0 (0) |
| No. (%) displaying only some movement of the legsa | 6 (20) | 9 (30) | 4 (13) | 10 (33) | 0 (0) |
| No. (%) displaying only weak/uncoordinated movementb | 1 (3) | 3 (10) | 3 (10) | 1 (3) | 0 (0) |
| No. (%) displaying normal movementc | 8 (27) | 3 (10) | 3 (10) | 0 (0) | 30 (100) |
| % displaying normal movement that ascended onto a fingerd | 75 | 67 | 100 | – | – |
| 2 d after exposure | |||||
| No. (%) completely motionless | 16 (53) | 17 (57) | 19 (63) | 26 (87) | 0 (0) |
| No. (%) displaying only some movement of the legsa | 5 (17) | 8 (27) | 5 (17) | 4 (13) | 0 (0) |
| No. (%) displaying only weak/uncoordinated movementb | 2 (7) | 2 (7) | 3 (10) | 0 (0) | 0 (0) |
| No. (%) displaying normal movementc | 7 (23) | 3 (10) | 3 (10) | 0 (0) | 30 (100) |
| % displaying normal movement that ascended onto a fingerd | 100 | 100 | 100 | – | – |
| 3 d after exposure | |||||
| No. (%) completely motionless | 18 (60) | 18 (60) | 19 (63) | 26 (87) | 0 (0) |
| No. (%) displaying only some movement of the legsa | 3 (10) | 4 (13) | 1 (3) | 2 (7) | 0 (0) |
| No. (%) displaying only weak/uncoordinated movementb | 1 (3) | 2 (7) | 6 (20) | 2 (7) | 0 (0) |
| No. (%) displaying normal movementc | 8 (27) | 6 (20) | 4 (13) | 0 (0) | 30 (100) |
| % displaying normal movement that ascended onto a fingerd | 62 | 83 | 100 | – | – |
| 4 d after exposure | |||||
| No. (%) completely motionless | 18 (60) | 19 (63) | 18 (60) | 26 (87) | 0 (0) |
| No. (%) displaying only some movement of the legsa | 0 (0) | 2 (7) | 3 (10) | 2 (7) | 0 (0) |
| No. (%) displaying only weak/uncoordinated movementb | 2 (7) | 1 (3) | 2 (7) | 1 (3) | 0 (0) |
| No. (%) displaying normal movementc | 10 (33) | 8 (27) | 7 (23) | 1 (3) | 30 (100) |
| % displaying normal movement that ascended onto a fingerd | 100 | 100 | 100 | 0 | – |
| 7 d after exposure | |||||
| No. (%) completely motionless | 18 (60) | 20 (67) | 20 (67) | 27 (90) | 0 (0) |
| No. (%) displaying only some movement of the legsa | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No. (%) displaying only weak/uncoordinated movementb | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No. (%) displaying normal movementc | 12 (40) | 10 (33) | 10 (33) | 3 (10) | 30 (100) |
| % displaying normal movement that ascended onto a fingerd | 100 | 100 | 100 | 100 | 97 |
Ticks capable of some movement of the legs but not able to right themselves or walk.
Ticks capable of righting themselves but not able to move in a coordinated way or orient toward a stimulus.
Ticks displaying normal movement and response to stimulus.
Ticks displaying normal movement that ascended onto a human finger when provided the opportunity.
Fig. 5.
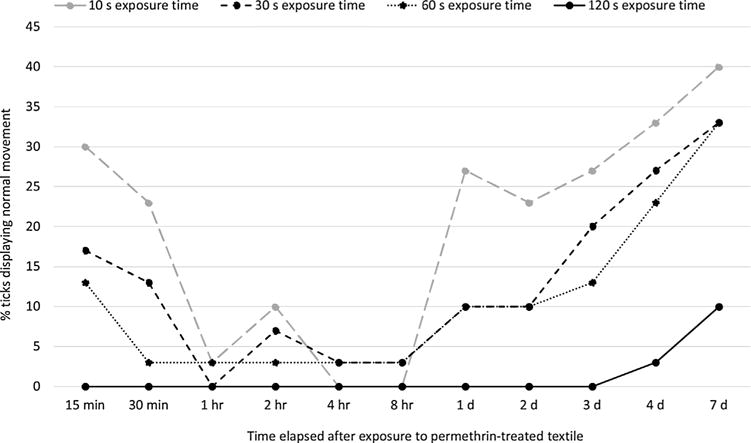
Percentages of laboratory-reared I. scapularis nymphs (CT 15) displaying normal movement at various points in time from 15 min to 7 d after exposure to permethrin-treated textile for durations ranging from 10 to 120 s. Control ticks held in contact with non-treated filter paper for 120 s uniformly displayed normal movement across all post-exposure observation time points.
Directly after contact with permethrin-treated textile, regardless of exposure time from 10 to 120 s, all nymphs (n = 120) displayed normal movement but none ascended onto a finger. At 15 and 30 min after contact, ticks displayed variable vigor with all ticks still capable of some movement but most ticks (53–100% across exposure times) unable to right themselves or walk. At these observation time points, the proportions of ticks capable only of some movement of the legs was higher after 120 s of exposure as compared with 10 s of exposure to treated textile (Fisher’s Exact 2-tailed Test; P < 0.001 for either 15 or 30 min after contact). Conversely, ticks displayed normal movement more commonly after 10 s exposure as compared with 120 s exposure (P ≤ 0.01 for either 15 or 30 min after contact), but none of the ticks displaying normal movement ascended onto a finger (Fig. 6).
Fig. 6.
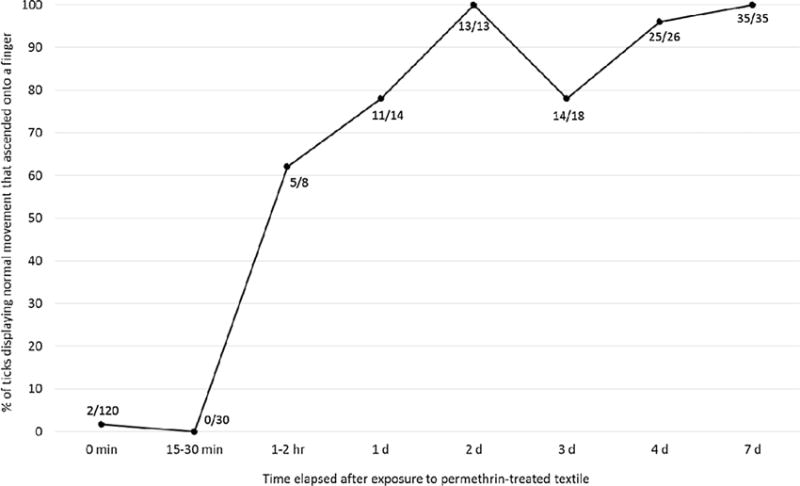
Percentages of laboratory-reared I. scapularis nymphs (CT 15) displaying normal movement that ascended onto a human finger at various points in time from 0 min to 7 d after exposure to permethrin-treated textile.
At 1–2 h after contact, the vast majority of ticks (> 80% regardless of exposure time) were capable of some movement of the legs but not able to right themselves or walk. However, in striking contrast to the 0–30 min time points, ∼60% of ticks (n = 8) that displayed normal movement at 1–2 h after exposure now ascended onto a finger. At 8 h after contact, most ticks (43–83% across exposure times) still were capable of some movement of the legs, but an increasing number (17–30% across exposure times) were now completely motionless. Very few (n = 2) ticks displayed normal movement at this post-exposure time point.
One day after contact, most ticks (50–67% across exposure times) were completely motionless. However, an increasing proportion (27%) of ticks from the group with the shortest (10 s) exposure time now displayed normal movement and 6 of the 8 active ticks ascended onto a finger. Significantly fewer (0%) ticks from the group with the 120 s exposure time, as compared with the 10 s exposure time, displayed normal movement 1 d post-exposure (P = 0.005). Results for tick vigor 2–3 d post-exposure were similar to those seen 1 d post-exposure, with the majority of ticks completely motionless (60–87% across exposure times) and normal movement seen only for ticks with exposure times ≤60 s.
Seven days after exposure to the permethrin-treated textile, all ticks either displayed normal movement, including willingness to ascend onto a finger, or were completely motionless. Ticks exposed to treated textile for 120 s were less likely (10%) to display normal movement 7 d later than those exposed for 10 s (40%) (P = 0.02).
3.6. Toxicity bioassay: vigor of laboratory-reared ticks of different generations and field-collected ticks at 1 and 24 h after 1-min contact with permethrin-treated textile
Continuous contact for 1 min with permethrin-treated textile resulted in uniformly low proportions (0–2%) of laboratory-reared nymphs displaying normal movement 1 h post-exposure, regardless of whether the ticks were of the first, second, or third laboratory generation (Table 5). Albeit based on a very small sample size, field-collected nymphs appeared to be more likely (30%) to display normal movement at the 1 h post-exposure time point. However, only 1 of the 3 field-collected nymphs displaying normal movement ascended onto a finger. At 24 h post-exposure, the proportion of nymphs displaying normal movement was similar (15–20%) for field-collected nymphs (CT-field) and first generation laboratory nymphs from the same general area (CT-15).
Table 5.
Results of a toxicity assay where I. scapularis nymphs of variable origin were held in continuous contact with a non-treated filter paper or textile surface or a permethrin-treated textile for 1 min and subsequently assessed for vigor at 1 and 24 h after exposure.
| Tick source and vigor | 1 h after exposure
|
24 h after exposure
|
||
|---|---|---|---|---|
| Non-treated control surface | Permethrin-treated textile | Non-treated control surface | Permethrin-treated textile | |
| Field-collected nymphs (CT-field) | ||||
| No. examined | 10 | 10 | 10 | 10 |
| No. (%) displaying any movement | 10 (100) | 3 (30) | 10 (100) | 2 (20) |
| No. (%) displaying normal movement and response to stimulusa | 10 (100) | 3 (30) | 10 (100) | 2 (20) |
| 1st laboratory generation nymphs (CT15)b | ||||
| No. examined | 40 | 40 | 40 | 40 |
| No. (%) displaying any movement | 40 (100) | 38 (95) | 40 (100) | 12 (30) |
| No. (%) displaying normal movement and response to stimulusa | 40 (100) | 1 (2) | 40 (100) | 6 (15) |
| 1st laboratory generation nymphs (MN15) | ||||
| No. examined | 10 | 10 | 20 | 20 |
| No. (%) displaying any movement | 10 (100) | 1 (10) | 20 (100) | 4 (20) |
| No. (%) displaying normal movement and response to stimulusa | 10 (100) | 0 (0) | 20 (100) | 1 (5) |
| 2nd laboratory generation nymphs (CT14) | ||||
| No. examined | 9 | 10 | 9 | 10 |
| No. (%) displaying any movement | 9 (100) | 2 (20) | 9 (100) | 2 (20) |
| No. (%) displaying normal movement and response to stimulusa | 9 (100) | 0 (0) | 9 (100) | 0 (0) |
| 3rd laboratory generation nymphs (CT13) | ||||
| No. examined | 10 | 10 | 10 | 10 |
| No. (%) displaying any movement | 10 (100) | 8 (80) | 10 (100) | 2 (20) |
| No. (%) displaying normal movement and response to stimulusa | 10 (100) | 0 (0) | 10 (100) | 1 (10) |
Excluding ticks capable of some movement of the legs but not able to right themselves or walk and ticks capable of righting themselves but not able to move in a coordinated way or orient toward a stimulus.
Including data from Table 4.
4. Discussion
To improve our knowledge of how permethrin-treated textiles impact the vigor and behavior of nymphal I. scapularis ticks, we developed a suite of new bioassays and a refined classification scheme for tick vigor after exposure to a permethrin-treated textile. Our first objective was to examine whether permethrin-treated textiles protect against nymphal ticks through non-contact spatial repellency or contact irritancy. Ticks approaching a textile impregnated with a strong non-contact spatial repellent (DEET) very rarely made physical contact with the treated textile (Tables 1 and 2). In striking contrast, ticks readily walked onto a permethrin-treated textile. We conclude that permethrin-treated textile appears to have minimal non-contact spatial repellency for I. scapularis, which agrees with field observations on the closely related I. pacificus tick (Lane, 1989).
Our next objective was to devise an assay mimicking a scenario where a host-seeking tick makes initial contact with a vertically oriented permethrin-treated clothing article, for example a pant leg or the arm of a long-sleeved shirt. Laboratory-reared ticks became visibly agitated, displaying a hot-foot effect, and escaped contact with the permethrin-treated textile by tumbling downwards until they dislodged themselves completely from a textile-covered assay card (Table 3; Fig. 4). This finding agrees with a study showing that humans having laboratory-reared I. scapularis nymphs introduced directly onto per-methrin-treated shoes were 74 times less likely to receive a tick bite as compared with humans having ticks introduced onto non-treated shoes (Miller et al., 2011). Albeit based on a small sample size, field-collected nymphs appeared to display a weaker contact irritancy response than laboratory-reared ones, leading to more prolonged contact with the permethrin-treated textile for the field-collected ticks. However, by 1 and 24 h post-exposure very few ticks displayed normal movement, thus presenting minimal risk to bite, regardless of whether they were reared in the laboratory or collected in the field (Table 3). We speculate that ticks stunned by contact with permethrin-treated garments are prone to be dislodged from the clothing and fall off the host because they are unable to actively grip the textile fibers.
The reason for a weaker contact irritancy response in field-collected ticks is not clear. One marked difference between the laboratory-reared and field-collected I. scapularis nymphs used in our assays is that the former ticks continuously were kept under optimal temperature and humidity conditions from the egg stage to the nymphal stage, whereas the field-collected ticks were exposed to highly variable temperature and humidity conditions, including having overwintered either as fed larvae or unfed nymphs. It is conceivable that tick development under natural environmental conditions may result in anatomical or physiological manifestations − for example in the thickness or chemical composition of the exocuticle or the cuticulin or wax layers of the epicuticle − resulting in slower absorption of the permethrin through the cuticle to reach its voltage-gated sodium channel target sites compared to ticks having developed under constant and optimal laboratory conditions. A related question is how the lipophilic permethrin contacted via a treated textile specifically enters the tick’s tarsi, legs, body, and capitulum through the cuticle or via sensilla to reach the target sites. The physiological age of the laboratory-reared nymphs (4–5 mo post-molt) and the field-collected nymphs (unknown) used in our assays also very likely differed, which may have impacted their susceptibility to permethrin. Previous studies on the closely related I. ricinus and Ixodes persulcatus tick species indicated positive associations between physiological age and susceptibility to various pesticides (Rupes et al., 1972a, 1972b, 1977; Uspensky and Repkina, 1974; Uspensky and Ioffe-Uspensky 2006). Moreover, positive associations between physiological age and susceptibility specifically to permethrin have been documented for hard ticks of other genera (Heller-Haupt and Varma, 1982). Finally, permethrin resistance also could be a factor if field-collected nymphs come from localized areas experiencing high enough pyrethroid pressure to conceivably result in resistance development.
Our final objective was to assess tick vigor and behavior at various time points after exposure to permethrin-treated textile (Table 4; Figs. 5 and 6). Immediately following a 10–120 s exposure to permethrin-treated textile, I. scapularis nymphs displayed normal movement and oriented toward a human finger stimulus. However, they did not ascend onto the finger when given the opportunity. We speculate that this failure at even attempting to locate a feeding site and bite resulted from the ticks already being impacted by the permethrin. As time progressed in the first hour after exposure, an increasing proportion of ticks became incapable of normal movement and instead were limited to weak and uncoordinated movement or only some movement of the legs, indicative of permethrin reaching its target sites and inhibiting movement. At 1 h after exposure, < 5% of ticks displayed normal movement. This pattern persisted through 8 h after exposure, at which point in time an increasing number of exposed ticks were completely motionless. One day (24 h) after exposure to the treated textile, a majority of ticks were completely motionless. Similar slowly developing toxic effects, termed “slow-death syndrome”, were previously described for I. persulcatus ticks topically exposed to the pesticides DDT and fenthin (Uspensky and Ioffe-Uspensky, 2006). However, ticks having recovered normal movement 1 d after exposure in our study most often ascended onto a finger when given the opportunity (and presumably also were capable of biting). Another notable finding is that the percent of ticks displaying normal movement continued to increase from 1 to 7 d after exposure as ticks continued to recover from sub-lethal permethrin exposures (Fig. 5). Similar recovery over time from sub-lethal pesticide exposure was described previously for I. persulcatus ticks (Uspensky and Ioffe-Uspensky, 2006).
Ticks exposed to permethrin-treated textile for 120 s were more impacted than ticks exposed for 10 s. Recovery to normal movement did not occur until 4–7 d after exposure for ticks with a 120 s exposure, whereas ticks with a 10 s exposure began to recover and display normal movement 1 d after exposure. In a real-life scenario, prolonged periods of time where ticks having fallen off a human host after contact with permethrin-treated textile are unable to move will undoubtedly increase the risk of mortality due to desiccation or predation. Similar studies on the vigor and behavior of field-collected ticks at various points in time after exposure to permethrin-treated textile would be of interest.
In addition to the source of ticks and their physiological age, some other issues should be discussed in the context of limitations of the bioassay results described here. Although our refined classification of tick vigor promotes standardized, more easily understandable determination of the physical status of a tick after exposure to a permethrin-treated textile, there is still potential ambiguity in the distinction between a tick that can right itself but is unable to move its legs in a coordinated way versus a tick displaying normal movement. We solved this by introducing a human finger to provide a strong stimulus for directed movement. Allowing ticks to ascend onto the tip of the finger provides further evidence of normal host-seeking behavior. In addition to potential differences in attractiveness of fingers belonging to different individuals, this could be problematic when using field-collected ticks with a high probability of being infected with human pathogens rather than non-infected laboratory colony ticks.
Another issue to consider is how our bioassays relate to a real-life scenario where host-seeking ticks encounter humans wearing perme-thrin-treated clothing on some part, or parts, of their body. The contact irritancy and toxicity assays provide a reasonable approximation of what a tick experiences if it makes initial contact with permethrin-treated clothing rather than bare skin. A scenario more difficult to address in a bioassay is when a tick makes initial contact with bare skin and subsequently approaches loose-fitting summer-weight permethrin-treated garments such as shorts or a T-shirt. In this case, the tick may walk underneath the treated textile and be contacted primarily from the dorsal side as the person moves and the clothing comes in and out of contact with the tick and the person’s skin. To some extent, this could be addressed in a variation of the toxicity assay where treated textile is placed in contact with the dorsal side of the tick for specified periods of time.
A final issue is that we cannot be certain that a previously permethrin-exposed tick that ascends onto a finger ultimately is capable of biting and feeding. The study by Miller et al. (2011) using human volunteers allowing themselves to receive tick bites showed that the majority of attached nymphs died within hours of their attachment on individuals wearing summer-weight permethrin-treated clothing, but it is not clear whether this resulted from delayed toxic effects from permethrin exposure before they attached or from being contacted by treated clothing after attaching. An animal model will ultimately be required to clarify whether ticks that display normal movement and ascend onto a human finger after exposure to permethrin-treated textile also are able to bite and feed.
Similar to the inadequate state of knowledge for non-contact spatial repellents (Eisen and Gray, 2016), there is much still to learn about how well realistic use of different types of permethrin-treated clothing articles protects against tick bites. Permethrin-treated military uniforms that cover most of the body were shown to reduce tick bites by > 95% in Germany (Faulde et al., 2008, 2014) and long-sleeved permethrin-treated uniforms for outdoor workers reduced tick bites by > 80% in the first year after treatment in the eastern United States (Vaughn et al., 2014). Similar prospective studies unfortunately still are lacking for summer-weight permethrin-treated clothing worn around the home in the summer, such as socks, shorts and T-shirts.
Acknowledgments
We thank Martin Williams of the Centers for Disease Control and Prevention (CDC) and Vanele Dacosta, Carina Doyle, Kimberly Lockwood, and Rebecca Warren of Western Connecticut State University for field and technical support. Dr. Jenna Bjork of the Minnesota Department of Health provided source material to start a tick colony. Work conducted at Western Connecticut State University was supported by CDC through TickNET and the Emerging Infections Program cooperative agreement (5U50CK000195-05).
Footnotes
Disclaimer
The findings and conclusions of this study are by the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- Bissinger BW, Roe RM. Tick repellents Past, present, and future. Pest Biochem Physiol. 2010;96:63–79. [Google Scholar]
- Carroll JF, Klun JA, Debboun M. Repellency of deet and SS220 applied to skin involves olfactory sensing by two species of ticks. Med Vet Entomol. 2005;19:101–106. doi: 10.1111/j.0269-283X.2005.00559.x. [DOI] [PubMed] [Google Scholar]
- Carroll JF, Cantrell CL, Klun JA, Kramer M. Repellency of two terpenoid compounds isolated from Callicarpa americana (Lamiaceae) against Ixodes scapularis and Amblyomma americanum ticks. Exp Appl Acarol. 2007;41:215–224. doi: 10.1007/s10493-007-9057-2. [DOI] [PubMed] [Google Scholar]
- Dautel H, Kahl O, Siems K, Oppenrieder M, Müller-Kuhrt L, Hilker M. A novel test system for detection of tick repellents. Entomol Exp Appl. 1999;91:431–441. [Google Scholar]
- Dietrich G, Dolan MC, Peralta-Cruz J, Schmidt J, Piesman J, Eisen RJ, Karchesy JJ. Repellent activity of fractioned compounds from Chamaecyparis nootka-tensis essential oil against nymphal Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 2006;43:957–961. doi: 10.1603/0022-2585(2006)43[957:raofcf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Panella NA. A review of arthropod repellents. In: Paluch GE, Coats JR, editors. Recent Developments in Invertebrate Repellents. American Chemical Society; Washington, D.C., USA: 2011. pp. 1–19. [Google Scholar]
- Dolan MC, Dietrich G, Panella NA, Montenieri JA, Karchesy JJ. Biocidal activity of three wood essential oils against Ixodes scapularis (Acari: Ixodidae), Xenopsylla cheopis (Siphonaptera: Pulicidae), and Aedes aegypti (Diptera: Culicidae) J Econ Entomol. 2007;100:622–625. doi: 10.1603/0022-0493(2007)100[622:baotwe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Eisen L, Gray JS. Lyme borreliosis prevention strategies: United States versus Europe. In: Braks MAH, Van Wierer SE, Takken W, Sprong H, editors. Ecology and Prevention of Lyme Borreliosis Wageningen. Academic Publishers; Wageningen, The Netherlands: 2016. pp. 429–450. [Google Scholar]
- Eisen RJ, Eisen L, Beard CB. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J Med Entomol. 2016;53:349–386. doi: 10.1093/jme/tjv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SR, Korch GW, Jr, Lawson MA. Comparative field evaluation of per-methrin and deet-treated military uniforms for personal protection against ticks (Acari) J Med Entomol. 1990;27:829–834. doi: 10.1093/jmedent/27.5.829. [DOI] [PubMed] [Google Scholar]
- Faulde M, Uedelhoven W. A new clothing impregnation method for personal protection against ticks and biting insects. Int J Med Microbiol. 2006;296(S1):225–229. doi: 10.1016/j.ijmm.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Faulde MK, Uedelhoven WM, Robbins RG. Contact toxicity and residual activity of different permethrin-based fabric impregnation methods for Aedes aegypti (Diptera: Culicidae), Ixodes ricinus (Acari: Ixodidae), and Lepisma saccharina (Thysanura: Lepismatidae) J Med Entomol. 2003;40:935–941. doi: 10.1603/0022-2585-40.6.935. [DOI] [PubMed] [Google Scholar]
- Faulde M, Scharninghausen J, Tisch M. Preventive effect of permethrin-impregnated clothing to Ixodes ricinus ticks and associated Borrelia burgdorferi s.l in Germany . Int J Med Microbiol. 2008;298(S1):321–324. [Google Scholar]
- Faulde MK, Rutenfranz M, Keth A, Hepke J, Rogge M, Görner A. Pilot study assessing the effectiveness of factory-treated long-lasting permethrin-impregnated clothing for the prevention of tick bites during occupational tick exposure in highly infested military training areas, Germany. Parasitol Res. 2014;114:671–678. doi: 10.1007/s00436-014-4232-y. [DOI] [PubMed] [Google Scholar]
- Grieco JP, Achee NL, Chareonviriyaphap T, Suwonkerd W, Chauhan K, Sardelis MR, Roberts DR. A new classification system for the actions of IRS chemicals traditionally used for malaria control. PLoS One. 2007;2:e716. doi: 10.1371/journal.pone.0000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halos L, Baneth G, Beugnet F, Bowman AS, Chomel B, Farkas R, Franc M, Guillot J, Inokuma H, Kaufman R, Jongejan F, Joachim A, Otranto D, Pfister K, Pollmeier M, Sainz A, Wall R. Defining the concept of ‘tick repellency’ in veterinary medicine. Parasitology. 2012;139:419–423. doi: 10.1017/S0031182011002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller-Haupt A, Varma MGR. The effect of age on susceptibility of two species of African ticks (Ixodidae) to synthetic pyrethroids. Trop Pest Manag. 1982;28:385–392. [Google Scholar]
- Jordan RA, Schulze TL, Dolan MC. Efficacy of plant-derived and synthetic compounds on clothing as repellents against Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 2012;49:101–106. doi: 10.1603/me10241. [DOI] [PubMed] [Google Scholar]
- Lane RS, Anderson JR. Efficacy of permethrin as a repellent and toxicant for personal protection against the Pacific Coast tick and the Pajaroello tick (Acari: Ixodidae and Argasidae) J Med Entomol. 1984;21:692–702. doi: 10.1093/jmedent/21.6.692. [DOI] [PubMed] [Google Scholar]
- Lane RS. Treatment of clothing with a permethrin spray for personal protection against the western black-legged tick, Ixodes pacificus (Acari: Ixodidae) Exp Appl Acarol. 1989;6:343–352. doi: 10.1007/BF01193304. [DOI] [PubMed] [Google Scholar]
- Maupin GO, Piesman J. Acaricide susceptibility of immature Ixodes scapularis (Acari: Ixodidae) as determined by the disposable pipette method. J Med Entomol. 1994;31:319–321. doi: 10.1093/jmedent/31.2.319. [DOI] [PubMed] [Google Scholar]
- Mead PS. Epidemiology of Lyme disease. Infect Dis Clin N Am. 2015;29:187–210. doi: 10.1016/j.idc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Miller NJ, Rainone EE, Dyer MC, González ML, Mather TN. Tick bite protection with permethrin-treated summer-weight clothing. J Med Entomol. 2011;48:327–333. doi: 10.1603/me10158. [DOI] [PubMed] [Google Scholar]
- Mount GA, Snoddy EL. Pressurized sprays of permethrin and deet on clothing for personal protection against the lone star tick and the American dog tick (Acari: Ixodidae) J Econ Entomol. 1983;76:529–531. doi: 10.1093/jee/76.3.529. [DOI] [PubMed] [Google Scholar]
- Panella NA, Dolan MC, Karchesy JJ, Xiong Y, Peralta-Cruz J, Khasawneh M, Montenieri JA, Maupin GO. Use of novel compounds for pest control: insecticidal and acaricidal activity of essential oil components from heartwood of Alaska yellow cedar. J Med Entomol. 2005;42:352–358. doi: 10.1093/jmedent/42.3.352. [DOI] [PubMed] [Google Scholar]
- Richards SL, Balanay JAG, Harris JW. Effectiveness of permethrin-treated clothing to prevent tick exposure in foresters in the central Appalachian region of the USA. Int J Environ Health Res. 2015;25:453–462. doi: 10.1080/09603123.2014.963033. [DOI] [PubMed] [Google Scholar]
- Rupes V, Chmela J, Ledvinka J, Novak K, Těmín K, Hozák A, Sládková D. The age of Ixodes ricinus ticks as a factor of their susceptibility to pp-DDT, imidan and carbaryl. Folia Parasitol. 1972a;19:217–226. [PubMed] [Google Scholar]
- Rupes V, Chmela J, Ledvinka J, Sládková D. Effects of age on the susceptibility of larvae and nymphs of Ixodes ricinus (L.) to insecticides. Folia Parasitol. 1972b;19:379–382. [PubMed] [Google Scholar]
- Rupes V, Chmela J, Ledvinka J, Privora M. Effectiveness of fenitrothion for area control of ticks Ixodes ricinus (L.) Folia Parasitol. 1977;24:63–72. [PubMed] [Google Scholar]
- Schreck CE, Posey K, Smith D. Durability of permethrin as a potential clothing treatment to protect against blood-feeding arthropods. J Econ Entomol. 1978;71:397–400. doi: 10.1093/jee/71.3.397. [DOI] [PubMed] [Google Scholar]
- Schreck CE, Snoddy EL, Mount GA. Permethrin and repellents as clothing impregnants for protection from the lone star tick. J Econ Entomol. 1980;73:436–439. [Google Scholar]
- Schreck CE, Mount GA, Carlson DA. Pressurized sprays of permethrin on clothing for personal protection against the lone star tick (Acari: Ixodidae) J Econ Entomol. 1982a;75:1059–1061. doi: 10.1093/jee/75.6.1059. [DOI] [PubMed] [Google Scholar]
- Schreck CE, Mount GA, Carlson DA. Wear and wash persistence of perme-thrin used as a clothing treatment for personal protection against the lone star tick (Acari: Ixodidae) J Med Entomol. 1982b;19:143–146. doi: 10.1093/jmedent/19.2.143. [DOI] [PubMed] [Google Scholar]
- Schreck CE, Snoddy EL, Spielman A. Pressurized sprays of permethrin or deet on military clothing for personal protection against Ixodes dammini (Acari: Ixodidae) J Med Entomol. 1986;23:396–399. doi: 10.1093/jmedent/23.4.396. [DOI] [PubMed] [Google Scholar]
- Schreck CE, Fish D, McGovern TP. Activity of repellents applied to skin for protection against Amblyomma americanum and Ixodes scapularis ticks (Acari: Ixodidae) J Am Mosq Control Assoc. 1995;11:136–140. [PubMed] [Google Scholar]
- Uspensky I, Ioffe-Uspensky I. Potential risk of pathogen transmission by acar-icide-poisoned ticks. Int J Med Microbiol. 2006;296(S1):217–224. doi: 10.1016/j.ijmm.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Uspensky IV, Repkina LV. Physiological age and sensitivity to DDT in Ixodes persulcatus P. Sch. ticks from a natural population. Parazitologiya. 1974;8:3–11. in Russian. [PubMed] [Google Scholar]
- Vaughn MF, Meshnick SR. Pilot study assessing the effectiveness of long-lasting permethrin-impregnated clothing for the prevention of tick bites. Vector-Borne Zoonotic Dis. 2011;11:869–875. doi: 10.1089/vbz.2010.0158. [DOI] [PubMed] [Google Scholar]
- Vaughn MF, Funkhouser SW, Lin FC, Fine J, Juliano JJ, Apperson CS, Meshnick SR. Long-lasting permethrin impregnated uniforms: a randomized-controlled trial for tick bite prevention. Am J Prev Med. 2014;46:473–480. doi: 10.1016/j.amepre.2014.01.008. [DOI] [PubMed] [Google Scholar]


