Abstract
Borrelia miyamotoi sensu lato relapsing fever group spirochetes are emerging as causative agents of human illness (Borrelia miyamotoi disease) in the United States. Host-seeking Ixodes scapularis ticks are naturally infected with these spirochetes in the eastern United States and experimentally capable of transmitting B. miyamotoi. However, the duration of time required from tick attachment to spirochete transmission has yet to be determined. We therefore conducted a study to assess spirochete transmission by single transovarially infected I. scapularis nymphs to outbred white mice at three time points post-attachment (24, 48, and 72 h) and for a complete feed (> 72–96 h). Based on detection of B. miyamotoi DNA from the blood of mice fed on by an infected nymph, the probability of spirochete transmission increased from 10% by 24 h of attachment (evidence of infection in 3/30 mice) to 31% by 48 h (11/35 mice), 63% by 72 h (22/35 mice), and 73% for a complete feed (22/30 mice). We conclude that (i) single I. scapularis nymphs effectively transmit B. miyamotoi relapsing fever group spirochetes while feeding, (ii) transmission can occur within the first 24 h of nymphal attachment, and (iii) the probability of transmission increases with the duration of nymphal attachment.
Keywords: Borrelia miyamotoi, Ixodes scapularis, Transmission, Vector
1. Introduction
The relapsing fever group spirochete, Borrelia miyamotoi, was first described from Ixodes persulcatus and the rodent Apodemus argenteus in Japan (Fukunaga et al., 1995), with subsequent records from Ixodes scapularis in the eastern United States (Scoles et al., 2001), Ixodes ricinus in Europe (Fraenkel et al., 2002), and Ixodes pacificus in the far western United States (Mun et al., 2006). Naturally infected I. scapularis have since been documented throughout most of the tick’s geographic range in the eastern United States (Barbour et al., 2009; Crowder et al., 2014; Hamer et al., 2014; Nelder et al., 2016). Associations with human illness – named Borrelia miyamotoi disease, which typically manifests with symptoms including fever, headache, chills, and myalgia – were first reported from Russia in 2011 (Platonov et al., 2011) and soon thereafter from the eastern United States (Chowdri et al., 2013; Gugliotta et al., 2013; Krause et al., 2013) and Europe (Hovius et al., 2013; Jahfari et al., 2014). Although it remains unclear how many human cases of Borrelia miyamotoi disease occur worldwide, this illness may be more common than previously recognized in both North America and Eurasia (Krause et al., 2014; Molloy et al., 2015; Jobe et al., 2016; Siński et al., 2016).
A recent phylogenetic study (Barbour, 2014) indicated sufficient genetic variability among spirochete isolates to justify the provisional use of B. miyamotoi sensu lato for a putative species complex comprised of the originally described B. miyamotoi sensu stricto from Japan (Fukunaga et al., 1995) and other species complex members yet to be formally characterized and named. The latter potentially includes the North American LB-2001 B. miyamotoi sensu lato isolate which originated from an I. scapularis tick (Barbour, 2014). The infected I. scapularis nymphs used in this study originated from a naturally infected North American female tick which passed spirochetes to her offspring. Because we have not yet characterized the spirochetes used in this study genetically, they are most appropriately classified as B. miyamotoi sensu lato but hereafter referred to as B. miyamotoi for simplicity.
Transmission of B. miyamotoi spirochetes via the bite of infected ticks was demonstrated previously for I. scapularis (Scoles et al., 2001) and I. ricinus (van Duijvendijk et al., 2016). This included transmission by transovarially infected I. scapularis nymphs (Scoles et al., 2001) and transovarially infected I. ricinus larvae (van Duijvendijk et al., 2016). However, studies have yet to determine the duration of time required from tick attachment to spirochete transmission. B. miyamotoi spirochetes are transmitted from infected Ixodes females to their offspring (Scoles et al., 2001; Richter et al., 2012; Rollend et al., 2013). We speculated that transovarially infected nymphs harbor disseminated spirochetes in their salivary glands prior to tick-attachment, and could therefore be capable of transmitting spirochetes shortly after attachment.
The primary aim of this study was to assess the probability of transmission of B. miyamotoi spirochetes by single infected I. scapularis nymphs occurring by different time points after attachment. To facilitate messaging to the public regarding the time of attachment required for spirochete transmission, we included a complete nymphal feed as well as three time points for a partial nymphal feed (24, 48, and 72 h) that are easy to understand in terms of checking for and removing attached ticks daily or every two or three days.
2. Materials and methods
2.1. Source of B. miyamotoi-infected I. scapularis ticks, and experimental mouse host
The I. scapularis ticks used in this transmission experiment were second generation transovarially B. miyamotoi-infected nymphs that originated from a single female (CT15-0840) collected in November 2014 from Fairfield County, CT and then allowed to feed to repletion on a New Zealand white rabbit (Charles River Laboratories, Wilmington, MA, USA). The spent female and her larval offspring were confirmed to be infected with B. miyamotoi by polymerase chain reaction (PCR) as described previously by Dolan et al. (2016). First generation transovarially infected larvae and nymphs were fed on naïve CD-1 outbred Mus musculus mice (Charles River Laboratories) and the resulting adults were fed on a white rabbit. Second generation transovarially infected larvae, originating from a single female, were then fed on two naïve mice to generate the infected nymphs used in this B. miyamotoi transmission experiment. PCR-based examination (see Section 2.3.) of a subset of the unfed nymphs showed 97% (37/38) to be infected with B. miyamotoi. Some of these unfed nymphs (n = 18) also had their salivary glands dissected out and tested for presence of B. miyamotoi separately from the remainder of their bodies. Prior to processing for PCR, excised salivary glands were double-washed in two separate drops of sterile phosphate-buffered saline (PBS) to minimize the risk of external spirochete contamination.
Female 1–3 month old CD-1 outbred mice were used as experimental hosts for the infected nymphs to confirm transmission of B. miyamotoi.
2.2. Challenge of naïve mice with single B. miyamotoi-infected I. scapularis nymphs and blood collection from experimental hosts
Single nymphal ticks were allowed to feed on naïve mice for either 24 h (n = 34 mice with successful recovery of the tick), 48 h (n = 37 mice), 72 h (n = 37 mice), or a complete nymphal feed lasting > 72–96 h (n = 35 mice). To facilitate removal or recovery of partially or fully fed ticks, the nymphs were contained within feeding capsules attached to the shaved dorsal-midline of the mice as described previously (Mbow et al., 1994; Soares et al., 2006). A single nymph was introduced into each capsule and sealed inside using a small mesh screen attached to the top of the capsule to prevent tick escape while allowing for air circulation. All recovered partially or fully fed nymphs were processed for detection of B. miyamotoi DNA by PCR as described in Section 2.3. Blood samples were collected from the mice before they were exposed to nymphal feeding (baseline sample), 9–11 days after nymphal feeding commenced (for detection of B. miyamotoi DNA in the blood by PCR as described in Section 2.4), and 8–10 weeks post-challenge (for examination of serological reactivity to B. miyamotoi as described in Section 2.5). Blood collections for detection of B. miyamotoi DNA were performed 9–11 days after nymphs first attached, based on preliminary studies (data not shown) indicating that this time period falls within a short optimal window of time when B. miyamotoi DNA can be detected in mouse blood before falling to undetectable levels thereafter.
2.3. PCR-based detection of B. miyamotoi DNA in nymphal ticks and salivary glands
Nucleic acids were isolated from ticks and tick salivary glands with a Mini-Beadbeater-96 (BioSpec Products, Inc., Bartlesville, OK, USA) and a QIAcube HT robot (Qiagen, Valencia, CA, USA) using the cador Pathogen Kit (Qiagen) as previously described (Dolan et al., 2016). The in-house multiplex PCR master mix (designated M55; see Table 1 for primer and probe sequences) included primers and probes for the following targets: the I. scapularis actin target (Hojgaard et al., 2014), which serves as a control for both the DNA purification and the PCR; an in-house 23S rDNA pan-Borrelia target (Johnson et al., 2017); the adenylosuccinate lyase (purB) target for B. miyamotoi (Graham et al., 2016); and the flagellar filament cap (fliD) of B. burgdorferi, which is absent in B. miyamotoi (Hojgaard et al., 2014). The multiplex PCR was performed using 10 μl solutions containing 5 μl iQ Multiplex Powermix (Bio-Rad, Hercules, CA, USA) and 4.8 μl DNA extract, with forward and reverse primers (0.2 μl) in a final concentration of 300 nM each, and probes in a final concentration of 200 nM each. The PCR cycling conditions were: DNA denaturation at 95 °C for 3 min followed by 40 cycles of 95 °C for 10 s, 58 °C for 10 s, and 60 °C for 45 s on a C1000 Touch thermal cycler with a CFX96™ real time system (Bio-Rad).
Table 1.
Primers and probes included in the in-house M55 multiplex PCR master mix.
| Primers and probes | Sequence (5′–3′)a | Refs. |
|---|---|---|
| fliD-F | TGGTGACAGAGTGTATGATAATGGAA | Hojgaard et al. (2014) |
| fliD-R | ACTCCTCCGGAAGCCACAA | Hojgaard et al. (2014) |
| fliD-probe | FAM-TGCTAAAATGCTAGGAGATTGTCTGTCGCC-BHQ1 | Hojgaard et al. (2014) |
| 23S-F | TCGGTGAAATTGAAGTATC | Johnson et al. (2017) |
| 23S-R | CARGCTATAGTAAAGGTTCA | Johnson et al. (2017) |
| 23S-probe | HEX-CGTCTAACCACAAGTAATCGGCATC-BHQ1 | Johnson et al. (2017) |
| purB-F | TCCTCAATGATGAAAGCTTTA | Graham et al. (2016) |
| purB-R | GGATCAACTGTCTCTTTAATAAAG | Graham et al. (2016) |
| purB-probe | CalRd610-TCGACTTGCAATGATGCAAAACCT-BHQ2 | Graham et al. (2016) |
| actin-F | GCCCTGGACTCCGAGCAG | Hojgaard et al. (2014) |
| actin-R | CCGTCGGGAAGCTCGTAGG | Hojgaard et al. (2014) |
| actin-probe | Q670-CCACCGCCGCCTCCTCTTCTTCC −BHQ3 | Hojgaard et al. (2014) |
BHQ1, BHQ2, BHQ3: Black Hole Quencher 1, 2 and 3 respectively; CalRd610: CalFluor Red 610; FAM: 6-carboxyfluorescein; HEX: hexachlorofluorescein phosphramidite; Q670: Quasar 670.
2.4. PCR-based detection of B. miyamotoi DNA in rodent blood
DNA was extracted from mouse bloods (200 μl blood samples collected in EDTA tubes) using a MagNA Pure 96 (Roche Diagnostics, Indianapolis, IN, USA) and a MagNA Pure 96 DNA and viral NA small volume kit. DNA was eluted in 100 μl of elution buffer. The B. miyamotoi glpQ PCR target was used for detection of spirochete DNA (modified from Ullmann et al., 2005). Primer sequences were BmglpQ-F GACAATATTCCTGTTATAATGC, BmglpQ-R CACTGAGATTTAGTGATTTAAGTTC. The probe sequence was BmglpQ-P FAM-CCCAGAAATTGACACAACCAC-BHQ1. Each reaction contained 5 mM MgCl2, 500 nM of each primer, 100 nM probe, 1× MasterMix (Quanta Biosciences, Inc., Gaithersburg, MD, USA), 5 μl extracted DNA, and DNase and RNase free water to a final volume of 20 μl. Cycling conditions on the Applied Biosystems® 7500 Fast DX (Thermo Fisher Scientific, Waltham, MA, USA) were 95 °C for 3 min to denature the DNA, then 50 cycles of 95 °C for 10 s and 57 °C for 30 s.
2.5. Examination of serological reactivity of mouse blood to B. miyamotoi
Whole cells from North American B. miyamotoi (strain CT13-2396, isolated from a transovarially infected nymphal tick originating from an infected I. scapularis female collected in Connecticut; National Center for Biotechnology Information accession number: PRJNA310783) were cultivated in modified Barbour-Stoenner-Kelly (BSK) medium (in-house BSK-R medium) and harvested by centrifuging at 10,000 g for 10 min at 4 °C. The resulting cell pellet was frozen, thawed, and re-suspended in TE buffer, sonicated, and diluted in TE buffer to a final protein concentration of 2.0 mg/ml. The sonicate was mixed with an equal volume of 2× Laemmli sample buffer with DTT (Bio-Rad), placed in a 95 °C heat block for 10 min, and run with 120 μl sonicate and sample buffer mixture on 10.5–14.0% gradient Criterion precast Tri- HCL gel (Bio-Rad) at 200 V for 65 min.
Separated proteins were transferred to a 0.2 μm nitrocellulose membrane using Trans-Blot Turbo Transfer pack (Bio-Rad) and Trans-Blot Turbo Blotting System (Bio-Rad). Membranes were soaked overnight in TBS with 1% casein blocker (Bio-Rad) and cut into 3 mm strips for immunoblotting. Strips were hydrated in 1% milk and TBS-t wash buffer, then incubated on a rocking platform in wash buffer with 20 μl serum from experimental mouse hosts used in the transmission experiment at a concentration of 1:200 for 30 min. Strips were then washed and incubated for 15 min in wash buffer and phosphatase-labeled goat anti mouse IgG (H + L) conjugate (1 mg/ml; KPL, Gaithersburg, MD, USA) at a concentration of 1:5000, followed by a final wash series. Strips were developed for 4 min using BCIP/NBT phosphatase substrate (KPL).
Serological reactivity against B. miyamotoi was examined for all mice that were fed on by an infected nymph but tested negative for B. miyamotoi DNA in blood drawn 9–11 days after the nymphal feed commenced (n = 78 mice). Serum from a mouse that tested positive for B. miyamotoi DNA in blood drawn 9–11 days after tick feeding commenced was used as a positive control. Additional testing to facilitate the interpretation of serological reactivity for this in-house assay included (i) post-tick exposure sera for a subset of 19 mice that tested positive for B. miyamotoi DNA in blood and (ii) paired pre- and post-tick exposure sera for a set of 10 mice out of 35 that demonstrated potential immunoblot reactivity (weak reactivity for the 10 tested mice and very weak reactivity for the remaining 25) in the initial testing of post-tick exposure sera.
2.6. Statistical evaluation
Probabilities of B. miyamotoi transmission based on detection of infection in mice (outcome variable) were compared across tick attachment durations (explanatory variable) of 24, 48, and 72 h, and complete feed for > 72–96 h using a generalized linear model assuming a binomial distribution with a log link. Resulting estimates of the ratios of transmission probabilities and 95% one-sided confidence intervals were computed, with Tukey’s method to adjust for multiple comparisons. Since interest was only in detecting an increase in transmission across durations, one-sided confidence intervals were calculated.
2.7. Regulatory compliance
Animal use and experimental procedures were in accordance with an approved protocol on file with the Centers for Disease Control and Prevention Division of Vector-Borne Diseases Animal Care and Use Committee.
3. Results
3.1. Salivary gland infection of unfed I. scapularis nymphs with B. miyamotoi
Out of 17 unfed nymphs with B. miyamotoi DNA detected in their bodies, 15 (88.2%) also had detectable levels of B. miyamotoi DNA present in their salivary glands.
3.2. Transmission of B. miyamotoi by single infected I. scapularis nymphs in relation to duration of feeding
The probability of B. miyamotoi transmission to mice exposed to feeding by a single infected nymph increased from 10% following 24 h of attachment (evidence of spirochete infection in 3/30 mice) to 31% by 48 h (11/35 mice), 63% by 72 h (22/35 mice), and 73% for a complete feed (22/30 mice) (Table 2). Statistically significant increases in the probabilities of transmission occurred from 24 h to either 72 h or a complete feed (ratios of transmission probabilities 6.29 (95% Lower Confidence Limit, 1.76) and 7.00 (95% LCL, 1.97), respectively), and from 48 h to either 72 h or a complete feed (ratios of transmission probabilities 2.00 (95% LCL, 1.06) and 1.23 (95% LCL, 1.19), respectively). The observed increases were not statistically significant from 24 to 48 h (ratio of transmission probability 3.14 (95% LCL, 0.81)) or from 72 h to a complete feed (ratio of transmission probability 1.11 (95% LCL, 0.75)).
Table 2.
Transmission outcomes for mice fed upon for different durations of time by a single B. miyamotoi-infected I. scapularis nymph.
| Duration of nymphal feeding | Evidence of B. miyamotoi DNA in mouse blooda
|
||
|---|---|---|---|
| No. mice examined | No. mice with evidence of infection | Percent of mice with evidence of infection | |
| 24 h | 30 | 3 | 10 |
| 48 h | 35 | 11 | 31 |
| 72 h | 35 | 22 | 63 |
| Complete feed (> 72–96 h) | 30 | 22 | 73 |
Blood collected 9–11 days after the nymphal tick started to feed.
All mice with evidence of B. miyamotoi DNA in their blood 9–11 days after an infected nymph started to feed had moderate to strong serological reactivity to B. miyamotoi 8–10 weeks later, with test strips displaying numerous distinct bands (Fig. 1). An additional 35 mice without PCR-detectable B. miyamotoi DNA in their blood 9–11 days after tick feeding displayed weak (n = 10) or very weak (n = 25) reactivity in the initial testing of their post-tick exposure sera, with test strips displaying one or a few weak or very weak bands. Subsequent examination of paired pre- and post-tick exposure sera for the 10 mice with weak reactivity yielded test strips with subtle differences in banding patterns suggesting that B. miyamotoi exposure may have occurred during the tick feed (Fig. 2). However, we do not consider the serology results for the 35 mice showing weak or very weak reactivity post-tick exposure to provide clear evidence of spirochete exposure, and these mice consequently were scored as negative for exposure to B. miyamotoi in the present study.
Fig. 1.
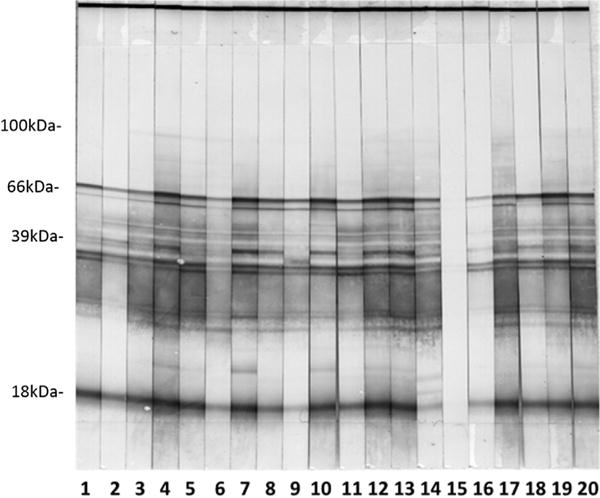
Serological reactivity post-tick exposure for 19 mice (strips 1–14 and 16–20) that tested positive for B. miyamotoi DNA in their blood. Strip 15 illustrates lack of reactivity post-tick exposure for a mouse that did not test positive for B. miyamotoi DNA in its blood.
Fig. 2.
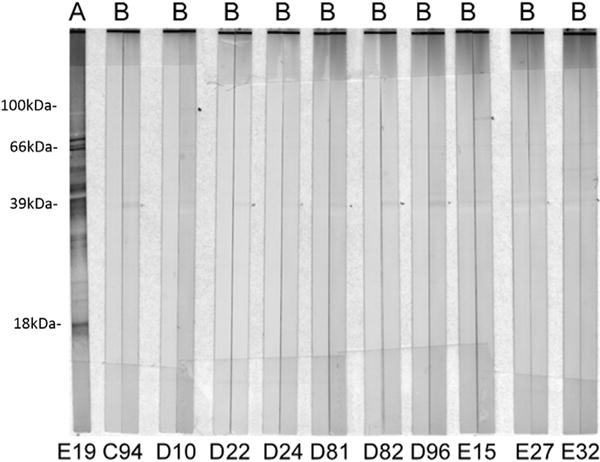
Examples of serological reactivity for mice (A) after exposure to B. miyamotoi-infected I. scapularis nymphs for mouse E19 testing positive for B. miyamotoi DNA in its blood and (B) before (left strip) and after (right strip) exposure to B. miyamotoi-infected I. scapularis nymphs for mice (C94, D10, D22, D24, D81, D82, D96, E15, E27, E32) testing negative for B. miyamotoi DNA in their blood.
4. Discussion
We document experimental transmission of B. miyamotoi relapsing fever group spirochetes during feeding by a single tick, and show that the probability of transmission increases with duration of tick attachment. Previous studies on experimental transmission by ticks of B. miyamotoi to mouse hosts used mass feeding, with mice showing evidence of B. miyamotoi infection after having been exposed to > 10 potentially infected nymphal ticks (Scoles et al., 2001) or to low numbers of infected larval ticks feeding simultaneously with > 100 non-infected larvae (van Duijvendijk et al., 2016). Here, we demonstrate that when B. miyamotoi-infected I. scapularis nymphs are allowed to feed to completion, their probability of transmitting spirochetes to an outbred mouse is > 70% (Table 2). Follow-up studies are needed to determine the probability of B. miyamotoi transmission occurring after partial or complete blood meals by single infected larval or adult female ticks. This is particularly important in the case of the larval stage since the peak seasonal distribution of human acute B. miyamotoi infections in the northeastern United States appears to extend into August, when I. scapularis larvae rather than nymphs are most active (Molloy et al., 2015; Telford et al., 2015). We also cannot rule out the possibility that nymphs infected as larvae through feeding on an infectious blood meal host may differ in their efficiency to transmit B. miyamotoi as compared with transovarially infected nymphs.
Because nearly all examined unfed nymphs harbored B. miyamotoi in their salivary glands, it was not surprising to find that spirochetes could be transmitted within the first 24 h of attachment by single infected nymphal ticks. This finding is in striking contrast to the Lyme disease spirochete, Borrelia burgdorferi, which does not appear to be transmitted by a single infected I. scapularis nymph within the first 24 h of attachment. Indeed, none of 79 exposed mice, examined across multiple studies, showed evidence of B. burgdorferi infection after 24 h of attachment by a single infected nymph (Piesman et al., 1987; des Vignes et al., 2001; Piesman and Dolan, 2002; Hojgaard et al., 2008). Moreover, transmission within the first 48 h of attachment by a single infected nymph also appears to occur more commonly for B. miyamotoi (31% of 35 mice infected; Table 2) as compared with B. burgdorferi (10% of 111 mice examined across multiple studies with evidence of spirochete infection; Piesman et al., 1987; des Vignes et al., 2001; Piesman and Dolan, 2002; Hojgaard et al., 2008). At later time points (≥72 h) the probability of transmission by single infected nymphs appears to be lower for B. miyamotoi as compared with B. burgdorferi (Table 2; Piesman et al., 1987; des Vignes et al., 2001). The shorter duration of nymphal attachment required for transmission of B. miyamotoi relapsing fever group spirochetes, as compared with Lyme disease spirochetes, needs to be incorporated into existing messages to the medical community and the public regarding the protection afforded by regular tick checks and tick removal to prevent transmission by infected attached ticks. However, it also should be emphasized that even though removal of attached ticks daily or every two days may be less effective in preventing transmission of B. miyamotoi, as compared with B. burgdorferi, the risk of B. miyamotoi transmission still increases distinctly with every day a tick is allowed to remain attached.
One shortcoming of this transmission experiment is that we did not examine the outcome for attachment durations shorter than 24 h. Although our confirmation of infection in the mice did not include culture of spirochetes from blood, microscopic detection of spirochetes in blood, or feeding by xenodiagnostic larvae to demonstrate presence of live spirochetes, we consider detection of B. miyamotoi DNA in rodent blood 1 week after the infected nymphs completed their feeding combined with distinct serological reactivity of the mice to B. miyamotoi to provide adequate evidence that the nymphs did transmit viable spirochetes while feeding. A final weakness is that we used ticks infected with a North American B. miyamotoi “strain” that has not yet been characterized genetically. However, we note that mice infected with these spirochetes showed strong serological reactivity against the well characterized B. miyamotoi strain CT13-2396. Another point of interest is the source of the weak and very weak serological reactivity seen post-tick exposure, but not pre-tick exposure, for some of the mice without evidence of B. miyamotoi DNA in their blood after having been fed on by infected nymphs. We are not certain whether these faint banding patterns resulted from exposure of the mice to low numbers of B. miyamotoi spirochetes that failed to produce a viable infection detectable by PCR or if they are artifacts of the in-house serological assay, perhaps relating to the tick saliva itself or to unknown commensal microorganisms commonly transmitted via the saliva. Notably, the weak bands were observed at or around 39 kD (Fig. 2), where we would expect to see a B. miyamotoi glycerophosphoryl diester phosphodiesterase (glpQ) band.
We conclude that single infected I. scapularis nymphs effectively transmit B. miyamotoi relapsing fever group spirochetes while feeding, that transmission can occur within the first 24 h of nymphal attachment, and that the probability of transmission increases with the duration of nymphal attachment.
Acknowledgments
We thank Mark Pilgard, and Martin Williams of the Centers for Disease Control and Prevention for technical support, and Dr. Neeta Connally of Western Connecticut State University for providing source material to start tick colonies.
Footnotes
Disclaimer
The findings and conclusions of this study are by the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG. Phylogeny of a relapsing fever Borrelia species transmitted by the hard tick Ixodes scapularis. Inf Genet Evol. 2014;27:551–558. doi: 10.1016/j.meegid.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdri HR, Gugliotta JL, Berardi VP, Goethert HK, Molloy PJ, Sterling SL, Telford SR. Borrelia miyamotoi infection presenting as human granulocytic anaplasmosis: a case report. Ann Intern Med. 2013;159:21–27. doi: 10.7326/0003-4819-159-1-201307020-00005. [DOI] [PubMed] [Google Scholar]
- Crowder CD, Carolan HE, Rounds MA, Honig V, Mothes B, Haag H, Nolte O, Luft BJ, Grubhoffer L, Ecker DJ, Schutzer SE, Eshoo MW. Prevalence of Borrelia miyamotoi in Ixodes ticks in Europe and the United States. Emerg Infect Dis. 2014;20:1678–1682. doi: 10.3201/eid2010.131583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- des Vignes F, Piesman J, Heffernan R, Schulze TL, Stafford KC, III, Fish D. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J Infect Dis. 2001;183:773–778. doi: 10.1086/318818. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Hojgaard A, Hoxmeier JC, Replogle AJ, Respicio-Kingry LB, Sexton C, Williams MA, Pritt BS, Schriefer ME, Eisen L. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii. Ticks Tick-Borne Dis. 2016;7:665–669. doi: 10.1016/j.ttbdis.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Fraenkel CJ, Garpmo U, Berglund J. Determination of novel Borrelia genospecies in Swedish Ixodes ricinus ticks. J Clin Microbiol. 2002;40:3308–3312. doi: 10.1128/JCM.40.9.3308-3312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, Nakao M. Genetic and phenotypic analysis of Borrelia miyamotoi sp nov isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol. 1995;45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- Graham CB, Pilgard MA, Maes SE, Hojgaard A, Eisen RJ. Paired real-time PCR assays for detection of Borrelia miyamotoi in North American Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) Ticks Tick-Borne Dis. 2016;7:1230–1235. doi: 10.1016/j.ttbdis.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugliotta JL, Goethert HK, Berardi VP, Telford SR., III Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med. 2013;368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer SA, Hickling GJ, Walker ED, Tsao JI. Increased diversity of zoonotic pathogens and Borrelia burgdorferi strains in established versus incipient Ixodes scapularis populations across the Midwestern United States. Infect Genet Evol. 2014;27:531–542. doi: 10.1016/j.meegid.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Hojgaard A, Eisen RJ, Piesman J. Transmission dynamics of Borrelia burgdorferi s.s. during the key third day of feeding by nymphal Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 2008;45:732–736. doi: 10.1603/0022-2585(2008)45[732:TDOBBS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hojgaard A, Lukacik G, Piesman J. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti: with two different multiplex PCR assays. Ticks Tick-Borne Dis. 2014;5:349–351. doi: 10.1016/j.ttbdis.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Hovius JWR, De Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, Oei A, Knol H, Narasimhan S, Hodiamont CJ, Jahfari S, Pals ST, Horlings HM, Fikrig E, Sprong H, Van Oers MHJ. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Herremans T, Platonov AE, Kuiper H, Karan LS, Vasilieva O, Koopmans MPG, Hovius JWR, Sprong H. High seroprevalence of Borrelia miyamotoi antibodies in forestry workers and individuals suspected of human granulocytic anaplasmosis in the Netherlands. New Microbes New Infect. 2014;2:144–149. doi: 10.1002/nmi2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe DA, Lovrich SD, Oldenburg DG, Kowalski TJ, Callister SM. Borrelia miyamotoi infection in patients from upper midwestern United States, 2014–2015. Emerg Infect Dis. 2016;22:1471–1473. doi: 10.3201/eid2208.151878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Graham CB, Hojgaard A, Breuner NE, Maes SE, Boegler KA, Replogle AJ, Kingry LC, Petersen JM, Eisen L, Eisen RJ. Isolation of the Lyme disease spirochete Borrelia mayonii from naturally infected rodents in Minnesota. J Med Entomol. 2017;54 doi: 10.1093/jme/tjx062. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, Barbour A, Fish D. Human Borrelia miyamotoi infection in the United States. N Engl J Med. 2013;368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Barbour AG, Platonov AE, Brancato J, Lepore T, Dardick K, Mamula M, Rollend L, Steeves TK, Diuk-Wasser M, Usmani-Brown S, Williamson P, Sarksyan DS, Fikrig E, Fish D, Tick Borne Diseases Group Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the Northeastern United States. Emerg Infect Dis. 2014;20:1183–1190. doi: 10.3201/eid2007.131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbow ML, Christe M, Rutti B, Brossard M. Absence of acquired resistance to nymphal Ixodes ricinus ticks in Balb/C mice, developing cutaneous reactions. J Parasitol. 1994;80:81–87. [PubMed] [Google Scholar]
- Molloy PJ, Telford SR, III, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med. 2015;163:91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- Mun J, Eisen RJ, Eisen L, Lane RS. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J Med Entomol. 2006;43:120–123. doi: 10.1093/jmedent/43.1.120. [DOI] [PubMed] [Google Scholar]
- Nelder MP, Russell CB, Sheehan NJ, Sander B, Moore S, Li Y, Johnson S, Patel SN, Sider D. Human pathogens associated with the blacklegged tick Ixodes scapularis: a systematic review. Parasites Vectors. 2016;9:265. doi: 10.1186/s13071-016-1529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Dolan MC. Protection against Lyme disease spirochete transmission provided by prompt removal of nymphal Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 2002;39:509–512. doi: 10.1603/0022-2585-39.3.509. [DOI] [PubMed] [Google Scholar]
- Piesman J, Mather TN, Sinsky RJ, Spielman A. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557–558. doi: 10.1128/jcm.25.3.557-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Debski A, Hubalek Z, Matuschka FR. Absence of Lyme disease spirochetes in larval Ixodes ricinus ticks. Vector-Borne Zoonotic Dis. 2012;12:21–27. doi: 10.1089/vbz.2011.0668. [DOI] [PubMed] [Google Scholar]
- Rollend L, Fish D, Childs JE. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick-Borne Dis. 2013;4:46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector-Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- Siński E, Welc-Falęciak R, Zajkowska J. Borrelia miyamotoi: A human tick-borne relapsing fever spirochete in Europe and its potential impact on public health. Adv Med Sci. 2016;61:255–260. doi: 10.1016/j.advms.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Soares CAG, Zeidner NS, Beard CB, Dolan MC, Dietrich G, Piesman J. Kinetics of Borrelia burgdorferi infection in larvae of refractory and competent tick vectors. J Med Entomol. 2006;43:61–67. doi: 10.1093/jmedent/43.1.61. [DOI] [PubMed] [Google Scholar]
- Telford SR, III, Goethert HK, Molloy PJ, Berardi VP, Chowdri HR, Gugliotta JR, Lepore TJ. Borrelia miyamotoi disease (BMD): Neither Lyme disease nor relapsing fever. Clin Lab Med. 2015;35:867–882. doi: 10.1016/j.cll.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann AJ, Gabitzsch ES, Schulze TL, Zeidner NS, Piesman J. Three multiplex assays for detection of Borrelia burgdorferi sensu lato and Borrelia miyamotoi sensu lato in field-collected Ixodes nymphs in North America. J Med Entomol. 2005;42:1057–1062. doi: 10.1093/jmedent/42.6.1057. [DOI] [PubMed] [Google Scholar]
- van Duijvendijk G, Coipan C, Wagemakers A, Fonville M, Ersöz J, Oei A, Földvári G, Hovius J, Takken W, Sprong H. Larvae of Ixodes ricinus transmit Borrelia afzelii and B. miyamotoi to vertebrate hosts. Parasites Vectors. 2016;9:97. doi: 10.1186/s13071-016-1389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


