Abstract
Background
Oxidative/nitrosative stress and endothelial dysfunction are hypothesized to be central to cancer therapeutics-related cardiac dysfunction (CTRCD). However, the relationship between circulating arginine-nitric oxide (NO) metabolites and CTRCD remains unstudied.
Objectives
To examine the relationship between arginine-NO metabolites and CTRCD in a prospective cohort of 170 breast cancer patients treated with doxorubicin ± trastuzumab.
Methods
Plasma levels of arginine, citrulline, ornithine, asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), and N-monomethylarginine (MMA) were quantified at baseline, 1 month, and 2 months following doxorubicin initiation. Determinants of baseline biomarker levels were identified using multivariable linear regression, and Cox regression defined the association between baseline levels and 1- or 2-month biomarker changes and CTRCD rate in 139 participants with quantitated echocardiograms at all time points.
Results
Age, hypertension, body mass index, and African American race were independently associated with ≥1 baseline citrulline, ADMA, SDMA, and MMA levels. Decreases in arginine and citrulline, and increases in ADMA were observed at 1 and 2 months (all P<0.05). Overall, 32 participants experienced CTRCD over a maximum follow-up of 5.4 years. Hazard ratios for ADMA and MMA at 2 months were 3.33 (95% confidence interval [CI] 1.12, 9.96) and 2.70 (95%CI 1.35, 5.41), respectively, and 0.78 (95%CI 0.64, 0.97) for arginine at 1 month.
Conclusions
In breast cancer patients undergoing doxorubicin therapy, early alterations in arginine-NO metabolite levels occurred, and early biomarker changes were associated with a greater CTRCD rate. Our findings highlight the potential mechanistic and translational relevance of this pathway to CTRCD.
Keywords: cardio-oncology, arginine metabolism, nitrosative stress, cardiotoxicity, doxorubicin, trastuzumab
Introduction
Anthracyclines and trastuzumab (Herceptin®) are highly effective in the treatment of HER2-positive breast cancer, but carry a significant risk of cancer therapeutics-related cardiac dysfunction (CTRCD) (1,2). Doxorubicin is associated with a dose-dependent risk of heart failure and cardiomyopathy, and doxorubicin and trastuzumab in combination result in an increased incidence of cardiotoxicity. Despite extensive research on CTRCD, there remains a critical need to develop new biomarkers that may yield better understanding of the involved mechanistic pathways, and, ultimately, more individualized prognosis and treatment strategies (3).
Oxidative stress has been established as a primary mechanism of doxorubicin-induced toxicity. Doxorubicin forms a semiquinone moiety that reduces oxygen to superoxide, which then reacts with several molecules including nitric oxide (NO). This results in the production of reactive oxygen and nitrogen species, disruption of cellular redox balance, and increased oxidative/nitrosative stress (4–6). Indeed, previous studies by our group suggest that higher circulating levels of oxidative stress, as quantified by myeloperoxidase, are associated with increased CTRCD risk in breast cancer patients (7). However, other studies, such as those showing that the oxidative effects of doxorubicin may be mediated through topoisomerase-IIβ (8), suggest that the involved pathways are likely to be multiple and complex.
Endothelial dysfunction further promotes CTRCD by disrupting normal paracrine interactions between endothelial cells and cardiomyocytes—through signaling factors such as NO, vascular endothelial growth factor (VEGF), and neuregulin-1 (9,10)—and by leading to increases in blood pressure and afterload (11). ErbB2 inhibition from trastuzumab may exacerbate these effects, resulting in worse oxidative stress and cardiomyocyte and endothelial cell dysfunction (10,12,13).
The arginine-NO metabolism pathway, which has been implicated in a variety of cardiovascular disease states and specifically in anthracycline-induced cardiotoxicity (14), plays a central role in both cellular oxidative/nitrosative stress and endothelial dysfunction (Central Illustration). NO is an essential molecular mediator of normally functioning endothelium, and reductions in NO bioavailability lead to DNA damage, lipid peroxidation, cardiomyocyte apoptosis, endothelial cell dysfunction, and reduced cardiac contractility (15,16). L-arginine, a key substrate in NO production, can be alternatively catabolized to urea or converted to N-monomethylarginine (MMA), which results in nitric oxide synthase (NOS) inhibition and less NO bioavailability. MMA is a precursor to 2 amino acid derivatives: asymmetric dimethylarginine (ADMA), which potently inhibits NOS, and symmetrical dimethylarginine (SDMA), which is inactive against NOS. Prior studies have demonstrated that increased levels of ADMA, as an inhibitor of endothelial NOS, is a predictor of cardiovascular mortality in individuals with coronary artery disease (17), myocardial infarction (18), and heart failure (19), and is an independent risk factor for heart failure, hypertension, coronary artery disease, diabetes, and renal dysfunction (20).
Central Illustration. Pathophysiology of NO Production, Arginine Metabolism, and Anthracycline-Induced Cardiotoxicity.
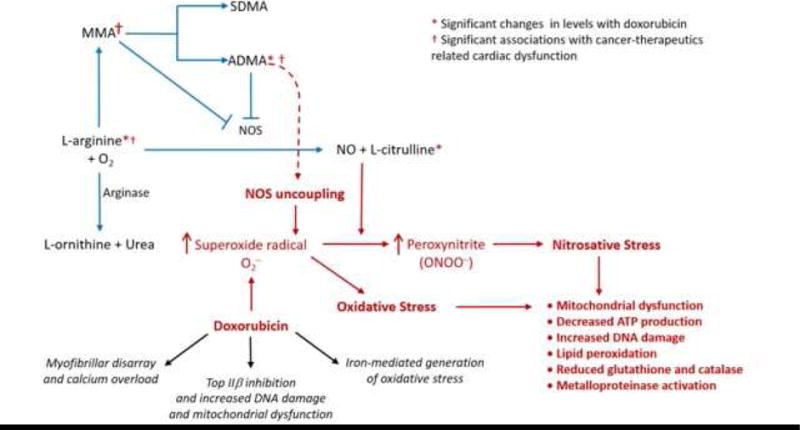
L-arginine (substrate) is oxidized by nitric oxide synthase (NOS) to form citrulline and NO. Alternatively, L-arginine can be catabolized by arginase to form ornithine or shunted to the methylation pathway to form N-monomethylarginine (MMA), symmetric dimethylarginine (SDMA) and asymmetric dimethylarginine (ADMA). Both MMA and ADMA inhibit NOS. Anthracycline-induced cardiotoxicity occurs through formation of superoxide radical, a potent cause of oxidative stress, which then interacts with a variety of molecules, including NO, to produce toxic peroxynitrites that lead to further nitrosative stress. Reductions in NO can also lead to anthracycline-induced cardiotoxicity by disrupting the beneficial physiologic effects that NO exerts on normal well-functioning endothelium. Doxorubicin also results in calcium overload and myofibrillar disarray, topoisomerase-IIβ inhibition which may further exacerbate oxidative stress via alterations in the transcriptome; and iron-mediated generation of oxidative stress.
However, despite the potential relevance of this pathway to CTRCD, to our knowledge, there have been no studies to date of circulating arginine-NO metabolite biomarkers in cardio-oncology. Thus, we sought to determine whether arginine-NO metabolites have the potential to function as informative biomarkers in breast cancer patients treated with doxorubicin. In this study, we examined the clinical determinants of baseline arginine-NO metabolite levels, characterized early changes in these metabolites with doxorubicin therapy, and determined whether these early changes were associated with CTRCD in a longitudinal prospective cohort of breast cancer patients.
Methods
Study Cohort
The study cohort was a subset of the Cardiotoxicity of Cancer Therapy study, an ongoing, prospective longitudinal cohort study of women with breast cancer recruited from the Rena Rowan Breast Cancer Center of the Abramson Cancer Center at the University of Pennsylvania (Philadelphia, Pennsylvania). Inclusion and exclusion criteria were minimal and have been previously described (21). Treatment regimens were determined by the treating oncologist, and consisted of either: 1) doxorubicin (240 mg/m2) and cyclophosphamide for a total of 4 cycles every 2 weeks, followed by paclitaxel either 4 cycles every 2 weeks, or weekly for 12 weeks; or 2) doxorubicin (240 mg/m2) and cyclophosphamide for a total of 4 cycles every 2 weeks, followed by paclitaxel and trastuzumab (Figure 1). Trastuzumab was prescribed for a total of 1 year at dosages per standard guidelines.
Figure 1. Biomarker Protocol According to Treatment Regimen.
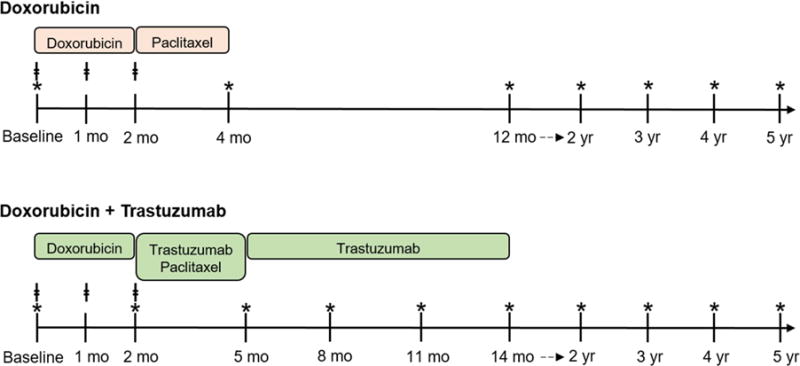
Participants were treated with either doxorubicin or doxorubicin + trastuzumab. ǂdenotes visits when blood samples were collected. *denotes visits when transthoracic echocardiograms were performed.
Each participant completed standardized questionnaires at baseline (prior to initiation of doxorubicin) and at each subsequent follow-up visit. Clinical data were verified via review of medical records. The current analysis was limited to participants enrolled between August 2010 and March 2014 with biomarkers measured at baseline and ≥2 echocardiograms performed and quantitated. The University of Pennsylvania Institutional Review Board approved the study, and all participants provided written informed consent.
Echocardiography and Cardiac Dysfunction Outcomes Definition
Transthoracic echocardiograms were performed by a dedicated sonographer at standardized intervals according to treatment regimen (Figure 1), digitally archived, and quantitated in a blinded manner using the TomTec Imaging Systems platform (Unterschleissheim, Germany) (21). Methods for determining left ventricular ejection fraction (LVEF) are described in detail in the Online Appendix. The primary outcome measure of CTRCD was a priori defined as a reduction in LVEF by ≥10% from baseline prior to chemotherapy, to an absolute value of <50%. Although there is no universally accepted definition of CTRCD, this definition has been commonly used in the cardio-oncology literature (22–24).
Measurements of Biomarkers
Blood samples were obtained at baseline, 1 month (following cycle 2 and immediately prior to cycle 3 of doxorubicin), and 2 months (following completion of doxorubicin) in all participants (Figure 1). Measurements of plasma levels of arginine, ornithine, citrulline, ADMA, SDMA, and MMA were performed by stable-isotope-dilution high performance liquid chromatography with online tandem mass spectrometry using the API 365 triple quadruple mass spectrometer (Applied Biosystems, Foster, California) with Ionics EP 10+ upgrade (Concord, Ontario, California) and Cohesive Technologies Aria LX Series high performance liquid chromatography multiplexing system (Franklin, Massachusetts); measurement techniques are described fully in the Online Appendix. Biomarker measurements were performed in a blinded manner independent from study investigators and clinical/echocardiographic data.
Statistical Methods
Participant characteristics at baseline were summarized using standard descriptive statistics. We used multivariable linear regression to test the association between baseline biomarker levels and clinical and demographic variables. These variables were prespecified and included age, race, hypertension, hyperlipidemia, body mass index (BMI), systolic blood pressure, tobacco use, statin use, and other cardiac medication use including angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and beta blockers. Because baseline biomarker levels tended to be non-normal, we transformed the data by dividing the baseline value by the median baseline value and then taking the log of this ratio. The exponentiated coefficients from the resulting regression model can thus be interpreted as the expected multiple of the median increase observed per unit change in the clinical variables. Additionally, because of the large numbers of tests being conducted, statistical significance was determined based on the Wald test adjusted for multiple comparisons based on the number of covariates in each model using the Holm method (25).
Next, to characterize biomarker changes in the overall cohort, we calculated the ratio of biomarker levels at 1 and 2 months relative to baseline, using the Wilcoxon signed-rank test to determine statistical significance. We then summarized time to first CTRCD event using Kaplan-Meier methods and used Cox models to determine the association between the hazard of first CTRCD and individual biomarkers. These survival analyses were restricted to the subset of participants who had quantitated echocardiography at all time points. In these analyses, 3 distinct Cox models, each corresponding to a separate biomarker assessment (baseline, 1 month, and 2 months), were constructed for the subcohort. We used the log1.5-ratio of the biomarker value at baseline (relative to the cohort median), 1 and 2 months (relative to baseline) as the independent variable, such that the resulting hazard ratio can be interpreted as the expected increase in the instantaneous risk of CTRCD from a 1.5-fold increase in the biomarker ratio (relative either to the median or to the baseline value, depending on the model). All models were adjusted for treatment regimen as well as any clinical or demographic variable found to be significantly associated (P <0.05) with baseline levels of any of the biomarkers. Additionally, to account for correlation between baseline values and subsequent biomarker changes, 1- and 2-month models were adjusted for the baseline biomarker level, using the same log1.5-ratio transformation.
Results
Study Population
Baseline cohort characteristics are summarized in Table 1. Briefly, our study cohort included 170 participants with a median age of 48 years and median BMI of 27 kg/m2; 60% were Caucasian and 29% were African-American. Cardiovascular risk factors such as hypertension, diabetes, and current/former smoking status were noted in 28%, 8%, and 41%, respectively. 133 participants (78%) received doxorubicin without trastuzumab, while 37 participants (22%) received combination therapy. Among those who had quantitated echocardiography at all time points, the median LVEF at baseline was 54% (IQR 50%, 56%). Out of 170 participants in the cohort, 19 individuals died due to progression of cancer during the first 4 years of follow-up. No individuals died of other causes.
Table 1.
Baseline patient characteristics (N = 170)
| Clinical Characteristic | N (%)* |
|---|---|
| Age, years [median (IQR)] | 48 (41, 56) |
| Race | |
| Caucasian | 102 (60) |
| African American | 50 (29) |
| Other or unknown | 18 (11) |
| BMI, kg/m2 [median (IQR)] | 27 (24, 32) |
| Breast cancer side | |
| Left | 78 (46) |
| Right | 81 (48) |
| Bilateral | 11 (6) |
| Breast cancer stage | |
| Stage 1 | 29 (17) |
| Stage 2 | 94 (55) |
| Stage 3 | 45 (27) |
| Stage 4 | 2 (1) |
| Left-sided Radiotherapy | 58 (34) |
| Chemotherapy regimen | |
| Doxorubicin | 133 (78) |
| Doxorubicin + Trastuzumab | 37 (22) |
| Hypertension | 47 (28)† |
| Diabetes | 13 (8) |
| Hyperlipidemia | 40 (24) |
| Tobacco use | |
| Current | 9 (5) |
| Former | 61 (36) |
| Never | 100 (59) |
| Cardiac medications | |
| ACE-inhibitor or ARB | 20 (12) |
| Beta-blocker | 15 (9) |
| Statin | 13 (8) |
| LVEF, % [median (IQR)] | 54 (50, 56)‡ |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; IQR, interquartile range; LVEF, left ventricular ejection fraction.
Values are N (%) or median (IQR) if specified.
1 participant had unknown hypertension status.
Based on the 139 individuals with quantitated echocardiography at all time points.
Associations with Baseline Arginine Metabolite Levels
The results of baseline biomarker levels regressed on demographic and clinical characteristics are shown in Table 2. Age was associated with higher levels of SDMA (adjusted P = 0.02). African-American race was associated with lower levels of ornithine, ADMA and MMA (adjusted P = 0.006, P <0.001, and P = 0.01, respectively). Additionally, the presence of hypertension was associated with higher levels of citrulline (adjusted P = 0.008), while increased BMI was associated with higher levels of ADMA (adjusted P = 0.03). Notably, non-African American (versus Caucasian) race, diabetes, hyperlipidemia, smoking status, cardiac medications, and statin use were not associated with any of the baseline biomarker levels (data not shown). No clinical variables were significantly associated with arginine levels.
Table 2.
Cross-sectional associations between baseline clinical characteristics and arginine-NO metabolites
| Metabolites (μmol/L) |
Median
value (IQR) |
Multiples of the median (95% CI) for clinical covariates† | |||
|---|---|---|---|---|---|
| Age (per 10 years) |
African American race‡ | Hypertension | BMI (per 5 units) |
||
| Arginine | 54 (43, 69) | 1.00 (0.94, 1.07) |
0.99 (0.85, 1.16) |
1.05 (0.87, 1.28) |
0.99 (0.94, 1.04) |
|
| |||||
| Citrulline | 25 (21, 29) | 1.05 (1.01, 1.09) |
0.92 (0.83, 1.01) |
1.24 (1.10, 1.40)* |
0.98 (0.95, 1.01) |
|
| |||||
| Ornithine | 65 (52, 78) | 1.03 (0.97, 1.09) |
0.78 (0.68, 0.90)* |
1.05 (0.89, 1.24) |
1.03 (0.98, 1.08) |
|
| |||||
| ADMA | 0.62 (0.57, 0.71) | 1.00 (0.98, 1.03) |
0.87 (0.83, 0.92)* |
1.05 (0.99, 1.12) |
1.03 (1.01, 1.05)* |
|
| |||||
| SDMA | 0.42 (0.37, 0.47) | 1.05 (1.02, 1.09)* |
0.97 (0.90, 1.04) |
1.08 (0.99, 1.19) |
0.99 (0.97, 1.02) |
|
| |||||
| MMA | 0.22 (0.18, 0.27) | 0.98 (0.94, 1.03) |
0.83 (0.74, 0.93)* |
1.12 (0.98, 1.28) |
0.99 (0.96, 1.03) |
Abbreviations: ADMA, asymmetric methylarginine; BMI, body mass index; CI, confidence interval; IQR, interquartile range; MMA, N-monomethylarginine; SDMA, symmetric methylarginine.
P<0.05 via the Wald test, adjusted for multiple comparisons based on the number of covariates in the model using the Holm method. Note that confidence intervals may not always align with P-values because the P-values are adjusted.
The log-ratio of each metabolite at baseline relative to the cohort median was the dependent variable in individual multivariable linear regression models, which included these clinical variables as covariates: age, race, hypertension, BMI, diabetes, hyperlipidemia, current tobacco, statin, and other cardiac medication use. Only variables that had a significant coefficient for ≥1 metabolite are shown above. The exponentiated coefficients presented here are interpreted as the expected multiple of the median change in biomarker value per unit change in the clinical covariate. As an example, a value of 1.05 for SDMA represents a 1.05-fold increase over the cohort median for each 10-year increase in age.
The coefficients for African American race are relative to Caucasian race.
Biomarker Changes with Doxorubicin Therapy for the Entire Cohort
Baseline concentrations of arginine-NO metabolite levels are shown in Table 2, and changes in metabolite levels at 1 and 2 months are shown in Table 3. At 1 month (immediately prior to the third cycle of doxorubicin for both treatment groups), decreased arginine and citrulline levels (both P<0.001), and increased ADMA levels (P<0.001) were observed. These changes persisted at 2 months after a cumulative doxorubicin dose of 240 mg/m2 for both treatment groups (all P<0.001). There were no statistically significant changes in ornithine, SDMA, or MMA at 1 or 2 months. 2-fold or more increases in biomarker levels at 2 months were observed in a small portion (<5%) of individual participants for arginine, citrulline, and ornithine, but not seen for ADMA, SDMA, and MMA.
Table 3.
Changes in arginine-NO metabolite levels at 1 and 2 months following doxorubicin initiation
| Biomarker (μmol/L) |
Ratio of Biomarker Level at 1 Month | Ratio of Biomarker Level at 2 Months | ||
|---|---|---|---|---|
| Relative to Baseline | Relative to Baseline | |||
| Median (IQR) | Ratio ≥1.5† | Median (IQR) | Ratio ≥1.5† | |
| Arginine | 0.86 (0.70, 1.12)* | 6.8% | 0.79 (0.64, 1.04)* | 9.9% |
|
| ||||
| Citrulline | 0.85 (0.66, 1.01)* | 3.1% | 0.80 (0.67, 1.02)* | 3.3% |
|
| ||||
| Ornithine | 0.94 (0.77, 1.20) | 10.5% | 0.99 (0.77, 1.27) | 13.2% |
|
| ||||
| ADMA | 1.05 (0.95, 1.15)* | 0% | 1.08 (0.99, 1.21)* | 4.0% |
|
| ||||
| SDMA | 1.01 (0.88, 1.16) | 0.6% | 1.00 (0.93, 1.14) | 0% |
|
| ||||
| MMA | 0.97 (0.84, 1.18) | 6.8% | 1.00 (0.82, 1.17) | 6.0% |
Abbreviations: ADMA, asymmetric dimethylarginine; IQR, interquartile range; MMA, N-monomethylarginine; SDMA, symmetric dimethylarginine
Median ratio differed significantly from 1.0 (P<0.05) via the Wilcoxon signed-rank test.
A ratio of 1.5 corresponds to the effect size for the hazard ratios presented in Table 4.
Patterns of CTRCD in the Overall Cohort
Quantitated echocardiograms were available at all time points in 139 individuals. Over a maximum follow-up time of 5.4 years (IQR 1.8, 4.1 years), 32 (23%) participants experienced CTRCD (Figure 2). Of those individuals experiencing CTRCD, 12 (38%) had hypertension, 10 (31%) had hyperlipidemia, 4 (13%) had diabetes, 8 (25%) were on cardiac medications, and the median BMI was 27 kg/m2 at baseline. Participants receiving doxorubicin alone experienced a lower CTRCD rate than those receiving doxorubicin with trastuzumab. Specifically, 18% of participants (19/108) receiving doxorubicin alone experienced CTRCD, with the majority of events occurring after therapy was completed (median 15.8 months; IQR 8.2, 30.7 months). In contrast, 42% of participants (13/31) receiving both therapies experienced CTRCD, with all events occurring during therapy (median 7.4 months; IQR 4.9, 9.4 months).
Figure 2. Occurrence of Cancer Therapeutics-Related Cardiac Dysfunction (CTRCD) over Time.
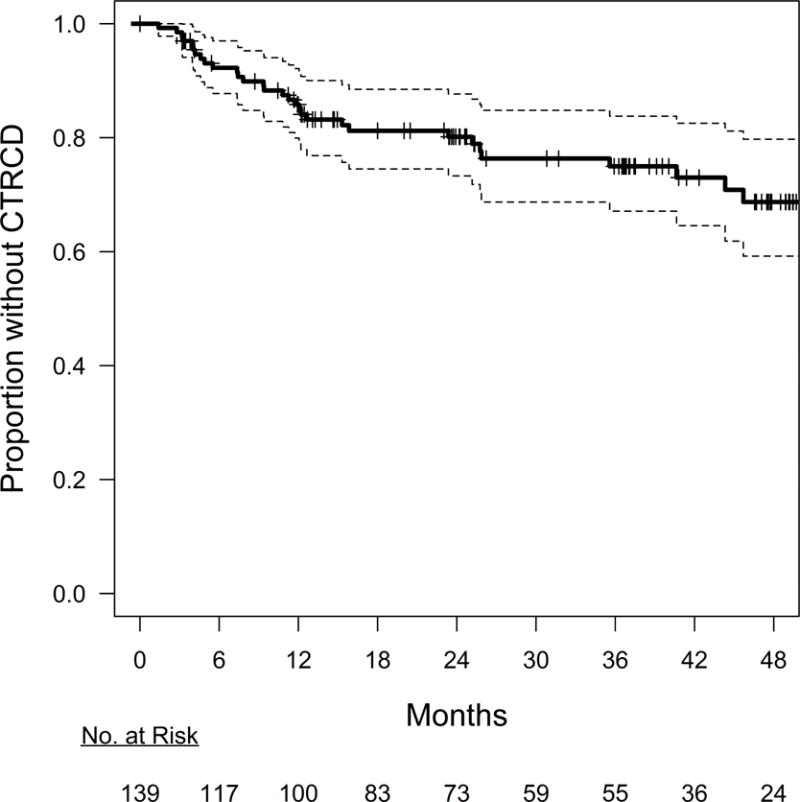
Kaplan-Meier plot illustrating the overall incidence of CTRCD in the cohort. The number of individuals at risk of CTRCD over time is shown below the plot. By 12.1 months (95% CI 7.8, 25.9), 15% of the cohort experienced CTRCD. The plot is based on the subset of the cohort with quantitated echocardiography at all time points (N = 139).
The median decline in LVEF at the time of CTRCD was 12% (IQR 11%, 13%), and the median absolute LVEF at the time of CTRCD was 43% (IQR 41%, 45%). Following CTRCD, 19 participants (59%) showed an improvement in LVEF of at least 5%. Overall, 14 of 32 participants (44%) experiencing CTRCD developed heart failure symptoms, as determined by the treating cardio-oncologist, and 12 of these participants (86%) were either prescribed a new cardiac medication or had a dose change on an existing cardiac medication. The remaining 2 participants refused cardiac treatment despite counseling. Seven participants (22%) experienced temporary dose interruptions and 1 patient (3%) discontinued therapy early as a result of their CTRCD.
Association between Individual Biomarkers and Cardiac Dysfunction
Table 4 shows the association between the time to first CTRCD event and the biomarkers at either baseline or at 1 or 2 months after normalization to baseline. In models adjusted for treatment regimen, age, race, hypertension, and BMI, baseline biomarker levels were not significantly associated with CTRCD. However, at 1 month, decreases in arginine were significantly associated with CTRCD, and at 2 months, increases in ADMA and MMA were associated with CTRCD (Figure 3). Specifically, each 1.5-fold increase in the arginine ratio at 1 month was associated with a decreased hazard ratio of 0.78 (95% confidence interval [CI] 0.64, 0.97), while each 1.5-fold exponential increase in ADMA and MMA was associated with hazard ratios of 3.33 (95%CI 1.12, 9.96) and 2.70 (95%CI 1.35, 5.41), respectively. This level of increase in biomarker values was observed in 7%, 4%, and 6% of the overall cohort for arginine, ADMA, and MMA, respectively. In the subcohort with quantitated echocardiography at all time points, missingness in biomarker values was 4% at 1 month and 10% at 2 months. Sensitivity analyses in which missing biomarker data were replaced with cross-sectional mean values at each time point did not substantially change model results (data not shown).
Table 4.
Risk of cancer therapeutics related cardiac dysfunction (CTRCD) according to baseline levels and changes in arginine-NO metabolite levels
| Biomarker (μmol/L) | Baseline (N = 138)† | 1 month (N = 124) | 2 months (N = 117) | |||
|---|---|---|---|---|---|---|
| Adjusted HR‡ (95% CI) | P-value | Adjusted HR‡ (95% CI) | P-value | Adjusted HR‡ (95% CI) | P-value | |
| Arginine | 0.93 (0.69, 1.25) | 0.61 | 0.78 (0.64, 0.97) | 0.02* | 1.23 (0.83, 1.83) | 0.29 |
|
| ||||||
| Citrulline | 1.04 (0.58, 1.87) | 0.90 | 0.83 (0.46, 1.50) | 0.53 | 1.35 (0.78, 2.35) | 0.28 |
|
| ||||||
| Ornithine | 1.13 (0.74, 1.73) | 0.57 | 1.59 (0.91, 2.77) | 0.10 | 1.70 (0.98, 2.97) | 0.06 |
|
| ||||||
| ADMA | 0.77 (0.25, 2.39) | 0.65 | 3.31 (0.88, 12.5) | 0.08 | 3.33 (1.12, 9.96) | 0.03* |
|
| ||||||
| SDMA | 0.61 (0.24, 1.55) | 0.30 | 0.94 (0.39, 2.27) | 0.90 | 0.91 (0.28, 3.01) | 0.88 |
|
| ||||||
| MMA | 0.90 (0.53, 1.53) | 0.69 | 0.61 (0.29, 1.27) | 0.18 | 2.70 (1.35, 5.41) | 0.005* |
Abbreviations: ADMA, asymmetric dimethylarginine; CI, confidence interval; HR, hazard ratio; SDMA, symmetric dimethylarginine; MMA, N-monomethylarginine
P<0.05 via the Wald test.
This analysis was restricted to the 139 participants with quantitated echocardiography at all time points. 1 participant was dropped from this analysis due to unknown hypertension status.
The estimated hazard ratio (HR) and associated 95% confidence interval (CI) in a model adjusted for treatment regimen, age, race, hypertension, and BMI are shown. Non-baseline models are also adjusted for baseline biomarker value. HRs are shown for the log1.5-ratio of the biomarker value at baseline relative to the cohort median and the log1.5-ratio of the biomarker value at 1 and 2 months relative to baseline. HRs can be interpreted as the expected relative increase in the instantaneous risk of CTRCD from a 1.5-fold increase in the biomarker ratio. For example, a 1.5-fold increase in the ADMA level from baseline to 2 months would be expected to lead to a 3.33-fold increase in the rate of CTRCD.
Figure 3. Cancer Therapy Related Cardiac Dysfunction (CTRCD) by Arginine-NO Metabolite Levels at 2 Months.
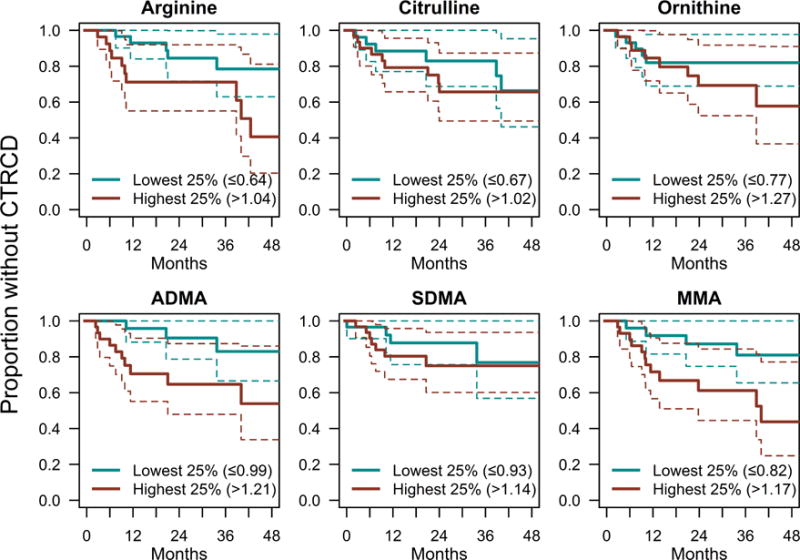
Kaplan-Meier plots of first CTRCD event for participants below the first quartile (lowest 25%), or above the third quartile (highest 25%), of the biomarker ratio (level at 2 months versus baseline level). Note that this figure is based on the subset of the cohort with quantitated echocardiography available at all time points (N = 139).
Discussion
Our study is the first to prospectively examine temporal changes in circulating arginine-NO metabolites in breast cancer patients receiving doxorubicin. We found the measurement of arginine-NO metabolites feasible, and levels were similar to those reported in other populations (26). Higher baseline levels of specific metabolites were associated with older age, hypertension, and higher BMI, which is consistent with previous studies showing these metabolites as general markers of cardiovascular disease risk (18,20,27). African Americans had lower baseline levels of ADMA and SDMA, which is consistent with previously described ethnic distributions that had demonstrated higher redox status in this population (28–30). However, African Americans also had a slightly greater degree of cardiovascular comorbidities—including more hypertension (52% vs. 20%), diabetes (16% vs. 4%), and larger BMI (median 31 vs. 25)—at baseline as compared to Caucasians. We also report decreases in arginine and citrulline and increases in ADMA across the entire cohort following doxorubicin administration, consistent with an adverse cardiovascular risk profile and the pathobiology of anthracycline cardiotoxicity.
The overall CTRCD rate in our cohort was similar to rates observed in other observational studies of breast cancer patients treated with anthracyclines with or without trastuzumab (1,31), and comparable to the rate in a recent randomized trial of trastuzumab patients (32). Participants with CTRCD tended to have mild left ventricular dysfunction, and the preponderance of those who were symptomatic were treated with cardiac medications. Importantly, those who experienced decreases in arginine or increases in ADMA or MMA following doxorubicin administration also experienced a significantly higher CTRCD rate. Baseline metabolite levels were not associated with CTRCD, suggesting that changes after doxorubicin treatment may reflect an acute cardiotoxic response. ADMA—which has the most consistent cardiotoxic profile in our study, showing significant overall increases at 1 and 2 months post-doxorubicin and consistently elevated point estimates for the association with CTRCD at both time points—is an established clinical marker of vascular endothelial dysfunction due to its inhibition of endothelial NOS (33). In vitro evidence suggests that ADMA further contributes to endothelial dysfunction through accelerated endothelial cell senescence and reduced motility (34,35). Lower arginine bioavailability has also been demonstrated to be associated with more severe myocardial dysfunction in patients with chronic heart failure (27).
Interestingly, ADMA and MMA both directly inhibit NOS, making our results consistent with NOS-dependent endothelial dysfunction being a primary mechanism for doxorobucin-related cardiotoxicity. This hypothesis is strengthened by data from murine models where cardiotoxic effects of doxorubicin treatment are mediated specifically through endothelial NOS (36). Moreover, it is also likely consistent with clinical studies by our group demonstrating the importance of arterial load to the development of CTRCD in breast cancer patients (21), as doxorubicin-mediated endothelial dysfunction would be expected to also worsen afterload through effects on the vasculature.
In vitro and in vivo data suggest that higher levels of ADMA and MMA can lead to uncoupling of endothelial NOS oxygen reduction from NO formation, leading to increased superoxide formation, peroxynitrite formation, and subsequent oxidative/nitrosative stress (37, 38). Indeed, one can speculate that doxorubicin-induced oxidative damage leads to a complex feedback loop where oxidative stress from low arginine levels in combination with other pathways leads to increased proteolysis and subsequent increases in ADMA and MMA levels, thus causing reduced NO concentrations, NOS decoupling, and even higher levels of oxidative/nitrosative injury and endothelial dysfunction. Previous studies in humans have also demonstrated increased endothelial NOS uncoupling with higher ADMA levels (39), lending credence to this hypothesis. However, the exact molecular mechanisms of CTRCD are likely to be quite complex and remain to be fully elucidated.
It is unclear from our findings whether the increased CTRCD risk from post-anthracycline changes in arginine-NO metabolite levels reflects a direct increase in CTRCD risk or an increase in susceptibility to CTRCD with subsequent trastuzumab therapy. In vitro and in vivo findings suggest that increases in oxidative stress and endothelial dysfunction with doxorubicin could be further worsened with trastuzumab (10,12,13), corroborating the notion of a 2-hit hypothesis (40). However, this study is underpowered to formally examine interactions between biomarker level and treatment group, and testing this hypothesis in clinical data remains an important area for further research.
The balance between the effects of arginine-NO metabolites on oxidative/nitrosative stress, endothelial dysfunction, and cardiotoxicity is highly complex. It not only involves substrate availability, but also cofactor and enzyme availability, substrate consumption, and transport system functionality. As such, we recognize that the precise causal role of arginine-NO metabolites in CTRCD can only be demonstrated by in vivo or in vitro studies, and with subsequent confirmation through pharmacologic manipulation of the involved pathways in humans. Future research to test hypotheses relevant to this pathway—including whether the use of statins, which have been shown in humans to decrease plasma ADMA levels (41), or nitrate supplementation, which has been shown in mice to decrease doxorubicin cardiotoxicity (42), might decrease the risk of CTRCD—is critically needed.
Additional research is also needed to formally test the prognostic potential of these biomarkers for predicting CTRCD events in individual patients receiving cardiotoxic chemotherapy. Substantial increases in arginine, ADMA, and MMA levels (i.e. >1.5 fold) following anthracycline administration were only observed in a small number of individuals (4–7%). Although the elevations in these metabolites were small, evidence from prior work suggests that even small modifications in ADMA levels can significantly alter vascular NO production and vascular resistance (43,44). However, it is likely that the arginine-NO pathway is only one of many important pathways for the development of CTRCD in breast cancer patients (Central Illustration), leading one to expect clinical prediction models based on these metabolites alone to be more specific than sensitive, and emphasizing the need for multi-marker algorithms to improve sensitivity. Moreover, research into newer markers of oxidative/nitrosative stress, including myeloperoxidase, isoprostanes, and redox couples; topoisomerase-IIβ activity; and fibrosis are essential to integrate and advance our understanding of the complexities of doxorubicin injury.
We acknowledge additional potential limitations of our study. First, while our study is 1 of the larger biomarker studies reported to date in this population, only 32 events occurred. As a result, our study was powered to detect only large associations and was limited in the ability to adjust for a large number of potential confounders, with some risk of overfitting of model estimates. Second, whether fasting may affect levels of NO metabolites is unknown, and our participants were not fasting prior to blood draw. Third, we focused only on left ventricular systolic dysfunction. The associations between these metabolites and other echocardiographic measures such as diastolic dysfunction in this cohort are unknown, although this is a topic for future research. Fourth, while we utilized the log-ratio transformation to help normalize biomarker data, not all biomarkers at all time points were fully normalized following this transformation (data not shown). As a result, there is the possibility of an increased Type II error rate for some analyses, although simulation studies suggest that use of linear regression is unbiased and provides adequate coverage with non-normal data at sample sizes comparable to our cohort (45). Finally, while the duration of follow-up with complete echocardiographic data is a major strength of the study (51% of individuals had at least 3 years of follow-up), the association between late biomarker changes and late CTRCD remains to be further elucidated.
In conclusion, for patients with breast cancer undergoing doxorubicin therapy, we determined that the CTRCD rate is associated with early changes in arginine-NO metabolites, specifically arginine, ADMA, and MMA. These findings suggest a plausible mechanistic pathway involving endothelial dysfunction and increased oxidative and nitrosative stress. Biomarkers of arginine-NO metabolism may have the potential to identify patients at high risk for cardiac dysfunction from these therapies, and additional research is warranted to further study their prognostic utility.
Supplementary Material
CONDENSED ABSTRACT.
In 170 breast cancer patients treated with doxorubicin ± trastuzumab, we examined how early changes in arginine-nitric oxide metabolites relate to cancer therapeutics-related cardiac dysfunction (CTRCD). Age, hypertension, body mass index, and African American race were associated with baseline citrulline, asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), and N-monomethylarginine (MMA) levels. Decreases in arginine and citrulline, and increases in ADMA occurred with doxorubicin. Increases in ADMA and MMA at 2 months, and decreases in arginine at 1 month, were associated with increased CTRCD risk over 5.4 years of maximum follow-up, highlighting the mechanistic and translational relevance of this pathway to CTRCD.
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge
New mechanistic biomarkers can enhance our understanding of the pathophysiology of doxorubicin and/or trastuzumab cardiac dysfunction, and better inform risk prediction. While our prior work suggests oxidative stress markers are associated with cardiac dysfunction, the role of additional key mediators of oxidative and nitrosative stress and endothelial dysfunction, specifically arginine-nitric oxide (NO) metabolites, remains to be determined.
Translational Outlook 1
Early changes in circulating levels of specific arginine-NO metabolites (arginine, ADMA, and MMA) are associated with the development of subsequent cardiac dysfunction. Additional prospective clinical research is needed to quantify the prognostic potential of these biomarkers as a component of multimarker statistical models.
Translational Outlook 2
Randomized trial evidence is needed to determine whether pharmacologic manipulation of this pathway could lead to reduced incidence or greater recovery from cancer therapeutics-related cardiac dysfunction.
Acknowledgments
Funding: This work was supported by NHLBI R01-HL118018 (Ky), McCabe Fellow Award (Philadelphia, PA, Ky), American Cancer Society Institutional Research Grant -78-002-30 (Atlanta, Georgia, Ky), NHLBI K23-HL095661 (Ky), R01-HL103931(Tang), and NICHD T32-HD060550 (Narayan). The funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
Abbreviations
- ADMA
asymmetric dimethylarginine
- BMI
body mass index
- CTRCD
cancer therapeutics-related cardiac dysfunction
- LVEF
left ventricular ejection fraction
- MMA
N-monomethylarginine
- NO
nitric oxide
- NOS
nitric oxide synthase
- SDMA
symmetric dimethylarginine
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Putt reports owning stock in Roche, and Ky reports consulting for Roche. None of the other authors have any relationships with industry or other relevant entities to disclose that are relevant to the content of this study.
Prior Presentations: Presented in part at the Global Cardio-Oncology Summit, Vancouver, Canada, September 23, 2016
Ethics Statement: The study was approved by the University of Pennsylvania Institutional Review Board, and all participants provided written informed consent.
References
- 1.Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60:2504–12. doi: 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 2.Bowles EJA, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenihan DJ, Cardinale DM. Late cardiac effects of cancer treatment. J Clin Oncol. 2012;30:3657–64. doi: 10.1200/JCO.2012.45.2938. [DOI] [PubMed] [Google Scholar]
- 4.Mukhopadhyay P, Rajesh M, Bátkai S, et al. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2009;296:1466–83. doi: 10.1152/ajpheart.00795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstein DM, Mihm MJ, Bauer JA. Cardiac peroxynitrite formation and left ventricular dysfunction following doxorubicin treatment in mice. J Pharmacol Exp Ther. 2000;294:396–401. [PubMed] [Google Scholar]
- 6.Fogli S, Nieri P, Breschi MC. The role of nitric oxide in anthracycline toxicity and prospects for pharmacologic prevention of cardiac damage. FASEB J. 2004;18:664–75. doi: 10.1096/fj.03-0724rev. [DOI] [PubMed] [Google Scholar]
- 7.Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–16. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–42. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 9.Wan A, Rodrigues B. Endothelial cell-cardiomyocyte crosstalk in diabetic cardiomyopathy. Cardiovasc Res. 2016;111:172–83. doi: 10.1093/cvr/cvw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn VS, Lenihan DJ, Ky B. Cancer therapy-induced cardiotoxicity: Basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc. 2014;3(2):e000665. doi: 10.1161/JAHA.113.000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol. 2009;157:527–36. doi: 10.1111/j.1476-5381.2009.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milano G, Raucci A, Scopece A, et al. Doxorubicin and trastuzumab regimen induces biventricular failure in mice. J Am Soc Echocardiogr. 2014;27:568–79. doi: 10.1016/j.echo.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Aghajanian H, Cho YK, Manderfield LJ, et al. Coronary vasculature patterning requires a novel endothelial ErbB2 holoreceptor. Nat Commun. 2016;7:12038. doi: 10.1038/ncomms12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf MB, Baynes JW. The anti-cancer drug, doxorubicin, causes oxidant stress-induced endothelial dysfunction. Biochim Biophys Acta. 2006;1760:267–71. doi: 10.1016/j.bbagen.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Toya T, Hakuno D, Shiraishi Y, Kujiraoka T, Adachi T. Arginase inhibition augments nitric oxide production and facilitates left ventricular systolic function in doxorubicin-induced cardiomyopathy in mice. Physiol Rep. 2014;2(9):e12130. doi: 10.14814/phy2.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond J, Balligand J. Nitric oxide synthase and cyclic GMP signaling in cardiac myocytes: From contractility to remodeling. J Mol Cell Cardiol. 2012;52:330–40. doi: 10.1016/j.yjmcc.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Meinitzer A, Seelhorst U, Wellnitz B, et al. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the ludwigshafen risk and cardiovascular health study) Clin Chem. 2007;53:273–83. doi: 10.1373/clinchem.2006.076711. [DOI] [PubMed] [Google Scholar]
- 18.Boger RH, Sullivan LM, Schwedhelm E, et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dückelmann C, Mittermayer F, Haider DG, Altenberger J, Eichinger J, Wolzt M. Asymmetric dimethylarginine enhances cardiovascular risk prediction in patients with chronic heart failure. Arterioscler Thromb Vasc Biol. 2007;27:2037–42. doi: 10.1161/ATVBAHA.107.147595. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Hou L, Xu D, et al. Effect of asymmetric dimethylarginine (ADMA) on heart failure development. Nitric Oxide. 2016;54:73–81. doi: 10.1016/j.niox.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narayan HK, Finkelman BS, French B, et al. Detailed echocardiographic phenotyping in breast cancer patients: Associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of followup. Circulation. 2017;135:1397–412. doi: 10.1161/CIRCULATIONAHA.116.023463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 23.Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: Clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–6. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 24.Zardavas D, Suter TM, Van Veldhuisen DJ, et al. Role of troponins I and T and N-terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early-stage human epidermal growth factor receptor 2—Positive breast cancer receiving trastuzumab: A herceptin adjuvant study cardiac marker substudy. J Clin Oncol. 2017;35:878–84. doi: 10.1200/JCO.2015.65.7916. [DOI] [PubMed] [Google Scholar]
- 25.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 26.Hov GG, Sagen E, Bigonah A, Åsberg A. Health-associated reference values for arginine, asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) measured with high-performance liquid chromatography. Scandinavian Journal of Clinical & Laboratory Investigation. 2007;67:868–76. doi: 10.1080/00365510701429836. [DOI] [PubMed] [Google Scholar]
- 27.Tang WHW, Shrestha K, Wang Z, Troughton RW, Klein AL, Hazen SL. Diminished global arginine bioavailability as a metabolic defect in chronic systolic heart failure. J Card Fail. 2013;19:87–93. doi: 10.1016/j.cardfail.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drew DA, Tighiouart H, Scott T, et al. Asymmetric dimethylarginine, race, and mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2014;9:1426–33. doi: 10.2215/CJN.00770114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sydow K, Fortmann SP, Fair JM, et al. Distribution of asymmetric dimethylarginine among 980 healthy, older adults of different ethnicities. Clin Chem. 2010;56:111–20. doi: 10.1373/clinchem.2009.136200. [DOI] [PubMed] [Google Scholar]
- 30.Mels CMC, Loots I, Schwedhelm E, Atzler D, Böger RH, Schutte AE. Nitric oxide synthesis capacity, ambulatory blood pressure and end organ damage in a black and white population: The SABPA study. Amino Acids. 2016;48:801–10. doi: 10.1007/s00726-015-2128-5. [DOI] [PubMed] [Google Scholar]
- 31.Drafts BC, Twomley KM, D’Agostino R, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6:877–85. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pituskin E, Mackey JR, Koshman S, et al. Multidisciplinary approach to novel therapies in cardio-oncology research (MANTICORE 101–Breast): A randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J Clin Oncol. 2016;35:870–7. doi: 10.1200/JCO.2016.68.7830. [DOI] [PubMed] [Google Scholar]
- 33.Griendling K, Touyz R, Zweier J, et al. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: A scientific statement from the american heart association. Circ Res. 2016;119(5):e39–75. doi: 10.1161/RES.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scalera F, Borlak J, Beckmann B, et al. Endogenous nitric oxide synthesis inhibitor asymmetric dimethyl L-arginine accelerates endothelial cell senescence. Arterioscler Thromb Vasc Biol. 2004;24:1816–22. doi: 10.1161/01.ATV.0000141843.77133.fc. [DOI] [PubMed] [Google Scholar]
- 35.Wojciak-Stothard B, Torondel B, Tsang LYF, et al. The ADMA/DDAH pathway is a critical regulator of endothelial cell motility. J Cell Sci. 2007;120:929–42. doi: 10.1242/jcs.002212. [DOI] [PubMed] [Google Scholar]
- 36.Deng S, Kruger A, Schmidt A, et al. Differential roles of nitric oxide synthase isozymes in cardiotoxicity and mortality following chronic doxorubicin treatment in mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:25–34. doi: 10.1007/s00210-009-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardounel AJ, Xia Y, Zweier JL. Endogenous methylarginines modulate superoxide as well as nitric oxide generation from neuronal nitric-oxide synthase: Differences in the effects of monomethyl-and dimethylarginine in the presence and absence of tetrahydrobiopterin. J Biol Chem. 2005;280:7540–9. doi: 10.1074/jbc.M410241200. [DOI] [PubMed] [Google Scholar]
- 38.Lin KY, Ito A, Asagami T, et al. Impaired nitric oxide synthase pathway in diabetes mellitus: Role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106:987–92. doi: 10.1161/01.cir.0000027109.14149.67. [DOI] [PubMed] [Google Scholar]
- 39.Antoniades C, Shirodaria C, Leeson P, et al. Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: Implications for endothelial function in human atherosclerosis. Eur Heart J. 2009;30:1142–50. doi: 10.1093/eurheartj/ehp061. [DOI] [PubMed] [Google Scholar]
- 40.Ky B, Vejpongsa P, Yeh ETH, Force T, Moslehi JJ. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ Res. 2013;113:754–64. doi: 10.1161/CIRCRESAHA.113.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serban C, Sahebkar A, Ursoniu S, et al. A systematic review and meta-analysis of the effect of statins on plasma asymmetric dimethylarginine concentrations. Sci Rep. 2015;5:9902. doi: 10.1038/srep09902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu S, Kukreja RC, Das A, Chen Q, Lesnefsky EJ, Xi L. Dietary nitrate supplementation protects against doxorubicin-induced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol. 2011;57:2181–9. doi: 10.1016/j.jacc.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Böger RH. Asymmetric dimethylarginine (ADMA) modulates endothelial function—therapeutic implications. Vasc Med. 2003;8:149–51. doi: 10.1191/1358863x03vm501ed. [DOI] [PubMed] [Google Scholar]
- 44.Wells SM, Holian A. Asymmetric dimethylarginine induces oxidative and nitrosative stress in murine lung epithelial cells. Am J Respir Cell Mol Biol. 2007;36:520–8. doi: 10.1165/rcmb.2006-0302SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Judkins DR, Porter KE. Robustness of ordinary least squares in randomized clinical trials. Stat Med. 2016;35:1763–73. doi: 10.1002/sim.6839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


