Abstract
Objective
The aim of this study is to determine the validity and reliability of neutrophil CD64 in identifying infected infants and to evaluate the impact of this marker on clinical care.
Study Design
Neutrophil CD64 index was incorporated in 371 infection evaluations in 234 infants (ages 1–293 days) from 2005 to 2009 and the impact of this change on clinical care was evaluated.
Results
The sensitivity of the neutrophil CD64 assay was 87% in identifying 31 episodes of culture positive sepsis and 83% in identifying 12 infants with ventilator-associated pneumonia. There was no difference in the mean number of antibiotic days in infants with a normal CD64 versus those with a normal complete blood count (CBC) (p = 0.89), but twofold more infants were identified as “not infected” by CD64 than by CBC.
Conclusion
CD64 had a high sensitivity for identifying infected infants while also decreasing the number of infants that were exposed to unnecessary antibiotic use.
Keywords: CD64, neonatal infection, sepsis, sepsis marker
The care of neonates has advanced significantly in recent decades with the introduction of surfactant, gentle ventilation strategies, and antenatal steroids.[1] [2] Despite these advances, morbidity and mortality in premature and other critically ill neonates remains unacceptably high. One of the most significant contributors to poor neonatal outcomes is neonatal infection.[3] [4] [5] [6]
Traditionally, neonatologists have relied on physical exam, blood culture, and complete blood counts (CBC) to evaluate and identify infected infants. Although blood cultures are considered the gold standard for diagnosing infection, the sensitivity of blood cultures in neonates is poor and the 24 to 48 hours required for blood cultures to grow is too long to be useful in the immediate evaluation of a potentially infected infant.[7] [8] [9] Moreover, the sensitivity and specificity of physical exam and CBC are also poor, making these tests unreliable in sepsis evaluations.[10] [11] [12] [13] [14] [15] [16] [17] [18] The poor sensitivity and specificity inherent to this traditional approach means that most infants that are evaluated for infection are started on antimicrobial therapy and will be needlessly exposed to the potential negative effects of antimicrobial exposure.
In 2007, options for improving the evaluation of potentially infected infants at Cincinnati Children’s Hospital Medical Center were investigated. Several candidate hematologic markers, including neutrophil CD64 index, C-reactive protein, and procalcitonin, were evaluated to determine which one would most likely improve the care of neonates. Because we desired a marker that would change quickly and therefore could be used to determine whether or not to initiate antibiotics during the initial assessment of the infant, C-reactive protein was ruled out because of the low sensitivity reported for this marker during the early stages of infection.[13] [19] [20] [21] [22] Neutrophil CD64 was subsequently selected over procalcitonin because studies suggested CD64 had a slightly higher sensitivity and there were concerns that procalcitonin may increase nonspecifically during the first few days of life and during neonatal stress caused by tissue trauma or neonatal respiratory distress syndrome.[19] [20] [23] [24] [25] [26] [27] [28] [29] Therefore, CD64 was implemented as part of the standard evaluation of neonates with suspected bacterial infection.
Neutrophil CD64 is a high affinity Fc receptor expressed at low levels on the neutrophil surface at baseline, which increases significantly when neutrophils are activated by infectious stimuli.[12] [28] [29] [30] [31] Several previous studies have determined that CD64 has a high sensitivity, specificity, negative predictive value, and positive predictive value in neonatal infection.[12] [28] [29] [31] [32] [33] [34] The purpose of the study was to evaluate the validity and reliability of CD64 and to measure the impact of this test on the care of potentially infected neonates in “real world” clinical practice.
Methods
This study was approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board and was conducted at Cincinnati Children’s newborn intensive care unit (NICU) between March 2005 and March 2009.
The CD64 assay was performed by incubating 50 ul peripheral blood with a saturated amount of anti-CD64 FITC/anti-CD163 PE cocktail followed by incubation with ammonium chloride to lyse RBCs. An internal relative control bead suspension is added before analysis on the FACSCalibur or FACSCanto II flow cytometers (BD Biosciences, San Jose, CA).
Each patient sample provides an internal negative control (lymphocytes) and internal positive control (monocytes). The CD64 index is then calculated using the ratio of the mean fluorescent intensity of the neutrophil population to that of the beads as described by the manufacturer (Trillium Diagnostics LLC, Brewer, ME).
During the first phase of the study, normal CD64 indices were established for our patient population. A neutrophil CD64 index was obtained from discarded blood in infants in the NICU who had a CBC evaluation for either infectious or noninfectious reasons.[32] [35] [36] All infants younger than 1 year who were admitted to the NICU and had a CBC evaluation were eligible for the study. Patients were excluded if they (1) were on antibiotics for more than 48 hours before obtaining the CD64 index or (2) had known immunologic disorder, leukemia, or lymphoma. CD64 indices were obtained within 24 hours of obtaining the CBC and measured in peripheral blood neutrophils. Although the majority of samples were obtained from different patients (164 total patients), a patient could be enrolled multiple times for each separate CBC evaluation performed, provided they were obtained at least 24 hours apart. Outcome data obtained during phase one of the study were (1) CD64 index, (2) culture result, and (3) antibiotic use. Infants with positive blood cultures were diagnosed as culture-proven sepsis according to the National Healthcare Safety Network (NHSN) definition for laboratory confirmed blood stream infections. Because the sensitivity of blood cultures in neonates is poor, infants were also diagnosed with clinical sepsis if they met the criteria defined by the NHSN for clinical sepsis. Data obtained from phase 1 of the study were not shared with the health care team. In addition, a CD64 index was obtained from discarded blood in noninfected older (i.e., age 1 to > 18 years) patients that met the same exclusion criteria.
During the second phase of the study, CD64 index was made available as part of the sepsis evaluation for infants younger than 1 year in the NICU. Health care providers in the NICU were trained on the optimal cutoff value of the CD64 index and the sensitivity, specificity, and negative/positive predictive value of the CD64 index in neonatal sepsis. Health care providers were instructed to consider the CD64 index along with the clinical history, physical exam, and other laboratory values when determining whether to start and how long to continue antibiotic therapy, but they were not given CD64 criteria that required starting or stopping antibiotic therapy. Sepsis evaluations included a CD64, CBC, two peripheral blood cultures, and if indicated endotracheal tube, urine, and cerebrospinal fluid cultures. Because of laboratory limitations, CD64 was not available on weekends and as a result, sepsis evaluations that occurred on weekends did not include a CD64 (i.e., CBC group) whereas those that occurred on weekdays did include a CD64 (i.e., CD64 group). CD64 results were typically available within 3 hours of the initial evaluation and the health care team was allowed to use the results in determining the infant’s plan of care. Patients were excluded if they had a known immunologic disorder, leukemia, or lymphoma. In addition, we also excluded healthy normal newborn infants that were admitted to the NICU through the emergency department for a 48-hour evaluation of fever without a source, as we were focused on the more complex and critically ill NICU population and did not want these relatively healthy infants to bias the results. Although the majority of samples were obtained from different patients, a patient could be enrolled multiple times for each separate sepsis evaluation, provided there was a minimum of 48 hours between each evaluation. Data obtained during phase 2 of the study were as follows: (1) birth weight, (2) gestational age, (3) age at time of testing, (4) CD64 index, (5) culture result (per unit protocol a minimum of 0.5 mL was obtained for two blood cultures in each patient), (6) location of infection, (7) antibiotic use, (8) CBC results, (9) use of inotropes, (10) respiratory support, and (11) length of stay.
Receiver operating characteristic (ROC) curves were constructed using the CD64 indices and the diagnosis of clinical or culture positive sepsis using SAS 9.3 (SAS Institute Inc., Cary, NC). The ROC curve is a plot of 1 specificity versus the sensitivity of the CD64 index at all observed values. The optimum cut off value of the CD64 index was identified with SAS based on maximizing the Youden index. Sensitivity, specificity, and standard deviations were calculated using Microsoft Excel software. For phase 2 analysis, comparisons of CD64 values or days of antibiotics between groups were performed using a Student t-test with a two-tailed distribution and confirmed equal variance. Statistical significance was established at a p value less than 0.05.
Results
A total of 182 evaluations (n = 108, age ≤ 12 months; n = 26, age = 1–12 years; n = 24, age = 12–18 years; and n = 24, age > 18 years) were performed during phase 1 study. The mean CD64 index (± standard deviation) in noninfected individuals in each age category was 1.61 (± 0.84), 1.26 (± 0.98), 0.81 (± 0.23), and 0.68 (± 0.19) for ages ≤ 12 months, 1 to 12 years, 12 to 18 years, and > 18 years, respectively. Results were generally available within 3 hours of obtaining the sample and were routinely obtained from 0.05 to 0.1 mL of blood. No variability was observed in CD64 indices when comparing samples that were stored in ethylenediaminetetraacetic acid versus sodium heparin.[37] In addition, no significant variability was observed in CD64 indices in control samples over time when samples were stored for 24 hours at room temperature (mean CD64 index was 0.51 at 0 hours and 0.57 after 24 hours in control samples from the > 18 years group). Finally, intra- and interassay runs found the CD64 index coefficient of variation was 7% (CD64 = 0.54 ± 0.04) in control samples.
A more in-depth analysis was performed on patients in the ≤ 12 months category. These infants ranged in gestational age at delivery from 25 to 40 weeks gestation and the chronologic age at the time of evaluation was 2 days to 12 months. Because a CD64 index was obtained in all infants who had a CBC, a CD64 index was obtained in both infants who were suspected of infection and proven negative, and in infants who had a CBC evaluation for noninfectious reasons. However, no difference was detected in CD64 indices between these two groups of noninfected infants (mean CD64 = 1.6, n = 14 proven not infected vs. 1.4, n = 84 controls, p = 0.52). A total of 11 infants in ≤ 12 months group were suspected of infection and proven positive (six infants had culture positive sepsis and five infants were diagnosed with clinical sepsis). The responsible organisms in the culture positive patients were Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, and Staphylococcus epidermidis.
The ROC curves for CD64 obtained during phase 1 study were calculated using the combined infected group of both culture positive and clinical sepsis in the ≤ 12 months category. Analysis of the ROC curve revealed an optimal cutoff value of CD64 index = 2.3 which produced a sensitivity and specificity of 100 and 93%, respectively.
In phase 2 of the study, 371 evaluations were performed in 234 infants (54% male and 46% female). Because of laboratory capacity limitations CD64 indices were not available 24 hours/day, therefore, 305 evaluations included a CD64 index, CBC, and culture (CD64 group) whereas 66 evaluations included CBC and culture only (CBC group). Gestational age ranged from 24 to 40 weeks with a mean of 32.5 weeks. The average age at time of testing was 59.4 days with a range of 1 to 293 days of life.
Overall 30 infants had positive blood cultures in the CD64 group and 10 infants had positive blood cultures in the CBC group. Because CD64 and CBC were used to direct clinical care and influenced decisions regarding antibiotic duration, the diagnosis of clinical sepsis was excluded from calculating sensitivity during phase 2 data analysis. Using a cutoff value for CD64 of 2.3 that was established in phase 1 of this study and previously published normal values for CBC indices (i.e., normal was defined as total white blood cell (WBC) = 5,000–20,000; absolute neutrophil count = 7,500–14,500; and immature:total neutrophil ratio < 0.2),[13] [14] [15] [16] [17] [18] the sensitivities of CD64 and the CBC indices of total WBC, absolute neutrophil count, and immature:total neutrophil ratio for detecting culture positive sepsis in patients in the CD64 group were 87, 50, 83, and 40%, respectively ([Figure 1]). Of the 30 infants, 4 infants with positive blood cultures in the CD64 group had normal CD64 index. In these infants, two had blood cultures that were positive for Staphylococcus epidermidis, one was diagnosed with Haemophilus influenza, and one had a positive blood culture that was collected from a central venous catheter.
Figure 1.
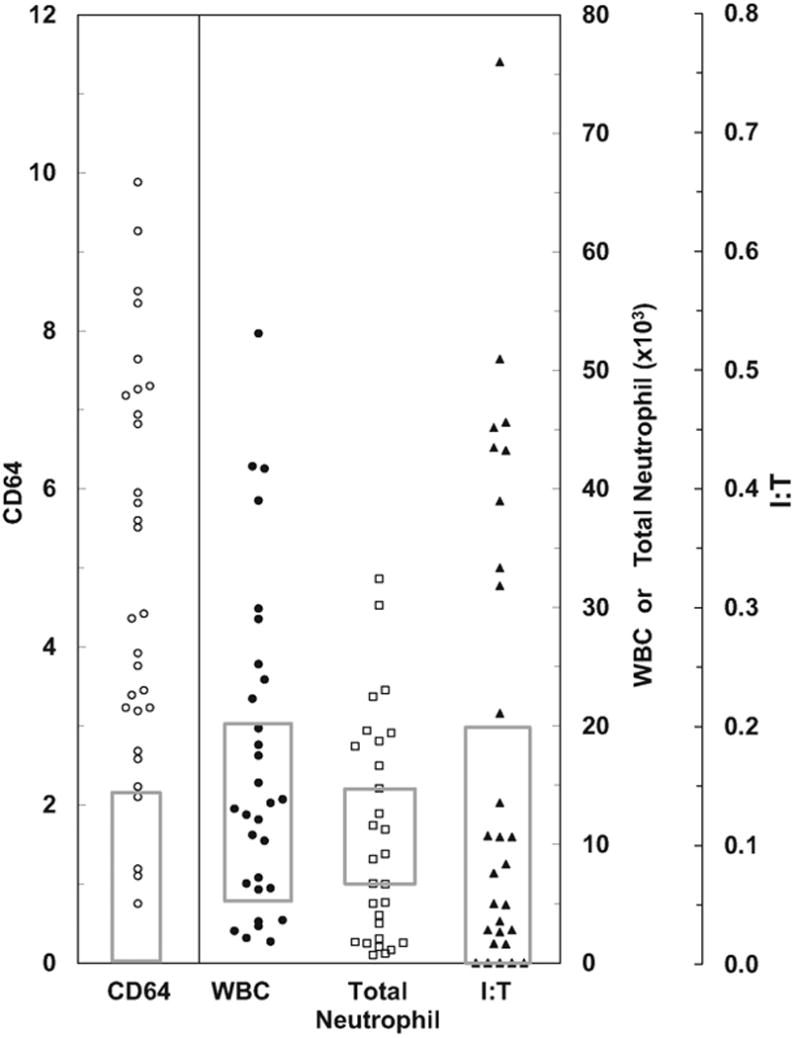
CD64 and white blood cell (WBC) indices in infants with culture positive sepsis. Thirty infants were identified with culture positive sepsis in our study group. CD64 and complete blood count (CBC) indices are reported for each infant with culture positive sepsis during phase 2 of this study. To determine the sensitivity of CD64 (CD64) and the CBC indices of total count, total neutrophil count (total neutrophil) and immature:total neutrophil ratio (I:T) in detecting culture positive sepsis, a CD64 normal range of < 2.3 and previously published normal values for CBC indices (indicated by gray boxes) were used.
To determine if the CD64 index correlated with disease severity, CD64 indices were compared in infants with positive blood cultures based on the need for mechanical ventilation or inotropic support ([Figure 2A, B]). When evaluating the entire population of culture positive infants, there was no significant difference in the CD64 index when comparing the need for mechanical ventilation (p = 0.87) or inotropic support (p = 0.90).
Figure 2.

CD64 values do not correlate with disease severity. CD64 indices were compared in infants with positive blood cultures based on the need for (A) mechanical ventilation or (B) inotropic support. When evaluating the entire population of culture positive infants as a whole, there was no significant difference in CD64 indices when comparing the need for mechanical ventilation (p = 0.87) or inotropic support (p = 0.90).
During phase 2, 16 infants in the study population were evaluated 20 times for ventilator-associated pneumonia (VAP). While all of these infants had a positive endotracheal tube culture and negative blood culture, only 12 evaluations met the National Healthcare Safety Network criteria for VAP. CD64 indices were elevated in 10 of these evaluations (83% sensitivity). In contrast, only 5 of the 12 infants with VAP had an abnormal CBC (42% sensitivity). CD64 indices were normal in five of the eight evaluations that were negative for VAP (63% specificity) ([Figure 3]).
Figure 3.
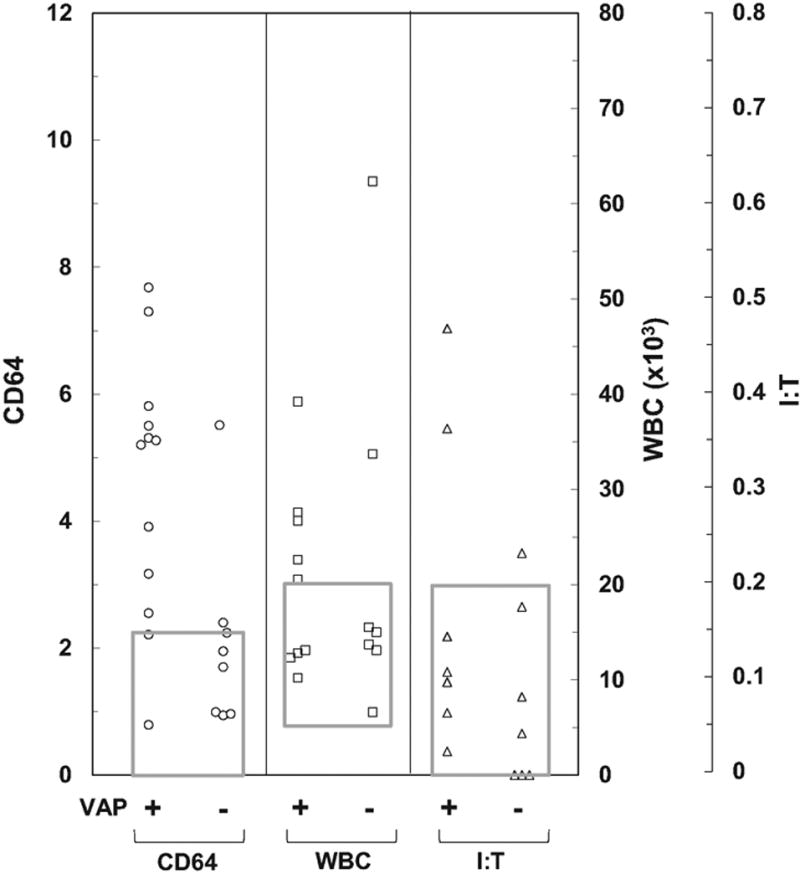
CD64 and complete blood count indices in infants with ventilator-associated pneumonia (VAP). Sixteen infants in the study population were specifically evaluated a total of 20 times for VAP. Twelve infants met the criteria for VAP (VAP +) and 8 were negative for VAP (VAP −). CD64 indices (CD64), white blood cell and I:T neutrophil ratios (I:T) were determined. WBC, white blood cell; I:T neutrophil ratio, immature:total neutrophil ratio.
One of the primary goals of this study was to improve the care of neonates by decreasing unnecessary antibiotic exposure. Therefore, we measured the duration of antibiotic exposure in infants with normal results in the CD64 group (i.e., CD64 < 2.3) versus those with normal results in the CBC group (i.e., a normal WBC, absolute neutrophil, and I:T ratio; [Table 1]) There was no significant difference in the mean number of antibiotic days in infants evaluated with a CD64 versus those evaluated with the traditional CBC (p = 0.89), but only 23% of the infants in the CBC group had a normal result, whereas 51% of the infants in the CD64 group had a normal result.
Table 1.
Duration of antibiotic use in infants evaluated with a CD64 versus CBC (days ± standard deviation)
| Normal result | Abnormal result | |
|---|---|---|
| CD64 group | 4.3 ± 4.7 (n = 156, 51%) | 7.7 ± 5.1 (n = 149, 49%) |
| CBC group | 4.5 ± 3.5 (n = 15, 23%) | 5.2 ± 5.2 (n = 51, 77%) |
| p Value | 0.89[a] | 0.006[a] |
Abbreviation: CBC, complete blood count
p values were calculated using a Student t-test.
When infants with elevated CD64 indices in the CD64 group were compared with infants with abnormal WBC indices in the CBC group, the CD64 group was treated with significantly longer antibiotics (p = 0.006) ([Table 1]). Ultimately, the difference of an abnormal versus normal result in the CD64 group was associated with a change of 3.4 antibiotic days versus a change of only 0.7 days in the CBC group.
Discussion
For decades, a CBC and blood culture were the standard practices in neonatology when evaluating an infant for suspected sepsis. However, mounting evidence continues to suggest that these tests are significantly limited in their ability to diagnose sepsis and that failing to evaluate sepsis quickly and accurately can place the infant at risk.[7] [8] [9] [13] [14] [15] [16] The purpose of this study was to evaluate the validity and reliability of neutrophil CD64 index in clinical practice to determine if this marker improved the care of potentially infected neonates.
During phase 1 of this study, normal values were established for CD64 in the study population. Although this study focused toward the neonatal population, there was concern that CD64 indices may vary with age; therefore, CD64 indices were initially measured in noninfected patients across a broad age range. The mean CD64 index did increase with decreasing age, but the relative change was minor in comparison to increases observed in infected neonates. Within the infant population, an appropriate cutoff value was established by ROC curve (CD64 index = 2.3) for the combined group of culture positive and clinical sepsis. This result was based on a relatively small number of patients, but it was only meant to validate the results of previous studies reporting a similar optimal cutoff and confirmed that this assay was reproducible at our institution.[12] [28] [29] [31] [32] [33] [34]
During phase 2 of this study, CD64 was made available as part of the sepsis evaluation in the NICU and the impact of this marker on neonatal care was monitored. Because CD64 indices were used to determine duration of antibiotic use, which is part of the definition of clinical sepsis, the sensitivity of CD64 for detecting clinical sepsis during phase 2 of the study could not be evaluated, but the sensitivity of CD64 for detecting culture positive sepsis could be evaluated. The observed CD64 sensitivity of 87% was higher than the sensitivity of the individual CBC indices, but slightly lower than the sensitivity predicted by the ROC curve in phase 1. The reason for this lower CD64 sensitivity during phase 2 is uncertain, but it may have been influenced by three of the culture positive patients with low CD64 indices that potentially represented contaminants and not true systemic infection: two were positive for Staphylococcus epidermidis and one had a positive culture that was obtained a presumptively colonized line. Despite this potential caveat, our observed CD64 sensitivity of 87% is consistent with previous results and a marked improvement from the sensitivity of the traditional CBC.
We originally hypothesized that CD64 indices would predict the severity of the infection; however, we found no correlation between CD64 indices and disease severity. It is possible that a correlation would be revealed if the many factors that influence disease severity in the critically ill neonate were eliminated via a multivariate analysis, but that analysis is beyond the scope of this study.
Although the CD64 index performed relatively well in diagnosing neonatal sepsis, when evaluating individual patients our data suggests that a normal CD64 did not lead to a decrease in the number of antibiotic days when compared with a normal CBC. It is important to note that there were no criteria within our protocol for stopping antibiotics and any observed change in antibiotic use was due solely to the impact of the test on clinical decision making by the care provider. Despite this limitation, twice as many of the evaluated infants were identified as “not infected” by normal CD64 versus a normal CBC (51 and 21%, respectively) suggesting that CD64 would ultimately lead to an overall decrease in unnecessary antibiotic use. In addition, a comparison of the number of antibiotic days in infants with abnormal and normal results in the CD64 group versus CBC group suggests that CD64 had a much greater impact on antibiotic use (i.e., 3.4 vs. 0.7 days, respectively) and that in general; neonatologists found CD64 to be more influential than CBC when evaluating potentially infected infants. This influence may have been even greater had we coupled CD64 with a quality improvement initiative focused toward using this marker to stop unnecessary antibiotic use. Given the high sensitivity of CD64, it would not be unreasonable to either wait until the CD64 result returned to start antibiotics or stop antibiotics in infants that are clinically stable when a normal CD64 index is returned (i.e., 3–4 hours after the evaluation) rather than completing the traditional 48-hour rule out while waiting for blood culture results.
It is important to note that our conclusions are limited by the lack of randomization between the CD64 and CBC groups although our infants were essentially quasi-randomized as the group determination was based on when the sepsis evaluation occurred (i.e., weekend vs. weekday). In addition, this study only compared CD64 to the traditional CBC, it did not compare the neutrophil CD64 index to other biomarkers such as C-reactive protein or procalcitonin.
In summary, these findings suggest that CD64 is a very sensitive marker for detecting neonatal sepsis and VAP and that this test decreased unnecessary antibiotic use when it was placed into neonatal clinic.
Acknowledgments
This work was supported by National Institutes of Health grant HL089505 (PSK).
References
- 1.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 2.St John EB, Carlo WA. Respiratory distress syndrome in VLBW infants: changes in management and outcomes observed by the NICHD Neonatal Research Network. Semin Perinatol. 2003;27(4):288–292. doi: 10.1016/s0146-0005(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, Gordon T, Korones SB, et al. Early-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129(1):72–80. doi: 10.1016/s0022-3476(96)70192-0. [DOI] [PubMed] [Google Scholar]
- 5.Ganatra HA, Stoll BJ, Zaidi AK. International perspective on early-onset neonatal sepsis. Clin Perinatol. 2010;37(2):501–523. doi: 10.1016/j.clp.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Brodie SB, Sands KE, Gray JE, et al. Occurrence of nosocomial bloodstream infections in six neonatal intensive care units. Pediatr Infect Dis J. 2000;19(1):56–65. doi: 10.1097/00006454-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Isaacman DJ, Karasic RB, Reynolds EA, Kost SI. Effect of number of blood cultures and volume of blood on detection of bacteremia in children. J Pediatr. 1996;128(2):190–195. doi: 10.1016/s0022-3476(96)70388-8. [DOI] [PubMed] [Google Scholar]
- 8.Benitz WE. Adjunct laboratory tests in the diagnosis of early-onset neonatal sepsis. Clin Perinatol. 2010;37(2):421–438. doi: 10.1016/j.clp.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Kumar Y, Qunibi M, Neal TJ, Yoxall CW. Time to positivity of neonatal blood cultures. Arch Dis Child Fetal Neonatal Ed. 2001;85(3):F182–F186. doi: 10.1136/fn.85.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonadio WA, Hennes H, Smith D, et al. Reliability of observation variables in distinguishing infectious outcome of febrile young infants. Pediatr Infect Dis J. 1993;12(2):111–114. doi: 10.1097/00006454-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Baraff LJ. Management of fever without source in infants and children. Ann Emerg Med. 2000;36(6):602–614. doi: 10.1067/mem.2000.110820. [DOI] [PubMed] [Google Scholar]
- 12.Ng PC. Diagnostic markers of infection in neonates. Arch Dis Child Fetal Neonatal Ed. 2004;89(3):F229–F235. doi: 10.1136/adc.2002.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da Silva O, Ohlsson A, Kenyon C. Accuracy of leukocyte indices and C-reactive protein for diagnosis of neonatal sepsis: a critical review. Pediatr Infect Dis J. 1995;14(5):362–366. doi: 10.1097/00006454-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Schelonka RL, Yoder BA, des Jardins SE, Hall RB, Butler J. Peripheral leukocyte count and leukocyte indexes in healthy newborn term infants. J Pediatr. 1994;125(4):603–606. doi: 10.1016/s0022-3476(94)70018-4. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg AG, Rosenfeld CR, Manroe BL, Browne R. Neonatal blood cell count in health and disease. II. Values for lymphocytes, monocytes, and eosinophils. J Pediatr. 1985;106(3):462–466. doi: 10.1016/s0022-3476(85)80681-8. [DOI] [PubMed] [Google Scholar]
- 16.Schmutz N, Henry E, Jopling J, Christensen RD. Expected ranges for blood neutrophil concentrations of neonates: the Manroe and Mouzinho charts revisited. J Perinatol. 2008;28(4):275–281. doi: 10.1038/sj.jp.7211916. [DOI] [PubMed] [Google Scholar]
- 17.Manroe BL, Rosenfeld CR, Weinberg AG, Browne R. The differential leukocyte count in the assessment and outcome of early-onset neonatal group B streptococcal disease. J Pediatr. 1977;91(4):632–637. doi: 10.1016/s0022-3476(77)80522-2. [DOI] [PubMed] [Google Scholar]
- 18.Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease. I. Reference values for neutrophilic cells. J Pediatr. 1979;95(1):89–98. doi: 10.1016/s0022-3476(79)80096-7. [DOI] [PubMed] [Google Scholar]
- 19.Chiesa C, Pellegrini G, Panero A, et al. C-reactive protein, interleukin-6, and procalcitonin in the immediate postnatal period: influence of illness severity, risk status, antenatal and perinatal complications, and infection. Clin Chem. 2003;49(1):60–68. doi: 10.1373/49.1.60. [DOI] [PubMed] [Google Scholar]
- 20.Guibourdenche J, Bedu A, Petzold L, et al. Biochemical markers of neonatal sepsis: value of procalcitonin in the emergency setting. Ann Clin Biochem. 2002;39(Pt 2):130–135. doi: 10.1258/0004563021901874. [DOI] [PubMed] [Google Scholar]
- 21.Ng PC, Cheng SH, Chui KM, et al. Diagnosis of late onset neonatal sepsis with cytokines, adhesion molecule, and C-reactive protein in preterm very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1997;77(3):F221–F227. doi: 10.1136/fn.77.3.f221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger C, Uehlinger J, Ghelfi D, Blau N, Fanconi S. Comparison of C-reactive protein and white blood cell count with differential in neonates at risk for septicaemia. Eur J Pediatr. 1995;154(2):138–144. doi: 10.1007/BF01991918. [DOI] [PubMed] [Google Scholar]
- 23.Monneret G, Labaune JM, Isaac C, Bienvenu F, Putet G, Bienvenu J. Procalcitonin and C-reactive protein levels in neonatal infections. Acta Paediatr. 1997;86(2):209–212. doi: 10.1111/j.1651-2227.1997.tb08870.x. [DOI] [PubMed] [Google Scholar]
- 24.Lapillonne A, Basson E, Monneret G, Bienvenu J, Salle BL. Lack of specificity of procalcitonin for sepsis diagnosis in premature infants. Lancet. 1998;351(9110):1211–1212. doi: 10.1016/S0140-6736(05)79165-0. [DOI] [PubMed] [Google Scholar]
- 25.Cardelli P, Ferraironi M, Amodeo R, et al. Evaluation of neutrophil CD64 expression and procalcitonin as useful markers in early diagnosis of sepsis. Int J Immunopathol Pharmacol. 2008;21(1):43–49. doi: 10.1177/039463200802100106. [DOI] [PubMed] [Google Scholar]
- 26.Chiesa C, Panero A, Rossi N, et al. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis. 1998;26(3):664–672. doi: 10.1086/514576. [DOI] [PubMed] [Google Scholar]
- 27.Lacour AG, Gervaix A, Zamora SA, et al. Procalcitonin, IL-6, IL-8, IL-1 receptor antagonist and C-reactive protein as identificators of serious bacterial infections in children with fever without localising signs. Eur J Pediatr. 2001;160(2):95–100. doi: 10.1007/s004310000681. [DOI] [PubMed] [Google Scholar]
- 28.Ng PC, Li G, Chui KM, et al. Neutrophil CD64 is a sensitive diagnostic marker for early-onset neonatal infection. Pediatr Res. 2004;56(5):796–803. doi: 10.1203/01.PDR.0000142586.47798.5E. [DOI] [PubMed] [Google Scholar]
- 29.Ng PC, Li K, Wong RP, Chui KM, Wong E, Fok TF. Neutrophil CD64 expression: a sensitive diagnostic marker for late-onset nosocomial infection in very low birthweight infants. Pediatr Res. 2002;51(3):296–303. doi: 10.1203/00006450-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Elghetany MT. Surface antigen changes during normal neutrophilic development: a critical review. Blood Cells Mol Dis. 2002;28(2):260–274. doi: 10.1006/bcmd.2002.0513. [DOI] [PubMed] [Google Scholar]
- 31.Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates: neutrophil CD64 as a diagnostic marker. Pediatrics. 2008;121(1):129–134. doi: 10.1542/peds.2007-1308. [DOI] [PubMed] [Google Scholar]
- 32.Streimish I, Bizzarro M, Northrup V, et al. Neutrophil CD64 as a diagnostic marker in neonatal sepsis. Pediatr Infect Dis J. 2012;31(7):777–781. doi: 10.1097/INF.0b013e318256fb07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genel F, Atlihan F, Gulez N, et al. Evaluation of adhesion molecules CD64, CD11b and CD62L in neutrophils and monocytes of peripheral blood for early diagnosis of neonatal infection. World J Pediatr. 2012;8(1):72–75. doi: 10.1007/s12519-011-0304-6. [DOI] [PubMed] [Google Scholar]
- 34.Dilli D, Oğuz SS, Dilmen U, Köker MY, Kızılgün M. Predictive values of neutrophil CD64 expression compared with interleukin-6 and C-reactive protein in early diagnosis of neonatal sepsis. J Clin Lab Anal. 2010;24(6):363–370. doi: 10.1002/jcla.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis BH, Bigelow NC. Comparison of neutrophil CD64 expression, manual myeloid immaturity counts, and automated hematology analyzer flags as indicators of infection or sepsis. Lab Hematol. 2005;11(2):137–147. doi: 10.1532/LH96.04077. [DOI] [PubMed] [Google Scholar]
- 36.Davis BH, Olsen SH, Ahmad E, Bigelow NC. Neutrophil CD64 is an improved indicator of infection or sepsis in emergency department patients. Arch Pathol Lab Med. 2006;130(5):654–661. doi: 10.5858/2006-130-654-NCIAII. [DOI] [PubMed] [Google Scholar]
- 37.Kingma P, Marmer D. Laboratory and Clinical Practice for Monitoring Sepsis with Neutrophil CD64 Index: Seven Year Experience at Cincinnati Children’s Hospital Medical Center. Glenview, IL: International Clinical Cytometry Society; 2011. [Google Scholar]


