Abstract
Familial dysbetalipoproteinemia (FD) is a genetic disorder associated with impaired postprandial lipid clearance. The effect of adding bezafibrate to standard lipid-lowering therapy on postprandial and fasting lipid levels in patients with FD is unknown. In this randomized placebo-controlled double-blind crossover trial, 15 patients with FD received bezafibrate and placebo for 6 weeks in randomized order in addition to standard lipid-lowering therapy (statin, ezetimibe, and/or lifestyle). We assessed post-fat load lipids, expressed as incremental area under the curve (iAUC) and area under the curve (AUC), as well as fasting levels and safety, and found that adding bezafibrate did not reduce post-fat load non-HDL-cholesterol (non-HDL-C) iAUC (1.78 ± 4.49 mmol·h/l vs. 1.03 ± 2.13 mmol·h/l, P = 0.57), but did reduce post-fat load triglyceride (TG) iAUC (8.05 ± 3.32 mmol·h/l vs. 10.61 ± 5.92 mmol·h/l, P = 0.03) and apoB (0.64 ± 0.62 g·h/l vs. 0.93 ± 0.71 g·h/l, P = 0.01). Furthermore, bezafibrate significantly improved AUC and fasting levels of non-HDL-C, TG, total cholesterol, HDL-C, and apoB. Bezafibrate was associated with lower estimated glomerular filtration rate (78.4 ± 11.4 ml/min/1.73 m2 vs. 86.1 ± 5.85 ml/min/1.73 m2, P = 0.002). In conclusion, in patients with FD, the addition of bezafibrate to standard lipid-lowering therapy resulted in improved post-fat load and fasting plasma lipids. Combination therapy of statin/fibrate could be considered as standard lipid-lowering treatment in FD.
Keywords: clinical trial, dyslipidemias, hypolipidemic drugs, diet effects/lipid metabolism, cholesterol, type III hyperlipoproteinemia, fibrates, fasting, non-high density lipoprotein cholesterol, triglycerides, familial dysbetalipoproteinemia
Familial dysbetalipoproteinemia (FD) is a genetic disorder characterized by accumulation of atherogenic lipoprotein remnants in the circulation (1). FD is associated with mutations in the APOE gene, which is involved in hepatic remnant clearance. Mutations in APOE can cause decreased hepatic receptor binding, which leads to reduced clearance and subsequent increased plasma levels of remnants. High remnant cholesterol and high levels of non-HDL-cholesterol (non-HDL-C) [non-HDL-C = total cholesterol (TC) minus HDL-C] are associated with increased CVD risk (2, 3). The prevalence of FD in the general population is approximately 1 in 850 individuals (4, 5).
The treatment target in FD is non-HDL-C instead of LDL-cholesterol (LDL-C) (6) because FD patients usually have low to normal LDL-C plasma levels and normal HDL-C concentration (7). LDL-C is low because the conversion of VLDL to LDL-C is decreased (8). The recommended treatment in guidelines for FD is statin/fibrate combination therapy because statins increase hepatic LDL uptake, reduce VLDL production, and decrease CVD risk, while fibrates reduce triglycerides (TGs) (9, 10). However, in clinical practice only 10% of the FD patients are treated with statin/fibrate combination (6), which is probably due to an ongoing debate about the efficacy of fibrate therapy in reducing clinical endpoints in patients with T2D (11) and the risk of adverse events, such as myalgia and rhabdomyolysis during statin/fibrate combination therapy (12). Furthermore, clinical trial evidence to substantiate treatment decisions in FD is scarce. Two clinical trials that included a total of 31 FD patients showed that statin/fibrate combination therapy decreased fasting levels of TC, TG, and VLDL-cholesterol (VLDL-C) in 12 patients that remained hypercholesterolemic on monotherapy with either fibrate or statin (13, 14). With regard to postprandial lipids, a study that evaluated the effect of statins on postprandial fat clearance in five FD patients showed significant reductions in both VLDL synthesis and absolute cholesterol absorption compared with no statin treatment, but no improvement in the delayed postprandial fat clearance (15). Fenofibrate has been shown to reduce postprandial TG compared with placebo in patients with T2D, but has not been investigated in FD (16).
To assess whether addition of bezafibrate (400 mg daily) to standard lipid-lowering therapy would reduce fasting and post-fat load lipid levels compared with placebo in FD, we performed a randomized placebo-controlled double-blind crossover study.
MATERIALS AND METHODS
Patients
Study subjects were recruited from the outpatient clinic of the Department of Vascular Medicine at the University Medical Center Utrecht for hyperlipidemia or primary/secondary prevention of vascular disease. In total, 15 patients were included. After genetic confirmation of FD, the treating physician approached the patients to participate in the study. Inclusion criteria were a genetically confirmed ε2ε2 genotype or autosomal dominant mutation in the apoE gene (APOE) in combination with (at least) one of the following clinical characteristics: (history of) presence of xanthoma, apoB/TC ratio <0.15, or use of lipid-lowering medication. All patients were over 18 years and received standard treatment for FD, which included lifestyle measures with or without pharmacological therapy with statin and/or ezetimibe. Women were only included if they were postmenopausal. Exclusion criteria were: current use of or intolerance to fibrates; use of oral anticoagulants; history of cholelithiasis or previous cholecystectomy; uncontrolled diabetes mellitus [glycated hemoglobin (HbA1c) >69 mmol/mol]; increased hepatic enzymes (>1.5 times upper limit of normal); impaired renal function [estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2]; increased levels of creatinine kinase (CK) (CK >3 × upper limit of normal); or use of a cytochrome P450 3A4 inhibitor. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. The Medical Ethics Review Committee of the institution approved the study and all participants provided written informed consent prior to study enrollment.
Study design
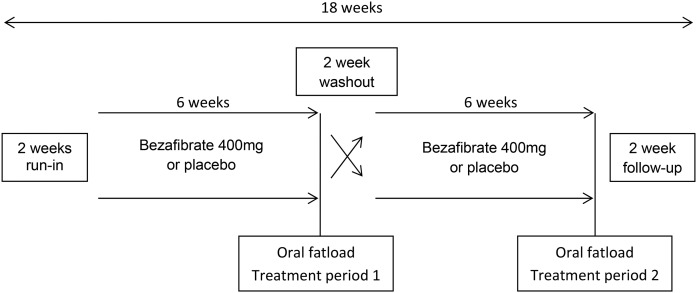
The study was a monocenter randomized double-blind placebo-controlled crossover study. Randomization for treatment order was performed in blocks of four. Patients and staff were blinded for treatment order and outcome measures. Participants received bezafibrate [400 mg (slow-release)] once daily in the morning for a period of 6 weeks and placebo once daily for a period of 6 weeks in random order (Fig. 1). A washout period of 2 weeks without study medication controlled for carryover effects because the half-life of bezafibrate is short (4 h) (17). Patients were instructed not to change their diet, alcohol use, medication use, or physical activity during the study.
Fig. 1.
Study design.
At the start of the first treatment period (baseline) and after both treatment periods, patients visited the hospital to receive an oral fat load after an overnight fast. The fat load consisted of unsweetened fresh cream with a fat content of 35% (mass/volume). Cream was administered at a dose of 110 g of fat per square meter of body surface area, with a maximum of 500 ml. Before and 2, 3, 4, and 6 h following the fat load, venous blood samples were collected. During this period patients were allowed to drink water. Endpoints were (incremental) post-fat load and fasting concentrations of non-HDL-C, TG, TC, HDL-C, apoB, insulin, glucose, and high sensitive C-reactive protein (hsCRP). Safety and tolerability were also assessed.
The sample size was based on an expected reduction in (incremental) postprandial non-HDL-C of 15% for bezafibrate compared with placebo and a median Cohen’s effect size. The 15% reduction was based on the observation that adding fenofibrate to atorvastatin led to an additional postprandial TG reduction of 25% (11, 18). For a parallel group design with a power of 80%, this resulted in a sample size (N) of 92 patients. To calculate the sample size for a crossover design, we used the formula [(1 − ρ) × N]/2 with 0.7 for ρ, the correlation between measurements (19). This resulted in a required sample size of 14 patients for this trial, but 15 patients were included to compensate for any loss-to-follow up during the study.
Definitions and measurements
CVD at baseline was defined as a history of coronary artery disease (angina pectoris, myocardial infarction, coronary artery bypass graft or percutaneous intervention), cerebrovascular disease accident (stroke or transient ischemic attack), peripheral arterial disease (leg claudication or peripheral revascularization), or abdominal aorta aneurysm. T2D was defined as self-reported presence of T2D, use of glucose-lowering agents, a fasting plasma glucose level ≥7.0 mmol/l, or HbA1c >48 mmol/mol at screening (20). Hypertension was defined as self-reported presence of hypertension, use of antihypertensive agents, or a high blood pressure (BP) at baseline (systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg). BP was measured once at the upper right and left arm using the appropriate cuff size. The mean of the two measurements was reported. BMI was calculated by dividing mass (in kilograms) by height (in meters) squared. Waist circumference (WC) was measured halfway between the lower costal margin and the iliac crest when standing. Metabolic syndrome was defined, using the ATP III criteria, as having at least three of the following metabolic abnormalities (21): WC >102 cm for males and >88 cm for females; fasting TGs ≥1.7 mmol/l (150 mg/dl); HDL-C <1.03 mmol/l (40 mg/dl) for males and HDL-c <1.29 mmol/l (50 mg/dl) for females; systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg; fasting plasma glucose ≥5.6 mmol/l. Insulin resistance was expressed as homeostasis model assessment parameter of insulin resistance (HOMA-IR). HOMA-IR was calculated as fasting serum glucose (mmol/l) × fasting serum insulin (mIU/l)/22.5 (22).
Laboratory analyses
Laboratory samples were analyzed on coded specimens without knowledge of treatment allocation. TC, TG, and fasting glucose were measured with a commercial enzymatic dry chemistry kit (Johnson & Johnson, New Brunswick, NJ) and HDL-C was measured with a commercial enzymatic kit (Boehringer, Mannheim, Germany). Non-HDL-C was calculated as TC minus HDL-C. HbA1c was measured using high-performance liquid chromatography on a HA-8180 analyzer (Menarini Diagnostics, Florence, Italy). Insulin was measured with an immunometric technique on an IMMULITE 1000 analyzer (Diagnostic Products Corporation, Los Angeles, CA). Serum hsCRP was measured by immunonephelometry (Nephelometer Analyzer BN II; Dade-Behring, Germany). Thyroid stimulating hormone measurements were done by a Dxi analyzer (Beckman Coulter, Woerden, The Netherlands). Creatinine, CK, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured with an enzymatic colorimetric assay (DxAU 5811; Beckman Coulter, Brea, CA). The eGFR was estimated with the modification of diet in renal disease (MDRD) formula (23).
Data analyses
Baseline variables were presented as mean with standard deviation or median with interquartile range (IQR) when appropriate. Categorical variables were shown as number with percentage. Post-fat load lipids were expressed as area under the curve (AUC) and incremental area under the curve (iAUC). AUC (in millimoles per hour per liter) was calculated with the trapezoid rule for post-fat load measurements (at 0, 2, 3, 4, and 6 h). The iAUC was calculated to adjust the AUC for the fasting value by subtracting six (hours) × the fasting value (at timepoint 0) from the AUC.
Differences in mean iAUC, AUC, and fasting values between the two treatment arms were calculated using a paired t-test or a Wilcoxon signed rank sum test in case of nonlinearity. Carryover and period effects were assessed with an independent samples t-test. There were no missing values. P < 0.05 was considered statistically significant. For the statistical analyses, R version 3.1.1 (R Development Core Team, Vienna, Austria) and SPSS version 21 (Chicago, IL) were used.
RESULTS
Baseline characteristics
Fifteen patients were included, 2 women and 13 men (Table 1). The mean age was 61.5 ± 10.0 years. One patient had a dominant type of FD (K146Q mutation) and 14 patients had a ε2ε2 genotype. Four patients had (a history of) xanthoma. Four patients had T2D, four patients had hypertension, and five patients had a history of CVD. Lipid-lowering medication consisted of statin only (N = 8) or statin plus ezetimibe (N = 5). Two patients were on a low-fat diet only. The BMI was 27.1 ± 2.0 kg/m2.
TABLE 1.
Baseline characteristics
| All Patients (n = 15) | |
| Age (years) | 61.5 ± 10.0 |
| Male sex (N, %) | 13 (87) |
| APOE genotype | |
| ε2ε2 | 14 (93) |
| Dominant | 1 (8) |
| History of xanthoma (N, %) | 4 (27) |
| CVD | 5 (33) |
| CAD (N, %) | 1 (7) |
| PAD (N, %) | 1 (7) |
| CVA (N, %) | 2 (13) |
| AAA (N, %) | 1 (7) |
| Current smoking (N, %) | 0 (0) |
| T2D (N, %) | 4 (27) |
| Hypertension (N, %) | 4 (27) |
| Metabolic syndrome (N, %) | 9 (60) |
| Family history of CVD (N, %) | 5 (33) |
| Lipid-lowering treatment | |
| Diet only (N, %) | 2 (13) |
| Statin only (N, %) | 8 (53) |
| Statin + ezetimibe (N, %) | 5 (33) |
| BMI (kg/m2) | 27.1 ± 2.0 |
| WC (cm) | 101 ± 7 |
| Systolic BP (mmHg) | 138 ± 13 |
| Diastolic BP (mmHg) | 86 ± 7 |
| HbA1c (mmol/mol) | 37.3 ± 4.0 |
| Creatinine (µmol/l) | 76 ± 13 |
| eGFR (ml/min/1.73 m2) | 85 ± 7 |
| CK (U/l) | 136 ± 47 |
| AST (U/l) | 35 ± 7 |
| ALT (U/l) | 34 ± 13 |
| Thyroid stimulating hormone (mIU/l) | 1.90 ± 1.16 |
CAD, coronary artery disease; PAD, peripheral artery disease; CVA, cerebrovascular accident; AAA, abdominal aorta aneurysm.
Post-fat load lipids
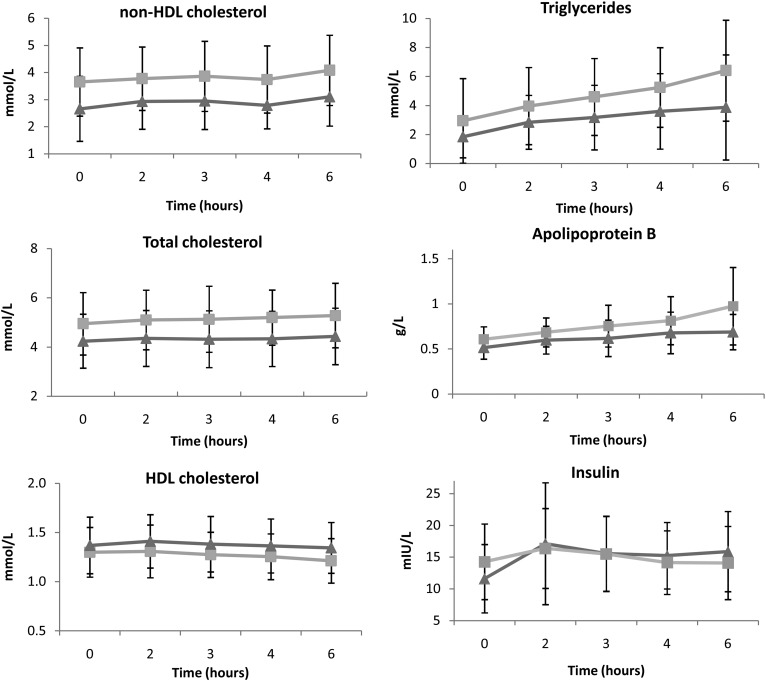
Addition of bezafibrate to standard lipid-lowering treatment did not reduce non-HDL-C iAUC compared with placebo (1.78 ± 4.49 mmol·h/l vs. 1.03 ± 2.13 mmol·h/l, P = 0.57), but did decrease TG iAUC (8.05 ± 3.32 mmol·h/l vs. 10.61 ± 5.92 mmol·h/l, P = 0.03) and apoB iAUC (0.64 ± 0.62 g·h/l vs. 0.93 ± 0.71 g·h/l, P = 0.01) (Fig. 2). Bezafibrate also increased insulin iAUC (21.9 ± 14.7 mIU·h/l vs. 4.0 ± 28.5 mIU·h/l, P = 0.03) and HOMA-IR (4.40 ± 4.11 vs. −0.37 ± 6.92, P = 0.03). No differences in iAUC of TC, HDL-C, glucose, or hsCRP were observed (Table 2). There were no carry-over (P = 0.23) or period effects (P = 0.50).
Fig. 2.
Post-fat load lipid levels (with 95% CI) after treatment with bezafibrate (triangles) and placebo (squares).
TABLE 2.
Post-fat load iAUC after 6 weeks of bezafibrate compared with placebo
| Placebo iAUC | Bezafibrate iAUC | P | |
| Non-HDL-C (mmol·h/l) | 1.03 ± 2.13 | 1.78 ± 4.49 | 0.5719 |
| TG (mmol·h/l) | 10.61 ± 5.92 | 8.05 ± 3.32 | 0.0266a |
| TC (mmol·h/l) | 1.18 ± 1.44 | 0.76 ± 0.69 | 0.3491 |
| HDL-C (mmol·h/l) | −0.16 ± 0.42 | 0.03 ± 0.24 | 0.0717 |
| apoB (g·h/l) | 0.93 ± 0.71 | 0.64 ± 0.62 | 0.0098a |
| Glucose (mmol·h/l) | −2.78 ± 2.26 | −1.88 ± 1.47 | 0.1565 |
| Insulin (mIU·h/l) | 4.0 ± 28.5 | 21.9 ± 14.7 | 0.0275a |
| HOMA-IR | −0.37 ± 6.92 | 4.40 ± 4.11 | 0.0304a |
| hsCRP (mg·h/l) | −0.54 ± 0.98 | −0.02 ± 1.14 | 0.3036 |
Data are expressed as mean ± SD.
P < 0.05.
The AUC of non-HDL-C was significantly reduced by addition of bezafibrate compared with placebo (16.4 ± 4.7 mmol·h/l vs. 22.2 ± 7.6 mmol·h/l, P = 0.002), as were TG AUC (15.8 mmol·h/l, IQR 14.0–22.2 mmol·h/l vs. 24.5 mmol·h/l, IQR 19.3–31.3 mmol·h/l, P < 0.001), TC AUC (24.9 ± 5.5 mmol·h/l vs. 29.8 ± 8.5 mmol·h/l, P = 0.007), and apoB AUC (3.55 ± 0.97 g·h/l vs. 4.53 ± 1.41 g·h/l, P < 0.001; Table 3). The HDL-C AUC was increased (8.33 ± 1.55 mmol·h/l vs. 7.34 ± 1.81 mmol·h/l, P < 0.001). No differences in the AUC of glucose, insulin, HOMA-IR, or hsCRP were observed.
TABLE 3.
Post-fat load AUC after 6 weeks of bezafibrate compared with placebo
| PlaceboAUC | BezafibrateAUC | P | |
| Non-HDL-C (mmol·h/l) | 22.2 ± 7.6 | 16.4 ± 4.7 | 0.0022a |
| TG (mmol·h/l) | 24.5 (19.3–31.3) | 15.8 (14.0–22.2) | 0.0001a |
| TC (mmol·h/l) | 29.8 ± 8.5 | 24.9 ± 5.5 | 0.0070a |
| HDL-C (mmol/l) | 7.34 ± 1.81 | 8.33 ± 1.55 | <0.0001a |
| ApoB (g·h/l) | 4.53 ± 1.41 | 3.55 ± 0.97 | 0.0007a |
| Glucose (mmol·h/l) | 31.98 ± 3.45 | 32.56 ± 3.47 | 0.3938 |
| Insulin (mIU·h/l) | 83.5 (63.0–115.2) | 81.4 (66.7–112.2) | 0.8469 |
| HOMA-IR | 21.2 (15.4–26.9) | 19.7 (15.5–25.8) | 0.9341 |
| hsCRP (mg·h/l) | 8.30 (6.13–18.42) | 8.80 (5.63–13.27) | 0.7615 |
Data are expressed as mean ± SD or median (IQR).
P < 0.05.
Fasting lipids
Treatment with bezafibrate significantly lowered fasting plasma concentrations of non-HDL-C (2.43 ± 0.96 mmol/l vs. 3.53 ± 1.35 mmol/l, P = 0.008), TG (1.50 mmol/l, IQR 1.20–1.90 mmol/l vs. 2.60 mmol/l, IQR 2.19–3.40 mmol/l; P = 0.001), TC (4.02 ± 0.91 mmol/l vs. 4.78 ± 1.42 mmol/l, P = 0.014), and apoB (0.49 ± 0.10 g/l vs. 0.60 ± 0.17 g/l, P = 0.002) compared with placebo (Table 4). HDL-C levels were significantly increased compared with placebo (1.38 ± 0.27 mmol/l vs. 1.25 ± 0.32 mmol/l, P = 0.002). Bezafibrate reduced fasting insulin levels compared with placebo (9.9 mIU/l, IQR 8.2–12.5 mIU/l vs. 12.0 mIU/l, IQR 10.8–17.5 mIU/l; P = 0.045), but did not affect fasting glucose or hsCRP levels.
TABLE 4.
Fasting concentrations after 6 weeks of bezafibrate compared with placebo
| Placebo | Bezafibrate | P | |
| Non-HDL-C (mmol/l) | 3.53 ± 1.35 | 2.43 ± 0.96 | 0.0079a |
| TG (mmol/l) | 2.60 (2.19–3.40) | 1.50 (1.20–1.90) | 0.0012a |
| TC (mmol/l) | 4.78 ± 1.42 | 4.02 ± 0.91 | 0.0144a |
| HDL-C (mmol/l) | 1.25 ± 0.32 | 1.38 ± 0.27 | 0.0017a |
| ApoB (g/l) | 0.60 ± 0.17 | 0.49 ± 0.10 | 0.0020a |
| Glucose (mmol/l) | 5.79 ± 0.77 | 5.74 ± 0.64 | 0.6796 |
| Insulin (mIU/l) | 12.0 (10.8–17.5) | 9.9 (8.2–12.5) | 0.0445a |
| HOMA-IR | 3.1 (2.7–4.3) | 2.9 (2.1–3.2) | 0.1726 |
| hsCRP (mg/l) | 1.40 (1.05–3.10) | 1.40 (0.95–2.40) | 1.00 |
Data are expressed as mean ± SD or median (IQR).
P-value <0.05.
Safety and tolerability
All 15 patients completed both treatment periods. Bezafibrate was generally well-tolerated. There were no serious adverse events and none of the patients discontinued treatment. Side effects occurred in seven patients (supplemental Table S1), of whom one developed myalgia in combination with CK 1,781 U/l. Bezafibrate was associated with higher serum creatinine compared with placebo (86.2 ± 14.7 umol/l vs. 76.1 ± 10.3 umol/l, P < 0.001) and an accompanying lower eGFR (78.4 ± 11.4 ml/min/1.73 m2 vs. 86.13 ± 5.85 ml/min/1.73 m2, P = 0.002). Other safety parameters did not differ between bezafibrate and placebo (Table 5).
TABLE 5.
Safety outcomes after 6 weeks of bezafibrate compared with placebo (n = 15)
| Placebo | Bezafibrate | P | |
| AST (U/l) | 34.1 ± 8.2 | 44.4 ± 40.2 | 0.3405 |
| ALT (U/l) | 30.7 ± 11.8 | 31.1 ± 9.6 | 0.9259 |
| CK (U/l) | 124 (103–143) | 139 (103–194) | 0.1182 |
| Creatinine (μmol/l) | 76.1 ± 10.3 | 86.2 ± 14.7 | 0.0005a |
| eGFR (ml/min/1.73 m2) | 86.13 ± 5.85 | 78.4 ± 11.4 | 0.0021a |
Data are expressed as mean ± SD or median (IQR).
P < 0.05.
DISCUSSION
In this study, we investigated adding bezafibrate to standard lipid-lowering therapy on fasting and post-fat load lipid levels in patients with FD. Adding bezafibrate did not reduce post-fat load nonHDL-C iAUC, but did reduce post-fat load iAUC of TG and apoB compared with placebo. Furthermore, bezafibrate significantly improved the post-fat load AUC, as well as fasting plasma levels of non-HDL-C, TG, TC, HDL-C, and apoB. Bezafibrate lowered fasting insulin levels and increased the iAUC, but not the AUC, of insulin and HOMA-IR. Post-fat load and fasting glucose and hsCRP were not affected. Finally, bezafibrate was associated with a reduced eGFR.
After an oral fat load, the production of chylomicrons (CMs) in the intestine and hepatic production of VLDLs is increased, which is reflected by postprandial hypertriglyceridemia that also includes remnants of these TG-rich lipoproteins, while TC and LDL-C change little (24). The transport of the biliary and administered cholesterol in CMs after the oral fat load was expected to be similar between the two groups, as suggested by the unchanged non-HDL-C iAUC. Nonfasting hypertriglyceridemia (>5 mmol/l) is associated with a 17-fold increased risk of myocardial infarction, a 5-fold increased risk of ischemic stroke, and a 4-fold increased risk of death in the general population (25). Nonfasting TG remains an independent risk factor for CVD, even after adjustment for TC, HDL-C, and insulin resistance, in contrast to fasting TG levels (26), probably because it reflects impaired clearance and remnant accumulation. Because TG is not present in atherosclerotic plaques, the association between plasma TG and CVD is more likely mediated by remnants and their cholesterol (27). Remnant lipoproteins are the residues of CMs and VLDL after lipolysis of their TG content. Like LDL particles, remnants contain apoB and are small enough to penetrate the vascular wall, to evoke an inflammatory reaction characteristic of atherosclerotic plaques (25). ApoB concentration is associated with CVD risk (3) because it reflects the number of LDL and remnant lipoproteins (28). FD patients have an impaired postprandial clearance of remnant lipoproteins, as expressed by high post-fat load TG and cholesterol levels (29). Fenofibrate is associated with a decrease in postprandial TG and apoB48 in hypertriglyceridemic subjects (16), which is in line with the present study where we show that addition of bezafibrate to standard lipid-lowering therapy reduced post-fat load levels of TG and apoB, indicating improved postprandial clearance of remnant lipoproteins.
Studies in FD patients that compared statin to fibrate therapy found that statins lower LDL-C levels and, to a lesser degree, TG, but do not increase HDL-C, while fibrates improve TG and HDL-C, but not LDL-C (30–32). Both statins and fibrates reduce apoB, although statins reduce apoB more effectively (33% vs. 17%) (32). Adding a fibrate to statin therapy in FD patients resulted in improved fasting levels of TC, TG, and HDL-C compared with statin monotherapy, but these changes were nonsignificant (13, 14), which was probably due to insufficient power because fibrate was only added in patients who did not sufficiently respond to statin monotherapy. In general, the European dyslipidemia guideline recommends initiating treatment for hypertriglyceridemia when TG levels are >2.3 mmol/l (10). The guideline mentions that, in FD, “most cases respond well to treatment with a statin or, if dominated by high TG, a fibrate” and that “often a combination of a statin and a fibrate may be needed” (10, p.3036–3037). Our study is the first that compared the effect of adding a fibrate to standard lipid-lowering therapy on post-fat load and fasting lipids in all FD patients and showed a significant beneficial effect on postprandial TG and apoB levels and on fasting non-HDL-C, TG, TC, HDL-C, and apoB levels. The lipid-lowering effect of bezafibrate that was observed is in line with a meta-analysis of 20 trials that included 25,655 patients (including 4,984 patients on bezafibrate) and showed that fibrates reduce (fasting) plasma TG and TC and increase HDL-C (33) and a postprandial study that found increased lipolysis and remnant removal by bezafibrate in patients with diabetes mellitus (34). Fasting non-HDL-C, TG, and apoB concentrations are associated with increased CVD risk (35) and the decrease in these biomarkers found in this study might therefore indicate CVD risk reduction by bezafibrate in FD.
Bezafibrate is a pan-PPAR agonist that affects lipid metabolism and also improves glucose uptake and reduces hepatic glucose production (36). Lipid metabolism is mainly influenced by PPAR-α agonism, while effects on glucose metabolism are mediated by PPAR-γ agonism (37). These findings are in line with our observation that fasting insulin was lower after bezafibrate treatment compared with placebo, as was observed in T2D patients (38). Interestingly, we also found increased incremental post-fat load insulin levels after bezafibrate therapy. T2D is associated with an impaired incretin response leading to reduced pancreatic insulin secretion shortly after a meal (first hour) (39, 40). A study in diabetic mice showed that bezafibrate improved the efficacy of incretin-based therapies leading to improved glycemic control and pancreatic β-cell morphology (41). The actual exposure of a patient to insulin is expressed by the AUC, which was the same between bezafibrate and placebo in our study. This is in line with a study in T2D patients (38), but in contrast to a study in mildly hypertriglyceridemic patients that found a decrease in insulin AUC (42).
The utility of adding fibrate to statin therapy to reduce CVD is debated due to the results of the ACCORD randomized trial, in which no reduction in major cardiovascular events was found in patients with T2D when fenofibrate was added to statin therapy (11). FD was not specifically identified in such studies to evaluate cardiovascular risk. A large meta-analysis of fibrate studies showed that fibrates decreased the risk of nonfatal myocardial infarction [hazard ratio (HR) 0.78, 95% CI 0.69–0.89], but not all-cause mortality (HR 1.05, 95% CI 0.95–1.15) (33), but effects of fibrate/statin combination therapy were not reported. Bezafibrate has been shown to reduce thoracic and abdominal aorta plaque volumes measured with MRI in patients with dyslipidemia and hypertriglyceridemia without CVD (17). Furthermore, meta-analyses in patients with high plasma TG or atherogenic dyslipidemia (high TG, low HDL-C) with and without diabetes and from both primary and secondary prevention showed that fibrates significantly decreased CVD in these subgroups (43, 44). This subgroup effect was also found in ACCORD (11). FD patients usually have high TG levels and it is therefore reasonable to hypothesize that bezafibrate added to standard lipid-lowering therapy reduces CVD risk in FD. This hypothesis should be tested in a randomized clinical trial.
In the present study, it was shown that bezafibrate was associated with a lower eGFR compared with placebo. This is a common finding in patients on fibrates and the decreased kidney function is reversible after cessation of therapy (45). The mechanism by which fibrates increase serum creatinine is not clearly understood. It is most likely due to a direct effect of fibrates on kidney function, because the rise in creatinine is accompanied by a rise in cystatin C, a marker for renal function. The decrease in kidney function might be due to decreased renal blood flow caused by a PPAR-α-mediated decrease in synthesis of vasodilatory prostaglandins (46). A study in patients with an eGFR within the normal or mildly impaired range found a decreased incidence of CVD of 3% (P < 0.001) with every 10 ml/min/1.73 m2 eGFR increase (47), indicating the beneficial effect of a higher eGFR. However, in patients with chronic kidney failure, fibrates were associated with a reduction in cardiovascular death (HR 0.60, 95% CI 0.38–0.98) and did not confer an increased risk of end-stage renal disease (HR 0.85, 95% CI 0.49–1.49) despite a decreased eGFR (−2.67 ml/min/1.73 m2, P = 0.01) (48). These studies indicate that, although kidney function should be closely monitored, mildly reduced kidney function per se is not a contra-indication for the use of fibrates. Besides this, four patients in the present study developed myalgia, with one of them having increased serum CK levels. The combination of fibrate and statin is associated with a higher incidence of myalgia and rhabdomyolysis (12), although the latter is rare. During statin/fibrate combination therapy, monitoring of kidney function, muscle-related side effects, and CK levels is recommended.
The strength of this study is that data were complete and all participants completed both treatment periods of the crossover study. A potential limitation of this study is the small sample size, although the crossover design greatly improves efficacy and reduces variation between groups. Nevertheless, the sample size limits the possibilities for subgroup analyses. Furthermore, no information on the TG and cholesterol content of VLDL was available and no direct LDL measurements were performed. Although this information could have improved the understanding of the specific lipid effects of bezafibrate and statins, these measurements are usually not available in routine clinical practice. This is also true for urea and cystatin C, which could have increased our understanding of the mechanism behind the eGFR lowering of bezafibrate. Lastly, the number of women in this trial was small and, therefore, generalization to female FD patients should be done with care.
In conclusion, in patients with FD, the addition of bezafibrate to standard lipid-lowering therapy resulted in lower fasting and post-fat load plasma lipids, which may significantly affect atherogenesis in FD. Combination therapy of statin/fibrate could be considered as standard lipid-lowering treatment in FD patients.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contribution of C. A. M. Joosten and I. P. Klaassen (trial nurses), G. Kranenburg (sub-investigator), S.P. Janssen (independent study physician), and M. W. Kuiper (study monitor). Furthermore, the authors would like to thank G. F. N. Berkelmans for his aid in the statistical analyses.
Footnotes
Abbreviations:
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- AUC
- area under the curve
- BP
- blood pressure
- CK
- creatinine kinase
- CM
- chylomicron
- eGFR
- estimated glomerular filtration rate
- FD
- familial dysbetalipoproteinemia
- HbA1c
- glycated hemoglobin
- HDL-C
- HDL-cholesterol
- HOMA-IR
- homeostasis model assessment parameter of insulin resistance
- HR
- hazard ratio
- hsCRP
- high sensitive C-reactive protein
- iAUC
- incremental area under the curve
- IQR
- interquartile range
- LDL-C
- LDL-cholesterol
- non-HDL-C
- non-HDL cholesterol
- TC
- total cholesterol
- TG
- triglyceride
- VLDL-C
- VLDL-cholesterol
- WC
- waist circumference
This was an investigator-initiated study not financially supported by a for-profit organization. The authors declare no conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Marais A. D., Solomon G. A., and Blom D. J.. 2014. Dysbetalipoproteinaemia: a mixed hyperlipidaemia of remnant lipoproteins due to mutations in apolipoprotein E. Crit. Rev. Clin. Lab. Sci. 51: 46–62. [DOI] [PubMed] [Google Scholar]
- 2.Varbo A., Benn M., Tybjaerg-Hansen A., Jorgensen A. B., Frikke-Schmidt R., and Nordestgaard B. G.. 2013. Remnant cholesterol as a causal risk factor for ischemic heart disease. J. Am. Coll. Cardiol. 61: 427–436. [DOI] [PubMed] [Google Scholar]
- 3.Boekholdt S. M., Arsenault B. J., Mora S., Pedersen T. R., LaRosa J. C., Nestel P. J., Simes R. J., Durrington P., Hitman G. A., Welch K. M., et al. 2012. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. 307: 1302–1309. [Erratum. 2012. JAMA. 307: 1915.] [DOI] [PubMed] [Google Scholar]
- 4.de Beer F., Stalenhoef A. F., Hoogerbrugge N., Kastelein J. J., Gevers Leuven J. A., van Duijn C. M., Havekes L. M., and Smelt A. H.. 2002. Expression of type III hyperlipoproteinemia in apolipoprotein E2 (Arg158 → Cys) homozygotes is associated with hyperinsulinemia. Arterioscler. Thromb. Vasc. Biol. 22: 294–299. [DOI] [PubMed] [Google Scholar]
- 5.Zannis V. I. 1986. Genetic polymorphism in human apolipoprotein E. Methods Enzymol. 128: 823–851. [DOI] [PubMed] [Google Scholar]
- 6.Koopal C., Retterstol K., Sjouke B., Hovingh G. K., Ros E., de Graaf J., Dullaart R. P., Bertolini S., and Visseren F. L.. 2015. Vascular risk factors, vascular disease, lipids and lipid targets in patients with familial dysbetalipoproteinemia: a European cross-sectional study. Atherosclerosis. 240: 90–97. [DOI] [PubMed] [Google Scholar]
- 7.Bennet A. M., Di Angelantonio E., Ye Z., Wensley F., Dahlin A., Ahlbom A., Keavney B., Collins R., Wiman B., de Faire U., et al. 2007. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 298: 1300–1311. [DOI] [PubMed] [Google Scholar]
- 8.Mahley R. W., Huang Y., and Rall S. C. Jr. 1999. Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia). Questions, quandaries, and paradoxes. J. Lipid Res. 40: 1933–1949. [PubMed] [Google Scholar]
- 9.Guyton J. R. 1999. Treatment of type III hyperlipoproteinemia. Am. Heart J. 138: 17–18. [DOI] [PubMed] [Google Scholar]
- 10.Catapano A. L., Graham I., De Backer G., Wiklund O., Chapman M. J., Drexel H., Hoes A. W., Jennings C. S., Landmesser U., Pedersen T. R., et al. 2016. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur. Heart J. 37: 2999–3058. [DOI] [PubMed] [Google Scholar]
- 11.Ginsberg H. N., Elam M. B., Lovato L. C., Crouse J. R. III, Leiter L. A., Linz P., Friedewald W. T., Buse J. B., Gerstein H. C., Probstfield J., et al. 2010. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 362: 1563–1574. [Erratum. 2010. N. Engl. J. Med. 362: 1748.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amend K. L., Landon J., Thyagarajan V., Niemcryk S., and McAfee A.. 2011. Incidence of hospitalized rhabdomyolysis with statin and fibrate use in an insured US population. Ann. Pharmacother. 45: 1230–1239. [DOI] [PubMed] [Google Scholar]
- 13.Feussner G., Eichinger M., and Ziegler R.. 1992. The influence of simvastatin alone or in combination with gemfibrozil on plasma lipids and lipoproteins in patients with type III hyperlipoproteinemia. Clin. Investig. 70: 1027–1035. [DOI] [PubMed] [Google Scholar]
- 14.Illingworth D. R., and O’Malley J. P.. 1990. The hypolipidemic effects of lovastatin and clofibrate alone and in combination in patients with type III hyperlipoproteinemia. Metabolism. 39: 403–409. [DOI] [PubMed] [Google Scholar]
- 15.Gylling H., Relas H., and Miettinen T. A.. 1995. Postprandial vitamin A and squalene clearances and cholesterol synthesis off and on lovastatin treatment in type III hyperlipoproteinemia. Atherosclerosis. 115: 17–26. [DOI] [PubMed] [Google Scholar]
- 16.Reyes-Soffer G., Ngai C. I., Lovato L., Karmally W., Ramakrishnan R., Holleran S., and Ginsberg H. N.. 2013. Effect of combination therapy with fenofibrate and simvastatin on postprandial lipemia in the ACCORD lipid trial. Diabetes Care. 36: 422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandini R., Assereto R., Castoldi D., Cunietti E., Garanzelli P., and Monzani W.. 1987. Normal and slow-release formulations of bezafibrate: a comparative pharmacokinetic study in man. Int. J. Clin. Pharmacol. Res. 7: 149–155. [PubMed] [Google Scholar]
- 18.Karpe F. 2002. Postprandial lipemia–effect of lipid-lowering drugs. Atheroscler. Suppl. 3: 41–46. [DOI] [PubMed] [Google Scholar]
- 19.Berry C. C., Moore P., and Dimsdale J. E.. 2006. Assessing the trade-offs between crossover and parallel group designs in sleep research. J. Sleep Res. 15: 348–357. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association. 2016. 2. Classification and diagnosis of diabetes. Diabetes Care. 39 (Suppl. 1): S13–S22. [Erratum. 2016. Diabetes Care. 39: 1653.] [DOI] [PubMed] [Google Scholar]
- 21.Grundy S. M., Cleeman J. I., Daniels S. R., Donato K. A., Eckel R. H., Franklin B. A., Gordon D. J., Krauss R. M., Savage P. J., Smith S. C. Jr., et al. 2005. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 112: 2735–2752. [DOI] [PubMed] [Google Scholar]
- 22.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., and Turner R. C.. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 23.Levey A. S., Bosch J. P., Lewis J. B., Greene T., Rogers N., and Roth D.. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 24.Kolovou G., and Ooi T. C.. 2013. Postprandial lipaemia and vascular disease. Curr. Opin. Cardiol. 28: 446–451. [DOI] [PubMed] [Google Scholar]
- 25.Nordestgaard B. G., and Freiberg J. J.. 2011. Clinical relevance of non-fasting and postprandial hypertriglyceridemia and remnant cholesterol. Curr. Vasc. Pharmacol. 9: 281–286. [DOI] [PubMed] [Google Scholar]
- 26.Mora S., Rifai N., Buring J. E., and Ridker P. M.. 2008. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 118: 993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordestgaard B. G., and Varbo A.. 2014. Triglycerides and cardiovascular disease. Lancet. 384: 626–635. [DOI] [PubMed] [Google Scholar]
- 28.Linton M. F., Yancey P. G., Davies S. S., Jerome W. G. J., Linton E. F., and Vickers K. C.. 2000. The role of lipids and lipoproteins in atherosclerosis. Accessed October 10, 2016, at https://www.ncbi.nlm.nih.gov/books/NBK343489/. [Google Scholar]
- 29.Weintraub M. S., Eisenberg S., and Breslow J. L.. 1987. Different patterns of postprandial lipoprotein metabolism in normal, type IIa, type III, and type IV hyperlipoproteinemic individuals. Effects of treatment with cholestyramine and gemfibrozil. J. Clin. Invest. 79: 1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao S. P., Smelt A. H., Van den Maagdenberg A. M., Van Tol A., Vroom T. F., Gevers Leuven J. A., Frants R. R., Havekes L. M., Van der Laarse A., and Van ’t Hooft F. M.. 1994. Plasma lipoproteins in familial dysbetalipoproteinemia associated with apolipoproteins E2(Arg158→Cys), E3-Leiden, and E2(Lys146→Gln), and effects of treatment with simvastatin. Arterioscler. Thromb. 14: 1705–1716. [DOI] [PubMed] [Google Scholar]
- 31.Zhao S. P., Smelt A. H., Leuven J. A., Vroom T. F., van der Laarse A., and van ’t Hooft F. M.. 1994. Changes of lipoprotein profile in familial dysbetalipoproteinemia with gemfibrozil. Am. J. Med. 96: 49–56. [DOI] [PubMed] [Google Scholar]
- 32.Kawashiri M. A., Kobayashi J., Nohara A., Noguchi T., Tada H., Nakanishi C., Inazu A., Mabuchi H., and Yamagishi M.. 2011. Impact of bezafibrate and atorvastatin on lipoprotein subclass in patients with type III hyperlipoproteinemia: result from a crossover study. Clin. Chim. Acta. 412: 1068–1075. [DOI] [PubMed] [Google Scholar]
- 33.Abourbih S., Filion K. B., Joseph L., Schiffrin E. L., Rinfret S., Poirier P., Pilote L., Genest J., and Eisenberg M. J.. 2009. Effect of fibrates on lipid profiles and cardiovascular outcomes: a systematic review. Am. J. Med. 122: 962.e1–962.e8. [DOI] [PubMed] [Google Scholar]
- 34.Attia N., Durlach V., Roche D., Paul J. L., Soni T., Zahouani A., Landron F., Labrousse F., Leutenegger M., and Girard-Globa A.. 1997. Post-prandial metabolism of triglyceride-rich lipoproteins in non-insulin-dependent diabetic patients before and after bezafibrate treatment. Eur. J. Clin. Invest. 27: 55–63. [DOI] [PubMed] [Google Scholar]
- 35.van den Berg M. J., van der Graaf Y., de Borst G. J., Kappelle L. J., Nathoe H. M., and Visseren F. L.. 2016. Low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, triglycerides, and apolipoprotein B and cardiovascular risk in patients with manifest arterial disease. Am. J. Cardiol. 118: 804–810. [DOI] [PubMed] [Google Scholar]
- 36.Tenenbaum A., Fisman E. Z., Boyko V., Benderly M., Tanne D., Haim M., Matas Z., Motro M., and Behar S.. 2006. Attenuation of progression of insulin resistance in patients with coronary artery disease by bezafibrate. Arch. Intern. Med. 166: 737–741. [DOI] [PubMed] [Google Scholar]
- 37.Tenenbaum A., and Fisman E. Z.. 2012. Balanced pan-PPAR activator bezafibrate in combination with statin: comprehensive lipids control and diabetes prevention? Cardiovasc. Diabetol. 11: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones I. R., Swai A., Taylor R., Miller M., Laker M. F., and Alberti K. G.. 1990. Lowering of plasma glucose concentrations with bezafibrate in patients with moderately controlled NIDDM. Diabetes Care. 13: 855–863. [DOI] [PubMed] [Google Scholar]
- 39.Mitrakou A., Kelley D., Mokan M., Veneman T., Pangburn T., Reilly J., and Gerich J.. 1992. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N. Engl. J. Med. 326: 22–29. [DOI] [PubMed] [Google Scholar]
- 40.Lautt W. W. 2007. Postprandial insulin resistance as an early predictor of cardiovascular risk. Ther. Clin. Risk Manag. 3: 761–770. [PMC free article] [PubMed] [Google Scholar]
- 41.Kang Z. F., Deng Y., Zhou Y., Fan R. R., Chan J. C., Laybutt D. R., Luzuriaga J., and Xu G.. 2013. Pharmacological reduction of NEFA restores the efficacy of incretin-based therapies through GLP-1 receptor signalling in the beta cell in mouse models of diabetes. Diabetologia. 56: 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tremblay-Mercier J., Tessier D., Plourde M., Fortier M., Lorrain D., and Cunnane S. C.. 2010. Bezafibrate mildly stimulates ketogenesis and fatty acid metabolism in hypertriglyceridemic subjects. J. Pharmacol. Exp. Ther. 334: 341–346. [DOI] [PubMed] [Google Scholar]
- 43.Lee M., Saver J. L., Towfighi A., Chow J., and Ovbiagele B.. 2011. Efficacy of fibrates for cardiovascular risk reduction in persons with atherogenic dyslipidemia: a meta-analysis. Atherosclerosis. 217: 492–498. [DOI] [PubMed] [Google Scholar]
- 44.Bruckert E., Labreuche J., Deplanque D., Touboul P. J., and Amarenco P.. 2011. Fibrates effect on cardiovascular risk is greater in patients with high triglyceride levels or atherogenic dyslipidemia profile: a systematic review and meta-analysis. J. Cardiovasc. Pharmacol. 57: 267–272. [DOI] [PubMed] [Google Scholar]
- 45.Mychaleckyj J. C., Craven T., Nayak U., Buse J., Crouse J. R., Elam M., Kirchner K., Lorber D., Marcovina S., Sivitz W., et al. 2012. Reversibility of fenofibrate therapy-induced renal function impairment in ACCORD type 2 diabetic participants. Diabetes Care. 35: 1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ncube V., Starkey B., and Wang T.. 2012. Effect of fenofibrate treatment for hyperlipidaemia on serum creatinine and cystatin C. Ann. Clin. Biochem. 49: 491–493. [DOI] [PubMed] [Google Scholar]
- 47.Eisen A., Hoshen M., Balicer R. D., Reges O., Rabi Y., Leibowitz M., Iakobishvili Z., and Hasdai D.. 2015. Estimated glomerular filtration rate within the normal or mildly impaired range and incident cardiovascular disease. Am. J. Med. 128: 1015– 1022.e2. [DOI] [PubMed] [Google Scholar]
- 48.Jun M., Zhu B., Tonelli M., Jardine M. J., Patel A., Neal B., Liyanage T., Keech A., Cass A., and Perkovic V.. 2012. Effects of fibrates in kidney disease: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 60: 2061–2071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.