Abstract
Recent cell culture and animal studies have suggested that expression of human apo C-III in the liver has a profound impact on the triacylglycerol (TAG)-rich VLDL1 production under lipid-rich conditions. The apoC-III Gln38Lys variant was identified in subjects of Mexican origin with moderate hypertriglyceridemia. We postulated that Gln38Lys (C3QK), being a gain-of-function mutation, promotes hepatic VLDL1 assembly/secretion. To test this hypothesis, we expressed C3QK in McA-RH7777 cells and apoc3-null mice to contrast its effect with WT apoC-III (C3WT). In both model systems, C3QK expression increased the secretion of VLDL1-TAG (by 230%) under lipid-rich conditions. Metabolic labeling experiments with C3QK cells showed an increase in de novo lipogenesis (DNL). Fasting plasma concentration of TAG, cholesterol, cholesteryl ester, and FA were increased in C3QK mice as compared with C3WT mice. Liver of C3QK mice also displayed an increase in DNL and expression of lipogenic genes as compared with that in C3WT mice. These results suggest that C3QK variant is a gain-of-function mutation that can stimulate VLDL1 production, through enhanced DNL.
Keywords: de novo lipogenesis, lipogenic gene expression, VLDL
Human apo C-III is a small (79 amino acids) exchangeable apolipoprotein that is composed mainly of amphipathic α-helices (1). The majority of apoC-III in the plasma is secreted from the liver and intestine as a constituent of VLDL and HDL (2). The plasma apoC-III concentration is positively correlated with the plasma triacylglycerol (TAG) concentrations, and elevated plasma apoC-III is invariably associated with hypertriglyceridemia (3). On the other hand, loss-of-function apoC-III mutations are associated with lowered plasma TAG and reduced risk of coronary disease (4) and ischemic vascular disease (5). Recent studies have shown that inhibition of apoC-III expression, using antisense oligonucleotides, results in lowered plasma TAG in rodents, nonhuman primates, and humans (6). Thus, attenuating apoC-III expression in the liver may offer a therapeutic advantage in the prevention and treatment of hypertriglyceridemia.
The hypertriglyceridemic effect of apoC-III expression has been attributed to at least three underlying mechanisms. First, plasma apoC-III attenuates TAG hydrolysis catalyzed by lipoprotein lipase, resulting in elevated plasma TAG (7). Second, high concentrations of apoC-III interfere with the clearance of TAG-rich lipoprotein remnants, through receptor-dependent or receptor-independent pathways, leading to hypertriglyceridemia (8). Third, overexpression of apoC-III in the liver promotes hepatic TAG-rich VLDL1 assembly and secretion under lipid-rich conditions (9), thus exacerbating hypertriglyceridemia. Cell culture and mouse studies of two loss-of-function apoC-III variants, Ala23Thr (10) and Lys58Glu (11), originally identified in subjects of hypotriglyceridemia, showed that the two mutations entirely abolished apoC-III ability in promoting VLDL1 assembly/secretion (12, 13). The Lys58Glu mutation apparently renders a lowered lipid-binding activity, thus resulting in diminished formation of lipid substrates, termed microsome-associated lipid droplets (MALDs), that presumably are utilized during VLDL1 assembly/secretion (13). On the other hand, the Ala23Thr mutation exhibits no impairment in lipid-binding, yet it fails to facilitate recruitment of lipid substrates into VLDL (12).
The apoC-III (Gln38Lys) variant was originally identified in large kindred of Mexican origin with moderate hypertriglyceridemia (14). Compared with unaffected relatives, individual heterozygous carriers for the Gln38Lys (C3QK) variant had ∼30% elevation in plasma TAG and normal levels of other lipids or lipoproteins (14). In the present study, we determined the functionality of the C3QK variant in McA-RH7777 cells and apoc3-null mice. Data obtained suggest that C3QK is a gain-of-function mutation that stimulates VLDL1 assembly/secretion as compared with C3WT, and the stimulatory effect is associated with increased de novo lipogenesis (DNL).
MATERIALS AND METHODS
Materials
Reagents and medium for cell cultures used in the studies were obtained from Invitrogen (Burlington, ON, Canada). The [2-3H]glycerol (9.6 Ci/mmol) was obtained from American Radiolabeled Chemicals (St. Louis, MO); [3H]acetic acid (0.1 Ci/mmol), [14C]2-deoxyglucose (2-DG) (60 mCi/mmol), and [35S]methionine/cysteine (1,000 Ci/mmol) were obtained from PerkinElmer (Woodbridge, ON, Canada); protein A-Sepharose™ CL-4B beads and HRP-linked anti-mouse and anti-rabbit IgG antibodies were obtained from GE Healthcare (Mississauga, ON); and HRP-linked anti-goat antibody was obtained from Sigma-Aldrich (Oakville, ON, Canada). Oleate, TAG, and phospholipid standards were from Avanti Polar Lipids (Albaster, AL). For Western blots, antibody against human apoC-III was obtained from Academy Biomedical Co., Inc. (Houston, TX); polyclonal goat anti-mouse apoB was obtained from Millipore (Billerica, MA); and polyclonal anti-mouse apoA-I and anti-mouse apoE antisera were obtained from BioDesign International (Saco, ME). Protease inhibitor cocktail and chemiluminescent substrates were obtained from Roche Diagnostics (Laval, PQ, Canada). Poloxamer 407 (P407) was a gift from BASF Corp. (Florham Park, NJ).
Preparation of expression plasmids and transfection
The coding sequence of human C3QK variant protein was synthesized (Life Technologies) flanked with 5′-EcoRI and 3′-HindIII restriction sites, and inserted into the pCMV5 vector predigested with EcoRI and HindIII restriction enzymes. The coding sequences of the cDNA constructs were verified by sequencing. Stably transfected McA-RH7777 cells expressing C3WT or C3QK were generated, and the cells were maintained in DMEM containing 10% FBS, 10% horse serum, and 200 µg/ml G418 as previously described (9).
Metabolic labeling of lipids
Cells (∼1.8 × 106 cells per 60 mm dish, in triplicate) were labeled with [3H]glycerol (5 µCi/ml) in DMEM supplemented with 20% FBS and 0.4 mM oleate for up to 2 h. For DNL experiments, cells were labeled with [3H]acetic acid (25 µCi/ml) for up to 2 h in DMEM supplemented with 20% FBS and either with 0.4 mM oleate (lipid-rich conditions) or without oleate (lipid-poor conditions). At the end of labeling, lipids were extracted from cell and conditioned media, respectively, and resolved by TLC, and radioactivity was quantified by scintillation counting as previously described (9).
Lipoprotein fractionation
Cells were labeled for 2 h with [35S]methionine/cysteine (100 µCi/ml in 100 mm dishes) in methionine/cysteine-free DMEM supplemented with 20% FBS and 0.4 mM oleate. The lipoproteins secreted into the medium were fractionated into VLDL1 (Sf > 100), VLDL2 (Sf 20–100), and other lipoproteins by cumulative rate flotation ultracentrifugation as previously described (9). The 35S-labeled apolipoproteins were resolved by using SDS-PAGE, and radioactivity was quantified by scintillation counting as previously described (15).
Mouse studies
Apoc3-null mice (B6.129-Apoc3tm1Unc/J), obtained from Jackson Laboratory (Bar Harbor, ME), were bred and maintained on chow diet at the University of Ottawa animal care facility according to the institutional animal care committee-approved protocol. For experimental purposes, male apoc3-null mice (12 weeks old) were fed a high-fat diet (TD.88137; Harlan Laboratories, Madison, WI) for 7 days with unlimited access to food. The mice were then injected, via tail vein, with adenovirus encoding C3WT, C3QK, or empty vector (109 pfu/mouse, mixed in 150 µl sterile saline). Forty-eight hours later, blood samples (about 50 µl) were collected from the saphenous vein, and apoC-III expression/secretion was determined by Western blot analysis. Mice that expressed the desired human apoC-III were fasted for 16 h and injected intraperitoneally with P407 (1 mg/g diluted in sterile saline). Blood samples were collected before (zero time point) and at 1 and 2 h after P407 injection. Plasma lipoproteins were fractionated by either ultracentrifugation (16) or size-exclusion chromatography (fast protein LC; FPLC) before lipid and apo determination as previously described (13).
Plasma biochemistry
Mice fed with normal chow diet were injected, via tail vein, with adenovirus (109 pfu per mouse) encoding apoC-III variants or empty vector. Three days later, mice were fasted for 8 h, and blood from the saphenous vein was collected into lithium heparin-coated tubes. Plasma separated from the blood was used in the colorimetric analysis to quantify TAG (BioVision K622-100), FA (BioVision K612-100), cholesterol (Chol), and cholesteryl ester (CE) (BioVision K623-100).
Size-exclusion chromatography
Plasma of three mice from each group (C3WT, C3QK, or vector) collected at 2 h after P407 injection were pooled and filtered by using Costar Spin-X columns (Fisher Scientific, Nepean, Canada) by centrifugation (12,000 g, 5 min). This filtered plasma (65 µl aliquot) was applied to a Superose 6 HR 10/30 column (Amersham Pharmacia Biotech Inc., Piscataway, NJ) on FPLC system (Gilson, Middleton, WI). Samples were eluted with a buffer containing 150 mM NaCl, 10 mM Na2HPO4, and 100 µM EDTA (pH 7.5), at a flow rate of 0.5 ml/min. Thirty 0.5 ml fractions (fraction numbers 11–40) were collected, each successive two fractions were combined, and lipoproteins in the combined fractions were concentrated by using hydrated fumed silica (Cab-O-Sil) as previously described (15). The silica-bound proteins were eluted into 100 µl of SDS-PAGE sample buffer (8 M urea, 2% SDS, and 10% β-mercaptoethanol) and resolved on SDS-PAGE, and then apoB-100, apoB-48, apoC-III, apoE, and apoA-I were detected by immunoblot analysis using appropriate antibodies.
Histology of mouse liver sections
Liver samples collected from the mice fed with high-fat diet were frozen fresh in Cryomatrix polymer complex (Thermo Scientific). The frozen blocks of liver were cut into thin sections by using a Cryostat (HM525 NX, Thermo Scientific) and then mounted on glass slides, followed by Oil Red O staining and covered with a glass coverslip. Intrahepatic lipid droplets stained by Oil Red O were visualized under a Zeiss AxioImager M2 microscope.
In vivo hepatic DNL assay
Mice fed chow diet were injected, via tail vein, with adenovirus (109 pfu per mouse) encoding apoC-III variants or empty vector. Three days later, mice were fasted for 8 h, and [3H]acetic acid (10 µCi/g body weight) was injected via tail vein. Three hours later, liver samples were collected, snap-frozen in liquid nitrogen, and stored at −80°C. Lipids extracted from the frozen liver samples were separated by TLC, and the 3H-labeld lipids was quantified by scintillation counting as previously described (9).
Hepatic lipogenesis gene expression analysis
Total liver RNA from mice injected with adenovirus (109 pfu per mouse) encoding apoC-III variants or empty vector was isolated with TriPure reagent (Roche), as per the manufacturer’s instructions. After removal of genomic DNA, the first-strand cDNA synthesis was performed by using a QuantiNova reverse transcription kit (Qiagen), and the template cDNA was diluted 1:20 with nuclease-free water. Relative mRNA expression was determined by quantitative RT-PCR using inventoried TaqMan assays (Srebf1; Mm00550338_m1, Srebf2; Mm01306292_m1, Fas; Mm00662319_m1, Scd1; Mm00772290_m1, Acc1; Mm00728460_s1, Acc2; Mm00624282_m1, Cd36; Mm00432403_m1, Hmgcr; Mm01282499_m1, Ldlr; Mm01177349_m1, Tbp; Mm00446973_m1, Pparg; Mm00440940_m1, Nr1h3 [liver X receptor a (LXRa)]; Mm00443451_m1, and βactin; Mm00607939_s1) combined with QuantiNova Probe PCR mix (Qiagen) on a Rotor-Gene-Q instrument (Qiagen). Relative expression was determined by using the ΔΔCt method (17), normalized to the average of both Tbp and βactin, and data were presented relative to vector control samples.
Next-generation sequencing and bioinformatics analysis
Genomic DNAs were isolated from whole blood of 1,557 samples of dyslipidemia phenotypes, and target-enriched genomic libraries of indexed and pooled samples were generated for 69 target candidate genes in lipid metabolism, including APOC3 on the LipidSeq Panel, as described (18, 19). Prepared sample libraries were assayed in the Illumina MiSeq personal sequencer (Illumina Inc., San Diego, CA) as described (18). FASTQ files from the MiSeq platform were processed individually by using a custom automated workflow in CLC Genomics Workbench version 8.5.1 (CLCbio, Aarhus, Denmark) for sequence mapping, variant calling, and target region coverage statistics. The variant annotation was performed by using ANNOVAR (https://www.qiagenbioinformatics.com/) with customized scripts. The frequency of the APOC3 p.Q58K variant was also assessed in the ExAC database totaling 60,706 sequenced samples from a wide range of ethnic groups and clinical phenotypes (http://exac.broadinstitute.org/).
Other assays
The Fat Western Lipid-Protein Overlay Assay to determine apoC-III-lipid binding was performed as previously described (13). Briefly, lipid samples (up to 80 µg) were spotted onto nitrocellulose membrane strips and incubated with conditioned medium (collected from cells expressing apoC-III proteins) overnight at 4°C. The apoC-III protein bound to lipid spots was detected by immunoblotting using an anti-hapoC-III antibody. Cell protein concentration was quantified by using the Bradford method (20).
Statistics
Student’s t-test and ANOVA statistical analysis of the data were performed by using GraphPad Prism program (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Expression of C3QK variant in McA-RH7777 cells results in increased VLDL1 secretion
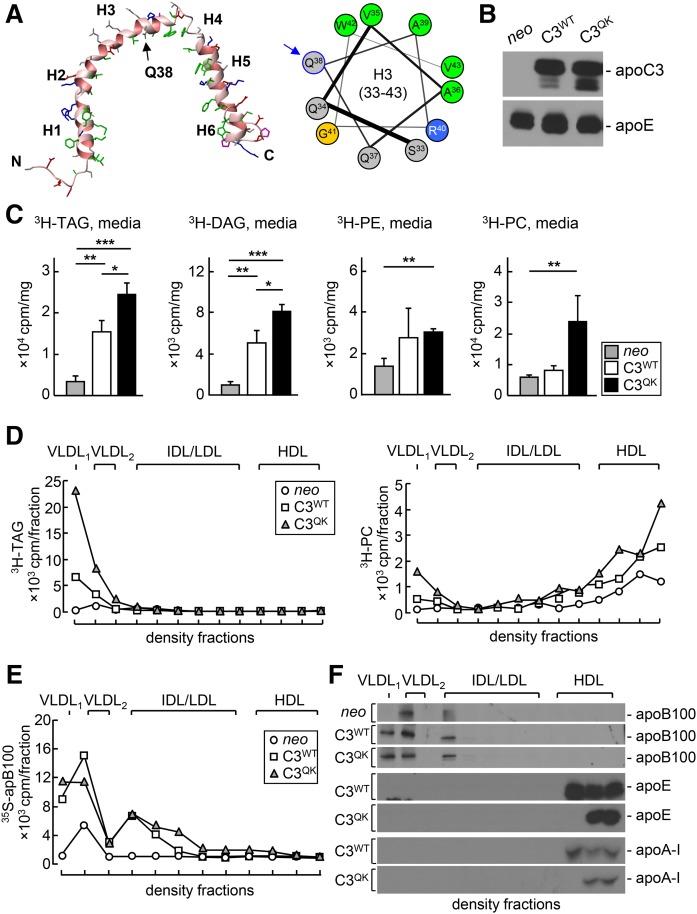
Human apoC-III is composed of six α-helices (1); the Gln38Lys mutation occurs within helix 3 (Fig. 1A). To determine the effect of Gln38Lys mutation on VLDL1 assembly/secretion, we contrasted expression of C3QK with that of C3WT. Stable cell lines expressing similar levels of C3QK or C3WT (Fig. 1B), when metabolically labeled with [3H]glycerol in the presence of 0.4 mM oleate for 2 h, showed that secretion of [3H]TAG, [3H]diacylglycerol, and [3H]phosphatidylcholine ([3H]PC) from C3QK cells was significantly higher (by 60, 60, and 190% respectively) than that from C3WT cells (Fig. 1C). Ultracentrifugal fractionation of lipoproteins secreted from the cells revealed that C3QK expression markedly stimulated [3H]TAG and [3H]PC secretion as VLDL1 (by 230%) (Fig. 1D). Ultracentrifugal fractionation of medium lipoproteins at 2 h postmetabolic labeling with [35S]methionine/cysteine (in the presence of 0.4 mM oleate) showed that the secreted [35S]apoB100 associated with VLDL1 to a similar extent in both C3QK cells and C3WT cells (Fig. 1E, F). However, C3WT cells secreted 30% more [35S]apoB100 that were associated with VLDL2 particles (Fig. 1E). The secreted [35S]apoE and [35S]apoA-I were mainly associated with HDL under density ultracentrifugal fractionation conditions (Fig. 1F). The combined [3H]lipid and [35S]apoB data suggest that expression of C3QK variant in McA-RH7777 cells results in secretion of TAG-rich VLDL1 particle without an increase in apoB100 secretion suggesting late addition of TAG posttranslocationally to apoB-100 during the assembly of lipid-rich VLDL1 particles.
Fig. 1.
ApoC-III Q38K variant is a gain-of-function mutation that enhances secretion of VLDL1-associated TAG and apoB-100. A: Six-helix (H1–H6) structure of human apoC-III (left) and position of Gln38 at the putative water-lipid interface of helix 3 (right). Apolar residues are colored in green, positively charged residues in blue, polar residues in gray, and Gly residue in yellow. The Q38K mutation introduces a positively charged Arg residue at the putative water-lipid interface, thus presumably rendering helix 3 a type A amphipathic helix (26). B: Immunoblots of human apoC-III and endogenous rat apoE in the medium of neo, C3WT, or C3QK cells. C: [3H]TAG in the medium of neo, C3WT, or C3QK cells labeled with [3H]glycerol for 2 h under lipid-rich conditions. * P < 0.05; ** P < 0.01; *** P < 0.001 (n = 3 dishes per cell line). D: [3H]TAG (left) and [3H]PC (right) in fractionated lipoproteins secreted from cells 2 h after labeling with [3H]glycerol. E: [35S]ApoB-100 in fractionated lipoproteins secreted from cells 3 h after labeling with [35S]methionine/cysteine. F: Fluorography of [35S]apoB-100, [35S]apoE, and [35S]apoA-I in fractionated lipoproteins.
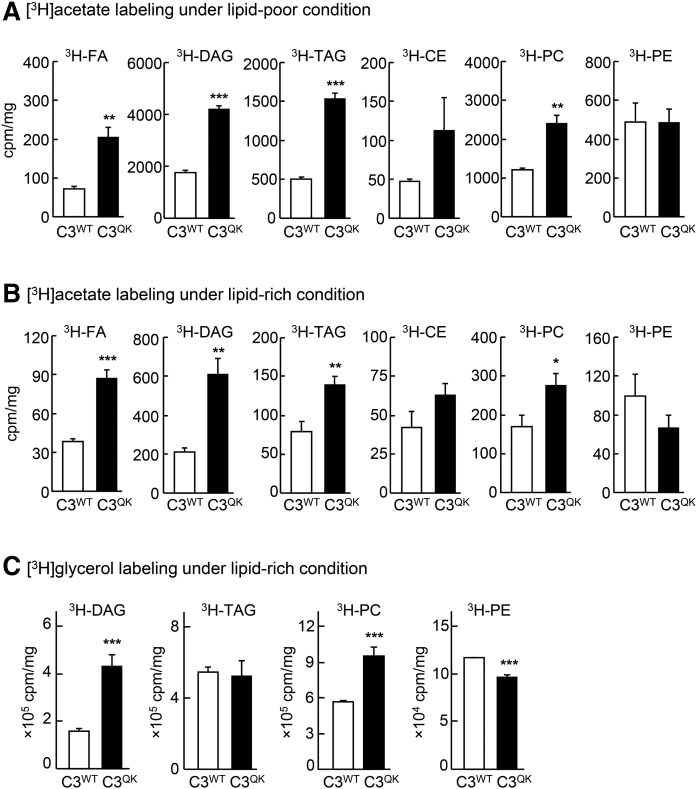
Expression of C3QK variant in McA-RH7777 cells enhances DNL
We next wanted to know the source of increased TAG in driving the TAG-rich VLDL1 secretion in cells expressing C3QK. Hence, we determined the effect of C3QK expression on the intracellular DNL by metabolic labeling with [3H]acetate under lipid-poor (medium devoid of exogenous FAs) or lipid-rich (medium supplemented with 0.4 mM oleate) conditions. Results showed that significantly increased incorporation of [3H]acetate into FAs, TAG, diacylglycerol (DAG), and phosphatidylcholine (PC) [but not phosphatidylethanolamine (PE)] occurred in C3QK cells under both lipid-poor (Fig. 2A) and lipid-rich (Fig. 2B) conditions. There was also a trend of increased incorporation of [3H]acetate into CE in C3QK cells, but the increase was not statistically significant as compared with that in C3WT cells (Fig. 2A, B). These results suggest that C3QK expression results in enhanced hepatic DNL. We also performed metabolic labeling, using [3H]glycerol under lipid-rich conditions (Fig. 2C), to assess the effect of C3QK expression on glycerolipid biosynthesis. Results from these experiments showed increased incorporation of [3H]glycerol into DAG and PC in C3QK cells as compared with that in C3WT cells, but the incorporation of [3H]glycerol into intracellular TAG was similar, and the incorporation of [3H]glycerol into PE was decreased (Fig. 2C). Together, these data suggest strongly that the enhanced VLDL1-TAG secretion (shown in Fig. 1) observed in C3QK cells is associated with enhanced hepatic lipogenesis.
Fig. 2.
Expression of C3QK mutant increases DNL. A, B: Cells were labeled with [3H]acetic acid (25 µCi/ml) for up to 2 h under lipid-poor (A) or lipid-rich (B) conditions. C: Cells were labeled with [3H]glycerol for up to 2 h under lipid-rich conditions. At the end of labeling, cell-associated lipids were quantified. Data are expressed as mean ± SD. * P < 0.05; ** P < 0.01; *** P < 0.001 (n = 3 dishes per cell line).
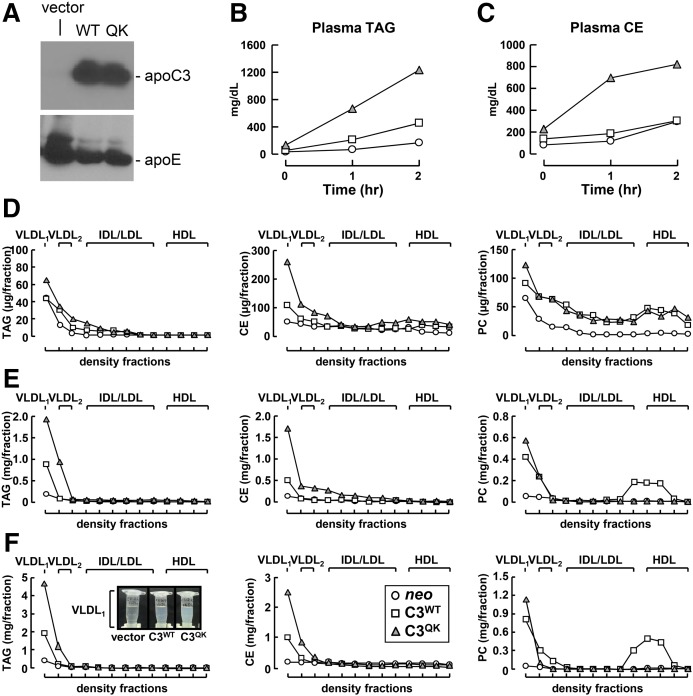
Expression of C3QK variant in apoc3-null mice results in hypertriglyceridemia
We next determined the effect of C3QK expression on lipoprotein production in vivo, using adenovirus-mediated gene transfer into apoc3-null mice fed with a high-fat diet for 7 days. Expression of C3WT and C3QK was confirmed by immunoblot analysis of the mouse plasma (Fig. 3A). Quantification of TAG and CE mass from the plasma collected from mice before injecting P407 (time zero) and at 1 and 2 h after P407 injection was carried out (Fig. 3B, C). Lipid analysis results showed that at the initial time point (time zero), there was no significant differences in TAG content among plasma from all three mice groups. But at 1 and 2 h time points, plasma from C3QK mice accumulated more TAG than plasma from C3WT or vector control mice (Fig. 3B). On the other hand, the plasma CE content was slightly higher in C3QK mice, even at time zero, and continued to accumulate more during the next 2 h of post-P407 injection time points than that in C3WT or vector control mice (Fig. 3C). Fractionation of plasma collected before P407 injection (at time zero) (Fig. 3D), at 1 h (Fig. 3E), and 2 h (Fig. 3F) after P407 injection into various lipoprotein particles using cumulative rate floatation centrifugation was carried out, followed by mass measurement of TAG, CE, and PC in various lipoprotein fractions. Lipid analysis confirmed increased VLDL1-TAG (by ∼2-fold) (Fig. 3D–F, left) and VLDL1-CE (by >2-fold) (Fig. 3D–F, middle) in C3QK plasma than that in C3WT plasma. There was also increased VLDL1-PC (by ∼40%) in C3QK plasma (Fig. 3D–F, right). At 2 h after P407 injection, the accumulation of lipid-rich VLDL1 was strikingly visible in C3QK mice plasma as compared with C3WT mice plasma (Fig. 3F, left, inset).
Fig. 3.
Expression of C3QK mutant in apoc3-null mice promotes TAG-rich VLDL1 production. Apoc3-null mice were fed with a high-fat diet for a week and then injected with adenovirus constructs encoding C3WT, C3QK, or empty vector. At 48 h after injection, mice were fasted for 12 h, and blood samples were collected (time zero) and then injected with P407 intraperitoneally. At 1 and 2 h after P407 injection, plasma samples from three mice per time point per group were collected, pooled, and analyzed for lipids. A: Immunoblot of C3WT or C3QK (top) along with endogenous mouse apoE (bottom). B: Total TAG in pooled plasma samples at 0, 1, and 2 h after P407 injection are shown. C: Total CE in pooled plasma samples at 0, 1, and 2 h after P407 injection are shown. D–F: Pooled plasma at time zero (D), at 1 h (E), and at 2 h (F) were fractionated by cumulative rate flotation ultracentrifugation, mass of TAG (left), CE (middle), and PC (right) in each fraction was quantified and plotted. The lipid-rich VLDL1 fractions collected at 2 h after P407 injection are shown in F, left (inset).
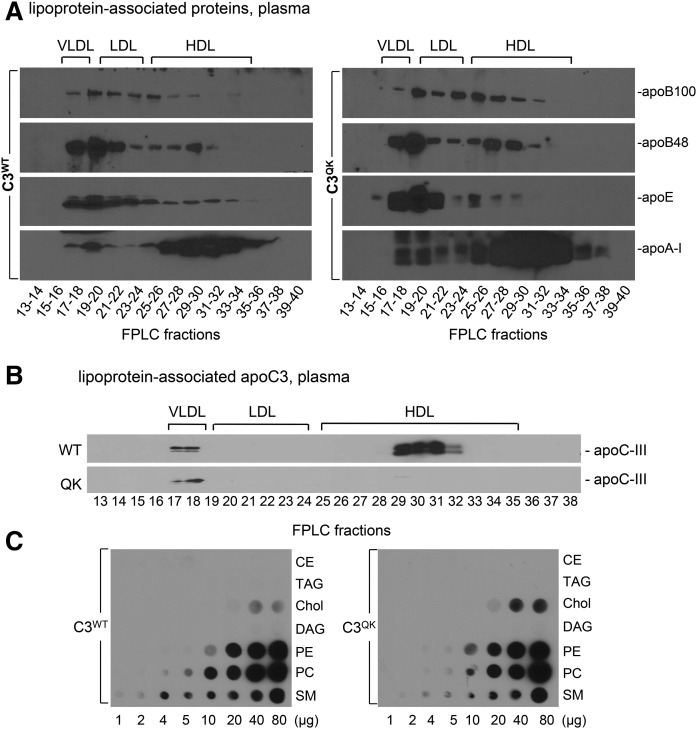
Expression of C3QK variant in apoc3-null mice increases the association of apoB-100 and apoE with large lipoprotein particles
The plasma lipoproteins were also fractionated by using size exclusion chromatography. Immunoblots of the fractionated plasma at 2 h after P407 injection of respective mouse apoB-100, apoB-48, apoE, and apoA-I are shown in Fig. 4A. Although size exclusion chromatography was unable to resolve VLDL1 from VLDL2 as effectively as density ultracentrifugation, a slight increase in apoB-100 signal associated with larger lipoproteins (VLDL and IDL) in C3QK plasma was discernible (e.g., FPLC fractions 19–20 in the apoB-100 blot in C3QK compared with C3WT). Notably, VLDL and IDL particles in C3QK plasma were also enriched with apoE (Fig. 4A, right). These in vivo data are the first indication that expression of C3QK in mice causes hypertriglyceridemia, which may explain the observed phenotypes in heterozygotes carrying the Gln38Lys variant in humans (14). Furthermore, fractionation of 2 h post-P407-injected mice plasma lipoproteins using size exclusion chromatography showed that apoC-III protein itself was almost exclusively found in association with VLDL in the C3QK mutant, whereas in C3WT, apoC-III protein was associated with both VLDL and HDL (Fig. 4B), indicative of an enhanced affinity of apoC-III from C3QK toward VLDL particles. We then determined in vitro lipid-binding properties of apoC-III using fat Western lipid-protein overlay assay (Fig. 4C). Compared with C3WT, C3QK bound to PC, PE, and SM in a similar manner (Fig. 4C). Neither C3WT nor C3QK bound to neutral lipids such as CE, TAG, or DG (Fig. 4C). However, C3QK showed a noticeable increase in binding to Chol as compared with C3WT (Fig. 4C). Additional fat Western lipid-protein overlay assay was performed by using a commercial membrane (Echelon Biosciences Inc.) bearing various phosphatidylinositides in addition to the major phospholipid species (supplemental Fig. S1). Overall, there was no major difference in lipid-binding properties between C3WT and C3QK.
Fig. 4.
Expression of C3QK mutant in apoc3-null mice promotes the association of apoB100, apoE, and apoC3 to plasma VLDL. Plasma collected from mice at 2 h after P407 injection were pooled and fractionated by size exclusion chromatography (FPLC). A: Western blot images of ApoB-100, apoB-48, apoE, and apoA-I (left, C3WT; right, C3QK). B: Western blots of apoC-III in FPLC fractionated plasma of C3WT and C3QK mice. C: Comparison of lipid-binding properties of apoC-III protein from C3WT and C3QK.
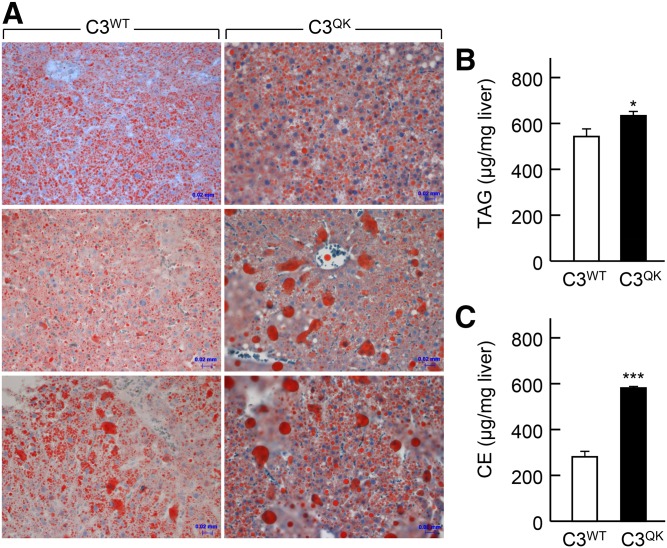
Expression of C3QK variant in apoc3-null mice results in steatosis
Under high-fat diet conditions, C3QK expression in mice resulted in steatosis as compared with C3WT. Accumulation of lipids in the C3QK liver specimens was apparent by Oil Red O staining of liver sections (Fig. 5A). Representative liver sections from three C3WT mice (Fig. 5A, left) and three C3QK mice (Fig. 5A, right) stained with Oil Red O are shown. Quantification of hepatic lipid mass showed that C3QK livers accumulated significantly more TAG (by 20%) (Fig. 5B) and CE (by 110%) (Fig. 5C), when compared with C3WT livers. These in vivo data offer the first indication that C3QK expression could also lead to a certain degree of hepatic steatosis under high fat diet condition.
Fig. 5.
Expression of C3QK mutant under high-fat diet condition in apoc3-null mice promotes hepatic steatosis. A: Oil Red O staining of liver sections of apoc3-null mice fed with a high-fat diet for 1 week and then injected with adenovirus constructs encoding C3WT or C3QK. Liver samples collected from the mice at 48 h after injection were frozen fresh and sectioned by using cryostat and then stained with Oil Red O and visualized under light microscope. Images of intrahepatic lipid droplets from three different mice liver sections per group are shown. Scale bar, 20 µm. B, C: Lipid mass measurements of liver TAG (B) and CE (C) from C3QK and C3WT mice are shown. Data are expressed as mean ± SD. * P < 0.05; *** P < 0.001 (n = 3–6 mice per group).
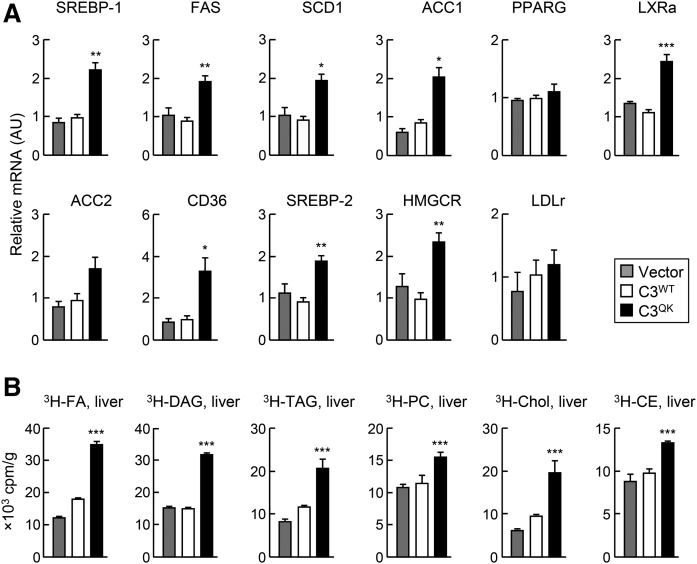
Expression of C3QK variant in apoc3-null mice increases hepatic lipogenic gene expression and hepatic DNL
We determined the expression of mRNA of various lipogenic genes using quantitative RT-PCR. The level of SREBP-1, SREBP-2, FAS, stearoyl-CoA desaturase, ACC1, LXRa, CD-36, and 3-hydroxy-3-methylglutaryl-CoA reductase mRNAs was significantly higher in C3QK mice as compared with C3WT or vector control mice (Fig. 6A). We also determined hepatic DNL in these mice using [3H]acetic acid (injected via tail vein) labeling for 3 h. Data presented in Fig. 6B indicate that 3H-radioactivity was incorporated significantly more into FA, DAG, TAG, PC, Chol, and CE in the livers of C3QK mice than in C3WT or vector control mice. These data suggest that C3QK expression in apoc3-null mice exerts a profound impact on hepatic lipogenesis.
Fig. 6.
Expression of C3QK in mice increases the expression of lipogenesis genes and hepatic DNL. A: Relative mRNA expression profile of genes involved in lipogenesis in the livers of mice injected with adenovirus constructs of vector control or C3WT or C3QK. Data are expressed as mean ± SD. * P < 0.05; ** P < 0.01; *** P < 0.001 (n = 3–5 mice per treatment). B: In vivo hepatic DNL in mice. Bar graph shows the radioactivity associated with the indicated lipids extracted from the livers of mice 3 h after tail vein injection of [3H] acetic acid. Data are expressed as mean ± SD. *** P < 0.001 (n = 3–5 mice per treatment).
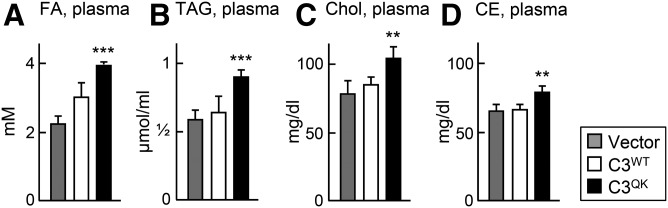
Expression of C3QK variant in apoc3-null mice causes hyperlipidemia
Mass measurement of plasma lipids showed that under chow diet conditions, there was a significant increase in fasting plasma concentrations of FA (by 30%) (Fig. 7A), TAG (by 40%) (Fig. 7B), Chol (by 24%) (Fig. 7C), and CE (by 18%) (Fig. 7D) in C3QK mice when compared with C3WT.
Fig. 7.
Expression of C3QK in mice causes hyperlipidemia. Twelve week old apoC3-null mice maintained on normal chow diet were injected with adenovirus constructs encoding C3WT or C3QK or empty vector. At 48 h after injection, mice were fasted for 8 h, blood was collected, and then levels of FA (A), TAG (B), Chol (C), and CE (D) in the plasma were quantified. Data are expressed as mean ± SD. ** P < 0.01; *** P < 0.001 (n = 5 mice per treatment).
Analysis of Gln38Lys mutation in human APOC3 gene
To determine the prevalence of APOC3 Gln38Lys (p.Q58K) in humans, we performed next-generation sequencing of 1,557 DNA samples from patients exhibiting dyslipidemia phenotypes, including hypertriglyceridemia, hypercholesterolemia, and combined hyperlipidemia, from Canadian of predominantly European origin, with some of Chinese, African, and South-Asian origin (18, 19). APOC3 p.Q58K was not found in these 1,557 dyslipidemia samples, nor was there any occurrence of this variant in the ExAC databases containing exome or genome sequence information on 60,706 individuals. Together, these data confirm the rarity of the APOC3 p.Q58K variant in humans.
DISCUSSION
The present study of the naturally occurring apoC-III Q38K variant provides new evidence for an intracellular role that human apoC-III plays in the assembly and secretion of hepatic TAG-rich VLDL1. Although apoC-III is a rather small protein of 79 amino acids, there are at least seven coding sequence variations reported to date. Three loss-of-function variants, namely, Lys58Glu (11), Ala23Thr (10), and Arg19X (21), are associated with a hypotriglyceridemia phenotype and may confer cardioprotective effect. Two missense mutations, Thr74Ala (22, 23) and Asp45Asn (24), have not been associated with lipid or lipoprotein abnormalities. Another newly identified missense mutation Asp25Val variant has been associated with renal insufficiency and hypotriglyceridemia (25). The Gln38Lys variant (14), on the other hand, was associated with hypertriglyceridemia, and the current study suggests Gln38Lys being a gain-of-function mutation in promoting VLDL1 production. However, genomic analysis of DNA samples of dyslipidemia patients, from various ethnic groups, as well as samples from normal controls (total of >65,000 individuals), did not detect the Gln38Lys variant. Thus, the Gln38Lys is a rare variant in the human population.
The present data, together with experimental evidence obtained from our previous studies of C3AT (12) and C3KE variants (13), have suggested three roles that apoC-III may play intrahepatocellularly. First, apoC-III is required for the formation of MALD, a putative lipid substrate of VLDL1 (13). Second, apoC-III promotes incorporation of the lipid substrates into VLDL during VLDL1 assembly/secretion (9). Third, expression of apoC-III (i.e., C3QK) enhances hepatic DNL (Figs. 2, 6), which is associated with hyperlipidemia, even under chow-diet conditions, upon C3QK expression (Fig. 7). Nearly all of these functions are lost in the loss-of-function variants C3KE (13); in contrast, the gain-of-function variant C3QK (this study) enhances these functions.
The mechanism by which apoC-III can play such a profound role in hepatic lipid metabolism is unclear. Regardless, the amphipathic nature of apoC-III α-helices appears to be critical to its function. The Gln38 residue resides in the putative water-oil interface of the amphipathic helix 3 (Fig. 1A). Substituting Gln38 with Lys introduces a positive charge at the interface, rendering helix 3 a perfect type A amphipathic helix (26). Previous studies have shown that changes in the net positive or negative charges of individual helices of apoC-III may affect its lipid binding properties (10, 13, 27). It has also been suggested that changes in charge distribution of apoC-III may exert an effect on the LPL-catalyzed lipolysis or receptor-mediated reuptake of lipoproteins (1, 10). Although under the present in vitro assay conditions, C3QK did not show gross changes in binding to lipid species, subtle differences in binding to Chol was noticed between C3WT and C3QK (Fig. 4C). Thus, charge distribution and the related amphipathicity of α-helices of apoC-III are important structural elements underlying its functions.
Results of the present study highlight the role that the Gln38Lys variant may play in lipogenesis. Overexpression of WT human apoC-III in mice resulting in certain degree of hepatosteatosis has been reported previously (28). Under our experimental conditions, hepatosteatosis was not consistently observed in apoc3-null mice transfected with adenovirus-encoded human apoC-III (i.e., C3WT). However, expression of the Gln38Lys variant (i.e., C3QK) in apoc3-null mice using the adenovirus vector invariably resulted in moderate levels of steatosis (Fig. 5B, C), indicating that this gain-of-function mutant could exert a potent lipogenic effect in the liver.
Overexpression of apoC-III in mice has also been shown to increase diet-induced obesity, which could be reversed by expression of physiological levels of CE transfer protein (29), and these mice were found predisposed to develop diet-induced hepatic steatosis and hepatic insulin resistance (28). On the other hand, mice deficient in apoC-III failed to develop hypertriglyceridemia, even after inducing diabetes with streptozotocin under chow-diet condition (30). However, apoC3 deficiency appears to promote high-fat diet-induced obesity in mice, presumably owing to enhanced hydrolysis of plasma TAG catalyzed by LPL, and the resulting FAs are subsequently taken up by adipose tissues and further aggravate insulin resistance due to decreased whole-body insulin sensitivity and unbridled endogenous glucose production (31). D-glucose and farnesoid X receptor (FXR) ligands additively regulate the expression of FXR and its target genes, small heterodimer partner and apoC3 in the liver of Zuker rats (32). Studies with mice lacking a liver-specific insulin receptor show that glucose induces apoC3 mRNA transcription via a mechanism involving the transcription factors carbohydrate response element binding protein and hepatocyte nuclear factor-4α (33). These studies together suggest that apoC-III may serve as a critical component in the complex interplay between lipid and carbohydrate metabolism.
In summary, the present study provides new experimental evidence for an intracellular function of apoC-III in regulating hepatic lipid metabolism by enhancing the hepatic DNL that provides the required lipid substrates (TAG, CE, Chol, PC, etc.) to be incorporated into the VLDL assembly process, resulting in the secretion of lipid-rich buoyant VLDL1 particles from the cells and mice expressing the gain-of function variant C3QK.
Supplementary Material
Acknowledgments
The authors thank Karen Massel for generating tetracysteine-tagged ApoC3 expression plasmids, Michele Geoffrion for assistance with FPLC, and Dong Li for liver histology analysis. The authors are grateful to Gina M. Peloso (Boston University) and Sekar Kathiresan (Massachusetts General Hospital, Harvard Medical School) for genomic analysis of APOC3 in human populations.
Footnotes
Abbreviations:
- CE
- cholesteryl ester, Chol, cholesterol
- DAG
- diacylglycerol
- DNL
- de novo lipogenesis
- FPLC
- fast protein LC
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- TAG
- triacylglycerol
This work was supported by Canadian Institutes of Health Research Operating Grant MOP 123279 (Z.Y.), Project Grant PJT148634, and New Investigator Award MSH141981 (M.D.F.).
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Gangabadage C. S., Zdunek J., Tessari M., Nilsson S., Olivecrona G., and Wijmenga S. S.. 2008. Structure and dynamics of human apolipoprotein CIII. J. Biol. Chem. 283: 17416–17427. [DOI] [PubMed] [Google Scholar]
- 2.Jong M. C., Hofker M. H., and Havekes L. M.. 1999. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler. Thromb. Vasc. Biol. 19: 472–484. [DOI] [PubMed] [Google Scholar]
- 3.Yao Z., and Wang Y.. 2012. Apolipoprotein C–III and hepatic triglyceride-rich lipoprotein production. Curr. Opin. Lipidol. 23: 206–212. [DOI] [PubMed] [Google Scholar]
- 4.Crosby J., Peloso G. M., Auer P. L., Crosslin D. R., Stitziel N. O., Lange L. A., Lu Y., Tang Z. Z., Zhang H., Hindy G., et al. 2014. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 371: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jørgensen A. B., Frikke-Schmidt R., Nordestgaard B. G., and Tybjaerg-Hansen A.. 2014. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 371: 32–41. [DOI] [PubMed] [Google Scholar]
- 6.Graham M. J., Lee R. G., Bell T. A. III, Fu W., Mullick A. E., Alexander V. J., Singleton W., Viney N., Geary R., Su J., et al. 2013. Antisense oligonucleotide inhibition of apolipoprotein C–III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ. Res. 112: 1479–1490. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg H. N., Le N. A., Goldberg I. J., Gibson J. C., Rubinstein A., Wang-Iverson P., Norum R., and Brown W. V.. 1986. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo 2. J. Clin. Invest. 78: 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sehayek E., and Eisenberg S.. 1991. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J. Biol. Chem. 266: 18259–18267. [PubMed] [Google Scholar]
- 9.Sundaram M., Zhong S., Bou Khalil M., Links P. H., Zhao Y., Iqbal J., Hussain M. M., Parks R. J., Wang Y., and Yao Z.. 2010. Expression of apolipoprotein C–III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J. Lipid Res. 51: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H., Labeur C., Xu C. F., Ferrell R., Lins L., Brasseur R., Rosseneu M., Weiss K. M., Humphries S. E., and Talmud P. J.. 2000. Characterization of the lipid-binding properties and lipoprotein lipase inhibition of a novel apolipoprotein C–III variant Ala23Thr. J. Lipid Res. 41: 1760–1771. [PubMed] [Google Scholar]
- 11.von Eckardstein A., Holz H., Sandkamp M., Weng W., Funke H., and Assmann G.. 1991. Apolipoprotein C–III(Lys58—-Glu). Identification of an apolipoprotein C–III variant in a family with hyperalphalipoproteinemia. J. Clin. Invest. 87: 1724–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundaram M., Zhong S., Bou Khalil M., Zhou H., Jiang Z. G., Zhao Y., Iqbal J., Hussain M. M., Figeys D., Wang Y., et al. 2010. Functional analysis of the missense APOC3 mutation Ala23Thr associated with human hypotriglyceridemia. J. Lipid Res. 51: 1524–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin W., Sundaram M., Wang Y., Zhou H., Zhong S., Chang C-C., Manhas S., Yao E. F., Parks R. J., McFie P. J., et al. 2011. Missense mutation in APOC3 within the C-terminal lipid-binding domain of human apoC-III results in impaired assembly and secretion of triacylglycerol-rich very low density lipoproteins. Evidence that apoC-III plays a major role in the formation of lipid precursors within the microsomal lumen. J. Biol. Chem. 286: 27769–27780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pullinger C. R., Malloy M. J., Shahidi A. K., Ghassemzadeh M., Duchateau P., Villagomez J., Allaart J., and Kane J. P.. 1997. A novel apolipoprotein C–III variant, apoC-III(Gln38→Lys), associated with moderate hypertriglyceridemia in a large kindred of Mexican origin. J. Lipid Res. 38: 1833–1840. [PubMed] [Google Scholar]
- 15.Vance D. E., Weinstein D. B., and Steinberg D.. 1984. Isolation and analysis of lipoproteins secreted by rat liver hepatocytes. Biochim. Biophys. Acta. 792: 39–47. [DOI] [PubMed] [Google Scholar]
- 16.Redgrave T. G., and Carlson L. A.. 1979. Changes in plasma very low density and low density lipoprotein content, composition, and size after a fatty meal in normo- and hypertriglyceridemic man. J. Lipid Res. 20: 217–229. [PubMed] [Google Scholar]
- 17.Livak K. J., and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 18.Hegele R. A., Ban M. R., Cao H., McIntyre A. D., Robinson J. F., and Wang J.. 2015. Targeted next-generation sequencing in monogenic dyslipidemias. Curr. Opin. Lipidol. 26: 103–113. [DOI] [PubMed] [Google Scholar]
- 19.Johansen C. T., Dube J. B., Loyzer M. N., MacDonald A., Carter D. E., McIntyre A. D., Cao H., Wang J., Robinson J. F., and Hegele R. A.. 2014. LipidSeq: a next-generation clinical resequencing panel for monogenic dyslipidemias. J. Lipid Res. 55: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 21.Pollin T. I., Damcott C. M., Shen H., Ott S. H., Shelton J., Horenstein R. B., Post W., McLenithan J. C., Bielak L. F., Peyser P. A., et al. 2008. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 322: 1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda H., Uzawa H., and Kamei R.. 1981. Unusual familial lipoprotein C–III associated with apolipoprotein C–III-O preponderance. Biochim. Biophys. Acta. 665: 578–585. [DOI] [PubMed] [Google Scholar]
- 23.Maeda H., Hashimoto R. K., Ogura T., Hiraga S., and Uzawa H.. 1987. Molecular cloning of a human apoC-III variant: Thr 74—-Ala 74 mutation prevents O-glycosylation. J. Lipid Res. 28: 1405–1409. [PubMed] [Google Scholar]
- 24.Lüttmann S., von Eckardstein A., Wei W., Funke H., Köhler E., Mahley R. W., and Assmann G.. 1994. Electrophoretic screening for genetic variation in apolipoprotein C–III: identification of a novel apoC-III variant, apoC-III(Asp45→Asn), in a Turkish patient. J. Lipid Res. 35: 1431–1440. [PubMed] [Google Scholar]
- 25.Valleix S., Verona G., Jourde-Chiche N., Nédelec B., Mangione P. P., Bridoux F., Mangé A., Dogan A., Goujon J. M., Lhomme M., et al. 2016. D25V apolipoprotein C–III variant causes dominant hereditary systemic amyloidosis and confers cardiovascular protective lipoprotein profile. Nat. Commun. 7: 10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segrest J. P., De L. H., Dohlman J. G., Brouillette C. G., and Anantharamaiah G. M.. 1990. Amphipathic helix motif: classes and properties. Proteins. 8: 103–117. [DOI] [PubMed] [Google Scholar]
- 27.Liu H., Talmud P. J., Lins L., Brasseur R., Olivecrona G., Peelman F., Vandekerckhove J., Rosseneu M., and Labeur C.. 2000. Characterization of recombinant wild type and site-directed mutations of apolipoprotein C–III: lipid binding, displacement of ApoE, and inhibition of lipoprotein lipase. Biochemistry. 39: 9201–9212. [DOI] [PubMed] [Google Scholar]
- 28.Lee H. Y., Birkenfeld A. L., Jornayvaz F. R., Jurczak M. J., Kanda S., Popov V., Frederick D. W., Zhang D., Guigni B., Bharadwaj K. G., et al. 2011. Apolipoprotein CIII overexpressing mice are predisposed to diet-induced hepatic steatosis and hepatic insulin resistance. Hepatology. 54: 1650–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salerno A. G., Silva T. R., Amaral M. E., Alberici L. C., Bonfleur M. L., Patricio P. R., Francesconi E. P., Grassi-Kassisse D. M., Vercesi A. E., Boschero A. C., et al. 2007. Overexpression of apolipoprotein CIII increases and CETP reverses diet-induced obesity in transgenic mice. Int. J. Obes.(Lond). 31: 1586–1595. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi T., Hirano T., Okada K., and Adachi M.. 2003. Apolipoprotein CIII deficiency prevents the development of hypertriglyceridemia in streptozotocin-induced diabetic mice. Metabolism. 52: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 31.Duivenvoorden I., Teusink B., Rensen P. C., Romijn J. A., Havekes L. M., and Voshol P. J.. 2005. Apolipoprotein C3 deficiency results in diet-induced obesity and aggravated insulin resistance in mice. Diabetes. 54: 664–671. [DOI] [PubMed] [Google Scholar]
- 32.Duran-Sandoval D., Mautino G., Martin G., Percevault F., Barbier O., Fruchart J. C., Kuipers F., and Staels B.. 2004. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes. 53: 890–898. [DOI] [PubMed] [Google Scholar]
- 33.Caron S., Verrijken A., Mertens I., Samanez C. H., Mautino G., Haas J. T., Duran-Sandoval D., Prawitt J., Francque S., Vallez E., et al. 2011. Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 31: 513–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.