Abstract
Upregulation of the hepatic endocannabinoid (EC) receptor [cannabinoid receptor-1 (CB1)] and arachidonoylethanolamide (AEA) is associated with nonalcoholic fatty liver disease (NAFLD). Male mice fed high-fat diet (HFD) ad libitum also exhibit NAFLD, increased hepatic AEA, and obesity. But, preference for HFD complicates interpretation and almost nothing is known about these effects in females. These issues were addressed by pair-feeding HFD. Similarly to ad libitum-fed HFD, pair-fed HFD also increased WT male and female mouse fat tissue mass (FTM), but preferentially at the expense of lean tissue mass. In contrast, pair-fed HFD did not elicit NAFLD in WT mice regardless of sex. Concomitantly, pair-fed HFD oppositely impacted hepatic AEA, 2-arachidonoyl glycerol, and/or CB1 in WT males versus females. In pair-fed HFD mice, liver FA binding protein-1 (Fabp1) gene ablation (LKO): i) exacerbated FTM in both sexes; ii) did not elicit liver neutral lipid accumulation in males and only slightly in females; iii) increased liver AEA in males, but decreased it in females; and iv) decreased CB1 only in males. Thus, pair-fed HFD selectively impacted hepatic ECs more in females, but did not elicit NAFLD in either sex. These effects were modified by LKO consistent with FABP1’s ability to impact EC and FA metabolism.
Keywords: mouse, liver, gene ablation
Increasingly prevalent worldwide, high-fat diets (HFDs; about 40% of energy) induce obesity and nonalcoholic fatty liver disease (NAFLD) (1). Apart from weight loss, there are currently no effective therapies for NAFLD (2). To date, knowledge about cellular and molecular defects in NAFLD is heavily based on studies of HFD-induced male rodent models (3). However, almost all of these are fed HFD ad libitum, even though rodents exhibit a strong preference to consume more HFD than normal chow (4). The prevalence of obesity and NAFLD/nonalcoholic steatohepatitis (NASH) is greater in women, yet little is known about HFD effects on hepatic lipid in female rodents (5). Thus, it is unclear whether the HFD-induced NAFLD in males, much less females, is due to the higher proportion of fat in the diet or to the increased intake of HFD.
While the biochemical basis for NAFLD is not completely understood, recent studies have established a link between HFD-induced NAFLD and the hepatic endocannabinoid (EC) system. Hepatic expression of the cannabinoid receptor-1 (CB1) is increased by HFD and in patients with NAFLD (6–8). Concomitant to HFD-induced obesity and NAFLD, hepatic arachidonoylethanolamide (AEA), but not 2-arachidonoyl glycerol (2-AG), is selectively increased in males, which, together with CB1’s higher affinity for AEA than 2-AG, results in hepatic activation of CB1 receptors, a requirement for development of NAFLD in male mice (6, 7, 9). Poorly aqueous-soluble AEA and 2-AG require a cytosolic “chaperone” for intracellular binding/trafficking between synthetic and degradative sites (10). The novel discovery that the liver FA binding protein-1 (FABP1, L-FABP) has high affinity not only for the precursor of AEA and 2-AG [i.e., arachidonic acid (ARA)] (11, 12), but also for AEA and 2-AG, suggested that FABP1 may account for the high first-pass hepatic clearance rate of the EC precursor, ARA, and likely first pass removal of plasma ECs for intracellular degradation (10, 13). Fabp1 gene ablation (LKO) increased serum total ARA (free and esterified ARA) along with AEA and 2-AG in a nonhepatic tissue, i.e., brain, of male mice (14). Interestingly, hepatic FABP1 is markedly upregulated by ad libitum feeding of HFD (15, 16) and in NAFLD (17, 18), but it is not clear whether FABP1 mitigates or antagonizes the HFD-induced NAFLD.
Taken together, these observations suggest that FABP1, itself, may impact the hepatic EC response to HFD. Therefore, this possibility was examined not only in livers of HFD pair-fed WT and LKO male mice but also in female mice, of which less is known. Pair feeding the HFD eliminates the potential complications of mouse preference for and increased consumption of a HFD (19).
MATERIALS AND METHODS
Animal care
Male and female inbred WT C57BL/6NCr mice were obtained from the National Cancer Institute (Frederick Cancer Research and Development Center, Frederick, MD). Fabp1 gene-ablated (LKO) mice were generated and backcrossed to WT C57BL/6NCr mice for more than the N10 generation, as described (20). For maintenance of the colony, mice were housed in barrier cages on ventilated racks at 12 h light/dark cycle, maintained at 25°C, and allowed ad libitum access to water at all times and standard rodent chow mix [5% calories from fat, D8604 Rodent Diet; Teklad Diets (Madison, WI)] before the study. Animal protocols were approved by the Institutional Animal Care and Use Committee at Texas A&M University. Mice were sentinel monitored quarterly and confirmed free of all known rodent pathogens.
Dietary study
WT male, WT female, LKO male, and LKO female mice aged 7 weeks were housed individually in Tecniplast Sealsafe IVC cages (external water bottles, wire lid holders for food pellets). All mice were then switched to a pelleted defined control diet (10 kcal% fat, #D12450B; Research Diets, Inc., New Brunswick, NJ) for 1 week for acclimation prior to either continuing on the control diet or the HFD, pair-fed similarly as described earlier (21). The defined control diet was free of phytol and phytoestrogen, which may impact sex differences and FABP1 expression (22, 23). The pair-fed HFD (#D12451; Research Diets, Inc.) was based on a defined diet modified to increase fat from 10 to 45 kcal%, concomitantly decreasing carbohydrate from 70 to 35 kcal% and maintaining protein constant at 20 kcal%. The complete FA profiles, taken from the Research Diets website, are shown in supplemental Table S1. This showed the following composition for the control chow: n-6 PUFA 18:2n-6 (precursor of 20:4n-6) and 20:4n-6 comprised 18.3 and 0.1 g/kg, respectively, while n-3 PUFA 18:3n-3, 20:5n-3, and 22:6n-3 comprised 2.2, 0, and 0 g/kg, respectively. The HFD had levels of C18:2n-6 and C20:4n-6 increased >3-fold to 56.7 and 0.5 g/kg, respectively, while the n-3 PUFAs, 18:3n-3, 20:5n-3, and 22:6n-3, were only slightly increased to 4.3, 0, and 0.2 g/kg, respectively.
The pair-feeding regimen was performed similarly as described earlier (21). Briefly, after the 1 week of adaptation, described above, the mice were split into four groups of 16 mice each: male LKO, male WT, female LKO, and female WT. On the first day of the feeding study, the food consumption of half of each group was measured by weight. On the second day the other half was pair-fed the defined HFD (45 kcal% fat, #D12451; Research Diets, Inc.) in the amount of the measured control group (1 day offset). Body weights and food intake for all mice were measured every day for the duration of the 12 week study and food consumption for the HFD was adjusted to be within a nonsignificant margin by weight and calories from the previous day of the control group. At the end of the dietary study, total body weight change and total food intake were used to determine energy conversion efficiency. In addition, at the end of the dietary study, mice were fasted overnight for whole-body phenotype analysis, euthanasia, serum collection, and liver collection, as described in the following sections.
Dual-energy X-ray absorptiometry to determine fat tissue mass and lean tissue mass
To obtain an in vivo measurement of whole-body fat tissue mass (FTM) and bone-free lean tissue mass (LTM), mice were anesthetized (at both the beginning and the end of the dietary study) with ketamine/xylazine (0.01 ml/g body weight; 10 mg ketamine per milliliter and 1 mg xylazine per milliliter in 0.9% saline solution) prior to analysis by dual-energy X-ray absorptiometry (DEXA) with a Lunar PIXImus densitometer (Lunar Corp., Madison, WI), as in (22). The PIXImus instrument was calibrated prior to the analysis using a phantom mouse of known bone mineral density and FTM, as in (22). Whole-body FTM and bone-free LTM were determined for the entire mouse, minus the head region, as described earlier (22).
Serum collection and analysis
At the end of the dietary study and after DEXA, as described above, blood was collected, serum prepared, and aliquots snap-frozen on dry ice and stored at −80°C. Serum protein was determined by Bradford micro-assay (#500-0001; Bio-Rad Laboratories, Hercules, CA). Stanbio diagnostic kits (Boerne, TX) were used to determine serum levels of the hepatic enzymes, aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Serum content of individual lipid classes was determined using commercially available kits from Wako Chemicals (Richmond, VA), including triacylglycerol (L-type Triglyceride M), free cholesterol, total cholesterol (cholesterol E), phospholipid, and NEFA (HR Series NEFA-HR), as described (24). Quantitation of serum lipids using these kits did not differ significantly from quantitation by solvent extraction/thin layer chromatography/colorimetry (24).
Liver gross morphology and histopathology
After cervical dislocation as above, livers were immediately excised, washed with ice-cold PBS, photographed, weighed, and sectioned into several parts. For histology, one part was excised near the porta hepatis, fixed in 10% neutral buffered formalin for 24 h, and then stored in 70% alcohol. The stored liver was then processed, embedded in paraffin, sectioned (4–5 μ thickness), and stained with hematoxylin and eosin for histological evaluation, with grading of hepatocyte fatty vacuolation, inflammation, and necrosis (if present), as in (25). Another part of the liver was placed in RNA stabilization buffer [RNAlater (Ambion, Austin, TX)] for storage at −20°C and quantitative (Q)RT-PCR analysis, as described in the following sections. The remainder of the liver was flash-frozen on dry ice, stored at −80°C, and used for lipid and Western blot analysis, as described in the following sections.
Liver lipid content and class composition
After liver samples (∼0.1 g) were thoroughly minced, 0.5 ml PBS (pH 7.4) was added, followed by homogenization using a motor-driven pestle (Tekmar Co, Cincinnati, OH) at 2,000 rpm. Liver homogenate protein concentration was determined by Bradford protein micro-assay (Bio-Rad Laboratories). Aliquots of liver homogenate were analyzed for lipid class composition using commercially available kits, as described above for serum lipids. Quantitation of liver lipids with these kits did not differ significantly from quantitation by solvent extraction/thin layer chromatography/colorimetry (24).
LC-MS analysis of liver N-acylethanolamides and 2-monoacylglycerols
LC-MS analysis of liver N-acylethanolamides (NAEs) and 2-monoacylglycerols (2-MGs) was performed as described earlier (14, 26).
QRT-PCR of liver gene transcripts in the liver EC system
TaqMan® specific gene expression probe primers from Life Technologies™ (Carlsbad, CA) were used to determine liver mRNA levels of: acetyl-CoA carboxylase-α (Acaca) (Mm01304285_m1); acyl-CoA oxidase-1 (Acox1) (Mm00443579_m1); 1-acylglycerol-3-phosphate-O-acyltransferase (Abhd5) [Mm00470734_m1; also called comparative gene identification-58 (CGI58) or lipid droplet binding protein]; CB1 (Cnr1) (Mm01212171_s1); cannabinoid receptor-2 (Cnr2) (Mm02620087_s1); carnitine palmitoyltransferase 1A (Cpt1a) (Mm00550438_m1); diacylglycerol lipase α (Dagla) (Mm00813830_m1); diacylglycerol lipase β (Daglb) (Mm00523381_m1); FA amide hydrolase (Faah) (Mm00515684_m1); FASN (Fasn) (Mm00662319_m1); 2-MG lipase (Mgll) (Mm00449274_m1); N-acylethanolamide-hydrolyzing acid amidase (Naaa) (Mm01341699_m1); N-acylphosphatidylethanolamide (NAPE) phospholipase D (Nape-pld) (Mm00724596_m1); PPARα (Ppara) (Mm00440939_m1); PPARβ (Pparb) (Mm00803184_m1); patatin-like phospholipase domain-containing protein 2 (Pnpla2) [Mm00503040_m1, also called adipose triglyceride lipase (ATGL)]; sterol carrier protein-2 (Scp2) (Mm01257982_m1); sterol regulatory element-binding transcription factor 1 (Srebf1) (Mm01138344_m1); and transient receptor potential cation channel subfamily V member 1 (Trvp-1) (Mm01246302_m1). Each sample reaction (20 μl total volume each) was performed on two replicates in 96-well optical reaction plates (Applied Biosystems®, Foster City, CA). The threshold cycle from each well was established using ABI Prism 7000 SDS software (Applied Biosystems®). The 18S RNA housekeeping gene was used for normalizing QRT-PCR data for mRNA expression of the above genes, as described earlier (14, 26, 27).
Western blotting
Aliquots of liver homogenates were subjected to SDS-PAGE followed by Western blotting, as described earlier (14, 26, 27). The following antibodies for Western blotting were obtained from Santa Cruz Biotechnology (Santa Cruz, CA): goat polyclonal anti-FAAH (sc-26427), anti-FA translocase protein (FATP)4 (sc-5834), anti-NAPE-PLD (sc-163117), anti-CB1 (sc-10066), anti-liver-type FABP1 (L-FABP; sc-16064), anti-acyl-CoA binding protein (ACBP) (sc-23474), monoclonal anti-mouse NAAA (sc-100470), monoclonal anti-mouse β-actin (sc-47778), rabbit polyclonal anti-monoacylglyceride lipase (MGL; sc-134789), anti-diacylglycerol lipase α (DAGLα sc-133307), and mouse monoclonal antibody to growth arrest and DNA damage inducible β (GADD45b; sc-377311). Mouse polyclonal anti-FATP5 (ab89008), rabbit polyclonal anti-FATP2 (ab83763), anti-cytochrome C oxidase 4 (COX4) (ab16056), and specific monoclonal anti-mouse heat shock protein 70 (HSP70) (ab2787) were from Abcam (Cambridge, MA). Mouse monoclonal GAPDH (MAB374) was from Millipore, Inc. (Billerica, MA). Rabbit polyclonal antibody recognizing 13.2 kDa sterol carrier protein-2 (SCP-2), 15 kDa pro-SCP-2, and 58 kDa SCP-x was described (25). To accommodate the molecular mass ranges of targeted proteins, as well as multiple targets on the same nitrocellulose blots, COX4 (14 kDa), GAPDH (37 kDa), or β-actin (43 kDa) was used as internal standard. Thus, relative protein levels were normalized to gel-loading control proteins, β-actin, GAPDH, or COX4; values were compared with male WT set to 1; data are presented as mean ± SEM (n = 8).
Statistical analysis
All values represent the mean ± SEM. Statistical analysis was performed by one-way ANOVA followed by Newman-Keuls post hoc analysis. Statistical differences of P < 0.05 were considered significant: *P < 0.05 for LKO versus WT; #P < 0.05 for female versus male.
RESULTS
Impact of pair-fed control diet or HFD on whole-body phenotype of WT and LKO mice
HFD alone selectively increased body weight in male, but not female, WT mice (Table 1). While LKO alone did not significantly alter the change in body weight, LKO conferred on HFD the ability to increase the body weight change (grams) in female mice (Table 1). While HFD-induced weight gain in WT mice was not attributable to increased total food consumption (kilocalories), LKO conferred on HFD the ability to slightly increase total food consumption (kilocalories) in both male and female mice (Table 1). When expressed in terms of energy conversion efficiency (i.e., percent weight change per kilocalorie of food consumed), HFD alone selectively increased energy conversion efficiency in WT male mice (Table 1). While LKO did not alter energy conversion efficiency in control-fed male or female mice, it conferred on HFD the ability to significantly increase energy conversion efficiency in both (Table 1).
TABLE 1.
Effect of LKO and HFD on whole-body phenotype of female versus male mice
| Parameter | Male | Female | ||||||
| WT | LKO | WT | LKO | |||||
| CO | HFD | CO | HFD | CO | HFD | CO | HFD | |
| Body weight change (g) | 7.2 ± 0.835 | 11.7 ± 1.159a | 6.4 ± 0.929 | 13.9 ± 1.364a | 5.1 ± 0.467 | 4.7 ± 0.275 | 4.1 ± 0.432 | 8.4 ± 1.023a,b |
| Initial body weight (g) | 21.1 ± 0.266 | 21.0 ± 0.328 | 21.3 ± 0.414 | 21.5 ± 0.35 | 16.1 ± 0.302 | 16.9 ± 0.210 | 17.0 ± 0.304 | 17.0 ± 0.232 |
| End body weight (g) | 28.4 ± 0.874 | 32.7 ± 1.349a | 27.7 ± 0.946 | 35.3 ± 1.46a | 21.3 ± 0.261 | 21.6 ± 0.338 | 21.3 ± 0.512 | 25.4 ± 1.09a,b |
| Total food consumption (kcal) | 972 ± 12 | 1,020 ± 20 | 943 ± 12 | 1,037 ± 16a | 893 ± 21 | 943 ± 25 | 886 ± 10 | 954 ± 17a |
| Energy conversion efficiency (%/kcal) | 0.035 ± 0.004 | 0.054 ± 0.004a | 0.032 ± 0.041 | 0.062 ± 0.005a | 0.036 ± 0.003 | 0.030 ± 0.001c | 0.027 ± 0.003 | 0.052 ± 0.006a,b |
| FTM change (%) | 38.8 ± 13.2 | 156.0 ± 26.5a | 156.4 ± 33.0b | 431.9 ± 47.0a,b | 2.1 ± 6.8c | 11.7 ± 3.0a,c | 56.7 ± 7.4b,c | 252.4 ± 34.2a,b,c |
| LTM change (%) | 13.8 ± 1.5 | 15.9 ± 2.3 | 2.7 ± 0.4b | 13.2 ± 1.8 | 15.4 ± 2.5 | 15.6 ± 1.1 | 1.4 ± 1.0b | 10.1 ± 2.3 |
Male and female WT and Fabp1-null (knockout; LKO) mice were pair-fed control diet (CO) (10 kcal%) or HFD (45 kcal%), as described in the Materials and Methods. All parameters were determined as described in the Materials and Methods. Food consumption and body weight, measured daily from the beginning to the end of the dietary study, were used to calculate body weight change (grams), total food consumption (kilocalories), and food conversion efficiency [body weight change (%)/total food consumed (kcal). At the beginning and end of the dietary study, mice were examined by DEXA to determine FTM change (percent) and LTM change (percent). Values represent average ± means ± SEM (n = 8).
By ANOVA, P ≤ 0.05, HFD versus control diet.
By ANOVA, P ≤ 0.05, LKO versus WT on same diet.
By ANOVA, P ≤ 0.05, female versus male of same genotype and diet.
To determine whether the above HFD- and LKO-induced increases in percent weight gain were associated with altered FTM change (percent) and/or LTM (percent), mice were analyzed at the beginning and end of the dietary study by DEXA, as described in the Materials and Methods. HFD alone selectively elicited a 4- and 5.6-fold increase in the percent FTM WT males and WT females, respectively (Table 1). Likewise, LKO alone increased the percent FTM by 4- and 27-fold in male and female control-fed mice, respectively (Table 1). HFD exacerbated the increase in percent FTM by 2.8- and 4.5-fold in LKO male and female mice (Table 1). Pair-fed HFD alone did not significantly alter LTM change (percent) in either male or female WT mice (Table 1). In contrast, LTM (percent) in LKO mice was inhibited in both male and female mice on control diet, while HFD increased the LTM change (percent) to be similar to that in the control-fed WT male and female mice (Table 1).
These data indicated that WT males were more sensitive to pair-fed HFD-induced body weight change (grams). LKO alone increased FTM change (percent) in both male and female control-fed mice, while retarding LTM change (percent).
Gross liver weight, gross morphology, histology, and serum enzyme markers of hepatotoxicity in WT and LKO mice pair-fed control diet or HFD
Neither pair-fed HFD nor LKO alone nor both together significantly altered liver weight (not shown) in either male or female WT mice. Thus, the increased weight gain in mice, especially LKO mice, fed HFD did not elicit hepatomegaly. Likewise, neither pair-fed HFD nor LKO alone nor both together significantly altered the gross morphology of the mice (supplemental Fig. S1C vs. supplemental Fig. S1A; Fig. 1G vs. Fig. 1E; supplemental Fig. S1B vs. supplemental Fig. S1A; Fig. 1F vs. Fig. 1E; supplemental Fig. S1D vs. supplemental Fig. S1C; Fig. 1H vs. Fig. 1G). On histologic evaluation, sections of liver from all groups were unremarkable (supplemental Fig. S2A–H). There was minimal hepatocyte vacuolation consistent with fatty change, including in control-fed WT male and female mice. Neither HFD alone (supplemental Fig. S2C vs. supplemental Fig. S2A; Fig. 2G vs. Fig. 2E) nor LKO alone (supplemental Fig. S2B vs. supplemental Fig. S2A; Fig. 2F vs. Fig. 2E) nor both together (supplemental Fig. S2D vs. supplemental Fig. S2A; Fig. 2H vs. Fig. 2E) altered the appearance of livers or induced any significant change in hepatocyte vacuolation scores in either male or female mice. There was no evidence of inflammation or hepatocyte necrosis to indicate liver damage. The absence of histologically detectable inflammatory changes was consistent with LKO having relatively little overall effect on levels of inflammatory cytokines (MCP-1, PAI-1, TNFα) and adipokines (adiponectin, resistin, leptin) in serum (not shown) or tissues (14, 26, 28). Finally, neither HFD nor LKO nor both HFD and LKO significantly altered serum AST (supplemental Fig. S3A) or ALT (supplemental Fig. S3B) values in either male or female mice. All serum AST and ALT values were well within the normal range of mouse values (blood chemistry and hematology in eight inbred strains of mice; Mouse Phenome Database: Eumorphia; http://phenome.jax.org).
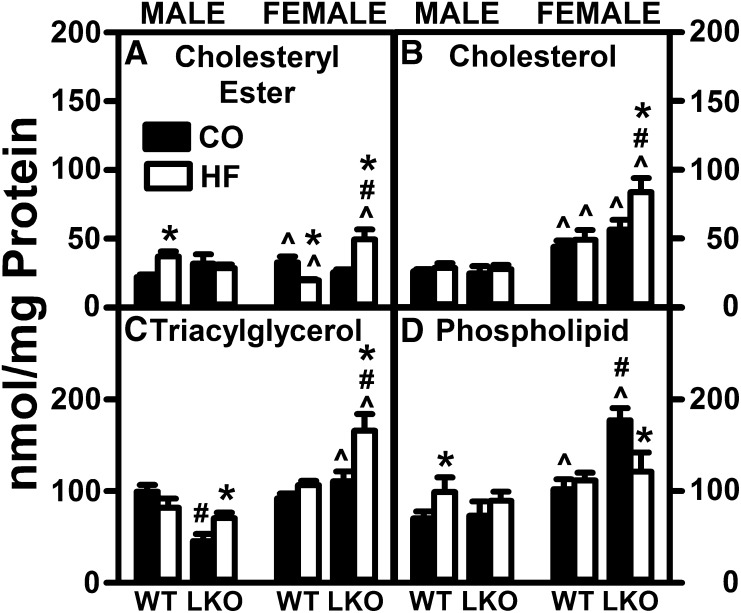
Fig. 1.
LKO and pair-fed HFD impact hepatic lipid accumulation in female and male mice. Male and female WT and Fabp1-null (knockout; LKO) mice on a C57BL/6N background were pair-fed control diet (black bars, CO) or HFD (open bars, HF), as described in the Materials and Methods. At the end of the dietary study, mice were fasted overnight and livers removed, flash-frozen, and stored at −80°C prior to liver lipid extraction and analysis, as described in the Materials and Methods. Levels of cholesteryl ester (A), unesterified free cholesterol (B), triacylglycerol (C), and phospholipid (D) were measured as in the Materials and Methods. Data are expressed as the mass (nanamoles per milligram protein) of each lipid class in the liver. Mean ± SEM (n = 8). By ANOVA, *P ≤ 0.05 HFD versus control diet; #P ≤ 0.05 LKO versus WT on same diet; ^P ≤ 0.05 female versus male of same genotype and diet.
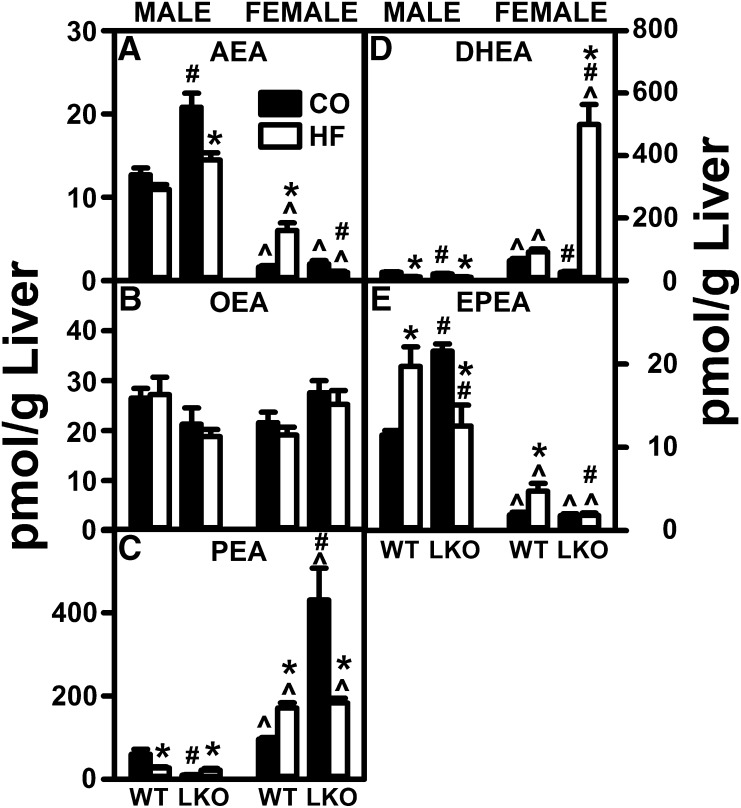
Fig. 2.
LKO diminishes the impact of a pair-fed HFD on liver NAE levels in male mice. Male and female WT and FABP1 LKO mice on a C57BL/6N background were pair-fed a control diet (black bars, CO) or HFD (open bars, HF), fasted overnight, livers removed, flash frozen, and stored at −80°C. NAEs were extracted and quantified by LC-MS analysis using deuterated internal standards, as described in the Materials and Methods. AEA (A), OEA (B), PEA (C), DHEA (D), and EPEA (E). Mean ± SEM (n = 8). By ANOVA, *P ≤ 0.05 HFD versus control diet; #P ≤ 0.05 LKO versus WT on same diet; ^P ≤ 0.05 female versus male of same genotype and diet.
These data suggested that, regardless of sex or genotype, neither LKO nor HFD nor both together elicited significant gross, histological, or enzyme markers indicative of hepatic lipid accumulation, inflammation, or toxicity.
Impact of pair-fed HFD and LKO on hepatic lipid phenotype
HFD alone did not significantly alter hepatic accumulation of total lipid or total neutral lipid in WT male or female mice as compared with their control-fed counterparts. Within the neutral lipid fraction, HFD alone differentially increased and decreased hepatic cholesteryl ester in WT males and females, respectively (Fig. 1A), but did not alter hepatic levels of free cholesterol (Fig. 1B) or triacylglycerol (Fig. 1C) in either male or female WT mice. Likewise, HFD alone did not change the hepatic level of total phospholipid in WT males and females, respectively (Fig. 1D). LKO alone did not impact hepatic accumulation of individual neutral lipid species, such as cholesteryl ester (Fig. 1A) or cholesterol (Fig. 1B), but selectively decreased hepatic triacylglyceride in males, but not females (Fig. 1C). LKO alone selectively increased hepatic phospholipid in control-fed females, but not males (Fig. 1D). In pair-fed HFD mice, LKO increased total neutral lipid only in females, attributable to increased levels of cholesteryl ester (Fig. 1A), free cholesterol (Fig. 1B), and triacylglycerol (Fig. 1C), but decreased liver total phospholipid selectively in females (Fig. 1D), resulting in no significant change in hepatic total lipid.
Thus, pair-fed HFD alone elicited small sex-dependent changes only in liver cholesteryl ester and phospholipid. LKO selectively conferred on pair-fed HFD females a modest increase in hepatic neutral lipids (especially triacylglycerol, free cholesterol, and cholesteryl ester). However, the extent of increases in these neutral lipids was markedly less than the 10- and 1.9-fold elevated hepatic triacylglycerol and cholesterol observed in human subjects with NAFLD (29).
Effect of pair-fed HFD and LKO on serum lipid phenotype
Overall, serum levels of almost all lipid classes were significantly lower in females than in males, regardless of pair-fed HFD or genotype (supplemental Table S2). Within each sex, pair-fed HFD alone had little, if any, effect on triacylglycerol, cholesterol, or cholesteryl ester in either male or female WT mice, as compared with their control-fed counterparts (supplemental Table S2). While serum phospholipid was decreased slightly (about 10%), this decrease was noted only in WT females pair-fed HFD (supplemental Table S2). In control-chow mice, LKO alone also did not impact serum triacylglycerol, cholesterol, or cholesteryl ester in either male or female WT mice, as compared with their control-fed counterparts (supplemental Table S2). Only serum phospholipid was decreased slightly (about 10%) in both male and female control-fed WT mice (supplemental Table S1). In HFD-fed mice, LKO decreased cholesterol in both sexes, while increasing cholesteryl ester only in males (supplemental Table S2). Serum NEFA levels were unchanged as a result of the LKO in both male and female mice (data not shown).
Taken together, these data indicated that pair-fed HFD alone, LKO alone, and LKO in the context of pair-fed HFD overall had very little effect on serum lipid content.
A pair-fed HFD elicits a sex-dependent increase in hepatic NAE and 2-MG levels in WT C57BL/6N mice
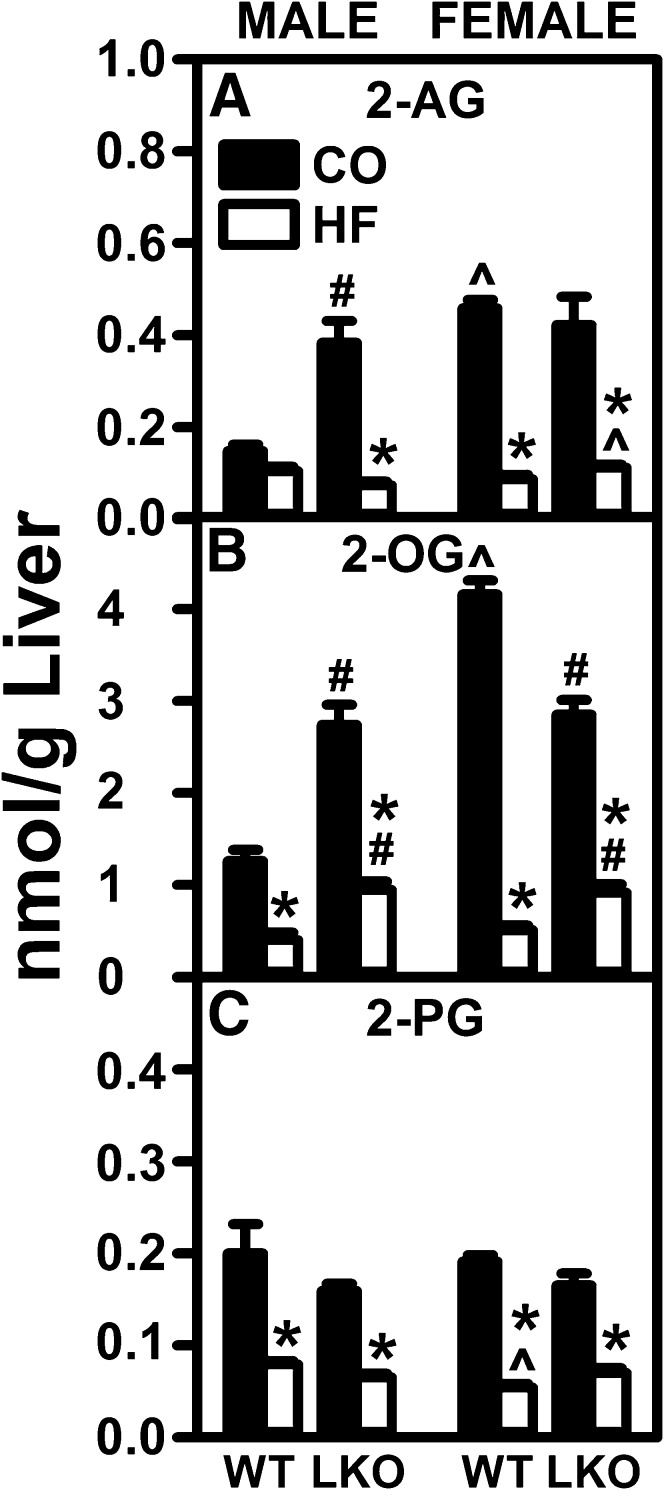
Pair-fed HFD alone selectively increased liver AEA level by nearly 3-fold in WT females, but this level was still only half that of their pair-fed HFD WT male counterparts (Fig. 2A). In contrast, pair-fed HFD alone had little effect on liver AEA level in WT males (Fig. 2A). Similarly, in WT females, the pair-fed HFD selectively increased liver levels of several non-ARA-containing NAEs [e.g., palmitoylethanolamide (PEA) (Fig. 2C) and eicosapentaenoylethanolamide (EPEA) (Fig. 2E)], but not others [e.g., oleoylethanolamide (OEA) (Fig. 2B) and docosahexaenoylethanolamide (DHEA) (Fig. 2D)]. In contrast, in WT males, the pair-fed HFD increased the liver level of only one non-ARA-containing NAE [i.e., EPEA (Fig. 2E)], but decreased that of PEA (Fig. 2C). In contrast to the impact of pair-fed HFD on hepatic NAE levels, the pair-fed HFD markedly decreased hepatic levels of all 2-MGs in males, and even more so in females (Fig. 3A–C).
Fig. 3.
LKO confers on HFD the ability to decrease liver 2-MGs in male mice. Male and female WT and FABP1 LKO mice on a C57BL/6N background were pair-fed a control diet (black bars, CO) or HFD (open bars, HF), as described in the Materials and Methods. All conditions were as in Fig. 2 except that 2-MGs were quantified by LC-MS analysis using deuterated internal standards (Cayman Chemical), as described in the Materials and Methods. Shown are 2-arachidonoylglycerol (2-AG) (A), 2-OG (B), and 2-palmitoylglycerol (2-PG) (C). Mean ± SEM (n = 8). By ANOVA, *P ≤ 0.05 HFD versus control diet; #P ≤ 0.05 LKO versus WT on same diet; ^P ≤ 0.05 female versus male of same genotype and diet.
Taken together, these findings indicated that the increased liver level of AEA (and most non-ARA-containing NAEs), along with the unaltered 2-AG level previously seen in males on an ad libitum-fed HFD (6, 7, 30, 31), may be attributable more to increased consumption of HFD than increased proportion of dietary fat. Interestingly, however, the pair-fed HFD study revealed for the first time that the higher proportion of fat increased AEA in females, but not males.
LKO differentially impacts the ability of a pair-fed HFD to alter hepatic NAE and 2-MG levels in male versus female mice
In mice pair-fed HFD, the LKO essentially blocked the ability of HFD to increase liver AEA in females and lowered AEA in males (Fig. 2A). Concomitantly, in females, LKO also blocked the ability of pair-fed HFD to increase hepatic levels of non-ARA NAEs, such as PEA (Fig. 2C) and EPEA (Fig. 2E), but not DHEA (Fig. 2D). In males, the LKO also decreased the ability of pair-fed HFD to increase liver AEA (Fig. 2A), while differentially impacting non-ARA-containing NAEs by not altering OEA (Fig. 2B) or DHEA (Fig. 2D), increasing PEA (Fig. 2C), and decreasing EPEA (Fig. 2E). The LKO also adversely impacted the effect of pair-fed HFD on hepatic 2-MGs in a sexually dimorphic manner. In female mice, the LKO only slightly impaired the ability of the pair-fed HFD to decrease hepatic levels of liver 2-AG (Fig. 3A), as well as the non-ARA 2-MGs, such as 2-oleoylglycerol (2-OG) or 2-palmitoylglycerol (2-PG) (Fig. 3B, C). In male mice, the LKO further enhanced the ability of the HFD to reduce hepatic levels of 2-AG (Fig. 3A), but not 2-OG (Fig. 3B) or 2-PG (Fig. 3C).
Thus, in general, the LKO counteracted some of the effects of a pair-fed HFD on hepatic AEA more in females, but 2-AG more in males.
LKO impact of a HFD on hepatic membrane FA transport/translocase proteins
Because LKO and sex both impacted the ability of a pair-fed HFD to induce hepatic levels of AEA and/or 2-AG, it was important to determine whether these findings were associated with concomitant upregulation of membrane proteins involved in FA uptake/translocation into hepatocytes. Three hepatic membrane-associated proteins (FATP5, FATP2, and FATP4) mediate the uptake/transmembrane transport of FAs, such as ARA, from which AEA and 2-AG are derived (13, 32).
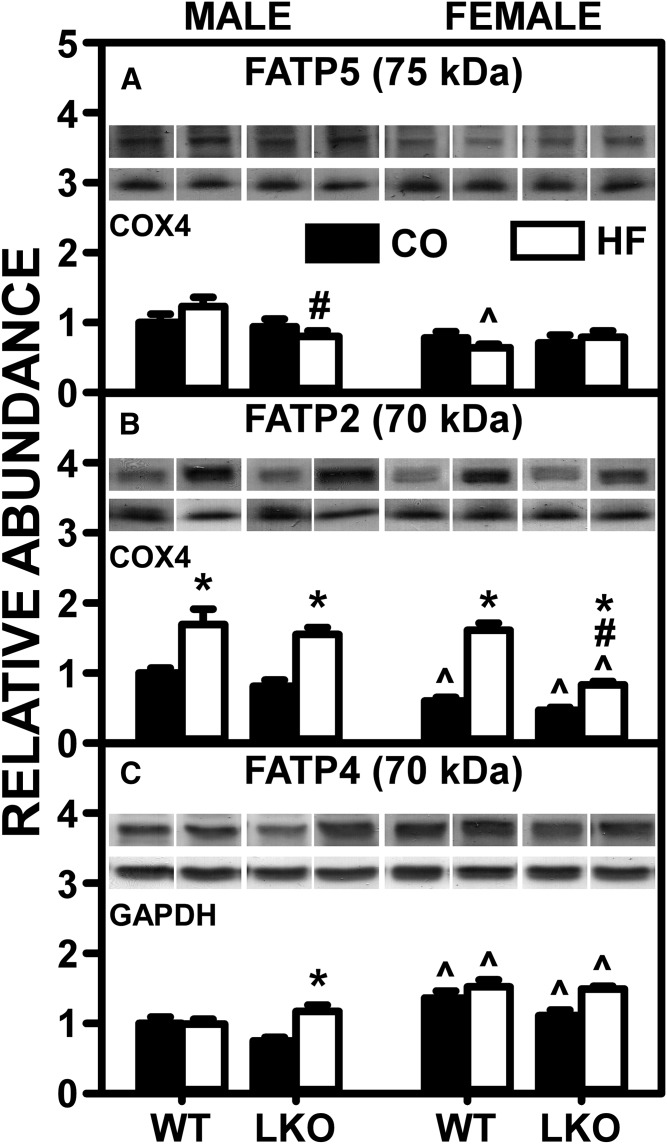
Western blotting determined that, in both male and female WT mice, the pair-fed HFD alone increased liver protein levels of only one major membrane FA transporter, FATP2 (Fig. 4B). In males, LKO differentially impacted the effect of pair-fed HFD on hepatic expression of membrane proteins in FA uptake/translocation, decreasing that of FATP5 (Fig. 4A) while increasing that of FATP4 (Fig. 4C). In females, the pair-fed HFD females LKO diminished the ability of HFD to increase hepatic levels of FATP5 (Fig. 4A).
Fig. 4.
Effect of LKO on ability of HFD to impact liver protein levels of membrane FA transport/translocase proteins involved in uptake of FA. Male and female WT and FABP1 LKO mice on a C57BL/6N background were pair-fed a control diet (black bars, CO) or HFD (open bars, HF), as described in the Materials and Methods. All conditions were as described in Fig. 2 except that SDS-PAGE followed by Western blotting on aliquots of liver homogenates were done, as described in the Materials and Methods, to determine levels of: FATP5 (A), FATP2 (B), and FATP4 (C). Relative protein levels were normalized to gel-loading control protein COX4 (FATP5 and FATP2) and β-actin (FATP4) and values compared with male WT set to 1. Mean ± SEM (n = 6–7). By ANOVA, *P ≤ 0.05 HFD versus control diet; #P ≤ 0.05 LKO versus WT on same diet; ^P ≤ 0.05 female versus male of same genotype and diet.
These data suggested that the pair-fed HFD-induced alterations in the hepatic AEA and 2-AG levels of WT and LKO mice did not correlate well with marked upregulation or downregulation of liver membrane FA transport/translocase proteins.
The effect of LKO on the impact of a HFD on liver cytosolic proteins that chaperone ARA, AEA, and 2-AG
The possibility that the hepatic changes in NAE and 2-MG may be associated with upregulation or downregulation of liver cytosolic proteins that chaperone FAs and/or ECs was considered. Liver expresses four families of intracellular cytosolic proteins that facilitate cytosolic transport of poorly aqueous-soluble lipidic molecules to intracellular organelles for metabolism: i) FABP1 binds not only ARA but also ARA esters, such as ARA-CoA, AEA, 2-AG, and most non-ARA-containing NAE and 2-MG (11, 13); ii) SCP-2 also binds ARA and ARA esters, such as ARA-CoA, AEA, and 2-AG (14, 33); iii) ACBP binds ARA-CoA, but not free ARA, AEA, or 2-AG (13, 34); and iv) HSP70 binds AEA (35).
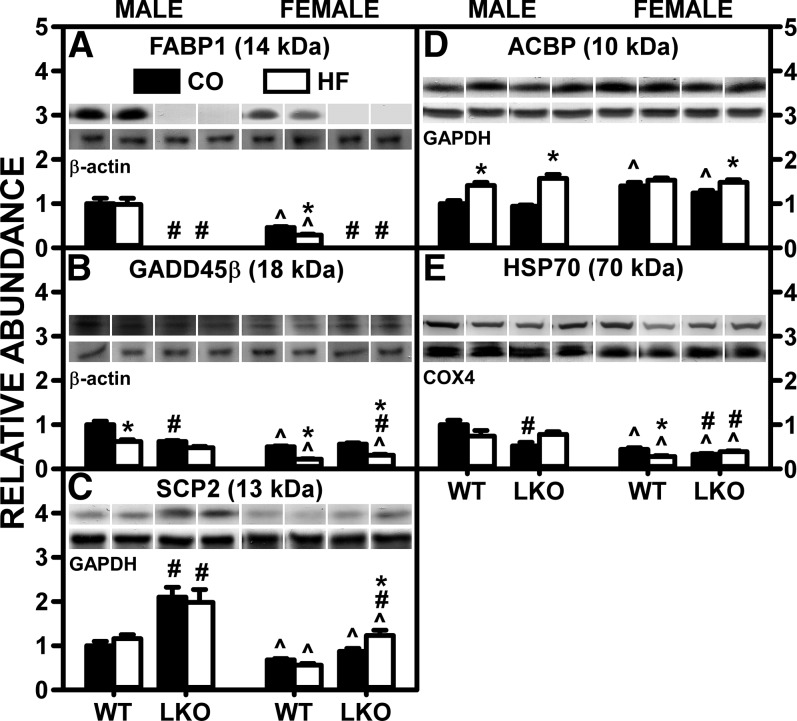
Although the pair-fed HFD alone did not significantly alter hepatic FABP1 level in WT male mice, it decreased it in WT females by nearly half (Fig. 5A). Concomitantly, the pair-fed HFD decreased the hepatic level of GADD45β in both males and females by 35% and 60%, respectively (Fig. 5B). GADD45β is a protein that physically forms a complex with FABP1, causing FABP1 redistribution from cytosol toward endoplasmic reticulum and/or plasma membranes, and thereby diminishes FABP1 function in facilitating FA uptake/cytosolic transport (36). In pair-fed HFD mice, LKO did not further impact GADD45β in males or females (Fig. 5B). These findings suggested that, despite the HFD-induced decrease in hepatic FABP1 level in WT females, the greater decrease in the inhibitory GADD45β in female WT mice may have compensated to maintain functional activity in females approaching that in males. Conversely, any changes in GADD45β in LKO mice were likely functionally ineffective due to a complete absence of FABP1.
Fig. 5.
LKO antagonizes the ability of HFD to decrease liver protein levels of cytosolic proteins that bind/chaperone ARA and/or ECs. Male and female WT and FABP1 LKO mice on a C57BL/6N background were pair-fed a control diet (black bars, CO) or HFD (open bars, HF), as described in the Materials and Methods. All conditions were as in Fig. 4 except that Western blot analysis was performed, as described in the Materials and Methods, to determine protein levels of: FABP1 (A), GADD45β (B), SCP-2 (C), ACBP (D), and HSP70 (E). Relative protein levels were normalized to gel-loading control protein β-actin (FABP1, GADD45β), GAPDH (SCP-2, ACBP), and COX4 (HSP70) and values compared with male WT set to 1. Mean ± SEM (n = 6–7). By ANOVA, *P ≤ 0.05 HFD versus control diet; #P ≤ 0.05 LKO versus WT on same diet; ^P ≤ 0.05 female versus male of same genotype and diet.
Regarding the other potential proteins involved in uptake/intracellular trafficking of lipidic ligands, the pair-fed HFD alone had no effect on hepatic SCP-2 (Fig. 5C) or ACBP (Fig. 5D) in either male or female WT mice, but decreased HSP70 in both male and female WT mice (Fig. 5E). LKO had no additional impact on the effect of pair-fed HFD on SCP-2 (Fig. 5C), ACBP (Fig. 5D), or HSP70 (Fig. 5E) in either males or females.
Taken together, these findings indicated that the HFD-induced increase in hepatic AEA (and most other NAEs) observed in female WT mice was attributable, in part, to a significant decrease in the FABP1 inhibitory protein, GADD45β, not counteracted by modest decreases in SCP-2 and HSP70. The lack of effect on AEA and a decrease in 2-AG in WT males were attributable, in part, to a higher intrinsic FABP1 level counteracted by more FABP1 inhibitory protein, GADD45β, as compared with those in WT females. LKO’s ability to block the pair-fed HFD-induced increase in hepatic AEA in females correlated largely with loss of FABP1 that was not compensated for by increased levels of SCP-2 and/or HSP70.
LKO: effect on the ability of a HFD to alter hepatic levels of proteins for AEA and 2-AG synthesis and degradation
Tissue levels of ECs are significantly determined by enzymes involved in EC synthesis (NAPEPLD and DAGLα) and degradation (FAAH, NAAA, and MGL) (37–39). Thus, it was important to determine the impact of a pair-fed HFD and LKO on hepatic protein levels of these enzymes.
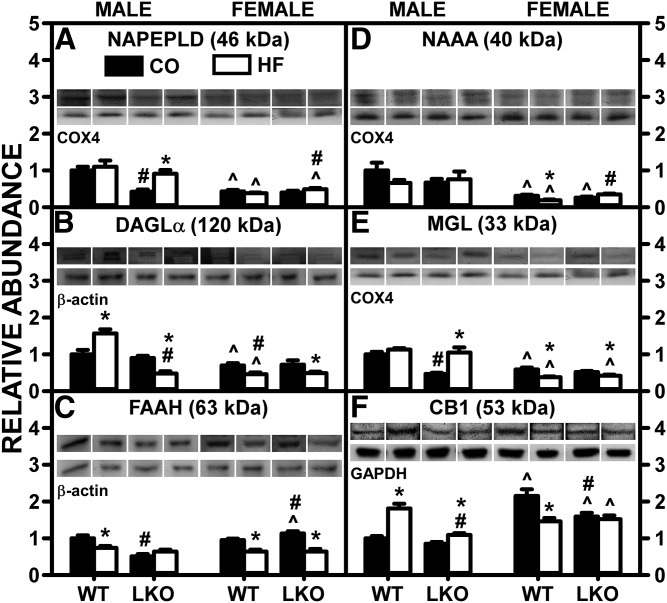
Western blotting showed that, in control-fed WT mice, HFD alone did not alter the hepatic level of NAPE-PLD in either males or females (Fig. 6A), while DAGLα was increased selectively only in male WT mice (Fig. 6B). In HFD-fed mice, LKO decreased the liver protein level of NAPE-PLD in males, but not females (Fig. 6A). Concomitantly, LKO blocked the ability of pair-fed HFD to increase DAGLα in males, while not altering the effect in females (Fig. 6B). Interestingly, these alterations in hepatic expression of protein measured by Western blotting poorly correlated with alterations in the respective hepatic mRNAs encoding these enzymes, by QRT-PCR (supplemental Fig. S4A–C). In WT mice, the pair-fed HFD decreased AEA degradative enzymes, such as FAAH (Fig. 6C) and NAAA (Fig. 6D), in both males and females, while the 2-MG degradative enzyme, MGL, was decreased only in females (Fig. 6E). In pair-fed HFD mice, LKO did not alter hepatic protein levels of FAAH (Fig. 6C), NAAA (Fig. 6D), or MGL (Fig. 6E) in either males or females. Again, these HFD- and LKO-induced alterations on liver protein levels of EC degradative enzymes correlated poorly with respective alterations in liver mRNA levels of Faah (supplemental Fig. S7D), Naaa (supplemental Fig. S7E), or Mgll (supplemental Fig. S7F).
Fig. 6.
LKO differentially impacts the ability of HFD to alter liver protein levels of membrane proteins involved in EC synthesis and degradation. Male and female WT and FABP1 LKO mice on a C57BL/6N background were pair-fed a control diet (black bars, CO) or HFD (open bars, HF), as described in the Materials and Methods. All conditions were as in Fig. 4 except that Western blot analysis was performed, as described in the Materials and Methods, to determine protein levels of: NAPE-PLD (A), DAGLα (B), FAAH (C), NAAA (D), MGL (E), and CB1 (F). Relative protein levels were normalized to gel-loading control protein β-actin (FAAH and DAGLα), GAPDH (CB1), and COX4 (NAPE-PLD, NAAA, and MGL) and values compared with male WT set to 1. Mean ± SEM (n = 6–7). By ANOVA, *P ≤ 0.05 HFD versus control diet; #P ≤ 0.05 LKO versus WT on same diet; ^P ≤ 0.05 female versus male of same genotype and diet.
These data indicated that the pair-fed HFD-induced increase in AEA and decrease in 2-AG in WT females correlated only in part with altered expression of the synthetic enzymes, NAPE-PLD and DAGLα, or of key degradative enzymes.
Effect of LKO on the ability of a HFD to induce hepatic CB1
Hepatic expression of the major cannabinoid receptor, CB1, is normally low, but was markedly upregulated by HFD in male mice (6–8). Therefore, the impact of LKO on the ability of HFD to increase hepatic levels of CB1 protein and Cnr1 mRNA expression was examined in male and female WT and LKO mice.
In WT mice, HFD nearly doubled the hepatic CB1 protein level in WT males, but decreased it in females (Fig. 6F). The decreased CB1 protein level in WT females was attributable, in part, to decreased Cnr1 mRNA in females (supplemental Fig. S5A). LKO diminished the ability of HFD to increase hepatic CB1 in males (Fig. 6F), attributable, in part, to decreased Cnr1 mRNA in LKO males (supplemental Fig. S5A). Conversely, in HFD-fed mice, LKO decreased the hepatic CB1 level in males, while not further altering it in females (Fig. 6F), an effect that correlated poorly with alterations in Cnr1 mRNA (supplemental Fig. S5A). For other cannabinoid receptors present at even lower levels in WT liver, Cnr2 mRNA was markedly increased in HFD-fed female LKO mice (supplemental Fig. S5B), while hepatic transcription of Trpv1 mRNAs was decreased in HFD-fed LKO males, but not females (supplemental Fig. S5C).
Thus, the hepatic CB1 protein level in male and female mice was differentially susceptible to HFD, i.e., increased and decreased respectively in WT mice; but in both sexes, LKO tended to diminish the impact.
Impact of LKO on the ability of HFD to alter hepatic expression of SREBP1-regulated enzymes in lipogenesis
Ad libitum-fed HFD increases AEA, the ligand activator of CB1, which in turn is known to induce de novo lipogenesis via stimulation of SREBP1c transcription of lipogenic genes, such as Acaca and Fasn (1, 6, 31). Concomitantly, ad libitum-fed HFD also increases SREBP1 (40). Therefore, the impact of pair-fed HFD and LKO on SREBP and its target genes was examined.
In contrast to ad libitum-fed WT males (40), pair-fed HFD did not increase, but instead decreased, Srebpf1 mRNA levels in male and female WT mice (supplemental Fig. S6A). Consequently, transcription of the SREBP1 target gene rate limiting in de novo lipogenesis, i.e., Acaca (supplemental Fig. S6), was reduced rather than increased in pair-fed HFD WT males and females (supplemental Fig. S6). SREBP1-mediated transcription of Fasn (another major SREBP1 target gene) was increased in pair-HFD WT males, but not WT females (supplemental Fig. S6C). LKO conferred on pair-fed HFD the ability to increase Srebf1 mRNA level in both male and female mice (supplemental Fig. S6A). However, this was not associated with increased transcription of the SREBP1 target genes, Acaca in either males or females (supplemental Fig. S6B) or Fasn in males, but increased that of Fasn in females (supplemental Fig. S6C). Pair-fed HFD alone had little effect on transcription of a key protein regulating hepatic lipid accumulation Pnpla2, encoding the key enzyme hydrolyzing triacylglycerol, in either male or female WT mice (supplemental Fig. S6D). However, pair-fed HFD alone increased Abhd5, encoding the coactivator protein of PNPLA2 in male, but not female, WT mice (supplemental Fig. S6E). LKO alone significantly increased Pnpla2, encoding the key enzyme hydrolyzing triacylglycerol in control-fed males, but not females (supplemental Fig. S6D). In pair-fed HFD mice, LKO decreased Pnpla2 in females and increased Abhd5 in males (supplemental Fig. S6E).
Taken together, these findings suggested that, overall, pair-fed HFD induced transcription of only a few (Fasn, Abhd5) lipogenic genes in WT male mice, but not female mice, consistent with the inability of pair-fed HFD to increase hepatic AEA or 2-AG levels (Figs. 2, 3). Likewise, overall, LKO did not confer on pair-fed HFD the ability to induce transcription of most lipogenic genes in male or female mice, consistent with the inability of pair-fed HFD to increase hepatic AEA or 2-AG levels, both of which were actually decreased rather than increased (Figs. 2, 3).
Effect of LKO on the ability of a HFD to alter the hepatic level of PPARα-regulated enzymes
Concomitant with AEA activation of CB1 inducing SREBP1-mediated transcription of lipogenic enzymes, CB1 activation decreases CPT1 (rate limiting enzyme in mitochondrial FA oxidation) and decreases FA oxidation (31, 41). Conversely, the non-ARA-containing NAEs (OEA, PEA), which do not bind CB1, nevertheless enter the nucleus to activate PPARα transcription of FA oxidative genes, such as Cpt1a, Acox1, and Ppara itself (1, 19, 42, 43). Because pair-feeding a HFD markedly decreased hepatic levels AEA and also altered non-ARA NAEs, effects mitigated by the LKO (Fig. 2), it was important to determine the net impact of such alterations in NAE profile on transcription of PPARα target enzymes.
Pair-fed HFD alone did not alter hepatic transcription of Ppara or Pparb (supplemental Fig. S7A, B) in either male or female WT mice. However, transcription of target enzymes, Acox1 and Cpt1a (supplemental Fig. S7C, D), was decreased in pair-fed HFD WT males, but not females. LKO alone also did not alter hepatic transcription of Ppara or Pparb (supplemental Fig. S7A, B) in either male or female WT mice. Consistent with this finding, transcription of only one target enzyme (Cpt1a) was increased in males, but not females (supplemental Fig. S7D), while Acox1 was unaltered in both males and females (supplemental Fig. S7C). In pair-fed HFD mice, LKO increased hepatic transcription of Ppara (supplemental Fig. S7A) in both male and female mice, as well as Pparb in females (supplemental Fig. S7B). However, it significantly increased only Cpt1a in males (supplemental Fig. S7D) and did not significantly alter Acox1 (supplemental Fig. S7C) or Cpt1a (supplemental Fig. S7D) in females.
Thus, although ad libitum feeding of a HFD to WT males decreased PPARα (40), pair-fed HFD did not decrease Ppara in either male or female WT mice, but nevertheless modestly decreased PPAR target enzymes in males (but not females). Taken together with the lack of AEA change in livers of pair-fed HFD WT mice, this would indicate that the lack of alteration in hepatic lipid accumulation therein was not associated with altered expression of FA oxidative enzymes. Likewise, the lack of major liver lipid accumulation in pair-fed HFD LKO mice was not associated with significant alterations in expression of FA oxidative enzymes overall.
DISCUSSION
The recently discovered connection between NAFLD and the liver EC system represents a major breakthrough in our understanding of factors regulating the EC system in hepatic fat accumulation. Several recent discoveries suggested a potential link between NAFLD, the EC system, and FABP1 (10, 16, 40, 44). Hepatic FABP1 protein level was strongly upregulated in human NAFLD (44), human FABP1 T94A expressors with NAFLD [reviewed in (10)], and animal models of NAFLD (16, 40). FABP1 is the major cytosolic EC and cannabinoid binding/chaperone protein in mouse liver (13). While ad libitum-fed HFD induces ECs and NAFLD in human and rodent models, whether this is due to the high proportion of dietary fat and/or preference for/increased consumption of HFD versus low fat diet is not completely clear. Ad libitum feeding HFD does not discriminate between the impact (if any) of the higher fat content of the HFD versus increased total food intake. Unfortunately, there is a paucity of pair-fed HFD translational/clinical and animal model studies to resolve this problem. Therefore, the current study examined the impact of the Fabp1 gene and pair-fed HFD on the hepatic EC system and NAFLD in C57BL/6N mice to provide the following new insights:
First, pair-fed HFD alone increased the proportion of FTM (percent FTM) in WT C57BL/6N mice. The finding of increased percent FTM in pair-fed HFD male mice was analogous to the effect of ad libitum-fed HFD, which induces obesity in male C57BL/6N mice (5, 45–47). Likewise, pair-fed HFD also increased the percent FTM in female C57BL/6N mice, analogous to the effect of ad libitum-fed HFD (albeit a lower percent fat than that used herein), which also increased percent FTM in female C57BL/6N mice (48). In view of the preference of both humans (49–52) and rodents (4, 19) for and higher consumption of a HFD compared to a low fat diet, the current finding that pair-fed HFD increased FTM in male and female C57BL/6N mice suggested that high dietary fat content alone, independent of dietary preference/consumption, increased FTM/obesity.
Second, pair-fed HFD alone did not induce hepatic fat accumulation in either male or female WT C57Bl/6N mice. The lack of effect of pair-fed HFD on hepatic fat level was consistent with pair-fed HFD not altering hepatic protein levels of FABP1 in males and even slightly decreasing it in WT females. FABP1 is known to enhance FA incorporation into glycerides (and less so cholesteryl esters), both of which accumulate in NAFLD [reviewed in (10)]. This was in marked contrast to ad libitum-fed HFD, which induced massive neutral lipid (triacylglycerol, cholesteryl ester) accumulation manifested as NAFLD in WT male mice (5, 45–47) and increased hepatic neutral lipid accumulation in WT female mice fed HFD ad libitum (53). Hepatic FABP1 is markedly upregulated by ad libitum feeding of HFD to WT male mice (15, 16, 40). Thus, dietary content of high fat alone, independent of dietary preference/consumption for HFD, did not elicit hepatic neutral lipid accumulation/NAFLD. It is important in future studies beyond the scope of the present investigation to determine the translational/clinical significance of this finding. While composition of the fat may also contribute (54), resolving this complex issue is beyond the scope of the current investigation.
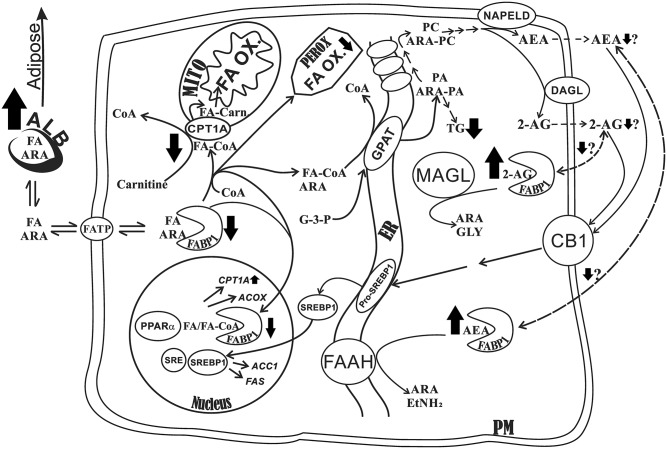
Third, LKO markedly increased the whole-body proportion of FTM (percent FTM) in both male and female control-fed mice, an effect exacerbated by pair-fed HFD. Remarkably, this increase was at the expense of LTM (percent LTM), which increased only slightly in both control-fed and HFD-fed mice. This apparent redirection of FAs toward adipose storage may be explained by the marked impact that FABP1 has on hepatic FA uptake/oxidation, as proposed in the schematic in Fig. 7. It has been shown in FABP1-overexpressing transformed cells, cultured primary hepatocytes from LKO mice, and in vivo with LKO mice that FABP1 enhances hepatic FA uptake and directly facilitates FA import via CPT1A into mitochondria (rate limiting) and into peroxisomes to directly enhance FA oxidation. FABP1 also enhances FA import into the nucleus for interacting with and targeting bound FAs to PPARα to induce transcription of FA oxidative enzymes, thereby also indirectly enhancing FA oxidation (55–60). In a variety of gene-ablated mice, hepatic FA oxidation correlated directly with hepatic FABP1 expression (55). Together, as outlined in the schematic in Fig. 7 (heavy black arrows), these cited studies showed that, in the absence of FABP1 (i.e., LKO), less FA is taken up, targeted to mitochondria and peroxisomes, and targeted to nuclei for upregulating FA oxidative enzymes. This would make more FA available for redirection/deposition toward adipose, consistent with the findings presented herein (Fig. 7, heavy black arrow). Consistent with this possibility, LKO did not elicit hepatic neutral lipid (triacylglycerol, cholesterol, and cholesteryl ester) accumulation in males and, overall, only slightly in females. Under both control diet and HFD, there was little impact on histological vacuolation or hepatic inflammation, as shown by histological examination. Although AEA and 2-AG are key ECs known to be associated with HFD and NAFLD via CB1 activation of an intracellular pathway that promotes de novo lipogenesis (6, 7, 31), the lack of hepatic lipid accumulation, especially in male LKO mice fed control diet, was not attributable to any decrease in AEA and 2-AG; both were instead increased (Fig. 7, heavy black arrow). The elevated AEA and 2-AG may be explained by the fact that FABP1 is the major hepatic cytosolic protein that binds and solubilizes the highly insoluble membrane-bound AEA and 2-AG (13). By binding AEA and 2-AG, FABPs enhance their reuptake and transport/targeting to degradative enzymes (FAAH and MAGL, respectively) (10, 61–63). LKO would inhibit AEA and 2-AG transport/targeting for degradation, thereby eliciting AEA and 2-AG accumulation, as shown herein. Concomitantly, however, the loss of FABP1 (LKO) would also be expected to decrease transport of AEA and 2-AG to the plasma membrane for efflux and subsequent activation of CB1 (Fig. 7, heavy black arrow). Finally, it is also important to note that FABP1 has high affinity for ARA and enhances ARA cellular uptake (56). Thus, LKO would be expected to decrease ARA uptake and thereby reduce ARA availability for incorporation into ARA-containing phosphatidylcholine (ARA-PC), the immediate precursor from which AEA and 2-AG are synthesized by NAPE-PLD and DAGL, respectively (Fig. 7, heavy black arrow) (14). Indeed, LKO increases serum total ARA and concomitantly increases brain levels of ARA and ARA-derived ECs (AEA, 2-AG) in other tissues (14). Also, as indicated by the data presented herein, HFD further impacts this overall picture by increasing dietary ARA mass (grams) availability by 5-fold, as compared with the control diet (supplemental Table S1), consistent with increased AEA and 2-AG in HFD-fed WT male mice. This effect of HFD was blocked by LKO. Finally, these effects were also sex dependent, consistent with WT female mice expressing significantly lower basal levels of FABP1 and other FA/EC cytosolic chaperone proteins than their male counterparts (13, 27, 64, 65).
Fig. 7.
Proposed schematic shows the impact of LKO on metabolism of FAs, EC (AEA, 2-AG) metabolism, and lipid accumulation in livers of male mice. Long-chain FAs, including ARA, dissociate from serum albumin for translocation across the plasma membrane via transporters, such as the long-chain FATP. The schematic shows the pathways wherein liver FA binding protein facilitates uptake and metabolism of long-chain FA, including ARA as well as the ARA-derived ECs, AEA and 2-AG. The heavy black arrows denote the impact of ablating the Fabp1 gene (i.e., LKO), with up arrows and down arrows denoting increase and decrease, respectively, while the thickness of the arrow indicates the strength of impact. Briefly, FABP1 facilitates the uptake of FAs (including ARA) and their cytosolic transport and targeting toward intracellular sites to: i) directly facilitate long-chain FA oxidation (FA-OX) via mitochondria (MITO) and peroxisomes (PEROX); ii) directly facilitate fatty acyl-CoA (FA-CoA) transacylation of glycerol-3-phosphate by glycerol-3-phosphate acyltransferase (GPAT) and lysophosphatidic acid to yield phosphatidic acid (PA) in the endoplasmic reticulum (ER); iii) indirectly facilitate FA oxidation by cotransporting bound FA and FA-CoA into the nucleus, interacting with peroxisome PPARα, transferring FA and FA-CoA to PPARα to induce transcription of multiple FA oxidative enzymes, including the rate limiting enzyme in mitochondrial long-chain FA oxidation (CPT1A) and peroxisomal long-chain FA oxidation [acyl-CoA oxidase (ACOX)]; iv) directly facilitate synthesis/release of ARA-containing ECs (AEA, 2-AG). By enhancing ARA uptake/transport to the ER and esterification to PA, FABP1 also provides increased availability of ARA for incorporation into several other phospholipids downstream of PA (e.g., ARA-PC), which are subsequently incorporated into the plasma membrane (PM). Therein, a series enzymes, including NAPE-PLD and diacylglycerol lipase (DAGL), mediate synthesis/release of AEA and 2-AG, respectively, from the ARA-PC precursor. The released AEA and 2-AG then bind/activate the plasma membrane CB1, which, by downstream signaling, induces release of SREBP-1 (SREBP1) from its ER-bound precursor pro-SREBP1. SREBP1 then traffics to the nucleus wherein it interacts with sterol regulatory element (SRE) protein to induce transcription of key enzymes in de novo FA synthesis/lipogenesis, including acetyl-CoA acyltransferase-1 (ACC1) and FAS; v) hepatic AEA and 2-AG levels are thought to be regulated not only by synthesis/release as in (iv) above, but also by reuptake for transport to intracellular degradative enzymes. Because AEA and 2-AG are very poorly aqueous soluble, this requires a chaperone/binding protein, a function primarily served by FABP1. In these degradative pathways, FABP1 is thought to facilitate reuptake of released AEA to facilitate its trafficking to fatty acyl ethanolamide hydrolase (FAAH) in the ER for degradation to ARA and ethanolamine (EtNH2) and to monoacylglycerol lipase (MAGL), a primarily cytosolic enzyme, for degradation of 2-AG to ARA and glycerol (GLY). Finally, it is important to note that the net impact of LKO (denoted by heavy black arrows) is to redirect serum FA and ARA away from the liver to make them more available for uptake by other tissues, such as adipose (shown herein by increased FTM) and earlier into brain (14).
Fourth, pair-feeding HFD markedly diminished the impact of an ad libitum-fed HFD on hepatic ECs associated with NAFLD, suggesting that hepatic fat accumulation in response to ad libitum-fed HFD (6, 7, 9) was attributable more to hyperphagia than to the proportion of fat. In contrast, the increased hepatic AEA level was attributable more to the proportion of fat in the HFD rather than hyperphagia in WT females. The fact that pair-fed HFD did not increase hepatic fat in WT males represented the balance of several factors: i) unaltered level of AEA and decreased 2-AG, the major CB1 endogenous agonist; ii) potentially increased hepatic PPARα activation of FA oxidative enzymes (PEA, a weak PPARα activator) (however, no increase in mRNA transcripts of FA oxidative genes was observed); and/or iii) the increased hepatic level of CB1 may have compensated for the reduced level of 2-AG. Consistent with this possibility, induction of SREBP1 transcription of target genes in de novo lipogenesis was observed only for Fasn, but not for Acaca (which encodes the rate limiting enzyme for de novo lipogenesis) in WT males. Neither Fasn nor Acaca were increased in WT females.
Fifth, pair-fed HFD did not differ from ad libitum-fed HFD in impacting the hepatic CB1 receptor level. NAFLD is mediated by activation of hepatic CB1 receptor, and the CB1 receptor is required for development of HFD-induced NAFLD (6, 7, 9). The pair-fed HFD markedly increased hepatic CB1 protein level in WT males, but decreased it in WT females. Although an ad libitum-fed HFD also increases hepatic CB1 in WT male mice (31), the effect of an ad libitum-fed HFD on hepatic CB1 in WT females is not known. Differential hepatic CB1 expression between male and female WT mice reveals increased hepatic CB1 in females in comparison to males. Although LKO decreased hepatic CB1 levels in female mice, CB1 expression remained significantly higher than in the male LKO mice. Furthermore, LKO significantly muted the response in hepatic CB1 levels to HFD pair-feeding in male and female mice.
In summary, as shown herein by pair-fed HFD and earlier by ad libitum-fed HFD, both elicited obesity/increased FTM, indicating contributions from both the fat content of the diet and hyperphagy. However, pair-fed HFD did not elicit NAFLD, suggesting that in previous studies of ad libitum-fed HFD, HFD-induced NAFLD was attributable to increased consumption of ad libitum-fed HFD rather than to the higher proportion of fat in the diet. Independent of hyperphagia, however, pair-fed HFD nevertheless significantly altered hepatic EC and CB1 receptor protein levels in a sex-dependent manner different from that in response to ad libitum-fed HFD. In addition, LKO differentially affected the ability of pair-fed HFD to alter hepatic ECs and CB1 receptor level, again in a sex-dependent manner. While hepatic lipid mass was not markedly altered, regardless of sex, LKO did exacerbate the increase in percent FTM in both males and females in response to pair-fed HFD (see schematic in Fig. 7). Together these and other findings indicated that the lack of FABP1 modulated the impact of a pair-fed HFD on the hepatic EC system by several mechanisms: i) Loss of FABP1 may reduce the hepatic uptake of ARA and γ-linolenic acid (γ-LNA; C18:2, n-6), which can be metabolized to ARA in liver. FABP1 has high affinity for ARA and γ-LNA (11, 12), is preferentially enriched in ARA and γ-LNA (12), and FABP1 overexpression enhances ARA uptake in cultured cells (56). ii) Lack of FABP1 may impede the ability of the very poorly aqueous-soluble high membrane-bound ECs, such as AEA and 2-AG, to traffic through the cytosol to sites of degradation. The novel discovery that both murine and human FABP1 have high affinity for ECs, such as NAEs (AEA, OEA, EPEA, DHEA) (10, 13) and 2-MGs (2-AG, 2-OG, 2-PG) (10, 13, 66), combined with the high concentration of murine FABP1 (2–3% of cytosolic protein) and the even higher concentration of human FABP1 (up to 10% of cytosolic protein) in liver (10, 13) suggest that FABP1 is the major hepatic EC binding protein [reviewed in (10, 13)]. As such, FABP1 may be the primary chaperone, binding and carrying ECs to intracellular sites for degradation, analogous to what has been shown for other FA binding protein family members (FABP3, -5, and -7) expressed in brain [reviewed in (10, 13)]. Importantly, the fact that the pair-fed HFD did not induce massive hepatic fat accumulation in the FABP1 gene-ablated mice on the NAFLD-susceptible C57BL/6N background strain suggests FABP1 as a potential therapeutic target for impacting the hepatic EC system in the treatment of NAFLD. Finally, while beyond the scope of the current investigation, the translational relevance of these findings will be explored in future studies of samples and cells from human patients, especially liver and cultured primary hepatocytes from human subjects expressing the FABP1 T94A variant versus the WT FABP1 T94T.
Supplementary Material
Footnotes
Abbreviations:
- Abhd5
- 1-acylglycerol-3-phosphate-O-acyltransferase
- Acaca
- acetyl-CoA carboxylase-α ACBP, acyl-CoA binding protein
- Acox1
- acyl-CoA oxidase-1
- AEA
- arachidonoylethanolamide
- 2-AG
- 2-arachidonoyl glycerol
- ALT
- alanine aminotransferase
- ARA
- arachidonic acid
- ARA-PC
- arachidonic acid-containing phosphatidylcholine
- AST
- aspartate aminotransferase
- CB1 (Cnr1)
- cannabinoid receptor-1
- Cpt1a
- carnitine palmitoyltransferase 1A
- Cnr2
- cannabinoid receptor-2
- COX4
- cytochrome C oxidase 4
- DAGLα
- diacylglycerol lipase α DEXA, dual-energy X-ray absorptiometry
- DHEA
- docosahexaenoylethanolamide
- EC
- endocannabinoid
- EPEA
- eicosapentaenoylethanolamide
- Faah
- FA amide hydrolase
- Fabp1 (L-FABP)
- liver FA binding protein-1
- FATP
- FA translocase protein
- FTM
- fat tissue mass
- HFD
- high-fat diet
- HSP70
- heat shock protein 70
- LKO
- FABP1 gene ablation
- γ-LNA
- γ-linolenic acid
- LTM
- lean tissue mass
- 2-MG
- 2-monoacylglycerol
- MGL
- monoacylglyceride lipase
- Mgll
- 2-monoacylglycerol lipase
- Naaa
- N-acylethanolamide-hydrolyzing acid amidase
- NAE
- N-acylethanolamide
- NAFLD
- nonalcoholic fatty liver disease
- NAPE
- N-acylphosphatidylethanolamide
- Nape-pld
- N-acylphosphatidylethanolamide phospholipase D
- OEA
- oleoylethanolamide
- 2-OG
- 2-oleoylglycerol
- PEA
- palmitoylethanolamide
- 2-PG
- 2-palmitoylglycerol
- Pnpla2
- patatin-like phospholipase domain-containing protein 2
- QRT-PCR
- quantitative RT-PCR
- SCP-2 (Scp2)
- sterol carrier protein-2
This work was supported, in part, by the US Public Health Service/National Institutes of Health Grant 5T35OD010991 (S.C., A.B.K.) and TxAgriLife Research (F.S., S.C., A.B.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare that they have no conflicts of interest with the contents of this article.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Naughton S. S., Mathai M. L., Hryciw D. H., and McAinch A. J.. 2013. Fatty acid modulation of the endocannabinoid system and the effect on food intake and metabolism. Int. J. Endocrinol. 2013: 361895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawano Y., and Cohen D. E.. 2013. Mechanisms of hepatic triglyceride accumulation in non-alcoholic liver disease. J. Gastroenterol. 48: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamath S., Chavez A. O., Gestaldelli A., Casiraghi F., Halff G. A., Abrahamian G. A., Davalli A. M., Bastarrachea R. A., Comuzzie A. G., Guardado-Mendoza R. , et al. 2011. Coordinated defects in hepatic long chain fatty acid metabolism and triglyceride accumulation contribute to insulin resistance in non-human primates. PLoS One. 6: e27617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douglass J. D., Zhou Y. X., Wu A., Zadrogra J. A., Gajda A. M., Lackey A. I., Lang W., Chevalier K. M., Sutton S. W., Zhang S-P., et al. 2015. Global deletion of monoacylglycerol lipase in mice delays lipid absorption and alters energy homeostasis and diet-induced obesity. J. Lipid Res. 56: 1153–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fengler V. H. I., Macheiner T., Kessler S. M., Czepukojc B., Gemperlein K., Muller R., Kiemer A. K., Magnes C., Haybaeck J., Lackner C., et al. 2016. Susceptibility of different mouse wild-type strains to develop diet induced NAFLD/AFLD associated liver disease. PLoS One. 11: e0155163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alswat K. A. 2013. The role of endocannabinoids system in fatty liver disease and therapeutic potential. Saudi J. Gastroenterol. 19: 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tam J., Liu J., Mukhopadhyay B., Cinar R., Godlewski G., and Kunos G.. 2011. Endocannabinoids in liver disease. Hepatology. 53: 346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regnell S. E. 2013. Cannabinoid 1 receptor in fatty liver. Hepatol. Res. 43: 131–138. [DOI] [PubMed] [Google Scholar]
- 9.Osei-Hyiaman D., Liu J., Zhou L., Godlewski G., Harvey-White J., Jeong W., Batkai S., Marsicano G., Lutz B., Buettner C., et al. 2008. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J. Clin. Invest. 118: 3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroeder F., McIntosh A. L., Martin G. G., Huang H., Landrock D., Chung S., Landrock K. K., Dangott L. J., Li S., Kaczocha M., et al. 2016. Fatty acid binding protein-1 (FABP1) and the human FABP1 T94A variant: roles in the endocannabinoid system and dyslipidemias. Lipids. 51: 655–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frolov A., Cho T. H., Murphy E. J., and Schroeder F.. 1997. Isoforms of rat liver fatty acid binding protein differ in structure and affinity for fatty acids and fatty acyl CoAs. Biochemistry. 36: 6545–6555. [DOI] [PubMed] [Google Scholar]
- 12.Murphy E. J., Edmondson R. D., Russell D. H., Colles S. M., and Schroeder F.. 1999. Isolation and characterization of two distinct forms of liver fatty acid binding protein from the rat. Biochim. Biophys. Acta. 1436: 413–425. [DOI] [PubMed] [Google Scholar]
- 13.Huang H., McIntosh A. L., Martin G. G., Landrock D., Chung S., Landrock K. K., Dangott L. J., Li S., Kier A. B., and Schroeder F.. 2016. FABP1: a novel hepatic endocannabinoid and cannabinoid binding protein. Biochemistry. 55: 5243–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin G. G., Chung S., Landrock D., Landrock K. K., Huang H., Dangott L. J., Peng X., Kaczocha M., Seeger D. R., Murphy E. J., et al. 2016. FABP1 gene ablation impacts brain endocannabinoid system in male mice. J. Neurochem. 138: 407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaemers I. C., Stallen J. M., Kunne C., Wallner C., van Werven J., Nederveen A., and Lamers W. H.. 2011. Lipotoxicity and steatohepatitis in an overfed mouse model for non-alcoholic fatty liver disease. Biochim. Biophys. Acta. 1812: 447–458. [DOI] [PubMed] [Google Scholar]
- 16.Baumgardner J. N., Shankar K., Hennings L., Badger T. M., and Ronis M. J.. 2008. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am. J. Physiol. Gastrointest. Liver Physiol. 294: G27–G38. [DOI] [PubMed] [Google Scholar]
- 17.Higuchi N., Kato M., Tanaka M., Miyazaki M., Takao S., Kohjima M., Kotoh K., Enjoji M., Nakamuta M., and Takayanagi R.. 2011. Effects of insulin resistance and hepatic lipid accumulation on hepatic mRNA expression levels of apoB, MTP, and L-FABP in non-alcoholic fatty liver disease. Exp. Ther. Med. 2: 1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlton M., Viker K., Krishnan A., Sanderson S., Veldt B., Kaalsbeek A. J., Kendrick M., Thompson G., Que F., Swain J., et al. 2009. Differential expression of lumican and fatty acid binding protein-1: new insights into the histologic spectrum of nonalcoholic fatty liver disease. Hepatology. 49: 1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silvestri C., and DiMarzo V.. 2013. The endocannabinoid system in energy homeostasis and the etiophathology of metabolic disorders. Cell Metab. 17: 475–490. [DOI] [PubMed] [Google Scholar]
- 20.Martin G. G., Danneberg H., Kumar L. S., Atshaves B. P., Erol E., Bader M., Schroeder F., and Binas B.. 2003. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid binding protein (L-FABP) gene. J. Biol. Chem. 278: 21429–21438. [DOI] [PubMed] [Google Scholar]
- 21.Nelson D. W., Gao Y., Spencer N. M., Banh T., and Yen C-L. E.. 2011. Deficiency of MGAT2 increases energy expenditure without high-fat feeding and protects genetically obese mice from excessive weight gain. J. Lipid Res. 52: 1723–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milligan S., Martin G. G., Landrock D., McIntosh A. L., Mackie J. T., Schroeder F., and Kier A. B.. 2017. Impact of dietary phytol on lipid metabolism in SCP2/SCPx/L-FABP null mice. Biochim. Biophys. Acta. 1862: 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storey S. M., Huang H., McIntosh A. L., Martin G. G., Kier A. B., and Schroeder F.. 2017. Impact of Fabp1/SCP-2/SCP-x gene ablation (TKO) on hepatic phytol metabolism in mice. J. Lipid. Res. 58: 1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin G. G., Atshaves B. P., Landrock K. K., Landrock D., Schroeder F., and Kier A. B.. 2015. Loss of L-FABP, SCP-2/SCP-x, or both induces hepatic lipid accumulation in female mice. Arch. Biochem. Biophys. 580: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackie J. T., Atshaves B. P., Payne H. R., McIntosh A. L., Schroeder F., and Kier A. B.. 2009. Phytol-induced hepatotoxicity in mice. Toxicol. Pathol. 37: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin G. G., Chung S., Landrock D., Landrock K., Dangott L. J., Peng X., Kaczocha M., Murphy E. J., Kier A. B., and Schroeder F.. 2016. Female mice are resistant to Fabp1 gene ablation induced alterations in brain endocannabinoid levels. Lipids. 51: 1007–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klipsic D., Landrock D., Martin G. G., McIntosh A. L., Landrock K. K., Mackie J. T., Schroeder F., and Kier A. B.. 2015. Impact of SCP-2/SCP-x gene ablation and dietary cholesterol on hepatic lipid accumulation. Am. J. Physiol. Gastrointest. Liver Physiol. 309: G387–G399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin G. G., Atshaves B. P., McIntosh A. L., Mackie J. T., Kier A. B., and Schroeder F.. 2009. Liver fatty acid binding protein gene ablation enhances age-dependent weight gain in male mice. Mol. Cell. Biochem. 324: 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puri P., Baillie R. A., Wiest M. M., Mirshahi F., Choudhury J., Cheung O., Sergeant C., Contos M. J., and Sanyal A. J.. 2007. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 46: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 30.Lichtman A. H., and Cravatt B. F.. 2005. Food for thought: endocannabinoid modulation of lipogenesis. J. Clin. Invest. 115: 1130–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purohit V., Rapaka R., and Shurtleff D.. 2010. Role of cannabinoids in the development of fatty liver (steatosis). AAPS J. 12: 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watkins P. A. 2008. Very long chain acyl-CoA synthetases. J. Biol. Chem. 283: 1773–1777. [DOI] [PubMed] [Google Scholar]
- 33.Frolov A., Cho T. H., Billheimer J. T., and Schroeder F.. 1996. Sterol carrier protein-2, a new fatty acyl coenzyme A-binding protein. J. Biol. Chem. 271: 31878–31884. [DOI] [PubMed] [Google Scholar]
- 34.Frolov A., and Schroeder F.. 1998. Acyl coenzyme A binding protein. Conformational sensitivity to long chain fatty acyl-CoA. J. Biol. Chem. 273: 11049–11055. [DOI] [PubMed] [Google Scholar]
- 35.Oddi S., Fezza F., Pasquoriello N., D’Agostino A., Catanzaro G., De Simone C., Rapino C., Finazzi-Agrò A., and Maccarrone M.. 2009. Molecular identification of albumin and Hsp70 as cytosolic anandamide-binding proteins. Chem. Biol. 16: 624–632. [DOI] [PubMed] [Google Scholar]
- 36.Fuhrmeister J., Zota A., Sijmonsma T. P., Seibert O., Cıngır Ş., Schmidt K., Vallon N., de Guia R. M., Niopek K., Berriel Diaz M. , et al. 2016. Fasting-induced liver GADD45β restrains hepatic fatty acid uptake and improves metabolic health. EMBO Mol. Med. 8: 654–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goparaju S. K., Ueda N., Yamaguchi H., and Yamamoto S.. 1998. Anandamide amidohydrolase reacting with 2-AG, another cannabinoid receptor ligand. FEBS Lett. 422: 69–73. [DOI] [PubMed] [Google Scholar]
- 38.Di Marzo V., Bisogno T., Sugiura T., Melck D., and De Petrocellis L.. 1998. The novel endogenous cannabinoid 2-AG is inactivated by neuronal- and basophil-like cells: connections with anandamide. Biochem. J. 331: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blankman J. L., Simon G. M., and Cravatt B. F.. 2007. Comprehensive profile of brain enzymes that hydrolyze endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 14: 1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang S. Y., He X. Y., and Schulz H.. 1987. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J. Biol. Chem. 262: 13027–13032. [PubMed] [Google Scholar]
- 41.Auguet T., Berlanga A., Guiu-Jurado E., Terra X., Martinez S., Aguilar C., Filiu E., Alibalic A., Sabench F., Hernández M., et al. 2014. Endocannabinoid receptors gene experssion in morbidly obese women with NAFLD. Biomed Res. Int. 2014: 502542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu J., Gaetani S., Ovelsi F., Verme J. L., Serrano A., de Fonseca F. R., Rosengarth A., Luecke H., Di Giacomo B., Tarzia G., et al. 2003. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPARa. Nature. 425: 90–93. [DOI] [PubMed] [Google Scholar]
- 43.Fu J., Oveisi F., Gaetani S., Lin E., and Piomelli D.. 2005. Oleoylethanolamide, an endogenous PPAR-a agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology. 48: 1147–1153. [DOI] [PubMed] [Google Scholar]
- 44.Guzmán C., Benet M., Pisonero-Vaquero S., Moya M., García-Mediavilla M. V., Martínez-Chantar M. L., González-Gallego J., Castell J. V., Sánchez-Campos S., and Jover R.. 2013. The human liver fatty acid binding protein (FABP1) gene is activated by FOXA1 and PPARα and repressed by C/EBPα: implicaiton in FABP1 down-regulation in nonalcoholic liver disease. Biochim. Biophys. Acta. 1831: 803–818. [DOI] [PubMed] [Google Scholar]
- 45.Podrini C., Cambridge E. L., Lelliott C. J., and White J. K.. 2013. High-fat feeding rapidly induces obesity and lipid derangements in C57BL/6N mice. Mamm. Genome. 24: 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo Y., Burrington C. M., Graff E. C., Zhang J., Judd R. L., Suksaranjit P., Kaewpoowat Q., Davenport S. K., O’Neill A. M., and Greene M. W.. 2016. Metabolic phenotype and adipose and liver features in a high-fat Western diet-induced mouse model of obesity-linked NAFLD. Am. J. Physiol. Endocrinol. Metab. 310: E418–E439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dahlhoff C., Desmarchelier C., Sailer M., Furst R. W., Haag A., Ulbrich S. E., Hummel B., Obeid R., Geisel J., Bader B. L., et al. 2013. Hepatic methionine homeostasis is conserved in C57BL/6N mice on high-fat diet despite major changes in hepatic one carbon metabolism. PLoS One. 8: e57387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McIntosh A. L., Atshaves B. P., Landrock D., Landrock K. K., Martin G. G., Storey S. M., Kier A. B., and Schroeder F.. 2013. Liver fatty acid binding protein gene-ablation exacerbates weight gain in high fat fed female mice. Lipids. 48: 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drewnowski A., and Greenwood M. R. C.. 1983. Cream and sugar: human preferences for high fat foods. Physiol. Behav. 30: 629–633. [DOI] [PubMed] [Google Scholar]
- 50.Drewnowski A., and Almiron-Roig E.. 2010. Human perceptions and preferences for fat rich foods. In Montmayeur J. P. and le Coutre J., editors. CRC Press/Taylor & Francis, Boca Raton, FL: 1–36. [PubMed] [Google Scholar]
- 51.Birch L. L. 1992. Children’s preferences for high fat foods. Nutr. Rev. 50: 249–255. [DOI] [PubMed] [Google Scholar]
- 52.Cooling J., and Blundell J. E.. 2001. High fat and low fat phenotypes: habitual eating of high and low fat foods not related to taste preference for fat. Eur. J. Clin. Nutr. 55: 1016–1021. [DOI] [PubMed] [Google Scholar]
- 53.Li T., Owsley E., Matozel M., Hsu P., Novak C. M., and Chiang J. Y. L.. 2010. Transgenic expression of cholesterol 7a-hydroxylase in liver prevents high fat diet induced obesity and insulin resistance in mice. Hepatology. 52: 678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monteiro J., Leslie M., Moghadasian M. H., Arendt B. M., Allard J. P., and Ma D. W. L.. 2014. The role of n-6 and n-3 polyunsaturated fatty acids in the manifestation of the metabolic syndrome in cardiovascular disease and NAFLD. Food Funct. 5: 426–435. [DOI] [PubMed] [Google Scholar]
- 55.Atshaves B. P., Martin G. G., Hostetler H. A., McIntosh A. L., Kier A. B., and Schroeder F.. 2010. Liver fatty acid binding protein (L-FABP) and dietary obesity. J. Nutr. Biochem. 21: 1015–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McIntosh A. L., Huang H., Atshaves B. P., Wellburg E., Kuklev D. V., Smith W. L., Kier A. B., and Schroeder F.. 2010. Fluorescent n-3 and n-6 very long chain polyunsaturated fatty acids: three photon imaging and metabolism in living cells overexpressing liver fatty acid binding protein. J. Biol. Chem. 285: 18693–18708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Storey S. M., McIntosh A. L., Huang H., Martin G. G., Landrock K. K., Landrock D., Payne H. R., Kier A. B., and Schroeder F.. 2012. Loss of intracellular lipid binding proteins differentially impacts saturated fatty acid uptake and nuclear targeting in mouse hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 303: G837–G850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hostetler H. A., Lupas D., Tan Y., Dai J., Kelzer M. S., Martin G. G., Woldegiorgis G., Kier A. B., and Schroeder F.. 2011. Acyl-CoA binding proteins interact with the acyl-CoA binding domain of mitochondrial carnitine palmitoyltransferase I. Mol. Cell. Biochem. 355: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hostetler H. A., McIntosh A. L., Atshaves B. P., Storey S. M., Payne H. R., Kier A. B., and Schroeder F.. 2009. Liver type fatty acid binding protein (L-FABP) interacts with peroxisome proliferator activated receptor-a in cultured primary hepatocytes. J. Lipid Res. 50: 1663–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrescu A. D., Huang H., Martin G. G., McIntosh A. L., Storey S. M., Landrock D., Kier A. B., and Schroeder F.. 2013. Impact of L-FABP and glucose on polyunsaturated fatty acid induction of PPARa regulated b-oxidative enzymes. Am. J. Physiol. Gastrointest. Liver Physiol. 304: G241–G256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaczocha M., Glaser S. T., and Deutsch D. G.. 2009. Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl. Acad. Sci. USA. 106: 6375–6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaczocha M. 2009. Role of fatty acid binding proteins and FAAH-2 in endocannabinoid uptake and inactivation. PhD Dissertation. Stony Brook University, Stony Brook, NY.
- 63.Leung K., Elmes M. W., Glaser S. T., Deutsch D. G., and Kaczocha M.. 2013. Role of FAAH-like anandamide transporter in anandamide inactivation. PLoS One. 8: e79355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atshaves B. P., Payne H. R., McIntosh A. L., Tichy S. E., Russell D., Kier A. B., and Schroeder F.. 2004. Sexually dimorphic metabolism of branched chain lipids in C57BL/6J mice. J. Lipid Res. 45: 812–830. [DOI] [PubMed] [Google Scholar]
- 65.Atshaves B. P., McIntosh A. L., Payne H. R., Mackie J., Kier A. B., and Schroeder F.. 2005. Effect of branched-chain fatty acid on lipid dynamics in mice lacking liver fatty acid binding protein gene. Am. J. Physiol. Cell Physiol. 288: C543–C558. [DOI] [PubMed] [Google Scholar]
- 66.Lagakos W. S., Guan X., Ho S-Y., Sawicki L. R., Corsico B., Murota K., Stark R. E., and Storch J.. 2013. L-FABP binds monoacylglycerol in vitro and in mouse liver cytosol. J. Biol. Chem. 288: 19805–19815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.