Abstract
Smith-Lemli-Opitz syndrome is a recessive disorder caused by mutations in 7-dehydrocholesterol reductase (DHCR)7 with a heterozygous (HET) carrier frequency of 1–3%. A defective DHCR7 causes accumulation of 7-dehydrocholesterol (DHC), which is a highly oxidizable and toxic compound. Recent studies suggest that several antipsychotics, including the highly prescribed pharmaceuticals, aripiprazole (ARI) and trazodone (TRZ), increase 7-DHC levels in vitro and in humans. Our investigation was designed to compare the effects of ARI and TRZ on cholesterol (Chol) synthesis in fibroblasts from DHCR7+/− human carriers and controls (CTRs). Six matched pairs of fibroblasts were treated and their sterol profile analyzed by LC-MS. Significantly, upon treatment with ARI and TRZ, the total accumulation of 7-DHC was higher in DHCR7-HET cells than in CTR fibroblasts. The same set of experiments was repeated in the presence of 13C-lanosterol to determine residual Chol synthesis, revealing that ARI and TRZ strongly inhibit de novo Chol biosynthesis. The results suggest that DHCR7 carriers have increased vulnerability to both ARI and TRZ exposure compared with CTRs. Thus, the 1–3% of the population who are DHCR7 carriers may be more likely to sustain deleterious health consequences on exposure to compounds like ARI and TRZ that increase levels of 7-DHC, especially during brain development.
Keywords: 7-dehydrocholesterol, fibroblasts, antipsychotics, 7-dehydrocholesterol reductase
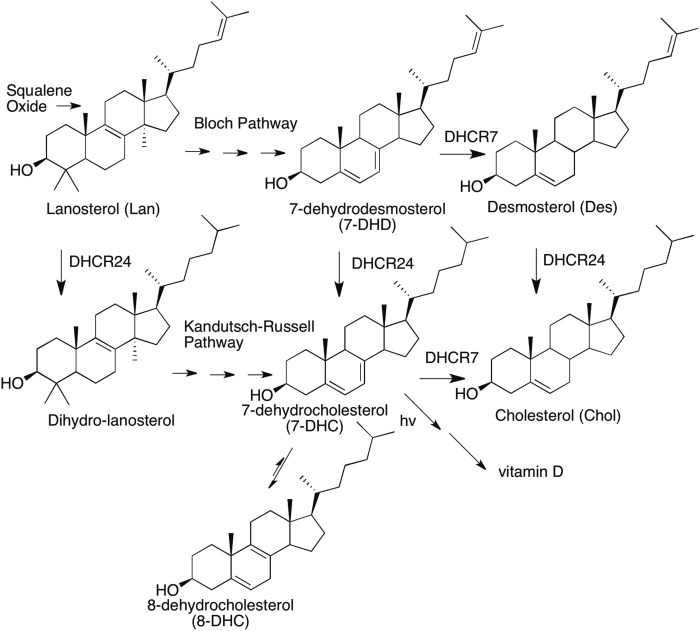
Smith-Lemli-Opitz syndrome (SLOS) is an autosomal recessive disorder caused by mutations of 7-dehydrocholesterol reductase (DHCR)7, the gene that encodes DHCR7, the enzyme that converts 7-dehydrocholesterol (DHC) to cholesterol (Chol); see Fig. 1 for selected sterols in the Chol biosynthesis pathway (1–8). There are nearly 200 mutations in DHCR7 that have been reported to date, most of them within a coding region of 1,425 open reading frame bases (9–13). Furthermore, a recent analysis of exome sequencing databases led to the conclusion that the carrier frequency of pathogenic DHCR7 mutations is 1–3% in the human population (14). Given the number of known DHCR7 mutations, most SLOS cases are compound heterozygous (HET) with different inherited maternal and paternal alleles. The incidence of clinical SLOS cases has been estimated to be 1 in 10,000–70,000 (8). Mildly affected patients may have minimal symptoms and severely affected individuals may suffer preterm demise, making ascertainment of clinical incidence problematic (15).
Fig. 1.
Chemical structures of selected sterols in the Chol biosynthesis pathway.
While there are many studies on SLOS patients to date, the health status of HET DHCR7+/− mutation carriers has been less extensively investigated. DHCR7+/− carriers are reported to have marginally higher plasma levels of 7-DHC than DHCR7+/+ controls (CTRs) (16) and animal studies also argue that a single mutant copy of Dhcr7 might affect homeostasis. The 7-DHC levels are increased in Dhcr7+/− HET mice, with the highest levels of 7-DHC found in the nervous system (17). Furthermore, the Dhcr7+/− mutant mice show increased dominance and elevated head-twitch response to a challenge with a 5-HT2A agonist (18).
Circulating blood levels of 7-DHC in CTR populations are very low, less than 0.5 µg/ul, but recent studies have shown that psychiatric patients taking either aripiprazole (ARI) or trazodone (TRZ) have greatly increased plasma levels of 7-DHC (19). In addition, a clinical report also noted that 15 of 22 individuals who were treated with either or both ARI and TRZ were misdiagnosed as SLOS patients based upon their 7-DHC plasma levels (20). Furthermore, an unbiased cell culture study of pharmacologically active compounds also identified over 5% of 700 7-DHC-elevating compounds (21). Finally, a recent comprehensive review of the effect of DHCR7 inhibitors on human health revealed that in utero exposure to DHCR7 inhibitors during the first trimester of pregnancy produces outcomes similar to those of known teratogens (22).
Exposure to DHCR7 inhibitors, such as ARI and TRZ, may have a significant impact on fetal health and development, especially because their use is widespread: ARI is a highly prescribed drug in the US, often used during pregnancy. The fact that the carrier frequency of DHCR7+/− mutations is high and significant exposures to drugs that affect this enzyme have been reported raises the question of whether there are groups of genetically distinct individuals who show increased vulnerability to exposure to DHCR7 inhibitors. Therefore, we monitored the response of DHCR7+/+ (WT) and DHCR7+/− HET fibroblasts to two compounds that strongly inhibit the enzymatic transformation of 7-DHC to Chol, ARI and TRZ. Our results suggest that exposure to ARI and TRZ may be deleterious to individuals who are HET carriers of a DHCR7 mutation. We note again that ARI and TRZ are only two of some 35 known pharmaceuticals that affect levels of 7-DHC in cell culture, so the studies reported here may point to a problem of broad scope.
MATERIALS AND METHODS
Materials
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO). HPLC grade solvents were purchased from Thermo Fisher Scientific Inc. (Waltham, MA). All cell culture reagents were from Mediatech (Manassas, VA), Life Technologies (Grand Island, NY), and Greiner Bio-One GmBH (Monroe, NC). All sterol standards, natural and isotopically labeled, used in this study are available from Kerafast, Inc. (Boston MA). Delipidated FBS was prepared as previously described and LC-MS was used to confirm that it did not have a detectable Chol level (23).
CTR and DHCR7-HET fibroblast genotyping
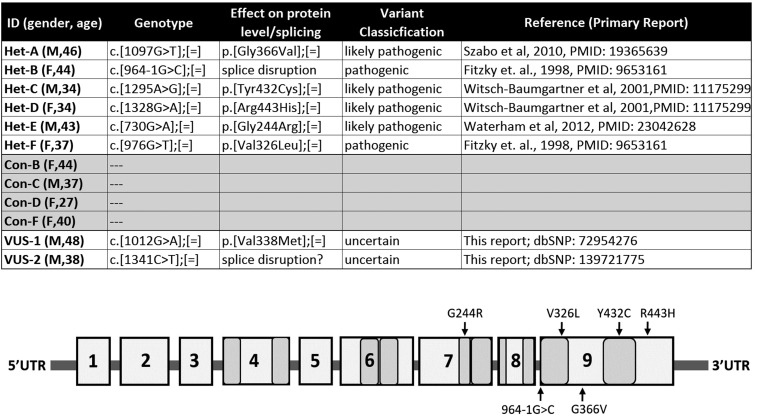
Molecular genetic analysis of the DHCR7 gene was performed as previously described (10). Briefly, after amplification of exons 3–9 (coding region) and exon/intron boundaries, bidirectional amplicon sequencing was performed using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. Samples were run on the ABI PRISM 310 genetic analyzer and data were analyzed using the Sequencing Analysis software (Applied Biosystems) (reference sequence: NM_001360.2). Sequence analysis of the DHCR7 gene detects approximately 96% of pathogenic variants. Variant classification was performed according to Richards et al. (24). In CTR samples (CTR 1–4), exclusively benign variants were detected [minor allele frequency (MAF) >5%, rs1044482, rs1790334, rs4316537, rs949177, rs736894, rs760241, rs909217]. In CTR sample variant of uncertain significance (VUS)-1, a synonymous/silent variant (MAF <0.01, rs13972775, c1341C>T, p.Asp447Asp) was detected in HET form. In CTR sample VUS-2, a nonsynonymous variant (MAF <0.01, rs72954276, c1012G>A, p.Val338Met) was detected in HET form. With the evidence that we have, both variants were classified as a VUS.
All DHCR7+/− fibroblasts were obtained from parents of biochemically and genetically confirmed SLOS patients. The affected patients were: a) compound HET c.[1097G>T];(964–1G>C): typical/classical phenotype [severity score 25 calculated according to Kelley and Hennekam (3)]; b) compound HET c.[1295A>G];[1328G>A]: mild (severity score 15); c) compound HET c.[730G>A];[976G>T]: typical/classical phenotype (severity score 40). In general, genotype/phenotype correlations are very weak in SLOS, as most of the patients are compound HET and there are only a low number of patients with the same genotype. Except for common mutations (∼60%), many are unique or infrequent. Patients with the same genotype can have different phenotypes; even intra-familial variability has been observed. A detailed genotypic description is presented in Fig. 2. All described mutations are classified as pathogenic or likely pathogenic DHCR7 variants.
Fig. 2.
Genotyping results of HFs. Parents of children with SLOS clinical phenotypes Het-A through Het-F were gender and age matched with CTR fibroblasts. Mutations in Het-A through Het-F are classified as pathogenic or likely pathogenic DHCR7 variants. Genotyping of CTR HFs revealed two variants of unknown significance that were not previously described in SLOS clinical cases.
Cell cultures, sterol extraction and LC-MS/MS measurements, and statistical analyses were described in detail in previous publications and they are in the supplemental information.
RESULTS
The biosynthesis of Chol is a complex process that proceeds from the isoprenoid, squalene, through its epoxide to the tetracyclic sterol precursor, lanosterol (Lan) (25). The post-lanosterol biosynthetic pathway to Chol consists of two parallel sequences, the Bloch and Kandutsch-Russell pathways, shown in Fig. 1. DHCR7 is an NADPH-dependent enzyme (25) that reduces the Δ7-8 double bond of 7-DHC or the corresponding Bloch pathway sterol, 7-dehydrodesmosterol. A cell with DHCR7 having reduced functional activity leads to elevated cellular levels of 7-DHC or 7-dehydrodesmosterol and, if a functioning DHCR24 is present in the cell, levels of 7-DHC can be used as the principal biomarker to identify a compromised DHCR7. Monitoring levels of 7-DHC of cells in culture therefore provides a straightforward method to determine whether DHCR7 activity is affected, either by a genetic mutation or by an enzyme inhibitor (17, 26–30).
DHCR7 genetic variants
A number of different cell types have been used to assess the effects of small molecules on Chol biosynthesis. While we have successfully used Dhcr7-deficient and WT Neuro2a cells (17, 21, 31) in the past for screening purposes, human patient-derived dermal fibroblasts represent an ideal model for follow-up experiments (32). As a result, for the purpose of assessing the effect of small molecule DHCR7 inhibitors on a cell having only one allele bearing a DHCR7 mutation, we chose human fibroblasts (HFs) from six DHCR7+/− HET parents of a SLOS offspring and six age- and sex-matched donors from a CTR population. These HET fibroblast cell lines were HET for pathogenic or likely pathogenic DHCR7 variants previously reported in SLOS patients. Details on these DHCR7+/− mutations are presented in Fig. 2. Subsequent to our analysis of the effect of ARI and TRZ on all 12 CTR and HET fibroblasts, we carried out genomic analysis of DHCR7 of the CTR HFs as well, and in two of the six CTR cell lines, we identified DHCR7 VUSs. One of these variants, VUS#1, is a synonymous/silent codon variant that could potentially affect splicing (Mutation Taster, www.mutationtaster.org; Human Splicing Finder, http://www.umd.be/HSF3/), while the other, VUS#2 (Val338Met), is at a residue that is not evolutionarily conserved. Importantly, neither of these variants has previously been associated with SLOS. Because of the outcomes of these post hoc sequence analyses, the mutant-CTR comparisons of sterol biosynthesis were performed and are reported in six DHCR7+/− and four DHCR7+/+ HF lines harboring no sequence variants. Data for the two VUS cells with DHCR7 VUSs are reported separately in the supplemental information.
Analysis of fibroblast sterols
Cellular levels of endogenous sterols in cultured fibroblasts were determined by methods previously reported for 7-DHC, desmosterol (Des), Lan, and Chol (21). Measurement of Chol synthesis in the presence of ARI or TRZ is confounded by the large amounts of pre-existing Chol that persist, even while Chol synthesis occurs in drug-treated fibroblasts. Nevertheless, ARI and TRZ have been shown to have a significant effect on sterol homeostasis, as measured by absolute levels of 7-DHC and Chol, by the ratio of these two sterols, 7-DHC/Chol, or by the fractional Chol/(7-DHC+Chol) determined in a cell (21).
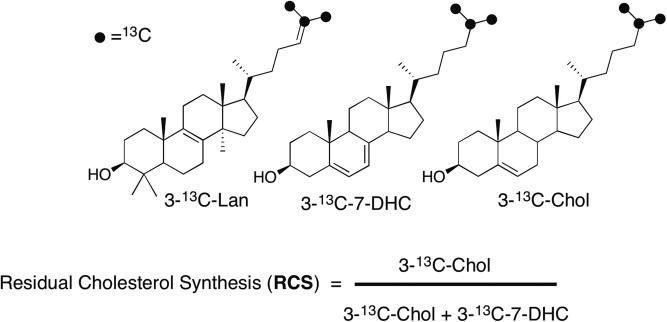
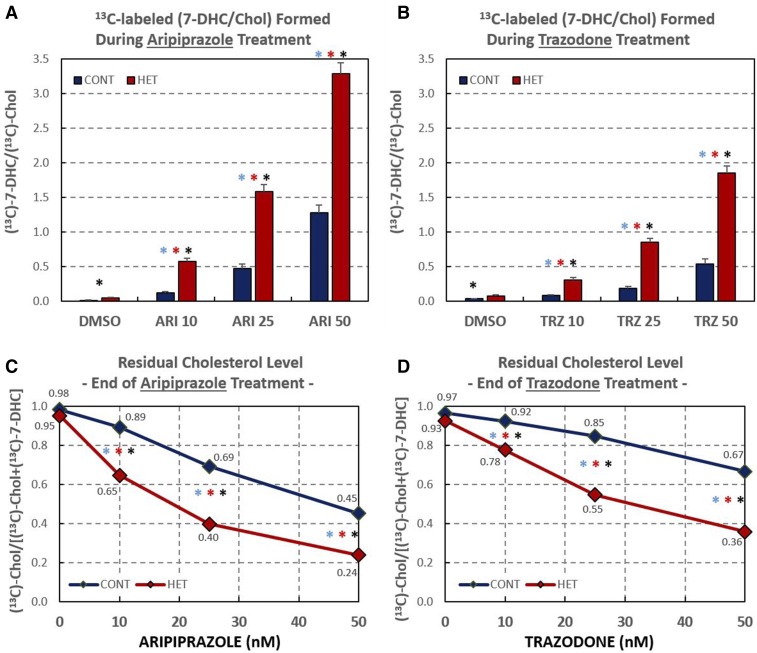
To isolate the effect of a drug on biosynthesis, methods making use of isotopically labeled sterol biosynthetic precursors have been developed to measure de novo or “residual Chol synthesis” (RCS) during a treatment regimen (27, 33). For the ARI and TRZ studies reported here, RCS was assessed by the use of an isotopically labeled Lan, 313C-Lan, bearing 13C at carbons C25, C26, and C27 of the sterol, which was added to fibroblasts during incubations with ARI or TRZ (see Fig. 3). RCS, defined in Fig. 3, reports the levels of 313C-7-DHC and 313C-Chol formed during fibroblast exposures to drugs. In practice, we report both the ratio of 313C-7-DHC/313C-Chol after exposure of fibroblasts to ARI and TRZ and the calculated RCS based on those isotopic sterol values. The structures of 313C-Lan, 313C-7-DHC, and 313C-Chol are presented in Fig. 3.
Fig. 3.
Chemical structures of 3-13C-labeled sterols and the formula used to calculate RCS.
Sterol levels can be measured in as few as 5,000 fibroblasts using the 4-phenyl-1,2,4-triazoline-3,5-dione procedures described in the supplemental information and in previous publications (21). At baseline, we found significant differences for sterol levels between HET and CTR fibroblasts for Chol, 7-DHC, and Des, while levels of 8-DHC and Lan did not meet the criteria for difference in these cells (see Table 1). Modestly elevated 7-DHC and reduced Chol levels have previously been reported in fibroblasts from obligate SLOS heterozygotes compared with CTR cells, but Des levels were not reported in those studies (16, 27).
TABLE 1.
Sterol levels in cultured CTR and DHCR7 HET HFs
| Chol | 7-DHC | 8-DHC | Des | Lan | |
| DHCR7+/+ | 59.6 ± 2.8 | 0.34 ± 0.08 | 0.81 ± 0.07 | 1.14 ± 0.08 | 0.16 ± 0.01 |
| DHCR7+/− | 48.3 ± 0.9 | 0.86 ± 0.08 | 1.03 ± 0.07 | 0.75 ± 0.07 | 0.14 ± 0.01 |
| P | 5.16E-05 | 4.03E-06 | 0.054 | 0.00041 | 0.478 |
Data are expressed in nanomoles per liter × 106 cells, mean ± SEM.
There is a growing body of evidence suggesting that DHCR7 is part of a larger complex that includes another sterol reductase, DHCR24 (34). It is of some interest that small molecules that increase levels of 7-DHC in cell culture do not cause a comparable increase in Des. If anything, changes in levels of 7-DHC and Des tend to occur in opposite directions. The difference is significant between DHCR7+/− fibroblasts and CTRs (see Table 1), but the effect of ARI and TRZ was not observed in fibroblasts (see supplemental information).
Human DHCR7+/− fibroblasts are preferentially affected by exposure to DHCR7 inhibitors
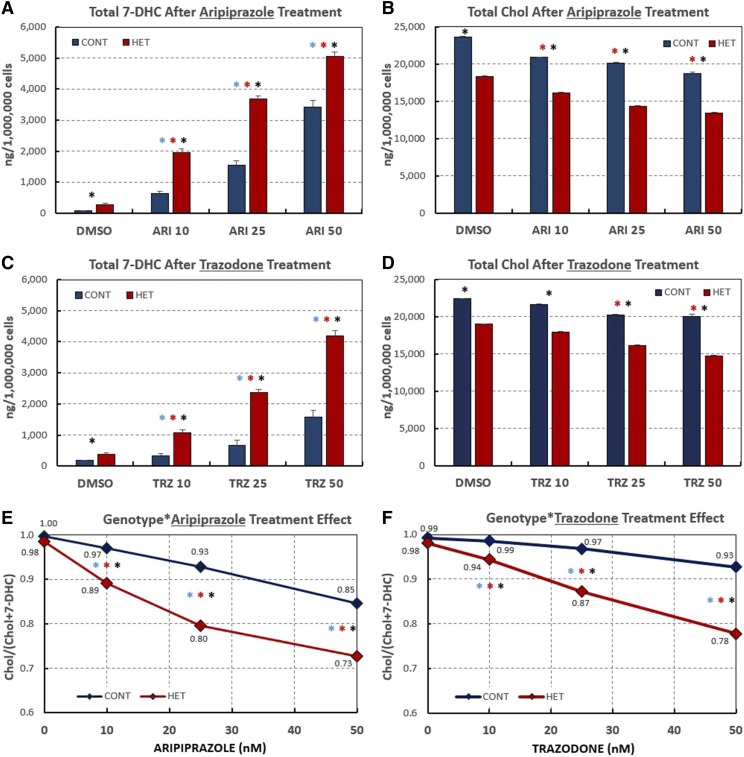
Subsequently, DHCR7+/− and CTR dermal HFs were treated with three different concentrations of either ARI or TRZ and cellular sterols were measured after 6 days in culture. Figure 4 summarizes the results for six DHCR7+/− HET and the four sequence-verified CTR fibroblasts. For all three concentrations tested, the treatments with ARI and TRZ resulted in significantly elevated cellular levels of 7-DHC in both HET and CTR cells (Fig. 4A, C). ARI appears to be more potent than TRZ in increasing 7-DHC in both CTR and HET HFs. In addition, while 7-DHC levels were increased in response to both treatments, Chol levels were significantly decreased in the same HET and CTR fibroblast cultures (Fig. 4B, D). Importantly, the decrease in the percentage of Chol present in the sterol profile is more pronounced in DHCR7+/− HET than in CTR fibroblasts (Fig. 4E, F), suggesting an increased vulnerability of DHCR7+/− HET to both tested compounds. Thus, ARI caused a decrease in the fraction of Chol present in the cells from nearly 1.0 (DMSO CTR) to 0.73 at 50 nM ARI, while this fraction dropped to only 0.85 for the CTR fibroblasts. The levels of Des and Lan were not significantly affected by treatment and are reported in the supplemental information. The data for all 12 of the individual cell lines (including the cells harboring DHCR7 variants of unknown significance) are also reported in the supplemental information.
Fig. 4.
Human DHCR7-HET fibroblasts are preferentially affected by exposures to DHCR7 inhibitors. Summary of 7-DHC and Chol levels in HF in response to various concentrations of ARI and TRZ. A, C: Graphs show average 7-DHC levels in nanograms per million cells for six DHCR7-HET and four CTR HFs. B, D: Graphs show average Chol levels in nanograms per million cells for six DHCR7-HET and four CTR HFs. E, F: Graphic representation of increasing amount of 7-DHC and decreasing amount of Chol in response to increasing amount of ARI (E) or TRZ (F) as measured by the ratio [Chol]/[Chol +7-DHC]. Asterisks above the bars show P values <0.01 (blue is the difference among CTR samples; red is the difference among HET samples; and black is the difference between CTR and HET samples. Supplemental Tables S1A and S1B contain companion data for Fig. 4 showing mean, SD, and SEM, as well as P values.
ARI and TRZ alter de novo Chol biosynthesis
To test to determine whether ARI and TRZ act by affecting the stability of Chol precursors or de novo lipid biosynthesis, we exposed CTR and HET fibroblasts to 500 nM of 313C-Lan at the same time that the cells were exposed to 10, 25, and 50 nM ARI or TRZ. This permitted assessment of the levels of newly synthesized Chol and its precursors by measuring the incorporation of isotopic label. After 6 days in culture, lipids were extracted and cellular levels of 313C-sterols were determined by the same methods used to analyze endogenous cellular sterols, with the exception that the masses monitored in the LC-MS protocol were 3 m/z units higher than the natural m/z values for Des, 7-DHC, and Chol. Isotopically labeled sterols made up approximately 10% of the total sterols present in the cells after 6 days of incubation. The HET and CTR cell lines showed a different biosynthesis profile as a function of ARI or TRZ concentration: for example, at 50 nM ARI, the ratio of 313C-7-DHC/313C-Chol found in HET cells was 3:1, while the same ratio was only 1.2:1 in CTR fibroblasts (see Fig. 5A). Indeed, the 313C-7-DHC to 313C-Chol ratio determined was found to be significantly higher for HET fibroblasts than the same ratio found in CTR cells at every concentration of ARI and TRZ studied.
Fig. 5.
ARI or TRZ alter residual Chol biosynthesis. Six DHCR7-HET and four CTR HFs were cultured in the presence of 500 nM 313C-Lan and different concentrations of ARI or TRZ. A, B: Graphs show the ratio of 313C-derived 7-DHC/313C-derived Chol; the 7-DHC is normalized to Chol. Asterisks above the bars show P values <0.01 (blue is the difference among CTR samples; red is the difference among HET samples; and black is the difference between CTR and HET samples). C, D: The calculated RCS in response to ARI or TRZ. Supplemental Tables S2A and S2B contain companion data for Fig. 5 showing mean, STDEV, and SEM, as well as P values.
ARI- and TRZ-induced de novo synthesized 313C-7-DHC and 313C-Chol depends on genotype
The drug exposure-dependent RCS is presented in Fig. 5C, D. In the absence of drug, CTR cells synthesized Chol more efficiently than HET cells, as evidenced by the higher RCS (∼0.97–0.98 for CTR cells and ∼0.93–0.95 for HET cells). Furthermore, the effect of ARI and TRZ on HET cells was larger than the effect of these drugs on CTR fibroblasts. Thus, on treatment with 10 nM ARI, the RCS for HET cells dropped to 0.65, while the RCS for CTR cells under the same treatment was 0.89. At 50 nM ARI, RCS drops to 0.24 for HET fibroblasts, a value lower than reported for the RCS in some SLOS fibroblasts (33). Chol biosynthesis was further impaired by 100 nM ARI, but inspection of the cells showed evidence of toxicity at these concentrations and our studies were thus limited to concentrations of 50 nM and below. The effects of ARI on RCS were almost twice the magnitude of TRZ for exposure to the same drug concentration, as seen by comparison of Figs. 5C and 4D.
The data for the two fibroblasts with single-copy DHCR7 VUSs are of some interest because these mutations have not previously been associated with SLOS (see supplemental Fig. S1). VUS#1, a cell line with a variant that potentially could disrupt DHCR7 splicing (c.[1341C>T];[=]), responds to treatment with ARI in a manner that parallels that of HET cells, rather than CTR cells. However, based on the response to TRZ, VUS#1 is more similar to CTR cells than HET cells. In contrast, the VUS#2 cell line responds to treatment with both ARI and TRZ like other CTR cells, suggesting that this genetic variant is unlikely to be pathogenic in the human population. This highlights our limited understanding of how the wide range of single-copy DHCR7 mutations affect function and underscores their potential importance on the health of HET individuals.
DISCUSSION
The findings of our study can be summarized in several main points: 1) ARI and TRZ treatments significantly elevate 7-DHC levels and alter the 7-DHC/Chol ratio in HFs regardless of genotype. 2) Response of HFs to both ARI and TRZ is dose-dependent. 3) DHCR7+/+ CTR and DHCR7+/− HET HFs respond differentially to both ARI and TRZ treatments in the human therapeutic range, with HET samples exhibiting a stronger response with elevated 7-DHC levels and an altered 7-DHC/Chol ratio. Importantly, this is not a “higher starting point, higher end point” finding. 4) Isotope experiments revealed that both ARI and TRZ act through altering de novo biosynthesis, rather than affecting the stability/turnover of Chol and its precursors. The effect of ARI and TRZ on RCS is more pronounced for HET samples than on CTR cells. 5) The primary action of ARI and TRZ at the concentrations studied is at the step of 7-DHC→Chol in the biosynthesis, as the rest of the Chol precursor profile is unaffected by these treatments.
Our studies were performed on HFs, yet these studies have clear implications for brain function: the Chol biosynthesis pathway is conserved across different tissue types. Although the human brain only accounts for about 2% of total body weight, it contains as much as 25% of Chol and Chol derivatives (35, 36). Importantly, Chol is synthesized by neurons; DHCR7, the last enzyme in the Chol biosynthesis pathway, is strongly expressed at high levels in neurons throughout the brain (37, 38). The function of Chol in the CNS goes beyond being a structural component of cellular membranes and lipid rafts; it is required for synapse and dendrite formation and axonal guidance, and serves as a precursor for various biosynthetic pathways. Thus, the impact of ARI and TRZ on human dermal fibroblasts and brain tissue is likely to be very similar at a level of biochemistry and primarily consist of 7-DHC elevation.
It is well-established that 7-DHC elevation in cells is a deleterious event. The 7-DHC is a highly reactive lipid molecule (39) and it undergoes spontaneous free radical peroxidation, producing over a dozen oxidation products (i.e., oxysterols) in vitro and in vivo (40, 41). These 7-DHC-derived oxysterols exert cytotoxicity, reduce cell proliferation, induce premature cell differentiation, and affect Hedgehog signal transduction (42). They also lead to a host of gene expression changes that are consistent across the human/mouse and in vivo/in vitro models (40, 43, 44).
There is evidence in humans and mice that single-allele DHCR7 mutations lead to elevation of 7-DHC levels (17, 27). Yet, our understanding of single copy mutations of DHCR7 on health remains mostly unknown to date. With nearly 200 different mutations in the human population and with a carrier rate of approximately 1–3% in the US (and 4% in Utah and 3% in European ancestry) (10, 22), their importance on health is potentially quite significant. A previous publication reports a correlation between birth weight and fetal DHCR7 gene/SNP combinations and Chol metabolism genes and preterm delivery (45). This view is also supported by animal experiments: assessment of behavioral differences between Dhcr7+/− HET and WT mice revealed that mutant mice were significantly more likely to win on the social dominance test and showed impairments in the response to 5-HT2A agonists (18).
Developmental defects are found in ∼3–5% of liveborn children (46). It is estimated that pharmaceuticals account for approximately 1% of teratogenic effects (47). Our results do not necessarily indicate that ARI and TRZ are unsafe for use in the general population. These drugs have been extensively tested and have proven themselves as very effective medications that help patients live more productive lives. ARI, marketed under the name of Abilify®, was the most prescribed medication in the US in 2013 (http://www.drugs.com/stats/abilify), with ∼2.5 million units sold quarterly, exceeding yearly sales of 6.9 billion dollars. However, it is noteworthy that the Food and Drug Administration classified both ARI and TRZ as “class C” compounds, stating in the Risk Summary: “Adequate and well controlled studies with ABILIFY have not been conducted in pregnant women. Administer ABILIFY during pregnancy only if the potential benefit justifies the potential risk to the fetus.” (https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021436s038,021713s030,021729s022,021866s023lbl.pdf) A recent report suggested that intrauterine exposure of rats to ARI may not be safe for the health of developing fetuses and offspring and that ARI exposure might significantly contribute to gastrointestinal congenital malformations (48). Human studies also highlighted the interaction between ARI/TRZ and Chol biosynthesis: ARI and TRZ treatment lead to elevated 7-DHC levels in the patient population, leading to a false-positive diagnosis of SLOS in patients treated with these two compounds (20).
It should be noted that 7-DHC elevation may not be limited to ARI and TRZ exposure. Previous screening of the National Institutes of Health Clinical Collection, consisting of 727 small molecules that have a history of use in human clinical trials, identified 30 compounds that significantly increased 7-DHC levels in Neuro2a cells (21). Many of these compounds (in addition to ARI and TRZ) have been classified as class C compounds by the Food and Drug Administration and are widely used in medicine, even during pregnancy. These data suggest that exposure to heterocyclic cationic amphiphiles, dependent on timing, duration, and concentration, could be harmful to the 1–3% of the human population who carry a single-copy of a DHCR7 missense mutation.
In summary, our approach is directly relevant to developing personalized medicine approaches, as understanding pharmacogenomic interactions is essential to ensure positive treatment outcomes. For example, genotyping patients for their ability to metabolize warfarin could avoid 85,000 serious bleeding events, 17,000 strokes, and one billion dollars in annual costs of care in the US alone (49). We argue that, in the era of precision medicine, potential differences in response to compounds that disrupt the Chol biosynthesis pathway must be respected, especially as their effect may be defined by both genetic makeup and life events at the same time. Thus, in the context of our studies, we suggest that treatment with 7-DHC-elevating substances (such as ARI and TRZ) raise issues for the population that carries single-allele disruptions of the DHCR7 gene. In addition, we propose that the vulnerability to 7-DHC-elevating compounds is perhaps most pronounced during pregnancy and brain development, especially when both the mother and the fetus carry a single potentially disruptive DHCR7 allele. This complex drug exposure × maternal genotype × fetus genotype × developmental time point interaction may elevate 7-DHC levels into a toxic range comparable to that seen in SLOS patients, resulting in deleterious developmental outcomes.
Supplementary Material
Acknowledgments
The authors thank the people who donated the biopsy samples used in the study, Emily Brown for assistance at the University of Nebraska Medical Center, and Eva Gombos and Laszlo Madar in Debrecen for sequencing HF samples.
Footnotes
Abbreviations:
- ARI
- aripiprazole
- Chol
- cholesterol
- CTR
- control
- Des
- desmosterol
- DHC
- dehydrocholesterol
- DHCR
- dehydrocholesterol reductase
- HET
- heterozygous
- HF
- human fibroblast
- Lan
- lanosterol
- MAF
- minor allele frequency
- RCS
- residual cholesterol synthesis
- SLOS
- Smith-Lemli-Opitz syndrome
- TRZ
- trazodone
- VUS
- variant of uncertain significance
This work was supported by Eunice Kennedy Shriver Institute of Child Health and Human Development Grant HD064727 (N.A.P.), National Institute of Environmental Health Sciences Grant ES024133 (N.A.P., Z.K.), National Institute of Mental Health Grants MH110636 and MH067234 (K.M.), and the Ministry of National Economy, Hungary Grant GINOP-2.3.2-15-2016-00039 (I.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Smith D. W., Lemli L., and Opitz J. M.. 1964. A newly recognized syndrome of multiple congenital anomalies. J. Pediatr. 64: 210–217. [DOI] [PubMed] [Google Scholar]
- 2.Tint G. S., Irons M., Elias E. R., Batta A. K., Frieden R., Chen T. S., and Salen G.. 1994. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N. Engl. J. Med. 330: 107–113. [DOI] [PubMed] [Google Scholar]
- 3.Kelley R. I., and Hennekam R. C.. 2000. The Smith-Lemli-Opitz syndrome. J. Med. Genet. 37: 321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herman G. E. 2003. Disorders of cholesterol biosynthesis: prototypic metabolic malformation syndromes. Hum. Mol. Genet. 12: R75–R88. [DOI] [PubMed] [Google Scholar]
- 5.Porter F. D. 2003. Human malformation syndromes due to inborn errors of cholesterol synthesis. Curr. Opin. Pediatr. 15: 607–613. [DOI] [PubMed] [Google Scholar]
- 6.Porter F. D. 2008. Smith-Lemli-Opitz syndrome: pathogenesis, diagnosis and management. Eur. J. Hum. Genet. 16: 535–541. [DOI] [PubMed] [Google Scholar]
- 7.Porter F. D., and Herman G. E.. 2011. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 52: 6–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanungo S., Soares N., He M., and Steiner R. D.. 2013. Sterol metabolism disorders and neurodevelopment—an update. Dev. Disabil. Res. Rev. 17: 197–210. [DOI] [PubMed] [Google Scholar]
- 9.Waterham H. R., and Hennekam R. C.. 2012. Mutational spectrum of Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. C. Semin. Med. Genet. 160C: 263–284. [DOI] [PubMed] [Google Scholar]
- 10.Balogh I., Koczok K., Szabo G. P., Torok O., Hadzsiev K., Csabi G., Balogh L., Dzsudzsak E., Ajzner E., Szabo L., et al. 2012. Mutational spectrum of Smith-Lemli-Opitz syndrome patients in Hungary. Mol. Syndromol. 3: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellingson M. S., Wick M. J., White W. M., Raymond K. M., Saenger A. K., Pichurin P. N., Wassif C. A., Porter F. D., and Babovic-Vuksanovic D.. 2014. Pregnancy in an individual with mild Smith-Lemli-Opitz syndrome. Clin. Genet. 85: 495–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oláh A. V., Szabó G. P., Varga J., Balogh L., Csábi G., Csákváry V., Erwa W., and Balogh I.. 2013. Relation between biomarkers and clinical severity in patients with Smith-Lemli-Opitz syndrome. Eur. J. Pediatr. 172: 623–630. [DOI] [PubMed] [Google Scholar]
- 13.Witsch-Baumgartner M., Schwentner I., Gruber M., Benlian P., Bertranpetit J., Bieth E., Chevy F., Clusellas N., Estivill X., Gasparini G., et al. 2008. Age and origin of major Smith-Lemli-Opitz syndrome (SLOS) mutations in European populations. J. Med. Genet. 45: 200–209. [DOI] [PubMed] [Google Scholar]
- 14.Cross J. L., Iben J., Simpson C. L., Thurm A., Swedo S., Tierney E., Bailey-Wilson J. E., Biesecker L. G., Porter F. D., and Wassif C. A.. 2015. Determination of the allelic frequency in Smith-Lemli-Opitz syndrome by analysis of massively parallel sequencing data sets. Clin. Genet. 87: 570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linck L. M., Hayflick S. J., Lin D. S., Battaile K. P., Ginat S., Burlingame T., Gibson K. M., Honda M., Honda A., Salen G., et al. 2000. Fetal demise with Smith-Lemli-Opitz syndrome confirmed by tissue sterol analysis. Prenat. Diagn. 20: 238–240. [PubMed] [Google Scholar]
- 16.Kelley R. I. 1995. Diagnosis of Smith-Lemli-Opitz syndrome by gas chromatography/mass spectrometry of 7-dehydrocholesterol in plasma, amniotic fluid and cultured skin fibroblasts. Clin. Chim. Acta. 236: 45–58. [DOI] [PubMed] [Google Scholar]
- 17.Liu W., Xu L., Lamberson C., Haas D., Korade Z., and Porter N. A.. 2014. A highly sensitive method for analysis of 7-dehydrocholesterol for the study of Smith-Lemli-Opitz syndrome. J. Lipid Res. 55: 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korade Z., Folkes O. M., and Harrison F. E.. 2013. Behavioral and serotonergic response changes in the Dhcr7-HET mouse model of Smith-Lemli-Opitz syndrome. Pharmacol. Biochem. Behav. 106: 101–108. [DOI] [PubMed] [Google Scholar]
- 19.Korade Ž., Liu W., Warren E. B., Armstrong K., Porter N. A., and Konradi C.. 2017. Effect of psychotropic drug treatment on sterol metabolism. Schizophr. Res. 187: 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall P., Michels V., Gavrilov D., Matern D., Oglesbee D., Raymond K., Rinaldo P., and Tortorelli S.. 2013. Aripiprazole and trazodone cause elevations of 7-dehydrocholesterol in the absence of Smith-Lemli-Opitz syndrome. Mol. Genet. Metab. 110: 176–178. [DOI] [PubMed] [Google Scholar]
- 21.Kim H-Y. H., Korade Z., Tallman K. A., Liu W., Weaver C. D., Mirnics K., and Porter N. A.. 2016. Inhibitors of 7-dehydrocholesterol reductase: screening of a collection of pharmacologically active compounds in Neuro2a cells. Chem. Res. Toxicol. 29: 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boland M. R., and Tatonetti N. P.. 2016. Investigation of 7-dehydrocholesterol reductase pathway to elucidate off-target prenatal effects of pharmaceuticals: a systematic review. Pharmacogenomics J. 16: 411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson K. M., Hoffmann G., Schwall A., Broock R., Aramaki S., Sweetman L., Nyhan W. L., Brandt I. K., Wappner R. S., Lehnert W., et al. 1990. 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured fibroblasts from patients with mevalonate kinase deficiency: differential response to lipid supplied by fetal bovine serum in tissue culture medium. J. Lipid Res. 31: 515–521. [PubMed] [Google Scholar]
- 24.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W. W., Hegde M., Lyon E., Spector E., et al. 2015. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17: 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nes W. D. 2011. Biosynthesis of cholesterol and other sterols. Chem. Rev. 111: 6423–6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canfrán-Duque A., Casado M. E., Pastor O., Sánchez-Wandelmer J., de la Peña G., Lerma M., Mariscal P., Bracher F., Lasunción M. A., and Busto R.. 2013. Atypical antipsychotics alter cholesterol and fatty acid metabolism in vitro. J. Lipid Res. 54: 310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honda A., Tint G. S., Salen G., Batta A. K., Chen T. S., and Shefer S.. 1995. Defective conversion of 7-dehydrocholesterol to cholesterol in cultured skin fibroblasts from Smith-Lemli-Opitz syndrome homozygotes. J. Lipid Res. 36: 1595–1601. [PubMed] [Google Scholar]
- 28.Horling A., Muller C., Barthel R., Bracher F., and Imming P.. 2012. A new class of selective and potent 7-dehydrocholesterol reductase inhibitors. J. Med. Chem. 55: 7614–7622. [DOI] [PubMed] [Google Scholar]
- 29.Lauth M., Rohnalter V., Bergström A., Kooshesh M., Svenningsson P., and Toftgård R.. 2010. Antipsychotic drugs regulate hedgehog signaling by modulation of 7-dehydrocholesterol reductase levels. Mol. Pharmacol. 78: 486–496. [DOI] [PubMed] [Google Scholar]
- 30.Polymeropoulos M. H., Licamele L., Volpi S., Mack K., Mitkus S. N., Carstea E. D., Getoor L., Thompson A., and Lavedan C.. 2009. Common effect of antipsychotics on the biosynthesis and regulation of fatty acids and cholesterol supports a key role of lipid homeostasis in schizophrenia. Schizophr. Res. 108: 134–142. [DOI] [PubMed] [Google Scholar]
- 31.Korade Z., Kim H-Y., Tallman K. A., Liu W., Koczok K., Balogh I., Xu L., Mirnics K., and Porter N. A.. 2016. The effect of small molecules on sterol homeostasis: measuring 7-dehydrocholesterol in Dhcr7-deficient Neuro2a cells and human fibroblasts. J. Med. Chem. 59: 1102–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kálmán S., Garbett K. A., Janka Z., and Mirnics K.. 2016. Human dermal fibroblasts in psychiatry research. Neuroscience. 320: 105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wassif C. A., Krakowiak P. A., Wright B. S., Gewandter J. S., Sterner A. L., Javitt N., Yergey A. L., and Porter F. D.. 2005. Residual cholesterol synthesis and simvastatin induction of cholesterol synthesis in Smith-Lemli-Opitz syndrome fibroblasts. Mol. Genet. Metab. 85: 96–107. [DOI] [PubMed] [Google Scholar]
- 34.Luu W., Hart-Smith G., Sharpe L. J., and Brown A. J.. 2015. The terminal enzymes of cholesterol synthesis, DHCR24 and DHCR7, interact physically and functionally. J. Lipid Res. 56: 888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dietschy J. M., and Turley S. D.. 2001. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 12: 105–112. [DOI] [PubMed] [Google Scholar]
- 36.Dietschy J. M., and Turley S. D.. 2004. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 45: 1375–1397. [DOI] [PubMed] [Google Scholar]
- 37.Korade Z., Mi Z., Portugal C., and Schor N. F.. 2007. Expression and p75 neurotrophin receptor dependence of cholesterol synthetic enzymes in adult mouse brain. Neurobiol. Aging. 28: 1522–1531. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki S., Kiyosue K., Hazama S., Ogura A., Kashihara M., Hara T., Koshimizu H., and Kojima M.. 2007. Brain-derived neurotrophic factor regulates cholesterol metabolism for synapse development. J. Neurosci. 27: 6417–6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu L., Davis T. A., and Porter N. A.. 2009. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc. 131: 13037–13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu L., Korade Z., Dale A., Rosado J., Liu W., Lamberson C. R., and Porter N. A.. 2011. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J. Lipid Res. 52: 1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu L., Korade Z., and Porter N. A.. 2010. Oxysterols from free radical chain oxidation of 7-dehydrocholesterol: product and mechanistic studies. J. Am. Chem. Soc. 132: 2222–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sever N., Mann R. K., Xu L., Snell W. J., Hernandez-Lara C. I., Porter N. A., and Beachy P. A.. 2016. Endogenous B-ring oxysterols inhibit the Hedgehog component smoothened in a manner distinct from cyclopamine or side-chain oxysterols. Proc. Natl. Acad. Sci. USA. 113: 1604984113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korade Z., Xu L., Shelton R., and Porter N. A.. 2010. Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J. Lipid Res. 51: 3259–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu L., Korade Z., Rosado D. A. Jr., Mirnics K., and Porter N. A.. 2013. Metabolism of oxysterols derived from nonenzymatic oxidation of 7-dehydrocholesterol in cells. J. Lipid Res. 54: 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steffen K. M., Cooper M. E., Shi M., Caprau D., Simhan H. N., Dagle J. M., Marazita M. L., and Murray J. C.. 2007. Maternal and fetal variation in genes of cholesterol metabolism is associated with preterm delivery. J. Perinatol. 27: 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finnell R. H. 1999. Teratology: general considerations and principles. J. Allergy Clin. Immunol. 103: S337–S342. [DOI] [PubMed] [Google Scholar]
- 47.Beckman D. A., and Brent R. L.. 1984. Mechanisms of teratogenesis. Annu. Rev. Pharmacol. Toxicol . 24: 483–500. [DOI] [PubMed] [Google Scholar]
- 48.Singh K. P., and Tripathi N.. 2014. Prenatal exposure of a novel antipsychotic aripiprazole: impact on maternal, fetal and postnatal body weight modulation in rats. Curr. Drug Saf. 9: 43–48. [DOI] [PubMed] [Google Scholar]
- 49.Carlson B. 2012. Vanderbilt pioneers bedside genetics. Biotechnol. Healthc. 9: 31–32. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.