Abstract
Lipid droplet (LD) coating proteins are essential for the formation and stability of intracellular LDs. Plin2 is an abundant LD coating protein in skeletal muscle, but its importance for muscle function is unclear. We show that myotubes established from Plin2−/− mice contain reduced content of LDs and accumulate less oleic acid (OA) in triacylglycerol (TAG) due to elevated LD hydrolysis in comparison with Plin2+/+ myotubes. The reduced ability to store TAG in LDs in Plin2−/− myotubes is accompanied by a shift in energy metabolism. Plin2−/− myotubes are characterized by increased oxidation of OA, lower glycogen synthesis, and reduced glucose oxidation in comparison with Plin2+/+ myotubes, perhaps reflecting competition between FAs and glucose as part of the Randle cycle. In accord with these metabolic changes, Plin2−/− myotubes have elevated expression of Ppara and Ppargc1a, transcription factors that stimulate expression of genes important for FA oxidation, whereas genes involved in glucose uptake and oxidation are suppressed. Loss of Plin2 had no impact on insulin-stimulated Akt phosphorylation. Our results suggest that Plin2 is essential for protecting the pool of skeletal muscle LDs to avoid an uncontrolled hydrolysis of stored TAG and to balance skeletal muscle energy metabolism.
Keywords: Plin2, triacylglycerol, lipid droplet, lipolysis and fatty acid metabolism, fatty acid/metabolism, insulin signaling, muscle
Energy to drive contraction of skeletal muscle fibers is obtained primarily by oxidation of glucose or FAs. Skeletal muscle cells store these two energy sources as cytosolic glycogen or triacylglycerol (TAG)-containing lipid droplets (LDs), respectively. Incorporation of glucose into glycogen and its degradation for energy utilization are relatively more understood processes than are storage and metabolism of muscular lipids. Aerobic glucose utilization occurs in the cytosol catalyzed by the glycolytic enzymes, whereas FAs are mainly utilized through mitochondrial β-oxidation. In addition to hormonal regulation and transcription input of these two separate oxidative pathways, competitive and feedback interactions among their various substrates and products also directs glucose/FA oxidative balance and may be mechanistically important for tissue insulin sensitivity (1). Likewise, a high content of intramyocellular TAG, suggestive of increased FA availability, is well known to correlate with reduced glucose disposal and insulin resistance in some individuals (2–6), but this is not absolute. Intramyocellular TAG content in endurance-trained individuals, which can be higher than in obese insulin-resistant individuals (7), does not affect insulin sensitivity or oxidative capacity, a phenomenon described as the athlete’s paradox (7–10). In addition, despite higher intramyocellular TAG levels, women are more insulin sensitive than are men (11). Therefore, new concepts have emerged to explain lipid-mediated muscular insulin resistance, which focus on abnormal lipid influx, storage, or build-up of lipid species arising from altered TAG lipolysis, rather than lipid content per se (12).
LDs in mammalian cells consist of an inner core of neutral lipids, such as TAGs, diacylglycerols (DAGs), or cholesteryl esters (CEs), surrounded by a single monolayer of phospholipids (PLs) with a protein coat (13, 14). The mechanisms for enzymatic degradation of the esterified neutral lipids in the LD core into FAs and glycerol in skeletal muscle are similar to those of many other tissues and involve active translocation of lipases to the LD surface. Thus, facilitated recruitment of proteins to the LD surface is the major mechanism regulating LD size and turnover. Adipose triglyceride lipase (ATGL) mediates the first step in TAG degradation (15), generating DAG, which is subsequently degraded by hormone-sensitive lipase (HSL) (16), followed by monoacylglycerol (MAG) cleavage by MAG lipase (17). Still, it is not understood mechanistically how lipases are actively recruited to the LD surface in muscle or how these enzymes can access the neutral lipids stored in the LD core through the protein and PL layer. Accumulating evidence suggests that perilipin (Plin) family proteins are involved [see reviews (8, 14, 18)]. As with other mammalian cell types, Plin proteins are among the most abundant LD coating proteins in skeletal muscle cells. The Plins derive from an ancient gene family that consists of five Plin genes in mammals, some with alternative splice variants (18–20). The Plin genes differ in their tissue expression and transcriptional regulation, and the encoded proteins differ in the number of residues, affinity to LDs, and stability when not bound to LDs. The fundamental understanding of Plin functions is based on the initial characterization of Plin1 in white adipose tissue. Plin1 interactions with lipases and their coactivators at the LD surface are differentially regulated by PKA phosphorylation (21) and serve as major regulatory steps controlling storage versus degradation of neutral lipids stored in the core of adipose LDs (22, 23). The other Plins are believed to regulate LD degradation in nonadipose tissues. This assumption is supported by the observations that removal of Plin1 (22, 23), Plin2 (24), Plin4 (25), or Plin5 (26) in mice results in reduced LD content in tissues where these proteins normally would be highly expressed. Overexpression of certain Plins increases the relative LD accumulation of specific neutral lipid species as opposed to others, which suggests diversity of Plin function (27).
Plin2 is an abundant LD coating protein in skeletal muscle where the majority of the LDs are covered by Plin2 (28). Interventions that increase muscle insulin sensitivity might be accompanied with an increase in Plin2 protein expression (29), suggesting that Plin2 might play a role in decreasing intramuscular lipid toxicity by promoting lipid storage. On the other hand, comparable muscular Plin2 protein content has been observed when comparing obese nondiabetic and obese diabetic subjects (30). To clarify the functional role of Plin2 in skeletal muscle, we established myoblast cultures from Plin2+/+ and Plin2−/− mice, differentiated these into myotubes, and compared myocellular lipid storage and energy metabolism. We observed enhanced lipolysis, reduced levels of TAG-containing LDs, and altered lipid and glucose oxidation rates in myotubes lacking Plin2 at the LD surface. Plin2−/− myotubes show a shift in metabolic energy utilization toward FA oxidation, consistent with suppression of glucose oxidation within the parameters of the Randle cycle. Our results suggest that Plin2 balances energy utilization of glucose and FAs by stabilizing and packaging excess FAs in LD stores.
MATERIALS AND METHODS
Materials
DMEM (DMEM-Glutamax™, 5.5 mM glucose) with sodium pyruvate, DMEM without phenol red, Ham’s F-10-Glutamax™ nutrient mixture (5.5 mM glucose), horse serum, heat-inactivated FBS, basic fibroblast growth factor, collagenase II, penicillin-streptomycin, and amphotericin B, Bodipy 493/503, Hoechst 33258, Hoechst 33342, ABI 6100 Nucleic Acid Prep-Station solutions, and Superscript III RT were from Thermo Fisher Scientific (Waltham, MA). Matrigel was purchased from BD Biosciences (Bedford, MA). Insulin (Actrapid®) was obtained from Novo Nordisk (Bagsvaerd, Denmark). BSA (essentially FA free), L-carnitine, Dulbecco’s PBS (DPBS, with Mg2+ and Ca2+), oleic acid (OA; 18:1, n-9), and glycogen were from Sigma-Aldrich (St. Louis, MO). [1-14C]oleic acid (58.2 mCi/mmol) and D-[14C-(U)]glucose (2.9 mCi/mmol) were from PerkinElmer NEN® (Boston, MA). Liberase Blendzyme 3 (0.038 WU/ml) and Complete Protease Inhibitor Cocktail were from Roche (Basel, Switzerland). Culture plates and flasks were from Corning (Corning, NY). Glass-bottom six-well plates were from MatTek (Ashland, MA). Ninety six-well Scintiplate®, UniFilter® micro plate, Isoplate® scintillation plate, and OptiPhase Supermix were obtained from PerkinElmer (Shelton, CT). Ultracentrifugation tubes were from Beckman Coulter Inc. (#344062; Brea, CA). TLC plates (Silica gel 60) were from Merck Millipore (Billerica, MA). Atglistatin was from Xcess Biosciences (San Diego, CA). CAY10499 was from Cayman Chemical (Ann Arbor, MI). TG PAP 150-kit (#61236) was obtained from BioMerieux (Marcy l’Etoile, France). SYBR Master Mix was from Kapa Biosystems (Wilmington, MA). Criterion or Mini-Protean® TGX™ gels (4–20%), Clarity™ Western ECL Substrate, and the goat anti-rabbit HRP-conjugated (HRP) secondary antibody #1706515 were purchased from Bio-Rad (Copenhagen, Denmark). Pierce™ BCA protein assay kit was from Thermo Fisher Scientific (Rockford, IL). Antibodies against total thymoma viral proto-oncogene/AKT serine/threonine kinase (Akt) (#9272, rabbit polyclonal antibody), phosphorylated Akt (pSer473, #9271, rabbit polyclonal antibody), Slc2a1/Glut1 (#12938, rabbit monoclonal antibody), Pkm (#3106, rabbit monoclonal antibody), Pdha1 (#3205, rabbit monoclonal antibody), and β-actin (#4970, rabbit monoclonal antibody) were from Cell Signaling Technology (Beverley, MA). Antibody against Pdha1-p300 (#ABS194, rabbit polyclonal antibody) was from Millipore. Antibodies against muscle-associated glycogen phosphorylase (Pygm) (#ab81901, rabbit polyclonal antibody) and Pdk4 (#214938, rabbit monoclonal antibody) were from Abcam (Cambridge, UK). Antibody against Gapdh (#sc-25778, rabbit polyclonal antibody) was obtained from Santa Cruz Biotechnology (Dallas, TX). The goat anti-rabbit HRP-conjugated secondary antibody #111-035-144 was from Jackson ImmunoResearch (Suffolk, UK). All chemicals used were of standard commercial high-purity quality.
Generation of the Plin2 null mice
A BAC clone containing the Plin2 genetic locus (AB2.2 ES cells, strain 129S7/SvEvBrd-Hprtb-m2, clone #bMQ-28H12) was modified with recombineering to generate a floxed Plin2 targeting vector. Because the Plin2 protein is rapidly degraded in the absence of LD targeting (31), LoxP sites were inserted in intron 3 and intron 6 of the Plin2 gene to flank exon sequences that are essential for targeting of the Plin2 protein to LD surfaces. With this design, any potential truncated Plin2 protein sequence translated from the genetically modified gene would be expected to untarget from LDs and be rapidly degraded. The specific details for generation of the targeting vector will be published elsewhere (Y.K.L., K.T.D., and A.R.K., unpublished observations). Standard homologous recombination in 129/SvJEv embryonic stem (ES) cells was used to target the Plin2 gene. Resulting chimeric mice were bred with C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) for germline transmission. The resulting Plin2 floxed line on a mixed B6.129/Sv background was subsequently crossed with mice expressing MMTV-Cre recombinase (Jackson Laboratory) to generate Plin2 null mice (Plin2−/−). The floxed Plin2 mice were generated in compliance with the guidelines given by the Animal Care and Use Committee of the National Institutes of Health under a Division of Intramural Research, National Institute of Diabetes and Digestive and Kidney Diseases, under approved animal study protocol K039-LCDB.
Animal experiments
Plin2+/+ and Plin2−/− mice were housed in a temperature-controlled (22°C) facility with a strict 12-h light/dark cycle. Male mice backcrossed for 10 generations into C57BL/6N (Janvier Labs, Le Genest-Saint-Isle, France) were given standard chow (#RM3-801190, SDS diets, consisting of 12% calories from fat, 27% from protein, and 61% from carbohydrate) until 12 weeks of age. Mice were euthanized by cervical dislocation at 8:00 to 10:00 AM, and tissues were snap-frozen in liquid nitrogen and stored at −80°C until further analysis. All animal use was approved and registered by the Norwegian Animal Research Authority (NARA, under animal study protocols FOTS IDs: #6305 and #6922) and conformed to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
Isolation of satellite cells
Female siblings (14 weeks) backcrossed into C57BL/6N for six generations were used for primary satellite cell isolation from the hind leg containing musulus gastrocnemius and musculus soleus. Primary muscle satellite cells (myoblasts) were isolated and purified, as has been previously described (32). Briefly, the muscle tissue was incubated with 2% collagenase II at 37°C for 90 min to enzymatically dissociate the cells. Satellite cells were liberated by further digestion with Blendzyme at 37°C for 30 min. To enrich myoblasts, we split the cell population in DPBS with no trypsin or EDTA. Further enrichment of myoblasts was obtained by preplating for 15 min on collagen-coated flasks. This tends to leave behind cells that stick quickly, which are predominantly fibroblasts. Fibroblasts tend to be very flat when grown on collagen, whereas myoblasts are more compact and smaller in diameter. In addition, the F-10-based primary myoblast growth medium gives myoblasts a growth advantage over fibroblasts. Plin2+/+ and Plin2−/− myotube cultures were considered established when fibroblasts were no longer visible in the cultures.
Cell culture and stimulation of myotubes
Purified myoblasts were proliferated on standard plasticware coated with collagen (0.01%). To enhance myotube formation, Matrigel (diluted 1:50 in DMEM) was used as coating when myoblasts were seeded to differentiate into myotubes. Mouse myoblasts were grown to subconfluence in DMEM/Ham’s F-10 (1:1, 5.5 mM glucose) supplemented with 20% FBS, 5 ng/ml basic fibroblast growth factor, 25 IU penicillin, 25 µg/ml streptomycin, and 1.25 μg/ml amphotericin B at 37°C in 5% CO2. At ∼80% confluence, growth medium was replaced by DMEM supplemented with 2% horse serum and antibiotics to induce fusion of myoblasts into multinucleated myotubes. Unless otherwise stated, cells differentiated for 4 days were used in experiments.
OA was bound to FA-free BSA with an OA:BSA ratio of 2.5:1 in all experiments (further referred to as OA). Control myotubes and OA-stimulated myotubes received the same concentration of BSA. The lipase inhibitors (Atglistatin and CAY10499) were solved in DMSO and diluted in medium to a final concentration of 0.1% DMSO. Control myotubes and lipase inhibitor-stimulated myotubes received the same concentration of DMSO.
To measure insulin response, we incubated myotubes differentiated for 3 days in medium containing OA (100 μM) for 24 h. Myotubes were subsequently incubated with glucose-free DMEM medium for 2 h in the presence of OA (100 μM), followed by 15-min incubation in DMEM (5.5 mM glucose) in the presence or absence of insulin (100 nM). Myotubes from two wells (six-well plate) were pooled in RIPA buffer, supplemented with Complete Protease Inhibitor cocktail, and stored at −80°C until further analysis. Protein content in cell extracts was measured by Pierce™ BCA protein assay kit.
Immunoblotting
Cell extracts were sonicated briefly, as has been described previously (33). Proteins (15 μg) were mixed with Laemmli buffer and separated on a 4%–20% Criterion or Mini-Protean® TGX™ gels, followed by blotting to nitrocellulose or PVDF membranes. Membranes were immunoblotted with the following antibodies: rabbit anti-mouse Plin2 [(31); #N510; 3 µg/ml], rabbit anti-mouse Plin3 [(34); 10 µg/ml], rabbit anti-mouse Plin4 and rabbit anti-mouse Plin5 [(33); 10 µg/ml], rabbit anti-human glucose transporter 1 (Slc2a1/Glut1, 1:1,000), rabbit anti-human pyruvate kinase (Pkm, 1:1,000), rabbit anti-human Pygm (1:1,000), rabbit anti-mouse pyruvate dehydrogenase kinase 4 (Pdk4, 1:1,000), rabbit anti-human muscle pyruvate dehydrogenase α 1 (Pdha1, 1:1,000), rabbit anti-human phosphorylated muscle pyruvate dehydrogenase α 1 (Pdha1-p300, 1:1,000), total Akt kinase (detects isoforms Akt1-3, 1:1,000), Ser473-phosphorylated Akt (1:1,000), GAPDH (Gapdh, 1:500), and β-actin (1:1,000), followed by HRP-labeled secondary antibodies (∼1:10,000). Immunoreactive bands were detected with Clarity™ Western ECL Substrate, visualized with ECL using Chemidoc XRS (Bio-Rad), and quantified with Image Lab software (version 4.0). The β-actin signal was used to normalize for protein loading.
Preparation and analysis of RNA
Cells were lysed in Nucleic Acid Purification Lysis Solution:PBS (1:1) and frozen at −80°C before isolation. Total RNA from cell extracts was isolated on a ABI 6100 Nucleic Acid Prep-Station with the preprogrammed “RNA-Cell method.” RNA quality and quantity were determined with NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Reverse transcription quantitative real-time PCR (RT-qPCR) was performed in two steps. First, total RNA (20 ng/µl) was reverse-transcribed into single-stranded complementary DNA with random hexamers and Superscript III RT (50°C for 60 min and 72°C for 15 min). Next, gene-specific regions (70–120 bp) were amplified from complementary DNA (10 ng) with assay primers (100 nM each) and KAPA SYBR Fast Master Mix (10-μl reaction, 95°C for 3 min, followed by 40 cycles; 95°C for 10 s and 60°C for 20 s) on the ABI 7900HT instrument (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA). Assay primers (Tm = 60) were designed with Primer-BLAST software (35). All assay primer pairs were designed to bind to adjacent exons spanned by a large intron with amplicon sizes ranging from 70 to 110 nt. Primers used are listed in Table 1. Data were analyzed with relative quantification (ΔΔCq method). Results are presented as gene expression in relation to endogenous control (2−ΔΔCq). TATA-box binding protein (Tbp) mRNA was verified to not differ in expression among groups or treatments and was used as endogenous control in all experiments.
TABLE 1.
Primers used for RT-qPCR in this study
| Gene | Forward Primer | Reverse Primer | Amplicon Size |
| Acadl | CGGAAGCTGCATAAGATGGGA | AGCTGGCAATCGGACATCTT | 75 |
| Acadm | TGGATTCATTGTGGAAGCCGA | CCTCTGGTGTCAGAGCATCG | 87 |
| Acadvl | GCATTTGGCCTGCAAGTACC | AATCTCTGCCAAGCGAGCAT | 78 |
| Acox1 | AATCTGGAGATCACGGGCACTT | GTCTTGGGGTCATATGTGGCAG | 95 |
| Cd36 | AGGCATTCTCATGCCAGTCG | TGTACACAGTGGTGCCTGTT | 119 |
| Cpt2 | TATGATGGCTGAGTGCTCCAA | CCGTAGAGCAAACAAGTGTCG | 91 |
| Fabp3 | GGACAGCAAGAATTTTGATGACTAC | TTGGTCATGCTAGCCACCTG | 78 |
| Gys1 | TTGGGGTCTTCCCCTCCTAC | GTGGAGATGCTGGGGATGC | 82 |
| Hk1 | GGACCACAGTTGGCGTAGA | CTCAGGGTCTTGTGGAACCG | 76 |
| Hk2 | CTTCCCTTGCCAGCAGAACA | TGACCACATCTTCACCCTCG | 95 |
| Pdha1 | CGTGGTTTCTGTCACTTGTGTG | CGTAGGGTTTATGCCAGCCT | 72 |
| Pdk4 | AAGATGCTCTGCGACCAGTA | CAATGTGGATTGGTTGGCCTG | 91 |
| Pkm | GAAACAGCCAAGGGGGACTAC | CACAAGCTCTTCAAACAGCAGAC | 108 |
| Plin2 (exon4-5) | GGGCTAGACAGGATGGAGGA | CACATCCTTCGCCCCAGTTA | 99 |
| Plin2 (exon7-8) | GTGGAAGAGAAGCATCGGCT | GGCGATAGCCAGAGTACGTG | 82 |
| Plin3 | CGAAGCTCAAGCTGCTATGG | TCACCATCCCATACGTGGAAC | 98 |
| Plin4 | ACCAACTCACAGATGGCAGG | AGGCATCTTCACTGCTGGTC | 109 |
| Plin5 | GGTGAAGACACCACCCTAGC | CCACCACTCGATTCACCACA | 115 |
| Ppara | ACTACGGAGTTCACGCATGT | GTCGTACACCAGCTTCAGCC | 74 |
| Pparg | TTGCTGTGGGGATGTCTCAC | AACAGCTTCTCCTTCTCGGC | 70 |
| Ppard | ACATGGAATGTCGGGTGTGC | CGAGCTTCATGCGGATTGTC | 108 |
| Ppargc1a | AGTCCCATACACAACCGCAG | CCCTTGGGGTCATTTGGTGA | 94 |
| Pygm | GAGTGGAGGACGTGGAAAGG | CCGAAGCTCAGGAATTCGGT | 77 |
| Slc2a1 | CTCGGATCACTGCAGTTCGG | CGTAGCGGTGGTTCCATGTT | 97 |
| Slc2a4 | CGACGGACACTCCATCTGTT | ACATAGCTCATGGCTGGAACC | 104 |
| Tbp | AGCCTTCCACCTTATGCTCAG | GCCGTAAGGCATCATTGGACT | 90 |
| Ucp2 | TTGGCCTCTACGACTCTGTCA | CAGGGCACCTGTGGTGCTA | 98 |
| Ucp3 | CTACAGAACCATCGCCAGGGA | GTCGTAGGTCACCATCTCAGCA | 109 |
Microscopy
Myoblasts seeded on Matrigel-coated six-well glass-bottom dishes were differentiated into myotubes for 3 days before switching to media supplemented with OA (100 µM) for 24 h. For live imaging, LDs were stained by incubating myotubes with Bodipy 493/503 (2 μg/ml) for 10 min, followed by nuclei staining with Hoechst 33258 (2.5 μg/ml) for 15 min. Live images were randomly taken in 25 positions per well with a ×20 objective on an Olympus IX81 inverted fluorescence Scan^R platform (Olympus Corporation, Tokyo, Japan), as has been previously described (36). After gating out aggregates and dead cells, each parameter was determined from an average 150 images per donor group (average of 96 ± 25 nuclei per image). For confocal pictures, myotubes were fixated for 1 h with 4% PFA in 100 mM of phosphate buffer (pH 7.4), washed and stained with Bodipy 493/503 (1 μM) and Hoechst 33342 (5 μM) for 20 min. Pictures were taken with a ×40 objective on a LSM 710 confocal microscope (Zeiss, Oberkochen, Germany).
FA accumulation and lipolysis assay
Accumulation (real-time uptake) and lipolysis (efflux) of FA were measured with scintillation proximity assay, as has been previously described (37). In this method, the radiolabeled substrates taken up will accumulate in the adherent cells and become concentrated close to the scintillator embedded in the plastic bottom of each well and provide a stronger radioactive signal than would the radiolabel dissolved in the medium alone. Cells were seeded and differentiated for 3 days in 96-well Scintiplate® coated with Matrigel. To determine OA uptake and accumulation, we gave myotubes fresh medium comprising DMEM without phenol red, supplemented with [1-14C]OA (0.5 µCi/ml) and unlabeled OA (final OA concentration 100 or 400 μM) in the presence of DMSO (0.1%) or the ATGL inhibitor Atglistatin [10 µM (38)]. Lipid accumulation was monitored regularly for the next 24 h on a 2450 MicroBeta2 scintillation counter (PerkinElmer, Shelton, CT). Thereafter, to measure FA efflux (lipolysis), the labeled myotubes were washed twice with PBS containing 0.5% BSA before the myotubes were incubated in DPBS supplemented with 10 mM HEPES, 0.5% BSA, and 100 µM glucose. The decline in accumulated [1-14C]OA was measured at 0, 2, 4, and 6 h.
Glucose and FA substrate oxidation assays
Cells were seeded and differentiated in 96-well plates coated with Matrigel solution and subjected to substrate oxidation assay, as has been described previously (37). To measure FA oxidation from prelabeled intracellular lipid pools, we incubated myotubes with [1-14C]OA (0.5 µCi/ml) and unlabeled OA (final OA concentration, 100 or 400 μM) for 24 h, combined with various treatments (0.1% DMSO, 10 μM CAY10499 or 10 μM Atglistatin). Myotubes were then washed twice with PBS containing 0.5% BSA and given DPBS media supplemented with 10 mM HEPES, 10 µM BSA, and 1 mM L-carnitine to capture CO2. To measure glucose oxidation, we gave myotubes D-[14C(U)]glucose (0.58 µCi/ml) and unlabeled glucose (final glucose concentration 200 µM) directly in the CO2-capturing media (DPBS supplemented with 10 mM HEPES, 10 µM BSA, and 1 mM L-carnitine). A 96-well UniFilter® microplate, activated for capture of CO2 by the addition of 1 M NaOH, was subsequently mounted on top of the 96-well plate, and the sandwich was incubated at 37°C for 4 h. [14C]CO2 captured in the filter was counted by liquid scintillation on a 2450 MicroBeta2 scintillation counter (PerkinElmer, Shelton, CT) as a measure of produced CO2. After CO2 capturing, myotubes were washed with PBS and lysed with 0.1 M NaOH before cell-associated radioactivity (accumulated substrate) was determined by liquid scintillation. Acid-soluble metabolites (ASMs) in the media were measured, as has been previously described (36). ASMs consist of β-oxidation and tricarboxylic acid cycle metabolites and reflect incomplete FA or glucose oxidation in the mitochondria.
Lipid droplet isolation
Cells from six 10-cm petri dishes were stimulated with 200 μM of OA for 24 h, washed twice with cold PBS, harvested in suspension buffer (25 mM tricine pH 7.8, 8.6% sucrose, and Complete Protease Inhibitor cocktail), mixed gently, and frozen at −80°C. To disrupt cells, samples were thawed slowly in ice-water slurry for ∼30 min and transferred to a precooled N2 cell- disruption vessel (#4639, Parr Instrument Co., Moline, IL). Samples were exposed to 600 psi N2 for 20 min, then released slowly (dropwise) into a 15-ml tube. The disrupted cell lysate was subsequently centrifuged for 10 min at 3,000 g at 4°C to remove nuclei and undisrupted cell debris. The suspension containing LDs was adjusted to 2 ml and transferred to the bottom of an ultracentrifugation tube, followed by a second layer consisting of 1.8 ml of wash buffer (20 mM HEPES pH 7.4, 100 mM KCl, 2 mM MgCl2, and 4% sucrose [w/v]), and a top layer of 0.4 ml of collection buffer (20 mM HEPES pH 7.4, 100 mM KCl, and 2 mM MgCl2). Tubes were balanced and centrifuged for 60 min in a SW60Ti rotor at 200,000 g at 4°C in a XL-90 Ultracentrifuge (Beckman Coulter Inc.). The top layer (∼0.4 ml) was isolated with a tube slicer prior to the collection of floating LDs.
Lipid composition of isolated LDs
Isolated LDs were mixed with ×2 volume of chloroform:heptane:methanol (4:3:2, v/v/v) and lipids extracted by thoroughly mixing for 1–2 min prior to centrifugation for 5 min at 2,000 g. The lower organic phase was carefully transferred into a glass tube and evaporated under N2 before lipids were dissolved in chloroform:methanol (2:1, v/v). TAG content in samples was determined with TG PAP 150 kit, adjusted to 250 ng TAG/µl, and stored at −20°C under argon. The TLC plate was fully developed in methanol:ethyl acetate (6:4, v/v) to remove impurities, then dried for 6–8 min at 40°C. Lipid samples (1 µg TAG) and lipid standard mix (equal weights of TAG, DAG, MAG, PL, FFA, CE, and free cholesterol) were spotted on the plate and air-dried briefly, and the plate was developed in heptane:diethyl ether:acetic acid (55:45:1, v/v/v). The plate was subsequently dried for 10 min at 40°C and developed with copper sulfate staining (39) by exposing the plate to a developing reagent consisting of 10% CuSO4 × 5H2O (w/v) and 8% H3PO4 in H2O (v/v) for ∼40 s. Excess solution was removed by decanting, and the back of the plate was cleaned with tissue paper. The plate was subsequently air-dried briefly, placed on a heating plate for 10 min at 60°C, and then for 10 min at 150°C. After being cooled down, the plate was scanned with an Epson Perfection V700 image scanner (Seiko Epson Corporation, Nagano, Japan).
Lipid distribution in cells
Myotubes were incubated with [1-14C]OA (0.5 µCi/ml) and unlabeled OA (final OA concentration 100 or 400 μM) for 24 h. The myotubes were washed twice with PBS and harvested with two additions of 125 μl dH2O. Cellular lipid distribution was analyzed as has been previously described (40). Briefly, homogenized cell fractions were extracted, lipids were separated by TLC with hexane:diethyl ether:acetic acid (65:35:1, v/v/v) as developing solvent, and radioactivity in excised bands was quantified by liquid scintillation (Packard TriCarb 1600, PerkinElmer, Shelton, CT).
Triacylglycerol measurements
Myotubes were incubated with OA alone (100 µM) or in the presence of Atglistatin (10 µM) for 24 h. Thereafter, the myotubes were washed with PBS and harvested in PBS or RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and 2 mM EDTA). Measurement of cellular TAG was performed with the TG PAP 150 kit according to the supplier’s protocol.
Glycogen synthesis
Myotubes were incubated in DMEM without glucose, supplemented with D-[14C(U)]glucose (0.5 μCi/ml), unlabeled glucose (final glucose concentration 1 mM), pyruvate (1 mM), and BSA (10 µM) in the presence or absence of insulin (100 nM) for 3 h to measure glycogen synthesis. The myotubes were washed twice with PBS and harvested in KOH (1 M). After protein measurements, glycogen (final concentration 20 mg/ml) and more KOH (final concentration 4 M) were added to the samples. Thereafter, D-[14C(U)]glucose incorporated into glycogen was measured as has been previously described (36).
Statistical methods
Values are presented as means ± SEM unless stated otherwise. The value n represents the number of experiments performed with at least duplicate samples. Two-tailed unpaired t tests were performed to determine the difference between groups (Plin2+/+ and Plin2−/−) with GraphPad Prism 5.0 Software (GraphPad Software Inc., San Diego, CA), whereas two-tailed paired t tests were performed to determine effects of treatments. Linear mixed-model analysis (SPSS 20.0.0.1, IBM SPSS Inc., Chicago, IL) was used to compare Plin2+/+ and Plin2−/− myotubes in the time-course FA accumulation and lipolysis experiments (scintillation proximity assay). P < 0.05 was considered statistically significant.
RESULTS
Establishment of myotube cultures lacking Plin2
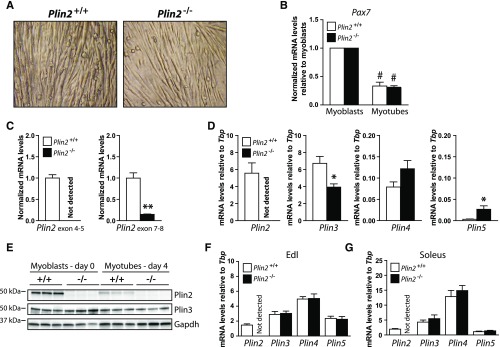
To investigate the functional role of Plin2 in myotubes, we first generated mice with homozygous disruption of the Plin2 gene by deleting exons 4, 5, and 6 that are essential for functional targeting of Plin2 to LD surfaces. A thorough phenotypic characterization of Plin2−/− mice will be published elsewhere (Y.K.L., K.T.D., and A.R.K, unpublished observations). Hind legs from female Plin2+/+ and Plin2−/− littermates, backcrossed into C57BL/6N for six generations, were used to isolate primary muscle satellite cells and establish Plin2+/+ and Plin2−/− myoblast cultures. The Plin2+/+ and Plin2−/− myoblast populations differentiated equally well into myotubes on the basis of the presence of multinuclear fiber-like cells, observed by microscopic inspection (Fig. 1A) and by the comparable reduction in mRNA expression of the nonmyotube satellite cell marker paired box 7 (Pax7) (Fig. 1B). The structural Plin2 gene differences in the Plin2+/+ and Plin2−/− myotubes were validated by RT-qPCR. Primers that recognize sequences within deleted exons 4 to 5 failed to amplify mRNA target sequences from Plin2−/− myotubes (Fig. 1C), confirming that these myotubes lack functional full-length Plin2 mRNA. Primers amplifying across the retained exon 7 to 8 junction showed lower expression of the truncated Plin2−/− mRNA (∼15%) compared with wild-type Plin2 mRNA (Fig. 1C). We next determined whether ablation of Plin2 was compensated for by increased expression of other Plin genes in myotubes. Plin3 mRNA expression was slightly (∼40%) reduced, Plin4 mRNA was unchanged, whereas Plin5 mRNA expression was increased (∼5-fold) in Plin2−/− compared with Plin2+/+ myotubes (Fig. 1D). Judged by mRNA levels in relation to Tbp, Plin4 and Plin5 mRNAs in the cultured myotubes were considerably lower (<1%) than Plin2 and Plin3 mRNAs in cultured wild-type myotubes.
Fig. 1.
Expression of Plins in muscle and established Plin2+/+ and Plin2−/− myotubes and myoblasts. Primary muscle satellite cells (myoblasts) were isolated from the hind leg of Plin2+/+ and Plin2−/− mice. A: Established Plin2+/+ and Plin2−/− myoblast cultures differentiated equally well into multinucleated myotubes. B: Expression of Pax7 mRNA in relation to the expression of TATA-box binding protein (Tbp) determined by RT-qPCR. The results are presented normalized to the expression levels in undifferentiated myoblasts. C: RT-qPCR with primers amplifying across the Plin2 exon 4–5 junction and the Plin2 exon 7–8 junction in relation to the expression of Tbp and normalized to the expression levels in Plin2+/+ myotubes, confirmed the absence of exon 4-6 Plin2 mRNA sequences in Plin2−/− myotubes. D: Expression of Plin2, Plin3, Plin4, and Plin5 mRNAs determined by RT-qPCR in relation to the expression of Tbp. Results in B–D are presented as means ± SEM (n = 3–6, *P < 0.05 and **P < 0.01 vs. Plin2+/+ myotubes, #P < 0.05 vs. myoblasts). E: Expression of Plin2 and Plin3 proteins in myoblasts (day 0) and differentiated myotubes (day 4). The membrane contains samples from three independent experiments (n = 3). F: Relative mRNA expression of Plin2, Plin3, Plin4, and Plin5 in extensor digitorum longus of chow-fed 12-week-old Plin2+/+ and Plin2−/− male mice. G: Relative mRNA expression of Plin2, Plin3, Plin4, and Plin5 in soleus. Gene expression levels in F and G were determined by RT-qPCR and are presented in relation to the expression of Tbp as means ± SEM (n = 9 in each group). Edl, extensor digitorum longus; Pax7, paired box 7.
Next, we analyzed Plin protein content. Plin2 immunosignals with an expected molecular mass of ∼50 kDa were observed in Plin2+/+ myoblasts and myotubes (Fig. 1E), whereas the signal was absent in Plin2−/− myotubes, which confirms correct genetic ablation of the Plin2 gene. We observed a significant decline in Plin2 protein levels when Plin2+/+ myoblasts were differentiated into myotubes, whereas Plin3 protein content was essentially unchanged by differentiation, regardless of genotype (Fig. 1E). Less distinct protein bands were observed for the very weakly transcribed Plin4 and Plin5 (results not shown). Hence, the lack of Plin2 in cultured Plin2−/− myotubes was not compensated for by elevated mRNA expression or accumulation of other Plin proteins.
We also examined mRNA levels of Plins in extensor digitorum longus and soleus muscle fibers dissected from chow-fed Plin2+/+ and Plin2−/− mice. Disruption of Plin2 did not alter expression of other Plin mRNAs (Fig. 1F, G). Furthermore, Plin2 and Plin3 mRNA levels were similar in myotubes and the two muscle fibers, whereas the expression of Plin4 and Plin5 mRNAs was more elevated in the muscle fibers than in the cultured myotubes. Thus, the Plin2+/+ and Plin2−/− myotube cultures represent an important parallel model to analyze Plin2 function for muscle metabolism, allowing for defined biochemical benchmarkings that are not readily accessible in situ.
Reduced accumulation of lipids in the absence of Plin2
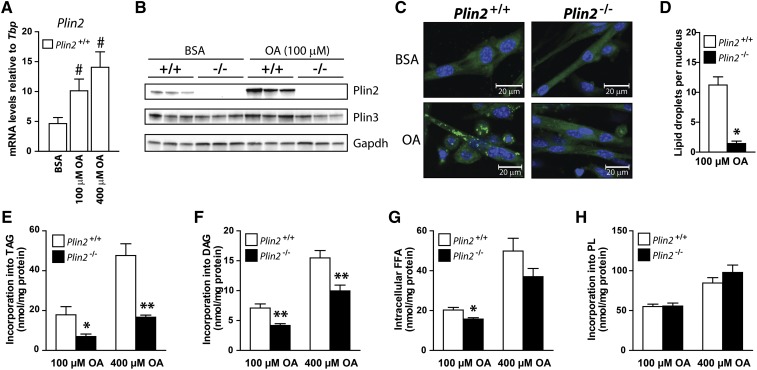
OA is easily taken up by cells and esterified into TAG that is incorporated into LDs, and thus, incubation with OA is an efficient strategy to promote LD formation and monitor relative intracellular lipid storage. Plin2+/+ myotubes cultured with 100 µM OA for 24 h increased Plin2 mRNA and protein content considerably compared with cells cultured with BSA (Fig. 2A, B) but had no effect on mRNA (results not shown) and protein expression of Plin3 (Fig. 2B). To determine whether removal of a functional Plin2 in myotubes affected the ability to store lipids, we incubated Plin2+/+ and Plin2−/− myotubes with 100 µM OA for 24 h before LDs were stained with Bodipy 493/503 (green) and nuclei stained with Hoechst 33258 (blue). A marked reduction in accumulated LDs was observed in Plin2−/− compared with Plin2+/+ myotubes (Fig. 2C). There were fewer quantified LDs per nucleus, observed under a ×20 objective, in Plin2−/− than in Plin2+/+ myotubes (Fig. 2D). Because smaller LDs are not necessarily labeled and recognized with automatic quantification, we also determined lipid distribution after incubation with [1-14C]OA for 24 h. Plin2−/− myotubes incorporated less OA into TAG (Fig. 2E) and DAG (Fig. 2F) and contained lower levels of FFAs (Fig. 2G) than did Plin2+/+ myotubes. Incorporation into phospholipids (Fig. 2H) and cholesteryl esters (results not shown) was unaffected by removal of Plin2. These observations demonstrate that Plin2−/− myotubes exposed to FAs store reduced levels of LDs compared with Plin2+/+ myotubes.
Fig. 2.
Lipid storage and distribution in Plin2+/+ and Plin2−/− myotubes. A–D: Myotubes were incubated for 24 h with BSA (40 µM) or OA (100 or 400 µM OA). A: Relative expression of Plin2 mRNA determined by RT-qPCR normalized to the expression of TATA-box binding protein (Tbp). Results are presented as means ± SEM (n = 3, #P < 0.05 vs. BSA). B: Expression of Plin2 and Plin3 proteins in myotubes. C: Lipid droplets (LDs) in Plin2+/+ and Plin2−/− myotubes were labeled with fluorescent dyes sequestering in neutral LDs (Bodipy 493/503, green) or nuclei (Hoechst 33342, blue). Representative confocal images are presented (×40 objective; inserted bars are 20 µm). D: Another set of images were acquired with a ×20 objective with an Olympus IX81 fluorescence microscope. Images were analyzed by Scan^R analytical software by comparing the number of stained LDs in relation to the number of nuclei per image, with an average total of 150 images per parameter. Results are presented as means ± SEM (n = 3, *P < 0.05 vs. Plin2 +/+). E–H: Myotubes were preincubated for 24 h with [1-14C]OA to label accumulated lipids. The content of radiolabeled TAG (E), DAG (F), FFA (G), and PL (H) in myotubes was determined by TLC and related to cellular protein content. The results are presented as means ± SEM (n = 6, *P < 0.05, **P < 0.01 vs. Plin2+/+).
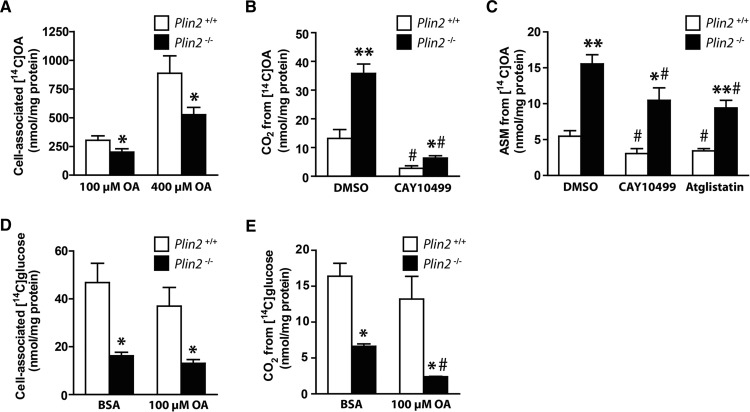
Absence of Plin2 reduced accumulation of lipids by increasing lipolysis
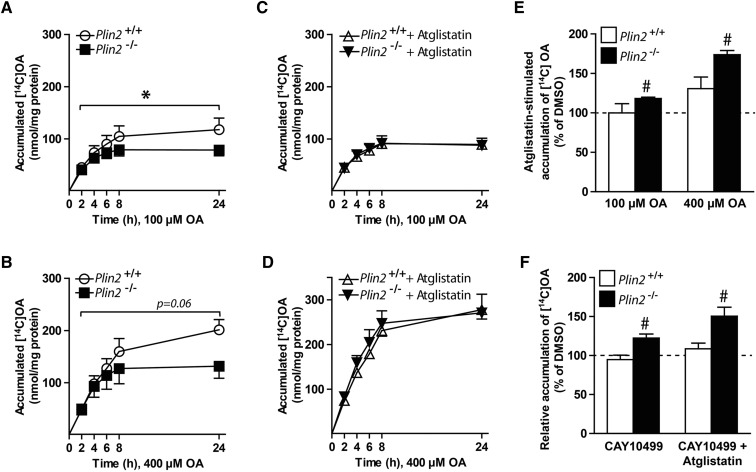
To mechanistically determine why myotubes lacking Plin2 accumulated less TAG-containing LDs, we followed OA accumulation in the myotubes for 24 h. During the first ∼4 h, Plin2+/+ and Plin2−/− myotubes accumulated similar levels of [1-14C]OA, but total accumulation after 24 h was lower in Plin2−/− myotubes incubated with 100 μM OA (Fig. 3A, P < 0.05) or 400 μM OA (Fig. 3B, P = 0.06) than in Plin2+/+ myotubes. In contrast, coincubation with Atglistatin (38), a reversible inhibitor of ATGL that catalyzes the first and rate-limiting step in lipolysis of TAG, increased accumulation of OA in Plin2−/− myotubes, in a manner similar to that of Plin2+/+ myotubes (Fig. 3C, D). Coincubation with Atglistatin increased cell-associated [1-14C]OA in Plin2−/− myotubes compared with DMSO with a more pronounced effect, with higher OA concentration (Fig. 3E). Myotubes cultured in 100 μM OA coincubated with the ATGL and HSL inhibitor CAY10499 (41, 42) alone or in combination with Atglistatin increased cell-associated [1-14C]OA only in Plin2−/− myotubes (Fig. 3F). These results suggest that there were constantly higher ATGL and HSL lipolytic activities in Plin2−/− myotubes than in Plin2+/+ myotubes.
Fig. 3.
Accumulation of oleic acid in Plin2+/+ and Plin2−/− myotubes. Myotubes were incubated with [1-14C]OA (100 or 400 µM) and accumulation over 24 h was determined with scintillation proximity assay. Accumulation was determined in presence of DMSO (0.1%) (A, B) or in presence of the adipose triglyceride lipase inhibitor (Atglistatin, 10 µM) (C, D). The results are presented as means ± SEM (n = 3, *P < 0.05 vs. Plin2+/+ across all points in time). E: The effect of Atglistatin on accumulation of [1-14C]OA assessed as an average of all time points from A–D. F: Cell-associated [1-14C]OA after 24 h incubation with 100 μM OA in presence of the lipase inhibitor (CAY10499, 10 μM) or CAY10499 combined with Atglistatin (10 μM). For E and F, the results are presented as means ± SEM normalized to DMSO treated Plin2+/+ myotubes (n = 3, #P < 0.05 vs. DMSO).
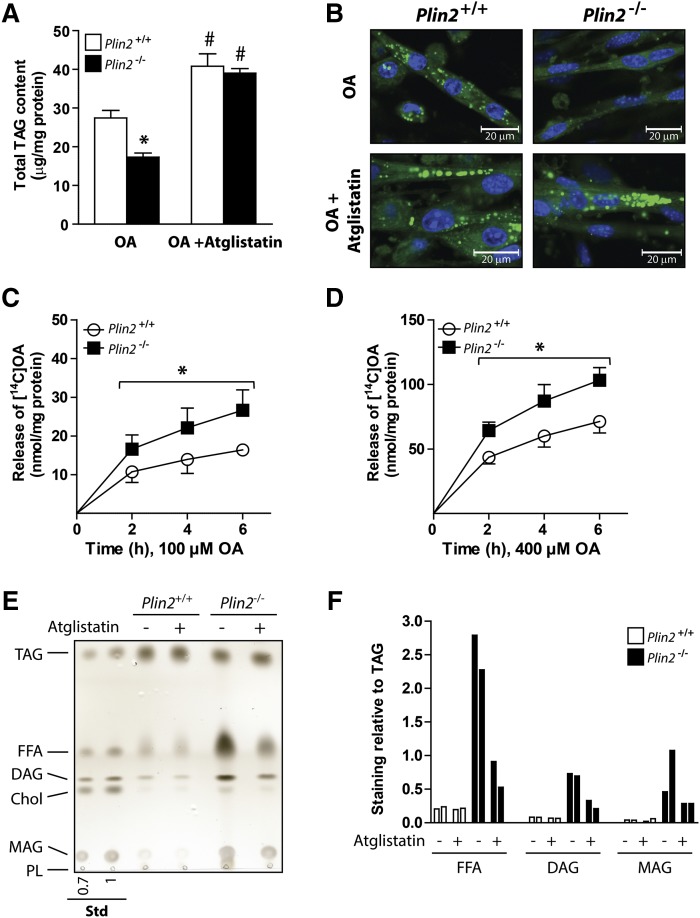
Lipolytic rates are difficult to normalize between two cell populations with differences in LD content. To overcome this, we utilized our established culture conditions using Atglistatin to minimize TAG differences between the Plin2+/+ and Plin2−/− myotubes. Whereas Plin2−/− myotubes cultured with 100 μM OA alone accumulated less TAG than did Plin2+/+ myotubes (Fig. 4A), a combination of OA and Atglistatin resulted in TAG levels (Fig. 4A) and LD content (Fig. 4B) in Plin2−/− myotubes that were similar to those in Plin2+/+ myotubes. These latter Plin2+/+ and Plin2−/− myotubes were then washed to remove the exogenous OA and Atglistatin before measurement of lipolysis. Lipolysis, measured as a loss of [1-14C]OA accumulated in the myotubes, was consistently overall higher in Plin2−/− myotubes than in Plin2+/+ myotubes (Fig. 4C, D). To compare the lipid composition of the stored LDs, we stimulated Plin2+/+ and Plin2−/− myotubes with 200 µM OA alone or coincubated with Atglistatin for 24 h prior to LD isolation. LD preparations containing the same amount of TAG (1 µg) were subsequently separated with TLC, and various lipid species were identified with copper sulfate staining. LDs isolated from OA-stimulated Plin2−/− myotubes stained stronger for lipolytic degradation products such as DAG, MAG, and FFA than did those from Plin2+/+ myotubes (Fig. 4E, F), whereas inhibition of lipolysis by coincubation with Atglistatin lowered the relative staining (Fig. 4F). These differences further supported the notion that disruption of Plin2 in cultured myotubes resulted in LDs prone to lipolytic attack.
Fig. 4.
Lipolysis in OA-loaded Plin2+/+ and Plin2−/− myotubes. Myotubes were incubated for 24 h with OA (100 μM) alone (0.1% DMSO) or in the presence of the adipose triglyceride lipase inhibitor Atglistatin (10 µM). A: Total triacylglycerol (TAG) content in Plin2+/+ and Plin2−/− myotubes. The results are presented as means ± SEM (n = 3, *P < 0.05 vs. Plin2+/+, #P < 0.05 vs. OA). B: Confocal pictures of Plin2+/+ and Plin2−/− myotubes. Fixated myotubes were labeled with fluorescent dyes sequestering in neutral lipid droplets (Bodipy 493/503, green) or in nuclei (Hoechst 33342, blue). C, D: Lipolysis (efflux) of OA after 24 h accumulation with [1-14C]OA (100 or 400 µM) in the presence of Atglistatin (10 µM). The results are presented as the release of accumulated [1-14C]OA to the medium at the various time points given as means ± SEM (n = 3–7, *P < 0.05 vs. Plin2+/+ across all time points). E: Cells were incubated with OA (200 μM) alone or in combination with Atglistatin (10 µM) for 24 h prior to isolation of lipid droplets and separation of lipid species with TLC. One representative of two independent experiments is shown. F: Staining intensities for the various bands in relation to the TAG signal (n = 2). Chol, cholesterol; Std, standard.
Absence of Plin2 increased FA oxidation
An important biological role for cytosolic LDs is to store energy-rich FAs that may be mobilized for energy production when needed. We were interested to determine whether the reduced LD stores in myotubes affected FA oxidation and thus incubated Plin2+/+ and Plin2−/− myotubes with [1-14C]OA for 24 h before CO2 production was captured over 4 h. Although cell-associated OA was lower in Plin2−/− myotubes than in Plin2+/+ myotubes (Fig. 5A), CO2 produced through oxidation of the stored intracellular lipids was higher in Plin2−/− than in Plin2+/+ myotubes when preincubated with 100 μM OA alone or in combination with the lipolysis inhibitor CAY10499 (Fig. 5B). Clearly, a large fraction of the produced CO2 originated from intracellular LDs in both Plin2+/+ and Plin2−/− myotubes, because CO2 production was drastically decreased in the presence of CAY10499. Similar results for increased CO2 production (i.e., FA oxidation) in Plin2−/− myotubes were obtained when cultures were preincubated with 400 μM OA (results not shown). Intermediary OA β-oxidation, measured as ASMs released from the myotubes into the cell media for 4 h, was also higher in Plin2−/− than in Plin2+/+ myotubes, regardless of treatment (Fig. 5C). ASMs were similarly decreased in the two myotube populations in the presence of the lipase inhibitors CAY10499 and Atglistatin (Fig. 5C).
Fig. 5.
Cell-associated radioactivity and oxidation of OA and glucose in Plin2+/+ and Plin2−/− myotubes. For measurement of cell-associated radioactivity and oxidation of fatty acids (A–C), myotubes were preincubated with [1-14C]OA (100 or 400 µM) alone (0.1% DMSO) or in the presence of CAY10499 (10 µM) or Atglistatin (10 µM) for 24 h and then subjected to FA substrate oxidation assay for 4 h. A: Total cell-associated [14C]radioactivity remaining in Plin2+/+ and Plin2−/− myotubes after 4 h. B: Released CO2 arising from accumulated [14C]radioactivity after 4 h. C: FA intermediary oxidation products measured as [14C]radiolabeled ASMs released from the myotubes into the cell media during 24 h incubation with [14C]OA. The results are presented as means ± SEM (n = 3–7, *P < 0.05 and **P < 0.01 vs. Plin2+/+, #P < 0.05 vs. DMSO). For measurement of cell-associated radioactivity and oxidation of glucose (D, E), myotubes were preincubated with BSA (40 μM, i.e., basal) or OA (100 µM) for 24 h, before myotubes were incubated with D-[14C(U)]glucose and subjected to glucose substrate oxidation assay for 4 h. D: Total cell-associated [14C]radioactivity accumulated in myotubes after 4 h. E: Released CO2 from oxidation of D-[14C(U)]glucose. Results are presented as means ± SEM (n = 3–6, *P < 0.05 vs. Plin2+/+, #P < 0.05 vs. BSA).
Absence of Plin2 in myotubes decreased both cell-associated glucose and glucose oxidation
Muscle contraction derives energy from stored glucose as glycogen and FA as TAG. Therefore, elevated FA oxidation in the absence of Plin2−/− may be coordinated with altered glucose metabolism. To measure glucose oxidation, we preincubated Plin2+/+ and Plin2−/− myotubes with BSA (40 µM) or OA (100 µM) for 24 h before CO2 production from D-[14C(U)]glucose was captured over 4 h. Cell-associated glucose was lower in Plin2−/− myotubes than in Plin2+/+ myotubes (Fig. 5D), as was the glucose oxidation (Fig. 5E). Furthermore, preincubation with OA for 24 h suppressed glucose oxidation approximately two-fold in Plin2−/− myotubes in relation to Plin2+/+ myotubes (Fig. 5E), consistent with an inverse correlation between energy derived from FA or glucose oxidation in myotubes. Collectively, these substrate oxidative assays reveal a shift in energy metabolism from utilization of glucose toward that of FAs in Plin2−/− myotubes.
Expression of genes involved in FA and glucose metabolism in the absence of Plin2
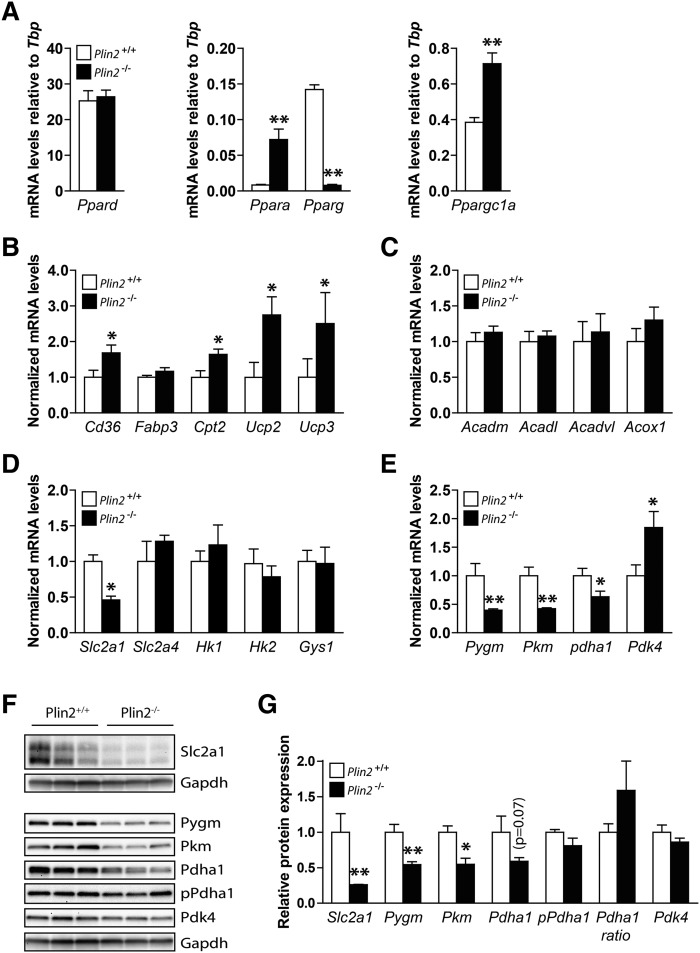
Prolonged changes in intracellular FA concentrations can directly affect the expression of several transcriptional factors and consequently the transcription of targeted gene families (43–45). First, we examined mRNA expression levels of Ppar members in Plin2+/+ and Plin2−/− myotubes (Fig. 6A), because these transcription factors are known to be activated by various lipid moieties (46, 47). Ppard, the predominant subtype in skeletal muscle, was expressed to similar levels in Plin2+/+ and Plin2−/− myotubes (Fig. 6A), whereas the subtypes Ppara and Pparg displayed completely opposite expression patterns (Fig. 6A). Ppara controls the expression of genes involved in FA oxidation (45) and was induced ∼10-fold in the Plin2−/− myotubes, with elevated FA levels compared with Plin2+/+ myotubes. Conversely, Pparg controls the expression of genes involved in FA storage and its expression was suppressed ∼15-fold in Plin2−/− myotubes compared with Plin2+/+ myotubes. Furthermore, the Ppar γ coactivator 1 α (Ppargc1a), a master regulator of mitochondrial biogenesis (48), was elevated ∼2-fold in Plin2−/− compared with Plin2+/+ myotubes (Fig. 6A). Others have reported that mice with transgenic overexpression of Ppara in muscle have increased FA oxidation and reduced glucose oxidation (49) and that overexpression of Ppargc1a in myotubes reduces glucose oxidation (50). The metabolic switches followed by overexpression of Ppara and Ppargc1a resemble what we observed in the Plin2−/− myotubes that also overexpress Ppara and Ppargc1a. These results imply that a Ppara/Ppargc1a component pathway is participatory in regulating FA versus glucose oxidative balance in myotubes lacking Plin2. Hence, we focused our continuing analyses on the expression of genes with linkage to FA and glucose oxidative pathways, which have been shown previously to be transcriptionally regulated by this transcription factor pathway.
Fig. 6.
Expression of genes involved in lipid metabolism in Plin2+/+ and Plin2−/− myotubes. Gene expression of various mRNAs in differentiated Plin2+/+ and Plin2−/− myotubes was analyzed by RT-qPCR and related to the expression of TATA-box binding protein (Tbp). A: Expression of transcription factors activated by FAs; Ppar α, γ and δ and Ppar γ coactivator 1 α (Ppargc1a). Results are presented as means ± SEM (n = 4, *P < 0.05 and **P < 0.01 vs. Plin2+/+). B: Expression of genes involved in lipid uptake and mitochondrial function; FA transporter/CD36 antigen (Cd36), Fabp3, Cpt2, and uncoupling proteins 2 and 3 (Ucp2 and Ucp3, respectively). C: Expression of genes catalyzing oxidation of FAs; mitochondrial Acadm, Acadl, Acadvl, and peroxisomal Acox1. D: Expression of genes involved in glucose uptake and storage; solute carrier family 2 member 1 and 4 (Slc2a1 and Slc2a4, respectively), hexokinase 1 and 2 (Hk1 and Hk2, respectively) and Gys1. E: Expression of genes involved in glycogen mobilization and glucose oxidation; muscle-associated glycogen phosphorylase(Pygm), muscle pyruvate kinase (Pkm), pyruvate dehydrogenase α 1 (Pdha1), and pyruvate dehydrogenase kinase 4 (Pdk4). Results in B–F are presented as means ± SEM normalized to the expression in Plin2+/+ myotubes (n = 5, *P < 0.05, **P < 0.01 vs. Plin2+/+). F: Protein content of Slc2a1, Pygm, Pkm, Pdha1, Pdha1 phosphorylated at Ser300 (pPdha1), and Pdk4 (n = 3). G: Protein content, related to Gapdh normalized to the expression levels in Plin2+/+ myotubes, or the ratio of pPdha1 against total Pdha1 (Pdha1 ratio). The results are presented as means ± SEM (n = 3, *P < 0.05 vs. Plin2+/+).
Among the genes important for FA oxidation, expression of plasma membrane FA transporter CD36 antigen (Cd36) and mitochondrial FA transporter carnitine palmitoyltransferase 2 (Cpt2) mRNAs were elevated in Plin2−/− compared with Plin2+/+ myotubes (Fig. 6B). Although no changes were observed for FA-binding protein 3 (Fabp3), higher mRNA expressions of uncoupling proteins 2 and 3 (Ucp2 and Ucp3), routinely associated with elevated FA oxidation, were also observed (Fig. 6B). Collectively, the expression of mRNAs for several enzymes oxidizing acyl-CoA to acetyl-CoA, such as the mitochondrial acyl-CoA dehydrogenases (Acadm, Acadl, Acadvl) and peroxisomal acyl-CoA oxidase 1 (Acox1), were unchanged in Plin2−/− compared with Plin2+/+ myotubes (Fig. 6C).
We next analyzed expression of genes involved in glucose oxidative pathways (Fig. 6D–F). Expression of solute carrier family 2 member 1 (Slc2a1), encoding for the basal glucose transporter 1 (Glut1), was ∼50% lower in Plin2−/− myotubes than in Plin2+/+ myotubes (Fig. 6D), whereas expression of Slc2a4, encoding for the insulin-responsive Glut4, was unaltered (Fig. 6D). Hexokinase 1 and 2 (Hk1 and Hk2), important for mobilization of glucose, and glycogen synthase 1 (Gys1) were also unaltered in expression (Fig. 6D). However, muscle-associated glycogen phosphorylase (Pygm), muscle pyruvate kinase (Pkm), and muscle pyruvate dehydrogenase α 1 (Pdha1), encoding for three enzymes that drive glucose oxidation via mobilization of pyruvate for the TCA cycle, were all less expressed in Plin2−/− than in Plin2+/+ myotubes (Fig. 6E). Pyruvate dehydrogenase kinase 4 (Pdk4), which phosphorylates Pdha1 at Ser300 and inhibits Pdha1 enzymatic activity, was expressed at higher levels in Plin2−/− than in Plin2+/+ myotubes (Fig. 6E).
We finally analyzed whether the altered gene transcript levels of the glucose transporter and the oxidative enzymes were reflected in protein level changes. Protein levels of Glut1, Pygm, Pkm, and to a lesser extent Pdha1 (P = 0.7), were all lower in Plin2−/− than in Plin2+/+ myotubes (Fig. 6F, G), and there was a further trended ratio for elevated levels of inactive phosphorylated Pdha1 (Ser300) to total Pdha1 (P = 0.1) in Plin2−/− than in Plin2+/+ myotubes. Taken together, the altered gene expressions in pathways for FA (Cd36) and glucose (Slc2a1) transport, glycogen degradation (Pygm) and glycolysis (Pkm, Phda1, and Pdk4) found in the Plin2−/− myotubes follows similar trends as for mice with muscle overexpression of Ppara (49) or Ppargc1a (50). Interestingly, all these models exhibit metabolic biases toward FA oxidation with a corresponding suppression of glucose oxidation. This change in oxidative balance may be further supported by the feedback inhibition of glucose oxidation by accumulated acyl-CoA substrates.
Absence of Plin2 had no impact on insulin-stimulated phosphorylation of Akt
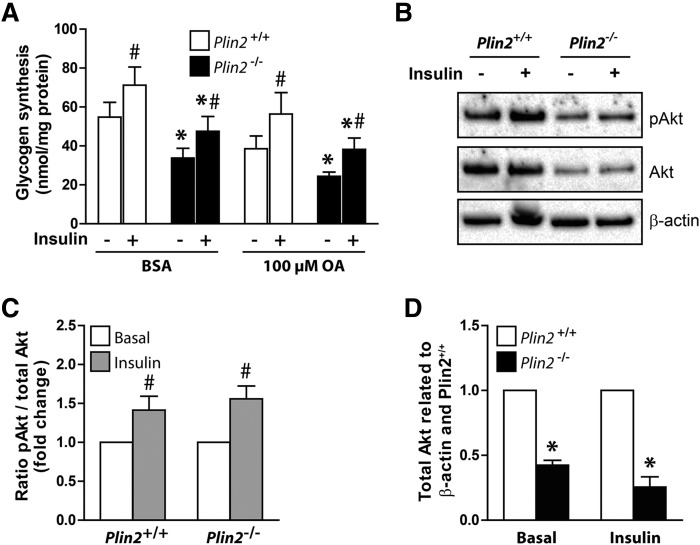
An important physiological role for skeletal muscle is whole-body glucose regulation by responding to insulin and thereby regulating active glucose transport into muscle cells. Mice with muscle-specific deletion of Pparg (51) or muscle-specific overexpression of Ppara (49) are both insulin resistant. Because Plin2−/− myotubes oxidized less glucose, had suppressed levels of Pparg, and had elevated levels of Ppara, we analyzed insulin response in Plin2+/+ and Plin2−/− myotubes. Compared with muscle tissue in vivo, cultured myotubes respond with only a modest increase in glucose uptake after insulin stimulation (52). Despite this reduced response, cultured myotubes provide a reliable model for testing factors affecting insulin signaling. First, we compared glycogen synthesis, which indirectly reflects cellular glucose uptake, after 24 h preincubation with either BSA (40 µM) or OA (100 µM), followed by incorporation of D-[14C(U)]glucose into glycogen for 3 h. The total amount of synthesized glycogen was lower in Plin2−/− than in Plin2+/+ myotubes (Fig. 7A) but the fold induction of glycogen synthesis by insulin stimulation was essentially the same (Fig. 7A). To further determine whether the altered FA and glucose metabolism in Plin2−/− myotubes affected insulin signaling, we determined insulin-stimulated mTORC2-mediated phosphorylation (Ser473) of the serine-threonine protein kinase Akt in the two cell populations. Akt phosphorylation in response to insulin was similar in Plin2+/+ and Plin2−/− myotubes (Fig. 7B, C), despite total Akt (Akt1-3) being expressed at lower levels in Plin2−/− myotubes than in Plin2+/+ myotubes (Fig. 7D). These findings suggest that although Plin2−/− myotubes have reduced basal glucose uptake compared with Plin+/+ myotubes, insulin signaling and insulin-stimulated glucose uptake are unaffected by removal of Plin2.
Fig. 7.
Effect of insulin in Plin2+/+ and Plin2−/− myotubes. A: Myotubes were preincubated with BSA (40 μM) or OA (100 µM) for 24 h. Glycogen synthesis was subsequently measured as incorporation of D-[14C(U)]glucose into glycogen in the absence or presence of insulin (100 nM) for 3 h. B: Myotubes were preincubated with OA (100 μM) for 24 h, then incubated in glucose-free medium supplemented with OA (100 μM) for 2 h, and subsequently incubated in medium containing glucose (5.5 mM) with or without insulin (100 nM) for 15 min. Cell samples were subjected for immunoblotting analysis with antibodies against total Akt (Akt1-3), pAkt (Ser473), and β-actin (housekeeping protein). Immunoblots from one representative experiment are shown. C: Ratio of pAkt/total Akt related to myotubes receiving no insulin. D: The content of total Akt related to β-actin normalized to expression levels in Plin2+/+ myotubes. The results are presented as means ± SEM (n = 4, *P < 0.05 vs. Plin2+/+ with the same treatment, #P < 0.05 vs. without insulin). pAkt, phosphorylated Akt.
DISCUSSION
Plin2 was the second Plin-family member identified as a LD surface-coating protein (53), but its function in whole-body lipid metabolism is still poorly understood. Most cell types transcribe a high pool of Plin2 mRNA but contain only a modest amount of the protein because of a rapid proteasomal degradation of the Plin2 pool that is not associated with LDs (31). Manipulation of Plin2 mRNA with temporal gene silencing (54) or overexpression of Plin2 (55) may therefore result in only modest changes in Plin2 protein levels. This phenomenon and a likely partial functional redundancy among some Plins (54) complicate studies on Plin2 function. The expression of Plin2 mRNA is induced and the produced Plin2 protein stabilized once LDs are formed in cells exposed to elevated levels of FAs. One example of this phenomenon is the large increase in Plin2 mRNA, Plin2 protein, and TAG-containing LDs in the liver of fasted mice (56). The significance of the fasting-induced hepatic Plin2 is unclear. Characterization of two separate Plin2 null mouse models demonstrates that lack of Plin2 protects against hepatosteatosis (24, 57, 58), emphasizing that Plin2 coating of LDs may not always be beneficial. The absence of Plin2 also suppresses obesity in mice on certain high-caloric diets (58), despite that Plin2 protein is normally not present on adipose LDs (53), arguing that a lack of Plin2 in other tissues has an impact on energy storage and metabolism.
The consequences for muscle energy metabolism upon Plin2 removal had not been fully investigated previously. To shed light on the function of Plin2 in skeletal muscle, we established myoblast cultures from Plin2+/+ and Plin2−/− mice. Given the known discrepancies in Plin2 mRNA and protein levels, our system with an absence of functional Plin2 differs from earlier studies based on siRNA knockdown or ectopic expression of Plin2 (54, 55). In a manner similar to that in the earlier studies based on the partial depletion of Plin2 (54, 55), we show in this study that myotubes that lack Plin2 have less accumulated TAG than wild-type myotubes. When Plin2−/− myotubes were exposed to Atglistatin, an inhibitor of ATGL that reduces lipolysis of TAG in LDs, TAG content was comparable to Plin2+/+ myotubes, whereas removal of the inhibitor resulted in a faster release of FAs from the accumulated TAG pool in Plin2−/−myotubes. The LDs retained in Plin2−/− myotubes furthermore consist of reduced amount of TAG in relation to a higher amount of lipolytic degradation products such as DAG, MAG, and FFA compared with Plin2+/+ myotubes. These results suggest that a lack of Plin2 on LDs does not affect FA uptake or incorporation into LDs per se, but its absence increases degradation of the TAG deposited within LDs through enhanced lipolysis. Our observations complement other studies, suggesting that overexpression of Plin2 in embryonic kidney cells reduced the interaction of lipases with LDs (59) or that a combined Plin2 and Plin3 knockdown increased lipolysis in hepatocytes (54). Consistent with a model in which reduced TAG in Plin2−/− myotubes is caused by elevated lipolytic activity, skeletal muscles of ATGL null mice show increased TAG accumulation and reduced lipolysis (60). In our study, inhibitors of ATGL (i.e., Atglistatin) or ATGL and HSL (i.e. CAY10499) enhanced LD content and elevated total accumulated TAG with considerably higher potency in Plin2−/− myotubes than in Plin2+/+ myotubes. These results support the notion that ATGL and HSL have limited access to LDs or have reduced activity in myotubes expressing Plin2 at the LD surface. Taken together, our results establish that one functional role for Plin2 in muscle is to protect TAG stored within LDs from lipases such as ATGL or HSL.
Metabolic consequences upon Plin2 removal may at least partly be caused by increased efflux of FAs from LDs in Plin2−/− myotubes. We observed a profound increase in OA oxidation and a reduction in glucose oxidation in Plin2−/− myotubes. In line with a functional role of Plin2 as a protector of TAG stored within LDs, we observed that Plin2−/− myotubes exposed to OA accumulated less DAG. Previous experiments in myotubes depleted of Plin2 by siRNA showed a more modest increase in palmitic acid (PA) oxidation but also an increase in DAG (55). The incomplete removal of the Plin2 protein in Plin2 siRNA knockdown myotubes compared with an absence of Plin2 in our Plin2−/− myotubes likely contributes to these discrepancies. The different types of labeled FAs used may also be significant. PA is accumulated to a lower extent into LDs than is OA in myotubes (61). The strong repression of FA oxidation observed in myotubes exposed to the dual ATGL and HSL lipase inhibitor (CAY10499) demonstrates that OA converted into CO2 derived mainly from LDs in our experiments. Consistent with LDs being an important source of substrate for FA oxidation, other investigators have reported that preservation of LDs by knockdown of the ATGL coactivator CGI-58 decreased lipolysis and FA oxidation while increasing glucose oxidation and incorporation into glycogen in cultured myotubes (62).
The enhanced release of FAs from the LDs observed in Plin2−/− myotubes may support inhibition of glucose oxidation and stimulation of FA oxidation via mutual inhibition of substrate metabolism in the glucose-FA cycle (43). A similar enhanced FA oxidation is found in cultured hepatocytes isolated from Plin2−/− mice (Y.K.L., K.T.D., and A.R.K., unpublished observations). Our data define a mechanistic function for Plin2 to regulate TAG stores and FA oxidation, in an antagonistic pathway that balances glucose oxidation. The observed alterations in expressions of genes facilitating glucose uptake and oxidation are consistent with the glucose-FA cycle described by Randle, which defines a metabolic competition between glucose and FAs, in which enhanced FA availability suppresses glucose oxidation. We observed decreased levels of proteins involved both in glucose uptake (Slc2a1/Glut1) and in glucose oxidation in Plin2−/− myotubes, consistent with studies examining how elevated circulating FAs inhibit glucose oxidation in human skeletal muscle (63). To facilitate such an energy substrate switch, transcription factors known to stimulate expression of genes driving FA oxidation such as Ppara and Ppargc1a (64) were expressed at higher levels in Plin2−/− myotubes, whereas Pparg, which stimulates expression of genes promoting lipid storage (65), were suppressed. Elevated expression of Ppara has previously been noted in livers of Plin2−/− mice (58), consistent with an opposite repression of Ppara expression in muscle overexpressing Plin2 (55). Interestingly, we found that our cultured Plin2−/− myotubes resemble the FA oxidative phenotype and repressed expression of glucose oxidative genes characteristic of muscle cells that were engineered to overexpress either Ppara (49) or Ppargc1a (50). We also observed enhanced expression of uncoupling proteins, which disconnects energy production from oxidative flux when FA levels are too elevated. Taken together, our data suggest an increased acyl-CoA to acetyl-CoA flux in Plin2−/− myotubes, as modeled in the Randle cycle, serving to metabolically inhibit and suppress expression of glycolytic enzymes and reduce glucose oxidation and favor FA oxidation.
With respect to insulin responsiveness, Plin2 loss-of-function studies in liver or whole animals have shown contradictory results (54, 57, 66, 67). In our study, Plin2+/+ and Plin2−/− myotubes had comparable insulin-stimulated responses judged by the increase in total Akt phosphorylation and glycogen synthesis, whereas glucose accumulation and oxidation were markedly decreased in Plin2−/− myotubes. Normal insulin signaling was also reported after Plin2 knockdown in C2C12 myotubes (55). It has been proposed that accumulation of lipotoxic intermediates in cells exposed to high levels of FAs may interfere with insulin signaling because of a mismatch among lipid storage, lipolysis, and oxidation (68). Enhanced FA oxidation in Plin2−/− myotubes may therefore act as a compensatory mechanism to handle the increased availability of FAs released from the more rapidly degrading LDs. It remains to be investigated further whether the reduced expressions of Akt proteins, measured with a pan-Akt antibody, mechanistically contribute to reduced glucose metabolism in Plin2−/− myotubes. The three different Akt isoforms (Akt1-3) have distinct roles, in which Akt2 is particularly involved in the maintenance of glucose homeostasis (69). Further studies are required to clarify whether a particular Akt protein is reduced in Plin2−/− myotubes and signals to reduce glucose uptake.
In summary, by characterizing myotubes established from Plin2+/+ and Plin2−/− mice, we demonstrate that loss of Plin2 results in myotubes with reduced accumulation of neutral lipids in LDs due to elevated lipolysis. Similar to what has been shown previously for Plin1 and Plin5 (21, 70), this establishes a functional role of Plin2 as a protector against lipolytic degradation of LDs. The increased efflux of FAs from LDs in Plin2−/− myotubes is likely to contribute to a metabolic shift in energy metabolism from utilization of glucose toward FAs. Such a shift may be facilitated by altered transcription of metabolic regulators in Plin2−/− myotubes. Our results demonstrate that Plin2 is essential for balancing the pool of LDs in cultured myotubes to avoid an uncontrolled hydrolysis of intracellular TAG and altered energy metabolism caused by increased release of FAs from LDs.
Acknowledgments
The authors thank Camilla Stensrud and Prabhat Khanal for technical assistance and members of the Rustan, Thoresen, and Dalen laboratories for scientific discussions. The authors thank the NORMIC-UiO imaging platform, Department of Biosciences, University of Oslo, for support, use of equipment, and excellent technical assistance.
Footnotes
Abbreviations:
- ASM
- acid-soluble metabolite
- Acox1
- acyl-CoA oxidase 1
- ATGL
- adipose triglyceride lipase
- Akt
- thymoma viral proto-oncogene/AKT serine/threonine kinase
- Cpt2
- carnitine palmitoyltransferase 2
- CE
- cholesteryl ester
- Cd36
- CD36 antigen/FA transporter
- DAG
- diacylglycerol
- Fabp3
- FA binding protein 3
- Glut1 and Glut4
- glucose transporter 1 and 4
- Pygm
- glycogen phosphorylase, muscle-associated
- Gys1
- glycogen synthase 1
- Hk1 and Hk2
- hexokinase 1 and 2
- HSL
- hormone sensitive lipase
- LD
- lipid droplet
- Acadl
- acyl-CoA dehydrogenase, long-chain
- Acadm
- acyl-CoA dehydrogenase, C-4 to C-12 straight chain
- Acadvl
- acyl-CoA dehydrogenase, very long chain
- MAG
- monoacylglycerol
- OA
- oleic acid
- Ppargc1a
- PPAR coactivator 1 alpha
- PL
- phospholipid
- Pdk4
- pyruvate dehydrogenase kinase isozyme 4
- Pdha1
- pyruvate dehydrogenase alpha 1
- Pkm
- pyruvate kinase muscle
- Plin
- perilipin
- Slc2a1 and Slc2a4
- solute carrier family 2 member 1 and 4
- TAG
- triacylglycerol Tbp, TATA box binding protein
- Ucp2 and Ucp3
- uncoupling protein 2 and 3
This work was supported by grants from the Medical Faculty at the University of Oslo, Henning and the Johan Throne-Holst Foundation (Y.L. and K.T.D.), the Intramural Research Programs of the National Institutes of Health (NIH), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (K.T.D. and A.R.K.), the Novo Nordisk Foundation (K.T.D.), Aktieselskabet Freia Chocolade Fabrik’s Medical Foundation, and Anders Jahre’s Foundation (K.T.D., A.C.R., G.H.T.). The authors declare that they have no conflicts of interest with the contents of this article.
REFERENCES
- 1.Hue L., and Taegtmeyer H.. 2009. The Randle cycle revisited: a new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 297: E578–E591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckardt K., Taube A., and Eckel J.. 2011. Obesity-associated insulin resistance in skeletal muscle: role of lipid accumulation and physical inactivity. Rev. Endocr. Metab. Disord. 12: 163–172. [DOI] [PubMed] [Google Scholar]
- 3.Samuel V. T., and Shulman G. I.. 2012. Mechanisms for insulin resistance: common threads and missing links. Cell. 148: 852–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan D. A., Lillioja S., Kriketos A. D., Milner M. R., Baur L. A., Bogardus C., Jenkins A. B., and Storlien L. H.. 1997. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 46: 983–988. [DOI] [PubMed] [Google Scholar]
- 5.Jacob S., Machann J., Rett K., Brechtel K., Volk A., Renn W., Maerker E., Matthaei S., Schick F., Claussen C. D., et al. 1999. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 48: 1113–1119. [DOI] [PubMed] [Google Scholar]
- 6.Goodpaster B. H., Theriault R., Watkins S. C., and Kelley D. E.. 2000. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 49: 467–472. [DOI] [PubMed] [Google Scholar]
- 7.van Loon L. J., Koopman R., Manders R., van der Weegen W., van Kranenburg G. P., and Keizer H. A.. 2004. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am. J. Physiol. Endocrinol. Metab. 287: E558–E565. [DOI] [PubMed] [Google Scholar]
- 8.Sztalryd C., and Kimmel A. R.. 2014. Perilipins: lipid droplet coat proteins adapted for tissue-specific energy storage and utilization, and lipid cytoprotection. Biochimie. 96: 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodpaster B. H., He J., Watkins S., and Kelley D. E.. 2001. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 86: 5755–5761. [DOI] [PubMed] [Google Scholar]
- 10.Amati F., Dube J. J., Alvarez-Carnero E., Edreira M. M., Chomentowski P., Coen P. M., Switzer G. E., Bickel P. E., Stefanovic-Racic M., Toledo F. G., et al. 2011. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 60: 2588–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Høeg L., Roepstorff C., Thiele M., Richter E. A., Wojtaszewski J. F., and Kiens B.. 2009. Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. J. Appl. Physiol. 107: 824–831. [DOI] [PubMed] [Google Scholar]
- 12.Coen P. M., and Goodpaster B. H.. 2012. Role of intramyocelluar lipids in human health. Trends Endocrinol. Metab. 23: 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brasaemle D. L. 2007. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48: 2547–2559. [DOI] [PubMed] [Google Scholar]
- 14.Bickel P. E., Tansey J. T., and Welte M. A.. 2009. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim. Biophys. Acta. 1791: 419–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 306: 1383–1386. [DOI] [PubMed] [Google Scholar]
- 16.Haemmerle G., Zimmermann R., Hayn M., Theussl C., Waeg G., Wagner E., Sattler W., Magin T. M., Wagner E. F., and Zechner R.. 2002. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 277: 4806–4815. [DOI] [PubMed] [Google Scholar]
- 17.Fredrikson G., Tornqvist H., and Belfrage P.. 1986. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim. Biophys. Acta. 876: 288–293. [DOI] [PubMed] [Google Scholar]
- 18.Kimmel A. R., and Sztalryd C.. 2016. The perilipins: major cytosolic lipid droplet-associated proteins and their roles in cellular lipid storage, mobilization, and systemic homeostasis. Annu. Rev. Nutr. 36: 471–509. [DOI] [PubMed] [Google Scholar]
- 19.Kimmel A. R., Brasaemle D. L., McAndrews-Hill M., Sztalryd C., and Londos C.. 2010. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J. Lipid Res. 51: 468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalen K. T., Dahl T., Holter E., Arntsen B., Londos C., Sztalryd C., and Nebb H. I.. 2007. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim. Biophys. Acta. 1771: 210–227. [DOI] [PubMed] [Google Scholar]
- 21.Sztalryd C., Xu G., Dorward H., Tansey J. T., Contreras J. A., Kimmel A. R., and Londos C.. 2003. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J. Cell Biol. 161: 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Botas J., Anderson J. B., Tessier D., Lapillonne A., Chang B. H., Quast M. J., Gorenstein D., Chen K. H., and Chan L.. 2000. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat. Genet. 26: 474–479. [DOI] [PubMed] [Google Scholar]
- 23.Tansey J. T., Sztalryd C., Gruia-Gray J., Roush D. L., Zee J. V., Gavrilova O., Reitman M. L., Deng C. X., Li C., Kimmel A. R., et al. 2001. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. USA. 98: 6494–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang B. H., Li L., Paul A., Taniguchi S., Nannegari V., Heird W. C., and Chan L.. 2006. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol. Cell. Biol. 26: 1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W., Chang B., Wu X., Li L., Sleeman M., and Chan L.. 2013. Inactivation of Plin4 downregulates Plin5 and reduces cardiac lipid accumulation in mice. Am. J. Physiol. Endocrinol. Metab. 304: E770–E779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuramoto K., Okamura T., Yamaguchi T., Nakamura T. Y., Wakabayashi S., Morinaga H., Nomura M., Yanase T., Otsu K., Usuda N., et al. 2012. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J. Biol. Chem. 287: 23852–23863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh K., Lee Y. K., Londos C., Raaka B. M., Dalen K. T., and Kimmel A. R.. 2012. Perilipin family members preferentially sequester to either triacylglycerol-specific or cholesteryl-ester-specific intracellular lipid storage droplets. J. Cell Sci. 125: 4067–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw C. S., Sherlock M., Stewart P. M., and Wagenmakers A. J.. 2009. Adipophilin distribution and colocalization with lipid droplets in skeletal muscle. Histochem. Cell Biol. 131: 575–581. [DOI] [PubMed] [Google Scholar]
- 29.Phillips S. A., Choe C. C., Ciaraldi T. P., Greenberg A. S., Kong A. P., Baxi S. C., Christiansen L., Mudaliar S. R., and Henry R. R.. 2005. Adipocyte differentiation-related protein in human skeletal muscle: relationship to insulin sensitivity. Obes. Res. 13: 1321–1329. [DOI] [PubMed] [Google Scholar]
- 30.Minnaard R., Schrauwen P., Schaart G., Jorgensen J. A., Lenaers E., Mensink M., and Hesselink M. K.. 2009. Adipocyte differentiation-related protein and OXPAT in rat and human skeletal muscle: involvement in lipid accumulation and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 94: 4077–4085. [DOI] [PubMed] [Google Scholar]
- 31.Xu G., Sztalryd C., Lu X., Tansey J. T., Gan J., Dorward H., Kimmel A. R., and Londos C.. 2005. Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J. Biol. Chem. 280: 42841–42847. [DOI] [PubMed] [Google Scholar]
- 32.Hessvik N. P., Boekschoten M. V., Baltzersen M. A., Kersten S., Xu X., Andersen H., Rustan A. C., and Thoresen G. H.. 2010. LXR{beta} is the dominant LXR subtype in skeletal muscle regulating lipogenesis and cholesterol efflux. Am. J. Physiol. Endocrinol. Metab. 298: E602–E613. [DOI] [PubMed] [Google Scholar]
- 33.Bindesbøll C., Berg O., Arntsen B., Nebb H. I., and Dalen K. T.. 2013. Fatty acids regulate perilipin5 in muscle by activating PPARδ. J. Lipid Res. 54: 1949–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miura S., Gan J. W., Brzostowski J., Parisi M. J., Schultz C. J., Londos C., Oliver B., and Kimmel A. R.. 2002. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J. Biol. Chem. 277: 32253–32257. [DOI] [PubMed] [Google Scholar]
- 35.Ye J., Coulouris G., Zaretskaya I., Cutcutache I., Rozen S., and Madden T. L.. 2012. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 13: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hessvik N. P., Bakke S. S., Fredriksson K., Boekschoten M. V., Fjorkenstad A., Koster G., Hesselink M. K., Kersten S., Kase E. T., Rustan A. C., et al. 2010. Metabolic switching of human myotubes is improved by n-3 fatty acids. J. Lipid Res. 51: 2090–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wensaas A. J., Rustan A. C., Lovstedt K., Kull B., Wikstrom S., Drevon C. A., and Hallen S.. 2007. Cell-based multiwell assays for the detection of substrate accumulation and oxidation. J. Lipid Res. 48: 961–967. [DOI] [PubMed] [Google Scholar]
- 38.Mayer N., Schweiger M., Romauch M., Grabner G. F., Eichmann T. O., Fuchs E., Ivkovic J., Heier C., Mrak I., Lass A., et al. 2013. Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat. Chem. Biol. 9: 785–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weerheim A. M., Kolb A. M., Sturk A., and Nieuwland R.. 2002. Phospholipid composition of cell-derived microparticles determined by one-dimensional high-performance thin-layer chromatography. Anal. Biochem. 302: 191–198. [DOI] [PubMed] [Google Scholar]
- 40.Gaster M., Rustan A. C., Aas V., and Beck-Nielsen H.. 2004. Reduced lipid oxidation in skeletal muscle from type 2 diabetic subjects may be of genetic origin: evidence from cultured myotubes. Diabetes. 53: 542–548. [DOI] [PubMed] [Google Scholar]
- 41.Grisouard J., Bouillet E., Timper K., Radimerski T., Dembinski K., Frey D. M., Peterli R., Zulewski H., Keller U., Muller B., et al. 2012. Both inflammatory and classical lipolytic pathways are involved in lipopolysaccharide-induced lipolysis in human adipocytes. Innate Immun. 18: 25–34. [DOI] [PubMed] [Google Scholar]
- 42.Iglesias J., Lamontagne J., Erb H., Gezzar S., Zhao S., Joly E., Truong V. L., Skorey K., Crane S., Madiraju S. R., et al. 2016. Simplified assays of lipolysis enzymes for drug discovery and specificity assessment of known inhibitors. J. Lipid Res. 57: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Randle P. J., Garland P. B., Hales C. N., and Newsholme E. A.. 1963. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1: 785–789. [DOI] [PubMed] [Google Scholar]
- 44.Jump D. B. 2004. Fatty acid regulation of gene transcription. Crit. Rev. Clin. Lab. Sci. 41: 41–78. [DOI] [PubMed] [Google Scholar]
- 45.Georgiadi A., and Kersten S.. 2012. Mechanisms of gene regulation by fatty acids. Adv. Nutr. 3: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poulsen L., Siersbaek M., and Mandrup S.. 2012. PPARs: fatty acid sensors controlling metabolism. Semin. Cell Dev. Biol. 23: 631–639. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura M. T., Yudell B. E., and Loor J. J.. 2014. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 53: 124–144. [DOI] [PubMed] [Google Scholar]
- 48.Lin J., Wu H., Tarr P. T., Zhang C. Y., Wu Z., Boss O., Michael L. F., Puigserver P., Isotani E., Olson E. N., et al. 2002. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 418: 797–801. [DOI] [PubMed] [Google Scholar]
- 49.Finck B. N., Bernal-Mizrachi C., Han D. H., Coleman T., Sambandam N., LaRiviere L. L., Holloszy J. O., Semenkovich C. F., and Kelly D. P.. 2005. A potential link between muscle peroxisome proliferator- activated receptor-alpha signaling and obesity-related diabetes. Cell Metab. 1: 133–144. [DOI] [PubMed] [Google Scholar]
- 50.Wende A. R., Huss J. M., Schaeffer P. J., Giguere V., and Kelly D. P.. 2005. PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism. Mol. Cell. Biol. 25: 10684–10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hevener A. L., He W., Barak Y., Le J., Bandyopadhyay G., Olson P., Wilkes J., Evans R. M., and Olefsky J.. 2003. Muscle-specific Pparg deletion causes insulin resistance. Nat. Med. 9: 1491–1497. [DOI] [PubMed] [Google Scholar]
- 52.Sarabia V., Lam L., Burdett E., Leiter L. A., and Klip A.. 1992. Glucose transport in human skeletal muscle cells in culture. Stimulation by insulin and metformin. J. Clin. Invest. 90: 1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brasaemle D. L., Barber T., Wolins N. E., Serrero G., Blanchette-Mackie E. J., and Londos C.. 1997. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J. Lipid Res. 38: 2249–2263. [PubMed] [Google Scholar]
- 54.Bell M., Wang H., Chen H., McLenithan J. C., Gong D. W., Yang R. Z., Yu D., Fried S. K., Quon M. J., Londos C., et al. 2008. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 57: 2037–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bosma M., Hesselink M. K., Sparks L. M., Timmers S., Ferraz M. J., Mattijssen F., van Beurden D., Schaart G., de Baets M. H., Verheyen F. K., et al. 2012. Perilipin 2 improves insulin sensitivity in skeletal muscle despite elevated intramuscular lipid levels. Diabetes. 61: 2679–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalen K. T., Ulven S. M., Arntsen B. M., Solaas K., and Nebb H. I.. 2006. PPARalpha activators and fasting induce the expression of adipose differentiation-related protein in liver. J. Lipid Res. 47: 931–943. [DOI] [PubMed] [Google Scholar]
- 57.Chang B. H., Li L., Saha P., and Chan L.. 2010. Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis, and improves whole body insulin resistance in leptin-deficient mice. J. Lipid Res. 51: 2132–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McManaman J. L., Bales E. S., Orlicky D. J., Jackman M., MacLean P. S., Cain S., Crunk A. E., Mansur A., Graham C. E., Bowman T. A., et al. 2013. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J. Lipid Res. 54: 1346–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Listenberger L. L., Ostermeyer-Fay A. G., Goldberg E. B., Brown W. J., and Brown D. A.. 2007. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J. Lipid Res. 48: 2751–2761. [DOI] [PubMed] [Google Scholar]
- 60.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737. [DOI] [PubMed] [Google Scholar]
- 61.Bakke S. S., Moro C., Nikolic N., Hessvik N. P., Badin P. M., Lauvhaug L., Fredriksson K., Hesselink M. K., Boekschoten M. V., Kersten S., et al. 2012. Palmitic acid follows a different metabolic pathway than oleic acid in human skeletal muscle cells; lower lipolysis rate despite an increased level of adipose triglyceride lipase. Biochim. Biophys. Acta. 1821: 1323–1333. [DOI] [PubMed] [Google Scholar]
- 62.Badin P. M., Loubiere C., Coonen M., Louche K., Tavernier G., Bourlier V., Mairal A., Rustan A. C., Smith S. R., Langin D., et al. 2012. Regulation of skeletal muscle lipolysis and oxidative metabolism by the co-lipase CGI-58. J. Lipid Res. 53: 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roden M., Price T. B., Perseghin G., Petersen K. F., Rothman D. L., Cline G. W., and Shulman G. I.. 1996. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Invest. 97: 2859–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nikolić N., Rhedin M., Rustan A. C., Storlien L., Thoresen G. H., and Strömstedt M.. 2012. Overexpression of PGC-1α increases fatty acid oxidative capacity of human skeletal muscle cells. Biochem. Res. Int. 2012: 714074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Azhar S. 2010. Peroxisome proliferator-activated receptors, metabolic syndrome and cardiovascular disease. Future Cardiol. 6: 657–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imai Y., Varela G. M., Jackson M. B., Graham M. J., Crooke R. M., and Ahima R. S.. 2007. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology. 132: 1947–1954. [DOI] [PubMed] [Google Scholar]
- 67.Varela G. M., Antwi D. A., Dhir R., Yin X., Singhal N. S., Graham M. J., Crooke R. M., and Ahima R. S.. 2008. Inhibition of ADRP prevents diet-induced insulin resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G621–G628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moro C., Bajpeyi S., and Smith S. R.. 2008. Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 294: E203–E213. [DOI] [PubMed] [Google Scholar]
- 69.Cho H., Mu J., Kim J. K., Thorvaldsen J. L., Chu Q., Crenshaw E. B. III, Kaestner K. H., Bartolomei M. S., Shulman G. I., and Birnbaum M. J.. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science. 292: 1728–1731. [DOI] [PubMed] [Google Scholar]
- 70.Pollak N. M., Jaeger D., Kolleritsch S., Zimmermann R., Zechner R., Lass A., and Haemmerle G.. 2015. The interplay of protein kinase A and perilipin 5 regulates cardiac lipolysis. J. Biol. Chem. 290: 1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]