Abstract
Objective
Paranasal sinus mucoceles are benign cystic lesions originating from sinus mucosa that can impinge on adjacent orbital structures, causing ophthalmic sequelae such as decreased visual acuity. Definitive treatment requires surgery. We present the first meta-analysis quantifying the effect of preoperative visual function and time to surgery on postoperative visual acuity outcomes.
Data Sources
PubMed, Ovid, Embase, Web of Science, and the Cochrane Library.
Methods
Two independent authors systematically reviewed articles describing outcomes after endoscopic sinus surgery for paranasal sinus mucoceles presenting with visual loss. Available data from case reports and series were combined to analyze the associations among preoperative visual acuity, time to surgery, and postoperative outcomes.
Results
Eighty-five studies were included that provided data on 207 patients. The average presenting visual acuity was 1.57 logMAR (logarithm of the minimum angle of resolution), and the average postoperative visual acuity was 0.21 logMAR, with visual improvement in 71.5% of cases. Preoperative visual acuity ≥1.52 logMAR correlated with postoperative improvement >1 logMAR (R = 0.4887, P<.0001). A correlation was found between a time to surgery <6 days and postoperative improvement (R = 0.297, P <.0001). Receiver operator curve analysis of these thresholds demonstrated a moderately accurate prognostic ability (area under the curve: 75.1 for preoperative visual acuity and 73.1 for time to surgery).
Conclusion
Visual loss resulting from paranasal sinus mucoceles is potentially reversible in most cases, even those presenting with poor vision. When possible, surgery should be performed promptly after diagnosis, but emergency surgery does not appear to be necessary for vision restoration.
Keywords: mucocele, endoscopic sinus surgery, vision loss, blindness, FESS, ESS, orbital complication
Paranasal sinus mucoceles are benign cystic lesions originating from sinus mucosa lined by respiratory epithelium. While their etiology is sometimes debated, they typically arise secondary to inflammation, trauma, scarring, or processes causing obstruction of sinus ostia and circulation. Obstruction leads to gradual accumulation of secretions within the lesion, causing gradual expansion.1 Mucocele contents can vary, ranging from clear mucus to thick purulent material, contributing to a distinct radiographic appearance. Additional predisposing factors to mucocele development include cranial dysplasias, fibro-osseous lesions, chronic rhinosinusitis, prior sinus surgery, facial trauma or fractures, and sinonasal manifestations of systemic disease.2 Diagnosis is usually made on imaging: computed tomography may illustrate an expansile soft tissue lesion with surrounding bony remodeling and thinning, and magnetic resonance imaging (MRI) may show variable degrees of proteinaceous fluid and delineate the relationship among the mucocele, brain tissue, the orbit, and other soft tissues.1 During early development, mucocele content is typically aqueous, resulting in hypointensity on T1-weighted MRI and hyperintensity on T2-weighted MRI. With time, proteinaceous material may accumulate, causing hyperintensity on T1- and T2-weighted images.3
Mucoceles most commonly occur in the frontal sinus but can also appear in the ethmoid, sphenoid, and maxillary sinuses. Due to their expansile nature and close proximity to the orbit, mucoceles can cause a variety of local ophthalmologic complications, including periorbital swelling and pain, proptosis or globe displacement, decreased ocular motility, diplopia, and optic neuropathy (including decreased color perception, visual acuity, and visual field loss).4 While frontal, anterior ethmoid, and maxillary sinus mucoceles show a predilection toward causing proptosis and periorbital pain, posterior ethmoid and sphenoid mucoceles more often cause changes in visual acuity and impaired ocular motility.5
While a number of surgical approaches have been employed in the management of mucoceles, the current treatment of choice is endoscopic sinus surgery to decompress and marsupialize these lesions.2 This surgical approach offers a quick, minimally invasive, well-tolerated, and highly successful strategy when compared with open approaches to mucoceles.6 Given the relative infrequency of mucoceles associated with vision loss, only a few case series exist in the literature documenting the presentation and outcomes of these lesions. There are very limited aggregate data regarding visual outcomes after intervention. Despite proposed grading systems to stratify patient risks,7 little is known about how factors such as degree of vision loss at presentation and time to surgery affect postoperative visual acuity outcomes. Because such questions have significant implications for patients and surgeons, we conducted a systematic review of the medical literature with a meta-analysis to evaluate visual outcomes after endoscopic surgical management of paranasal sinus mucoceles associated with vision loss.
Methods
A systematic review was performed according to the PRISMA statement guidelines (Preferred Reporting Items for Systematic Reviews and Meta-analyses),8 with details as follows.
Literature Search
PubMed, Ovid, Embase, Web of Science, and the Cochrane Library were searched on June 1, 2016, with the following search terms:
PubMed: (mucocele OR (sinus mucocele) OR (paranasal sinus mucocele)) AND ((vision) OR (visual) OR (vision loss) OR (visual acuity) OR (optic neuropathy) OR (optic neuritis)) AND (“humans”[MeSH Terms] AND English[lang] AND “adult”[MeSH Terms])
Ovid: ((mucocele or sinus mucocele or paranasal sinus mucocele) and (vision or visual or vision loss or visual acuity or optic neuropathy or optic neuritis)).mp
Embase: ‘mucocele’/exp OR mucocele OR (sinus AND (‘mucocele’/exp OR mucocele)) OR (paranasal AND sinus AND (‘mucocele’/exp OR mucocele)) AND (‘vision’/exp OR vision OR visual OR (‘vision’/exp OR vision AND loss) OR (visual AND acuity) OR (optic AND (‘neuropathy’/exp OR neuropathy)) OR (optic AND (‘neuritis’/exp OR neuritis))) AND [english]/lim AND ([young adult]/lim OR [adult]/lim OR [middle aged]/lim OR [aged]/lim OR [very elderly]/lim)
Web of Science: ((mucocele OR (sinus mucocele) OR (paranasal sinus mucocele)) AND ((vision) OR (visual) OR (vision loss) OR (visual acuity) OR (optic neuropathy) OR (optic neuritis)))
Cochrane Library: (mucocele OR (sinus mucocele) OR (paranasal sinus mucocele)) AND ((vision) OR (visual) OR (vision loss) OR (visual acuity) OR (optic neuropathy) OR (optic neuritis))
The search was limited to adults (>18 years of age) and articles written in English.
Study Selection
Studies were selected according to a 2-step process: screening the article titles and abstracts for relevance, followed by inclusion or exclusion based on predefined criteria. Inclusion criteria consisted of diagnosis of mucocele, documented preoperative visual acuity, endoscopic surgical management, and documented postoperative visual acuity. Studies without a diagnosis of mucocele, those without associated vision loss, those based on external surgical approaches or observation, or those without reported preoperative or postoperative visual acuity were excluded from the current analysis. Two authors (L.M.Z. and V.R.R.) independently performed the screening and selection process, and any disagreements about article inclusion or exclusion were discussed until consensus was reached. For articles not available through online university databases, attempts were made to obtain these through extensive interlibrary searches. Additional articles were selected and screened from the references of included articles. The study selection process is summarized in Figure 1.
Figure 1.
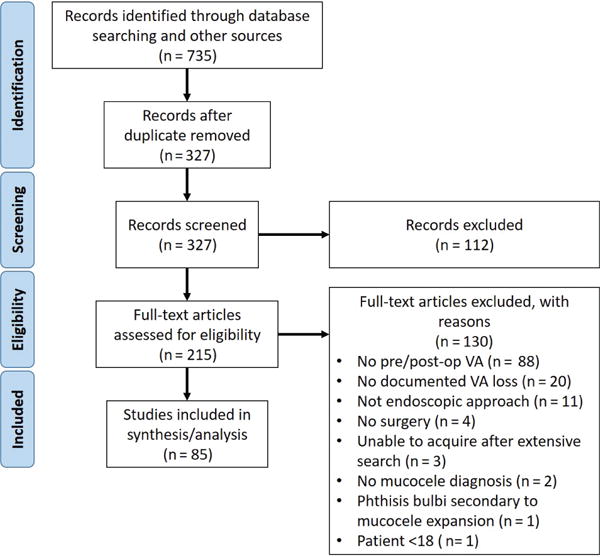
PRISMA flowchart for systematic review and meta-analysis article selection. VA, visual acuity.
Data Abstractions and Study Quality Assessment
When available, data extracted from the studies included patient age and sex, predisposing factors, mucocele location, pre- and postoperative visual acuity, additional reported symptoms, duration of symptoms, whether the authors or patients characterized the vision loss as acute or gradual, type of surgery performed, time from onset of symptoms to surgery, and operative findings. To analyze visual acuity data in a standardized and clinically accepted fashion, all visual acuity measures were standardized and converted to logMAR (logarithm of the minimum angle of resolution). This standardized notation ensures that appropriate comparisons and calculations can be made across the entire range of visual acuities measured in this study, taking into the account the inherent logarithmic pattern of letter size change in a standard Snellen chart (see Holladay9 for review and examples of calculations). Translation from common measurement systems is illustrated in Table 1. Qualitative measures, such as “counting fingers” and “hand motions,” were also converted with previously defined methods.10 It is important to note that we report the logMAR change toward visual acuity improvement to present our analysis in a more intuitive fashion. For example, a patient who exhibits improved vision from 3 to 2 on the logMAR scale would have a mathematical change of −1. However, in our study, we report this as 1 logMAR change toward improvement to make our analysis clearer, particularly with correlations. When cases were reported with bilateral vision loss, we recorded and analyzed the eye with the worst preoperative visual acuity because most reports did not provide consistent data on the second affected eye. Cases of visual acuity loss in the second eye often lacked postoperative visual acuity measurements, detailed radiographic and surgical descriptions, or clear evidence of mucocele as the etiology of vision loss. To assess study quality, we classified the levels of evidence in accordance with published guidelines.11
Table 1.
Visual Acuity Scale Conversion.
| Foot | Meter | Decimal | logMAR |
|---|---|---|---|
| No light perception | 3.00 | ||
| Light perception | 2.70 | ||
| Hand motion | 2.30 | ||
| Finger counting | 1.90 | ||
| 20/200 | 6/60 | 0.1 | 1.00 |
| 20/120 | 6/36 | 0.17 | 0.78 |
| 20/80 | 6/24 | 0.25 | 0.60 |
| 20/60 | 6/18 | 0.33 | 0.48 |
| 20/40 | 6/12 | 0.5 | 0.30 |
| 20/30 | 6/9 | 0.8 | 0.18 |
| 20/20 | 6/6 | 1.0 | 0.00 |
| 20/16 | 6/4.8 | 1.25 | −0.10 |
| 20/12.5 | 6/3.8 | 1.60 | −0.20 |
Abbreviation: logMAR, logarithm of the minimum angle of resolution.
Statistical Analysis
All statistical analysis was executed in GraphPad Prism 6 (La Jolla, California) or MATLAB (MathWorks Inc, Natick, Massachusetts). Correlation analysis was performed with Spearman and Pearson correlations, based on linearity of the data. To evaluate the effects of clinically relevant predictive factors (eg, time to surgery and preoperative visual acuity), we employed a receiver operator curve (ROC) analysis. This analysis was performed in several steps: a visual acuity change threshold was chosen, and the ROC analysis was performed with respect to a predictive variable (eg, time to surgery); another visual acuity change threshold was chosen, and the analysis was repeated. In this way, we tested every possible visual acuity “improvement” threshold and assessed the corresponding area under curve (AUC) for the ROC analysis, yielding statistically significant threshold values.
Results
Literature Search
Eighty-five studies were included that fulfilled inclusion and exclusion criteria, reporting on 207 patients (see Supplemental Table S1 in the online version of the article). Our analysis focuses on visual outcomes and time to surgical intervention, as other extracted data were not consistently reported. Associated data were irregularly published regarding predisposing factors for mucoceles, other associated symptoms, and operative findings. All the included studies were either individual case reports or case series, constituting a level of evidence of 4 and a grade of C.11
Data Set Characteristics
The average age of a patient in our study was 51.4 years (median, 52; range, 11–90), with males representing 46.4% of the data set. Thirty-one percent of patients had a documented history of facial trauma and/or prior surgery. Mucoceles classified as sphenoidal, ethmoidal, or sphenoethmoidal constituted 81.2% of the data set. Only 11.6% of mucoceles arose from the frontal, frontoethmoid, and maxillary sinuses. Precise paranasal sinus localization was not reported for 7.3% of patients. The predominance of sphenoidal and ethmoidal lesions in our study is consistent with past reports that mucoceles in these locations have a predilection toward causing decreased visual acuity.5
Overview of Visual Acuity Changes
The mean presenting visual acuity was 1.57 logMAR units (range, 0.046–3), and posttreatment was 0.21 (range, −0.17 to 3). Visual acuity improvement was achieved in 71.5% of cases after surgical intervention; visual acuity stabilization occurred in 27.1% of cases; and only 1.4% of cases resulted in worse postoperative visual acuity (Figure 2). Final reported visual acuity was recorded an average of 52 days postoperatively (median, 21; range, 1–365). However, we noted infrequent and irregular reporting of this value among included articles. Mucoceles arising from the sphenoid and/or ethmoid presented with a worse preoperative visual acuity than those arising from the frontal, frontoethmoid, maxillary, or unspecified sinuses on average (mean, 1.68 vs 1.10 logMAR units, P = .0015). While the average time to surgery between these groups did not differ (P > .9), postoperative improvement in visual acuity was significantly greater in the sphenoid/ethmoid group (0.85 vs 0.57 logMAR units, P = .044).
Figure 2.
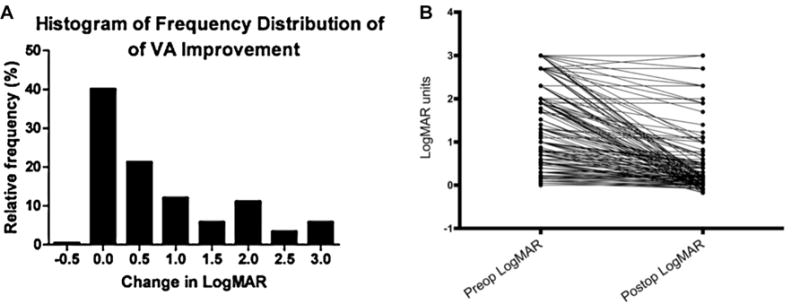
Frequency distribution of visual acuity (VA) change: (A) after surgery and (B) for each patient in our analysis.
Predictive Variable Analysis: Time to Surgery and Preoperative Visual Acuity
The 2 parameters most consistently reported in the literature were (1) time from symptom onset to surgical intervention and (2) pre- and postoperative visual acuities. Correlation was seen between poor preoperative visual acuity and degree of postoperative improvement (R = 0.3813, P < .0001), suggesting that vision is recoverable even in instances of severe vision loss (Figure 3A). Time to surgery after symptom onset exhibited an inverse correlation with visual improvement after surgical intervention (R = −0.3142, P < .0001), suggesting that more prompt surgical intervention can improve visual outcome (Figure 3B, Table 2). Note that symptom onset was defined as the patient’s subjective appreciation of change in visual acuity. Thus, the reported “time to surgery” is the sum of the time from symptom onset to presentation and the time from presentation to surgery. Interestingly, we also found a weak inverse correlation between preoperative visual acuity and duration of symptoms prior to presentation (R = −0.2429, P = .0013), suggesting that a more rapid deterioration of vision (shorter symptom duration) was associated with worse visual acuity at presentation (higher logMAR).
Figure 3.
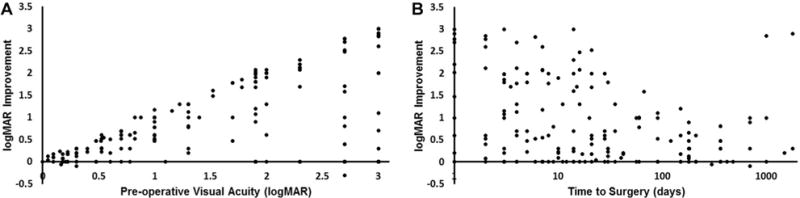
Correlation analysis of visual improvement as a function of (A) preoperative visual acuity and (B) time to surgery. logMAR, logarithm of the minimum angle of resolution.
Table 2.
Correlation of Preoperative VA and Time to Surgery with Postoperative Gain in VA.
| Variable | Correlation with VA Improvement, R (P Value) |
|---|---|
| Preoperative VA | 0.38125 (<.0001)a |
| Time to surgery | −0.3142 (<.0001)b |
Abbreviation: VA, visual acuity.
Pearson correlation used given the linear relationship of interval data.
Spearman correlation used given the nonlinear relationship of interval data.
With these findings, we set out to establish statistically significant thresholds for time to surgery and preoperative visual acuity that could best predict postoperative visual acuity improvement. To analyze the data in this manner, we defined “improvement” as a degree of postoperative visual acuity change >1 logMAR unit; visual acuity changes ≤1 logMAR were considered “not improved.” This cutoff was determined by testing every possible visual acuity change threshold while generating an ROC analysis with AUC for each trial with respect to preoperative visual acuity and time to surgery (Supplemental Table S2 in the online version of the article). After visual acuity improvement was defined, individual ROC analyses were performed for time to surgery and preoperative visual acuity (Figure 4). We calculated an optimal cutoff of preoperative visual acuity as ≥1.52 logMAR units such that the threshold could predict visual acuity improvement with a specificity of 88% and a sensitivity of 65%; the AUC value for this cutoff was 75.1. Similarly, we calculated the optimal cutoff for time to surgery to be <6 days, with an AUC value of 73.1 and sensitivity and specificity of 80% and 50%, respectively (Table 3). An AUC value of 1 represents a perfect test, while an AUC value of 0.5 represents completely random chance. AUC values of 0.7 to 0.9, like our analysis, indicate a moderately accurate test.12
Figure 4.
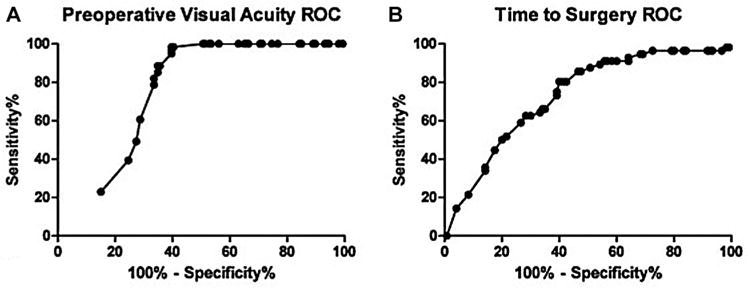
Receiver operator curve (ROC) analysis demonstrating optimal statistical thresholds for the predictive factors of (A) preoperative visual acuity and (B) time to surgery
Table 3.
Calculated Optimal Cutoffs for Time to Surgery and Preoperative VA per ROC Analysis.
| Variable | AUC, % | Calculated Threshold | Sensitivity, % | Specificity, % |
|---|---|---|---|---|
| Preoperative VA | 75.1 | 1.52 logMAR | 65 | 88 |
| Time to surgery | 73.1 | 6 d | 80 | 50 |
Abbreviations: AUC, area under curve; logMAR, logarithm of the minimum angle of resolution; ROC, receiver operator curve; VA, visual acuity.
Finally, we repeated the correlation analysis with these calculated cutoffs. This yielded an even stronger correlation between preoperative visual acuity and postoperative improvements with the ≥1.52 logMAR cutoff (R = 0.4887, P < .001). Similarly, a correlation (R = 0.297, P < .0001) was found between time to surgery and postoperative improvement with a cutoff of <6 days, suggesting that earlier intervention resulted in better visual outcomes. These results are summarized in Table 4. Compared with those receiving surgery at ≥6 days, patients receiving surgery in <6 days had significantly worse preoperative visual acuity (1.53 vs 1.91, P = .016) but also demonstrated a significantly greater improvement in visual acuity (0.68 vs 1.30, P < .0002). These postoperative changes led to similar final visual acuities between the groups (0.85 vs 0.61, P = .165). We tested the possibility for a confounding relationship between preoperative visual acuity and time to surgery. These do not correlate together, can be interpreted not to confound each other, and may represent true independent predictors of outcome.
Table 4.
Correlation of Preoperative VA and Time to Surgery with Postoperative Gain in VA Based on Calculated Cutoffs from ROC Analysis.
| Variable | Correlation with VA Improvement >1 logMAR, R (P Value) |
|---|---|
| Preoperative VA, ≥1.52 logMAR | 0.4887 (<.0001)a |
| Time to surgery, <6 d | 0.297 (<.0001)b |
Abbreviations: logMAR, logarithm of the minimum angle of resolution; ROC, receiver operator curve; VA, visual acuity.
Pearson correlation used given the linear relationship of interval data.
Spearman correlation used given the nonlinear relationship of interval data.
Discussion
The key finding in our study was that visual acuity improvement is attainable with treatment of paranasal sinus mucoceles, even when extreme vision loss is encountered at presentation. Further analysis of the compounded data indicates that the most improvement in vision outcomes (measured by change in visual acuity) occurs among those with preoperative visual acuities greater than or equal to 1.52 logMAR and those who underwent surgery within 6 days of initial presentation. In more clinically familiar terms, patients who presented with visual acuities approximately equal to 20/650 or worse (counting fingers, hand movements, etc) and had operative management within 6 days were most likely to have a visual acuity improvement of 1 logMAR, an improvement comparable to going from 20/200 to 20/20.
Our study is the first, to our knowledge, that utilizes a large data set to determine clinically applicable cutoff values for predictive variables. As a result of the summative data from numerous case reports and series, we demonstrate statistical significance in areas where many previous reports have been unable. Previous reports did not find correlations between preoperative visual acuity and postoperative improvement13–18; however, the authors of these studies consistently mention that their study populations were underpowered for appropriate statistical analysis or accurate trending of data. These previous studies also present conflicting information about the correlation between the timing of surgery and postoperative visual acuity outcomes. While most studies failed to find statistically significant correlations, Yoon et al15 reported that shorter intervals from symptom onset to surgery correlated with worse postoperative visual acuity outcomes, an intuitive finding given their explanation. The authors noted that sudden visual losses (prompting more urgent surgical intervention) correspond to severe damage, while gradual visual acuity deterioration represents more slow-growing masses, potentially prompting more conservative initial management. Such analysis and interpretation of symptom onset and pathogenesis have been reported in the literature,19,20 with hypotheses that sudden visual acuity loss potentially results from spread of infection or inflammation from the mucocele to the optic nerve, whereas gradual development of visual acuity loss occurs due to progressive ischemic changes caused by pressure from the slowly growing mucocele.13 Supporting these mechanisms, Loo et al reported that cases with acute infectious inflammatory changes, as evidenced by the operative finding of mucopyocele, had poorer outcomes.17 However, Kim et al suggested that the severity of disease could not be correlated with duration of symptoms, a point that was supported by the fact that most reports were unable to find significant correlations between time to surgery and postoperative visual acuity.16 Of note, we detected a weak inverse correlation between duration of symptoms and preoperative visual acuity, providing some support for the notion that more rapid visual acuity deterioration is associated with a more severe pathophysiologic process.
Our systematic review and meta-analysis, therefore, add significantly to our understanding of the disease process and expected surgical outcomes. Here, we were able to provide additional statistical power through inclusion of more subjects, a key weakness of past reports. In doing so, we were able to demonstrate a previously indeterminate correlation between preoperative visual acuity and postoperative outcomes. It is possible that the greater associated improvement in patients with worse preoperative visual acuities is due largely to the inherently larger room for improvement in a reversible disease process. The use of a logMAR scale allowed for inclusion of patients with severe vision loss (eg, hand-motion only) through normalization of data such that the degree of improvement in visual acuity could be determined to be significantly greater for those with worse preoperative visual acuity. Interestingly, prior reports suggest that a short duration of symptoms may correlate with worse outcomes, hypothetically as a result of different pathophysiologic mechanisms.15,19,20 Measuring the true time to surgery from onset of the disease is not possible, given that the time from disease onset to disease manifestation and clinic presentation is often unclear. However, the data that we present are valuable to understand that, regardless of the different processes behind rapid and gradual visual acuity deterioration, more prompt surgical intervention can improve outcomes by minimizing or preventing either pressure or inflammatory damage caused by mucoceles. A cutoff of 6 days was statistically most ideal according to our analysis, although most patients recovered some degree of vision regardless of time to surgery. This statistical finding does not necessarily represent a requisite time for surgery; rather, it demonstrates that an emergency intervention may not be mandatory and that urgent intervention (within 1 week) may be planned. Note that we are not suggesting a rigid guideline or cutoff to determine candidacy for surgical timing or preoperative visual acuity. For example, while a preoperative visual acuity greater than or equal to 1.52 logMAR had the best outcomes, this should not be used as a test for deciding when to intervene. Indeed, the management of any case requires the surgeon’s judgment and individual considerations of each case.
We recognize that a visual acuity improvement threshold of 1 logMAR represents a statistically significant definition, but it may not be a clinically significant cutoff in all cases. Indeed, a common threshold for visual acuity “improvement” cited in the ophthalmology literature is 15 letters (or 3 lines) on a Snellen chart,21 translating to a change of approximately 0.3 logMAR. This threshold produces a much lower level of statistical significance (Table S2, available online only). However, the improvement threshold of 1 logMAR holds clinical significance in our study’s patient population, given the severity of vision loss included in this meta-analysis. For patients presenting with visual acuity loss due to paranasal sinus mucoceles, we found an average preoperative visual acuity of 1.57 logMAR, or 20/740 Snellen. An improvement of 0.3 logMAR would result in visual acuity of 20/370, a relatively minor change in functional capacity for these patients. Since most of the patients in our data set had severe visual impairment on presentation, a threshold of 1 logMAR is particularly relevant to patients in this population, as it represents a significant functional improvement. While this threshold may bear less importance for those with minor visual impairments from paranasal sinus mucoceles, it is clinically informative for most patients with this condition.
The most notable limitation with the present study is the heterogeneity of source literature. Each author chose to make note of different symptoms and did not always report uniformly detailed medical histories, physical examinations, or operative findings. Thus, in this type of study, we are unable to examine other potentially relevant factors, such as motility impairment, color perception, visual field assessments, evidence of optic neuropathy, or optic canal dehiscence. These additional factors are potential confounding variables that the provider should consider when planning for intervention. Furthermore, the patients represented in this study were managed in different years and in many health care systems across numerous countries. Altogether, such a cohort may not fit the typical data set included in a meta-analysis. However, the data collected for the present study represent all available information in the literature and may therefore carry more general “real world” utility. Future research will be useful in further delineating prognostic data, accounting for confounding variables such as those mentioned here, provided that authors document ophthalmologic findings comprehensively. A retrospective or prospective study with appropriate controls and consistent documentation of key prognostic factors—including additional symptoms, detailed physical examination, operative findings, and presence of infection—will be challenging to complete with sufficient statistical power but may be useful to affirm the thresholds that we determined in this work. Finally, we recognize the potential publication bias of cases preferentially reporting more severe visual acuity loss or more acute worsening. Again, this concern could be eliminated in an appropriately controlled prospective study.
Conclusion
We performed a systematic review and meta-analysis of vision outcomes after endoscopic surgical management of paranasal sinus mucoceles presenting with vision loss. Our data illustrate improvement of vision loss in the majority of these patients, regardless of the severity of vision loss at presentation. Additionally, it appears that prompt surgery (<6 days) is ideal to achieve optimal visual outcomes.
Supplementary Material
Acknowledgments
Funding source: This work was supported by the National Institute On Deafness And Other Communication Disorders of the National Institutes of Health under Grant Number K23DC014747 (VRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions
Leonid M. Zukin, study design; data acquisition, analysis, and interpretation; drafting and revising the work; Eric M. Hink, data analysis and interpretation; drafting and revising the manuscript; Sophie Liao, data analysis and interpretation; drafting and revising the manuscript; Anne E. Getz, data analysis and interpretation; drafting and revising the manuscript; Todd T. Kingdom, data analysis and interpretation; drafting and revising the manuscript; Vijay R. Ramakrishnan, study concept and design; data analysis and interpretation; drafting and revising the work.
Disclosures
Competing interests: Vijay R. Ramakrishnan, consultant—Medtronic, Inc.
Sponsorships: None.
Supplemental Material
Additional supporting information is available in the online version of the article.
References
- 1.Devars du Mayne M, Moya-Plana A, Malinvaud D, et al. Sinus mucocele: natural history and long-term recurrence rate. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:125–130. doi: 10.1016/j.anorl.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Capra GG, Carbone PN, Mullin DP. Paranasal sinus mucocele. Head Neck Pathol. 2012;6:369–372. doi: 10.1007/s12105-012-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho BV, Lopes ICC, Correa JB, et al. Typical and atypical presentations of paranasal sinus mucocele at computed tomography. Radiol Bras. 2013;46:372–375. [Google Scholar]
- 4.Sadiq SA, Lim MK, Jones NS. Ophthalmic manifestations of paranasal sinus mucoceles. Int Ophthalmol. 2009;29:75–79. doi: 10.1007/s10792-008-9194-6. [DOI] [PubMed] [Google Scholar]
- 5.Tseng CC, Ho CY, Kao SC. Ophthalmic manifestations of paranasal sinus mucoceles. J Chin Med Assoc. 2005;68:260–264. doi: 10.1016/S1726-4901(09)70147-9. [DOI] [PubMed] [Google Scholar]
- 6.Serrano E, Klossek J, Percodani J, et al. Surgical management of paranasal sinus mucoceles: a long-term study of 60 cases. Otolaryngol Head Neck Surg. 2004;131:133–140. doi: 10.1016/j.otohns.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Al Anazy FH, Al Dousary SH. Ophthalmic manifestations of paranasal sinus disease: a clinical grading system. Int Forum Allergy Rhinol. 2012;2:331–335. doi: 10.1002/alr.21029. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997;13:388–391. doi: 10.3928/1081-597X-19970701-16. [DOI] [PubMed] [Google Scholar]
- 10.Schulze-Bonsel K, Feltgen N, Burau H, et al. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg Visual Acuity Test. Invest Ophthalmol Vis Sci. 2006;47:1236–1240. doi: 10.1167/iovs.05-0981. [DOI] [PubMed] [Google Scholar]
- 11.Liu JC, Stewart MG. Teaching evidence-based medicine in otolaryngology. Otolaryngol Clin North Am. 2007;40:1261–1274. doi: 10.1016/j.otc.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Fischer JE, Bachmann LM, Jaeschke R. A readers’ guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med. 2003;29:1043–1051. doi: 10.1007/s00134-003-1761-8. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y, Kim K, Lee J, et al. Paranasal sinus mucoceles with ophthalmologic manifestations: a 17-year review of 96 cases. Am J Rhinol Allergy. 2011;25:272–275. doi: 10.2500/ajra.2011.25.3624. [DOI] [PubMed] [Google Scholar]
- 14.Sleep TJ, Hodgkins PR, Honeybul S, et al. Visual function following neurosurgical optic nerve decompression for compressive optic neuropathy. Eye. 2003;17:571–578. doi: 10.1038/sj.eye.6700439. [DOI] [PubMed] [Google Scholar]
- 15.Yoon TM, Kim K, Lim SC. Visual outcome after endoscopic decompression surgery for sphenoid sinus disease: how we do it. Clin Otolaryngol. 2008;33:472–494. doi: 10.1111/j.1749-4486.2008.01722.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y, Kim K, Lee J, et al. Paranasal sinus mucocele with ophthalmologic manifestations: a 17-year review of 96 cases. Am J Rhinol Allergy. 2011;25:272–275. doi: 10.2500/ajra.2011.25.3624. [DOI] [PubMed] [Google Scholar]
- 17.Loo JL, Looi AL, Seah LL. Visual outcomes in patients with paranasal mucoceles. Ophthal Plast Reconstr Surg. 2009;25:126–129. doi: 10.1097/IOP.0b013e318198e78e. [DOI] [PubMed] [Google Scholar]
- 18.Lee LA, Huang CC, Lee TJ. Prolonged visual disturbance secondary to isolated sphenoid sinus disease. Laryngoscope. 2004;114:986–990. doi: 10.1097/00005537-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Moriyama H, Heska H, Tachibana T, et al. Mucoceles of ethmoid and sphenoid sinus with visual disturbance. Arch Otolaryngol Head Neck Surg. 1992;118:142–146. doi: 10.1001/archotol.1992.01880020034012. [DOI] [PubMed] [Google Scholar]
- 20.Yumoto E, Hyodo M, Kawakita S, et al. Effect of sinus surgery on visual disturbance caused by spheno-ethmoid mucoceles. Am J Rhinol. 1997;11:338–343. doi: 10.2500/105065897781286025. [DOI] [PubMed] [Google Scholar]
- 21.Beck RW, Maquire MG, Bressler NM, et al. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology. 2007;114:1804–1809. doi: 10.1016/j.ophtha.2007.06.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


