Abstract
Background
Age‐related cataract is the principal cause of blindness and visual impairment in the world. Phacoemulsification is the main surgical procedure used to treat cataract. The comparative effectiveness and safety of different‐sized incisions for phacoemulsification has not been determined.
Objectives
The aim of this systematic review was to assess the effectiveness and safety of smaller versus larger incisions for phacoemulsification in age‐related cataract. The primary outcome of this review was surgically induced astigmatism at three months after surgery.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 10), MEDLINE Ovid (1946 to 28 October 2016), Embase Ovid (1947 to 28 October 2016), PubMed (1948 to 28 October 2016), LILACS (Latin American and Caribbean Health Sciences Literature Database) (1982 to 28 October 2016), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com; last searched 13 May 2013), ClinicalTrials.gov (www.clinicaltrials.gov; searched 28 October 2016), and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 28 October 2016). We did not use any date or language restrictions in the electronic searches for trials.
Selection criteria
We included randomized controlled trials (RCTs) comparing different‐sized incisions in people with age‐related cataract undergoing phacoemulsification.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 26 RCTs with a total of 2737 participants (3120 eyes). These trials were conducted in Bosnia and Herzegovina, China, France, India, Italy, Korea, Spain, Switzerland, and Turkey. Half of the 26 trials were conducted in China. We judged all trials as mostly at unclear to low risk of bias. The included RCTs compared four different‐sized incisions:<= 1.5 mm, 1.8 mm, 2.2 mm, and approximately 3.0 mm. These incisions were performed using three different techniques: coaxial and biaxial microincision phacoemulsification (C‐MICS and B‐MICS) and standard phacoemulsification. Not all studies provided data in a form that could be included in this review. Five studies had three arms.
Fifteen trials compared C‐MICS (2.2 mm) with standard phacoemulsification (about 3.0 mm). Very low‐certainty evidence suggested less surgically induced astigmatism in the C‐MICS group at three months compared with standard phacoemulsification (mean difference (MD) ‐0.19 diopters (D), 95% confidence interval (CI) ‐0.30 to ‐0.09; 996 eyes; 8 RCTs). There was low‐certainty evidence that both groups achieved similar best‐corrected visual acuity (MD 0.00 logMAR, 95% CI ‐0.02 to 0.02; 242 eyes; 3 RCTs). There was low‐certainty evidence of little or no difference in endothelial cell loss and central corneal thickness comparing C‐MICS with standard phacoemulsification (MD ‐7.23 cells/mm2, 95% CI ‐78.66 to 64.20; 596 eyes; 4 RCTs) and (MD ‐0.68 μm, 95% CI ‐3.26 to 1.90; 487 eyes; 5 RCTs).
Nine trials compared C‐MICS (1.8 mm) with standard phacoemulsification (about 3.0 mm). Very low‐certainty evidence suggested less astigmatism at three months in the C‐MICS group compared with standard phacoemulsification group (MD ‐0.23 D, 95% CI ‐0.34 to ‐0.13; 561 eyes; 5 RCTs). Low‐certainty evidence suggested little or no difference in best‐corrected visual acuity, endothelial cell loss, and central corneal thickness in the two groups at three months (MD ‐0.02 logMAR, 95% CI ‐0.03 to ‐0.00; 192 eyes; 3 RCTs), (MD 7.56 cells/mm2, 95% CI ‐67.65 to 82.77; 380 eyes; 5 RCTs), and (MD ‐1.52 μm, 95% CI ‐6.29 to 3.25; 245 eyes; 3 RCTs).
Six studies compared C‐MICS (1.8 mm) with C‐MICS (2.2 mm). There was low‐certainty evidence that astigmatism, visual acuity, and central corneal thickness were similar in the two groups at three months (MD 0.04 D, 95% CI ‐0.09 to 0.16; 259 eyes; 3 RCTs), (MD 0.01 logMAR, 95% CI ‐0.01 to 0.04; 200 eyes; 3 RCTs), and (MD 0.45 μm, 95% CI ‐2.70 to 3.60; 100 eyes; 1 RCT). Very low‐certainty evidence suggested higher endothelial cell loss in the 1.8 mm group (MD 213.00 cells/mm2, 95% CI 11.15 to 414.85; 70 eyes; 1 RCT).
Four studies compared B‐MICS (<= 1.5 mm) with standard phacoemulsification (about 3.0 mm). Astigmatism was similar in the two groups at three months (MD ‐0.01 D, 95% CI ‐0.03 to 0.01; 368 eyes; 2 RCTs; moderate‐certainty evidence). There was low‐certainty evidence on visual acuity, suggesting little or no difference between the two groups (MD ‐0.02 logMAR, 95% CI ‐0.04 to ‐0.00; 464 eyes; 3 RCTs). Low‐certainty evidence on endothelial cell loss and central corneal thickness also suggested little or no difference between the two groups (MD 55.83 cells/mm2, 95% CI ‐34.93 to 146.59; 280 eyes; 1 RCT) and (MD 0.10 μm, 95% CI ‐14.04 to 14.24; 90 eyes; 1 RCT).
None of the trials reported on quality of life. One trial reported that no participants experienced endophthalmitis or posterior capsule rupture; they also reported little or no difference between incision groups regarding corneal edema (risk ratio 1.02, 95% CI 0.40 to 2.63; 362 eyes).
Authors' conclusions
Phacoemulsification with smaller incisions was not consistently associated with less surgically induced astigmatism compared with phacoemulsification with larger incisions. Coaxial microincision phacoemulsification may be associated with less astigmatism than standard phacoemulsification, but the difference was small, in the order of 0.2 D, and the evidence was uncertain. Safety outcomes and quality of life were not adequately reported; these should be addressed in future studies.
Plain language summary
Different‐sized incisions for cataract surgery in people with age‐related cataract
Review aim The aim of this Cochrane Review was to find out if the size of the incision (cut in the eye) during cataract surgery results in a difference in outcome of cataract surgery. We found 26 studies that answered this question.
Key messages Some, but not all, surgical techniques using smaller incisions were associated with less astigmatism; however, the differences were small and the evidence was uncertain. There was little evidence to suggest any important effects on vision. There were limited data on adverse effects and no evidence on the effects of different‐sized incisions on quality of life.
What was studied in this review? As people age the clear lens in the eye can become cloudy, which is known as a cataract. An operation can be performed to remove the cataract and replace the cloudy lens with a clear artificial lens. This surgery is safe and restores sight in almost all cases. Age‐related cataract is one of the leading causes of blindness worldwide.
One problem that can occur after cataract surgery is that due to the surgery the front of the eye is no longer a perfectly curved shape. This can result in blurred or distorted vision and is known as astigmatism. The vision problems arising from astigmatism can be corrected with spectacles. It is commonly believed that the smaller the cut or incision made in the eye during cataract surgery, the less chance of astigmatism.
Key results We searched for studies that compared different‐sized incisions for cataract surgery in people with age‐related cataract. This review includes 26 studies from Europe and Asia.
We found the following results.
• Some, but not all, surgical techniques using smaller incisions were associated with less astigmatism, however the differences were small and the evidence was uncertain (low‐ and very low‐certainty evidence). • In general, there may be little or no difference in visual acuity based on whether a smaller or larger incision is made (low‐certainty evidence). • There were no consistent effects on other signs such as thickness of the cornea (front of the eye) and number of cells in the front of the eye (low‐ and very low‐certainty evidence). • Adverse effects were not reported by most of the included studies. • None of the studies reported on the quality of life of participants.
How up‐to‐date is this review? We searched for studies published up to 28 October 2016.
Summary of findings
Summary of findings for the main comparison. Larger coaxial microincision cataract surgery (C‐MICS) versus standard phacoemulsification.
| Larger C‐MICS compared with standard phacoemulsification for age‐related cataract | ||||||
|
Patient or population: adults with age‐related cataract Settings: eye clinics Intervention: larger C‐MICS with 2.2‐millimeter incision Comparison: standard phacoemulsification with about 3.0‐millimeter incision | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard phacoemulsification | Larger C‐MICS | |||||
|
Mean postoperative surgically induced astigmatism Follow‐up: 3 months |
The mean surgically induced astigmatism was 0.7 to 1.34 diopters. | The mean surgically induced astigmatism in the intervention groups was 0.19 diopters lower (0.30 to 0.09 diopters lower). | ‐ | 996 (8 RCTs) | ⊕⊝⊝⊝ very lowa,b | A lower diopter value is a better clinical outcome. |
|
Mean postoperative best‐corrected visual acuity Follow‐up: 3 months |
The mean best‐corrected visual acuity was 0.05 to 0.11 logMAR. | The mean best‐corrected visual acuity in the intervention groups was 0.00 logMAR lower (0.02 logMAR lower to 0.02 logMAR higher). | ‐ | 242 (3 RCTs) | ⊕⊕⊝⊝ lowc | |
|
Mean endothelial cell loss Follow‐up: 3 months |
The mean of endothelial cell loss ranged across control groups was 2054.0 to 2339.0 cells/mm2. | The mean change of endothelial cell loss in the intervention groups was 7.23 cells/mm2 lower (78.66 cells/mm2 lower to 64.20 cells/mm2 higher). | ‐ | 596 (4 RCTs) |
⊕⊕⊝⊝ lowd | Little or no difference between groups is a clinically positive result. |
|
Central corneal thickness Follow‐up: 3 months |
The mean change of central corneal thickness was 9.24 μm. The mean central corneal thickness ranged across control groups from 546.0 to 580.0 μm. |
The mean change of central corneal thickness in the intervention groups was 0.68 μm lower (3.26 μm lower to 1.90 μm higher). | ‐ | 487 (5 RCTs) |
⊕⊕⊝⊝ lowd | Hwang 2016 did not report the standard deviation, but reported that the mean % decrease in central corneal thickness was 1.00 in the 2.2‐millimeter group and 0.31 in the 2.75‐millimeter group. Little or no difference between groups is a clinically positive result. |
|
Adverse events (corneal edema) Follow‐up: 3 months |
46 per 1000 | 47 per 1000 (19 to 122) |
RR 1.02 (0.40 to 2.63 |
362 (1 RCT) |
Wang 2009 reported "no intraoperative complications." | |
| Quality of life | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||
aDowngraded two levels for risk of bias, as one study was at high risk of selection and attrition bias, while another study was at high risk of reporting bias. bDowngraded one level for unexplained statistical heterogeneity. cDowngraded two levels for risk of bias, as the studies were at unclear risk of selection and attrition bias. dDowngraded two levels for risk of bias, as the studies were at unclear risk of selection, performance, detection, and attrition bias.
Summary of findings 2. Smaller coaxial microincision cataract surgery (C‐MICS) versus standard phacoemulsification.
| Smaller C‐MICS compared with standard phacoemulsification for age‐related cataract | ||||||
|
Patient or population: adults with age‐related cataract Settings: eye clinics Intervention: smaller C‐MICS with 1.8‐millimeter incision Comparison: standard phacoemulsification with about 3.0‐millimeter incision | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard phacoemulsification | Smaller C‐MICS | |||||
|
Mean postoperative surgically induced astigmatism Follow‐up: 3 months |
The mean postoperative surgically induced astigmatism ranged across control groups from 0.03 to 0.94 diopters. | The mean postoperative surgically induced astigmatism in the intervention groups was 0.23 lower (0.34 diopters to 0.13 diopters lower). | ‐ | 561 (5 RCTs) | ⊕⊝⊝⊝ very lowa,b | A lower diopter value is a better clinical outcome. |
|
Mean postoperative best‐corrected visual acuity Follow‐up: 2 to 3 months |
The mean postoperative best‐corrected visual acuity ranged across control groups from 0.05 to 0.06 logMAR units. | The mean postoperative best‐corrected visual acuity in the intervention groups was 0.02 logMAR units lower (0.03 logMAR units lower to 0.00 logMAR units). | ‐ | 192 (3 RCTs) | ⊕⊕⊝⊝ lowc | |
|
Mean change of endothelial cell loss Follow‐up: 3 months |
The mean change of endothelial cell loss ranged across control groups from 2231.22 to 3077.0 cells/mm2. | The mean change of endothelial cell loss in the intervention groups was 7.56 cells/mm2 higher (67.65 cells/mm2 lower to 82.77 cells/mm2 higher). | ‐ | 380 (5 RCTs) | ⊕⊕⊝⊝ lowa | |
|
Mean change of central corneal thickness Follow‐up: 3 months |
The mean change of central corneal thickness ranged across control groups from 3.74 to 63.5 μm. | The mean change of central corneal thickness in the intervention groups was 1.52 μm lower (6.29 μm lower to 3.25 μm higher). |
‐ | 245 (3 RCTs) | ⊕⊕⊝⊝ lowa | |
|
Adverse events Follow‐up: 3 months |
None of the trials reported on adverse events. | |||||
| Quality of life | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||
aDowngraded two levels for high risk of selection, performance, and detection bias. bDowngraded two levels for unexplained statistical heterogeneity, as the I2 was greater than 70%. cDowngraded two levels for unclear risk of selection, performance, detection, attrition, and reporting bias.
Summary of findings 3. Smaller coaxial microincision cataract surgery (C‐MICS) versus larger C‐MICS.
| Smaller coaxial microincision cataract surgery (C‐MICS) compared with larger C‐MICS for age‐related cataract | ||||||
|
Patient or population: adults with age‐related cataract Settings: eye clinics Intervention: smaller C‐MICS with 1.8‐millimeter incision Comparison: larger C‐MICS with 2.2‐millimeter incision | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Larger C‐MICS | Smaller C‐MICS | |||||
|
Mean postoperative surgically induced astigmatism Follow‐up: 3 months |
The mean postoperative surgically induced astigmatism ranged across control groups from 0.48 to 1.08 diopters. | The mean postoperative surgically induced astigmatism in the intervention groups was 0.04 diopters higher (0.09 diopters lower to 0.16 diopters higher). | ‐ | 259 (3 RCTs) | ⊕⊕⊝⊝ lowa | A lower diopter value is a better clinical outcome. |
|
Mean postoperative best‐corrected visual acuity Follow‐up: 3 months |
The mean postoperative best‐corrected visual acuity ranged across control groups from ‐0.06 logMAR units to 0.27 logMAR units. | The mean postoperative best‐corrected visual acuity in the intervention groups was 0.01 logMAR units higher (0.01 logMAR units lower to 0.04 logMAR units higher). | ‐ | 200 (3 RCTs) | ⊕⊕⊝⊝ lowa | |
|
Mean change of endothelial cell loss Follow‐up: 3 months |
The mean change in endothelial cell loss was 2303.0 cells/mm2. | The mean change in endothelial cell loss was 213.00 cells/mm2 higher (11.15 to 414.85 cells/mm2 higher). | ‐ | 70 (1 RCT) |
⊕⊝⊝⊝ very lowb,c | |
|
Mean central corneal thickness Follow‐up: 3 months |
The mean change in central corneal thickness was 2.99 μm. | The mean change in central corneal thickness was 0.45 μm higher (2.70 μm lower to 3.60 μm higher). | ‐ | 100 (1 RCT) | ⊕⊕⊝⊝ lowc | |
|
Adverse events Follow‐up: end of trial |
None of the trials reported on adverse events. | |||||
| Quality of life | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||
aDowngraded two levels for unexplained statistical heterogeneity, as the I2 was greater than 70%. bDowngraded one level for risk of bias, as the studies were at unclear risk of selection, performance, detection, and attrition bias. cDowngraded two levels for imprecision of results, as only one trial reported endothelial cell loss at three months' follow‐up and it is possible that the optimal information size has not been reached.
Summary of findings 4. Biaxial microincision cataract surgery (B‐MICS) versus standard phacoemulsification.
| B‐MICS compared with standard phacoemulsification for age‐related cataract | ||||||
|
Patient or population: adults with age‐related cataract Settings: eye clinics Intervention: B‐MICS with equal to or smaller than 1.5‐millimeter incision Comparison: standard phacoemulsification with about 3.0‐millimeter incision | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard phacoemulsification | B‐MICS | |||||
|
Mean postoperative surgically induced astigmatism Follow‐up: 3 months |
The mean postoperative surgically induced astigmatism ranged across control groups from 0.12 diopters to 0.44 diopters. | The mean postoperative surgically induced astigmatism in the intervention groups was 0.01 diopters lower (0.03 diopters lower to 0.01 diopters higher). | ‐ | 368 (2 RCTs) | ⊕⊕⊕⊝ moderatea | A lower diopter value is a better clinical outcome. |
|
Mean postoperative best‐corrected visual acuity Follow‐up: 3 months |
The mean postoperative best‐corrected visual acuity ranged across control groups from 0.11 logMAR units to 0.97 logMAR units. | The mean postoperative best‐corrected visual acuity in the intervention groups was 0.02 logMAR units lower (0.04 logMAR units lower to 0.00 LogMAR units). | ‐ | 464 (3 RCTs) | ⊕⊕⊝⊝ lowb | |
|
Mean endothelial cell loss Follow‐up: 3 months |
The mean endothelial cell loss in the control groups was 2410 cells/mm2. | The mean endothelial cell loss in the intervention groups was
55.83 cells/mm2 higher (34.93 cells/mm2 lower to 146.59 cells/mm2 higher). |
‐ | 280 (1 RCT) | ⊕⊕⊝⊝ lowc | |
|
Postoperative central corneal thickness Follow‐up: 3 months |
The mean change in central corneal thickness in the control group was 546 μm. | The mean change of central corneal thickness in the intervention groups was 0.10 μm higher (14.04 μm lower to 14.24 μm higher). |
‐ | 90 (1 RCT) | ⊕⊕⊝⊝ lowc | |
|
Adverse events Follow‐up: end of study |
None of the trials reported on adverse events. | |||||
| Quality of life | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||
aDowngraded one level for risk of bias, as the trials were at unclear risk of selection and reporting bias. bDowngraded two levels for unexplained statistical heterogeneity, as the I2 was greater than 70%. cDowngraded two levels for imprecision, as only one trial reported endothelial cell loss at three months' follow‐up and it is possible that the optimal information size has not been reached.
Background
Description of the condition
Cataract, or opacification of the lens, is the most common cause of visual impairment worldwide, and poses one of the greatest public health challenges of the 21st century (Asbell 2005; Resnikoff 2004). The opacified lens reduces the light reaching the retina, finally resulting in defective vision. No gender, racial, or ethnic group is immune to age‐related opacification of the lens, known as age‐related cataract (ARC). Age‐related cataract, which constitutes 90% of all cataracts, usually presents in the eyes of people older than 40 years (Schlote 2006). Age‐related cataract is divided into three major types, on the basis of slit‐lamp examination: nuclear, cortical, and posterior subcapsular (PSC) cataracts. Each of the three can occur alone or in combination, and all of them can progress to total opacification of the lens (Shiels 2010). Density and location of the lens opacity are the two main factors that determine the effect on vision. To date, no method has been confirmed to halt the formation of a cataract lens.
The World Health Organization (WHO) conducted a global review of surveys in 2004 that demonstrated that ARC remained the leading cause of blindness globally, accounting for 48% of world blindness, and that it affected individuals of both high‐income and middle‐ and low‐income countries (Resnikoff 2004). Without extra intervention, the global blind population will increase to 76 million in 2020 (Frick 2003). The cataract surgery rate (CSR), the number of cataract operations per million population per annum, is a useful measurement of eye care delivery in different settings; it differs between high‐income and middle‐ and low‐income countries.
In Sweden, the CSR was between 8000 and 9000 operations per million population per year from 2000 to 2009 (Behndig 2011), but in Africa the CSR was only about 200 operations per million population per year (WHO 2017). In India about 0.5 million cataract surgeries were performed from 1981 to 1982, but the number had increased to 4.8 million by the year 2006 (Aravind 2008). An even higher increase in the number of cataract surgeries is anticipated in low‐income countries under VISION 2020, the global initiative for the elimination of avoidable blindness (Foster 2005). Despite the increasing number of cataract surgeries, ARC continues to be a leading public health issue because of aging populations and increased life expectancies.
Description of the intervention
Currently, surgery to remove the opacification remains the primary and only treatment for ARC. Cataract surgery has changed dramatically over the past 50 years. Among various surgical techniques for cataract management, phacoemulsification using a clear corneal incision has become the gold standard (Kelman 1967). In phacoemulsification, the surgeon uses an ultrasonic handpiece to emulsify the opacified lens and aspirate the consequent small fragments. Compared with earlier cataract surgeries with large invasive incisions and a large postoperative astigmatic error, phacoemulsification requires only a small incision and thus improves surgical outcomes, with fewer intraoperative and postoperative complications and rapid visual rehabilitation. Continuous advancement in instrumentation, techniques, and intraocular lenses (IOLs), to replace the natural lens, has allowed surgeons to use much smaller incision sizes.
The incision of standard phacoemulsifications measures about 3.0 mm in length. This incision size allows the silicone sleeve‐covered phacoemulsification handpiece to fit snugly through the surgically induced corneal wound. The covered silicone sleeve is intended to prevent thermal injury to the cornea with the goal of improving effectiveness and safety of phacoemulsification. Two other approaches, coaxial microincision cataract surgery (C‐MICS) and biaxial microincision cataract surgery (B‐MICS), have been developed (Osher 2007a; Osher 2007b). These procedures allow surgeons to operate through even smaller incisions (Paul 2005). Coaxial microincision cataract surgery allows surgeons to perform irrigation, ultrasound, and aspiration through one incision about 2 mm in length. Biaxial microincision cataract surgery accomplishes irrigation and aspiration separately through two corneal incisions less than 1.5 mm in length (Lam 2009; Weikert 2006). These two approaches may result in intraoperative changes such as the increased use of cumulative dissipated energy (CDE), total use of balanced salt solution (BSS), and prolonged surgical time. To date, Infiniti Vision System (Alcon Laboratories) and Stellaris Vision Enhancement System (Bausch & Lomb) are the two main surgical systems used in the phacoemulsification cataract surgery already described.
How the intervention might work
The goal of cataract surgery is to improve vision by replacing the opacified lens with an artificial intraocular lens called the IOL. After the cataract lens is removed, an IOL is implanted inside the 'bag' of the lens capsule to correct the induced refractive error due to removal of the natural lens. The very small incisions achieved with modern techniques (B‐MICS and C‐MICS) are expected to lower surgically induced astigmatism (SIA) and allow more rapid visual rehabilitation postoperatively, when compared with current phacoemulsification.
Why it is important to do this review
As phacoemulsification techniques and foldable IOLs have developed rapidly in recent decades, the incision size for phacoemulsification has been reduced from around 3.0 mm with standard phacoemulsification to around 2.0 mm with C‐MICS and even smaller ‐ measuring less than 1.5 mm ‐ with B‐MICS (Cavallini 2007; Luo 2012). Smaller clear corneal cataract surgical incisions may be associated with better postoperative visual acuity, reduced SIA, improved wound healing, earlier stabilization of refractive error resulting in shorter recovery time, more stable corneal biomechanical properties, and lower risk of endophthalmitis (intraocular infection and inflammation) (Chee 2005). However, B‐MICS and C‐MICS require more advanced equipment and may result in longer surgical time, increased use of CDE, and consequently greater endothelial cell loss during surgery. An active area of study therefore involves determining the optimal incision size for performance of phacoemulsification. To date, no systematic review of randomized trials has been conducted to assess the effectiveness and safety of incisions of different sizes for phacoemulsification surgery in eyes with age‐related cataract.
Objectives
The aim of this systematic review was to assess the effectiveness and safety of smaller versus larger incisions for phacoemulsification in ARC. The primary outcome of this review was surgically induced astigmatism at three months after surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs) in this review.
Types of participants
We included trials of participants affected by ARC with no ocular condition other than cataract. We included only studies with participants age 50 years or older. Ocular pathologic conditions included high myopia, glaucoma, short axis eyes, etc. We did not impose restrictions with regard to participants' gender or ethnicity.
Types of interventions
We included trials that compared at least two of the following four interventions (major systems included but not limited to Infiniti Vision System and Stellaris Vision Enhancement System).
Standard phacoemulsification with about 3.0 mm incision
Larger coaxial microincision cataract surgery (C‐MICS) with 2.2‐millimeter incision
Smaller coaxial microincision cataract surgery (C‐MICS) with 1.8‐millimeter incision
Biaxial microincision cataract surgery (B‐MICS) with equal to or smaller than 1.5‐millimeter incision
We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. We excluded trials when the intervention did not meet our inclusion criteria, such as when the initial incision was made on the steepest meridian or expanded for IOL implant, or the manual microincision cataract surgery did not use phacoemulsification to emulsify the opacified lens, etc.
Types of outcome measures
Primary outcomes
Mean SIA (in diopters; D) at three months after surgery, measured with corneal topography and assessed by the vector method.
Secondary outcomes
Surgically induced astigmatism data when measured at 12 months where clinically important difference is from 0.8 D to 0.1 D.
Postoperative best corrected visual acuity (BCVA) on the LogMAR scale at three and 12 months where clinically important difference is 0.1 logMAR units.
Postoperative changes of endothelial cell loss (ECL) and central corneal thickness (CCT), from measurements made preoperatively and at three and 12 months after surgery, where little or no difference between baseline and follow‐up is clinically advantageous outcome. Endothelial cell loss measured with non‐contact specular microscopy (cell/mm2 or percentage decrease) and mean CCT measured with ultrasonic pachymeter (μm or percentage increase).
-
Intraoperative parameters recorded at the end of surgery, which are indicative of surgical skill; a lower value is interpreted as having higher surgical skill. The intraoperative parameters are:
use of cumulative dissipated energy is the percentage of power utilized during the ultrasound period (CDE, %);
total use of balanced salt solution (BSS, mL);
surgical time (minutes);
phacoemulsification time (seconds).
Postoperative and intraoperative complications, including endophthalmitis, posterior capsule rupture (PCR), etc.
Quality of life as measured by any validated instruments at three and 12 months.
Follow‐up
We considered outcomes at three months and one year after surgery. As studies may not report outcomes at precisely these time points, we defined the following time periods.
Three months: from four weeks to less than six months
Twelve months: from six months to less than 18 months
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for RCTs and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 28 October 2016.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 10) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 28 October 2016) (Appendix 1)
MEDLINE Ovid (1946 to 28 October 2016) (Appendix 2)
Embase Ovid (1947 to 28 October 2016) (Appendix 3)
PubMed (1948 to 28 October 2016) (Appendix 4)
LILACS (Latin American and Caribbean Health Sciences Literature Database (1982 to 28 October 2016) (Appendix 5)
metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com; last searched 13 May 2013) (Appendix 6)
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 28 October 2016) (Appendix 7)
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp; searched 28 October 2016) (Appendix 8)
Searching other resources
We searched the references of included studies for additional relevant studies, without restriction in terms of language or date of publication.
Data collection and analysis
Selection of studies
Two review authors independently reviewed the titles and abstracts identified through the electronic and manual searches. We classified the titles and abstracts as 'definitely include,', 'unsure,' or 'definitely exclude.' We retrieved full‐text articles for those classified as 'definitely include' or 'unsure' and reassessed them according to the inclusion criteria of the review. We consulted a third review author whenever disagreements arose. For non‐English and non‐Mandarin articles, we used Cochrane worldwide resources to identify translators for specific languages. All publications from studies meeting the inclusion criteria underwent assessment for risk of bias and data extraction. We contacted the authors of articles to obtain further information as needed. We excluded studies classified as 'definitely exclude' after full‐text retrieval, and recorded reasons for exclusions in the 'Characteristics of excluded studies' tables.
Data extraction and management
For English and Mandarin full texts, two review authors independently extracted the study description and data for the primary and secondary outcomes on to paper data collection forms developed in collaboration with Cochrane Eyes and Vision. We resolved discrepancies by discussion to reach consensus. For full texts in Korean, one review author (YK) extracted the data. One review author entered data into Review Manager 5 (RevMan 2014), and a second review author verified the data entry.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of included studies as part of the data extraction process. We followed the tools for assessing risk of bias set forth in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We examined the studies for the following 'Risk of bias' criteria: random sequence generation; allocation concealment; masking of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting; funding source; and other sources of bias. We labeled each quality criterion as high risk of bias, low risk of bias, or unclear risk of bias (lack of information or uncertainty over the potential for bias). We recorded the assessments in the 'Characteristics of included studies' tables.
Measures of treatment effect
Continuous data
For continuous outcomes, we analyzed the treatment effect as mean difference (MD) with 95% confidence intervals (CI). Continuous outcomes included SIA, BCVA, ECL, CCT, CDE use, amount of BSS, surgical time, and phacoemulsification time. None of the continuous outcomes used different scales, therefore we did not calculate standardized mean differences (SMDs); however, if appropriate, we would do so in future updates of this review.
Dichotomous data
We reported the treatment effect for dichotomous outcomes using risk ratio (RR).
Unit of analysis issues
The unit of analysis was the participant. None of the included trials had assigned individual eyes of a participant to different interventions (standard phacoemulsification, C‐MICS, or B‐MICS). Sample sizes in some included trials suggested paired‐eye trials because the number of eyes included was twice the number of participants (Capella 2010; Hwang 2016; Musanovic 2012; Zhu 2014). We referred to Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions as a guide for intraperson correlation between eyes (Higgins 2011b).
Dealing with missing data
We referred to guidelines in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions for information on handling missing data (Higgins 2011b). We attempted to contact study investigators to obtain missing data or to clarify reported information that was unclear. When the investigators did not respond within six weeks or after three attempts at contact, we extracted data as available from the published report.
Assessment of heterogeneity
We examined clinical, methodological, and statistical heterogeneity of the included studies. We used the I2 statistic (%) to determine the proportion of variation in effect estimation, and concluded that an I2 statistic above 50% suggested substantial statistical heterogeneity (Higgins 2003).
Assessment of reporting biases
All meta‐analyses included fewer than 10 trials, therefore we did not use funnel plots to identify any publication biases. If more trials are added to future versions of the review, and the meta‐analysis includes more than 10 trials, we intend to use funnel plots to identify potential publication bias.
Data synthesis
We summarized data from trials that had reported comparable outcome measures at similar follow‐up times. In the absence of clinical, methodological, and substantial statistical heterogeneity, we performed meta‐analysis. We used the fixed‐effect model for meta‐analysis of three or fewer trials. We used a random‐effects model for meta‐analysis of outcome data from more than three trials.
Sensitivity analysis
We intended to conduct sensitivity analyses to determine the impact of excluding studies with lower methodological quality, including exclusion of industry‐funded studies and unpublished studies. However, data were insufficient to conduct such analyses.
Strength of evidence
We rated the strength of evidence using the GRADE approach (GRADEpro GDT 2015; Guyatt 2011), as recommended in the Cochrane Handbook (Schünemann 2011). We used the domains of risk of bias, directness of the evidence, inconsistency, imprecision, and publication bias.
'Summary of findings' table
We presented our results in a 'Summary of findings' table for four comparisons (Types of interventions). We presented the following six outcomes at three months' follow‐up.
SIA data when measured
Postoperative BCVA on the LogMAR scale
Mean ECL measured with non‐contact specular microscopy (cell/mm2 or percentage decrease)
Mean CCT measured with ultrasonic pachymeter (μm or percentage increase)
Adverse events
Quality of life as measured by any validated instruments
Results
Description of studies
Results of the search
As of 28 October 2016, the electronic search yielded 6808 records (Figure 1). There were 2607 records after removal of duplicates. The search of trial registries yielded 439 registered trials. There were 287 unique registered trials after removal of duplicates. We retrieved 72 full‐text articles, of which we included 26 studies and excluded 46 studies with the reasons shown in the Characteristics of excluded studies table.
1.
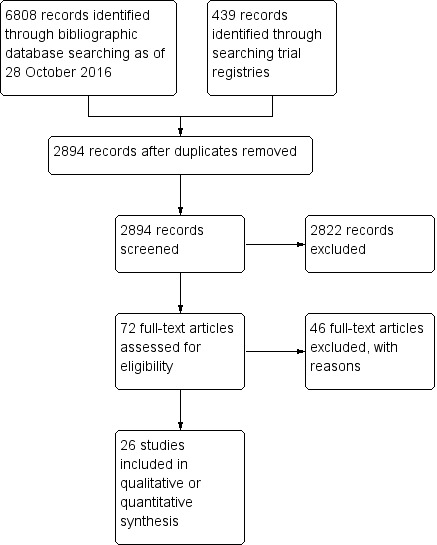
Study flow diagram.
Included studies
Twenty‐six RCTs met the inclusion criteria of this review. Five of the 26 trials compared three interventions. Further details can be found in the Characteristics of included studies table.
Size of studies
There were 26 RCTs with a total of 2737 participants (3120 eyes). The number of participants in each trial ranged from 30, in Mastropasqua 2011, to 362, in Shan 2016.
Location of studies
One trial was conducted in Bosnia and Herzegovina (Musanovic 2012), 13 trials in China (Hui 2016; Li 2016; Lin 2013; Luo 2012; Mao 2008; Shan 2016; Shi 2013; Wang 2009; Yao 2008; Yao 2011; Yu 2016; Zhang 2014; Zhu 2014), one trial in France (Febbraro 2015), one trial in India (Vasavada 2013a), one trial in Italy (Mastropasqua 2011), five trials in Korea (Hwang 2008; Hwang 2016; Jeong 2013; Li 2011; Moon 2011), two trials in Spain (Capella 2010; Morcillo‐Laiz 2009), one trial in Switzerland (Dosso 2008), and one trial in Turkey (Can 2010).
Comparisons
Five trials had three arms (Can 2010; Febbraro 2015; Luo 2012; Moon 2011; Yu 2016). Can 2010 was included in all pair‐wise comparisons, except the smaller C‐MICS versus standard phacoemulsification comparison. Febbraro 2015, Luo 2012, and Moon 2011 were included in all pair‐wise comparisons except B‐MICS compared with standard phacoemulsification. Yu 2016 was only included in the comparison between the smaller C‐MICS and standard phacoemulsification, as the third arm was microincisions with 2.0 mm incision, which is not of interest to this review. Follow‐up time of these trials ranged from one to six months.
Fifteen trials compared the larger C‐MICS with standard phacoemulsification (Can 2010; Febbraro 2015; Hwang 2008; Hwang 2016; Jeong 2013; Li 2011; Li 2016; Lin 2013; Luo 2012; Moon 2011; Musanovic 2012; Shan 2016; Shi 2013; Wang 2009; Zhu 2014).
Nine trials compared the smaller C‐MICS with standard phacoemulsification (Capella 2010; Dosso 2008; Febbraro 2015; Hui 2016; Luo 2012; Moon 2011; Yao 2011; Yu 2016; Zhang 2014).
Six trials compared the smaller C‐MICS with the larger C‐MICS (Can 2010; Febbraro 2015; Luo 2012; Mastropasqua 2011; Moon 2011; Vasavada 2013a).
Four trials compared B‐MICS with standard phacoemulsification (Can 2010; Mao 2008; Morcillo‐Laiz 2009; Yao 2008).
We did not make the following comparisons, as none of the included trials reported them.
B‐MICS versus the larger C‐MICS
B‐MICS versus the smaller C‐MICS
Type of IOL used
Five trials did not report the type of IOL inserted (Can 2010; Li 2011; Musanovic 2012; Shan 2016; Zhu 2014).
The types of IOL used for the standard phacoemulsification were: AKREOS MI60 IOL (Bausch & Lomb), Lentis L‐303 IOL (WaveLight GmbH), Adapt AO IOL (Bausch & Lomb, Rochester, NY, USA), SA60AT IOL (AcrySof; Alcon, USA), SN60WF IOL (Alcon), SENSER IOL (AMO), Y601075 (AJL, A ´lava, Spain), Staar KS‐1 (Canon), PY‐60 (Hoya, Japan), and ZEISS SPHERIS 209M.
The types of IOL used for the larger C‐MICS were: AcrySof Natural SN60WF IOL (Alcon, Fort Worth, TX, USA), SA60AT IOL (AcrySof; Alcon, USA), MI60 IOL (Bausch & Lomb, Rochester, NY, USA), and AcrySof IQ IOL (Alcon, Fort Worth, TX, USA).
A limited number of IOLs could be used for the smaller C‐MICS: AKREOS MI60 IOL (Bausch & Lomb), Lentis L‐303 IOL (WaveLight GmbH), AcrySof SN60WF IOL (Alcon), and ZEISS ASPHINA 509M.
The types of IOL used for B‐MICS, which was the smallest‐sized incision, were: Acri.Smart46s (Acti.Tec), Acri.Smart 48S, and MI60 IOL (Bausch & Lomb).
Excluded studies
We excluded 46 full‐text articles and documented our reasons for exclusion in the Characteristics of excluded studies tables.
Risk of bias in included studies
See 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary table (Figure 3).
2.
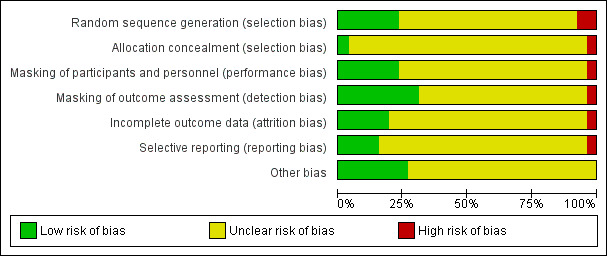
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
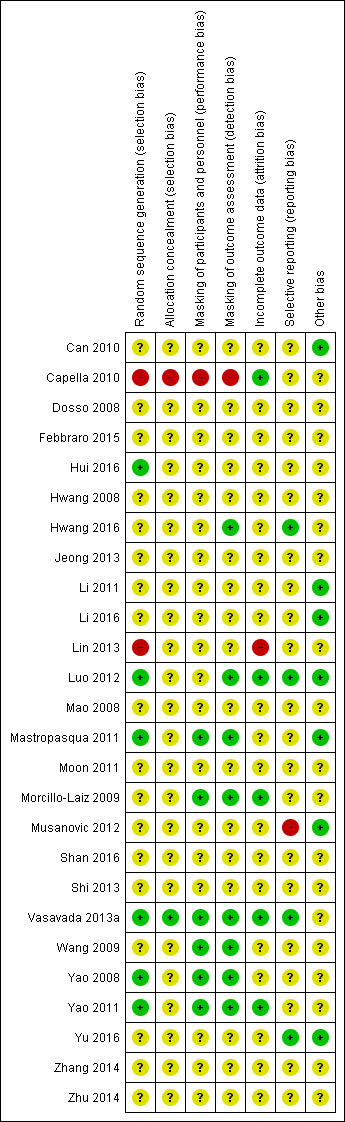
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged one trial to be at high risk, six trials at low risk, and 19 trials at unclear risk of selection bias based on the reporting of random sequence generation. When the study investigators reported the method of randomization, we judged the study to be at low risk of selection bias. We judged Capella 2010 to be at high risk of bias because it was reported as a "prospective and randomised study" (p269), but the authors noted through email correspondence that the treatment assignment was by "alternating one‐by‐one."
We judged the following six trials to be at low risk of bias. Luo 2012 used a random number generator, and participants were assigned by the remainder after dividing by 3. Participants in Mastropasqua 2011 were randomly assigned to group 1 or group 2 the day before surgery by block randomization (randomly assigned by computer‐generated numbers). After contacting the study investigator, Vasavada 2013a replied that "randomization was done using computer generated random numbers." Hui 2016, Yao 2008, and Yao 2011 used "random numbers table" for sequence generation. Study investigators of the remaining trials mentioned that participants were randomized but did not specify the methods for random sequence generation, therefore we judged them to be at unclear risk of bias.
We judged Capella 2010 to have inadequate allocation concealment; if the treatment was assigned by alternating one‐by‐one, participants might be able to guess their or the next treatment assignment. We judged Vasavada 2013a to have sufficient allocation concealment as the authors had informed us through email correspondence that the random number sequence was stored in a sequentially number and sealed envelope in a dedicated box in the operating room. The remaining trials did not provided sufficient information to determine whether treatment allocation was adequately concealed before randomization and were therefore judged to be at unclear risk of bias.
Masking (performance bias and detection bias)
We judged one trial to be high risk, six trials to be at low risk, and 19 trials at unclear risk of performance and detection bias. The authors of the trial judged to be at high risk of performance and detection bias reported through email that the participants, personnel, and outcome assessors were not masked. Trials that we judged to be at low risk of performance and detection bias explicitly reported masking of participants, personnel, and outcome assessors. Mastropasqua 2011 reported that participants and examiners performing preoperative and postoperative controls were masked to the surgical technique in each case. Morcillo‐Laiz 2009 was a double‐blind clinical trial, and neither the participants nor the examiners knew which technique had been applied. Vasavada 2013a reported only that the trial was "double blind" (participants and outcome assessors) in the article; after contacting the study investigator, we received the following reply: "the patients and outcome assessors were blind to the technique used, however, the surgeon was not. The outcome assessor was another ophthalmologist, and not the operating surgeon." Wang 2009 reported that participants and examiners were masked to the group assignment. In Yao 2008 and Yao 2011, participants were masked to their treatment groups; surgeons could not be masked, and standard surgical procedures were followed that were unlikely to result in bias. Study investigators of Luo 2012 mentioned that only the technician and surgeon were masked during postoperative examinations, so we judged this trial to be at low risk of detection bias and unclear risk of performance bias. Hwang 2016 did not report whether the participants were masked, but the image examiner was masked, so we judged this trial to be at low risk of detection bias and unclear risk of performance bias.
Incomplete outcome data
We judged one trial to be at high risk of bias, five at low risk of bias, and the remaining 20 trials at unclear risk of bias. The five trials judged to be at low risk of bias reported that either no participants or no more than 10% of participants were lost to follow‐up. Capella 2010 reported that 3% of 37 participants were lost to follow‐up; Morcillo‐Laiz 2009 reported that 10% of 94 participants were lost to follow‐up; and in Yao 2011 9% of 89 participants were lost to follow‐up. We judged Lin 2013 to be at high risk of bias as participants were excluded postrandomization. Luo 2012 and Vasavada 2013a reported that no participants were lost to follow‐up and were judged to be at low risk of attrition bias.
Selective reporting
We judged one trial to be at high risk of reporting bias. In the methods description of Musanovic 2012, four outcomes were described; two of them, corneal keratometry and corneal astigmatism, were not reported in the results section.
We judged four trials to be at low risk of reporting bias. Four studies were registered (Hwang 2016; Luo 2012; Vasavada 2013a; Yu 2016). The outcomes that were described in the registry search were consistent with those reported in the papers.
We could not obtain the protocol or trial registration number for the remaining 21 trials and judged them to be at unclear risk of reporting bias. The authors of one trial noted through email correspondence that they did not publish the protocol or register the trial.
Other potential sources of bias
We judged seven trials to be at low risk of other potential sources of bias because they were not funded by device manufacturers or had declared no conflict of interest (Can 2010; Li 2011; Li 2016; Luo 2012; Mastropasqua 2011; Musanovic 2012; Yu 2016). The remaining 19 trials did not report their source of funding or conflict of interest, therefore we judged them to be at unclear risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
1. The larger C‐MICS versus standard phacoemulsification
Fifteen trials reported outcomes for this comparison (Can 2010; Febbraro 2015; Hwang 2008; Hwang 2016; Jeong 2013; Li 2011; Li 2016; Lin 2013; Luo 2012; Moon 2011; Musanovic 2012; Shan 2016; Shi 2013; Wang 2009; Zhu 2014). Shi 2013 included both eyes of 34 of 98 participants (35%) in the study, but they did not report to which group the eyes were allocated and did not account for intraperson correlation in their analysis. The larger C‐MICS refers to the C‐MICS with 2.2‐millimeter incision.
Surgically induced astigmatism (SIA)
Surgically induced astigmatism was reported in nine out of the 15 trials at three months' follow‐up (Febbraro 2015; Hwang 2016; Lin 2013; Moon 2011; Musanovic 2012; Shan 2016; Shi 2013; Wang 2009; Zhu 2014). None of the trials reported on SIA at one year postoperatively. Moon 2011 reported the mean SIA in both groups, but did not report the standard deviation (SD) or standard error and also did not state clearly what the reported exact P value was testing. The mean SIA in the larger C‐MICS group was 0.54 D, while it was 0.72 D in the standard phacoemulsification group.
The small difference suggested from eight trials may not have clinical importance (Analysis 1.1; mean difference (MD) ‐0.19 D, 95% confidence interval (CI) ‐0.30 to ‐0.09; 996 eyes; I2 = 73%). We judged the evidence at three months to be very low‐certainty due to unexplained statistical heterogeneity (downgraded one level) and high risk of bias, as one study was at high risk of selection and attrition bias, while another study was at high risk of reporting bias (downgraded two levels).
1.1. Analysis.
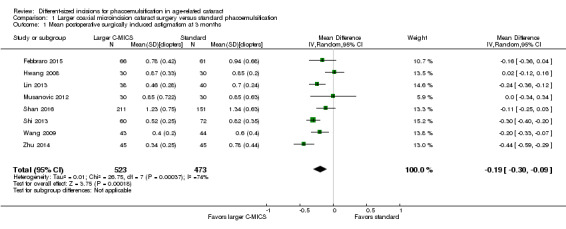
Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 1 Mean postoperative surgically induced astigmatism at 3 months.
Best‐corrected visual acuity (BCVA)
Seven of 15 trials reported BCVA at three months' follow‐up (Can 2010; Hwang 2008; Hwang 2016; Lin 2013; Moon 2011; Shan 2016; Wang 2009). Hwang 2008, Lin 2013, and Shan 2016 did not report the units of the BCVA measurement, which could be either decimal or logMAR units, therefore we did not include their data in the meta‐analysis. We also could not include Wang 2009's BCVA data as it was reported as the percentages of participants that had greater or equal to 1.0 D, 0.8 D, or 0.5 D. The authors of Wang 2009 also noted that "postoperative uncorrected distance visual acuity tended to be better with the smaller incisions, but this trend did not reach statistical significance (P = 0.07)."
At three months after surgery, three trials showed little or no difference between the larger C‐MICS group and the standard phacoemulsification group (Analysis 1.2; MD 0.00 logMAR units, 95% CI ‐0.02 to 0.02; 242 eyes; I2 = 29%). We judged the certainty of the evidence to be low due to unclear risk of selection bias and attrition bias (downgraded two levels).
1.2. Analysis.
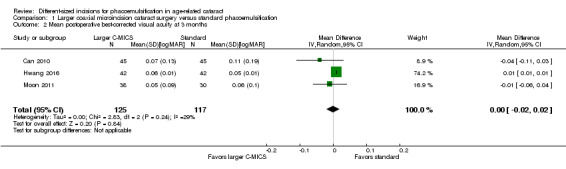
Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 2 Mean postoperative best‐corrected visual acuity at 3 months.
Endothelial cell loss (ECL)
Four of 15 trials reported mean ECL at three months' follow‐up (Li 2011; Moon 2011; Shan 2016; Zhu 2014). No significant difference was found between the larger C‐MICS and standard phacoemulsification (Analysis 1.3; MD ‐7.23 cells/mm2, 95% CI ‐78.66 to 64.20; 596 eyes; 4 trials; I2 = 0%). We judged the certainty of the evidence to be low due to risk of bias, as the studies were at unclear risk of selection, performance, detection, and attrition bias (downgraded two levels).
1.3. Analysis.
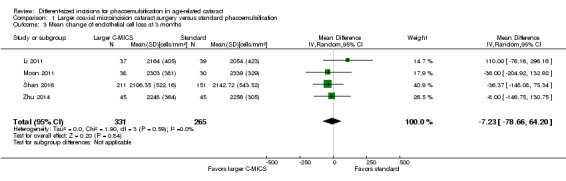
Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 3 Mean change of endothelial cell loss at 3 months.
Central corneal thickness (CCT)
Six of 15 trials reported this outcome at any time point (Can 2010; Hwang 2016; Jeong 2013; Li 2011; Wang 2009; Zhu 2014). Hwang 2016 did not report the standard deviation, but reported the mean % increase in CCT: the mean % increase in CCT was 1.00% in the larger C‐MICS group and 0.31% in the standard phacoemulsification group. Four of the five studies included in Analysis 1.4 reported the mean CCT at three months (Can 2010; Li 2011; Wang 2009; Zhu 2014). In contrast, Jeong 2013 reported the mean change in CCT. Since the reported the baseline mean CCT was similar in Jeong 2013, we decided it was appropriate to include their results in Analysis 1.4; at baseline the mean CCT was 530.02 (32.61) μm for the larger C‐MICS group and 534.86 (31.23) μm for the standard phacoemulsification group, therefore we included their data in the meta‐analysis.
1.4. Analysis.
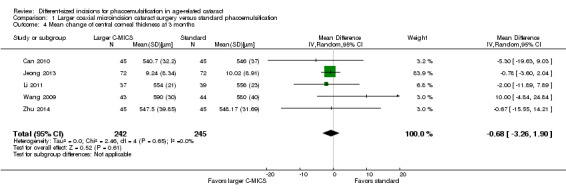
Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 4 Mean change of central corneal thickness at 3 months.
Five trials showed little or no difference in CCT between the larger C‐MICS and standard phacoemulsification groups at three months' postoperatively (Analysis 1.4; MD ‐0.68 μm, 95% CI ‐3.26 to 1.90; 487 eyes; I2 = 0%). We judged the certainty of the evidence to be low due to risk of bias, as the studies were at unclear risk of selection, performance, detection, and attrition bias (downgraded two levels).
Intraoperative parameters
Six of 15 trials described intraoperative parameters (Can 2010; Jeong 2013; Li 2011; Luo 2012; Shan 2016; Wang 2009).
Studies comparing the larger C‐MICS with standard phacoemulsification showed little or no difference in intraoperative use of cumulative dissipated energy (CDE) (Analysis 1.5; MD ‐0.30%, 95% CI ‐1.33 to 0.72; 784 participants; 6 trials), intraoperative use of balanced salt solutions (BSS) (Analysis 1.6; MD 2.87 mL, 95% CI ‐2.26 to 8.00; 80 eyes; 1 trial), surgical time (Analysis 1.7; MD ‐0.05 minutes, 95% CI ‐0.26 to 0.16; 314 eyes; 3 trials; I2 = 46%), and phacoemulsification time (Analysis 1.8; MD ‐0.96 seconds, 95% CI ‐3.48 to 1.56; 608 eyes; 4 trials; I2 = 55%). The duration of surgical time and phacoemulsification time depends on the skills and preferences of different surgeons. We judged the certainty of the evidence to be low as we downgraded for unexplained statistical heterogeneity (downgraded two levels).
1.5. Analysis.
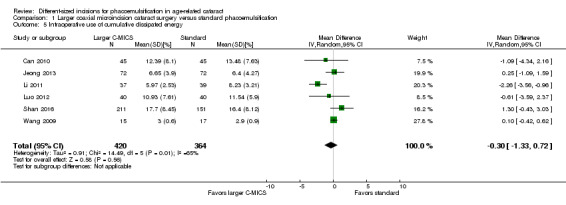
Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 5 Intraoperative use of cumulative dissipated energy.
1.6. Analysis.
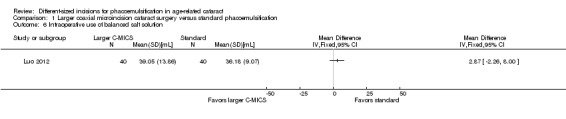
Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 6 Intraoperative use of balanced salt solution.
1.7. Analysis.
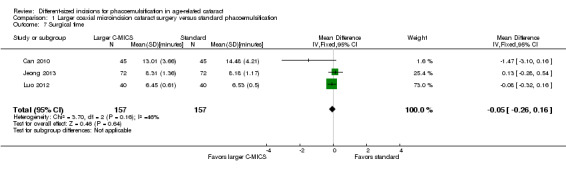
Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 7 Surgical time.
1.8. Analysis.
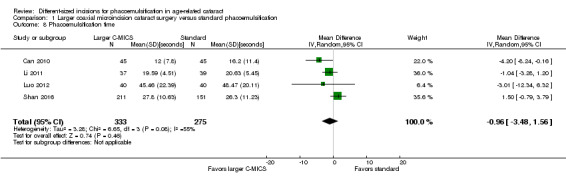
Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 8 Phacoemulsification time.
Adverse events
One of 15 trials reported that none of the participants experienced endophthalmitis or posterior capsule rupture (Shan 2016). The same trial reported little or no difference in the risk ratio (RR) of corneal edema (Analysis 1.9; RR 1.02, 95% CI 0.40 to 2.63; 362 eyes).
1.9. Analysis.
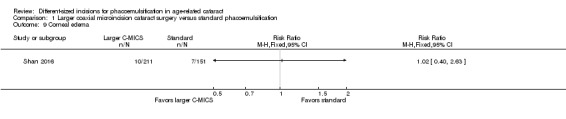
Comparison 1 Larger coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 9 Corneal edema.
Wang 2009 reported "no intraoperative complications."
Quality of life
None of the 15 trials reported on quality of life.
2. The smaller C‐MICS versus standard phacoemulsification
Nine trials compared these surgical approaches with respect to one or more outcomes of interest (Capella 2010; Dosso 2008; Febbraro 2015; Hui 2016; Luo 2012; Moon 2011; Yao 2011; Yu 2016; Zhang 2014). The smaller C‐MICS refers to the C‐MICS with 1.8‐millimeter incision.
Surgically induced astigmatism (SIA)
Seven of nine trials reported this outcome at three months' follow‐up (Capella 2010; Febbraro 2015; Hui 2016; Moon 2011; Yao 2011; Yu 2016; Zhang 2014). Two trials did not provide sufficient data to be included in a meta‐analysis (Moon 2011; Yu 2016). Yu 2016 reported the group means and exact P value at three months' follow‐up, and did not report the standard deviation by group, therefore the data could not be included in the meta‐analysis. Moon 2011 reported the mean SIA in both groups, but did not report the group standard deviation. The mean SIA was 0.43 D in the smaller C‐MICS group, and 0.72 D in the standard phacoemulsification group.
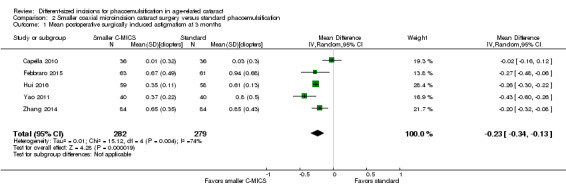
Meta‐analysis of five trials favored the smaller C‐MICS group over standard phacoemulsification (Analysis 2.1; MD ‐0.23 D, 95% CI ‐0.34 to ‐0.13; 561 eyes; I2 = 74%). The certainty of evidence was very low as we downgraded for unexplained statistical heterogeneity and high risk of selection, performance, and detection biases.
2.1. Analysis.
Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 1 Mean postoperative surgically induced astigmatism at 3 months.
Best‐corrected visual acuity (BCVA)
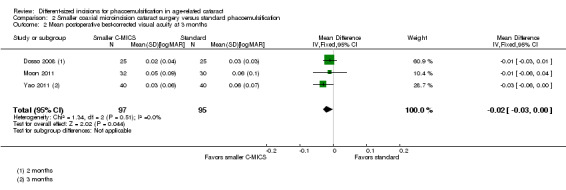
Three of nine trials reported this outcome at two to three months' follow‐up (Dosso 2008; Moon 2011; Yao 2011). The smaller C‐MICS group showed slightly better to no difference in BCVA results than standard phacoemulsification (Analysis 2.2; MD ‐0.02 logMAR unit, 95% CI ‐0.03 to ‐0.00; 192 eyes; I2 = 0%). The certainty of the evidence was low as we downgraded for high risk of bias as two studies were at unclear risk of selection, performance, detection, attrition, and reporting bias (downgraded two levels).
2.2. Analysis.
Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 2 Mean postoperative best‐corrected visual acuity at 3 months.
Endothelial cell loss (ECL)
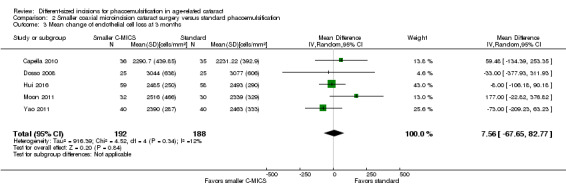
Five of nine trials reported ECL after surgery (Capella 2010; Dosso 2008; Hui 2016; Moon 2011; Yao 2011). At three months' postoperatively, meta‐analysis of five trials showed little or no difference between the smaller C‐MICS and standard phacoemulsification groups (Analysis 2.3; MD 7.56 cells/mm2, 95% CI ‐67.65 to 82.77; 380 eyes; I2 = 12%). We judged the certainty of the evidence to be low as we downgraded for high risk of selection, performance, and detection bias (downgraded two levels).
2.3. Analysis.
Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 3 Mean change of endothelial cell loss at 3 months.
Central corneal thickness (CCT)
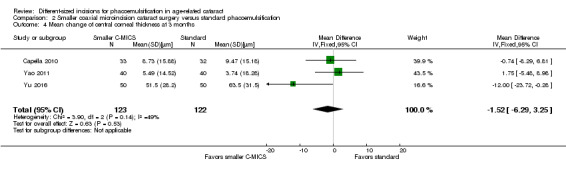
Three of nine trials reported CCT change (Capella 2010; Dosso 2008; Yao 2011). The meta‐analysis of the three trials showed little or no difference between the smaller C‐MICS and standard phacoemulsification groups at three months' postoperatively (Analysis 2.4; MD ‐1.52 μm, 95% CI ‐6.29 to 3.25; 245 eyes; I2 = 49%). We judged the certainty of the evidence to be low due to high risk of bias, including selection, performance, and detection bias (downgraded two levels).
2.4. Analysis.
Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 4 Mean change of central corneal thickness at 3 months.
Intraoperative parameters
Three of nine trials reported intraoperative parameters (Dosso 2008; Luo 2012; Yao 2011). The smaller C‐MICS group was slightly favored over the standard phacoemulsification group in CDE (Analysis 2.5; MD ‐4.25%, 95% CI ‐5.43 to ‐3.07; 360 eyes; 2 trials; I2 = 78%). The evidence showed little or no difference between groups for the use of BSS (Analysis 2.6; MD ‐1.93 mL, 95% CI ‐6.32 to 2.46; 130 eyes; 2 trials; I2 = 85%), surgical time (Analysis 2.7; MD ‐0.14 minutes, 95% CI ‐0.35 to 0.07; 130 eyes; 2 trials; I2 = 93%), and phacoemulsification time (Analysis 2.8; MD 8.20 seconds, 95% CI 2.88 to 13.52; 130 eyes; 2 trials; I2 = 97%). Authors of Yao 2011 reported effective phacoemulsification time, which is a different measure than that reported by the authors of Dosso 2008 and Luo 2012. We were not able to identify a citation to convert effective phacoemulsification time to phacoemulsification time. The authors of Yao 2011 reported the mean effective phacoemulsification time was 6.71 seconds (SD = 4.14) in the smaller C‐MICS group and 6.84 seconds (SD = 4.01) in the standard phacoemulsification group, showing little or no difference between the groups (MD ‐0.13 seconds, 95% CI ‐1.92 to 1.66; 40 eyes). We downgraded the certainty of the evidence to very low for unexplained statistical heterogeneity (downgraded two levels) and wide confidence intervals (downgraded one level).
2.5. Analysis.
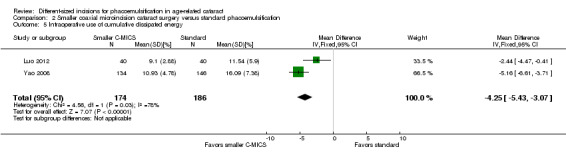
Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 5 Intraoperative use of cumulative dissipated energy.
2.6. Analysis.
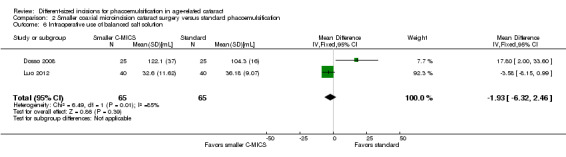
Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 6 Intraoperative use of balanced salt solution.
2.7. Analysis.
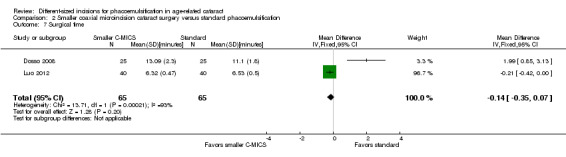
Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 7 Surgical time.
2.8. Analysis.
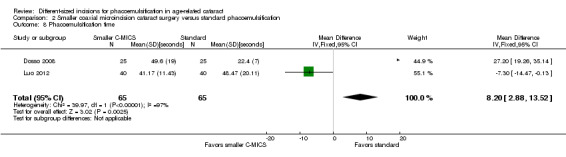
Comparison 2 Smaller coaxial microincision cataract surgery versus standard phacoemulsification, Outcome 8 Phacoemulsification time.
Adverse events
None of the nine trials reported on adverse events.
Quality of life
None of the nine trials reported on quality of life.
3. The smaller C‐MICS versus the larger C‐MICS
Six trials compared these surgical techniques (Can 2010; Febbraro 2015; Luo 2012; Mastropasqua 2011; Moon 2011; Vasavada 2013a). Since C‐MICS is used for two incision sizes and for clarity, we have referred to the smaller incision as C‐MICS with 1.8‐millimeter incision and the larger incision as C‐MICS with 2.2‐millimeter incision.
Surgically induced astigmatism (SIA)
Four of the six trials reported this outcome at three months' follow‐up (Febbraro 2015; Mastropasqua 2011; Moon 2011; Vasavada 2013a). We did not include Moon 2011 in the meta‐analysis as they reported the mean SIA in both groups, but did not report data to calculate SDs. The mean SIA was 0.43 D in the C‐MICS (1.8 mm) group, and 0.54 D in the larger C‐MICS group.
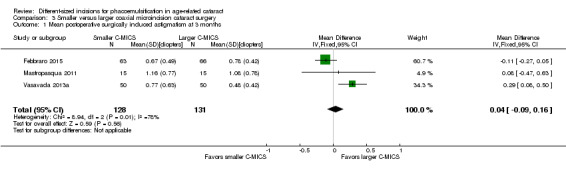
The meta‐analysis showed little or no difference in SIA between the smaller and larger C‐MICS groups in three trials (Analysis 3.1; MD 0.04 D, 95% CI ‐0.09 to 0.16; 259 eyes; I2 = 78%). We judged the evidence to be of low‐certainty as we downgraded for unexplained statistical heterogeneity (downgraded two levels).
3.1. Analysis.
Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 1 Mean postoperative surgically induced astigmatism at 3 months.
Best‐corrected visual acuity (BCVA)
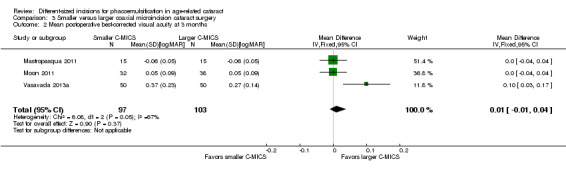
Three of the six trials reported this outcome (Mastropasqua 2011; Moon 2011; Vasavada 2013a). The meta‐analysis showed little or no difference in BCVA at three months' postoperatively (Analysis 3.2; MD 0.01 logMAR, 95% CI ‐0.01 to 0.04; 200 eyes; I2 = 67%). Moon 2011 reported a SD of 0.9 in both groups, which is abnormally high and therefore presumed to be a typo; we entered an SD of 0.09 instead. We judged the evidence to be of low‐certainty, downgrading for unexplained statistical heterogeneity (downgraded two levels).
3.2. Analysis.
Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 2 Mean postoperative best‐corrected visual acuity at 3 months.
Endothelial cell loss (ECL)
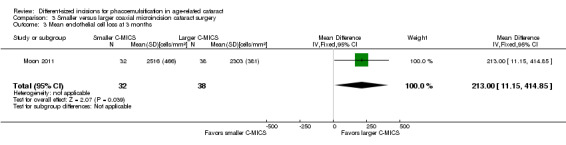
Moon 2011 was the only trial to report the mean ECL and showed less ECL in the larger C‐MICS group than in the smaller C‐MICS group (Analysis 3.3; MD 213.00 cells/mm2, 95% CI 11.15 to 414.85; 70 eyes). We judged the evidence to be of very low‐certainty, downgrading for risk of bias as the study was judged to be at unclear risk of selection, performance, detection, attrition, and reporting bias (downgraded one level) and imprecision, as only one trial reported ECL at three months' follow‐up (downgraded two levels).
3.3. Analysis.
Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 3 Mean endothelial cell loss at 3 months.
Central corneal thickness (CCT)
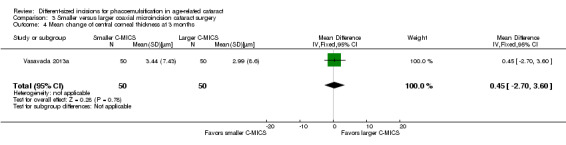
One of the six trials reported CCT (Vasavada 2013a). The mean change of CCT comparing smaller C‐MICS with larger C‐MICS was 3.44 ± 7.43 μm versus 2.99 ± 8.6 μm at three months' postoperatively, respectively. The difference in change of CCT was trivial or nonexistent comparing the two groups at three months' postoperatively (Analysis 3.4; MD 0.45 μm, 95% CI ‐2.70 to 3.60; 100 eyes). We judged the evidence to be of low‐certainty, downgrading for imprecision, as only one trial reported CCT at three months' follow‐up (downgraded two levels).
3.4. Analysis.
Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 4 Mean change of central corneal thickness at 3 months.
Intraoperative parameters
Four of five trials reported intraoperative parameters (Can 2010; Luo 2012; Mastropasqua 2011; Vasavada 2013a). Meta‐analysis showed little or no difference in the use of CDE (Analysis 3.5; MD ‐0.33%, 95% CI ‐3.72 to 3.07; 300 eyes; 4 trials; I2 = 82%) or the use of BSS (Analysis 3.6; MD 1.04 mL, 95% CI ‐2.45 to 4.53; 210 eyes; 3 trials; I2 = 82%). Two trials reported the mean surgical time for each group (Luo 2012; Vasavada 2013a). Meta‐analysis showed surgical times to be slightly longer in the smaller C‐MICS group compared with larger C‐MICS group (Analysis 3.7; MD 0.33 minutes, 95% CI 0.12 to 0.55; 180 eyes; I2 = 99%). The study investigators of Luo 2012 were the only ones to report mean phacoemulsification times and showed there was little or no difference in phacoemulsification time between the two groups (Analysis 3.8; MD ‐4.29 seconds, 95% CI ‐12.08 to 3.50; 80 eyes; I2 = 0%). We judged the evidence to be of low‐certainty, downgrading for unexplained statistical heterogeneity (downgraded one level) and imprecision of results (downgraded one level).
3.5. Analysis.
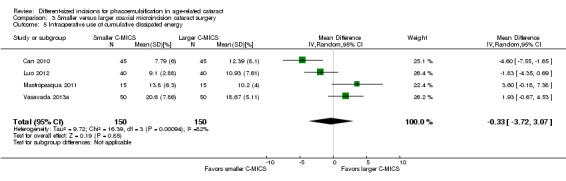
Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 5 Intraoperative use of cumulative dissipated energy.
3.6. Analysis.
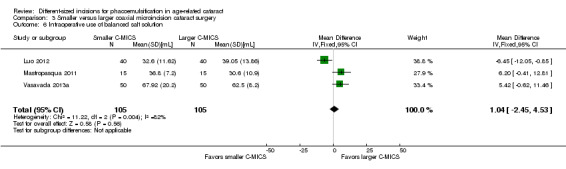
Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 6 Intraoperative use of balanced salt solution.
3.7. Analysis.
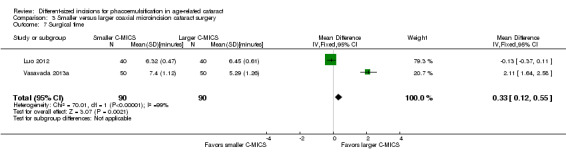
Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 7 Surgical time.
3.8. Analysis.
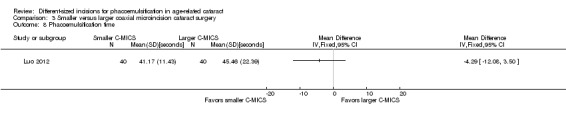
Comparison 3 Smaller versus larger coaxial microincision cataract surgery, Outcome 8 Phacoemulsification time.
Adverse events
None of the six trials reported on adverse events.
Quality of life
None of the six trials reported on quality of life.
4. B‐MICS versus standard phacoemulsification
We found four trials that compared these procedures (Can 2010; Mao 2008; Morcillo‐Laiz 2009; Yao 2008).
Surgically induced astigmatism (SIA)
Two of four trials reported this outcome at different postoperative time points (Morcillo‐Laiz 2009; Yao 2008). Meta‐analysis showed little or no difference between the B‐MICS group and the standard phacoemulsification group at three months (Analysis 4.1; MD ‐0.01 D, 95% CI ‐0.03 to 0.01; 368 eyes; I2 = 0%). We judged the evidence to be of moderate‐certainty, downgrading only for risk of bias, as the trials were judged to be at unclear risk of selection and reporting bias (downgraded one level).
4.1. Analysis.
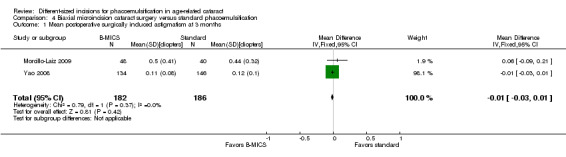
Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 1 Mean postoperative surgically induced astigmatism at 3 months.
Only Morcillo‐Laiz 2009 reported SIA at six months' postoperatively; the mean SIA comparing the B‐MICS group and the standard phacoemulsification group was 0.45 ± 0.35 D versus 0.41 ± 0.23 D, respectively. The trial results showed little or no difference between the B‐MICS group and the standard phacoemulsification group (Analysis 4.2; MD 0.04 D, 95% CI ‐0.08 to 0.16; 86 eyes).
4.2. Analysis.
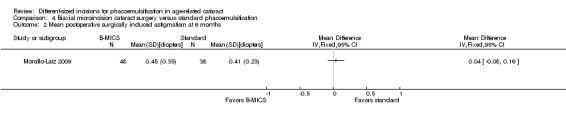
Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 2 Mean postoperative surgically induced astigmatism at 6 months.
Best‐corrected visual acuity (BCVA)
Three of four trials reported this outcome at three months (Can 2010; Morcillo‐Laiz 2009; Yao 2008). The meta‐analysis of the three trials favored B‐MICS over standard phacoemulsification, but the difference was trivial (Analysis 4.3; MD ‐0.02 logMAR units, 95% CI ‐0.04 to ‐0.00; 464 eyes; I2 = 71%). We judged the evidence to be of low‐certainty, downgrading for unexplained statistical heterogeneity (downgraded two levels).
4.3. Analysis.
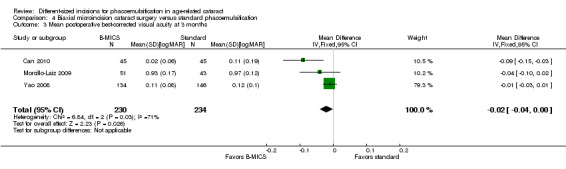
Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 3 Mean postoperative best‐corrected visual acuity at 3 months.
Endothelial cell loss (ECL)
One of four trials reported this outcome three months' postoperatively (Yao 2008). Evidence from study investigators of Yao 2008 reported that the mean ECL slightly favored B‐MICS over standard phacoemulsification, but this was clinically trivial (Analysis 4.4; MD 55.83 cells/mm2, 95% CI ‐34.93 to 146.59; 280 eyes). We graded the evidence as of low‐certainty, downgrading for imprecision and because only one trial reported ECL at three months' follow‐up (downgraded two levels).
4.4. Analysis.
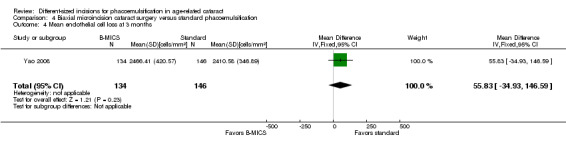
Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 4 Mean endothelial cell loss at 3 months.
Central corneal thickness (CCT)
One of four trials reported this outcome (Can 2010). Can 2010 found no clinically important difference between the two surgical approaches three months' postoperatively (Analysis 4.5; MD 0.10 μm, 95% CI ‐14.04 to 14.24; 90 eyes). We judged the evidence to be of low‐certainty, downgrading for imprecision and because only one trial reported CCT at three months' follow‐up (downgraded two levels).
4.5. Analysis.
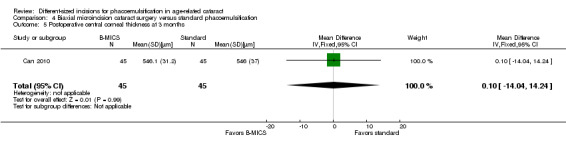
Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 5 Postoperative central corneal thickness at 3 months.
Intraoperative parameters
Two of four trials reported these outcomes (Can 2010; Yao 2008). Less CDE was used in the B‐MICS group than in the standard phacoemulsification group (Analysis 4.6; MD ‐5.27%, 95% CI ‐6.58 to ‐3.97; 370 eyes; 2 trials; I2 = 0%). The mean surgical time, reported by one study, was longer in the B‐MICS group compared with the standard phacoemulsification group (Analysis 4.7; MD 4.31 minutes, 95% CI 2.03 to 6.59; 90 eyes); however, the phacoemulsification time in the B‐MICS group was less than in the standard phacoemulsification group in two studies (Analysis 4.8; MD ‐5.58 seconds, 95% CI ‐9.52 to ‐1.63; 370 eyes; I2 = 0%).
4.6. Analysis.
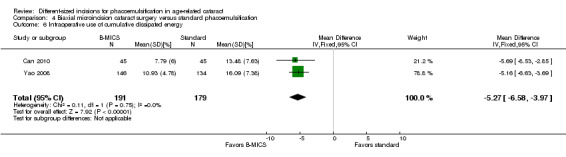
Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 6 Intraoperative use of cumulative dissipated energy.
4.7. Analysis.
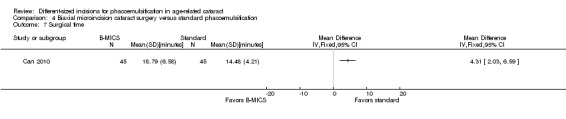
Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 7 Surgical time.
4.8. Analysis.
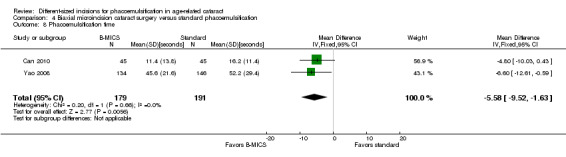
Comparison 4 Biaxial microincision cataract surgery versus standard phacoemulsification, Outcome 8 Phacoemulsification time.
We judged the evidence to be of low‐certainty, downgrading for imprecision of results and inconsistency (downgraded two levels).
Adverse events
None of the four trials reported on adverse events.
Quality of life
None of the four trials reported on quality of life.
Discussion
Summary of main results
Comparison between larger C‐MICS and standard phacoemulsification
Fifteen trials compared these interventions, but half of these trials or fewer reported data for each outcome of interest in this review (Table 1). The larger C‐MICS group was favored over the standard phacoemulsification group for SIA and BCVA at three months' follow‐up, but all differences were clinically small. The ECL, CCT change, and four intraoperative parameters showed little or no difference between the two groups. One trial reported on corneal edema, and another trial reported that there were no complications.
Comparison between smaller C‐MICS and standard phacoemulsification
Nine trials compared these phacoemulsification approaches. The smaller C‐MICS was favored over standard phacoemulsification for SIA and BCVA, but the differences were small (Table 2). There was little or no difference with respect to ECL and CCT change. Of the four intraoperative parameters, only CDE favored the smaller C‐MICS. The included trials did not report on adverse events.
Comparison between smaller C‐MICS and larger C‐MICS
Six trials reported on this comparison. We found little or no difference in SIA, BCVA, and CCT change at three months' follow‐up (Table 3). The surgical time in the larger C‐MICS showed a slight advantage over the smaller C‐MICS. However, other intraoperative parameters including CDE, phacoemulsification time, and the use of BSS showed little or no difference. None of the trials reported on adverse events.
Comparison between B‐MICS and standard phacoemulsification
Four trials reported on this comparison. We found no difference or a clinically unimportant difference in SIA, BCVA, ECL, and CCT at three months' follow‐up (Table 4). The surgical time was longer in the B‐MICS group than in the standard phacoemulsification group, whereas phacoemulsification time and CDE in the B‐MICS group was less than in the standard phacoemulsification group. None of the included trials reported on adverse events.
Overall completeness and applicability of evidence
We included 26 RCTs, of which half were conducted in China. Meta‐analysis was possible for the primary review outcome comparing all four different‐sized incisions: <= 1.5 mm, 1.8 mm, 2.2 mm, and approximately 3.0 mm. However, the meta‐analysis results were inconsistent when comparing all four different‐sized incisions, and the study methods were poorly reported. The low‐ to very low‐certainty evidence did not consistently show that phacoemulsification with smaller incisions was associated with less surgically induced astigmatism compared with phacoemulsification with larger incisions. Poor reporting of the methods of all included studies resulted in a judgement of mostly unclear risk of bias.
Potential biases in the review process
We followed standard Cochrane methods and have no potential biases to report.
Agreements and disagreements with other studies or reviews
Shentu 2016 conducted a meta‐analysis of 15 RCTs, reported as of May 2015, to compare the larger C‐MICS with 2.2‐millimeter incision and standard phacoemulsification. In this review, the larger C‐MICS had less SIA at one week and one month postoperatively; no between‐group differences were detected in BSS use, CDE, BCVA, or CCT change. Their findings were consistent with our review except for that of BCVA. Seventeen studies included in this review were not included in Shentu 2016 (Capella 2010; Febbraro 2015; Hui 2016; Hwang 2008; Jeong 2013; Li 2016; Lin 2013; Mao 2008; Mastropasqua 2011; Moon 2011; Musanovic 2012; Shan 2016; Shi 2013; Vasavada 2013a; Yao 2008; Yu 2016; Zhang 2014).
Our review findings that the astigmatic effects were the same for the smaller and larger C‐MICS are consistent with the conclusions of Febbraro 2015. Also, we agree with Lee 2009 that there were no significant differences in SIA, postoperative BCVA, or amount of BSS used between these two groups.
A meta‐analysis of 11 RCTs, Yu 2012, evaluated the outcomes of B‐MICS versus standard phacoemulsification and showed CDE and phacoemulsification time to be significantly less in the B‐MICS group, which is consistent with our review. However, their review showed less SIA in the B‐MICS group, which is not consistent with our review. The difference in conclusions might be the result of the inclusion of different trials. We excluded eight RCTs that were included in Yu 2012; our reasons for exclusion are listed in the Characteristics of excluded studies table (Alio 2005; Crema 2007; Denoyer 2008; Kahraman 2007; Kurz 2006; Kurz 2009; Mencucci 2006). Also, Mao 2008, which was included in this review, was not included in Yu 2012.
Authors' conclusions
Implications for practice.
This review was limited by the very low‐ to moderate‐certainty evidence that did not show a consistent benefit in smaller incisions with respect to surgically induced astigmatism. Furthermore, the lack of adequately reported adverse events does not inform patients or ophthalmologists of the potential harm of any of the procedures relative to others. Ultimately, the choice of surgical technique should be based on the patient's medical history, patient and physician preferences, and the skill of the surgeon.
Implications for research.
There is a need for better‐reported randomized controlled trials and randomized controlled trials that collect and report data on adverse events when comparing different‐sized incisions for age‐related cataract. Participants would be followed longer (at least three months) after surgery to investigate the rate of recovery for endothelial cells and the rehabilitation of visual acuity. Furthermore, better reporting of the randomization method, how the allocation was concealed before randomization, masking (participants, personnel, and outcome assessors), complete outcome data, methods, trial registry number, and source of funding is necessary. Availability of trial protocols and registrations should also be reported. In addition, study investigators must explicitly report all adverse events during and after surgery so as to assess the safety of using different‐sized incisions for phacoemulsification.
Acknowledgements
We acknowledge the Cochrane Eyes and Vision (CEV) Information Specialist for developing the search strategy and executing the electronic searches. We also acknowledge Ann Ervin and the CEV editorial team, and the peer reviewers (Matt Wade and Milan C Mathew), for their support and comments during preparation of this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Cataract explode all trees #2 MeSH descriptor Cataract Extraction explode all trees #3 MeSH descriptor Phacoemulsification explode all trees #4 MeSH descriptor Capsulorhexis explode all trees #5 (Cataract* near/4 (extract* OR aspirat* OR operat* OR remov* OR surg* OR excis* OR implant*)) #6 (lens* near/4 (extract* OR aspirat* OR operat* OR remov* OR surg* OR excis* OR implant* OR emulsif*)) #7 (Cataract Extraction* OR Phakectom* OR Zonulolys* OR catarectom*) #8 (pha*oemulsif* OR pha?o OR Capsulor*hexis OR lensectom*) #9 Microphaco* OR miniphaco* #10 Coaxial OR co‐axial OR biaxial OR bimanual OR Microcoaxial OR micro‐coaxial OR MICS OR C‐MICS OR B‐MICS OR MISICS OR SICS #11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10) #12 Incision* OR microincision* #13 MeSH descriptor Microsurgery, this term only with qualifier: MT #14 MeSH descriptor Surgical Procedures, Minimally Invasive, this term only #15 (#12 OR #13 OR #14) #16 (#11 AND #15)
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Cataract/ 13. exp cataract extraction/ 14. exp Phacoemulsification/ 15. exp Capsulorhexis/ 16. (Cataract* adj4 (extract* or aspirat* or operat* or remov* or surg*or excis* or implant*)).tw. 17. (lens* adj4 (extract* or aspirat* or operat* or remov* or surg* or excis* or implant* or emulsif*)).tw. 18. (Phakectom* or Zonulolys* or catarectom*).tw. 19. (pha*oemulsif* or pha?o or Capsulor*hexis or lensectom*).tw. 20. (Microphaco* or micro‐phaco or miniphaco* or mini‐phaco*).tw. 21. (Coaxial or co‐axial or biaxial or bimanual or Microcoaxial or micro‐coaxial).tw. 22. (C‐MICS or B‐MICS or MISICS or SICS or MICS).tw. 23. 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 24. (Incision* or microincision*).tw. 25. Microsurgery/mt [Methods] 26. Surgical Procedures, Minimally Invasive/ 27. 24 or 25 or 26 28. 11 and 23 and 27
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'cataract'/exp #34 (cataract* NEAR/4 (extract* OR aspirat* OR operat* OR remov* OR surg* OR excis* OR implant*)):ab,ti #35 (lens* NEAR/4 (extract* OR aspirat* OR operat* OR remov* OR surg* OR excis* OR implant* OR emulsif*)):ab,ti #36 'cataract extraction'/exp #37 'cataract extraction':ab,ti OR 'cataract extractions':ab,ti OR phakectom*:ab,ti OR zonulolys*:ab,ti OR catarectom*:ab,ti #38 'phacoemulsification'/exp #39 pha*oemulsif*:ab,ti OR phaco:ab,ti OR phako:ab,ti OR capsular*hexis:ab,ti OR lensectom*:ab,ti #40 'capsulorhexis'/exp #41 microphaco*:ab,ti OR miniphaco*:ab,ti #42 coaxial:ab,ti OR 'co axial':ab,ti OR biaxial:ab,ti OR bimanual:ab,ti OR microcoaxial:ab,ti OR 'micro coaxial':ab,ti #43 'c mics':ab,ti OR 'b mics':ab,ti OR misics:ab,ti OR sics:ab,ti OR mics:ab,ti #44 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 #45 'incision'/exp #46 incision*:ab,ti OR microincision*:ab,ti #47 'microsurgery'/de #48 #45 OR #46 OR #47 #49 #32 AND #44 AND #48
Appendix 4. PubMed search strategy
1. ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) 2. (Cataract*[tiab] AND (extract*[tiab] OR aspirat*[tiab] OR operate*[tiab] OR operati*[tiab]OR remov*[tiab] OR surge*[tiab] OR surgi*[tiab] OR excis*[tiab] OR implant*[tiab])) NOT Medline[sb] 3. (lens*[tiab] AND (extract*[tiab] OR aspirat*[tiab] OR operate*[tiab] OR operati*[tiab]OR remov*[tiab] OR surge*[tiab] OR surgi*[tiab] OR excis*[tiab] OR implant*[tiab])) NOT Medline[sb] 4. (Phakectom*[tiab] OR Zonulolys*[tiab] OR catarectom*[tiab]) NOT Medline[sb] 5. (phaco*[tiab] OR phako*[tiab] OR Capsulorhexis[tiab] OR Capsulorrhexis[tiab] OR lensectom*[tiab]) NOT Medline[sb] 6. (Microphaco*[tiab] OR micro‐phaco[tiab] OR miniphaco*[tiab]) NOT Medline[sb] 7. (Coaxial[tiab] OR co‐axial[tiab] OR biaxial[tiab] OR bimanual[tiab] OR Microcoaxial[tiab] OR micro‐coaxial[tiab]) NOT Medline[sb] 8. (C‐MICS[tiab] OR B‐MICS[tiab] OR MISICS[tiab] OR SICS[tiab] OR MICS[tiab]) NOT Medline[sb] 9. #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 10. (Incision*[tiab] OR microincision*[tiab]) NOT Medline[sb] 11. #1 AND #9 AND #11
Appendix 5. LILACS search strategy
(MH:C11.510.245$ OR MH:E04.540.825.249$ OR E04.943.875$ OR phacoemulsif$ OR phakoemulsif$ OR facoemulsif$ OR phaco$ OR phako$ OR capsulorhexis OR capsulorrhexis OR phakectom$ OR lensectom$ OR zonulolys$ OR catarectom$ OR cataract$ OR catarata$ OR lens$ OR Microphaco* OR miniphaco* OR Coaxial OR "co‐axial" OR biaxial OR bimanual OR Microcoaxial OR "C‐MICS" OR "B‐MICS" OR MISICS OR SICS OR MICS) AND (MH:E04.494$ OR MH:E05.591.580$ OR E04.502$ OR Incision$ OR microincision$ OR microsurger$)
Appendix 6. metaRegister of Controlled Trials search strategy
(cataract OR Phacoemulsification OR Capsulorhexis OR phaco* OR phako* OR microphaco* OR Coaxial OR co‐axial OR biaxial OR bimanual OR Microcoaxial OR MISICS OR SICS OR MICS) AND (incision* OR microincision* OR microsurgery)
Appendix 7. ClinicalTrials.gov search strategy
(Cataract OR Phacoemulsification OR Capsulorhexis OR phaco OR phako OR microphaco OR Coaxial OR "co‐axial" OR biaxial OR bimanual OR Microcoaxial OR "C‐MICS" OR "B‐MICS" OR MISICS OR SICS OR MICS) AND (incision OR microincision OR microsurgery)
Appendix 8. WHO ICTRP search strategy
Cataract AND incision OR cataract AND microincision OR cataract AND microsurgery OR Phacoemulsification AND incision OR Phacoemulsification AND microincision OR Phacoemulsification AND microsurgery OR Phakoemulsification AND incision OR Phakoemulsification AND microincision OR Phakoemulsification AND microsurgery OR Capsulorhexis AND incision OR Capsulorhexis AND microincision OR Capsulorhexis AND microsurgery OR Capsulorrhexis AND incision OR Capsulorrhexis AND microincision OR Capsulorrhexis AND microsurgery OR Lensectomy AND incision OR Lensectomy AND microincision OR Lensectomy AND microsurgery OR phaco AND incision OR phaco AND microincision OR phaco AND microsurgery OR phako AND incision OR phako AND microincision OR phako AND microsurgery OR microphaco AND incision OR microphaco AND microincision OR microphaco AND microsurgery OR Coaxial AND incision OR Coaxial AND microincision OR Coaxial AND microsurgery OR "co‐axial" AND incision OR "co‐axial" AND microincision OR "co‐axial" AND microsurgery OR biaxial AND incision OR biaxial AND microincision OR biaxial AND microsurgery OR bimanual AND incision OR bimanual AND microincision OR bimanual AND microsurgery OR Microcoaxial AND incision OR Microcoaxial AND microincision OR Microcoaxial AND microsurgery
Phakectomy AND incision OR Phakectomy AND microincision OR Phakectomy AND microsurgery OR Zonulolyses AND incision OR Zonulolyses AND microincision OR Zonulolyses AND microsurgery OR Zonulolysis AND incision OR Zonulolysis AND microincision OR Zonulolysis AND microsurgery OR Catarectomy AND incision OR Catarectomy AND microincision OR Catarectomy AND microsurgery OR "C‐MICS" AND incision OR "C‐MICS" AND microincision OR "C‐MICS" AND microsurgery OR "B‐MICS" AND incision OR "B‐MICS" AND microincision OR "B‐MICS" AND microsurgery OR MISICS AND incision OR MISICS AND microincision OR MISICS AND microsurgery OR SICS AND incision OR SICS AND microincision OR SICS AND microsurgery OR MICS AND incision OR MICS AND microincision OR MICS AND microsurgery
Data and analyses
Comparison 1. Larger coaxial microincision cataract surgery versus standard phacoemulsification.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean postoperative surgically induced astigmatism at 3 months | 8 | 996 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.30, ‐0.09] |
| 2 Mean postoperative best‐corrected visual acuity at 3 months | 3 | 242 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.02, 0.02] |
| 3 Mean change of endothelial cell loss at 3 months | 4 | 596 | Mean Difference (IV, Random, 95% CI) | ‐7.23 [‐78.66, 64.20] |
| 4 Mean change of central corneal thickness at 3 months | 5 | 487 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐3.26, 1.90] |
| 5 Intraoperative use of cumulative dissipated energy | 6 | 784 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.33, 0.72] |
| 6 Intraoperative use of balanced salt solution | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Surgical time | 3 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.26, 0.16] |
| 8 Phacoemulsification time | 4 | 608 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐3.48, 1.56] |
| 9 Corneal edema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Smaller coaxial microincision cataract surgery versus standard phacoemulsification.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean postoperative surgically induced astigmatism at 3 months | 5 | 561 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.34, ‐0.13] |
| 2 Mean postoperative best‐corrected visual acuity at 3 months | 3 | 192 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.03, ‐0.00] |
| 3 Mean change of endothelial cell loss at 3 months | 5 | 380 | Mean Difference (IV, Random, 95% CI) | 7.56 [‐67.65, 82.77] |
| 4 Mean change of central corneal thickness at 3 months | 3 | 245 | Mean Difference (IV, Fixed, 95% CI) | ‐1.52 [‐6.29, 3.25] |
| 5 Intraoperative use of cumulative dissipated energy | 2 | 360 | Mean Difference (IV, Fixed, 95% CI) | ‐4.25 [‐5.43, ‐3.07] |
| 6 Intraoperative use of balanced salt solution | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐1.93 [‐6.32, 2.46] |
| 7 Surgical time | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.35, 0.07] |
| 8 Phacoemulsification time | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | 8.20 [2.88, 13.52] |
Comparison 3. Smaller versus larger coaxial microincision cataract surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean postoperative surgically induced astigmatism at 3 months | 3 | 259 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.09, 0.16] |
| 2 Mean postoperative best‐corrected visual acuity at 3 months | 3 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.04] |
| 3 Mean endothelial cell loss at 3 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 213.00 [11.15, 414.85] |
| 4 Mean change of central corneal thickness at 3 months | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.45 [‐2.70, 3.60] |
| 5 Intraoperative use of cumulative dissipated energy | 4 | 300 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐3.72, 3.07] |
| 6 Intraoperative use of balanced salt solution | 3 | 210 | Mean Difference (IV, Fixed, 95% CI) | 1.04 [‐2.45, 4.53] |
| 7 Surgical time | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.12, 0.55] |
| 8 Phacoemulsification time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Biaxial microincision cataract surgery versus standard phacoemulsification.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean postoperative surgically induced astigmatism at 3 months | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 2 Mean postoperative surgically induced astigmatism at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Mean postoperative best‐corrected visual acuity at 3 months | 3 | 464 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.04, ‐0.00] |
| 4 Mean endothelial cell loss at 3 months | 1 | 280 | Mean Difference (IV, Fixed, 95% CI) | 55.83 [‐34.93, 146.59] |
| 5 Postoperative central corneal thickness at 3 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐14.04, 14.24] |
| 6 Intraoperative use of cumulative dissipated energy | 2 | 370 | Mean Difference (IV, Fixed, 95% CI) | ‐5.27 [‐6.58, ‐3.97] |
| 7 Surgical time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Phacoemulsification time | 2 | 370 | Mean Difference (IV, Fixed, 95% CI) | ‐5.58 [‐9.52, ‐1.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Can 2010.
| Methods |
Study design: parallel‐group RCT Number randomized: 135 eyes of 96 participants in total; 45 eyes of 32 participants in each of the 3 groups Exclusions after randomization: none reported Number analyzed: not reported, assumed to be: 135 eyes of 96 participants in total; 45 eyes of 32 participants in each of the 3 groups Unit of analysis: unclear as trial investigators did not report how 39 participants with both eyes included were analyzed Unit of randomization: unclear as trial investigators did not report whether 39 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no |
|
| Participants |
Country: Turkey Mean age (SD): 64.5(NR) overall: 61.5 (8.1) for the B‐MICS with 1.2 to 1.4 trapezoidal/1.8‐millimeter incision group 65.8 (13.2) for the larger C‐MICS group 66.2 (12.6) for the standard phacoemulsification with 2.8‐millimeter incision group Gender: 50 men and 46 women overall: 14 men (44%) and 18 women (56%) in the B‐MICS with 1.2 to 1.4 trapezoidal/1.8‐millimeter incision group 17 men (55%) and 14 women (45%) in the larger C‐MICS group 19 men (58%) and 14 women (42%) in the standard phacoemulsification with 2.8‐millimeter incision group Inclusion criteria: not reported Exclusion criteria: previous ocular surgery or eye disease that might affect the final visual acuity (amblyopia, corneal scar, glaucoma, retinal or macular disorders, etc.) Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: B‐MICS with 1.2 to 1.4 trapezoidal/1.8‐millimeter incision group Intervention 2: larger C‐MICS group Intervention 3: standard phacoemulsification with 2.8‐millimeter incision group The type of IOL inserted was not reported. Length of follow‐up: Planned: not reported Actual: 90 days |
|
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: VA, pachymetric differences, SIA, phacoemulsification time, CDE (i.e. average ultrasound power), and effective phaco time (time required had 100% power been used throughout), complications Adverse events reported: yes, 1 eye with capsule rupture in 2.8‐millimeter group Intervals at which outcomes assessed: 1 day, 7 days, 30 days, and 90 days |
|
| Notes |
Full study name: Coaxial, microcoaxial, and biaxial microincision cataract surgery prospective comparative study Type of study: published Funding sources: not reported Disclosures of interest: No author has a financial or proprietary interest in any material or method mentioned. Study period: November 2006 to September 2008 Trial registration: not reported We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | The study did not report whether participants were masked. |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not report how many participants were followed at each time point. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Low risk | Source of funding not reported. Study investigators declared "no author has a financial or proprietary interest in any material or method mentioned" (p740). |
Capella 2010.
| Methods |
Study design: paired‐eye RCT Number randomized: 74 eyes of 37 participants; 37 eyes of 37 participants per group Exclusions after randomization: none reported Number analyzed: 72 eyes of 36 participants at 3 months; 36 eyes of 36 participants per group Unit of analysis: 1 eye of each participant Unit of randomization: both eyes of each participant included; 1 eye is randomized to smaller C‐MICS and the other eye to standard phacoemulsification Losses to follow‐up: 1 participant at 3 months How were missing data handled? excluded from analysis Reported power calculation: no Unusual study design: the study used paired‐eye design, but did not used paired analysis. |
|
| Participants |
Country: Spain Mean age (SD): 72.97 (NR) years overall; age not reported by group Age range: 52 to 84 years Gender: 12 men (32%) and 25 women (68%) overall; number per group not reported Inclusion criteria: over 50 years of age, bilateral senile cataracts intervened for cataract surgery in both eyes between September 2008 and April 2010 Exclusion criteria: corneal pathology, previous ocular trauma or surgery, infectious and/or inflammatory ocular pathology, and astigmatism exceeding 3 diopters Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: smaller C‐MICS Intervention 2: standard phacoemulsification with 2.8‐millimeter incision Akreos MI60 IOL (Bausch & Lomb) was inserted for both groups. Length of follow‐up: Planned: 3 months Actual: 3 months |
|
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined by the study report: SIA, ECL, CCT, and foveal thickness Adverse events reported: no Intervals at which outcomes assessed: preoperatively and day 1, 1 week, 1 month, and 3 months after surgery |
|
| Notes |
Full study name: Comparative study of coaxial microincision cataract surgery and standard phacoemulsification Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: September 2008 to April 2010 Trial registration: not reported We emailed the authors and included data provided through email correspondence. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The published reported stated that it was a prospective randomized study, but through email correspondence on 24 January 2017, the authors reported assigning treatment by "alternating one‐by‐one." From the published reported: "prospective and randomised study" (p269) |
| Allocation concealment (selection bias) | High risk | The published reported stated that it was a prospective randomized study, but through email correspondence the authors reported assigning treatment by "alternating one‐by‐one." |
| Masking of participants and personnel (performance bias) | High risk | We were informed through email correspondence that participants and personnel were not masked. |
| Masking of outcome assessment (detection bias) | High risk | We were informed through email correspondence that outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 out of 37 participants (less than 3%) lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Trial registration not reported. We were informed through email correspondence that the protocol was not published and the trial was not registered. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
Dosso 2008.
| Methods |
Study design: parallel‐group RCT
Number randomized: 50 eyes of 50 participants total; 25 eyes of 25 participants in each group
Exclusions after randomization: none reported
Number analyzed: not reported, assumed to be 50 eyes of 50 participants total and 25 eyes of 25 participants in each group
Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no |
|
| Participants |
Country: Switzerland
Mean age: 75 years overall: 75 years for the C‐MICS group 75 years for the standard phacoemulsification group Gender: 22 men (41%) and 28 women (59%) in total: 8 men (32%) and 17 women (68%) in the smaller C‐MICS group 14 men (53%) and 11 women (47%) in the standard phacoemulsification group Inclusion criteria: people who had nuclear or corticonuclear cataract of grade 2 to 4 according to the Lens Opacities Classification System III scale Exclusion criteria: people who had corneal pathology, inflammatory eye disease, glaucoma, previous ocular surgery or trauma, and endothelial cell density less than 1500 cells/mm2 Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: smaller C‐MICS with 1.6‐millimeter incision; enlarged to 1.8 mm
Intervention 2: standard phacoemulsification with 2.8‐millimeter incision
Lentis L‐303 IOL (WaveLight GmbH) was inserted for both groups. Length of follow‐up: Planned: not reported Actual: 8 weeks |
|
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: BCVA, CCT, and ECL Adverse events reported: no Intervals at which outcomes assessed: preoperatively and postoperatively at 1 and 8 weeks |
|
| Notes |
Full study name: Outcomes of coaxial microincision cataract surgery versus conventional coaxial cataract surgery
Type of study: published
Funding sources: not reported
Disclosures of interest: Dr Di Nardo is an employee of Oertli Instrumente. No other author has a financial or proprietary interest in any material or method mentioned.
Study period: January 2007 to March 2007 Trial registration: not reported We attempted to contact the authors but the email was returned undeliverable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The participants were randomly assigned to have coaxial MICS or conventional coaxial phacoemulsification.”; but sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported. Protocol not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
Febbraro 2015.
| Methods |
Study design: parallel‐group RCT Number randomized: 190 eyes of 151 participants in total: 63 eyes of 52 participants in the smaller C‐MICS group 66 eyes of 52 participants in the larger C‐MICS group 61 eyes of 47 participants in the standard phacoemulsification group Exclusions after randomization: none reported Number analyzed: 190 eyes of 151 participants in total: 63 eyes of 52 participants in the smaller C‐MICS group 66 eyes of 52 participants in the larger C‐MICS group 61 eyes of 47 participants in the standard phacoemulsification group Unit of analysis: mixed "As the original dataset included both unilateral and bilateral cases, we reported for each comparison the estimates obtained considering all evaluated eyes (unilateral and bilateral cases) and then repeated the analysis after restricting the data set to one eye per patient by choosing one eye randomly (via a computer‐generated algorithm) in bilateral cases (data not shown)." (p262) Unit of randomization: 1 or both eyes per participant were assigned to the same treatment group. Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no |
|
| Participants |
Country: not reported, assumed to be in France (all surgeries were performed by the same surgeon (JLF))
Mean age: 75.1 (9.2) overall: 76.6 (7.6) in the smaller C‐MICS group 73.9 (8.4) in the larger C‐MICS group 74.7 (11.3) in the standard phacoemulsification group Age range: not reported Gender: 93 women (62%) and 58 men (38%) overall: 35 women (67%) and 17 men (33%) in the smaller C‐MICS group 37 women (71%) and 15 men (29%) in the larger C‐MICS group 21 women (47%) and 26 men (53%) in the standard phacoemulsification group Inclusion criteria: people undergoing cataract surgery Exclusion criteria: "eyes with associated corneal, vitreous, or retinal pathologies" (p262) |
|
| Interventions |
Intervention 1: smaller C‐MICS with MI 60 IOL (Bausch & Lomb, Rochester, NY, USA) inserted Intervention 2: larger C‐MICS with AcrySof Natural SN60WF IOL (Alcon, Fort Worth, TX, USA) inserted Intervention 3: standard phacoemulsification (3.2‐millimeter incision) with Adapt AO IOL (Bausch & Lomb, Rochester, NY, USA) inserted We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. Length of follow‐up: Planned: not reported Actual: 1 month |
|
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: keratometric astigmatism (using the same autokeratometer (Tonoref II; Nidek, Aichi, Japan); the Tonoref II measures the corneal curvature based on the projection onto the cornea of 4 near‐infrared rays, scalar comparison of astigmatism, with‐the‐wound change, and against‐the‐wound change Adverse events reported: no Intervals at which outcomes assessed: 1 month |
|
| Notes |
Full study name: Astigmatic equivalence of 2.2‐mm and 1.8‐mm superior clear corneal cataract incision
Type of study: published full text
Funding sources: not reported
Disclosures of interest: not reported
Study period: not reported Trial registration: not reported We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None of the participants were reported to have been lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Trial registry number and protocol were not reported, therefore we could not compare outcomes reported in the published full text and the trial registry/protocol. |
| Other bias | Unclear risk | Trial register number and funding sources were not reported. |
Hui 2016.
| Methods |
Study design: parallel‐group RCT
Number randomized: 117 eyes of 85 participants in total: 59 eyes of 43 participants in the smaller C‐MICS group 58 eyes of 42 participants in the standard phacoemulsification group Exclusions after randomization: none reported Number analyzed: 85 participants with 117 eyes in total: 59 eyes of 43 participants in the smaller C‐MICS group 58 eyes of 42 participants in the standard phacoemulsification group Unit of analysis: unclear as trial investigators did not report how 32 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 32 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no |
|
| Participants |
Country: China
Mean age (SD): 64.1 (8.6) overall: 64.8 (8.5) in the smaller C‐MICS group 63.3 (8.8) in the standard phacoemulsification group Gender: 40 men (47%) and 45 women (53%) overall: 21 men (49%) and 22 women (51%) in the smaller C‐MICS group 19 men (45%) and 23 women (55%) in the standard phacoemulsification group Inclusion criteria: “(1)根据LOCSIII分级标准,晶状体核硬度为II˜III级;(2)角膜屈光度<1.0 diopters;(3)眼轴22 ˜ 24.5mm;(4)角膜内皮细胞密度>1500 个/mm2。” (p1829) (1) people with cataract nucleus graded II‐III, according to the Emery‐Little classification; (2) corneal diopter less than 1.0 diopters; (3) axial lengths 22 to 24.5 mm; (4) endothelial cell density more than 1500 cells/mm2 Exclusion criteria: “(1)角膜变性、葡萄膜炎、青光眼、高度近视眼、视神经 病变、视网膜病变、年龄相关性黄斑变性、眼外伤病史、准分子激光手术和其他内眼手术史;(2) 全身严重疾病史不能耐受手术者。” (p1829) (1) corneal degeneration, uveitis, glaucoma, high myopia, optic neuropathy, retinopathy, age‐related macular degeneration, history of eye injury, history of LASIK; (2) history of systemic disorders that could not tolerate operation Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: smaller C‐MICS with Akreos MI60 IOL inserted Intervention 2: standard phacoemulsification with 3.2‐millimeter incision with Adapt‐AO IOL inserted Planned: not reported Actual: 90 days |
|
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: uncorrected VA, average ultrasound energy, effective phacoemulsification time, ECL, and corneal topography Adverse events reported: yes, temporary increase in intraocular pressure Intervals at which outcomes assessed: 1 day, 7 days, 30 days, 90 days for uncorrected VA, 90 days for other outcomes |
|
| Notes |
Full study name: 同轴 1. 8mm 微切口白内障超声乳化吸除术的临床研究 [Clinical effects of coaxial 1.8 mm microincision phacoemulsification] Type of study: published full text Funding sources: 陕西省科学技术研究发展计划项目 [Shaanxi Province Science and Technology Research and Development Project (No. 2012K16‐11(5))] Disclosures of interest: not reported Trial registry number: not reported Study period: December 2012 to March 2015 We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “将患者按数字表法随机分为两组" [Participants were randomized into 2 groups using random number tables.] (p1829) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing data were reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for comparison. |
| Other bias | Unclear risk | No disclosures of interest, no trial registry number, no protocol |
Hwang 2008.
| Methods |
Study design: parallel‐group RCT Number randomized: 60 eyes of 55 participants overall: 30 eyes of 28 participants in the larger C‐MICS group 30 eyes of 27 participants in the standard phacoemulsification group Number randomized: 55 participants of 60 eyes overall: 30 eyes of 28 participants in the larger C‐MICS group 30 eyes of 27 participants in the standard phacoemulsification group Exclusions after randomization: none reported Number analyzed: unclear, assumed to be: 60 eyes of 55 participants overall: 30 eyes of 28 participants in the larger C‐MICS group 30 eyes of 27 participants in the standard phacoemulsification group Unit of analysis: unclear, as trial investigators did not report how 5 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 5 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no |
|
| Participants |
Country: South Korea Overall mean age: not reported Mean age (SD): 69.7 (NR) overall: 70.6 (9.2) in the larger C‐MICS group 68.8 (7.6) in the standard phacoemulsification group Age range: not reported Gender: 13 men and 15 women in the larger C‐MICS group; 11 men and 16 women in the standard phacoemulsification group Inclusion criteria: not reported Exclusion criteria: "patients with corneal disease, intraocular inflammation, glaucoma, and those who had previously undergone corneal surgery or surgery that could affect high‐order aberrations were excluded" (p1598) |
|
| Interventions |
Intervention 1: larger C‐MICS Intervention 2: standard phacoemulsification (2.8 mm) We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. SA60AT IOL (AcrySof; Alcon, USA) was inserted for both groups. Length of follow‐up: Planned: protocol not available Actual: 3 months |
|
| Outcomes |
Primary outcome, as defined in study reports: SIA
Secondary outcomes, as defined in study reports: Postoperative change of corneal higher‐order aberrations in each group at 1 month or 3 months after surgery Corneal higher‐order aberrations between the 2 groups preoperatively, at 1 month or 3 months after surgery Adverse events reported: not reported Intervals at which outcomes assessed: baseline, 1 month and 3 months |
|
| Notes |
Type of study: published full text Funding sources: Inje University Disclosures of interest: not reported Trial registry number: not reported Study period: July 2006 to December 2006 We attempted to contact the authors but the email was returned undeliverable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
Hwang 2016.
| Methods |
Study design: paired‐eye RCT
Number randomized: 42 participants with 42 eyes in total: 42 eyes of 42 participants in the larger C‐MICS group 42 eyes of 42 participants in the standard phacoemulsification group Exclusions after randomization: none reported Number analyzed: 42 participants with 42 eyes in total: 42 eyes of 42 participants in the larger C‐MICS group 42 eyes of 42 participants in the standard phacoemulsification group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none reported How were missing data handled? not reported Reported power calculation: none reported Unusual study design: the study randomized participants and included both eyes of the same participant in the same intervention, but they did not account for intraperson correlation. |
|
| Participants |
Country: Korea
Mean age (SD): 65.20 (NR) overall: 64.52 (10.65) in the larger C‐MICS group 65.87 (12.91) in the standard phacoemulsification group Gender: not reported Inclusion criteria: “patients with nuclear density from Grade 3–4, a dilated pupil of 7.0 mm or larger, and a corneal endothelial cell count (ECC) >2000 cells/mm2.” (p190) Exclusion criteria: “patients with history of uveitis or other intra‐ocular inflammation diseases, topical prostaglandin users, and intraoperative complications such as posterior lens capsule rupture and lens dislocation” (p190) Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: larger C‐MICS group
Intervention 2: standard phacoemulsification (2.75 mm) group MI60 IOL (Bausch & Lomb, Rochester, NY, USA) was inserted for both groups. Length of follow‐up: Planned: not reported Actual: 2 months |
|
| Outcomes |
Primary outcome, as defined in study reports: "1. Intraoperative measurements included: 1.1. Ultrasound time (UST) 1.2. Mean cumulative dissipated ultrasound energy (CDE) 1.3. Total balanced salt solution (BSS) use 2. Clinical measurements included preoperative, 1week postoperative, 1month postoperative and 2month postoperative: 2.1. Best corrected visual acuity (BCVA) 2.2. Central corneal thickness (CCT) 2.3. Endothelial cell count (ECC)" Secondary outcomes, as defined in study reports: enzymelinked immunosorbent assay (ELISA) and reverse transcription polymerase chain reaction (RTPCR) were performed for IL‐1alpha, IL‐6, VEGF, and PGE2 preoperatively and at 1 week postoperatively. Adverse events reported: not reported Intervals at which outcomes assessed: 1 day, 1 week, 1 month, and 2 months |
|
| Notes |
Full study name: Comparison of macular thickness and inflammatory cytokine levels after microincision versus small incision coaxial cataract surgery Type of study: published full text Funding sources: "Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (No. 2012R1A1A1038648) and the Institute of Clinical Medicine Research of Bucheon St. Mary’s Hospital, Research Fund, BCMC14AA07" (p194) Disclosures of interest: not reported Trial registry number: ISRCTN 69290731 Study period: not reported We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Low risk | “One examiner was masked as to whether the images were from the microincision group or the small incision group.” (p191) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None of the participants were reported to have been lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the outcomes in the registry have been reported. |
| Other bias | Unclear risk | Disclosures of interest were not reported. |
Jeong 2013.
| Methods |
Study design: parallel‐group RCT Number randomized: 144 eyes of 96 participants overall: 72 eyes of NR participants in the larger C‐MICS group 72 eyes of NR participants in the standard phacoemulsification group Exclusions after randomization: none reported, assumed to be none Number analyzed: 144 eyes of 96 participants overall: 72 eyes of NR participants in the larger C‐MICS group 72 eyes of NR participants in the standard phacoemulsification group Unit of analysis: unclear as trial investigators did not report how 48 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 48 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled?: no missing data reported Reported power calculation: no |
|
| Participants |
Country: South Korea Mean age (SD): 71.49 (NR) overall: 72.30 (10.85) in C‐MICS group 70.67 (8.27) in standard phacoemulsification group Age range: not reported Gender: not reported Inclusion criteria: people with cataract; no specific description Exclusion criteria: "patients with a history of preoperative corneal surgery, patients with diabetic retinopathy or iritis, and those who were previously diagnosed with keratopathy were excluded. The posterior capsular rupture or vitreous loss was excluded from the study." (p709) |
|
| Interventions |
Intervention 1: larger C‐MICS Intervention 2: standard phacoemulsification with 2.8‐millimeter incision We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. MI‐60 IOL (Bausch & Lomb, Rochester, NY, USA) was inserted for both groups. Length of follow‐up: Planned: not available Actual: 1 month |
|
| Outcomes |
Primary outcome, as defined in study reports: total UST, CDE, average torsional amplitude, fluid amount, case time, CCT increase
Secondary outcomes, as defined in study reports: ultrasound dynamic parameters measured during surgery and edema Adverse events reported: yes, burning of the cornea, no postoperative infection or persistent inflammation Intervals at which outcomes assessed: baseline, 1 day, and 1 month |
|
| Notes |
Type of study: published full text Funding sources: not reported Disclosures of interest: not reported Trial registry number: not reported Study period: not reported We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Authors may have not reported an outcome due to difficulty assigning it. "To assess corneal stress after surgery, it may be helpful to measure the density of endothelial cells and measure the thickness and recovery pattern of the corneal incision site. In the present study, it was difficult to measure endothelial cells in the presence of central corneal edema 1 day after surgery. Therefore, there is a limitation that it is excluded." |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
Li 2011.
| Methods |
Study design: parallel‐group RCT Number randomized: 76 eyes of 71 participants in total: 37 eyes of NR participants in the larger C‐MICS group 39 eyes of NR participants in the standard phacoemulsification group Exclusions after randomization: none reported Number analyzed: not reported, assumed to be: 76 eyes of 71 participants total; 37 eyes in the larger C‐MICS group 39 eyes in the standard phacoemulsification group Unit of analysis: unclear as trial investigators did not report how 5 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 5 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no |
|
| Participants |
Country: Korea Mean age: overall 68.53 years: 66.83 years in the larger C‐MICS group 69.25 years in the standard phacoemulsification group Age range: 57 to 85 overall Gender: 39 men (55%) and 32 women (45%) overall; number per group not reported Inclusion criteria: age‐related cataracts included eyes with grade II‐IV nuclear cataracts (Lens Opacities Classification System (LOCS) III) Exclusion criteria: “presence of pre‐existing corneal disease or degeneration, an incision that required enlarging to insert an IOL, and complicated or excessively prolonged surgery” Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: larger C‐MICS Intervention 2: standard phacoemulsification with 2.8‐millimeter incision They type of IOL was not reported; "in both the 2.2 and 2.8 mm groups, the IOL type and the IOL injector system were matched, except for the incision size, to minimize bias." (p201) Length of follow‐up: Planned: 1 month Actual: 1 month |
|
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: CDE, UST, ECL, and CCT Adverse events reported: not reported Intervals at which outcomes assessed: 1 day before surgery, and postoperatively at 1 day, 1 week, and 1 month |
|
| Notes |
Full study name: Early changes in corneal edema following torsional phacoemulsification using anterior segment optical coherence tomography and Scheimpflug photography Type of study: published Funding sources: Korea Healthcare Technology R&D Project, Ministry for Health Welfare & Family Affairs, Republic of Korea (A 090573) Disclosures of interest: not reported Study period: May to July 2009 Trial registration: not reported We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study mentioned that participants were randomized, but did not specify methods for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not reported. Trial registration not reported. |
| Other bias | Low risk | Source of funding reported, conflicts of interest not reported. "This work was supported by the Korea Healthcare Technology R&D Project, Ministry for Health Welfare & Family Affairs, Republic of Korea (A 090573)." (p203) |
Li 2016.
| Methods |
Study design: parallel‐group RCT
Number randomized: 90 eyes of 90 participants in total; 45 eyes of 45 participants in each group
Exclusions after randomization: none reported
Number analyzed: 90 eyes of 90 participants in total; 45 eyes of 45 participants in each group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no Unusual study design: none |
|
| Participants |
Country: China
Mean age (SD): 64.1 (8.6) overall: 51.5 (4.2) in C‐MICS 2.2 mm and IOL implantation group 50.2 (3.9) in standard phacoemulsification 3.0 mm and IOL implantation group Age range: 45 to 62 overall: 45 to 62 in coaxial C‐MICS 2.2 mm and IOL implantation group 46 to 61 in standard phacoemulsification 3.0 mm and IOL implantation group Gender: 49 men and 41 women overall: 25 men (56%) and 20 women (44%) in C‐MICS 2.2 mm and IOL implantation group 24 men (53%) and 21 women (47%) in standard phacoemulsification 3.0 mm and IOL implantation group Inclusion criteria: "patients receiving phacoemulsification cataract extraction surgeries" (p81) Exclusion criteria: "other eye diseases, severe autoimmune system diseases, and systemic connective tissue diseases" (p81) Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: C‐MICS 2.2 mm and IOL implantation Intervention 2: standard phacoemulsification 3.0 mm and IOL implantation Akreos MI60 IOL was inserted for both groups. Length of follow‐up: Planned: not reported Actual: 30 days |
|
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: dry eye symptom score, break‐up time, Schirmer's 1 test, and corneal fluorescein staining score Adverse events reported: yes, temporary increase in intraocular pressure Intervals at which outcomes assessed: 10, 20, 30 days |
|
| Notes |
Full study name: Changes of the ocular surface and tear film after clear corneal incision phacoemulsification with different incision sizes Type of study: published full text Funding sources: "海南省卫生厅科研项目(No.琼卫2012PT‐28)作者单位:(570102)中国海南省海口市,海南医学院附属医院眼科" (p80) Research of Hainan Provincial Health Department (No.琼卫2012PT‐28) Disclosures of interest: not reported Study period: May 2013 to May 2014 Trial registration: not reported We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not reported. Trial registration not reported. |
| Other bias | Low risk | Source of funding reported, conflicts of interest not reported "海南省卫生厅科研项目(No. 琼卫2012PT‐28) 作者单位:(570102)中国海南省海口市,海南医学院附属医院 眼科" (p80) |
Lin 2013.
| Methods |
Study design: parallel‐group RCT
Number randomized: 78 eyes of 56 participants in total: 38 eyes of NR participants in the larger C‐MICS group 40 eyes of NR participants in the standard phacoemulsification (3.0‐millimeter incision) group Exclusions after randomization: none reported Number analyzed: not reported, assumed to be: 78 eyes of 56 participants in total: 38 eyes of NR participants in the larger C‐MICS group 40 eyes of NR participants in the standard phacoemulsification (3.0‐millimeter incision) group Unit of analysis: unclear as trial investigators did not report how 22 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 22 participants were assigned to the same or different groups Losses to follow‐up: not reported How were missing data handled? not reported Reported power calculation: no Unusual study design: the study randomized participants and included both eyes of the same participant in the same intervention, but they did not account for intraperson correlation. |
|
| Participants |
Country: China
Mean age: 71.3 years overall: not reported by group
Gender: not reported
Inclusion criteria: “诊断为老年性白内 障,患者年龄大于50 岁,裂隙灯下观察见患眼晶状体混浊。 术前检查角膜内皮 细胞计数大于1200 个/mm2。” (p1465) age‐related cataract with age over 50 with preoperation endothelial cell count larger than 1200/mm2 Exclusion criteria: “排除增殖期糖尿病视网膜病变、青光眼、老年性黄斑变性等眼部疾病及既 往有内眼手术史的患者。 术中出现后囊膜破裂、玻璃体溢 出等并发症者、不能完成 3mo 随访者予以剔除。 ” (p1465) diabetic retinopathy, glaucoma, age‐related macular degeneration, previous operation, posterior capsule rupture during surgery, vitreous loss in the surgery Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: C‐MICS (2.2‐millimeter incision) Intervention 2: standard phacoemulsification (3.0‐millimeter incision) SN60WF IOL (Alcon) was inserted for both groups. Length of follow‐up: Planned: not reported Actual: 3 months |
|
| Outcomes | Primary and secondary outcomes were not differentiated. Outcomes, as defined in the study reports: uncorrected VA, BCVA, corneal astigmatism, SIA |
|
| Notes |
Full study name: 同轴微切口白内障超声乳化术后角膜散光的临床观察 Observation of corneal astigmatism induced by 2.2‐millimeter microincision coaxial phacoemulsification Type of study: published full text Funding sources: 佛山市卫生局医学科研立项课题 (No. 2011162) Medical scientific research program of Foshan board of health (No. 2011162) Disclosures of interest: not reported Trial registry number: not reported Study period: January to September 2011 We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “将患者按就诊顺序登记并按随机表法分成两组,分别施行 2郾 2mm 同轴微切口白内障超声乳化联合 IOL 植入术及 3.0mm 常规白内障超声乳化联合 IOL 植入术,其中 2.2mm 组 38 眼,3. 0mm 组 40 眼。 “排除增殖期糖尿病视网膜病变、青光眼、老年性黄斑变性等眼部疾病及既 往有内眼手术史的患者。 术中出现后囊膜破裂、玻璃体溢 出等并发症者、不能完成 3mo 随访者予以剔除。 ” (p1465) Randomized table was used to distribute the participants into different groups. Diabetic retinopathy, glaucoma, age‐related macular degeneration, previous operation, posterior capsule rupture during surgery, vitreous loss in the surgery. Postrandomization exclusions exist. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants were excluded postrandomization. |
| Selective reporting (reporting bias) | Unclear risk | Trial registry number and protocol were not available for comparison. |
| Other bias | Unclear risk | Funding sources and disclosures of interests were not reported. |
Luo 2012.
| Methods |
Study design: parallel‐group RCT Number randomized: 120 eyes of 120 participants total; 40 eyes of 40 participants per group Exclusions after randomization: none Number analyzed: 120 eyes of 120 participants total; 40 eyes of 40 participants per group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none reported How were missing data handled? no missing data Reported power calculation: no Unusual study design: no |
|
| Participants |
Country: China Mean age: overall mean age not reported; 73.95 years for 1.8 mm; 71.37 years for 2.2 mm; 72.48 years for 3.0 mm Gender: 58 men and 62 women overall; 21 men (53%) and 19 women (47%) in the smaller C‐MICS group 18 men (45%) and 22 women (55%) in the C‐MICS (2.2‐millimeter incision) group 19 men (47%) and 21 women (53%) in the standard phacoemulsification (3.0‐millimeter incision) group Inclusion criteria: age between 55 and 85 years, the presence of nuclear or corticonuclear cataract of grades 2.0 to 4.0 (Lens Opacities Classification System III), a transparent central cornea, pupil dilation 7 mm at the time of preoperative examination, and a preoperative central endothelial cell count of 1500 cells/mm2 Exclusion criteria: previous intraocular surgery, glaucoma, pseudoexfoliation, uveitis, high myopia, and diabetes mellitus Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: smaller C‐MICS group with Akreos MI 60 IOL (Bausch & Lomb) inserted Intervention 2: C‐MICS (2.2‐millimeter incision) group with SN60WF IOL (Alcon) inserted Intervention 3: standard phacoemulsification (3.0‐millimeter incision) group with SA60AT IOL (Alcon) inserted We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. Length of follow‐up: Planned: 3 months Actual: 3 months |
|
| Outcomes |
Primary outcome, as defined in study reports: UST, surgical time, CDE and the total BSS volume, intraoperative measurements of corneal incision size during surgery, change in maximal clear corneal incision thickness as evaluated by AS‐OCT, BCVA, central cornea ECL, and SIA
Secondary outcomes, as defined in study reports: primary and secondary outcomes not differentiated
Adverse events reported: no Intervals at which outcomes assessed: postoperatively at 1 day, 1 week, 1 month, 3 months |
|
| Notes |
Full study name: Clinical evaluation of three incision size–dependent phacoemulsification systems Type of study: published Funding sources: Natural Science Foundation of China (30973277) and the Key Projects for Hospital Clinical Disciplines Fund of the Chinese Ministry of Health in 2010–2012 (Project No.175 in Document 439 of the Planning and Finance Secretary of Ministry of Health, China) Disclosures of interest: “The authors indicate no financial conflict of interest.” Study period: July 2010 to January 2011 Trial registration: NCT01429532 (ClinicalTrials.gov) We attempted to contact the authors but the email was returned undeliverable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A number between 1 and 120, generated by a random number generator, was assigned to each subject. The number was divided by 3. If the remainder was 1, the patient was assigned to Group I; if the remainder was 2, the patient was assigned to Group II; and if the number was divisible by 3, the patient was assigned to Group III." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Low risk | "Both the technician and the surgeon were masked during postoperative examinations to the participants' group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All participants completed all follow‐up visits." |
| Selective reporting (reporting bias) | Low risk | This clinical trial has been registered at www.clinicaltrials.gov, registration number NCT01429532. All outcomes were reported. |
| Other bias | Low risk | Source of funding and conflicts of interest reported. "Publication of this clinical study was supported by the Natural Science Foundation of China (30973277)" (p838) "The authors indicate no financial conflict of interest." (p838) |
Mao 2008.
| Methods |
Study design: parallel‐group RCT Number randomized: 160 eyes of 160 participants total; 80 eyes of 80 participants in each group Exclusions after randomization: none reported Number analyzed: 160 eyes of 160 participants; 80 eyes of 80 participants in each group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none at 1 month How were missing data handled? not reported Reported power calculation: no Unusual study design: no |
|
| Participants |
Country: China Mean age (SD): 69 (NR) overall; not reported by group Age range: 48 to 82 years Gender: 84 men (52%) and 76 women (48%) overall; number per group not reported Inclusion criteria: age‐related cataract Exclusion criteria: people with glaucoma, diabetes, or other eye diseases were excluded Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: B‐MICS (1.4‐millimeter incision) group with Acri.Smart46s (Acti.Tec) IOL inserted Intervention 2: standard phacoemulsification (3.0‐millimeter incision) group with SENSER IOL (AMO) inserted. We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. Length of follow‐up: Planned: not reported Actual: 1 month |
|
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: uncorrected VA and SIA Adverse events reported: yes, corneal edema Intervals at which outcomes assessed: preoperatively and postoperatively at 1 day, 1 week, and 1 month |
|
| Notes |
Full study name: Analysis of the visual quality after bimanual phacoemulsification via micro‐incision Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: August 2005 to February 2006 Trial registration: not reported We attempted to contact the authors but the email was returned undeliverable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
Mastropasqua 2011.
| Methods |
Study design: parallel‐group RCT Number randomized: 30 eyes of 30 participants; 15 eyes of 15 participants per group Exclusions after randomization: not reported Number analyzed: not reported Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none How were missing data handled? not reported Reported power calculation: no Unusual study design? none |
|
| Participants |
Country: Italy Mean age (SD): 69.8 (NR) overall: 70.11 (NR) smaller C‐MICS group 69.44 (NR) C‐MICS (2.2‐millimeter incision) group Gender: not reported Inclusion criteria: age 65 to 75 years, axial length 23.0 to 24.0 mm, corneal astigmatism less than 3.00 diopters, nuclear cataract of grade 4 (nuclear opalescence ‐ NO4, Lens Opacities Classification System III), and corneal endothelial cell count greater than 1200/mm2 Exclusion criteria: anterior segment pathological alterations such as keratoconus, chronic uveitis, zonular dialysis, pseudoexfoliation syndrome, glaucoma, and diabetes mellitus; other ocular pathologies impairing visual function; previous anterior or posterior segment surgery; and intraoperative or postoperative complications Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: smaller C‐MICS Intervention 2: C‐MICS (2.2‐millimeter incision) AcrySof SN60WF IOL (Alcon) was inserted for both groups. Length of follow‐up: Planned: not reported Actual: 90 days |
|
| Outcomes |
Primary outcome, as defined in study reports: uncorrected VA and BCVA, keratometric astigmatism, endothelial cell count, and corneal thickness at incision site. The amount and axis of astigmatic change induced by the cataract surgery were assessed by calculating the SIA. Power vector analysis of keratometric astigmatic change between preoperative and postoperative values was performed.
Secondary outcomes, as defined in study reports: intraoperative parameters including mean torsional time, CDE, and BSS used
Adverse events reported: not reported Intervals at which outcomes assessed: postoperatively at 1 day, 7 days, 30 days, and 90 days |
|
| Notes |
Full study name: Microcoaxial torsional cataract surgery 1.8 mm versus 2.2 mm: functional and morphological assessment Type of study: published Funding sources: not reported Disclosures of interest: the authors have no financial or proprietary interest in the materials Study period: not reported Trial registration: not reported We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned to group 1 or group 2 the day before surgery by block randomization (randomly assigned by computer‐generated numbers)" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Low risk | "Participants and examiners performing preoperative and postoperative controls were masked to the surgical technique used in each case" |
| Masking of outcome assessment (detection bias) | Low risk | "Participants and examiners performing preoperative and postoperative controls were masked to the surgical technique used in each case" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Low risk | Source of funding not reported. "The authors have no financial or proprietary interest in the materials presented herein." (p114) |
Moon 2011.
| Methods |
Study design: parallel‐group RCT Number randomized: 100 eyes of 89 participants overall: 32 eyes of NR participants in the smaller C‐MICS group 38 eyes of NR participants in C‐MICS (2.2‐millimeter incision) group 30 eyes of NR participants in standard phacoemulsification with 2.8‐millimeter incision group Exclusions after randomization: none reported Number analyzed: 100 eyes of 89 participants overall: 32 eyes of NR participants in the smaller C‐MICS group 38 eyes of NR participants in C‐MICS (2.2‐millimeter incision) group 30 eyes of NR participants in standard phacoemulsification with 2.8‐millimeter incision group Unit of analysis: unclear as trial investigators did not report how 11 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 11 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no |
|
| Participants |
Country: South Korea Mean age (SD): 65.2 (NR) overall: 64.5 (10.5) years old in the smaller C‐MICS group 61.8 (15.3) years old in C‐MICS (2.2‐millimeter incision) group 69.4 (10.0) years old in standard phacoemulsification with 2.8‐millimeter incision group Age range: not reported Gender: 30 men and 59 women total; number per group not reported Inclusion criteria: "the subjects with corneal astigmatism less than 2.25 diopters were examined" (p408) Exclusion criteria: "the patients who underwent corneal surgery including refractive surgery, eyes that can not measure the corneal curvature due to corneal opacity, surgery that affects corneal astigmatism during surgery, or other types of intraocular lenses were excluded from the study." (p408) |
|
| Interventions |
Intervention 1: smaller C‐MICS with MI60 IOL (Bausch & Lomb, Rochester, NY, USA) Intervention 2: C‐MICS (2.2‐millimeter incision) with AcrySof IQ IOL (Alcon, Fort Worth, TX, USA) Intervention 3: standard phacoemulsification (2.8‐millimeter incision) with Akreos AO IOL (Bausch & Lomb) We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. Length of follow‐up: Planned: no protocol available Actual: baseline, 1 and 3 months |
|
| Outcomes |
Primary outcome, as defined in study reports: SIA and high‐order aberrations (coma, trefoil, and spherical aberration)
Secondary outcomes, as defined in study reports: coma–root mean square (RMS) and trefoil‐RMS were evaluated at 1 month after the cataract operation.
Adverse events reported: no Intervals at which outcomes assessed: baseline, 1 and 3 months |
|
| Notes |
Type of study: published full text Funding sources: not reported Disclosures of interest: not reported Study period: March 2009 to August 2009 Trial registry number: not reported We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Unclear risk | Funding sources, disclosures of interests were not reported. |
Morcillo‐Laiz 2009.
| Methods |
Study design: parallel‐group RCT Number randomized: 94 eyes of 64 participants total; 43 eyes in standard phacoemulsification group, 51 eyes in B‐MICS group Exclusions after randomization: none Number analyzed: 94 eyes of 64 participants; 43 eyes in standard phacoemulsification group, 51 in B‐MICS group Unit of analysis: unclear as trial investigators did not report how 30 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 30 participants were assigned to the same or different groups Losses to follow‐up: standard phacoemulsification group had none reported at 1 month, 3 participants at 3 months and 5 participants at 6 months; B‐MICS had none reported at 1 month, 3 participants at 3 months, and 3 participants at 6 months. How were missing data handled? not reported Reported power calculation: no Unusual study design? no |
|
| Participants |
Country: Spain Mean age (SD): 70.53 (NR) overall: 69.02 (NR) years for standard phacoemulsification 72.04 (NR) years for B‐MICS Gender: 24 men (37%) and 40 women (63%) overall; number not reported by group Inclusion criteria: people scheduled for elective cataract surgery Exclusion criteria: topographic astigmatism 42.0 diopters, intraoperative use of corneal sutures, or corneal disease Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: B‐MICS (1.5‐ to 2‐millimeter incision) with Acri.Smart 48S IOL (Acri.Tec, Hennigsdorf, Germany) inserted Intervention 2: standard phacoemulsification (2.8‐millimeter incision) with Y601075 (AJL, A ´lava, Spain) Length of follow‐up: Planned: not reported Actual: 6 months |
|
| Outcomes |
Primary outcome, as defined in study reports: SIA and BCVA Secondary outcomes, as defined in study reports: not reported Adverse events reported: not reported Intervals at which outcomes assessed: preoperatively and postoperatively at 1, 3, and 6 months |
|
| Notes |
Full study name: Surgically induced astigmatism after biaxial phacoemulsification compared to coaxial phacoemulsification Type of study: published Funding sources: not reported Disclosures of interest: no Study period: not reported Trial registration: not reported We attempted to contact the authors but the email was returned undeliverable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study mentioned that participants were randomized, but did not specify methods for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Low risk | "Neither the patient nor the examiner knew which technique had been applied."; double‐blind clinical trial |
| Masking of outcome assessment (detection bias) | Low risk | "Neither the patient nor the examiner knew which technique had been applied." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 out of 94 participants (9.6%) were lost to follow‐up at 6 months. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
Musanovic 2012.
| Methods |
Study design: paired‐eye RCT Number randomized: 60 eyes of 30 participants in total; 30 eyes of 30 participants per group Exclusions after randomization: not reported Number analyzed: not reported, assumed to be 60 eyes of 30 participants in total; 30 eyes of 30 participants per group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: not reported How were missing data handled? not reported Reported power calculation: no Unusual study design: none |
|
| Participants |
Country: Bosnia and Herzegovina Mean age (SD): 63.6 (NR) overall: 65.13 (NR) in standard phacoemulsification (3.0‐millimeter incision) group 62.06 (NR) in the larger C‐MICS group Gender: not reported Inclusion criteria: people from everyday operational program cataract surgery at the Eye Clinic of University Clinical Center Tuzla in the period of October 2009 to December 2009. People planned for cataract surgery and who met the inclusion criteria of the study were included. Exclusion criteria: not reported Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: standard phacoemulsification (3.0‐millimeter incision) Intervention 2: larger C‐MICS The type of IOL inserted was not reported. Length of follow‐up: Planned: not reported Actual: postoperatively at 1, 7, and 30 days; extended to 90 days for SIA |
|
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: uncorrected distance VA, corneal keratometry, and corneal astigmatism and SIA were assessed 1, 7, and 30 days after cataract surgery. Adverse events reported: not reported Intervals at which outcomes assessed: postoperatively at 1, 7, and 30 days; extended to 90 days for SIA |
|
| Notes |
Full study name: Corneal astigmatism after micro‐incision cataract operation Type of study: published Funding sources: not reported Disclosures of interest: none Study period: October 2009 to December 2009 Trial registration: not reported We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | The methods describe 4 outcomes; 2 of them, corneal keratometry and corneal astigmatism, were not found in the results section. |
| Other bias | Low risk | Source of funding not reported. "Conflict of interest: none declared." (p128) |
Shan 2016.
| Methods |
Study design: parallel‐group RCT Number randomized: 362 eyes of 362 participants in total: 211 eyes of 211 participants in C‐MICS 2.2‐millimeter group 151 eyes of 151 participants in standard phacoemulsification 2.8‐millimeter group Exclusions after randomization: none reported Number analyzed: 362 eyes of 362 participants in total: 211 eyes of 211 participants in C‐MICS 2.2‐millimeter group 151 eyes of 151 participants in standard phacoemulsification 2.8‐millimeter group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: none |
|
| Participants |
Country: China
Mean age: not reported
Gender: not reported
Inclusion criteria: “择 2014‐07/2015‐03 在宝鸡市人民医院眼 科接受透明角膜切口白内障超声乳化+后房型折叠式人 工晶状体植入术的年龄相关性白内障患者 362 例 362 眼 术前矫正视力 0. 3 ˜ 光感。 眼压、光定 位和色觉检查均正常。” (p98) age‐related cataract, undertaking clear corneal incision phacoemulsification + implantation of posterior chamber foldable intraocular lens Exclusion criteria: “排除标准:高度近视、角膜病变、中央角膜内皮细胞臆1800个/mm2、眼底严重病变、青光眼、糖尿病眼底病变、葡萄膜炎、既往眼外伤 或眼手术史患者。” (p98) high myopia, keratopathy, central corneal endothelial cell < 1800/mm2, fundus diseases, glaucoma, diabetic retinopathy, uveitis, trauma, previous operation |
|
| Interventions |
Intervention 1: C‐MICS 2.2‐millimeter group Intervention 2: standard phacoemulsification 2.8‐millimeter group The type of IOL inserted was not reported. Length of follow‐up: Planned: not reported Actual: 1 month |
|
| Outcomes |
Primary outcome, as defined in study reports: uncorrected VA, BCVA, corneal astigmatism, corneal endothelial cell counting, ultrasonic energy, phacoemulsification time
Adverse events reported (Y/N): yes, corneal edema Intervals at which outcomes assessed: 1 week and 1 month |
|
| Notes |
Full study name: 同轴 2.2 mm 与 2.8 mm 切口白内障超声乳化手术疗效 评价 [Evaluation on curative effect of coaxial 2.2‐ and 2.8‐millimeter incision phacoemulsification for cataract] Type of study: published full text Funding sources: not reported Disclosures of interest: not reported Study period: July 2014 to March 2015 Trial registry number: not reported We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing data reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Unclear risk | Funding sources, disclosures of interests were not reported. |
Shi 2013.
| Methods |
Study design: parallel‐group RCT Number randomized: 132 eyes of 98 participants overall: 60 eyes of NR participants in C‐MICS (2.2‐millimeter incision) group 72 eyes of NR participants in standard phacoemulsification (3.0‐millimeter incision) group Exclusions after randomization: none Number analyzed: not reported Unit of analysis: unclear as trial investigators did not report how 34 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 34 participants were assigned to the same or different groups Losses to follow‐up: not reported How were missing data handled? not reported Reported power calculation: no Unusual study design: none |
|
| Participants |
Country: China Mean age (SD): 72 (NR) overall: 71 (NR) in C‐MICS (2.2‐millimeter incision) 73 (NR) in standard phacoemulsification (3.0‐millimeter incision) Gender: not reported Inclusion criteria: people with cataract with endothelial cell > 2000/mm2 Exclusion criteria: people with corneal degeneration, uveitis, or glaucoma Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: C‐MICS (2.2‐millimeter incision) with AcrySof IQ IOL inserted Intervention 2: standard phacoemulsification (3.0‐millimeter incision) with AcrySof Natural IOL inserted Length of follow‐up: Planned: 3 months Actual: 3 months |
|
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: average ultrasound power, BCVA, SIA, ECL, incidence of capsule rupture and corneal edema Adverse events reported: yes; posterior capsular rupture and corneal edema Intervals at which outcomes assessed: postoperatively at 1 day, 1 month, and 3 months |
|
| Notes |
Full study name: Clinical evaluation on the coaxial microincision cataract surgery in hard nuclear cataracts Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: October 2011 to October 2012 Trial registration: NCT01385878 (ClinicalTrials.gov) We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. The study included both eyes of some participants in the same group, but did not account for intraperson correlation. |
Vasavada 2013a.
| Methods |
Study design: parallel‐group RCT Number randomized: 100 eyes of 100 participants in total; 50 eyes of 50 participants per group Exclusions after randomization: not reported Number analyzed: 100 eyes of 100 participants in total; 50 eyes of 50 participants per group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none How were missing data handled? not reported Reported power calculation: yes: “A sample size of 100 participants was computed ... A difference of 0.175 log units or more was considered ... A sample size of 50 participants in each group would have an 80% power to capture the above‐mentioned difference between the 2 groups” (p566‐7) Unusual study design: no |
|
| Participants |
Country: India Mean age (NR): 63.54 (NR) years overall: 64.42 (NR) years in the smaller C‐MICS group 62.67 (NR) years in C‐MICS (2.2‐millimeter incision) group Gender: 50 men (50%) and 50 women (50%) overall: 24 men (48%) and 26 women (52%) in the smaller C‐MICS group 26 men (52%) and 24 women (48%) in C‐MICS (2.2‐millimeter incision) group Inclusion criteria: people with uncomplicated ARC. People having nuclear or corticonuclear cataracts of grade 2 to 4 according to the Lens Opacities Classification System III were included. Ages eligible for study: 50 to 80 years Exclusion criteria: glaucoma, shallow anterior chamber (anterior chamber depth < 2.1 mm), pupil dilation less than 6.0 mm, extremely dense cataract, posterior polar cataract, subluxated cataract, white mature cataract, diabetic retinopathy, high myopia (defined as axial length > 25 mm), uveitis, or previous ocular trauma or surgery Equivalence of baseline characteristics (Y/N): yes |
|
| Interventions |
Intervention 1: corneal incision system with the Stellaris PC system (Bausch & Lomb) using longitudinal ultrasound (C‐MICS 1.8‐millimeter incision) with MI60 IOL (Bausch & Lomb) inserted Intervention 2: corneal incision system with the Infiniti Vision system (Alcon) using torsional ultrasound (C‐MICS 2.2‐millimeter) with AcrySof SN60WF IOL (Alcon) inserted Length of follow‐up: Planned: 3 months Actual: 3 months |
|
| Outcomes |
Primary outcome, as defined in study reports Ingress of trypan blue from the ocular surface into the anterior chamber, SIA at 3 months Intraoperative outcome measures: total surgical time, CDE, volume of BSS, incision enlargement, trypan blue ingress into the anterior chamber Postoperative outcome measures: anterior segment optical coherence tomography ‐ incision morphology, localized Descemet membrane detachment (postoperatively at 1 day, 1 week, or 1 month), endothelial gaping or misalignment (postoperative at 1 day and 1 week), epithelial gaps (postoperatively at 1 day and 1 week), anterior segment inflammation (postoperatively at 1 day, 1 week, or 1 month), corneal endothelial morphology (postoperatively at 3 months), CCT (postoperatively at 1 day, 1 week, or 1 month), corneal clarity (postoperatively at 1 day, 1 week, and 1 month) Secondary outcomes, as defined in study reports: not reported Adverse events reported: not reported Intervals at which outcomes assessed: postoperatively at 1 day, 7 days, 1 month, and 3 months |
|
| Notes |
Full study name: Incision integrity and postoperative outcomes after microcoaxial phacoemulsification performed using 2 incision‐dependent systems Type of study: published Funding sources: not reported Disclosures of interest: Iladevi Cataract & IOL Research Centre receives occasional travel support from Alcon Laboratories, Inc. No author has a financial or proprietary interest in any material or method mentioned. Study period: September 2011 to February 2012 Trial registration: NCT01385878 (ClinicalTrials.gov) We emailed the authors and included data provided through email correspondence. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study mentioned that participants were randomized, but did not specify methods for random sequence generation. We contacted the study investigator, and received the following reply: "Randomization was done using computer generated random numbers." |
| Allocation concealment (selection bias) | Low risk | From an email correspondence on 24 January 2017: "we prepared sequenced and sealed envelopes containing one of the two options which were stored in a dedicated box in the operating room. An unscrubbed nurse (1 of 2) opened the envelope and informed the operating surgeon of the incision size and phacoemulsification system to be used just prior to beginning of the surgery." |
| Masking of participants and personnel (performance bias) | Low risk | Both participants and outcome assessors were masked. We contacted the study investigator, and received the following reply: "The patients and outcome assessors were blinded to the technique used, however the surgeon was not. The outcome assessor was another ophthalmologist, and not the operating surgeon." |
| Masking of outcome assessment (detection bias) | Low risk | "This prospective randomized patient‐ and analyzer‐masked clinical trial comprised participants with uncomplicated age‐related cataract"; we contacted the study investigator, and received the following reply: "The patients and outcome assessors were blinded to the technique used, however the surgeon was not. The outcome assessor was another ophthalmologist, and not the operating surgeon." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "No participants were lost to follow‐up." |
| Selective reporting (reporting bias) | Low risk | The study is registered with ClinicalTrials.gov (NCT01385878). The outcomes described in the protocol are consistent with those in the paper. |
| Other bias | Unclear risk | "Iladevi Cataract & IOL Research Centre receives occasional travel support from Alcon Laboratories, Inc. No author has a financial or proprietary interest in any material or method mentioned." (p563) |
Wang 2009.
| Methods |
Study design: parallel‐group RCT Number randomized: 83 participants (129 eyes) in total: NR participants (43 eyes) in C‐MICS (2.2‐millimeter incision) group NR participants (42 eyes) in standard phacoemulsification (2.6‐millimeter incision) group NR participants (44 eyes) in standard phacoemulsification (3.0‐millimeter incision) group Exclusions after randomization: none reported Number analyzed: 83 participants (129 eyes) in total: NR participants (43 eyes) in C‐MICS (2.2‐millimeter incision) group NR participants (42 eyes) in standard phacoemulsification (2.6‐millimeter incision) group NR participants (44 eyes) in standard phacoemulsification (3.0‐millimeter incision) group Unit of analysis: unclear as trial investigators did not report how 46 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 46 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled?: no missing data reported Reported power calculation: no |
|
| Participants |
Country: China Overall mean age: 72 years Mean age (SD): 69 (9) in C‐MICS (2.2‐millimeter incision) group 69 (7) in standard phacoemulsification (2.6‐millimeter incision) group 71 (8) in standard phacoemulsification (3.0‐millimeter incision) group Overall age range: 49 to 83 years Gender: 33 men (40%) and 50 women (60%) Inclusion criteria: people diagnosed with age‐related cataract Exclusion criteria: "patients with corneal disease, glaucoma, uveitis, age‐related maculopathy, high myopia, or history of ocular trauma or surgery were excluded." (p665) Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: C‐MICS (2.2‐millimeter incision) Intervention 2: standard phacoemulsification (2.6‐millimeter incision) (excluded from review) Intervention 3: standard phacoemulsification (3.0‐millimeter incision) AcrySof Natural SN60AT IOL (Alcon) was inserted for both groups. Length of follow‐up: Planned: protocol not available Actual: 3 months |
|
| Outcomes |
Outcomes, as defined in study reports: SIA, BCVA, CDE, CCT, anterior chamber depth, ultrasound time
Adverse events reported: authors reported no intraoperative complications. Intervals at which outcomes assessed: 1 and 3 months |
|
| Notes |
Full study name: The effect of micro‐incision and small‐incision coaxial phaco‐emulsification on corneal astigmatism Type of study: published full text Funding sources: not reported Disclosures of interest: not reported Trial registry number: not reported Study period: September 2006 to February 2007 We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomized into three groups: 43 eyes into the 2.2‐mm group, 42 eyes into the 2.6‐mm group and 44 eyes into the 3.0‐mm group.” (p665) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Low risk | "Patients and examiners were masked to the group assignment.” (p665) |
| Masking of outcome assessment (detection bias) | Low risk | "Patients and examiners were masked to the group assignment.” (p665) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing data reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
Yao 2008.
| Methods |
Study design: parallel‐group RCT Number randomized: 280 eyes total: 146 eyes in B‐MICS group 134 eyes in standard phacoemulsification group Exclusions after randomization: none Number analyzed: 280 eyes total: 146 eyes in B‐MICS group 134 eyes in standard phacoemulsification group Unit of analysis: unclear as number of participants in each group was not reported Unit of randomization: unclear as number of participants in each group was not reported Losses to follow‐up: not reported How were missing data handled? not reported Reported power calculation: no Unusual study design: no |
|
| Participants |
Country: China Mean age : 69 years overall: 69 years in B‐MICS group 69 years in standard phacoemulsification group Age range: 51 to 86 years Gender: 126 men (45%) and 154 women (55%) overall: 64 men (44%) and 82 women (56%) in B‐MICS group 62 men (46%) and 72 women (54%) in standard phacoemulsification group Inclusion criteria: not reported Exclusion criteria: people with corneal diseases, glaucoma, and uveitis or surgery history of eye were excluded. Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: B‐MICS (1.5‐millimeter incision) group with Acri.Smart46s (Acti.Tec) IOL inserted Intervention 2: standard phacoemulsification (3.0‐millimeter incision) group with Staar KS‐1 (Canon) IOL inserted We considered standard phacoemulsification with incisions ranging from 2.75 to 3.2 mm to be about 3.0 mm. Length of follow‐up: Planned: 3 months Actual: 3 months |
|
| Outcomes |
Primary outcome, as defined in study reports: phacoemulsification time, VA, CCT, ECL, SIA
Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: postoperatively at 1 day and 3 months |
|
| Notes |
Full study name: Clinical evaluation on the bimanual microincision cataract surgery Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: January 2006 to May 2007 Trial registration: not reported Data were clarified by an author of this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study used 'random numbers table' for sequence generation" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Low risk | "Participants were masked to their treatment groups" |
| Masking of outcome assessment (detection bias) | Low risk | "Outcome assessors were masked to their treatment groups" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
Yao 2011.
| Methods |
Study design: parallel‐group RCT Number randomized: 89 eyes of 89 participants in total: 45 eyes of 44 participants in the smaller C‐MICS group 44 eyes of 44 participants in standard phacoemulsification (3.0‐millimeter incision) group Exclusions after randomization: 1 eye of 1 participant in microincision cataract surgery (1.8‐millimeter incision) group Number analyzed: 80 eyes of 80 participants total; 40 eyes in each group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: 9 participants How were missing data handled? not reported Reported power calculation: no Unusual study design: no |
|
| Participants |
Country: China Mean age: 72 years overall; number not reported by group Gender: 29/80 men (36%) and 51/80 women (64%) overall; number not reported by group Inclusion criteria: ARC with astigmatism < 2.00 diopters Exclusion criteria: people with corneal diseases, uveitis, glaucoma, age‐related macular degeneration, high myopia, eye trauma, or surgery history of eyes were excluded. Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: smaller C‐MICS with Akreos MI 60 IOL inserted Intervention 2: standard phacoemulsification (3.0‐millimeter incision) with Akreos Adapt IOL inserted Length of follow‐up: Planned: 3 months Actual: 3 months |
|
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: average ultrasound power, effective phacoemulsification time, uncorrected VA, BCVA, ECL, SIA Adverse events reported: no Intervals at which outcomes assessed: postoperatively at 1 day, 1 week, 1 month, and 3 months |
|
| Notes |
Full study name: Clinical evaluation on the coaxial 1.8 mm microincision cataract surgery Type of study: published Funding sources: National Eleventh Five‐Year Technology Support Program (2006BAI02B04), Zhejiang Key Creative Group of Technology (2009R50039) Disclosures of interest: not reported Study period: July 2009 to May 2010 Trial registration: not reported Data were clarified by an author of this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study used random numbers table for sequence generation" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Low risk | "Participants were masked to their treatment groups" |
| Masking of outcome assessment (detection bias) | Low risk | "Outcome assessors were masked to their treatment groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 out of 89 participants (10%) lost to follow‐up, but reasons not reported. 4 cases dropped out at 1 month, 5 extra cases dropped out at 3 months. |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
Yu 2016.
| Methods |
Study design: parallel‐group RCT Number randomized: 150 eyes of 150 participants in total; 50 eyes of 50 participants in each group Exclusions after randomization: none reported Number analyzed: not reported, assumed to be 150 eyes of 150 participants in total; 50 eyes of 50 participants in each group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no |
|
| Participants |
Country: China
Mean age (SD) : 70.44 (NR) overall: 70.30 (6.67) in the smaller C‐MICS group 70.68 (6.98) in microincision (2.0‐millimeter) group 70.34 (7.23) in standard phacoemulsification group Gender: not reported Inclusion criteria: “ages between 50 and 80y, with no medication history or other eye disease.” (p400) Exclusion criteria: “diabetes or other diseases which may influence the biomechanical properties of the cornea were also excluded” (p400) Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: smaller C‐MICS group with Akreos MI 60 IOL (Bausch & Lomb) inserted
Intervention 2: microincision (2.0 mm) (excluded from review) Intervention 3: standard phacoemulsification (3.0‐millimeter incision) group with PY‐60 IOL implantation (Hoya, Japan) inserted Length of follow‐up: Planned: not reported Actual: 3 months |
|
| Outcomes | Outcomes, as defined in study reports: intraoperative data and postoperative outcomes including SIA, corneal incision thickness, wavefront aberrations and modulation transfer function of cornea were obtained. Adverse events reported: not reported | |
| Notes |
Full study name: A comparable study of clinical and optical outcomes after 1.8, 2.0 mm microcoaxial and 3.0 mm coaxial cataract surgery Type of study: published full text Funding sources: supported by the Key Program of the National Natural Science Foundation of China (No. 81130018); National Twelfth Five‐Year Plan Foundation of China (No. 2012BAI08B01); Zhejiang Key Innovation Team Project of China (No. 2009R50039); Zhejiang Key Laboratory Fund of China (No. 2011E10006) Disclosures of interest: "none" (p404) Trial registry number: ChiCTR‐TRC‐12002565 Study period: not reported Data were clarified by an author of this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | "The postoperative follow‐up was performed by the same independent examiner, who did not perform any of the surgeries." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up was not reported. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes in the trial registry were reported. |
| Other bias | Low risk | Reported no conflict of interest and government‐funded trial |
Zhang 2014.
| Methods |
Study design: parallel‐group RCT
Number randomized: 160 participants with 168 eyes in total: 84 eyes of 80 participants in the smaller C‐MICS group 84 eyes of 83 participants in the standard phacoemulsification group Exclusions after randomization: none reported Number analyzed: not reported, assumed to be 160 participants with 168 eyes in total: 84 eyes of 80 participants in the smaller C‐MICS group 84 eyes of 83 participants in the standard phacoemulsification group Unit of analysis: unclear as trial investigators did not report how 8 participants with both eyes included were analyzed Unit of randomization: unclear whether both eyes of the 8 participants were assigned to the same or different groups Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no Unusual study design: none |
|
| Participants |
Country: China
Mean age : 68.5 overall: 67.5 in the smaller C‐MICS group 69.8 in the standard phacoemulsification group Age range: 50 to 83 years overall: 50 to 83 years in the smaller C‐MICS group 52 to 81 years in the standard phacoemulsification group Gender: 83 women and 77 men overall: 45 women and 35 men in the smaller C‐MICS group 38 women and 42 men in the standard phacoemulsification group Inclusion criteria: “age‐related cataract, lens nucleus hardness LOCS level 2‐5” (p671) Exclusion criteria: “history of eye trauma, corneal scar, glaucoma, diabetic retinopathy, macular degeneration” (p671) Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: smaller C‐MICS group with ZEISS ASPHINA 509M IOL inserted Intervention 2: standard phacoemulsification with 3.2‐millimeter incision group with ZEISS SPHERIS 209M IOL inserted Length of follow‐up: Planned: protocol not available Actual: 1 day, 1 week, and 1 month |
|
| Outcomes |
Outcomes, as defined in study reports: postoperative astigmatism, uncorrected VA
Secondary outcomes, as defined in study reports: none
Adverse events reported: not reported Intervals at which outcomes assessed: 1 day, 1 week, 1 month |
|
| Notes |
Full study name: Effect of 1.8 coaxial micro‐incision cataract phacoemulsification on corneal astigmatism Type of study: published full text Funding sources: not reported Disclosures of interest: not reported Trial registry number: not reported Study period: January 2012 to June 2012 We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available. Trial registration not reported. |
| Other bias | Unclear risk | Source of funding and conflicts of interest not reported. |
Zhu 2014.
| Methods |
Study design: paired‐eye RCT
Number randomized: 90 eyes of 45 participants in total; 45 eyes of 45 participants in each group
Exclusions after randomization: none reported
Number analyzed: 90 eyes of 45 participants in total; 45 eyes of 45 participants in each group Unit of analysis: 1 eye per participant Unit of randomization: 1 eye per participant Losses to follow‐up: none reported How were missing data handled? no missing data reported Reported power calculation: no Unusual study design: the study randomized participants and included both eyes of the same participant in the same intervention, but they did not account for intraperson correlation. |
|
| Participants |
Country: China Mean age : 67.7 overall: 66.8 years in the larger C‐MICS group 68.4 years in the standard phacoemulsification 3.0‐millimeter group Age range: 39 to 83 years overall: 39 to 80 years in the larger C‐MICS group 48 to 83 years in the standard phacoemulsification 3.0‐millimeter group Gender: 24 men (53%) and 21 women (47%) in total and by group Inclusion criteria: "patients with age‐related cataract undertaking phacoemulsification and intraocular lens implantation" (p1434) Exclusion criteria: none reported Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: larger C‐MICS Intervention 2: standard phacoemulsification with 3.0‐millimeter incision The type of IOL inserted was not reported. Length of follow‐up: Planned: not reported Actual: 3 months |
|
| Outcomes | Primary and secondary outcomes not differentiated. Outcomes, as defined in study reports: VA, corneal endothelial cell counting, CCT, SIA Intervals at which outcomes assessed: 1 day, 1 week, 1 month, 3 months |
|
| Notes |
Full study name: 不同切口同轴白内障超声乳化术的疗效比较 [Comparison of 2.2‐millimeter microincision and 3.0‐millimeter incision coaxial phacoemulsification] Type of study: published full text Funding sources: not reported Disclosures of interest: not reported Trial registry number: not reported Study period: January 2012 to June 2013 We attempted to contact the authors but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up or missing data were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not reported. Trial registration not reported. |
| Other bias | Unclear risk | Funding sources and disclosure of interest not reported. |
ARC: age‐related cataract AS‐OCT: anterior segment optical coherence tomography B‐MICS: biaxial microincision cataract surgery BCVA: best‐corrected visual acuity BSS: balanced salt solution C‐MICS: coaxial microincision cataract surgery CCT: central corneal thickness CDE: cumulative dissipated energy ECL: endothelial cell loss IL‐1alpha: interleukin 1 alpha IL6: interleukin 6 IOP: intraocular lens LOCS: Lens Opacities Classification System NR: not reported PGE2: prostaglandin E2 RCT: randomized controlled trial SD: standard deviation SIA: surgically induced astigmatism UST: ultrasound time VA: visual acuity VEGF: vascular endothelial growth factor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alio 2005 | RCT, but included the wrong intervention as the B‐MICS group's final incision size was 1.7 ± 0.21 mm, which overlapped with 2 of our comparison groups |
| Alio 2010 | RCT, but included the wrong intervention as B‐MICS group's final incision size range was from 1.6 to 1.8 mm, which overlapped with 2 of our comparison groups |
| Bhargava 2016 | RCT, but included the wrong intervention as the authors compared phacoemulsification versus manual small‐incision cataract surgery without phacoemulsification, removing cataract mechanically |
| Can 2011 | Not an RCT; this was a cohort study, and wrong intervention as incisions were enlarged before IOL implantation |
| Can 2012 | RCT, but wrong intervention as incisions were enlarged before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. |
| Cavallini 2007 | RCT, but wrong intervention as incisions were enlarged before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final size overlapped with our comparison groups. |
| Chee 2010 | RCT, but wrong intervention as the incisions in standard phacoemulsification group were 2.65 mm, not in the range of 2.75 to 3.2 mm |
| Crema 2007 | RCT, but wrong intervention as the incisions in B‐MICS group were enlarged to 2.8 mm before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. |
| Denoyer 2008 | RCT, but wrong intervention as the incisions in B‐MICS group were 1.7 mm |
| Devendra 2014 | RCT, but wrong intervention as 2.8‐millimeter incision was enlarged to 5.2 mm at the end of surgery |
| Dick 2012 | RCT, but wrong participants as incisions were enlarged in B‐MICS group to 1.8 mm before IOL implantation. The final incision size overlapped with the smaller C‐MICS group. |
| Elkady 2009 | RCT, but wrong intervention as incisions were enlarged before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. |
| Feng 2015 | RCT, but wrong intervention as incisions were enlarged before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. |
| Franchini 2006 | RCT, but wrong intervention as single stitch was made in all participants, which affected the surgically induced astigmatism |
| Gangwani 2011 | RCT, but wrong comparison as both intervention groups used the same incision size |
| Hashemian 2007 | RCT, but the conference abstract did not report the incision sizes created during microcoaxial and conventional phacoemulsification. Author contact information was not available through the conference website or other publications in PubMed. |
| Hayashi 2014 | RCT, but wrong comparison as the final incision size for the standard phacoemulsification group was 2.65 mm, which was not within our range of 2.75 mm to 3.2 mm |
| Hayashi 2016 | RCT, but wrong comparison as both intervention groups used the same incision size |
| Jain 2015 | RCT, but wrong intervention as incision was performed at the steep axis, which affects the evaluation of surgically induced astigmatism |
| Jeon 2010 | RCT, but wrong intervention as the incision size in the standard phacoemulsification group was 4.0 mm, which was not within our range of 2.75 mm to 3.2 mm |
| Jiang 2005 | RCT, but wrong intervention as incision was enlarged to 2.0 mm or 3.0 mm for IOL implant |
| Kahraman 2007 | This was a case series, not an RCT, and wrong intervention as the incision for the B‐MICS (1.4‐millimeter) group was enlarged to 3.2 mm before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision size was enlarged, and the size overlapped with our comparison groups. |
| Kaya 2007 | RCT, but wrong intervention as the incisions in B‐MICS group were enlarged to 2.0 mm for IOL implantation |
| Kim 2011 | RCT, but wrong comparison as the study authors compared pulse, burst, and continuous phacoemulsification mode |
| Kim 2013 | RCT, but wrong comparison as the intervention involved suturing the corneal incision, which affected the evaluation of surgically induced astigmatism |
| Kochhar 2014 | RCT, but wrong comparison as intervention was manual small‐incision cataract extraction without phacoemulsification |
| Kurz 2006 | RCT, but wrong intervention as the incision size was enlarged from 1.5 mm to 1.7 mm during IOL insertion |
| Kurz 2009 | RCT, but wrong intervention as the incision was enlarged from 1.5 mm to 1.7 mm in the B‐MICS group before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision was larger than 1.5 mm. |
| Lee 2009 | RCT, but wrong population as the participants "had nuclear or corticonuclear cataract of grade II to IV according to the Lens Opacities Classification System III (LOCS III) scale" (p875) |
| Masket 2009 | Not an RCT; quasi‐RCT as "patients were randomized by date of surgery; on odd dates, patients received a 3‐mm incision and on even dates a 2.2‐mm incision was used for the first eye." (p22) |
| Mencucci 2006 | RCT, but wrong intervention as both incisions enlarged to 2.75 mm before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. |
| NCT02642211 | RCT, but wrong comparison as the intervention was manual small‐incision cataract surgery |
| Park 2012 | Not an RCT; study did not report how participants were allocated to treatment groups. However, it was described as a "prospective, observer‐masked study" (p56). |
| Shen 2014 | RCT, but wrong intervention as the study compared 2.5‐millimeter (out the range of 2.75‐ to 3.2‐millimeter) mini‐incision coaxial phacoemulsification with 1.8‐millimeter microincision cataract surgery |
| Song 2014 | RCT, but wrong comparison as the intervention was manual small‐incision cataract extraction without phacoemulsification; the cataract was removed using irrigating vectis |
| Suasnavas 2010 | Not an RCT; this was a case series of people with nuclear or corticonuclear cataracts |
| Titiyal 2006 | RCT, but the incision size was not reported and the authors could be not be reached |
| Tong 2008 | RCT, but wrong intervention as incision size was enlarged before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. |
| Vasavada 2013b | Animal study (rabbit eyes) |
| von Sonnleithner 2015 | RCT, but wrong comparison as participants were assigned to different IOL groups |
| Wang 2012 | RCT, but wrong intervention as incisions were enlarged before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision was enlarged, and the final incision size overlapped with our comparison groups. |
| Wei 2012 | RCT, but wrong comparison as the incisions were enlarged to 3.5 mm before IOL implantation. The incision made for phacoemulsification procedure was not large enough for the IOL implantation, therefore the incision size was enlarged, and the final incision size overlapped with our comparison groups. |
| Wilczynska 2010 | Authors did not report how participants were assigned to treatment groups, and wrong intervention compared (B‐MICS incision size was 1.7 mm). |
| Wylegala 2009 | RCT, but we attempted to contact the authors as it was unclear if the incision had been enlarged |
| Yao 2006 | RCT, but wrong comparison as the B‐MICS incision size was 1.7 mm |
| Zhou 2012 | RCT, but wrong intervention as the surgery was performed on the steepest corneal meridians, which affects the evaluation of surgically induced astigmatism by usually decreasing the astigmatism |
ARC: age‐related cataract B‐MICS: biaxial microincision cataract surgery C‐MICS: coaxial microincision cataract surgery CCT: controlled clinical trial IOL: intraocular lens RCT: randomized controlled trial
Differences between protocol and review
In the protocol, we planned to impute the missing data using statistical methods. We decided that with so little missing data in the included studies any imputation would not change the conclusions of the review.
In the protocol, we stated that "we will determine whether data synthesis can be done depending on the heterogeneity calculated. If the I2 statistic suggests substantial statistical heterogeneity, we will present results in a narrative summary. If the I2 statistic is less than 50% (not indicating substantial heterogeneity), we will combine study results." We decided that the I2 statistic alone should not be the basis for our model selection. We used both clinical and methodological heterogeneity of the included studies to determine the appropriate model for meta‐analysis.
In the protocol, we planned to study intraoperative parameters including use of cumulative dissipated energy, total use of balanced salt solution, and surgical time. However, we found that phacoemulsification time is also an important parameter of interest to surgery. We therefore added phacoemulsification time as a new outcome in the review.
In the protocol, our primary and secondary outcomes were to be reported at day one, days five to seven, one month, and six weeks to two months, as data were available. However, we decided to report the primary and secondary outcomes at three and 12 months to be consistent with other Cochrane Reviews on age‐related cataract (Ang 2014; de Silva 2014; Mathew 2012; Riaz 2006; Riaz 2013); these are also the most clinically relevant postoperative time points. As studies may not have reported outcomes at precisely these time points, we considered data collection within the following time periods: three months' (from four weeks to six months) and 12 months' (from six months to less than 18 months) postoperatively.
In the protocol, we did not specify use of the GRADE approach and presenting of 'Summary of findings' tables for four comparisons, as this was not the Cochrane standard at the time.
Contributions of authors
Co‐ordinating the review: CJ
-
Data collection for the review update
Designing search strategies: CEV Information Specialist
Undertaking searches: CEV Information Specialist
Screening search results: CJ, XC, XW, AL, Sarah Money (SM), Yuanxi Jia (YJ)
Organizing retrieval of papers: AL
Screening retrieved papers against inclusion criteria: CJ, XC, AL
Appraising quality of papers: CJ, XC, AL, YJ
Extracting data from papers: CJ, XC, AL, YJ, YK
Writing to authors of papers for additional information: CJ, XW
Obtaining and screening data on unpublished studies: CJ, AL
-
Data management for the review
Entering data into Review Manager 5: AL, XW
Analysis of data: CJ, XC, AL
-
Interpretation of data
Providing a methodological perspective: AL, XW
Providing a clinical perspective: CJ, XC, WX, KY
Providing a policy perspective: CJ, XC
Providing a consumer perspective: CJ, XC
Writing the review: CJ, XC, AL
Providing general advice on the review: CJ, XC, AL, WX, KY
Securing funding for the review: N/A
Performing previous work that was the foundation of the current study: N/A
Guarantor for review: CJ
Sources of support
Internal sources
No sources of support supplied
External sources
Chongfei Jin is funded by Specialized Research Fund for the Doctoral Program of Higher Education of China (20100101120127), China.
-
National Eye Institute, National Institutes of Health, USA.
Xue Wang is funded by the Cochrane Eyes and Vision ‐ US Project through the National Eye Institute Grant 1 U01 EY020522‐01
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Chongfei Jin: is a coauthor of an included study (Yao 2011) Xinyi Chen: no conflicts of interest Andrew Law: no conflicts of interest Yunhee Kang: no conflicts of interest Xue Wang: no conflicts of interest Wen Xu: no conflicts of interest Ke Yao: is a coauthor of three included studies (Yao 2008; Yao 2011; Yu 2016)
The first two authors contribute equally to this paper.
New
References
References to studies included in this review
Can 2010 {published data only}
- Can I, Takmaz T, Yildiz Y, Bayhan HA, Soyugelen G, Bostanci B. Coaxial, microcoaxial, and biaxial microincision cataract surgery: prospective comparative study. Journal of Cataract and Refractive Surgery 2010;36(5):740‐6. [DOI] [PubMed] [Google Scholar]
Capella 2010 {published data only}
- Capella MJ, Barraquer E. Comparative study of coaxial microincision cataract surgery and standard phacoemulsification [Estudio comparativo entre cirugia de catarata por microincision coaxial y facoemulsificacion estandar]. Archivos de la Sociedad Espanola de Oftalmologia 2010;85(8):268‐73. [DOI] [PubMed] [Google Scholar]
Dosso 2008 {published data only}
- Dosso AA, Cottet L, Burgener ND, Nardo S. Outcomes of coaxial microincision cataract surgery versus conventional coaxial cataract surgery. Journal of Cataract and Refractive Surgery 2008;34(2):284‐8. [DOI] [PubMed] [Google Scholar]
Febbraro 2015 {published data only}
Hui 2016 {published data only}
- Hui N, Yu L, Wang CY, Yang XG. Clinical effects of coaxial 1.8 mm microincision phacoemulsification. International Eye Science 2016;16(10):1828‐31. [Google Scholar]
Hwang 2008 {published data only}
- Hwang SJ, Choi SK, Oh SH, Lee JH, Kim JH, Lee DH. Surgically induced astigmatism and corneal higher order aberrations in microcoaxial and conventional cataract surgery. Journal of the Korean Ophthalmological Society 2008; Vol. 49, issue 10:1597‐602.
Hwang 2016 {published data only}
Jeong 2013 {published data only}
- Jeong JH, Lee HJ, Lee SH. Comparison of phacodynamic effects on postoperative corneal edema between 2.8 mm and 2.2 mm microcoaxial torsional phacoemulsification. Journal of the Korean Ophthalmological Society 2013; Vol. 54, issue 5:709‐15.
Li 2011 {published data only}
- Li YJ, Kim HJ, Joo CK. Early changes in corneal edema following torsional phacoemulsification using anterior segment optical coherence tomography and Scheimpflug photography. Japanese Journal of Ophthalmology 2011;55(3):196‐204. [DOI] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li K, Lin ZX, Li L. Changes of the ocular surface and tear film after clear corneal incision phacoemulsification with different incision sizes. International Eye Science 2016; Vol. 16, issue 1:80‐3.
Lin 2013 {published data only}
- Lin YJ, Liang XJ, He JX, Zhao SY, Yang XY, Zeng S. Observation of corneal astigmatism induced by 2.2 mm micro‐incision coaxial phacoemulsification. International Eye Science 2013;13(7):1464‐6. [Google Scholar]
Luo 2012 {published data only}
- Luo L, Lin H, He M, Congdon N, Yang Y, Liu Y. Clinical evaluation of three incision size‐dependent phacoemulsification systems. American Journal of Ophthalmology 2012;153(5):831‐9. [DOI] [PubMed] [Google Scholar]
Mao 2008 {published data only}
- Mao ZH, Zhang GB, Chen W, Liu L, He XH. Analysis of the visual quality after bimanual phacoemulsification via micro‐incision [双手微小切口白内障超声乳化术术后视觉质量分析]. International Journal of Ophthalmology 2008;8(7):1373‐4. [Google Scholar]
Mastropasqua 2011 {published data only}
- Mastropasqua L, Toto L, Vecchiarino L, Nicola M, Mastropasqua R. Microcoaxial torsional cataract surgery 1.8 mm versus 2.2 mm: functional and morphological assessment. Ophthalmic Surgery, Lasers and Imaging 2011;42(2):114‐24. [DOI] [PubMed] [Google Scholar]
Moon 2011 {published data only}
- Moon SJ, Lee DJ, Lee KH. Induced astigmatism and high‐order aberrations after 1.8‐mm, 2.2‐mm and 3.0‐mm coaxial phacoemulsification incisions. Journal of the Korean Ophthalmological Society 2011; Vol. 52, issue 4:407‐13.
Morcillo‐Laiz 2009 {published data only}
- Morcillo‐Laiz R, Zato MA, Munoz‐Negrete FJ, Arnalich‐Montiel F. Surgically induced astigmatism after biaxial phacoemulsification compared to coaxial phacoemulsification. Eye 2009;23(4):835‐9. [DOI] [PubMed] [Google Scholar]
Musanovic 2012 {published data only}
- Musanovic Z, Jusufovic V, Halibasica M, Zvornicanin J. Corneal astigmatism after micro‐incision cataract operation. Medicinski Arhiv 2012;66(2):125‐8. [DOI] [PubMed] [Google Scholar]
Shan 2016 {published data only}
- Shan WQ, Lei XP, Tang Y, Gao LN, Ren YG, Zhao X, et al. Evaluation on curative effect of coaxial 2.2 mm and 2.8 mm incision phacoemulsification for cataract. International Eye Science 2016;16(1):97‐9. [Google Scholar]
Shi 2013 {published data only}
- Shi Q, Zhou Y, Chu L, Feng Y. Clinical evaluation on the coaxial microincision cataract surgery in hard nuclear cataracts [微切口超声乳化手术在硬核白内障病例中的效果评价]. International Eye Science 2013;13(5):934‐6. [Google Scholar]
Vasavada 2013a {published data only}
- Vasavada V, Vasavada AR, Vasavada VA, Srivastava S, Gajjar DU, Mehta S. Incision integrity and postoperative outcomes after microcoaxial phacoemulsification performed using 2 incision‐dependent systems. Journal of Cataract and Refractive Surgery 2013;39(4):563‐71. [DOI] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang J, Zhang EK, Fan WY, Ma JX, Zhao PF. The effect of micro‐incision and small‐incision coaxial phaco‐emulsification on corneal astigmatism. Clinical and Experimental Ophthalmology 2009;37(7):664‐9. [DOI] [PubMed] [Google Scholar]
Yao 2008 {published data only}
- Yao K, Tang XJ, Huang XD, Ye PP. Clinical evaluation on the bimanual microincision cataract surgery [双手法微切口超声乳化白内障吸除联合人工晶状体植入术的临床效果评价]. Chinese Journal of Ophthalmology 2008;44(6):525‐8. [PubMed] [Google Scholar]
Yao 2011 {published data only}
- Yao K, Wang W, Wu W, Tang XJ, Li ZC, Jin CF. Clinical evaluation on the coaxial 1.8 mm microincision cataract surgery [同轴1.8mm 微切口超声乳化白内障手术临床效果评价]. Chinese Journal of Ophthalmology 2011;47(10):903‐7. [PubMed] [Google Scholar]
Yu 2016 {published data only}
- Yu YB, Zhu YN, Wang W, Zhang YD, Yu YH, Yao K. A comparable study of clinical and optical outcomes after 1.8, 2.0 mm microcoaxial and 3.0 mm coaxial cataract surgery. International Journal of Ophthalmology 2016;9(3):399‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang JZ, Chen JH, Huang BJ, Jiang Y, Zhuang YZ, Li XX, et al. Effect of 1.8 mm coaxial micro‐incision cataract phacoemulsification on corneal astigmatism. International Eye Science 2014;14(4):670‐2. [Google Scholar]
Zhu 2014 {published data only}
- Zhu JG, Cao Y, Xu QH. Comparison of 2.2 mm micro incision and 3.0 mm incision coaxial phacoemulsification. International Eye Science 2014; Vol. 14, issue 8:1433‐5.
References to studies excluded from this review
Alio 2005 {published data only}
- Alio J, Rodriguez‐Prats JL, Galal A, Ramzy M. Outcomes of microincision cataract surgery versus coaxial phacoemulsification. Ophthalmology 2005;112(11):1997‐2003. [DOI] [PubMed] [Google Scholar]
Alio 2010 {published data only}
- Alio JL, Elkady B, Ortiz D. Corneal optical quality following sub 1.8 mm micro‐incision cataract surgery vs. 2.2 mm mini‐incision coaxial phacoemulsification. Middle East African Journal of Ophthalmology 2010;17(1):94‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhargava 2016 {published data only}
- Bhargava R, Kumar P, Sharma SK, Arora Y. Phacoemulsification versus manual small incision cataract surgery in patients with Fuchs heterochromic iridocyclitis. Asia‐Pacific Journal of Ophthalmology 2016;5(5):330‐4. [DOI] [PubMed] [Google Scholar]
Can 2011 {published data only}
- Can I, Bayhan HA, Celik H, Ceran BB. Anterior segment optical coherence tomography evaluation and comparison of main clear corneal incisions in microcoaxial and biaxial cataract surgery. Journal of Cataract and Refractive Surgery 2011;37(3):490‐500. [DOI] [PubMed] [Google Scholar]
Can 2012 {published data only}
- Can I, Bayhan HA, Celik H, Ceran BB. Comparison of corneal aberrations after biaxial microincision and microcoaxial cataract surgeries: a prospective study. Current Eye Research 2012;37(1):18‐24. [DOI] [PubMed] [Google Scholar]
Cavallini 2007 {published data only}
- Cavallini GM, Campi L, Masini C, Pelloni S, Pupino A. Bimanual microphacoemulsification versus coaxial miniphacoemulsification: prospective study. Journal of Cataract and Refractive Surgery 2007;33(3):387‐92. [DOI] [PubMed] [Google Scholar]
Chee 2010 {published data only}
- Chee SP, Ti SE, Lim L, Chan AS, Jap A. Anterior segment optical coherence tomography evaluation of the integrity of clear corneal incisions: a comparison between 2.2‐mm and 2.65‐mm main incisions. American Journal of Ophthalmology 2010;149(5):768‐76. [DOI] [PubMed] [Google Scholar]
Crema 2007 {published data only}
- Crema AS, Walsh A, Yamane Y, Nose W. Comparative study of coaxial phacoemulsification and microincision cataract surgery. One‐year follow‐up. Journal of Cataract and Refractive Surgery 2007;33(6):1014‐8. [DOI] [PubMed] [Google Scholar]
Denoyer 2008 {published data only}
- Denoyer A, Denoyer L, Marotte D, Georget M, Pisella PJ. Intraindividual comparative study of corneal and ocular wavefront aberrations after biaxial microincision versus coaxial small‐incision cataract surgery. British Journal of Ophthalmology 2008;92(12):1679‐84. [DOI] [PubMed] [Google Scholar]
Devendra 2014 {published data only}
- Devendra J, Agarwal S, Singh P. A comparative study of clear corneal phacoemulsification with rigid IOL versus SICS; the preferred surgical technique in low socio‐economic group patients of rural areas. Journal of Clinical and Diagnostic Research 2014; Vol. 8, issue 11:Vc01‐03. [DOI] [PMC free article] [PubMed]
Dick 2012 {published data only}
- Dick HB. Controlled clinical trial comparing biaxial microincision with coaxial small incision for cataract surgery. European Journal of Ophthalmology 2012;22(5):739‐50. [DOI] [PubMed] [Google Scholar]
Elkady 2009 {published data only}
- Elkady B, Pinero D, Alio JL. Corneal incision quality: microincision cataract surgery versus microcoaxial phacoemulsification. Journal of Cataract and Refractive Surgery 2009;35(3):466‐74. [DOI] [PubMed] [Google Scholar]
Feng 2015 {published data only}
- Feng XC, Pan WM, Guo L, Xie JR, Li HY. Research on refractive status characteristics and anterior chamber depth after cataract surgery. International Eye Science 2015; Vol. 15, issue 7:1194‐6.
Franchini 2006 {published data only}
- Franchini A, Frosini S, Boddi V. Standard coaxial phaco vs microincision cataract surgery: a corneal endothelium study. International Journal of Ophthalmology 2006;6(4):769‐74. [Google Scholar]
Gangwani 2011 {published data only}
Hashemian 2007 {published data only}
- Hashemian SJ. Microcoaxial phacoemulsification vs. conventional phacoemulsification. American Academy of Ophthalmology; 2007 Nov 10‐13; New Orleans (LA) 2007:204.
Hayashi 2014 {published data only}
Hayashi 2016 {published data only}
- Hayashi K, Ogawa S, Yoshida M, Yoshimura K. Wound stability and surgically induced corneal astigmatism after transconjunctival single‐plane sclerocorneal incision cataract surgery. Japanese Journal of Ophthalmology 2016;61(1):113‐23. [DOI] [PubMed] [Google Scholar]
Jain 2015 {published data only}
Jeon 2010 {published data only}
- Jeon S, Na KS, Kim MS. The effect of manipulation of corneal incision on astigmatism during the cataract surgery. Journal of the Korean Ophthalmological Society 2010; Vol. 51, issue 4:510‐5.
Jiang 2005 {published data only}
- Jiang Y, Luo L, Liu Y, Wu M, Zhang X, Liu Y. Ultrasonic power application in bimanual microphacoemulsification [双手微切口白内障超声乳化术的超声能量应用研究]. Chinese Ophthalmic Research 2005;23(5):528‐31. [Google Scholar]
Kahraman 2007 {published data only}
- Kahraman G, Amon M, Franz C, Prinz A, Abela‐Formanek C. Intraindividual comparison of surgical trauma after bimanual microincision and conventional small‐incision coaxial phacoemulsification. Journal of Cataract and Refractive Surgery 2007;33(4):618‐22. [DOI] [PubMed] [Google Scholar]
Kaya 2007 {published data only}
- Kaya V, Ozturker ZK, Ozturker C, Yasar O, Sivrikaya H, Agca A, et al. ThinOptX vs AcrySof: comparison of visual and refractive results, contrast sensitivity, and the incidence of posterior capsule opacification. European Journal of Ophthalmology 2007;17(3):307‐14. [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim EC, Byun YS, Kim MS. Microincision versus small‐incision coaxial cataract surgery using different power modes for hard nuclear cataract. Journal of Cataract and Refractive Surgery 2011;37(10):1799‐805. [DOI] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim JH, Kim TI, Kim EK, Lee HK. The morphological changes in main corneal incision (2.2 mm vs. 2.8 mm) evaluated using anterior segment optical coherence tomography. Journal of the Korean Ophthalmological Society 2013; Vol. 54, issue 6:877‐86.
Kochhar 2014 {published data only}
- Kochhar S, Bansal A, Ahuja A, Gupta VS. Comparative study of optical coherence tomography documented macular changes following uncomplicated cataract surgery. Clinical and Experimental Ophthalmology 2014; Vol. 42:78.
Kurz 2006 {published data only}
- Kurz S, Krummenauer F, Gabriel P, Pfeiffer N, Dick HB. Biaxial microincision versus coaxial small‐incision clear cornea cataract surgery. Ophthalmology 2006;113(10):1818‐26. [DOI] [PubMed] [Google Scholar]
Kurz 2009 {published data only}
- Kurz S, Krummenauer F, Thieme H, Dick HB. Optical coherence tomography of macular thickness after biaxial vs coaxial microincision clear corneal cataract surgery. European Journal of Ophthalmology 2009;19(6):990‐7. [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee KM, Kwon HG, Joo CK. Microcoaxial cataract surgery outcomes: comparison of 1.8 mm system and 2.2 mm system. Journal of Cataract and Refractive Surgery 2009;35(5):874‐80. [DOI] [PubMed] [Google Scholar]
Masket 2009 {published data only}
- Masket S, Wang L, Belani S. Induced astigmatism with 2.2‐ and 3.0‐mm coaxial phacoemulsification incisions. Journal of Refractive Surgery 2009;25(1):21‐4. [DOI] [PubMed] [Google Scholar]
Mencucci 2006 {published data only}
- Mencucci R, Ponchietti C, Virgili G, Giansanti F, Menchini U. Corneal endothelial damage after cataract surgery: microincision versus standard technique. Journal of Cataract and Refractive Surgery 2006;32(8):1351‐4. [DOI] [PubMed] [Google Scholar]
NCT02642211 {published data only}
- NCT02642211. IOP changes associated with SICS and phako. clinicaltrials.gov/ct2/show/NCT02642211 (first received 19 December 2015).
Park 2012 {published data only}
- Park YG, Chung SH, Joo CK. Comparison of microcoaxial with standard clear corneal incisions in torsional handpiece cataract surgery. Ophthalmologica 2012;227(1):55‐9. [DOI] [PubMed] [Google Scholar]
Shen 2014 {published data only}
- Shen MH, Fang CK. Long‐term effect of different incisions on the tear film after phacoemulsification. International Eye Science 2014;14(2):300‐3. [Google Scholar]
Song 2014 {published data only}
- Song ZY, Chen FH, Cheng F, Yan AM, Qiu X, Lou XF. Effect of micro‐incision on corneal wavefront aberration and tear film in phacoemulsification. International Eye Science 2014; Vol. 14, issue 12:2207‐9.
Suasnavas 2010 {published data only}
- Suasnavas AC, Estrella MJ. Cataract surgery induced astigmatism with phacoemulsification and 2.2 mm versus 2.75 mm incision: a prospective study [Cirugía de catarata con facoemulsificación y astigmatismo inducido con incisión 2.2 vs. 2.75 mm: estudio prospectivo]. Metro Ciencia 2010;19(1):5‐9. [Google Scholar]
Titiyal 2006 {published data only}
- Titiyal JS, More PD, Vajpayee RB, Tandon R, Sharma N. Comparative evaluation of bimanual microincision cataract surgery with coaxial phacoemulsification surgery. American Academy of Ophthalmology; 2006 Nov 11‐14 Las Vegas (NV). 2006; Vol. 35:204.
Tong 2008 {published data only}
- Tong N, He JC, Lu F, Wang Q, Qu J, Zhao YE. Changes in corneal wavefront aberrations in microincision and small‐incision cataract surgery. Journal of Cataract and Refractive Surgery 2008;34(12):2085‐90. [DOI] [PubMed] [Google Scholar]
Vasavada 2013b {published data only}
- Vasavada AR, Johar K Sr, Praveen MR, Vasavada VA, Arora AI. Histomorphological and immunofluorescence evaluation of clear corneal incisions after microcoaxial phacoemulsification with 2.2 mm and 1.8 mm systems. Journal of Cataract and Refractive Surgery 2013;39(4):617‐23. [DOI] [PubMed] [Google Scholar]
von Sonnleithner 2015 {published data only}
- Sonnleithner C, Bergholz R, Gonnermann J, Klamann MK, Torun N, Bertelmann E. Clinical results and higher‐order aberrations after 1.4‐mm biaxial cataract surgery and implantation of a new aspheric intraocular lens. Ophthalmic Research 2015;53(1):8‐14. [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang Y, Xia Y, Liu X, Zheng D, Luo L, Liu Y. Comparison of bimanual and micro‐coaxial phacoemulsification with torsional ultrasound. Acta Ophthalmologica 2012;90(2):184‐7. [DOI] [PubMed] [Google Scholar]
Wei 2012 {published data only}
- Wei YH, Chen WL, Su PY, Shen EP, Hu FR. The influence of corneal wound size on surgically induced corneal astigmatism after phacoemulsification. Journal of the Formosan Medical Association 2012;111(5):284‐9. [DOI] [PubMed] [Google Scholar]
Wilczynska 2010 {published data only}
- Wilczynska O, Wilczynski M, Omulecki W. Surgically induced astigmatism after bimanual phacoemulsification through microincision and after standard phacoemulsification [Niezbornosc indukowana chirurgicznie po bimanualnej fakoemulsyfikacji przez mikrociecie oraz po standardowej fakoemulsyfikacji zacmy]. Klinika Oczna 2010;112(4‐6):115‐9. [PubMed] [Google Scholar]
Wylegala 2009 {published data only}
- Wylegala E, Rebkowska‐Juraszek M, Dobrowolski D, Woyna‐Orlewicz A. Influence of 3.0 mm incision coaxial phacoemulsification and microincision cataract surgery (MICS) on corneal thickness [Wplyw fakoemulsyfikacji zacmy technika koaksjalna z ciecia 3.0 mm i metoda malego ciecia (MICS) na grubosc rogowki]. Klinika Oczna 2009;111(7‐9):207‐11. [PubMed] [Google Scholar]
Yao 2006 {published data only}
- Yao K, Tang X, Ye P. Corneal astigmatism, high order aberrations, and optical quality after cataract surgery: microincision versus small incision. Journal of Refractive Surgery 2006;22(9 Suppl):S1079‐82. [DOI] [PubMed] [Google Scholar]
Zhou 2012 {published data only}
- Zhou Y, Chang P, Wang D, Zhao Y. Influence of different length corneal limbal incisions on the anterior and posterior corneal astigmatism after phacoemulsification [两种不同长度角膜缘切口对白内障超声乳化术后角膜前后表面散光影响的对比]. Chinese Journal of Experimental Ophthalmology 2012;30(6):543‐7. [Google Scholar]
Additional references
Ang 2014
- Ang M, Evans JR, Mehta JS. Manual small incision cataract surgery (MSICS) with posterior chamber intraocular lens versus extracapsular cataract extraction (ECCE) with posterior chamber intraocular lens for age‐related cataract. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD008811.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aravind 2008
- Aravind S, Haripriya A, Sumara Taranum BS. Cataract surgery and intraocular lens manufacturing in India. Current Opinion in Ophthalmology 2008;19(1):60‐5. [DOI] [PubMed] [Google Scholar]
Asbell 2005
- Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S. Age‐related cataract. Lancet 2005;365(9459):599‐609. [DOI] [PubMed] [Google Scholar]
Behndig 2011
- Behndig A, Montan P, Stenevi U, Kugelberg M, Lundström M. One million cataract surgeries: Swedish National Cataract Register 1992‐2009. Journal of Cataract and Refractive Surgery 2011;37(8):1539‐45. [DOI] [PubMed] [Google Scholar]
Chee 2005
- Chee SP, Bacsal K. Endophthalmitis after microincision cataract surgery. Journal of Cataract and Refractive Surgery 2005;31(9):1834‐5. [DOI] [PubMed] [Google Scholar]
de Silva 2014
- Silva SR, Riaz Y, Evans JR. Phacoemulsification with posterior chamber intraocular lens versus extracapsular cataract extraction (ECCE) with posterior chamber intraocular lens for age‐related cataract. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD008812.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2005
- Foster A, Resnikoff S. The impact of Vision 2020 on global blindness. Eye 2005;19(10):1133‐5. [DOI] [PubMed] [Google Scholar]
Frick 2003
- Frick KD, Foster A. The magnitude and cost of global blindness: an increasing problem that can be alleviated. American Journal of Ophthalmology 2003;135(4):471‐6. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org. GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org, 2015.
Guyatt 2011
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64:380‐2. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kelman 1967
- Kelman CD. Phaco‐emulsification and aspiration. A new technique of cataract removal. A preliminary report. American Journal of Ophthalmology 1967;64(1):23‐35. [PubMed] [Google Scholar]
Lam 2009
- Lam DS, Rao SK, Wong VW, Yao K, Abhay R, Vasavada DF, et al. Ophthalmic Surgery Cataract II Advanced Phaco Surgery. Hong Kong: Bon Vision Limited, 2009. [Google Scholar]
Mathew 2012
- Mathew MC, Ervin AM, Tao J, Davis RM. Antioxidant vitamin supplementation for preventing and slowing the progression of age‐related cataract. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD004567.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osher 2007a
- Osher RH, Injev VP. Microcoaxial phacoemulsification Part 1: laboratory studies. Journal of Cataract and Refractive Surgery 2007;33(3):401‐7. [DOI] [PubMed] [Google Scholar]
Osher 2007b
- Osher RH. Microcoaxial phacoemulsification Part 2: clinical study. Journal of Cataract and Refractive Surgery 2007;33(3):408‐12. [DOI] [PubMed] [Google Scholar]
Paul 2005
- Paul T, Braga‐Mele R. Bimanual microincisional phacoemulsification: the future of cataract surgery?. Current Opinion in Ophthalmology 2005;16(1):2‐7. [DOI] [PubMed] [Google Scholar]
Resnikoff 2004
- Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bulletin of the World Health Organization 2004;82(11):844‐51. [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riaz 2006
- Riaz Y, Mehta JS, Wormald R, Evans JR, Foster A, Ravilla T, et al. Surgical interventions for age‐related cataract. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD001323.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riaz 2013
- Riaz Y, Silva SR, Evans JR. Manual small incision cataract surgery (MSICS) with posterior chamber intraocular lens versus phacoemulsification with posterior chamber intraocular lens for age‐related cataract. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008813.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schlote 2006
- Schlote T, Rohrbach J, Grueb M, Mielke J. Pocket Atlas of Ophthalmology. New York: Thieme, 2006. [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shentu 2016
- Shentu X, Zhang X, Tang X, Yu X. Coaxial microincision cataract surgery versus standard coaxial small‐incision cataract surgery: a meta‐analysis of randomized controlled trials. PLOS ONE 2016;11(1):e0146676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shiels 2010
- Shiels A, Bennett TM, Hejtmancik JF. Cat‐Map: putting cataract on the map. Molecular Vision 2010;16:2007‐15. [PMC free article] [PubMed] [Google Scholar]
Weikert 2006
- Weikert MP. Update on bimanual microincisional cataract surgery. Current Opinion in Ophthalmology 2006;17(1):62‐7. [DOI] [PubMed] [Google Scholar]
WHO 2017
- World Health Organization. Blindness: Vision 2020 ‐ control of major blinding diseases and disorders. The Global Initiative for the Elimination of Avoidable Blindness. Fact sheet N°214. Available at www.who.int/mediacentre/factsheets/fs214/en (accessed 14 September 2017).
Yu 2012
- Yu JG, Zhao YE, Shi JL, Ye T, Jin N, Wang QM, et al. Biaxial microincision cataract surgery versus conventional coaxial cataract surgery: meta‐analysis of randomized controlled trials. Journal of Cataract and Refractive Surgery 2012;38(5):894‐901. [DOI] [PubMed] [Google Scholar]


