Abstract
Background
Preventing hospitalization and improving event-free survival are primary goals of heart failure (HF) treatment according to current European Society of Cardiology guidelines; however, substantial uncertainty remains in our ability to predict risk and improve outcomes. Although caregivers often assist patients to manage their HF, little is known about their influence on clinical outcomes.
Aims
To quantify the influence of patient and caregiver characteristics on patient clinical-event risk in HF.
Methods
This was a secondary analysis of data using a sample of Italian adults with HF and their informal caregivers (n = 183 patient-caregiver dyads). HF patients were followed over 12 months for the following clinical events: hospitalization for HF, emergency room visit for HF or all-cause mortality. Influence of baseline caregiver- and patient-level factors (patient and caregiver age; dyad relationship type; patient NYHA Class, cognition, and comorbidities; and caregiver strain, mental health status, and contributions to HF self-care) on patient risk of death or hospitalization/emergency room use was quantified using Cox proportional hazards regression.
Results
Over the course of follow-up, 32.8% of patients died, 19.7% were hospitalized for HF and 10.4% visited the emergency room. Higher caregiver strain, better caregiver mental health status and greater caregiver contributions to HF self-care maintenance were associated with significantly better event-free survival. Worse patient functional class and greater caregiver contributions to patient self-care management were associated with significantly worse patient event-free survival.
Conclusion
Considering caregiving factors together with patient factors significantly increases our understanding of patient clinical event-risk in HF.
MeSH Keywords: Heart Failure, Hospitalization, Mortality, Caregivers
Introduction
A rapidly increasing number of adults are being diagnosed with heart failure (HF), a disorder characterized by significant symptom burden, poor quality of life (QOL) and premature mortality.1, 2 Frequent hospitalization and premature death are distressing for patients and families and costly to healthcare systems. As such, preventing hospitalization and improving event-free survival are primary treatment goals of the current European Society of Cardiology guidelines.2 Despite being the focus of a primary treatment goal, the burden of hospitalization and mortality remains high in HF. Over half of patients admitted for HF will be readmitted within a year,3 and 27% will die during that same time.4 The prognosis for HF is worse than most cancers,5 and most patients with advanced HF have repeated hospitalizations and die within 2 years.6, 7 Despite a heavy burden of events, there remains a substantial amount of uncertainty around our ability to predict clinical outcomes in HF,6, 8 and half of nursing interventions that aim to reduce hospitalization or death are unsuccessful.9 Thus, there is an imminent need to improve our capacity to predict and ameliorate clinical-event risk in HF.
Despite the fact that HF patients often have informal (unpaid) caregivers to assist them in managing their disease,10 caregiver factors are rarely included in risk models or interventions.8, 9 Including interpersonal (i.e. caregiver and relational) factors may be one promising avenue for elucidating unexplained variability in clinical event-risk in HF: patient and caregiver outcomes have been shown to be transactional (e.g. one member's characteristics influencing the other member and vice versa) in HF and other chronic illness contexts,11-13 and clinical event-risk has been associated with caregiver characteristics in HF.14-16 Thus, the objective of this analysis is to build on previous work by quantifying the impact of interpersonal characteristics on patient clinical-event risk in a larger sample of HF patient-caregiver dyads, and integrating important caregiver contributions to disease management.
Methods
Study Design
This was a secondary analysis of a subset of data from a multi-site observational study of Italian community-dwelling HF patients and their informal caregivers. The study methods have been described previously.17, 18 Patients were enrolled from outpatient cardiovascular clinics across 28 Italian provinces, and were eligible if: 1) they were at the clinic for a routine HF appointment; 2) they had an echocardiogram-confirmed diagnosis of HF; 3) clinical evidence of HF was present as outlined by European Society of Cardiology guidelines19 and 4) they were willing and able to provide informed consent. Patients less than 18 years of age were excluded, as were those with a recent (≤ 3 months prior) acute coronary event or clear evidence of dementia. If the patient's adult primary informal caregiver had accompanied them to the appointment, the caregiver was offered enrollment as well. For the cross-sectional parent study, data was collected at the time of enrollment. Data for this analysis is derived from a subset of data from an ancillary study, in which patients and caregivers provided additional consent for patient clinical events follow-up over the course of one year.
Ethical Approval
This investigation conformed with the principles outlined in the Declaration of Helsinki, ethics committees at each site approved the research protocol and written informed consent was obtained from all participants. Data for this analysis was appropriately de-identified and the Institutional Review Board of the first and senior authors approved this analysis as being exempt from additional human subjects review.
Measurement
Demographics and clinical characteristics
Demographic data (age, gender, marital status, education level, employment and how patient and caregiver were related) were collected from patients and caregivers using self-report questionnaires. Self-report questionnaires also included several study instruments; those germane to this analysis are described in the following section. Questionnaires were administered in a private space at the clinic at the time of enrollment by trained research nurses. The patient's medical record was also abstracted for clinical characteristics (left ventricular ejection fraction, New York Heart Association (NYHA) Class, duration of HF, hospitalizations within the past year, number of medications, serum sodium, serum hemoglobin, home oxygen use and comorbid conditions).20
Clinical events
The clinical events of interest in this study were as follows: a composite of all-cause mortality, hospitalization for HF, or emergency room visits due to HF exacerbation, whichever event came first. Clinical events data were collected via phone interviews with patients and/or their caregivers. In preparation for analysis, survival times (time to first clinical event) were calculated for each study participant.
Patient cognitive impairment
Cognitive impairment was measured using the Mini-Mental State Examination (MMSE).21 The MMSE is designed for brief cognitive evaluation in the clinical setting, and involves questions and activities that collectively test orientation, memory, attention and calculation and language. Scores on each portion of the exam are summed for a total possible score of 30, with higher scores indicating better cognition. The MMSE is one of the most widely used instruments for testing cognition, and has been validated for use in an Italian population.22
Caregiver strain
The Caregiver Burden Inventory23 was used to assess strain in caregivers. It is a multidimensional instrument with 24 items and 5 subscales. This instrument has been validated for use in an Italian population.24 The summed Physical and Developmental subscales were used for this analysis. Within an Italian population, the 9 items from these two subscales load together in psychometric analysis,24 providing cumulative information on strain related to caregiving in terms of physical stress and feelings of being “off time” in life expectations and hopes. Furthermore, including both developmental and physical strain is supported by dyadic caregiving theory, which highlights the additional influence of development (i.e. life course) on patient and caregiver adjustment to illness.11 Together, this combined subscale includes items such as: “I wish I could escape from this situation,” “My social life has suffered,” “I expected that things would be different at this point in my life,” “My health has suffered,” And “Caregiving has made me physically ill.” Scores for this subscale range from 0-36, with higher scores indicating greater strain; subscale reliability in this sample was good (Cronbach's α = 0.93).
Caregiver mental health status
The mental component summary scale of the Short Form-1225 was used to assess caregiver mental health status. Scores are normed and standardized to range from 0-100, with higher scores indicating better mental-health status. The SF-12 has been validated for use in Italy, has good reliability (Cronbach's α > 0.90) and has been widely used in caregiving research in general and HF caregiving research in particular.25-28 To examine the comparative difference in survival by caregiver mental health status, tertiles of caregiver mental health were generated and modeled as categorical predictors.
Patient and caregiver contributions to heart failure self-care
HF self-care is conceptualized as the daily maintenance (e.g. adhering to medications, exercise, restricting sodium, monitoring for HF symptoms) and symptom management (i.e. timely and appropriate response to HF symptoms when they occur) behaviors that patients must engage in order to maintain clinical stability.29, 30 Caregivers often assist patients with both maintenance and management behaviors.31, 32 Patient and caregiver contributions to HF self-care were assessed using the validated Italian Self-Care of HF Index version 6.2 (SCHFI) and the parallel caregiver contributions version (CC-SCHFI), respectively.33, 34 The SCHFI and CC-SCHFI have separate subscales for self-care maintenance and management behaviors, with varied item response scales. Scores for each subscale are standardized to range from 0-100, with higher scores indicating better self-care. While the SCHFI (patient version) asks the respondent how often they engage in their own care, the CC-SCHFI (caregiver version) asks how often the respondent recommends that the patient engages in care (or does the behavior for the patient if the patient is unable) on the same items. Factor score determinancy reliability coefficients were acceptable (0.78 – 0.90).
Analysis
Standard descriptive statistics (Means and standard deviations or n and proportions) were used to describe the sample. Cox proportional hazards modeling was used to quantify clinical event risk (all-cause mortality, hospitalization for HF or emergency room visit for HF). Due to limitations in sample size, a modified backwards stepwise approach was used to select covariates for a parsimonious model with a goal of no more than 10 predictors. First, patient HF-related clinical covariates identified in an event-risk analysis using the larger, patient-only subset of this data (manuscript under review)35 were entered into a backward stepwise Cox regression. Entered covariates included NYHA class (III/IV versus I/II), hospitalization in the past year (yes/no), number of medications, left ventricular ejection fraction, serum sodium, serum hemoglobin, home oxygen use (yes/no), HF-specific QOL (Minnesota Living with Heart Failure Physical and Emotional subscales36), duration of HF (months), and patient cognition. NYHA Class and patient cognition were retained in the model (p < 0.20 retention). Next, patient and caregiver controls and caregiver/dyadic variables of interest were entered into the model: patient/caregiver age, gender, education and employment; patient comorbidities; dyad relationship type (spousal/non-spousal); caregiver perceived social support (subscale of the Carers of Older People in Europe Index37); caregiver strain; caregiver mental and physical health status (SF-12 mental and physical component summary scores25) and patient and caregiver contributions to HF self-care maintenance and management. Variables without significant individual or global effect were removed to arrive at a parsimonious model. There was no evidence of multicollinearity among final model covariates. Global and individual covariate tests using Schoenfeld residuals were non-significant, and were graphically confirmed to verify that the proportional hazards assumption had not been violated.38 All analyses were performed using Stata MPv14 (College Station, TX); results from the proportional hazards model are reported using hazard ratios (HR) and 95% confidence intervals (CI).
Results
Of the 575 patients who agreed to participate in clinical events follow-up, 183 also had caregivers who participated in baseline data collection for the parent study. Characteristics of these 183 patient-caregiver dyads are presented in Table 1. In short, patients were older on average than their caregivers, and a slight majority of patients were male, while caregivers were largely female. Most commonly, caregivers were adult children of patients, and the second most common relationship was spousal. A little over half of patients were NYHA Class III or IV at baseline and had been hospitalized in the year prior to enrollment. Over the course of 12 months of follow-up, approximately one-third of patients had died, one-fifth had been hospitalized for HF, and one-tenth had visited the emergency room for HF.
Table 1. Characteristics of the Sample.
| Patients Mean ± SD or n (%) |
Caregivers Mean ± SD or n (%) |
|
|---|---|---|
| Age (years) | 75.6 ± 11.7 | 57.2 ± 14.3 |
| Gender (female) | 85 (46.5%) | 107 (67.3%) |
| Educationa | 46 (25.1%) | 87 (51.2%) |
| Employed | 23 (12.6%) | 89 (52.1%) |
| Spousal Caregiver | - | 51 (32.5%) |
| NYHA Class III/IV | 94 (51.4%) | - |
| EF (%) | 43.9 ± 12.4 | - |
| HF Duration (months) | 60.7 ± 49.4 | - |
| Prior hospitalizationb | 100 (54.6%) | - |
| Comorbiditiesc | 3.18 ± 1.4 | - |
| Cognition (MMSE) | 22.8 ± 7.1 | - |
| Caregiver Strain | - | 10.2 ± 9.5 |
| Mental Health Status | - | 48.5 ± 9.4 |
| Physical Health Status | - | 46.4 ± 8.8 |
| Self-Care Maintenance | 56.7 ± 16.5 | 58.5 ± 19.2 |
| Self-Care Management | 50.0 ± 21.6 | 56.7 ± 19.8 |
| Clinical Events Distribution: | ||
| Alive Without Event | 68 (37.2%) | - |
| Death (All-Cause) | 60 (32.8%) | - |
| HF Hospitalization | 36 (19.7%) | - |
| HF ED Visit | 19 (10.4%) | - |
Education: High School, Professional School, or University versus not
Hospitalization (yes/no) in year prior to enrollment
Comorbidities: Charlson Comorbidity Index score
NYHA Class: New York Heart Association Class; EF: ejection fraction; HF: heart failure; MMSE: Mini Mental State Exam; ED: emergency department
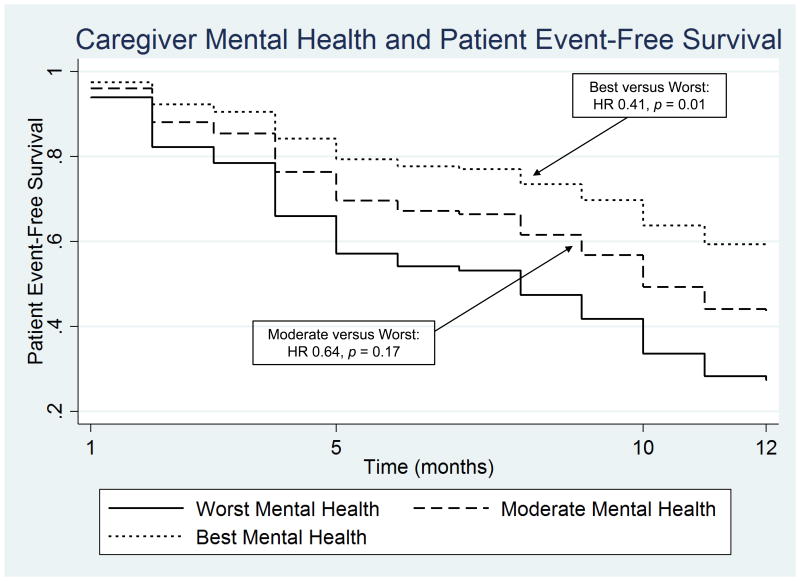
Results from the Cox proportional hazards model are displayed in Table 2. The final multivariate model included patient and caregiver age, patient NYHA Class (III/IV versus I/II), patient cognition, patient comorbidities, caregiving relationship type (non-spousal versus spousal), caregiver strain, caregiver mental health status and caregiver contributions to self-care maintenance and management. Higher caregiver strain, better caregiver mental health status, and greater caregiver contributions to HF self-care maintenance (daily adherence behaviors) at baseline were associated with significantly lower risk of patient death or hospitalization during follow-up. In contrast, higher (worse) patient NYHA Class and greater caregiver contributions to patient self-care management (response to HF symptoms when they occur) at baseline were associated with significantly higher patient clinical event-risk during follow up. Patients with caregivers in the highest tertile of self-reported mental health (opposed to the lowest tertile) had significantly lower risk of clinical events over time (Figure 1).
Table 2. Determinants of Patient Clinical Event-Risk.
| HR | 95% CI | p - value | |
|---|---|---|---|
| Patient Age | 1.00 | 0.98 – 1.03 | 0.96 |
| Caregiver Age | 1.00 | 0.98 – 1.03 | 0.79 |
| Patient NYHA Class III/IV | 2.14 | 1.28 – 3.59 | <0.01 |
| Patient Cognition (MMSE) | 0.98 | 0.94 – 1.02 | 0.30 |
| Patient Comorbidities (Charlson) | 1.06 | 0.90 – 1.25 | 0.49 |
| Non-Spousal Caregiver | 0.73 | 0.33 – 1.63 | 0.44 |
| Caregiver Strain | 0.94 | 0.91 – 0.97 | <0.001 |
| Caregiver Mental Health Status | 0.97 | 0.94 – 0.99 | 0.02 |
| Caregiver Contribution to Self-Care Maintenance | 0.99 | 0.97 – 0.99 | 0.04 |
| Caregiver Contributions to Self-Care Management | 1.01 | 1.00 – 1.03 | 0.04 |
HR: hazards ratio; CI: confidence interval; NYHA: New York Heart Association; MMSE: Mini-Mental State Exam
Figure 1.
Discussion
In this analysis of HF patients and their informal caregivers, we found that, in addition to patient functional class, multiple caregiver factors predicted patient clinical event-risk. We will focus our discussion on the significant caregiver determinants identified in the model, namely, caregiver strain, caregiver mental health and caregiver contributions to HF self-care. We will then discuss implications for research and practice and address study limitations.
Caregiver Strain
We found that higher caregiver strain was significantly associated with lower patient clinical event-risk, meaning patients with better event-free survival had caregivers who reported more strain at baseline. Interestingly, two previous studies in HF caregiving have identified associations between higher caregiving strain and greater risk of a patient event over time,14, 16 however, both the patient population and the instruments used to measure strain were substantially different. In particular, these prior studies almost exclusively consisted of patients who were hospitalized or previously hospitalized at time of enrollment, and the caregiver strain measures did not focus primarily on physical or developmental impacts of caregiving. In contrast, our study enrolled only community-dwelling patients, with nearly half having no prior hospitalization, and we specifically examined physical and developmental caregiving strain. As prior hospitalization has been associated with greater strain,15, 39 it is not surprising that our sample would yield different results. Furthermore, the association between greater physical/developmental strain in caregivers and fewer patient clinical events may be indicative of caregivers sacrificing their own health to provide care for the HF patient, a phenomenon that has been observed in advanced HF patients receiving VAD therapy,40 as well as more broadly across chronic illness contexts.41 Thus, for caregivers of relatively stable outpatients, our findings demonstrate a potential trade-off between patient and caregiver clinical event risk, given that caregiver strain is itself an independent predictor of caregiver morbidity and mortality.42, 43
Caregiver Mental Health
In contrast to the physical and developmental strain of caregiving, we found that better self-reported caregiver mental health status was associated with lower patient clinical event risk, or conversely, that worse caregiver mental health was associated with worse patient event-free survival. This is consistent with previous work, which has found associations between greater caregiver depression at baseline and higher HF patient clinical event-risk over time.14 It is possible that caregivers experience psychological distress as a function of caring for a patient that they perceive to be clinically worsening; thus, caregivers may be an early litmus test for impending loss of patient clinical stability. It is also possible that caregivers with worse mental health are impaired in their ability to provide care, resulting in higher patient event-risk. For example, feelings of distress, anger or depression in caregivers has been associated with significantly worse HF patient medication adherence.44 In either case, there may be value in including caregivers in regular clinic visits, as they may provide additional information to assist in patient clinical events prognostication, and identification and intervention on caregiver psychological distress may have dual patient-caregiver benefit.
Caregiver Contributions to Heart Failure Self-Care
We found that higher caregiver contributions to self-care maintenance were associated with better patient event-free survival, while higher caregiver contributions to self-care management were associated with worse patient event-free survival. To our knowledge, this is the first study in HF to demonstrate an association between caregiver engagement in HF self-care behaviors and patient clinical event-risk, with HRs in our model reflecting a 10% decrease or increase in mortality for each 10 point shift in caregiver contributions to self-care maintenance or management, respectively (scales range from 0-100). Interestingly, patient self-care contributions had no individual or global effect in our model. This is contrary to previous work done in a North American population,29 which may be evidence of cultural effects (i.e. Italian versus American) or modeling effects (i.e. individual patient contributions become less significant when caregiver factors are controlled).
From recent observational and interventional research, we know that caregivers play a major role in HF self-care,31 that the patient-caregiver relationship influences self-care engagement,32, 45, 46 and that dyadic interventions to improve self-care may be more efficacious than individual approaches.47, 48 Thus, it is not surprising that caregiver self-care contributions significantly predicted patient event-free survival in this analysis. Specifically, our analysis demonstrates that that caregiver engagement in the day-to-day adherence behaviors required to maintain clinical stability (self-care maintenance) may be protective against clinical events for patients, while greater caregiver engagement in symptom response behaviors (self-care management) may be a signal of increasing frequency of HF symptoms and impending decompensation. Moreover, the risk associated with caregivers' increased need to contribute to self-care management was evenly balanced with the protective benefit of caregivers' self-care maintenance contributions in our model (i.e. almost identical effect sizes). Thus, the hazards associated with patients' increased need for assistance with self-care management is, in essence, neutralized when caregivers help patients with adherence to day-to-day HF self-care maintenance behaviors.
Implications
This study has several implications for research and practice. Broadly, from both a research and clinical perspective, this analysis provides preliminary evidence that caregiver characteristics are associated with patient clinical event-risk in HF. Considering patients that are otherwise similar (age, functional class, cognition, comorbid burden), caregiver factors may provide additional information to refine our ability to predict clinical events. For example, caregiver psychological distress may be a sign that a patient is deteriorating, and similarly, increasing caregiver investment in self-care management behaviors may signal increasing or poorly controlled symptoms that require timely adjustment to medical management. Thus, identification in the clinical setting of increasing caregiver distress or contributions to HF symptom management may signal an important window of opportunity for patient and family intervention, and could potentially be used to trigger goals of care discussions, shared decision-making, advanced care planning, palliative care consultation, psychotherapeutic support services, or other potential interventions for the patient and caregiver. Furthermore, our analysis demonstrates that the support caregivers provide in terms of assistance with daily adherence behaviors (self-care maintenance) and investment in other caregiving activities that may result in caregiver strain have a measurable influence on patient clinical outcomes.
Our analysis also demonstrates potential trade-offs between caregiver and patient outcomes. Higher caregiver strain predicting better patient event-free survival is particularly concerning, given the increased risk of morbidity and mortality that comes with caregiver strain. Thus, there may be both ethical and clinical rationale for providing supportive services to caregivers. From an ethical perspective, healthcare systems rely on informal caregivers to assist patients with disease management in the community setting, which may subsequently make family members vulnerable to adverse outcomes as a result of assuming a caregiving role.41, 43 From a clinical perspective, supporting caregivers has the potential to improve both patient and caregiver outcomes. If caregiver factors impact patient clinical outcomes, as our analysis and others have demonstrated,14, 16 it may be beneficial to include caregivers (if patient and caregiver consent) in regular clinic visits. Caregivers may detect physical declines that the patient is not perceiving (e.g. due to compromised somatic perception or mild cognitive impairment related to HF49, 50) and thus may be able to provide additional insight to how the patient is progressing clinically in the community setting. This may also be a time that providers can identify and refer caregivers that may need extra support, given that struggling caregivers may not readily advocate for themselves.41
Limitations
This analysis has limitations. Ideally, our model would control for all patient-level factors that are known predictors of clinical event-risk (e.g. diagnostic, laboratory and treatment characteristics) before adding caregiver factors, however, our relatively small sample size limited the number of covariates we could include. Larger samples are needed to further examine the relationships we identified. Second, clinical events were measured by self-report, as the study personnel did not have long term access to patients' medical records; as such, it is possible that clinical events were underreported.51 Third, given that this was a single-country sample, results may not be generalizable outside of Italy, and thus replication in other regions is needed.
Conclusions
We identified multiple caregiver factors at baseline that significantly predicted patient clinical event risk over one year. In particular, higher caregiver strain and greater caregiver contributions to day-to-day HF self-care maintenance behaviors were associated with better patient event-free survival. Additionally, worse caregiver mental health status and greater caregiver engagement in HF symptom-response behaviors (self-care management) were associated with higher risk of patient clinical events. This analysis demonstrates that caregivers have a measurable impact on patient event-risk, and that concerning trade-offs may exist between patient and caregiver clinical outcomes. Future work is needed in larger samples and other regions to further examine the relationships identified in this study, and to test potential interventions to optimize clinical outcomes for patients and caregivers together.
Acknowledgments
Funding: The authors acknowledge the Center of Excellence for Nursing Scholarship, Rome, for funding the parent study. This work was supported by the National Institutes of Health/ National Institute of Nursing Research (1F31NR014760, T32NR012715). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
Footnotes
Declaration of Conflicting Interests: The authors declare that there is no conflict of interest
Contributor Information
Julie T Bidwell, 3455 SW US Veterans Hospital Road, Mail Code SN-ORD, Portland, OR 972391, United States of America.
Ercole Vellone, Department of Biomedicine and Prevention, University of Rome “Tor Vergata”, Via Montpellier, 1 – 00133, Rome, Italy.
Karen S Lyons, 3455 SW US Veterans Hospital Road, Mail Code SN-ORD, Portland, OR 97239, United States of America.
Fabio D'Agostino, Department of Biomedicine and Prevention, University of Rome “Tor Vergata”, Via Montpellier, 1 – 00133, Rome, Italy.
Barbara Riegel, Claire M. Fagin Hall, 418 Curie Boulevard, Philadelphia, PA 19104, United States of America.
Marco Paturzo, Department of Biomedicine and Prevention, University of Rome “Tor Vergata”, Via Montpellier, 1 – 00133, Rome, Italy.
Shirin O Hiatt, 3455 SW US Veterans Hospital Road, Mail Code SN-ORD, Portland, OR 97239, United States of America.
Rosaria Alvaro, Department of Biomedicine and Prevention, University of Rome “Tor Vergata”, Via Montpellier, 1 – 00133, Rome, Italy.
Christopher S Lee, 3455 SW US Veterans Hospital Road, Mail Code SN-ORD, Portland, OR 97239, United States of America.
References
- 1.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Rev Esp Cardiol (Engl Ed) 2016;69:1167. doi: 10.1016/j.rec.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Avaldi VM, Lenzi J, Castaldini I, et al. Hospital readmissions of patients with heart failure: the impact of hospital and primary care organizational factors in Northern Italy. PLoS One. 2015;10:e0127796. doi: 10.1371/journal.pone.0127796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alter DA, Ko DT, Tu JV, et al. The average lifespan of patients discharged from hospital with heart failure. J Gen Intern Med. 2012;27:1171–9. doi: 10.1007/s11606-012-2072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3:315–22. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 6.Russell SD, Miller LW, Pagani FD. Advanced heart failure: a call to action. Congest Heart Fail. 2008;14:316–21. doi: 10.1111/j.1751-7133.2008.00022.x. [DOI] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 8.Rahimi K, Bennett D, Conrad N, et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail. 2014;2:440–6. doi: 10.1016/j.jchf.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Allen JK, Dennison CR. Randomized trials of nursing interventions for secondary prevention in patients with coronary artery disease and heart failure: systematic review. J Cardiovasc Nurs. 2010;25:207–20. doi: 10.1097/JCN.0b013e3181cc79be. [DOI] [PubMed] [Google Scholar]
- 10.Hwang B, Luttik ML, Dracup K, Jaarsma T. Family caregiving for patients with heart failure: types of care provided and gender differences. J Card Fail. 2010;16:398–403. doi: 10.1016/j.cardfail.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Berg CA, Upchurch R. A developmental-contextual model of couples coping with chronic illness across the adult life span. Psychol Bull. 2007;133:920–54. doi: 10.1037/0033-2909.133.6.920. [DOI] [PubMed] [Google Scholar]
- 12.Bidwell JT, Lyons KS, Lee CS. Caregiver well-being and patient outcomes in heart failure: A meta-analysis. J Cardiovasc Nurs. doi: 10.1097/JCN.0000000000000350. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunbar SB, Clark PC, Quinn C, Gary RA, Kaslow NJ. Family influences on heart failure self-care and outcomes. J Cardiovasc Nurs. 2008;23:258–65. doi: 10.1097/01.JCN.0000305093.20012.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooley PJ, Butler G, Howlett JG. The relationship of quality of life, depression, and caregiver burden in outpatients with congestive heart failure. Congest Heart Fail. 2005;11:303–10. doi: 10.1111/j.1527-5299.2005.03620.x. [DOI] [PubMed] [Google Scholar]
- 15.Saunders MM. Family caregiver support and hospitalizations of patients with heart failure. Home Healthc Nurse. 2008;26:624–32. doi: 10.1097/01.NHH.0000341226.40640.ad. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz KA, Elman CS. Identification of factors predictive of hospital readmissions for patients with heart failure. Heart Lung. 2003;32:88–99. doi: 10.1067/mhl.2003.15. [DOI] [PubMed] [Google Scholar]
- 17.Cocchieri A, Riegel B, D'Agostino F, et al. Describing self-care in Italian adults with heart failure and identifying determinants of poor self-care. Eur J Cardiovasc Nurs. 2015;14:126–36. doi: 10.1177/1474515113518443. [DOI] [PubMed] [Google Scholar]
- 18.Vellone E, Chung ML, Cocchieri A, Rocco G, Alvaro R, Riegel B. Effects of self-care on quality of life in adults with heart failure and their spousal caregivers: testing dyadic dynamics using the actor-partner interdependence model. J Fam Nurs. 2014;20:120–41. doi: 10.1177/1074840713510205. [DOI] [PubMed] [Google Scholar]
- 19.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–69. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Grigoletto F, Zappala G, Anderson DW, Lebowitz BD. Norms for the Mini-Mental State Examination in a healthy population. Neurology. 1999;53:315–20. doi: 10.1212/wnl.53.2.315. [DOI] [PubMed] [Google Scholar]
- 23.Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798–803. doi: 10.1093/geront/29.6.798. [DOI] [PubMed] [Google Scholar]
- 24.Marvardi M, Mattioli P, Spazzafumo L, et al. The Caregiver Burden Inventory in evaluating the burden of caregivers of elderly demented patients: results from a multicenter study. Aging Clin Exp Res. 2005;17:46–53. doi: 10.1007/BF03337720. [DOI] [PubMed] [Google Scholar]
- 25.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Evangelista LS, Dracup K, Doering L, Westlake C, Fonarow GC, Hamilton M. Emotional well-being of heart failure patients and their caregivers. J Card Fail. 2002;8:300–5. doi: 10.1054/jcaf.2002.128005. [DOI] [PubMed] [Google Scholar]
- 27.Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1171–8. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 28.Martensson J, Dracup K, Canary C, Fridlund B. Living with heart failure: depression and quality of life in patients and spouses. J Heart Lung Transplant. 2003;22:460–7. doi: 10.1016/s1053-2498(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 29.Lee CS, Moser DK, Lennie TA, Riegel B. Event-free survival in adults with heart failure who engage in self-care management. Heart Lung. 2011;40:12–20. doi: 10.1016/j.hrtlng.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riegel B, Lee CS, Dickson VV. Self care in patients with chronic heart failure. Nat Rev Cardiol. 2011;8:644–54. doi: 10.1038/nrcardio.2011.95. [DOI] [PubMed] [Google Scholar]
- 31.Buck HG, Harkness K, Wion R, et al. Caregivers' contributions to heart failure self-care: a systematic review. Eur J Cardiovasc Nurs. 2015;14:79–89. doi: 10.1177/1474515113518434. [DOI] [PubMed] [Google Scholar]
- 32.Lee CS, Vellone E, Lyons KS, et al. Patterns and predictors of patient and caregiver engagement in heart failure care: a multi-level dyadic study. Int J Nurs Stud. 2015;52:588–97. doi: 10.1016/j.ijnurstu.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Vellone E, Riegel B, Cocchieri A, et al. Psychometric testing of the Self-Care of Heart Failure Index Version 6.2. Res Nurs Health. 2013;36:500–11. doi: 10.1002/nur.21554. [DOI] [PubMed] [Google Scholar]
- 34.Vellone E, Riegel B, Cocchieri A, et al. Validity and reliability of the caregiver contribution to self-care of heart failure index. J Cardiovasc Nurs. 2013;28:245–55. doi: 10.1097/JCN.0b013e318256385e. [DOI] [PubMed] [Google Scholar]
- 35.Lee CS, Cocchieri A, Paturzo M, et al. Patterns of self-care and clinical events in a cohort of adults with heart failure: 1 year follow-up. doi: 10.1016/j.hrtlng.2017.09.004. [under review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rector TS, Kubo SH, Cohn JN. Patient's self-assessment of their congestive heart failure. Part 2: Content, reliability, and validity of a new measure, the Minnesota Living with Heart Failure Questionnaire. Heart Failure. 1987;3:198–209. [Google Scholar]
- 37.Balducci C, Mnich E, McKee KJ, et al. Negative impact and positive value in caregiving: validation of the COPE index in a six-country sample of carers. Gerontologist. 2008;48:276–86. doi: 10.1093/geront/48.3.276. [DOI] [PubMed] [Google Scholar]
- 38.Schoenfeld D. The asymptotic properties of nonparametric tests for comparing survival distributions. Biometrika. 1981;68:316–9. [Google Scholar]
- 39.Hwang B, Fleischmann KE, Howie-Esquivel J, Stotts NA, Dracup K. Caregiving for patients with heart failure: impact on patients' families. Am J Crit Care. 2011;20:431–41. doi: 10.4037/ajcc2011472. quiz 42. [DOI] [PubMed] [Google Scholar]
- 40.Baker K, Flattery M, Salyer J, Haugh KH, Maltby M. Caregiving for patients requiring left ventricular assistance device support. Heart Lung. 2010;39:196–200. doi: 10.1016/j.hrtlng.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Wolff JL, Spillman BC, Freedman VA, Kasper JD. A National Profile of Family and Unpaid Caregivers Who Assist Older Adults With Health Care Activities. JAMA Intern Med. 2016;176:372–9. doi: 10.1001/jamainternmed.2015.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beach SR, Schulz R, Yee JL, Jackson S. Negative and positive health effects of caring for a disabled spouse: longitudinal findings from the caregiver health effects study. Psychol Aging. 2000;15:259–71. doi: 10.1037//0882-7974.15.2.259. [DOI] [PubMed] [Google Scholar]
- 43.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–9. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 44.Foebel AD, Hirdes JP, Heckman GA. Caregiver status affects medication adherence among older home care clients with heart failure. Aging Clin Exp Res. 2012;24:718–21. doi: 10.3275/8475. [DOI] [PubMed] [Google Scholar]
- 45.Bidwell JT, Vellone E, Lyons KS, et al. Determinants of Heart Failure Self-Care Maintenance and Management in Patients and Caregivers: A Dyadic Analysis. Res Nurs Health. 2015;38:392–402. doi: 10.1002/nur.21675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buck HG, Kitko L, Hupcey JE. Dyadic heart failure care types: qualitative evidence for a novel typology. J Cardiovasc Nurs. 2013;28:E37–46. doi: 10.1097/JCN.0b013e31827fcc4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung ML, Lennie TA, Mudd-Martin G, Moser DK. Adherence to a low-sodium diet in patients with heart failure is best when family members also follow the diet: a multicenter observational study. J Cardiovasc Nurs. 2015;30:44–50. doi: 10.1097/JCN.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunbar SB, Clark PC, Reilly CM, et al. A trial of family partnership and education interventions in heart failure. J Card Fail. 2013;19:829–41. doi: 10.1016/j.cardfail.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jurgens CY. Somatic awareness, uncertainty, and delay in care-seeking in acute heart failure. Res Nurs Health. 2006;29:74–86. doi: 10.1002/nur.20118. [DOI] [PubMed] [Google Scholar]
- 50.Lee CS, Gelow JM, Bidwell JT, et al. Blunted responses to heart failure symptoms in adults with mild cognitive dysfunction. J Cardiovasc Nurs. 2013;28:534–40. doi: 10.1097/JCN.0b013e31826620fa. [DOI] [PubMed] [Google Scholar]
- 51.Ansah EK, Powell-Jackson T. Can we trust measures of healthcare utilization from household surveys? BMC Public Health. 2013;13:853. doi: 10.1186/1471-2458-13-853. [DOI] [PMC free article] [PubMed] [Google Scholar]