Abstract
Low availability of oxygen can lead to stalled wound healing processes and chronic wounds. To address local hypoxia and to better understand direct cellular benefits, a perfluorocarbon (PFC) conjugated chitosan (MACF) hydrogel that delivers oxygen was created and applied for the first time to in vitro cultures of human dermal fibroblasts and human epidermal keratinocytes under both normoxic (21% O2) and hypoxic (1% O2) environments. Results revealed that local application of MACF provided 233.8 ± 9.9 mmHg oxygen partial pressure after 2 h then maintained equilibrium oxygen levels that were approximately 17 mmHg partial pressure greater than untreated controls. Cell culture experiments showed that MACF oxygenating gels improved cellular functions involved in wound healing such as cell metabolism, total DNA synthesis and cell migration under hypoxia in both fibroblasts and keratinocytes. Adenosine triphosphate (ATP) quantification also revealed that MACF treatments improved cellular ATP levels significantly over controls under both normoxia and hypoxia (p<0.005). In total, these studies provide new data to indicate that supplying local oxygen via MACF hydrogels under hypoxic environments improves key wound healing cellular functions.
Keywords: Chitosan, perfluorocarbons, oxygen delivery, wound healing, ATP
Introduction
Chronic wounds affect 6.5 million patients in the United States with treatment costs around US$25 billion annually.31 Increasing health care costs, an aging population and increasing incidence of diabetes all contribute to an expanding chronic wound population. The underlying problems of chronic wounds are varied but typically include factors such as local tissue hypoxia, bacterial colonization and age related factors. These deficiencies often lead to stalled wound healing and a wound that becomes mired in a self-sustaining inflammatory phase of wound healing.22, 29 Previous studies have shown that local tissue oxygen level plays a vital role in wound healing process, as this reparative process requires more oxygen than normal tissue for the increased demands of cell proliferation, bacterial defense, angiogenesis and extracellular matrix (ECM) deposition.29, 30, 34 In chronic wounds, oxygen partial pressures (PO2) in subcutaneous tissue may decrease to as low as 5 mmHg, whereas in normal non-diabetic wounds/tissues it only decreases to near 30 mmHg from 40 mmHg arterial pressure in healthy tissue/skin.4, 11 In fact, decreases in oxygen levels may lead to non-healing chronic wounds, as studies in ischemic rabbit ear models have shown that a decreased PO2 level from 40 to 28–30 mmHg led to an 80 % deceleration in wound healing.1 Other studies have also shown that even a moderate decrease in tissue oxygenation level leads to significant increases in the chance of infection, which is confirmed by in vitro studies that have shown neutrophils lose their bacterial elimination capability below 40 mmHg PO2.10 Oxygen is also important for cell metabolism and energy production as it plays a key role in adenosine triphosphate (ATP) synthesis through oxidative phosphorylation. This energy source is consumed in wound healing processes such as cell migration and proliferation. Finally, oxygen is important toward the generation of local defense-oriented reactive oxygen species (ROS), which are produced via oxygen dependent NADPH-linked oxygenase processes for eliminating foreign bodies from the wound site.29, 30, 34
Current research mainly addresses oxygen deficiencies by employing oxygen generators like peroxides embedded into biomaterial scaffolds.27 These approaches can supply beneficial levels of oxygen, but shortcomings such as finite oxygen supply, local pH changes, and local heating and toxicity responses, when applied in vivo, make them poorly suited for chronic wound therapies.9 Oxygen delivery research has also focused on perfluorocarbons (PFCs). PFCs possess a CxFy formula where all the hydrogen atoms in the hydrocarbon are replaced with fluorine atoms. PFCs are unique due to the 9 electrons packed in proportionally small space as compared to a typical hydrocarbon structure.28 The presence of a dense electron cloud, higher ionization potential, and greater electron affinity altogether gives rise to the useful properties that include biological inertness, hydrophobicity and high oxygen gas dissolving capacities. At standard temperature and pressure PFCs can increase O2 solubility in water by 20 fold, from 2.2 mmHg to 44 mm Hg.13, 19, 33 This ability of PFC’s to dissolve high amounts of O2 for delivery to places with low O2 tensions is the primary motivation for using PFCs in most clinical applications. There is considerable interest in using PFCs as O2 carriers in a variety of biomedical and bioprocess systems as blood substitutes.15, 33 Previous studies conducted on HepG2 cells encapsulated into alginate with oxygen releasing PFCs have shown improved results in terms of cell viability and growth rate when compared with the same cell-encapsulated in alginate alone.13 The main issue with the majority of PFCs, in terms of controlled biomedical applications, is that they are highly hydrophobic and immiscible with water, requiring that they be emulsified or formed into a colloidal suspension to use in aqueous environments. Our group has extended the biomedical usefulness of PFCs via the creation of fluorinated methacrylated chitosan (MACF), which is synthesized via nucleophilic substitution reaction where PFCs are conjugated to the free amines of chitosan.38 Furthermore, in this previous work we were able to show that the type and size of PFC substitution affected oxygen transport properties. In terms of a dermal wound healing application, we selected the aliphatic substitution of C7F15, which has demonstrated the best oxygen delivery to date. Chitosan is abundant in nature, biocompatible, non-toxic and is already used in many biomedical applications including wound healing.12, 17, 25, 26, 38 Chitosan is useful in bioconjugation reactions as it contains free amines. Further, chitosan degrades via native hydrolases, such a as lysozyme, and the degradation kinetics can be tuned via deacetylation percentage.12 Preliminary studies conducted in our lab on MACF hydrogels have shown beneficial results, including showing that MACF can be saturated and release oxygen to improve cellular functions in vitro using NIH 3T3 cells.38 More recently, in vivo studies conducted in a rat excisional wound healing model showed that oxygenating hydrogel sheets of MACF improved re-epithelization, collagen metabolism and deposition.25
Given the importance of oxygen in cellular wound healing processes, the main objective of this study was to further investigate and evaluate oxygenating MACF’s influence on vital in vitro human cellular wound healing processes under normoxic and hypoxic conditions. It is well established that sustained hypoxia decelerates cellular wound healing processes, mainly due to low cellular oxygen availability.29, 30, 34 Therefore, we hypothesized that the application of oxygenated MACF hydrogels, with the ability to supplement oxygen to deficient environments, would recover hypoxia decelerated cellular processes. To test this hypothesis, the cellular wound healing processes of cell migration, cell proliferation, protein synthesis, metabolism, and total ATP synthesis were evaluated for the first time in human skin cells (both dermal fibroblasts and epidermal keratinocytes) under normoxia (21% O2) and hypoxia (1% O2) upon treatment with locally oxygenating MACF hydrogels as compared to base material and no treatment controls.
Materials and Methods
Preparation of fluorinated methacrylamide chitosan and methacrylamide chitosan polymers
Fluorinated methacrylamide chitosan (MACF) and methacrylamide chitosan were synthesized (Fig. 1) and characterized as previously described.25, 38 Briefly, to prepare MACF first 3 wt% chitosan (ChitoClear 43010, Primex, Siglufjordur, Iceland) was dissolved in 2 vol% acetic acid:water. Fluorinated groups were added to chitosan by adding 0.14 M pentadecafluorooctanoyl chloride (Sigma-Aldrich, Saint Louis, MO, USA). Next, the resulting polymer was modified with methacrylic anhydride (Sigma-Aldrich) to add methacrylate groups to the polymer to create MACF. For purification, MACF or MAC solutions were placed in dialysis membranes (12,000–14,000 Da MW cut-off; Spectra/Por, Spectrum Labs, Rancho Dominguez, CA, USA) and dialyzed against deionized water for 3 d with 3 changes of water each day followed by lyophilizing (Labconco, Kansas City, MO, USA) to yield dry MACF or MAC polymer.38 Finally, small samples of each were dissolved in 2 vol% deuterated acetic acid/D2O and 1H and 19F nuclear magnetic resonance (NMR; Varian 500 MHz, Varian, Inc., Palo Alto, CA, USA) were conducted to find percent methacrylation and percent fluorination respectively as previously described. 25, 38
Figure 1.
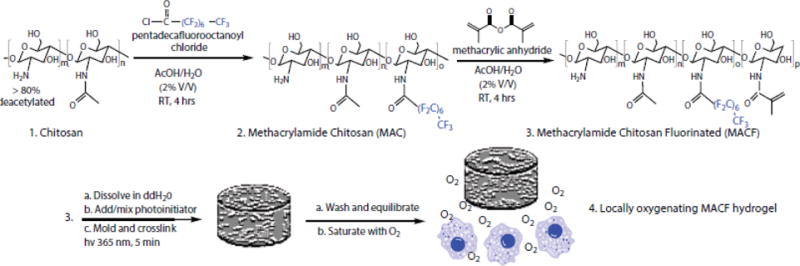
Synthesis of MACF polymer followed by hydrogel creation and application as a locally oxygenating hydrogel to enhance cellular functions.
MACF/MAC hydrogel preparation and oxygen saturation
To prepare hydrogels, first the dry polymer (MAC/MACF) is dissolved at 2.5 w/v% in ultra-pure water (MilliQ Direct 8 system at 18 M ohm resistance, Millipore, Billerica, MA, USA), then sterilized by autoclaving (liquid cycle, 10 min at 137°C). Next, a photo-initiator solution consisting of 300 mg/ml 1-hydroxycyclohexyl phenyl ketone (Sigma-Aldrich) in 1-vinyl-2-pyrrolidinone (Sigma-Aldrich) was added to the polymer solution at 10 μl per g of solution and thoroughly mixed and degassed at 3000 rpm for 2 min (Speed Mixer DAC 150 FVZ, Hauschild Engineering, Hamm, Germany). Hydrogels were formed by transferring 300 μl solution to a 96 well-plate (Corning Inc., Corning, NY, USA) followed by exposing UV light (365 nm, 16–19 mW/cm2) for 5 min. Next, hydrogels were washed thoroughly with phosphate buffered saline (PBS) with 3 changes per day for 3 d to remove all the unreacted polymer and photo-initiator solution. The dimensions of the final hydrogels were approximately 6 mm in diameter and 10 mm in height.
To prepare MACF hydrogels to be oxygenated, first washed gels were equilibrated in media (human keratinocyte media (American Type Culture Collection (ATCC), Manassas, VA, USA) or human fibroblast media (Life Technologies; Carlsbad, CA, USA)) overnight at 4°C. Next, they were saturated with oxygen by bubbling the solution with pure gaseous O2 to MACF gels for 30 min at 5 PSI as previously described.25
Oxygen release measurements
To quantify oxygen release of oxygenated MACF gels, first MACF hydrogels were saturated with O2 as described in section 2.2. Next, oxygenated MACF and MAC hydrogels were transferred to a 24 well-plate filled with 1 ml of culture media in inserts (6.5 mm transwell with 8.0μm pore polycarbonate membrane insert, Corning, Corning, NY, USA). Samples were then transferred and maintained in a normoxic incubator at 37°C. The oxygen concentration in the media was continuously monitored with oxygen dot sensors (FireSting O2, Pyro Science, Aachen, Germany) connected to a fiber-optic oxygen meter (Pyro Science). As controls, oxygen concentration in media were also monitored for MAC gels and media alone under the same conditions.
Cell culture
Neonatal human dermal fibroblasts (nHDFs) used for these studies were harvested from human neonatal foreskins obtained from Akron General Hospital after approval and in accordance with the IRB. nHDFs were expanded in Dulbecco’s modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and 100 μg/ml penicillin–streptomycin (all Life Technologies). For experiments media containing DMEM, 1% FBS and 100 μg/ml penicillin–streptomycin was used. Neonatal human epidermal keratinocytes (nHEK) used for these studies were obtained from ATCC and expanded and cultured for experiments in dermal cell basal medium containing the keratinocyte growth kit along with penicillin/streptomycin/amphotericin and phenol red (all ATCC). nHEK were expanded and passaged according to the standard protocol given by the company. Both cell types were expanded, passaged and grown in a normoxic incubator (18–19% O2, 5% CO2 and 76 – 77 %N2 at 37°C). A hypoxic environment (1% O2, 5% CO2 and 94% N2 at 37°C) was used for studies and created by using a modified tri-gas incubator (Thermo Fisher Scientific, Waltham, MA, USA). This tri-gas incubator was continuously purged with N2 and CO2 gas to obtain a 1% O2 level. O2 and CO2 levels are monitored and maintained at 1% and 5%, respectively with the help of custom added sensors/controllers. Oxygen was monitored using a TR250Z thermal oxygen sensor (CO2 Meter, Inc.; Ormond Beach, FL, USA) with a DTB4848 PID controller (Delta Controls Surrey, British Columbia, Canada) and Internal CO2 sensor is calibrated by using a COZIR wide range sensor development kit (COZIR-WR 20% CO2 sensor Dev.CM-0123 optical oxygen sensor, CO2 Meter, Inc.). Media used for growing the hypoxia cultured cells was equilibrated to hypoxic environment prior to use 2, 32 by maintaining the media in sterile T75 flasks for 24 h in the hypoxia incubator.
Cell proliferation studies
nHDFs and nHEKs were seeded in 24 well-plates at 8,000 cells/cm2. A hemocytometer was used when seeding to ensure that the number of cells per well was consistent. The cells were allowed to attach and spread overnight at 37°C in a normoxic incubator. Well-plates were then split into normoxic and hypoxic culture as described above and the treatments (MACF and MAC) were applied to wells in both environments alongside the no treatment control wells. Cells were left alone for 3 d (72 h). Thereafter, images were taken using an optical microscope to evaluate cellar morphology (Olympus IX-81, Tokyo, Japan). Next, wells were analyzed with biochemical assays, as described next.
PrestoBlue assay
To evaluate the cell metabolism (viability), first, 1 ml of 10 % PrestoBlue (Life Technologies,) in serum free growth media is applied on the cells and then allowed to incubate for 90 min at 37°C. Next, supernatant from each well is transferred to a 96 black well-plate (Greiner Bio-one, upper Austria, Germany). The chemical basis of PrestoBlue is resazurin (7-hydroxy-3H-phenoxazin-3-one-10-oxide), which is metabolically reduced to resorufin (7- hydroxy-3H-phenoxazin-3-one). This results in an absorbance shift that is proportional to the metabolic rate.16 Fluorescence values were determined in the well-plate at an excitation wavelength of 560 nm and emission of 590 nm using a spectrophotometer (Infinite M200, Tecan, Grödig, Austria). Sample values were compared against a dithiothreitol (DTT) standard curve to obtain the absolute values of cell viability in terms of equivalent DTT concentration, which is a direct measure of chemical reduction potenital.16
Sample digestion for endpoint assays
Following the PrestoBlue assay, the wells containing cells had media replaced with 1 ml of 25 mg/ml lysozyme dissolved in PBS and digested at pH 5 for 24 h at 60°C with through mixing by pipetting once every 3 to 4 h.16 The resulting solutions were transferred to 2 ml tubes and stored at 4 °C for up to 1 wk for the assays below.
Micro BCA assay for total protein
Total protein was quantified by using the Micro BCA protein assay kit (Thermo Fisher) according to the manufacturer’s protocol. Briefly, aliquots from the digest samples were diluted in PBS (15 μl of sample is added to 1000 μl of PBS). Next, 150 μl of each diluted digest sample was added to a clear 96 well-plate followed by 150 μl of micro BCA reagent. Samples were then incubated at 37°C for 2 h, then absorbance was measured at 562 nm (Tecan Infinite M200). A standard curve was created with serial dilution of bovine serum albumin (BSA) (VWR International, Radnor, PA, USA) in PBS. Absolute values of total protein per sample were determined via comparison with the standard curve.
PicoGreen assay for total DNA
The Quant-iT PicoGreen dsDNA kit (Life Technologies) was used to determine the total double-stranded DNA (dsDNA), which can directly be correlated to total cell number. First, aliquots of digests were diluted (100 μl of digested sample in 1000 μl of PBS). Next, 150 μl of the resulting samples were added to a black 96 well-plate in triplicate followed by 150 μl of Quant-it PicoGreen reagent and incubated for 10 min at room temperature in the dark.16 Next, fluorescence values (excitation 480 nm, emission 520 nm) were determined using a spectrophotometer (Tecan Infinite M200). Measured fluorescence values were compared against a standard curve created using supplied λDNA standards to find the total dsDNA concentration in each well.
ATP quantification
ATP quantification was determined in live cultures using a bio luminescence ATP determination kit (Thermo Fisher Scientific, A22066). The assay employs luciferin that reacts with ATP in the presence of luciferase, which in turn is converted to oxyluciferin. Light is emitted in proportion to the amount of ATP in the cells.2 The assay begins with preparing the standard reaction solution by mixing the components together: 20X TE buffer, ultrapure H2O, DTT, D-luciferin and firefly luciferase in appropriate quantities as stated in the company’s protocol. ATP standard solutions were prepared by diluting supplied ATP in ultrapure H2O and the assay standard reaction solution. Thereafter, 500 μl of the standard reaction solution is added to each sample in a clear 24 well-plate and luminescence values were recorded (Tecan Infinite M200). Finally, the absolute ATP values of the unknown samples were found by comparison with known values from the standard curve.
Scratch assay to study cell migration
To study the effect of supplying additional oxygen using MACF gels on cell migration in vitro, a scratch assay was conducted on nHDFs and nHEKs.6, 18 First, 24 well-tissue culture plates were coated with 1 ml of sterile 0.2 mg/ml collagen type 1 in 1X PBS for 2 h at 37°C and then washed once with sterile PBS. Next, wells were seeded with 40,000 cells/cm2, as determined by hemocytometer. After 2 d of culture in normoxia (21% O2) or hypoxia (1% O2), the resulting confluent cell monolayers were scraped in the middle of the well using a 100 μl micropipette tip to create a scratch (Around 1 mm in width). Wells were then washed one time with media to remove debris created due to scratching. Next, fresh media was added to the wells, the treatments were applied, and cells were allowed to freely migrate. Images of the scratches were taken at t=0 (prior to applying treatments) and t= 24 h respectively using a phase contrast microscope (Olympus IX81). Recorded images were used to determine the width of the scratch using ImageJ software (National Institutes of Health, Bethesda, Maryland) and the resulting percentage of cell migration was calculated for each treatment using the following equation: percentage of cell migration = Wi−Wf/Wi *100, where Wi= width of scratch at t= 0 h and Wf= width of scratch at t= 24 h.
Statistics
All statistical analyses were performed using JMP pro 12 (SAS Institute, Cary, NC). One-way ANOVA with Tukey’s post hoc analysis were performed to detect significant differences between groups. An α level of 0.05 was used to determine significance between groups. Capital letters are used to denote significance when multiple significant differences were detected. Different letters imply that a significant difference exists between groups, as is reported by JMP.
Results
MAC and MACF were successfully synthesized and purified. 19F NMR showed 38 to 42% PFC substitution and 1H NMR showed 19 to 23 % degree of substitution for methacrylation, which is similar to what has been previously reported. 38
Effect of oxygenated MACF gel on cell media
The PO2 level in the media with oxygenated MACF gels, MAC gels and their comparison with only media are shown in Figure 2. PO2 levels were elevated in media with oxygenated MACF gels for all the time points measured, with the highest values observed at 2 h (233.8 ± 9.9 mmHg) and due to the large gradient in concentration that existed upon outset of the experiment between saturated gels and the media. Oxygenated MACF hydrogels resulted in significantly more oxygen in wells over the 48 h time period as compared to the two controls (p<0.0001). The PO2 levels in media with oxygenated MAC gels were also significantly higher than the no media, but still less than MACF exposed media at all time points (p<0.0001).
Figure 2.
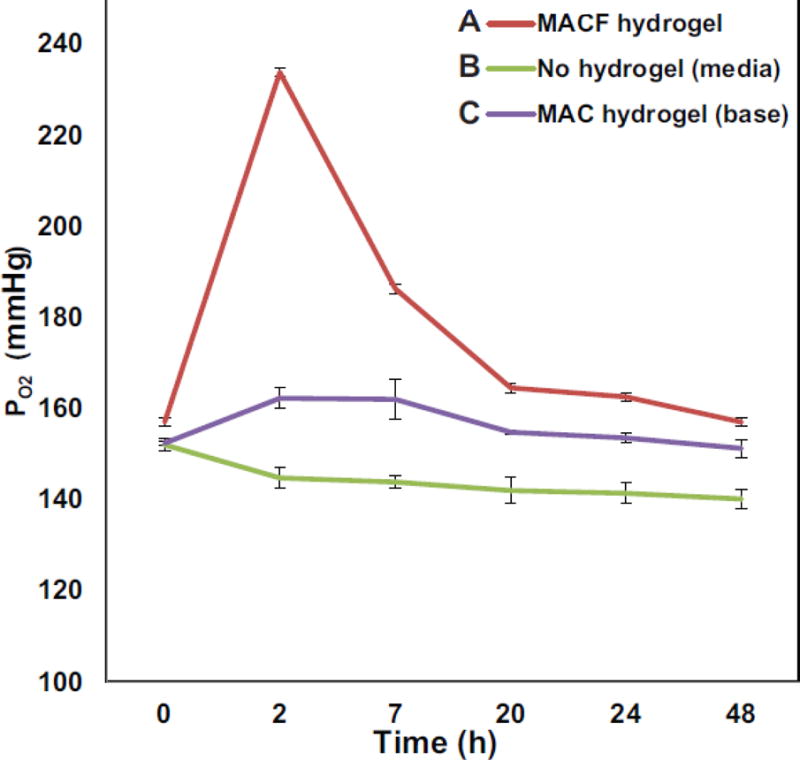
Oxygenating MACF hydrogels locally elevate PO2 level in culture media as compared to controls. Dissolved PO2 was measured at the bottom of the well of a culture plate with suspended hydrogel treatments over 48 h. All samples were maintained in a 5% CO2 incubator at 37°C. Data reported as mean ± SD with n=4.
Effect of oxygenated MACF gels on key cellular processes
Both nHDFs and nHEKs exhibited regular morphology they were uniform throughout the surface (Suppl. Figs. 1,2). PrestoBlue assay, which is a direct measure of mitochondrial metabolic activity, showed that both nHDFs and nHEKs were increasingly active in the presence of oxygenating MACF hydrogels under both normoxic and hypoxic environments (Fig. 3) and significantly higher than controls (p < 0.0001). Additionally, these results show that overall cell metabolic activity decreased in low oxygen concentrations (hypoxia significantly lower than normoxia; p < 0.0001), however, cells retained their metabolic activity upon the application of supplemental local oxygen through oxygenated MACF gels, such that activity was statistically the same under MACF treatment in both normoxia and hypoxia (p > 0.05). Supplying oxygen through MACF gels did not significantly alter the total protein content in both nHDFs and nHEKs under both normoxic and hypoxic environments and were the same as no gel treatment controls (data not shown, p > 0.05). Assaying for total dsDNA for both cell types (Fig.4) showed similar trends to the PrestoBlue results, mainly that supplemental oxygen via MACF hydrogels increased total dsDNA in both normoxic and hypoxic environments as compared to controls (p < 0.0001). Increased total dsDNA as compared to controls is indicative of cell proliferation. Further, total dsDNA levels for oxygenating MACF treatments for both cell types in hypoxia were either the same as normoxia no gel controls for nHEKs (Fig. 4B, p < 0.005) or greater than normoxia no gel controls for nHDFs (Fig 4A, p < 0.0001). Assaying total ATP levels (Fig. 5), revealed that MACF treatment most impacted ATP levels under hypoxia, and MACF treatment significantly increased ATP levels as compared to the other hypoxia controls for both cell types (p<0.0001). Interestingly, nHEKs had approximately ten times the concentration ATP as compared to nHDFs. PrestoBlue assays were performed at t=0 for each experiment and showed that the metabolic activity was not significantly different (p > 0.05) across all groups, confirming similar seeding densities for all treatment groups.
Figure 3.
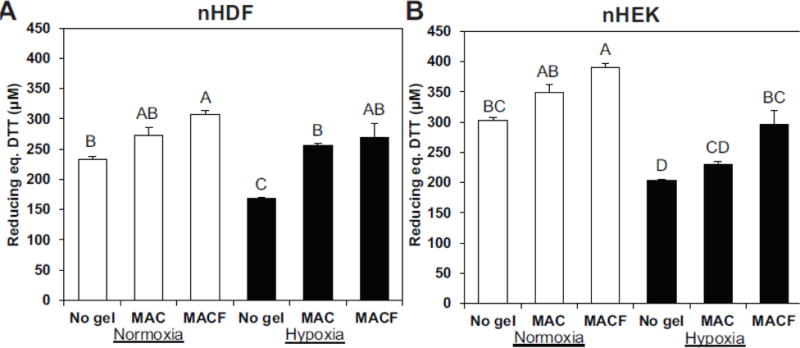
The effect of oxygenating MACF hydrogels on cell metabolism, as determined by the PrestoBlue assay at day 3. A. Neonatal human dermal fibroblasts (nHDFs) and B. neonatal human epidermal keratinocytes (nHEK) cultured for 3 d with treatments. MACF improved cell metabolism under both hypoxic (1% O2) and normoxic (21% O2) environments, as evidenced by increased reducing power per sample (equivalent DTT concentration). Letters are significantly different from one another by single-factor ANOVA (p < 0.0001). Data are reported as mean ± SD with n = 3–4.
Figure 4.
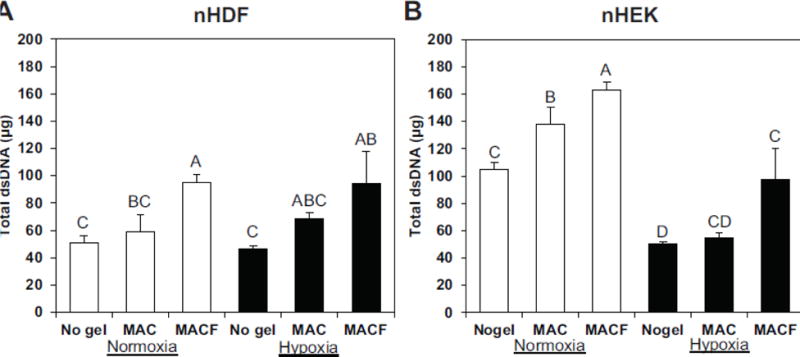
The effects of oxygenating MACF hydrogels on total dsDNA as measured by PicoGreen assay after 3 days of treatment exposure. MACF increased total dsDNA under both hypoxic (1% O2) and normoxic (21% O2) environments. Letters are significantly different from one another by single-factor ANOVA (nHDF, p < 0.005; nHEK, p < 0.0001). Data are reported as mean ± SD with n = 3–4.
Figure 5.
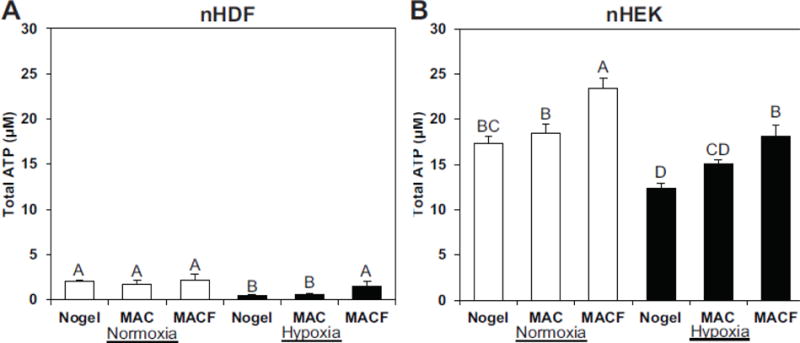
The effects of oxygenating MACF hydrogels on total live cellular ATP levels measured via luminescent assay after 3 days of treatment exposure. MACF increased total ATP under both hypoxic (1% O2) and normoxic (21% O2) environments. Letters are significantly different from one another by single-factor ANOVA (A, p < 0.005; B, p < 0.0001). Data are reported as mean ± SD with n = 3–4.
Effect of oxygenated MACF on cell migration
The results of scratch testing are presented in Figure 6. By 24 h scratch closure was complete in all the treatments under normoxia in both human skin cell types, whereas in hypoxia they were not closed completely, as is shown in representative phase contrast images (Fig. 6). Oxygenated MACF gels improved the impaired cell migration under hypoxia in both nHDFs and nHEKs as compared to the other two hypoxia groups of MAC and no gel (p<0.0001).
Figure 6.
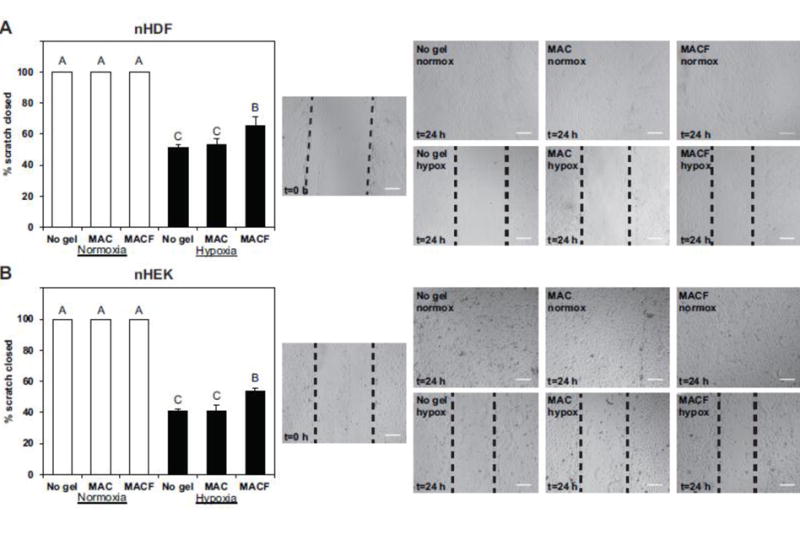
Oxygenated MACF improves cell migration under hypoxia. Fibroblasts (A) and keratinocytes (B) where gown to confluency then scratched in vitro, imaged, then cultured with treatments under either normoxia (21% O2) or hypoxia (1% O2). At 24 h cultures were once again imaged. Quantification is shown on the left, white bars represent normoxia and black bars hypoxia results. All data n = 3–4, mean ± SD Letters are significantly different from one another by one-factor ANOVA (p < 0.0001). Representative images are presented on the right, with scale bars = 200 μm.
Discussion
Fibroblasts and keratinocytes are the major cell types present in the dermis and epidermis of the human skin, performing vital cellular wound healing functions, including cell migration and proliferation.7, 21 Based on this importance, nHDF and nHEK cells were chosen for this study. Impairment of skin cell functions is a root cause for non-healing chronic wounds, leading to poor neo-vascularization, granulation and re-epithelization. Experimental evidence supports that an underlying reason for impaired cellular functions in chronic wounds is lack of oxygen.7, 29, 34 Thus, supplying sufficient oxygen directly to chronic wounds could improve wound healing, which is supported by current clinical practice employing hyperbaric oxygen therapy.23, 35 Oxygenated MACF hydrogels provide a potential alternative to locally supply oxygen to wounds, with the added benefits of a hydrogel, such as moist wound healing.39 Previous studies conducted by our lab on MACF have shown that this PFC incorporated chitosan polymer is able to be saturated with oxygen repeatedly, supply it as necessary and maintain a higher than ambient oxygen concentration at equilibrium.17, 25, 38 The results from Figure 2 expand upon this showing that MACF treatment yields an equilibrium oxygen partial pressure of 15–17 mmHg above media only values. This number is biologically significant as previous in vivo studies demonstrate that in acute and chronic wounds increasing oxygen partial pressures by 5 to 10 mmHg can considerably enhance wound healing responses.25 It is worth noting that in both nHDF and nHEK cultures, the base material MAC showed improved results in hypoxia when compared with no gel treatments in the three biochemical assays presented (Figs. 3–5). This is most likely due to oxygen surface absorption from the preprocessing steps, suggesting that the base chitosan material alone likely has a minor affinity for oxygen as well.
Overall this study revealed that oxygenated MACF gels improved important cellular functions under hypoxia (1% O2) as evidenced by improved in vitro metabolism, proliferation, and migration in nHDFs and nHEKs (Figs. 3–6). Hypoxia is often created to mimic the environment in a chronic wound and to study impaired cellular processes in vitro. Oxygen levels lower than 5% are generally considered hypoxic, but previous in vitro studies with oxygen levels between 2% and 5% did not show any significant differences in cellular functions such as proliferation and ATP synthesis when compared with normoxic treatments.24, 32 However, oxygen levels 2% and lower have shown significantly decelerated cellular functions in vitro including diminished proliferation and ATP synthesis.2 Further, oxygen levels approaching 0.5% causes cells to shift to a resting state leading to both reduced production and utilization of cellular ATP.2 Based on these reports, a condition of 1% oxygen was selected for our in vitro studies to best recreate hypoxic conditions that place cellular functions into a deficit. Results of our studies show the impact of a rigorous hypoxia protocol on important cell functions such as metabolism (Fig.3), proliferation (Fig. 4), ATP levels (Fig.5) and migration (Fig. 6). These results were expected as they have been reported in similar hypoxia studies.2, 24, 40 Most importantly, application of oxygenated MACF gels were able to fully or partially recover hypoxia-impaired cellular processes (Figs 3–6).
Energy for the bulk of cellular functions is provided by ATP, and wound healing process have enhanced energy demands.5, 30 Interestingly, assaying ATP levels in our experiments shows that MACF treatments enhance ATP in cells (Fig. 5), providing cells with higher energy to perform their functions even in a hypoxic environment. It is known from the literature that hypoxia leads to decreased oxidative phosphorylation leading to lowered ATP production in cells, which in turn leads to deceleration of the cellular functions like proliferation and migration2, 14. In our studies, hypoxia reduced ATP levels in nHDFs and nHEKs to approximately 30% and 70% (based on comparison of means) of their original values, respectively (Fig. 5). However, upon application of MACF gels, ATP levels in nHDFs and nHEKs again increased and recovered to approximately 72% and 110% (based on means) of their original values, respectively. These ATP results highlight the specific benefits of using oxygenated MACF gels to enhance cellular energy stores, as oxygenated MACF hydrogels improves the local availability of oxygen (Fig. 2).
Oxygenated MACF gels have several benefits over other oxygenating approaches such as oxygen generating biomaterials employing calcium peroxide,27 hydrogen peroxide20 and sodium per carbonate.37 Chiefly, as discussed in the introduction, these generation approaches have downsides which may lead to the further death of cells. Additionally, these oxygen generating biomaterials cannot be replenished, whereas MACF gels can be resaturated with oxygen as long as the material is present.38 More recent oxygenating biomaterial approaches have included hollow oxygen loaded microspheres5 that circumvent reaction issues. However, they once again are not easily replenished and do not sustain a long term equilibrium concentration of oxygen that is greater than the surroundings like MACF (Fig. 2).
With any biomaterial approach it is important to discuss any potential toxicity concerns. Our previous studies have shown that this MACF material is biodegradable and nontoxic in vitro17, 38 and in vivo.26 The common concern with PFCs mostly lie in terms of long-term environmental exposure tied to large scale polymer industries and consumer products, not controlled biomedical applications. Several PFC products have been FDA approved in the past including Fluosol-DA. This product was used in over 15,000 patients, unfortunately the product failed because of storage issues, not toxicity or safety issues.3 It is also important to note that most current PFC concerns are tied to intravenous applications at high concentrations, and despite this application method, work has shown that clinical PFC treatments do not result in permanent tissue alterations.8, 36 In terms of our approach with MACF, the conjugation chemistry to chitosan polysaccharide chains stabilizes the PFCs substantially and greatly reduces lipophilic nature and thus long-term concerns.
Conclusion
This study demonstrates that MACF hydrogels can be saturated with oxygen and release this oxygen to local surroundings, while supporting a higher than ambient equilibrium oxygen concentration for at least three days. We show for the first time that oxygenating MACF recovered energetic wound healing cellular processes, namely cell metabolism, proliferation, and cell migration that were impaired under hypoxic conditions in both human fibroblast and keratinocytes. Additionally, cellular ATP synthesis, which is reduced under hypoxic conditions by nearly 70 %, also recovered remarkably with oxygenated MACF treatment. Finally, this study confirms previous work that this PFC incorporated chitosan hydrogel is nontoxic and provides a simple solution for treating oxygen deficient biological environments.
Supplementary Material
Acknowledgments
We would like to acknowledge funding from the National Institute of General Medical Sciences of the National Institutes of Health under award number R15GM104851. We would like to thank Steve Roberts for design and construction of the tri-gas incubator, Judy Fulton from Akron General Hospital/Serena Group for tissue donation and valuable feedback, as well as thank group members Ashley Wilkinson, Andrew McClain, Mahmoud Farrag, Pritam Patil and Trevor Ham for assistance with various techniques, biomolecular assays and interpretation.
Abbreviations, symbols, terminology
- ATP
Adenosine triphosphate
- BSA
Bovine serum albumin
- DMEM
Dulbecco’s modified eagle medium
- dsDNA
double-stranded DNA
- DTT
Dithiothreitol
- ECM
Extracellular matrix
- MAC
Methacrylamide chitosan
- MACF
Fluorinated methacrylamide chitosan
- nHDF
Neonatal human dermal fibroblast
- nHEK
Neonatal human epidermal keratinocytes
- NMR
Nuclear magnetic resonance
- PO2
Oxygen partial pressure
- PBS
Phosphate buffered saline
- PFCs
Perfluorocarbons
- ROS
Reactive oxygen species
Footnotes
Author Contributions
S.A. and N.D.L. designed the study, analyzed data, and wrote the manuscript. S.A. performed all experiments, while I.K.B. established techniques and performed initial experiments for PrestoBlue, PicoGreen, and microBCA experiments.
Conflict of Interest
Sridhar Akula, Ivy Brosch and Nic D. Leipzig declare that they have no conflicts of interest.
References
- 1.Ahn ST, Mustoe TA. Effects of ischemia on ulcer wound healing: a new model in the rabbit ear. Ann Plast Surg. 1990;24:17–23. doi: 10.1097/00000637-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Baracca A, Sgarbi G, Padula A, Solaini G. Glucose plays a main role in human fibroblasts adaptation to hypoxia. Int J Biochem Cell Biol. 2013;45:1356–1365. doi: 10.1016/j.biocel.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Castro CI, Briceno JC. Perfluorocarbon-based oxygen carriers: review of products and trials. Artif Organs. 2010;34:622–634. doi: 10.1111/j.1525-1594.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang N, Goodson WH, 3rd, Gottrup F, Hunt TK. Direct measurement of wound and tissue oxygen tension in postoperative patients. Ann Surg. 1983;197:470–478. doi: 10.1097/00000658-198304000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook CA, Hahn KC, Morrissette-McAlmon JB, Grayson WL. Oxygen delivery from hyperbarically loaded microtanks extends cell viability in anoxic environments. Biomaterials. 2015;52:376–384. doi: 10.1016/j.biomaterials.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cory G. Scratch-wound assay. Methods Mol Biol. 2011;769:25–30. doi: 10.1007/978-1-61779-207-6_2. [DOI] [PubMed] [Google Scholar]
- 7.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 8.Flaim SF. Pharmacokinetics and side effects of perfluorocarbon-based blood substitutes. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:1043–1054. doi: 10.3109/10731199409138801. [DOI] [PubMed] [Google Scholar]
- 9.Gholipourmalekabadi M, Zhao S, Harrison BS, Mozafari M, Seifalian AM. Oxygen-Generating Biomaterials: A New, Viable Paradigm for Tissue Engineering? Trends Biotechnol. 2016;34:1010–1021. doi: 10.1016/j.tibtech.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Hohn DC, MacKay RD, Halliday B, Hunt TK. Effect of O2 tension on microbicidal function of leukocytes in wounds and in vitro. Surg Forum. 1976;27:18–20. [PubMed] [Google Scholar]
- 11.Kalani M, Brismar K, Fagrell B, Ostergren J, Jorneskog G. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care. 1999;22:147–151. doi: 10.2337/diacare.22.1.147. [DOI] [PubMed] [Google Scholar]
- 12.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62:3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Khattak SF, Chin KS, Bhatia SR, Roberts SC. Enhancing oxygen tension and cellular function in alginate cell encapsulation devices through the use of perfluorocarbons. Biotechnol Bioeng. 2007;96:156–166. doi: 10.1002/bit.21151. [DOI] [PubMed] [Google Scholar]
- 14.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Krafft MP. Fluorocarbons and fluorinated amphiphiles in drug delivery and biomedical research. Adv Drug Deliv Rev. 2001;47:209–228. doi: 10.1016/s0169-409x(01)00107-7. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence PG, Patil PS, Leipzig ND, Lapitsky Y. Ionically Cross-Linked Polymer Networks for the Multiple-Month Release of Small Molecules. ACS Appl Mater Interfaces. 2016;8:4323–4335. doi: 10.1021/acsami.5b10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Wijekoon A, Leipzig ND. Encapsulated neural stem cell neuronal differentiation in fluorinated methacrylamide chitosan hydrogels. Ann Biomed Eng. 2014;42:1456–1469. doi: 10.1007/s10439-013-0925-0. [DOI] [PubMed] [Google Scholar]
- 18.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 19.Lowe KC, Davey MR, Power JB. Perfluorochemicals: their applications and benefits to cell culture. Trends Biotechnol. 1998;16:272–277. doi: 10.1016/s0167-7799(98)01205-0. [DOI] [PubMed] [Google Scholar]
- 20.Mallepally RR, Parrish CC, McHugh MA, Ward KR. Hydrogen peroxide filled poly(methyl methacrylate) microcapsules: potential oxygen delivery materials. Int J Pharm. 2014;475:130–137. doi: 10.1016/j.ijpharm.2014.08.052. [DOI] [PubMed] [Google Scholar]
- 21.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 22.Mustoe T. Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg. 2004;187:65S–70S. doi: 10.1016/S0002-9610(03)00306-4. [DOI] [PubMed] [Google Scholar]
- 23.Niinikoski JH. Clinical hyperbaric oxygen therapy, wound perfusion, and transcutaneous oximetry. World J Surg. 2004;28:307–311. doi: 10.1007/s00268-003-7401-1. [DOI] [PubMed] [Google Scholar]
- 24.Oberringer M, Meins C, Bubel M, Pohlemann T. In vitro wounding: effects of hypoxia and transforming growth factor beta1 on proliferation, migration and myofibroblastic differentiation in an endothelial cell-fibroblast co-culture model. J Mol Histol. 2008;39:37–47. doi: 10.1007/s10735-007-9124-3. [DOI] [PubMed] [Google Scholar]
- 25.Patil PS, Fountas-Davis N, Huang H, Michelle Evancho-Chapman M, Fulton JA, Shriver LP, Leipzig ND. Fluorinated methacrylamide chitosan hydrogels enhance collagen synthesis in wound healing through increased oxygen availability. Acta Biomater. 2016;36:164–174. doi: 10.1016/j.actbio.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patil PS, Leipzig ND. Fluorinated Methacrylamide Chitosan Sequesters Reactive Oxygen Species to Relieve Oxidative Stress while Delivering Oxygen. J Biomed Mater Res A. 2017 doi: 10.1002/jbm.a.36079. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci U S A. 2012;109:4245–4250. doi: 10.1073/pnas.1113560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riess JG, Krafft MP. Fluorinated materials for in vivo oxygen transport (blood substitutes), diagnosis and drug delivery. Biomaterials. 1998;19:1529–1539. doi: 10.1016/s0142-9612(98)00071-4. [DOI] [PubMed] [Google Scholar]
- 29.Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163:257–268. doi: 10.1111/j.1365-2133.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 30.Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. 2009;17:1–18. doi: 10.1111/j.1524-475X.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiqui A, Galiano RD, Connors D, Gruskin E, Wu L, Mustoe TA. Differential effects of oxygen on human dermal fibroblasts: acute versus chronic hypoxia. Wound Repair Regen. 1996;4:211–218. doi: 10.1046/j.1524-475X.1996.40207.x. [DOI] [PubMed] [Google Scholar]
- 33.Spahn DR. Current status of artificial oxygen carriers. Adv Drug Deliv Rev. 2000;40:143–151. doi: 10.1016/s0169-409x(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 34.Tandara AA, Mustoe TA. Oxygen in wound healing–more than a nutrient. World J Surg. 2004;28:294–300. doi: 10.1007/s00268-003-7400-2. [DOI] [PubMed] [Google Scholar]
- 35.Thackham JA, McElwain DL, Long RJ. The use of hyperbaric oxygen therapy to treat chronic wounds: A review. Wound Repair Regen. 2008;16:321–330. doi: 10.1111/j.1524-475X.2008.00372.x. [DOI] [PubMed] [Google Scholar]
- 36.Tremper KK, Anderson ST. Perfluorochemical emulsion oxygen transport fluids: a clinical review. Annu Rev Med. 1985;36:309–313. doi: 10.1146/annurev.me.36.020185.001521. [DOI] [PubMed] [Google Scholar]
- 37.Ward CL, Corona BT, Yoo JJ, Harrison BS, Christ GJ. Oxygen generating biomaterials preserve skeletal muscle homeostasis under hypoxic and ischemic conditions. PLoS One. 2013;8:e72485. doi: 10.1371/journal.pone.0072485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wijekoon A, Fountas-Davis N, Leipzig ND. Fluorinated methacrylamide chitosan hydrogel systems as adaptable oxygen carriers for wound healing. Acta Biomater. 2013;9:5653–5664. doi: 10.1016/j.actbio.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 39.Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–294. doi: 10.1038/193293a0. [DOI] [PubMed] [Google Scholar]
- 40.Xia YP, Zhao Y, Tyrone JW, Chen A, Mustoe TA. Differential activation of migration by hypoxia in keratinocytes isolated from donors of increasing age: implication for chronic wounds in the elderly. J Invest Dermatol. 2001;116:50–56. doi: 10.1046/j.1523-1747.2001.00209.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


