Abstract
Recent studies have demonstrated a critical role for nerves in enabling tumor progression. The association of nerves with cancer cells is well established for a variety of malignant tumors, including pancreatic, prostate and the head and neck cancers. This association is often correlated with poor prognosis. A strong partnership between cancer cells and nerve cells leads to both cancer progression and expansion of the nerve network. This relationship is supported by molecular pathways related to nerve growth and repair. Peripheral nerves form complex tumor microenvironments, which are made of several cell types including Schwann cells. Recent studies have revealed that Schwann cells enable cancer progression by adopting a de-differentiated phenotype, similar to the Schwann cell response to nerve trauma. A detailed understanding of the molecular and cellular mechanisms involved in the regulation of cancer progression by the nerves is essential to design strategies to inhibit tumor progression.
Keywords: Nerve, Cancer, Perineural invasion, Schwann cell, Tumor microenvironment, Migration, Invasion
Introduction
The normal function of the peripheral nervous system is to connect the central nervous system to the limbs and organs. Nerves convey signals between the brain and spinal cord with the rest of the body. Interestingly, nerves also stimulate cancer progression. The denervation of organs in several murine cancer models has been demonstrated to impair cancer formation [1–4]. It is known that non-malignant cells surrounding cancer cells, termed the tumor microenvironment, modulate cancer development. Nerves, composed of a variety of cells including neurons and Schwann cells, form a unique type of tumor microenvironment. Schwann cells are a major component of the peripheral nerves and have been recently identified as cells that promote cancer spread. In this review, we present findings that highlight the stimulatory role of the nerves in cancer invasion and discuss more specifically the role of the Schwann cells in this process. First, we describe how nerves and their supporting cells, and more specifically the Schwann cells, form a specific tumor microenvironment. We then describe the functional relationship between cancer cells and nerve cells, and explore the potential role of Schwann cells in cancer development. Finally, we review cellular and molecular mechanisms of Schwann cell-mediated interactions between nerves and cancer cells.
Nerves and Schwann cells form a unique tumor microenvironment
Nerves consist of large caliber nerve trunks that branch into smaller nerves and ultimately into single nerve fibers (Fig. 1a). Cancer cells can be found inside or outside the nerves (Fig. 1b). Once cancer cells invade inside nerves, the nerve cells form the tumor microenvironment. In this situation, nerves can be damaged and destroyed, as observed by electron microscopy. Cancer cells and nerve cells intermingle, and in some cases, there is a complete loss of the neural elements [5]. However, before cancer cells invade nerves, nerves may still communicate and interact with nearby cancer cells. Several nerve cell types can contribute to cancer progression. In both cases, Schwann cells, closely associated with neurons, can interact with cancer cells. In this section, we describe first perineural invasion (PNI), a condition in which cancer cells infiltrate inside or around nerves, and we identify the cellular components involved in cancer progression. Then, we describe the terminal nerve fibers and their role as part of the tumor microenvironment.
Fig. 1 .
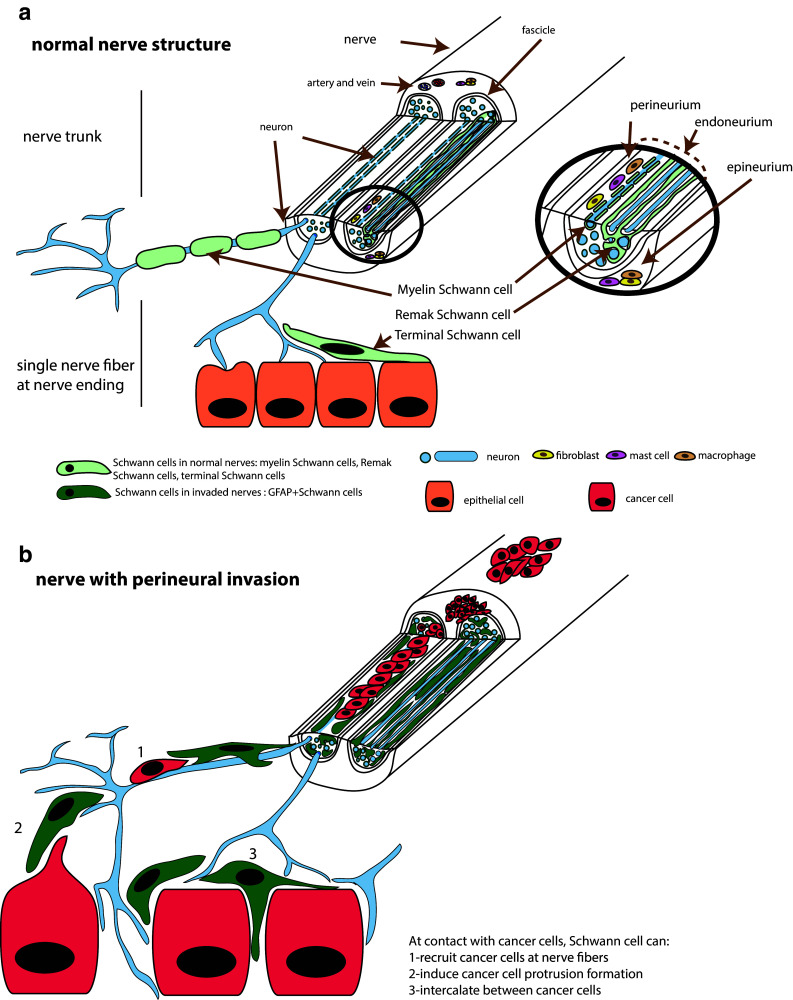
Schematic representation of nerve structure and Schwann cells distribution. (a) Normal nerve structure showing a large caliber nerve trunk and single nerve fibers at contact with epithelial cells. Myelin, Remak and terminal Schwann cells are shown (b) Nerve with perineural invasion. Cancer cells are present around or in the nerve. At contact with cancer cells, Schwann cells can recruit cancer cells at nerve fibers, induce cancer cell protrusion formation and intercalate between cancer cells
Perineural Invasion
Several reviews have described perineural invasion (PNI) [6–10]. PNI is the clinical identification of cancer cells invading in, through, or around nerves [11]. PNI is found more commonly in cancers of highly innervated organs. Depending on the level of pathologic scrutiny, PNI may be found in up to 100% of pancreatic cancers, up to 80% of head and neck cancers, up to 75% of prostate cancers, and up to 33% of colorectal cancer [7]. PNI is associated with a wide variety of clinical manifestations, with patients experiencing pain, paresthesias, numbness, or paralysis. PNI is a marker of poor prognosis and is associated with increased recurrence rates following therapy, and decreased patient survival rates. PNI is considered both as a form of local progression and as a form of metastasis, since nerve invasion may extend proximally to reach the central nervous system. In patients with pancreatic ductal adenocarcinoma, the invasion of cancer cells into nerves occurs both in the intrapancreatic nerves [12], as well as outside of the pancreas in extra pancreatic nerve plexuses. The presence of tumor invasion in nerves both inside and outside of the pancreas is significantly correlated with decreased survival [12–14]. Cancer cells may be found invading the distant retroperitoneal nerves of animal models of pancreatic cancer [15, 16]. The tracking of cancer cells along nerves to the spinal cord, proximal to the site of origin, is also seen in mouse models of pancreatic cancer [17].
Cells composing the nerve
Cancer cells may be seen around the nerve sections or in the three different layers of the nerves, the endoneurium, the perineurium, and the epineurium (Fig. 1). The innermost layer of the nerve is the endoneurium. The invasion of cancer cells in the endoneurium, named intraneural invasion, is associated with a worse prognosis than the invasion around or in the other layer of the nerves [18, 19]. A variety of cells from the nerve can facilitate cancer progression. In a normal, non-invaded nerve, the endoneurium consists mainly of axons and Schwann cells (Fig. 1a). It also contains mast cells, resident macrophages, fibroblasts, and blood vessels. Different subtypes of Schwann cells are present in the endoneurium. Myelin Schwann cells form a lamellar lipid myelin sheath around some axons. A myelin unit formed by a Schwann cell creates an internode separated by nodes of Ranvier, the uncovered portions of the axon membrane necessary for the saltatory transmission of the action potentials. Another type of Schwann cell, the Remak cell, associates with unmyelinated axons and forms Remak bundles [20]. These cells form overlapping chains, associate and elongate along axons. One Remak cell can be associated with 1–20 or more axons in a Remak bundle [21]. Myelin Schwann cells myelinate α- and γ-motor axons and Aα-, Aβ-, and Aδ-sensory axons. The non-myelinating Remak Schwann cells associate with C sensory and autonomic axons [21]. Thus, both the myelinated axons and non-myelinated axons are associated with Schwann cells.
The layer that protects and surrounds the endoneurium is the perineurium. It is a laminated cylindrical layering of cells thought to be of fibroblast origin [22] and that are interconnected by tight junctions and gap junctions forming layers separated by collagen fibrils (type IV). Live imaging in drosophila revealed that these cells actually originate from glial cells of the central nervous system [23]. The outermost layer of the peripheral nerve trunk is the epineurium and includes a collagen tissue sheath, a plexus of blood vessels, lymphatic vessels, resident macrophages, fibroblasts, mast cells, and sometimes adipose tissue. The epineurium is self-innervated. There are small unmyelinated axons from the endoneurium of the parent nerve trunk that travel outward into the connective tissue of the epineurium, and some of them control epineural vessels.
Several cell types from the nerves are likely to participate in PNI and might influence each other in their activity. Macrophages and the fibroblasts are able to contribute to cancer invasion in other microenvironments. Endoneurial macrophages have also been reported to play a role in PNI [24]. In addition, neurons from autonomic and sensory innervation of organs, and specific neurotransmitters have been strongly implicated in the regulation of cancer development and progression [1–4].
Recently, the role of Schwann cells in the context of cancer progression has been examined [25, 26]. The close association of Schwann cells with cancer cells has been reported in PNI from human specimens of pancreatic ductal carcinoma by electron microscopy [5] and more recently by immunofluorescence in pancreatic cancer specimens [25, 26] as well as other types of cancers including colon cancer [26], thyroid cancer, salivary duct carcinoma, and skin squamous cell carcinoma [25].
Nerve fibers in the tumor microenvironment
Interactions between the cancer cells and the neurons not only occur inside the trunk of the nerve, as seen in PNI specimens, but also at the terminal ends of nerves. The presence of Schwann cells at terminal end of single nerve fibers and in contact with pancreatic cells has been documented by scanning and transmission electron microscopy in both endocrine and exocrine rat pancreatic tissue [27, 28]. This interaction is likely maintained when epithelial cells transform into cancer cells. Isolated Schwann cells have been detected around murine and human pancreatic intraepithelial neoplasia (PanIN) lesions, the precursor formation of invasive ductal adenocarcinoma of the pancreas [26], suggesting a role for Schwann cells at early stages of cancer progression.
The density of nerves in malignant tissue is higher than in normal tissue for several types of cancer. Nerve fibers infiltrate tumors in human specimens of pancreatic cancer [29] and invasive breast cancer in human [30]. The infiltration of nerve fibers in tumor has also been observed in mice model of pancreatic cancer [17], prostate cancer [1] and skin cancer [3]. Sensory nerve fibers are detected in tumors of a mouse model of pancreatic ductal adenocarcinoma and appear to engulf the cancers cells [17]. Interestingly, neuroplastic changes are observed at early stages of cancer development, suggesting the involvement of nerves in cancer formation prior to cancer cell invasion. Single cutaneous sensory nerve fibers are also found associated with cancer cells in a mouse model of basal cell carcinoma [3]. An increase of nerve fibers has also been observed in prostate cancer in a metastatic mouse model, where prostate cancer cells were injected in bones. Prostate cancer cells in the bone induce sprouting of sensory nerve fibers [31–33]. In addition to the increase of nerve fiber density, these fibers have a highly disorganized appearance. The sprouting of nerve fibers is intermingled among the prostate cancer cells and the stromal cells [32]. Terminal single nerve fibers might, therefore, have pro-tumorigenic effects in highly innervated organs that are distinct from the process of perineural invasion.
Nerves and cancer growth
Nerves promote cancer cell growth and migration
Previous studies have characterized the interactions between nerve cells and cancer cells to better understand PNI and identify new targets for therapy. In vitro assays that aim to recreate cellular and molecular interactions in PNI include the co-culture of cancer cells with neurites that grow from the neurons of extracted mice dorsal root ganglia (DRG). These experiments demonstrate reciprocal interactions between cancer cells and the neurites. Nerves and cancer cells stimulate each other to promote growth [34]. Cancer cells are recruited to neurites and migrate along the nerve fiber toward the DRG center [34]. Recently, time-lapse movies have demonstrated cancer cell migration along neurites and active participation by Schwann cells in recruiting cancer cells to neurites [25]. The study of the mechanisms of PNI has led to the understanding that the nerve was not only a preferred location for cancer cells, but also that the nerve promotes cancer initiation and growth of the tumor.
Nerves promote cancer progression
The role of nerves in cancer progression was demonstrated in four recent studies using experimental models of denervation. Denervation leads to reduction of tumor growth in models of prostate, gastric, pancreatic, and skin cancer [1–4]. In a mouse model of prostate cancer, chemical or surgical ablation of hypogastric nerves results in diminished tumorigenesis [1]. In this study, the authors proposed that the sympathetic innervation regulates the initial neoplastic development and that parasympathetic innervation is involved in tumor proliferation, invasion, and metastasis formation. Similarly, in mouse models of gastric cancer, stomach denervation by surgical vagotomy or pharmacological denervation using botox treatment reduces gastric tumor incidence and growth. These authors propose a role for the cholinergic nerves in cancer initiation and progression [2]. These data support a previous study showing that chemical denervation using benzalkonium chloride decreased tumorigenesis in a rat model of gastric cancer [35]. In a mouse model of pancreatic ductal adenocarcinoma, the ablation the sensory neurons of the autonomic nervous system reduces the initiation and progression of the cancer [4]. Treatment with capsaicin, a drug that suppresses sensory innervation induced a delay in precancerous PanIN formation. Finally, in a mouse model of basal cell carcinoma, cutaneous sensory nerve fibers were also found to be associated with cancer cells. Denervation led to a slower progression of the basal cell carcinomas and a decrease of hedgehog activity in the area of the tumor formation [3].
Interestingly, a gain of function experiment also demonstrates a role for innervation in cancer development using an elegant in vivo optogenetic mouse model measuring human high-grade glioma proliferation. The activation of neurons induces an increase of high-grade glioma growth [36]. The presynaptic protein neuroligin-3 is implicated in this process. The authors suggest that neuroligin-3 is released from the activated neurons or from the associated oligodendroglial precursor cells.
The above experiments demonstrate a key role for peripheral innervation in cancer progression. However, a role for neuron-associated cells, such as Schwann cells, in cancer progression was not addressed by these studies. The denervation in these experimental models might also have removed associated glial cells in the tumor microenvironment. In fact, it has been shown that the denervation of the sympathetic nerves to the biceps femoris muscle and bone marrow reveals a significant decrease in GFAP expressing Schwann cells [37].
Inhibitory effects of nerves on cancer
Interestingly, there is also evidence that nerves may, conversely, have inhibitory effects on cancer growth and progression. Some denervation experiments have shown a stimulatory effect on cancer progression. The chemical denervation of skin using pentobarbital led to a reduction of tumor growth in mice [38]. In addition, the physical denervation of the spleen induces an increase of tumor development in colorectal cancer, an effect that is dependent on TFF2. TFF2 is an anti-inflammatory peptide that suppresses colon carcinogenesis, whose secretion by T cells is regulated by the vagus nerve [39].
A report by Kaminishi et al. [40] similarly proposed that partial gastrectomy and denervation may promote progression of gastric cancer in the gastric remnant. However, this result differs with a population-based study of over 7000 patients showing no statistical relationship between vagotomy and any change in risk of subsequent gastric cancer development [41]. More importantly, a recent study [2] demonstrated a supportive effect of vagal innervation on gastric cancer progression using transgenic and surgical mouse models. It is possible that the inhibitory effects previously reported in the Kaminishi study may have been related in part to delayed gastric emptying that could increase the exposure time of orally administered chemical carcinogens to the gastric mucosa.
Schwann cell function and cancer invasion
A striking feature of Schwann cells is the wide variation in their functional ability. These abilities include the conduction of nervous impulses along axons, nerve repair [20], nerve development, trophic support for neurons, production of extracellular matrix, modulation of neuromuscular synaptic activity [42], antigen presentation [43], in addition to the promotion of cancer invasion [25, 26, 44, 45]. Demir et al. show that Schwann cells migrate toward pancreatic and colon cancer cells [26]. This process involves NGF and its low affinity receptor p75NTR. The authors proposed that the attraction of Schwann cells toward the cancer cells and their precursor cells occurs before cancer invasion at the nerve [26]. Our work shows that Schwann cells increase cancer invasion, and this occurs after contact [25]. Schwann cells trigger cancer invasion using specific cellular mechanisms. The microscopic in vitro study of the interaction between Schwann cells and cancer cells has shown that Schwann cells are able to degrade matrix around cancer cells, stimulate cancer cell protrusion after contact, guide cancer cells during invasion, and are able to intercalate between cancer cells to disperse them [25].
Schwann cells are able to degrade the matrix in Matrigel. They form tunnels or tracks that they coat with laminin. Cancer cells can use these tracks during invasion. The remodeling of the matrix by Schwann cells has also been described in nerve biology. Schwann cells express MMP2 and MMP9 that facilitate neurite growth [46]. The presence of a tunnel formed by Schwann cells is not sufficient for cancer invasion. The physical contact between the cancer cells and the Schwann cells is necessary for cancer invasion in Matrigel. The heterocellular contact leads to directed cancer cell migration by the formation of cancer cell protrusion at site of contact and toward the Schwann cell [25]. This process is particular in the biology of cellular migration. Two migrating cells that collide together are known to cease or change the direction of their migration, in a process named contact inhibition of locomotion that was originally described by Abercrombie over 40 years ago [47]. Contact inhibition of locomotion is known to be defective in cancer cells. Interestingly, we noted that cancer cells actually move toward Schwann cells after making contact. The ability of Schwann cells to intercalate between other types of cells has not only been observed with cancer cells, but also with axons during the development of peripheral nerves. Schwann cells intercalating between axons allows axon isolation from large axon bundles, a step of the development of a peripheral nerve at which myelinization begins [48]. Therefore, Schwann cell behavior with cancer may recapitulate its behavior in a developmental context.
Two other recent studies proposed a role for Schwann cell paracrine function in PNI [44, 45]. The release of BDNF by Schwann cells increases cancer cell epithelial–mesenchymal transition (EMT) process in a salivary adenoid cystic carcinoma cell line. The Schwann cell condition medium induces a change of morphology into the mesenchymal type and increases migration and invasion ability of the salivary adenoid cystic carcinoma cell line SACC-83. This is accompanied by an upregulation of mesenchymal markers N-cadherin and vimentin and downregulation of epithelial marker E-cadherin. The authors show the involvement of BDNF and its receptor TrkB in this process [44]. In another study, conditioned medium from Schwann cell lines was shown to increase the integrin-dependent invasion of prostate and pancreatic cancer cell lines. Interestingly, the conditioned medium of a Schwann cell type that is unable to form myelin was inefficient in enabling cancer cell invasion [45].
Myelin Schwann cells dedifferentiate and become activated GFAP-positive Schwann cells when interacting with cancer cells
The variety of Schwann cell functions is mainly due to the plasticity of the Schwann cells that allow them to convert from one subtype to another one. This has been well described in the context of nerve repair [20]. After nerve injury, the process of nerve repair leads myelinating Schwann cells to convert into unmyelinating repair Schwann cells, which are capable of supporting axon regeneration and axon guidance [20]. Schwann cells from Remak bundles also convert into repair Schwann cells. This process is called de-differentiation, activation, or trans-differentiation of Schwann cells [20]. The conversion induces a large-scale change in gene expression. For myelinated Schwann cells, the downregulation of genes such as myelin transcription factor Egr2 (Krox20), structural proteins such as myelin protein zero (P0), myelin basic protein (MBP), and membrane-associated glycoprotein (MAG) accompanies the upregulation of genes expressed in the pre-myelinating Schwann cells such as GFAP, NCAM, L1-CAM, and p75 [20]. Interestingly, each of these molecules has been implicated in PNI [26, 49–51]. Specific expression repair Schwann cell genes that are necessary for nerve regeneration include GDNF, artemin, NT3, NGF, VEGF, erythropoietin, and N-cadherin. Nerve repair Schwann cells also activate an innate immune response by up-regulating the expression of cytokines including tumor necrosis factor alpha, interleukin-1α, interleukin-1β, leukaemia inhibitory factor (LIF), and CCL2, which leads to macrophage recruitment. The recruited macrophages provide an additional source of cytokines, promote vascularization of the nerve, and co-operate with Schwann cells to clear myelin debris [52].
The Schwann cells involved in cancer invasion share strong similarities with the Schwann cells involved during nerve repair. An increase of the number of GFAP-positive Schwann cells occurs when nerves are injured or invaded by cancer cells. The Schwann cells associated with cancer cells in human pancreatic [25, 26], colon [26], thyroid, and salivary gland cancer surgical specimens are GFAP-positive Schwann cells [25]. In patients with pancreatic adenocarcinoma, nerves near the tumor were found enriched in GFAP as compared to nerves without tumor in matched patients [25]. Similar observations were made in murine sciatic nerves after injection of pancreatic cancer cells [25], in murine pancreatic cancer models, and in murine colon cancer models [26]. These data suggest that a de-differentiation program of Schwann cells occurs at the vicinity of cancer in a similar manner than during nerve repair. Interestingly, Schwann cells were specifically found in the precancerous PanIN lesions and they were less numerous in colon cancer specimens than in pancreatic lesions [26], suggesting that Schwann cells function in cancer invasion might occur at early stage of cancer development and the extent of the de-differentiation program depends on the type of cancer. The repair Schwann cells and Schwann cells observed in cancer invasion share the same ability for guiding other cells or processes. In nerve repair, Schwann cells induce axon sprouting during the axonal guidance process. At contact with cancer cells, Schwann cells induce cancer cell protrusions and guide cancer cell movement toward the Schwann cell [25].
Induction of Schwann cell de-differentiation and GFAP expression
In nerve repair, the signal for the Schwann cell de-differentiation and activation originates from the crushed or the damaged neurons, but has not been clearly identified. Axonal damage might trigger the de-differentiation and activation of the Schwann cells through the release of neuregulin-1 (NRG1) by remaining neurons. NRG1 modulates multiple behaviors of Schwann cells, including survival, proliferation, motility, and myelination [53]. NRG1 induces Schwann cell demyelination in response to nerve injury by activation of its receptor erbB2 on Schwann cells [54].
A recent study proposed that hypoxia activates Schwann cells and is linked to the presence of interleukin-6 produced by cancer cells in response to hypoxic conditions [51]. GFAP signal in nerves is associated with markers of hypoxia in patients with pancreatic cancer. Hypoxic conditions or co-culture with pancreatic cancer cells induces Schwann cell activation. This effect varies among cancer cell lines, and is increased in the presence of T cells. Blockade of IL-6 using a specific antibody reduces the amount of GFAP Schwann cells. A reduced number of Schwann cells associated with PanIN lesions have been observed in transgenic pancreatic cancer mouse models crossed with two different types of IL-6 signaling deficient mice. The increase of GFAP in Schwann cells is reminiscent of the activation of astrocytes that occur with stroke and tumor growth [55]. Schwann cells activated by cancer cells also developed star like processes that are similar to those observed in activated astrocytes [51]. Activation of astrocytes is observed in murine models of pancreatic cancer [4, 56], as well as in the spinal cord in patients with pancreatic cancer and correlates with the presence of PNI [56]. Interestingly, Demir et al. also describe a decrease of pain sensation associated with activated Schwann cells in patients with pancreatic cancer and pancreatic cancer mice models [51]. Pain is an important feature of PNI [7]. Pain generation in patients with PDAC can be due to the invasion of cancer cells within the nerve and subsequent damage. Pain can also be due to an increase in nerve fiber growth resulting from the cancer [7]. It remains unclear how Schwann cell activation by cancers relates to pain sensation. The decrease of Schwann cell activation observed in IL-6 knock out mice is concomitant with an increase of activation of astroglia and microglia in the spinal cord, and the spinal cells involved in nociception [51].
Interaction of Schwann cells with the other non-cancer cells in the stroma
Schwann cells are likely to interact with other cell types in the stroma of the cancer microenvironment to regulate cancer progression. Schwann cells can modulate the action of its neighbor cells as well as be regulated by them. Immune cells infiltrate tumor and modulate cancer invasion. In PDAC, the inflammatory infiltrate includes cytotoxic T cells, mast cells, and macrophages [57, 58]. This infiltration even occurs in preinvasive pancreatic lesions [57]. Cancer cells induce recruitment of immune cells, which in turn accelerate the process of tumorigenesis. Several molecules released by immune cells, including IL-6 and IL-17, have been implicated in this process [59, 60]. IL-6 induces Schwann cell activation and T cells enhance the activation [51]. In addition, Schwann cells may recruit immune cells in sites of PNI since they are capable of recruiting macrophages during nerve repair [52]. Interestingly, the formation of blood vessels after nerve injury has been shown to assist with subsequent Schwann cell activity. Schwann cells were shown to use blood vessels induced by macrophage released VEGF-A as a track to repair axons after nerve injury. [61].
Another important cellular component of the tumor microenvironment that regulates cancer progression in several types of cancer are fibroblasts. Cancer-associated fibroblasts contribute to cancer invasion by secreting factors that activate cancer cells and remodel the matrix [62–65]. One study implicates a role for a subset of these fibroblasts, the pancreatic stellate cells, in promoting PNI. The process involves the activation of the hedgehog signaling pathway in the fibroblasts induced by cancer cells [66]. The authors proposed that the stellate cells promote cancer migration. In addition, a cooperative role for fibroblasts and Schwann cells has been reported in nerve injury [67]. Fibroblasts have been shown to contribute to Schwann cell-induced axonal growth during nerve repair upon contact. The process involves the activation of EphB2 receptors on Schwann cells by ephrin-B on fibroblasts [67].
Interestingly, two studies report an antitumorigenic effect by stromal cells in PDAC [68, 69]. Inhibiting the hedgehog signaling in a mice model of PDAC perturbs the tumor microenvironment by reducing the stromal content of the tissue including fibroblasts and leukocytes. This also induced an acceleration of tumor growth and increased metastasis, suggesting that some stromal constituents impede tumor progression. Similarly, depletion of αSMA+ myofibroblasts (the pancreatic stellate cells) in a mouse model of pancreatic ductal adenocarcinoma (PDAC) leads to more invasive and undifferentiated tumors and diminished survival [69]. Fibroblasts and immune cells were depleted in these two models, but these studies do not report on whether nerve density and nerve invasion were affected.
Neurons are important cellular players in regulating cancer progression, and their functions are closely associated with Schwann cells. Several types of fibers releasing a variety of neurotransmitters are involved in cancer progression [1, 4]. Schwann cells are known to affect neuronal excitability and synaptic transmission [70]. It remains to be determined whether such an influence occurs during nerve-related cancer invasion. The close cooperation between neurons and Schwann cells in response to injury and in inducing axonal growth during nerve repair might also occur during PNI. It is possible that damaged neurons send injury signals sensed by Schwann cells, which in turn becomes activated and perform repair and guidance functions.
Molecules involved in PNI and their relation to Schwann cells
Efforts have been made to identify the molecules involved in neural invasion to decipher the mechanisms of PNI for therapeutic purposes. Several reviews on the mechanisms of PNI have listed a large set of these molecules [6, 7, 71]. They include neurotrophic factors, cytokines, chemokines, proteinases, and some cell surface markers (Fig. 2). Many of these molecules are implicated in nerve development or nerve repair, which includes nerve growth, cell differentiation, and neuron guidance. The activation of these mechanisms is consistent with the observations of increased nerve growth in pancreatic, prostate and breast cancers [1, 17, 30, 72]. Importantly, some of these molecules not only target nerve cells, but also directly affect cancer cells to stimulate growth or migration. In addition, cancer cells can also produce some of these presumed nerve molecules, which are actually expressed in a wide variety of cell types. We discuss both the direct and indirect effects of these factors on cancer invasion and identify the implication of the specific cell types involved, with a particular attention to Schwann cells.
Fig. 2.
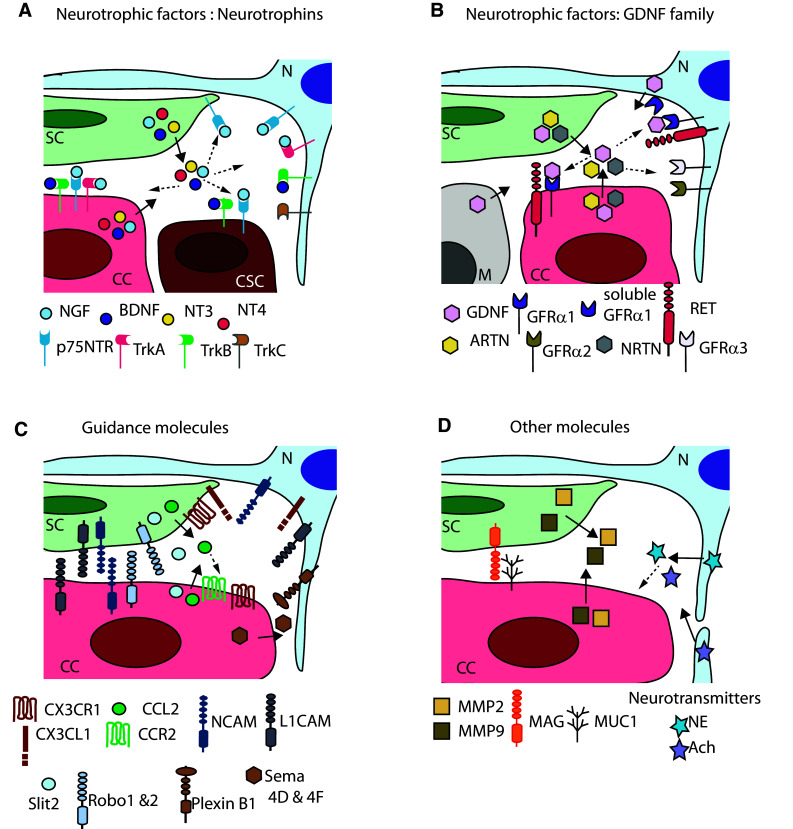
Molecules involved in the interactions between cancer cells and nerve cells. a Neurotrophic factors, neurotrophins (NGF, BDNF, NT3, and NT4) and their receptors (p75NTR, TrkA, TrkB, and Trkc). b Neurotrophic factors of the GDNF family (GDNF, ARTN, and NRTN) and their receptors (RET, GFRα1, GFRα2, and GFRα3). c Guidance molecules (CX3CL1 and CX3CR1, CCL2, and CCR2, NCAM1, L1CAM, Slit2, and Robo 1&2, PlexinB1, and Sema 4D&4F). d Other molecules [MMP2 and MMP9, MAG and MUC1, neurotransmitters norepinephrine (NE), and acetylcholine (Ach)]. N neuron, SC Schwann cell, CC cancer cell, CSC cancer stem cell, M macrophage. Full arrows show release of soluble factors by specific cell types. Dotted arrows point cell types that express corresponding receptors
Neurotrophic factors
Several studies have reported a major role for the neurotrophic factors in supporting PNI. They comprise the molecules from the GDNF family and the neurotrophins (NGF family) (Fig. 2a, b). In several types of cancer, a significant proportion of patients show alterations in genes coding for the neurotrophic factors. Using cBioPortal [73, 74], we found (unpublished) that a total of 48% of pancreatic cancer patients from a recent study carried on 109 patients show an alteration in genes coding for the neurotrophin family or their receptors (NGF, NGFR coding for p75NTR, NTRK1 coding for TrkA, BDNF, NTRK2 coding for TrkB, NTF3 coding for NF-3, NTRK3 coding for TrkC, and NTF4 coding for NF-4) [75]. Similarly, the modification of these genes is seen in 48% of patients with neuroendocrine prostate cancer [76], in 67% of patients with breast cancer, where cells were grown as xenograft in mice [77], and in 27% of patients with breast invasive carcinoma in another study [78]. In all these studies, among the different reported gene alterations including mutations, deletions, and amplifications, the main gene alteration observed was a gene amplification, and the most affected gene was NTRK1 coding for TrkA.
NGF/TrkA/p75NTR
NGF regulates neural development and differentiation. The role of NGF and its high affinity receptor TrkA and low affinity receptor p75NTR in cancer growth is well established [79]. NGF and its receptors modulate cancer cell growth and expansion in several cancer types. The implication of NGF in the interactions between the nerve cells and the cancer cells has been reported in pancreatic cancer [17, 72, 80] and other types of cancer such as the breast cancer [30]. NGF stimulates axonal growth during neural development and nerve repair. It is produced by a variety of cell types that include Schwann cells, neurons, fibroblasts, muscle cells, and macrophages. The NGF production by Schwann cells increases during nerve repair [20], consistent with the central Schwann cell role in this process.
The expression levels of NGF and TrkA in pancreatic cancer correlate with poor prognosis, with the level of PNI [72, 80–83], with the increase of innervation at tumor site [72], and with the level of metastasis in lymph nodes [82]. NGF and TrkA levels also correlate with the level of PNI in basal cell and cutaneous squamous cell carcinoma [84], oral squamous cell carcinoma [85], and adenoic cystic carcinoma [86]. A high density of nerve fibers and elevated levels of NGF are also observed in breast cancer and are associated with aggressiveness behavior [30]. The increase of NGF expression in tumor compared to control tissue is observed in cancer cells. NGF levels are higher in the cytoplasm of pancreatic cancer cells than in normal acinar and ductal cells [72, 80].
TrkA detected by immunohistochemistry is localized in the perineurium of pancreatic cancer nerves [80]. TrkA is also expressed at higher levels in pancreatic ductal adenocarcinoma cells as compared with normal acinar and ductal pancreatic cells, as seen by immunohistochemistry. Among these patients, about 40% of the patients express a high level of NGF and 25% of patients express higher p75NTR in PDAC cells as compared to normal cells [83]. P75NTR is a low affinity receptor for NGF that also binds other neurotrophins. TrkA is associated with aggressiveness and increased cancer cell proliferation, while p75NTR is associated with a milder prognosis [81].
However, P75NTR is associated with PNI in pancreatic cancer [87] and cutaneous squamous cell carcinoma [84, 88]. In specimens of cutaneous squamous cell carcinoma, p75NTR is found in both the perineurium of the nerves and the cancer cells near the nerves [84, 88]. While p75NTR supports tumor development in some of cancer types including glioma and breast cancer [89, 90], p75NTR also behaves as a tumor suppressor in other cancer types such as gastric, bladder, and prostate cancers by blocking cell cycle progression and inducing apoptosis [91–93]. P75NTR is also expressed by repair Schwann cells [20, 94]. Mice embryo lacking p75NTR show a defect in Schwann cell migration with an inhibition of axon growth [95]. Depleting p75NTR from the Schwann cells and preventing the binding of NGF to p75NTR using the small-molecule inhibitor RO.08.2750 reduce the migration of Schwann cells toward cancer cells [26]. This suggests a role for NGF and p75NTR in promoting the nerve infiltration via Schwann cells migration.
BDNF/TrkB and NT-3/TrkC
Other members of the neurotrophin family include BDNF and neurotrophin-4/5 that preferentially bind to receptor TrkB, and neurotrophin-3 that preferentially binds to TrkC. These neurotrophins are also produced at sites of nerve repair and found to play a major role in cancer development, mainly by inducing cell proliferation. TrkB expression correlates with PNI in pancreatic cancer [96] and in salivary adenoid cystic carcinoma [97]. Although there is a higher than normal expression of BDNF in cancer cells [83, 87, 98], there is no correlation with PNI in pancreatic cancer patients [83] or adenoid cystic carcinoma patients [98]. However, it has been proposed that BDNF released by Schwann cells induces epithelial–mesenchymal transition in a salivary adenoid cystic carcinoma cell line [44]. A recent study tested the effect of AZD1332, an inhibitor for TrkA, B and C, on pancreatic cancer growth in in vitro and in vivo assays. Although an inhibitory effect was observed in vitro, the drug was inefficient in vivo at inhibiting cancer growth [99], demonstrating further the importance of the microenvironment on tumor growth.
In one study, NGF, BDNF, NT3, and NT4 mRNAs were found higher in pancreatic cancer tissues than in control tissues. Laser capture microdissection experiments also revealed a higher level of mRNAs of these four neurotrophins and their receptors in the nerves in comparison with the other cell types. In contrast to the other neurotrophins, NT-3 was only present in nerves and absent in the cancer cells [87]. In another study, the expression of NGF, BDNF, and NT3 in the Schwann cells of patients with pancreatic cancer was measured; 73% of patients expressed NT-3 in their Schwann cells, as compared to 27% for NGF and 27% for BDNF [83].
Neurotrophins have also been reported to modulate several aspects of the biology of cancer stem cells in several types of cancers such as head and neck squamous cell carcinoma, melanoma, and breast cancer [100]. Cancer stem cells are a subtype of cancer cells that share the ability of self-renewal and differentiation with normal stem cells, and play a central role in tumor initiation and metastasis. Cancer stem cells are also resistant to the conventional therapies and are thought to be responsible for recurrence [101]. P75NTR is reported to be a functionally active marker of cancer stem cells of head and neck squamous cell carcinoma. The loss of p75NTR in these cells induces an inhibition of both cell proliferation and tumor formation. Blocking antibody treatment to p75NTR diminishes NGF induced signaling and decreased tumor formation [102]. Similarly, p75NTR knock-down in melanoma cancer cells prevents tumor formation in a xenograft mouse model. The loss of p75NTR in these cells is associated with a loss of stemness marker [103]. P75NTR is also present in a population of breast cancer stem cells, in which it mediates the self-renewal effects of NGF [104]. BDNF and its receptor TrkB also promote the self-renewal of cancer stem cells. TrkB is present in the cancer stem cells of recurrent triple negative breast cancer and mediates the effect of BDNF in promoting their expansion. BDNF acts in a paracrine manner, and it is secreted by another population of cancer cells upon stimulation by paclitaxel [105]. The ability of cancer stem cells to self-renew and differentiate is regulated by neurotrophins and its receptors. The direct effect of nerves and glial cells in this process remains to be studied.
GDNF family
The GDNF family of neurotrophic factors has been strongly implicated in the interaction between cancer cells and nerve cells [106–109]. The GDNF family includes GDNF, neurturin (NRTN), artemin (ARTN), and persephin (PSPN). These factors bind co-receptors of the GFRα family (GDNF to GFRα1, NRTN to GFRα2, ARTN to GFRα3, and PSPN to GFRα), and these complexes then bind and activate the transmembrane receptor tyrosine kinase RET. GDNF is also a ligand for the neural call adhesion molecule (NCAM) [110]. These neurotrophic factors and receptors play a major role in nerve growth and nerve repair by mediating neuronal differentiation, survival, and growth. The levels of transcripts for GDNF and its receptors GFRα1 and RET are increased after sciatic nerve lesion in mice [111]. GDNF is produced by neurons and Schwann cells.
The expression of GDNF in bile duct carcinoma correlates with PNI [108]. In pancreatic cancer specimens, there is higher expression of RET and GFRα1 in tumors as compared with control tissues [106]. GDNF produced by nerves attracts pancreatic cancer cells expressing its receptors RET and GFRα1 and supports PNI in both co-culture and animal models [106]. Interestingly, the presence of GFRα1 is not required in cancer cells, since GFRα1 can be released by the nerve cells [107]. Tumor activated macrophages located near PNI sites also produce higher GDNF levels than resting macrophages [24].
The other members of the GDNF family ARTN and NRTN might also be involved in PNI of pancreatic ductal adenocarcinoma. Their expression in cancer cells correlates with nerve invasion in patients [112, 113]. Furthermore, ARTN and its receptor GFRα3 expression levels increase in tissue of chronic pancreatitis and more specifically in the Schwann cells of the nerve fibers of the specimens [114].
Guidance molecules: NCAM/L1CAM, semaphorin, Slit2, CCL2/CCR2, CX3CL1
Several molecules involved in cell guidance have been implicated in the process of PNI. They include axonal guidance molecules, such as slit, semaphorins, ephrin, and cell adhesion molecules, as well as chemokines such as CCL2 and CX3CL1 (Fig. 2c). Their expression is often deregulated in human cancers, and they can act as oncogenes and mediates metastasis, cell migration, and neovascularization [115–117].
NCAM and L1CAM
The cell adhesion molecules involved in axonal guidance are membrane proteins that mediate adhesion between nerve cells and induce intracellular signaling. They include the members of the immunoglobulin family, NCAM and L1CAM [118]. L1CAM expression in pancreatic cancer is high and correlates with PNI [49]. NCAM expression in cancer cells correlates with PNI in specimens from a variety of cancer including skin, prostate, pancreas, and gallbladder cancer [84, 119–123]. High expression of NCAM is found also in the nerve of prostate cancer specimens with PNI [124]. Schwann cells expressing NCAM associate with cancer cell in specimens of pancreatic ductal adenocarcinoma [25]. NCAM KO mice injected with cancer cells in sciatic nerve have lower PNI than wild-type mice. Furthermore, the loss of NCAM expression in Schwann cells inhibited Schwann cell-dependent cancer invasion in in vitro models of cancer cell invasion by inhibiting protrusion formation in cancer cells and impairing the directionality of cell migration.
Semaphorins
Two types of semaphorins have been implicated in PNI in prostate cancer. In one study, Semaphorin-4D and Plexin B1 have been shown to promote PNI. The production of Sema4D by prostate cancer cells mediates neurite outgrowth and chemotaxis of nerve cells expressing plexin-B1 [125]. In the other study, Semaphorin-4F expression level in the human prostate cancer cells correlates with PNI diameter and nerve density [126].
Slit2
Mechanisms of chemorepulsion might also occur in the interaction between nerve cells and cancer cells to promote cancer invasion. Slit2, a chemorepellent ligand and its receptor roundabout Robo-1 and Robo-2, are involved in axonal guidance and nerve branching [127, 128]. Slit and Robo regulate the migration of neuronal cells [129] and glial cells [130] including the Schwann cells [131], as well as tumor cells [132]. Schwann cells express Slit2 and its receptors Robo1 and Robo2. Slit 2-repels the migration of Schwann cells [131]. Slit2 and Robo have also been implicated in PNI of pancreatic cancer [133, 134]. Slit2 expression is lower in pancreatic cancer cell as compared to normal pancreatic cells. The low expression of Slit2 in the pancreatic cancer cells allows the bidirectional migration of tumor and nerve cells. When cancer cells overexpress Slit 2, their migration toward Schwann cells is inhibited. Similarly, the migration of Schwann cells toward cancer cells is higher when cancer cells do not express Slit2 [133]. Another recent study demonstrates that Slit2 is expressed in cancer-associated fibroblast (CAF) in PDAC. Slit2 produced by CAF increases neurite outgrowth in an in vitro model and stimulates Schwann cell proliferation and migration via an N-cadherin/β-catenin signaling mechanism [134].
EphA2/ephrinA1
Another pair of axonal guidance molecules, EphA2 receptor, and its ligand ephrinA1 might be involved in PNI. Their expression level correlates with PNI in adenoid cystic carcinoma of salivary gland [135]. High expression of EphA2 is associated with a poor prognosis, increased metastasis, and decreased survival in other cancer types [136, 137]. Schwann cells express EphA2 and other EphA receptors [138], but their role in PNI has not been studied.
CCL2–CCR2
Other molecules involved in cell guidance include the chemokine CCL2 and its receptors CCR2. CCL2 acts as a chemoattractant for monocytes and basophils at sites of inflammation or infection. CCL2 and CCR2 are involved in a variety of cancers including breast, prostate, ovarian, and bladder cancer [117]. They mediate tumor growth and angiogenesis by affecting both cancer cells and stromal cells such as fibroblasts and immune cells. More specifically, CCL2 and CCR2 signaling has been found to support PNI in prostate cancer, using both animal models and review of human surgical specimens [139]. The expression of CCR2 in cancer cells allows cancer cell migration toward dorsal root ganglia that release CCL2.
In nerve repair, the release of CCL2 by Schwann cells contributes to macrophage recruitment, which facilitates nerve repair by clearing myelin debris [52]. Macrophages play an important role in cancer invasion and metastasis [140]. There is also a recruitment of macrophages to site of PNI, through CSF1 secretion by cancer cells. In addition, CCR2 deficient mice have reduced macrophage recruitment and less nerve invasion after cancer cell injection in sciatic nerves [24]. In breast cancer, not only the tumor cells but also the stromal cells produce CCL2 to recruit inflammatory monocytes at site of metastasis [141]. During nerve repair, Schwann cells release other cytokines that recruit macrophages. They include tumor necrosis factor (TNF)-α, LIF, interleukin (IL)-1α, IL-1β, and LIF [52]. These molecules might similarly be released by Schwann cells at sites of cancer invasion and play a role in PNI.
CX3CL1–CX3CR1
The expression of soluble or membrane bound chemokine CX3CL1, and its receptor CX3CR1, has been reported in perineural invasion specimens in pancreatic cancer [142], in gastric carcinoma [143], and in squamous cell carcinoma of the tongue [144]. Marchesi et al. proposed that pancreatic cancer cells highly express CX3CR1 and migrate toward CX3CL1 produced by nerves. CX3CR1 is also expressed in Schwann cells [144]. It is unknown if CX3CR1 from Schwann cell plays a role in PNI.
Other molecules: Myelin associated glycoprotein (MAG)/MUC1
The interaction between Schwann cells and cancer cells via two other molecules MAG and MUC1 has been reported [145] and is proposed to play a role in PNI. MAG is an immunoglobulin-like lectin expressed in Schwann cells that binds to sialylated glycoconjugates and mediates interaction between myelin Schwann cells and neurons. MAG is an inhibitor of neurite growth after nerve injury [146] that is also expressed in pancreatic cancer cells [145]. MAG from Schwann cells binds to MUC1, a transmembrane protein that is highly expressed and aberrantly glycosylated in pancreatic cancer cells and cell from other cancer types [145]. Elevated levels of MUC1 are associated with metastasis and poor prognosis in pancreas, gallbladder and colon cancer patients [147–149]. MUC1 has been shown to be involved in proliferation, metabolism, invasion, and angiogenesis [150]. Although it remains unclear how MAG/MUC1 binding mechanistically regulates PNI, the association of these two molecules forms a direct link between Schwann and cancer cells in PNI.
Conclusion
Studies have clearly demonstrated an important role for nerves in cancer progression. Targeting stromal components of the tumor microenvironment is now a recognized strategy to inhibit cancer development. It is, therefore, essential to determine the precise cellular and molecular mechanisms involved in the process of nerve-dependent cancer progression. A variety of neutrotrophic factors, chemokines, and adhesion molecules have been identified in this process. Schwann cell involvement in this process has only recently been reported. The behavior of Schwann cells during cancer invasion shares a strong similarity with their activity in nerve repair. However, many questions remain. How do the different reported molecular mechanisms act together? Do they act simultaneously or sequentially, and how are they coordinated? Are the different mechanisms specific to different cancer types? How are Schwann cells activated during cancer invasion? What are the specific interactions between Schwann cells and the other cells in the tumor microenvironment that regulate cancer invasion? It is important to identify the specific functions of each of the cell types of the nerves and consider the nerves as a multicellular complex microenvironment rather than a single element. A comprehensive understanding of the role of nerves and their cellular contents in cancer progression should lead to an identification of novel targets and therapeutic strategies to inhibit cancer invasion by nerves.
References
- 1.Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 2.Zhao C-M, Hayakawa Y, Kodama Y, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115–250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson SC, Eberl M, Vagnozzi AN, et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16:400–412. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saloman JL, Albers KM, Li D, et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci. 2016 doi: 10.1073/pnas.1512603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bockman DE, Büchler M, Beger HG. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology. 1994;107:219–230. doi: 10.1016/0016-5085(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 6.Amit M, Na’ara S, Gil Z. Mechanisms of cancer dissemination along nerves. Nat Rev Cancer. 2016 doi: 10.1038/nrc.2016.38. [DOI] [PubMed] [Google Scholar]
- 7.Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. doi: 10.1038/nrc3131. [DOI] [PubMed] [Google Scholar]
- 8.Demir IE, Ceyhan GO, Liebl F, et al. Neural invasion in pancreatic cancer: the past, present and future. Cancers. 2010;2:1513–1527. doi: 10.3390/cancers2031513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demir IE, Friess H, Ceyhan GO. Nerve-cancer interactions in the stromal biology of pancreatic cancer. Front Physiol. 2012;3:97. doi: 10.3389/fphys.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liebig C, Ayala G, Wilks JA, et al. Perineural invasion in cancer. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 11.Batsakis JG. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol. 1985;94:426–427. doi: 10.1177/000348948509400523. [DOI] [PubMed] [Google Scholar]
- 12.Petrou A, Soonawalla Z, Silva M-A, et al. Prognostic indicators following curative pancreatoduodenectomy for pancreatic carcinoma: a retrospective multivariate analysis of a single centre experience. J BUON. 2016;21:874–882. [PubMed] [Google Scholar]
- 13.Nakao A, Harada A, Nonami T, et al. Clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas. 1996;12:357–361. doi: 10.1097/00006676-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Mitsunaga S, Hasebe T, Kinoshita T, et al. Detail histologic analysis of nerve plexus invasion in invasive ductal carcinoma of the pancreas and its prognostic impact. Am J Surg Pathol. 2007;31:1636–1644. doi: 10.1097/PAS.0b013e318065bfe6. [DOI] [PubMed] [Google Scholar]
- 15.Eibl G, Reber HA. A xenograft nude mouse model for perineural invasion and recurrence in pancreatic cancer. Pancreas. 2005;31:258–262. doi: 10.1097/01.mpa.0000175176.40045.0f. [DOI] [PubMed] [Google Scholar]
- 16.Pour PM, Egami H, Takiyama Y. Patterns of growth and metastases of induced pancreatic cancer in relation to the prognosis and its clinical implications. Gastroenterology. 1991;100:529–536. doi: 10.1016/0016-5085(91)90226-B. [DOI] [PubMed] [Google Scholar]
- 17.Stopczynski RE, Normolle DP, Hartman DJ, et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Can Res. 2014;74:1718–1727. doi: 10.1158/0008-5472.CAN-13-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amit M, Binenbaum Y, Trejo-Leider L, et al. International collaborative validation of intraneural invasion as a prognostic marker in adenoid cystic carcinoma of the head and neck. Head Neck. 2015;37:1038–1045. doi: 10.1002/hed.23710. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee D, Katz MH, Rashid A, et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2012;36:409–417. doi: 10.1097/PAS.0b013e31824104c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol (Lond) 2016 doi: 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zochodne DW (2008) Neurobiology of peripheral nerve regeneration
- 22.Bunge MB, Wood PM, Tynan LB, et al. Perineurium originates from fibroblasts: demonstration in vitro with a retroviral marker. Science. 1989;243:229–231. doi: 10.1126/science.2492115. [DOI] [PubMed] [Google Scholar]
- 23.Kucenas S, Takada N, Park H-C, et al. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat Neurosci. 2008;11:143–151. doi: 10.1038/nn2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavel O, Shomron O, Shabtay A, et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Can Res. 2012;72:5733–5743. doi: 10.1158/0008-5472.CAN-12-0764. [DOI] [PubMed] [Google Scholar]
- 25.Deborde S, Omelchenko T, Lyubchik A, et al. Schwann cells induce cancer cell dispersion and invasion. J Clin Invest. 2016;126:1538–1554. doi: 10.1172/JCI82658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demir IE, Boldis A, Pfitzinger PL, et al. Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J Natl Cancer Inst. 2014;106:dju184. doi: 10.1093/jnci/dju184. [DOI] [PubMed] [Google Scholar]
- 27.Sunami E, Kanazawa H, Hashizume H, et al. Morphological characteristics of Schwann cells in the islets of Langerhans of the murine pancreas. Arch Histol Cytol. 2001;64:191–201. doi: 10.1679/aohc.64.191. [DOI] [PubMed] [Google Scholar]
- 28.Ushiki T, Ide C. Autonomic nerve networks in the rat exocrine pancreas as revealed by scanning and transmission electron microscopy. Arch Histol Cytol. 1988;51:71–81. doi: 10.1679/aohc.51.71. [DOI] [PubMed] [Google Scholar]
- 29.Ceyhan GO, Bergmann F, Kadihasanoglu M, et al. Pancreatic neuropathy and neuropathic pain—a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136(177–186):e1. doi: 10.1053/j.gastro.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 30.Pundavela J, Roselli S, Faulkner S, et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol Oncol. 2015;9:1626–1635. doi: 10.1016/j.molonc.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jimenez-Andrade JM, Bloom AP, Stake JI, et al. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci. 2010;30:14649–14656. doi: 10.1523/JNEUROSCI.3300-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jimenez-Andrade JM, Ghilardi JR, Castañeda-Corral G, et al. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain. 2011;152:2564–2574. doi: 10.1016/j.pain.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantyh WG, Jimenez-Andrade JM, Stake JI, et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171:588–598. doi: 10.1016/j.neuroscience.2010.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayala GE, Wheeler TM, Shine HD, et al. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate. 2001;49:213–223. doi: 10.1002/pros.1137. [DOI] [PubMed] [Google Scholar]
- 35.Polli-Lopes AC, Zucoloto S, de Queirós Cunha F, et al. Myenteric denervation reduces the incidence of gastric tumors in rats. Cancer Lett. 2003;190:45–50. doi: 10.1016/S0304-3835(02)00584-0. [DOI] [PubMed] [Google Scholar]
- 36.Venkatesh HS, Johung TB, Caretti V, et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell. 2015;161:803–816. doi: 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamazaki S, Nakauchi H. Bone marrow Schwann cells induce hematopoietic stem cell hibernation. Int J Hematol. 2014;99:695–698. doi: 10.1007/s12185-014-1588-9. [DOI] [PubMed] [Google Scholar]
- 38.Pawlowski A, Weddell G. Induction of tumours in denervated skin. Nature. 1967;213:1234–1236. doi: 10.1038/2131234a0. [DOI] [Google Scholar]
- 39.Dubeykovskaya Z, Si Y, Chen X, et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat Commun. 2016;7:10517. doi: 10.1038/ncomms10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaminishi M, Shimizu N, Shimoyama S, et al. Denervation promotes the development of cancer-related lesions in the gastric remnant. J Clin Gastroenterol. 1997;25(Suppl 1):S129–S134. doi: 10.1097/00004836-199700001-00022. [DOI] [PubMed] [Google Scholar]
- 41.Lundegårdh G, Ekbom A, McLaughlin JK, Nyrén O. Gastric cancer risk after vagotomy. Gut. 1994;35:946–949. doi: 10.1136/gut.35.7.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colomar A, Robitaille R. Glial modulation of synaptic transmission at the neuromuscular junction. Glia. 2004;47:284–289. doi: 10.1002/glia.20086. [DOI] [PubMed] [Google Scholar]
- 43.Wekerle H, Schwab M, Linington C, Meyermann R. Antigen presentation in the peripheral nervous system: Schwann cells present endogenous myelin autoantigens to lymphocytes. Eur J Immunol. 1986;16:1551–1557. doi: 10.1002/eji.1830161214. [DOI] [PubMed] [Google Scholar]
- 44.Shan C, Wei J, Hou R, et al. Schwann cells promote EMT and the Schwann-like differentiation of salivary adenoid cystic carcinoma cells via the BDNF/TrkB axis. Oncol Rep. 2015;35:427–435. doi: 10.3892/or.2015.4366. [DOI] [PubMed] [Google Scholar]
- 45.Sroka IC, Chopra H, Das L, et al. Schwann cells increase prostate and pancreatic tumor cell invasion using laminin binding A6 integrin. J Cell Biochem. 2015 doi: 10.1002/jcb.25300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferguson TA, Muir D. MMP-2 and MMP-9 increase the neurite-promoting potential of Schwann cell basal laminae and are upregulated in degenerated nerve. Mol Cell Neurosci. 2000;16:157–167. doi: 10.1006/mcne.2000.0859. [DOI] [PubMed] [Google Scholar]
- 47.Abercrombie M. Contact inhibition and malignancy. Nature. 1979;281:259–262. doi: 10.1038/281259a0. [DOI] [PubMed] [Google Scholar]
- 48.Cravioto H. The role of Schwann cells in the development of human peripheral nerves. An electron microscopic study. J Ultrastruct Res. 1965;12:634–651. doi: 10.1016/S0022-5320(65)80053-3. [DOI] [PubMed] [Google Scholar]
- 49.Ben Q-W, Wang J-C, Liu J, et al. Positive expression of L1-CAM is associated with perineural invasion and poor outcome in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2010;17:2213–2221. doi: 10.1245/s10434-010-0955-x. [DOI] [PubMed] [Google Scholar]
- 50.Deborde S, Perret E, Gravotta D, et al. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demir IE, Tieftrunk E, Schorn S, et al. Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut. 2016 doi: 10.1136/gutjnl-2015-309784. [DOI] [PubMed] [Google Scholar]
- 52.Jessen KR, Mirsky R, Lloyd AC. Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol. 2015;7:a020487. doi: 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fricker FR, Bennett DL. The role of neuregulin-1 in the response to nerve injury. Future Neurol. 2011;6:809–822. doi: 10.2217/fnl.11.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guertin AD, Zhang DP, Mak KS, et al. Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci. 2005;25:3478–3487. doi: 10.1523/JNEUROSCI.3766-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 56.Imoto A, Mitsunaga S, Inagaki M, et al. Neural invasion induces cachexia via astrocytic activation of neural route in pancreatic cancer. Int J Cancer. 2012;131:2795–2807. doi: 10.1002/ijc.27594. [DOI] [PubMed] [Google Scholar]
- 57.Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Can Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 58.Demir IE, Schorn S, Schremmer-Danninger E, et al. Perineural mast cells are specifically enriched in pancreatic neuritis and neuropathic pain in pancreatic cancer and chronic pancreatitis. PLoS One. 2013;8:e60529. doi: 10.1371/journal.pone.0060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 60.McAllister F, Bailey JM, Alsina J, et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 2014;25:621–637. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cattin A-L, Burden JJ, Van Emmenis L, et al. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell. 2015 doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaggioli C, Hooper S, Hidalgo-Carcedo C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Publ Group. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 63.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 64.Vong S, Kalluri R. The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes Cancer. 2011;2:1139–1145. doi: 10.1177/1947601911423940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merika EE, Syrigos KN, Saif MW. Desmoplasia in pancreatic cancer. Can we fight it? Gastroenterol Res Pract. 2012;2012:781765–781810. doi: 10.1155/2012/781765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X, Wang Z, Ma Q, et al. Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer. Clin Cancer Res. 2014;20:4326–4338. doi: 10.1158/1078-0432.CCR-13-3426. [DOI] [PubMed] [Google Scholar]
- 67.Parrinello S, Napoli I, Ribeiro S, et al. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bakst RL, Wong RJ. Mechanisms of perineural invasion. J Neurol Surg B Skull Base. 2016;77:96–106. doi: 10.1055/s-0036-1571835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ceyhan GO, Schäfer K-H, Kerscher AG, et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann Surg. 2010;251:923–931. doi: 10.1097/SLA.0b013e3181d974d4. [DOI] [PubMed] [Google Scholar]
- 73.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Witkiewicz AK, McMillan EA, Balaji U, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. doi: 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beltran H, Prandi D, Mosquera JM, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22:298–305. doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eirew P, Steif A, Khattra J, et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature. 2015;518:422–426. doi: 10.1038/nature13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ciriello G, Gatza ML, Beck AH, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163:506–519. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Demir IE, Tieftrunk E, Schorn S, et al. Nerve growth factor and TrkA as novel therapeutic targets in cancer. Biochim Biophys Acta. 2016;1866:37–50. doi: 10.1016/j.bbcan.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Zhu Z, Friess H, diMola FF, et al. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol. 1999;17:2419–2428. doi: 10.1200/JCO.1999.17.8.2419. [DOI] [PubMed] [Google Scholar]
- 81.Dang C, Zhang Y, Ma Q, Shimahara Y. Expression of nerve growth factor receptors is correlated with progression and prognosis of human pancreatic cancer. J Gastroenterol Hepatol. 2006;21:850–858. doi: 10.1111/j.1440-1746.2006.04074.x. [DOI] [PubMed] [Google Scholar]
- 82.Ma J, Jiang Y, Jiang Y, et al. Expression of nerve growth factor and tyrosine kinase receptor A and correlation with perineural invasion in pancreatic cancer. J Gastroenterol Hepatol. 2008;23:1852–1859. doi: 10.1111/j.1440-1746.2008.05579.x. [DOI] [PubMed] [Google Scholar]
- 83.Sakamoto Y, Kitajima Y, Edakuni G, et al. Expression of Trk tyrosine kinase receptor is a biologic marker for cell proliferation and perineural invasion of human pancreatic ductal adenocarcinoma. Oncol Rep. 2001;8:477–484. doi: 10.3892/or.8.3.477. [DOI] [PubMed] [Google Scholar]
- 84.Chen-Tsai CP, Colome-Grimmer M, Wagner RF. Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75NGFR, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg. 2004;30:1009–1016. doi: 10.1111/j.1524-4725.2004.30306.x. [DOI] [PubMed] [Google Scholar]
- 85.Kolokythas A, Cox DP, Dekker N, Schmidt BL. Nerve growth factor and tyrosine kinase A receptor in oral squamous cell carcinoma: is there an association with perineural invasion? J Oral Maxillofac Surg. 2010;68:1290–1295. doi: 10.1016/j.joms.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 86.Kobayashi K, Ando M, Saito Y, et al. Nerve growth factor signals as possible pathogenic biomarkers for perineural invasion in adenoid cystic carcinoma. Otolaryngol Head Neck Surg. 2015;153:218–224. doi: 10.1177/0194599815584762. [DOI] [PubMed] [Google Scholar]
- 87.Ketterer K, Rao S, Friess H, et al. Reverse transcription-PCR analysis of laser-captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin Cancer Res. 2003;9:5127–5136. [PubMed] [Google Scholar]
- 88.Lewis Kelso R, Colome-Grimmer MI, Uchida T, et al. p75(NGFR) immunostaining for the detection of perineural invasion by cutaneous squamous cell carcinoma. Dermatol Surg. 2006;32:177–183. doi: 10.1111/j.1524-4725.2006.32032.x. [DOI] [PubMed] [Google Scholar]
- 89.Verbeke S, Meignan S, Lagadec C, et al. Overexpression of p75(NTR) increases survival of breast cancer cells through p21(waf1) Cell Signal. 2010;22:1864–1873. doi: 10.1016/j.cellsig.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 90.Johnston ALM, Lun X, Rahn JJ, et al. The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS Biol. 2007;5:e212. doi: 10.1371/journal.pbio.0050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jin H, Pan Y, Zhao L, et al. p75 neurotrophin receptor suppresses the proliferation of human gastric cancer cells. Neoplasia. 2007;9:471–478. doi: 10.1593/neo.07175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khwaja F, Tabassum A, Allen J, Djakiew D. The p75(NTR) tumor suppressor induces cell cycle arrest facilitating caspase mediated apoptosis in prostate tumor cells. Biochem Biophys Res Commun. 2006;341:1184–1192. doi: 10.1016/j.bbrc.2006.01.073. [DOI] [PubMed] [Google Scholar]
- 93.Tabassum A, Khwaja F, Djakiew D. The p75(NTR) tumor suppressor induces caspase-mediated apoptosis in bladder tumor cells. Int J Cancer. 2003;105:47–52. doi: 10.1002/ijc.11038. [DOI] [PubMed] [Google Scholar]
- 94.Taniuchi M, Clark HB, Schweitzer JB, Johnson EM. Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J Neurosci. 1988;8:664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bentley CA, Lee KF. p75 is important for axon growth and Schwann cell migration during development. J Neurosci. 2000;20:7706–7715. doi: 10.1523/JNEUROSCI.20-20-07706.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sclabas GM, Fujioka S, Schmidt C, et al. Overexpression of tropomysin-related kinase B in metastatic human pancreatic cancer cells. Clin Cancer Res. 2005;11:440–449. [PubMed] [Google Scholar]
- 97.Jia S, Wang W, Hu Z, et al. BDNF mediated TrkB activation contributes to the EMT progression and the poor prognosis in human salivary adenoid cystic carcinoma. Oral Oncol. 2015;51:64–70. doi: 10.1016/j.oraloncology.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 98.Kowalski PJ, Paulino AFG. Perineural invasion in adenoid cystic carcinoma: its causation/promotion by brain-derived neurotrophic factor. Hum Pathol. 2002;33:933–936. doi: 10.1053/hupa.2002.128249. [DOI] [PubMed] [Google Scholar]
- 99.Johnson MD, Stone B, Thibodeau BJ, et al. The significance of Trk receptors in pancreatic cancer. Tumour Biol. 2017;39:1010428317692256. doi: 10.1177/1010428317692256. [DOI] [PubMed] [Google Scholar]
- 100.Chopin V, Lagadec C, Toillon R-A, Le Bourhis X. Neurotrophin signaling in cancer stem cells. Cell Mol Life Sci. 2016;73:1859–1870. doi: 10.1007/s00018-016-2156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Publ Group. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 102.Murillo-Sauca O, Chung MK, Shin JH, et al. CD271 is a functional and targetable marker of tumor-initiating cells in head and neck squamous cell carcinoma. Oncotarget. 2014;5:6854–6866. doi: 10.18632/oncotarget.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Redmer T, Welte Y, Behrens D, et al. The nerve growth factor receptor CD271 is crucial to maintain tumorigenicity and stem-like properties of melanoma cells. PLoS One. 2014;9:e92596. doi: 10.1371/journal.pone.0092596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tomellini E, Touil Y, Lagadec C, et al. Nerve growth factor and proNGF simultaneously promote symmetric self-renewal, quiescence, and epithelial to mesenchymal transition to enlarge the breast cancer stem cell compartment. Stem Cells. 2015;33:342–353. doi: 10.1002/stem.1849. [DOI] [PubMed] [Google Scholar]
- 105.Yin B, Ma ZY, Zhou ZW, et al. The TrkB+ cancer stem cells contribute to post-chemotherapy recurrence of triple-negative breast cancers in an orthotopic mouse model. Oncogene. 2015;34:761–770. doi: 10.1038/onc.2014.8. [DOI] [PubMed] [Google Scholar]
- 106.Gil Z, Cavel O, Kelly K, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102:107–118. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He S, Chen C-H, Chernichenko N, et al. GFRα1 released by nerves enhances cancer cell perineural invasion through GDNF-RET signaling. Proc Natl Acad Sci. 2014 doi: 10.1073/pnas.1402944111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iwahashi N, Nagasaka T, Tezel G, et al. Expression of glial cell line-derived neurotrophic factor correlates with perineural invasion of bile duct carcinoma. Cancer. 2002;94:167–174. doi: 10.1002/cncr.10169. [DOI] [PubMed] [Google Scholar]
- 109.Liu H, Li X, Xu Q, et al. Role of glial cell line-derived neurotrophic factor in perineural invasion of pancreatic cancer. Biochim Biophys Acta. 2012;1826:112–120. doi: 10.1016/j.bbcan.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 110.Paratcha G, Ledda F, Ibáñez CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113:867–879. doi: 10.1016/S0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- 111.Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9:1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 112.Gao L, Bo H, Wang Y, et al. Neurotrophic factor artemin promotes invasiveness and neurotrophic function of pancreatic adenocarcinoma in vivo and in vitro. Pancreas. 2015;44:134–143. doi: 10.1097/MPA.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang K, Demir IE, D’Haese JG, et al. The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis. 2014;35:103–113. doi: 10.1093/carcin/bgt312. [DOI] [PubMed] [Google Scholar]
- 114.Ceyhan GO, Bergmann F, Kadihasanoglu M, et al. The neurotrophic factor artemin influences the extent of neural damage and growth in chronic pancreatitis. Gut. 2007;56:534–544. doi: 10.1136/gut.2006.105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marchesi F, Locatelli M, Solinas G, et al. Role of CX3CR1/CX3CL1 axis in primary and secondary involvement of the nervous system by cancer. J Neuroimmunol. 2010;224:39–44. doi: 10.1016/j.jneuroim.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 116.Mehlen P, Delloye-Bourgeois C, Chédotal A. Novel roles for slits and netrins: axon guidance cues as anticancer targets? Nat Publ Group. 2011;11:188–197. doi: 10.1038/nrc3005. [DOI] [PubMed] [Google Scholar]
- 117.Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ. Targeting the CCL2–CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7:28697–28710. doi: 10.18632/oncotarget.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 119.Kameda K, Shimada H, Ishikawa T, et al. Expression of highly polysialylated neural cell adhesion molecule in pancreatic cancer neural invasive lesion. Cancer Lett. 1999;137:201–207. doi: 10.1016/S0304-3835(98)00359-0. [DOI] [PubMed] [Google Scholar]
- 120.McLaughlin RB, Montone KT, Wall SJ, et al. Nerve cell adhesion molecule expression in squamous cell carcinoma of the head and neck: a predictor of propensity toward perineural spread. Laryngoscope. 1999;109:821–826. doi: 10.1097/00005537-199905000-00026. [DOI] [PubMed] [Google Scholar]
- 121.Seki H, Tanaka J, Sato Y, et al. Neural cell adhesion molecule (NCAM) and perineural invasion in bile duct cancer. J Surg Oncol. 1993;53:78–83. doi: 10.1002/jso.2930530205. [DOI] [PubMed] [Google Scholar]
- 122.Shang J, Sheng L, Wang K, et al. Expression of neural cell adhesion molecule in salivary adenoid cystic carcinoma and its correlation with perineural invasion. Oncol Rep. 2007;18:1413–1416. [PubMed] [Google Scholar]
- 123.Vural E, Hutcheson J, Korourian S, et al. Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2000;122:717–720. doi: 10.1016/S0194-5998(00)70203-8. [DOI] [PubMed] [Google Scholar]
- 124.Li R, Wheeler T, Dai H, Ayala G. Neural cell adhesion molecule is upregulated in nerves with prostate cancer invasion. Hum Pathol. 2003 doi: 10.1016/s0046-8177(03)00084-4. [DOI] [PubMed] [Google Scholar]
- 125.Binmadi NO, Yang YH, Zhou H, et al. Plexin-B1 and semaphorin 4D cooperate to promote perineural invasion in a RhoA/ROK-dependent manner. AJPA. 2012;180:1232–1242. doi: 10.1016/j.ajpath.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Ding Y, He D, Florentin D, et al. Semaphorin 4F as a critical regulator of neuroepithelial interactions and a biomarker of aggressive prostate cancer. Clin Cancer Res. 2013;19:6101–6111. doi: 10.1158/1078-0432.CCR-12-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- 128.Ma L, Tessier-Lavigne M. Dual branch-promoting and branch-repelling actions of Slit/Robo signaling on peripheral and central branches of developing sensory axons. J Neurosci. 2007;27:6843–6851. doi: 10.1523/JNEUROSCI.1479-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu W, Wong K, Chen J, et al. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kinrade EF, Brates T, Tear G, Hidalgo A. Roundabout signalling, cell contact and trophic support confine longitudinal glia and axons in the Drosophila CNS. Development. 2001;128:207–216. doi: 10.1242/dev.128.2.207. [DOI] [PubMed] [Google Scholar]
- 131.Wang Y, Teng H-L, Huang Z-H. Repulsive migration of Schwann cells induced by Slit-2 through Ca2+-dependent RhoA-myosin signaling. Glia. 2013;61:710–723. doi: 10.1002/glia.22464. [DOI] [PubMed] [Google Scholar]
- 132.Yiin J-J, Hu B, Jarzynka MJ, et al. Slit2 inhibits glioma cell invasion in the brain by suppression of Cdc42 activity. Neuro Oncol. 2009;11:779–789. doi: 10.1215/15228517-2009-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Göhrig A, Detjen KM, Hilfenhaus G, et al. Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer. Can Res. 2014;74:1529–1540. doi: 10.1158/0008-5472.CAN-13-1012. [DOI] [PubMed] [Google Scholar]
- 134.Secq V, Leca J, Bressy C, et al. Stromal SLIT2 impacts on pancreatic cancer-associated neural remodeling. Cell Death Dis. 2015;6:e1592. doi: 10.1038/cddis.2014.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shao Z, Zhu F, Song K, et al. EphA2/ephrinA1 mRNA expression and protein production in adenoid cystic carcinoma of salivary gland. J Oral Maxillofac Surg. 2013;71:869–878. doi: 10.1016/j.joms.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 136.Kinch MS, Moore M-B, Harpole DH. Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res. 2003;9:613–618. [PubMed] [Google Scholar]
- 137.Miyazaki T, Kato H, Fukuchi M, et al. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer. 2003;103:657–663. doi: 10.1002/ijc.10860. [DOI] [PubMed] [Google Scholar]
- 138.Afshari FT, Kwok JC, Fawcett JW. Astrocyte-produced ephrins inhibit Schwann cell migration via VAV2 signaling. J Neurosci. 2010;30:4246–4255. doi: 10.1523/JNEUROSCI.3351-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.He S, He S, Chen C-H, et al. The chemokine (CCL2–CCR2) signaling axis mediates perineural invasion. Mol Cancer Res. 2015;13:380–390. doi: 10.1158/1541-7786.MCR-14-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 141.Qian B-Z, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marchesi F, Piemonti L, Fedele G, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Can Res. 2008;68:9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- 143.Lv C-Y, Zhou T, Chen W, et al. Preliminary study correlating CX3CL1/CX3CR1 expression with gastric carcinoma and gastric carcinoma perineural invasion. World J Gastroenterol. 2014;20:4428–4432. doi: 10.3748/wjg.v20.i15.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Doumas S, Paterson JC, Norris PM, et al. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) in squamous cell carcinoma of the tongue: markers of nerve invasion? Oral Maxillofac Surg. 2015;19:61–64. doi: 10.1007/s10006-014-0455-4. [DOI] [PubMed] [Google Scholar]
- 145.Swanson BJ, McDermott KM, Singh PK, et al. MUC1 Is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Can Res. 2007;67:10222–10229. doi: 10.1158/0008-5472.CAN-06-2483. [DOI] [PubMed] [Google Scholar]
- 146.Mukhopadhyay G, Doherty P, Walsh FS, et al. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 147.Nakamori S, Ota DM, Cleary KR, et al. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology. 1994;106:353–361. doi: 10.1016/0016-5085(94)90592-4. [DOI] [PubMed] [Google Scholar]
- 148.Lüttges J, Feyerabend B, Buchelt T, et al. The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 2002;26:466–471. doi: 10.1097/00000478-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 149.Kashiwagi H, Kijima H, Dowaki S, et al. DF3 expression in human gallbladder carcinoma: significance for lymphatic invasion. Int J Oncol. 2000;16:455–459. doi: 10.3892/ijo.16.3.455. [DOI] [PubMed] [Google Scholar]
- 150.Nath S, Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014;20:332–342. doi: 10.1016/j.molmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


