Abstract
Despite the dramatic improvement in the overall survival for patients diagnosed with Wilms’ tumor (WT), the outcomes for those that experience relapse have remained disappointing. We describe the outcomes of 253 patients with relapsed WT who received high-dose chemotherapy (HDT) followed by autologous hematopoietic stem cell transplant (HCT) between 1990 and 2013, and reported to the Center for International Blood and Marrow Transplantation Research (CIBMTR). The 5-year estimates for event free survival (EFS) and overall survival (OS) were 36% (95% CI; 29 – 43%) and 45% (95% CI; 38 – 51%) respectively. Relapse of primary disease was the cause of death in 81% of the population. EFS, OS, relapse and transplant-related mortality (TRM) showed no significant differences when broken down by disease status at transplant, time from diagnosis to transplant, year of transplant or conditioning regimen. Our data suggest that HDT followed by autologous HCT for relapsed WT is well tolerated and outcomes are similar to those reported in the literature. Since attempts to conduct a randomized trial comparing maintenance chemotherapy with consolidation versus high-dose chemotherapy followed by stem cell transplant have failed, one should balance the potential benefits with the yet unknown long-term risks. Since disease recurrence continues to be the most common cause of death, future research should focus on the development of consolidation therapies for those patients achieving complete response to therapy.
INTRODUCTION
Each year approximately 500 children in the United States are diagnosed with Wilms tumor (WT).1 The outcomes for children with WT have improved dramatically with the advent of multi-modal therapy, and survival rates are currently approaching 90%.2 With improved chemotherapy the overall relapse rate for children with WT has decreased to less than 15%.3–5 However, long term survival for patients who relapse and are re-treated with chemotherapy and surgery alone have been disappointing, with approximately 50% of patients dying of disease by 4 years.6–9
The identification of prognostic factors associated with risk for treatment failure after relapse has allowed for risk-adapted treatment strategies. In the most recent National Wilms’ Tumor Study - 5 (NWTS-5) children who relapsed after minimal initial therapy with vincristine and actinomycin have a greater than 80% 4-year overall survival (OS) rate.10 However, the 4-year OS rate was less than 50% for those who relapsed after receiving these two drugs plus doxorubicin and radiation.11
Due to the poor long-term outcomes of patients with relapsed WT, many investigators have used high dose therapy (HDT) followed by autologous HCT in a non-randomized fashion and reports usually consist of small institutional series or review articles. Since the first European Bone Marrow Transplant (EBMT) report, the number of relapsed WT patients transplanted in the EBMT registry has continued to grow.12 When compared with historical controls of patients treated with conventional chemotherapy, these trials obtained better outcomes, with 3- or 4-year OS rates ranging from 60 to 73%.13–17
Taken together, these studies suggest a potential survival advantage of HDT with autologous HCT over conventional chemotherapy in patients with relapsed WT and a good response to re-induction therapy, although increased toxicity is a major concern. However, this small advantage may be a result of the small number of patients enrolled in each study and a possible selection bias.
In order to investigate the role of HDT followed by autologous HCT in pediatric patients with relapsed WT, we sought to utilize the Center for International Blood and Marrow Transplant Research (CIBMTR) database to describe outcomes of patients transplanted from 1990 to 2013 and reported to CIBMTR.
METHODS
Patients
Data were obtained from the CIBMTR, a working group of more than 500 transplant centers worldwide that provide patient, disease, and transplant characteristics including outcomes for consecutive transplantations to a statistical center coordinated through the National Marrow Donor Program (NMDP). All HCTs have basic information collected; selected transplants have more detailed information collected. For this, only information from the basic level forms was available. Patients or guardians provided written informed consent for data submission and research participation in accordance with the Declaration of Helsinki. The Institutional Review Boards of the Medical College of Wisconsin and NMDP approved this study.
Eligibility Criteria
Patients with recurrent WT treated with autologous HCT between 1990–2013 who were younger than 21 years old at the date of initial diagnosis were eligible for inclusion. Patients were excluded if they were untreated prior to transplant, or were treated at a center with low follow-up (the percentage of patients who were not dead nor lost to follow-up was less than 20% at 5-years post-transplant).
Endpoints
The primary objective of this study was to describe event-free survival (EFS) with the secondary outcomes being OS, post-HCT relapse, and transplant-related mortality (TRM). EFS was defined as survival without relapse or disease progression. Relapse was defined as the recurrence of WT. TRM was defined as death in the first 28 days’ post-transplant or death in continuous remission. OS was defined as survival from all causes.
Statistical analysis
The probabilities of EFS and OS were calculated using the Kaplan Meier estimator.18–20 Ninety-five percent confidence intervals were calculated using log transformation. EFS was defined as the time interval between HCT and relapse, disease progression, or death whichever occurs first. Patients surviving in remission were censored at the date of last follow-up. OS was defined as the time interval between HCT and death from any cause. Patients surviving were censored at the date of last follow-up. The cumulative incidences of relapse and TRM were estimated using the cumulative incidence function with TRM as the competing risk for relapse and relapse as the competing risk for TRM.
In multivariate analysis, the Cox proportional hazards model was used to identify significant risk factors. Variables tested in the Cox proportional hazards regression model included disease status at transplantation (first pre-transplant relapse versus second or greater pre-transplant relapse or continuous remission versus persistent/progressive disease versus missing), time from diagnosis to transplantation (< 12 months versus 12–18 months versus > 18 months), graft type (bone marrow [BM] versus peripheral blood stem cells [PBSC]), year of transplant (1990–1994 versus 1995–1999 versus 2000–2004 versus 2005–2013) and conditioning regimen (alkylating agents [cyclophosphamide+melphalan, busulfan+cyclophosphamide, busulfan+melphalan, melphalan, cyclophosphamide+thiotepa, cyclophosphamide] versus platinum agents [carboplatin+melphalan, cyclophosphamide+carboplatin, carboplatin]). The assumption of proportional hazards for each factor was tested using time-dependent covariates. When the test indicated differential effects over time (non-proportional hazards), models were constructed breaking the post-transplant time course into two periods, using the maximized partial likelihood method to find the most appropriate breakpoint. The proportionality assumptions were further tested. A stepwise model selection approach was used to identify all significant risk factors. Factors that were significant at a 5% level were kept in the final model. Two-way interactions were tested for all variables that remained in the final model. Analysis was performed using SAS version 9.33 (Cary, NC).
Results
Patient, disease and transplant characteristics of 253 children with WT by transplant period and disease status at transplantation are shown in Tables 1 and 2. The median patient age at HCT was 6 years (range 1–23), with 135 (53%) patients being female. According to the available data, 62% (152/253) of the patients were transplanted while in remission and 25% (62/253) of the patients were transplanted with disease present. Among patients transplanted in remission, 67% (106/158) of the patients were in CR2 or greater and 20% were in PR at time of transplant. The median time from diagnosis to transplant was 18 months (range 5–208). Fifty-one percent of the patients were transplanted 18 months or longer after their diagnosis, 73 (29%) were transplanted 12–18 months after diagnosis and 51 (20%) were transplanted less than 12 months after diagnosis.
Table 1.
Characteristics of patients who underwent autologous transplant for Wilms Tumor from 1990–2013, as registered to the CIBMTR, by Year of transplant.
| Characteristics of patients: | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2013 |
|---|---|---|---|---|
| Number of patients (total: 253) | 33 | 67 | 52 | 101 |
| Number of centers | 15 | 38 | 34 | 53 |
| Region of center | ||||
| US | 32 (97) | 53 (79) | 36 (69) | 80 (79) |
| Canada | 0 | 6 (9) | 3 (6) | 9 (9) |
| International | 1 (3) | 8 (12) | 13 (25) | 12 (12) |
| Patient-related | ||||
| Age at diagnosis, median(range), years | 5 (2–12) | 4 (<1–16) | 5 (<1–21) | 4 (<1–19) |
| Age at diagnosis, years | ||||
| 0–4 | 19 (58) | 40 (60) | 27 (52) | 61 (60) |
| 5–9 | 13 (39) | 20 (30) | 19 (37) | 32 (32) |
| 10+ | 1 (3) | 7 (10) | 6 (12) | 8 (8) |
| Age at transplant, median(range), years | 6 (3–14) | 6 (1–18) | 7 (2–22) | 7 (2–23) |
| Age at transplant, years | ||||
| 0–4 | 9 (27) | 23 (34) | 11 (21) | 26 (26) |
| 5–9 | 20 (61) | 29 (43) | 29 (56) | 58 (57) |
| 10+ | 4 (12) | 15 (22) | 12 (23) | 17 (17) |
| Gender | ||||
| Male | 14 (42) | 34 (51) | 25 (48) | 43 (43) |
| Female | 19 (58) | 32 (48) | 26 (50) | 58 (57) |
| Missing | 0 | 1 (1) | 1 (2) | 0 |
| Remission status | ||||
| In remission | 10 (30) | 34 (51) | 32 (62) | 82 (81) |
| Disease present | 15 (45) | 20 (30) | 13 (25) | 14 (14) |
| Missing | 8 (24) | 13 (19) | 7 (13) | 5 (5) |
| Disease status | ||||
| CR2 | 5 (15) | 5 (7) | 22 (42) | 53 (52) |
| CR3+ | 0 | 1 (1) | 6 (12) | 14 (14) |
| CR unknown * | 1 (3) | 16 (24) | 3 (6) | 0 |
| REL1 | 8 (24) | 9 (13) | 4 (8) | 7 (7) |
| REL2 | 1 (3) | 0 | 3 (6) | 1 (<1) |
| REL3 | 0 | 2 (3) | 0 | 1 (<1) |
| REL unknown | 0 | 1 (1) | 6 (12) | 1 (<1) |
| Progression | 1 (3) | 4 (6) | 0 | 4 (4) |
| Stable disease | 5 (15) | 4 (6) | 0 | 0 |
| Partial response * | 4 (12) | 12 (18) | 1 (2) | 15 (15) |
| Missing | 8 (24) | 13 (19) | 7 (13) | 5 (5) |
| Transplant-related | ||||
| Time from diagnosis to transplant, median(range), months | 13 (5–120) | 20 (6–108) | 18 (5–132) | 19 (10–208) |
| Time from diagnosis to transplant, months | ||||
| < 12 | 15 (45) | 15 (22) | 12 (23) | 9 (9) |
| 12–18 | 6 (18) | 17 (25) | 13 (25) | 37 (37) |
| ≥ 18 | 12 (36) | 35 (52) | 27 (52) | 55 (54) |
| Graft type | ||||
| BM | 23 (70) | 19 (28) | 5 (10) | 7 (7) |
| PBSC | 10 (30) | 46 (69) | 45 (87) | 94 (93) |
| Missing | 0 | 2 (3) | 2 (4) | 0 |
| Regimen groups ** | ||||
| Alkylating agents | 20 (61) | 52 (78) | 25 (48) | 45 (45) |
| Platinum agents | 7 (21) | 4 (6) | 13 (25) | 51 (50) |
| Missing | 6 (18) | 11 (16) | 14 (27) | 5 (5) |
| Follow-up of survivors, median (range), months | 109 (17–241) | 55 (1–198) | 97 (3–147) | 26 (3–94) |
Abbreviations: CR = Complete remission, REL = Relapse, BM = Bone marrow, PBSC = Peripheral Blood Stem Cells, CY = Cyclophosphamide, LPAM = Melphalan, BU = Busulfan, THIO = Thiotepa, CARB = Carboplatinum.
Determined patients not in CR1 or PR1 based on duration of time from diagnosis to transplant.
Alkylating agents are defined as CY+LPAM, BU+CY, BU+LPAM, LPAM, CY+THIO, CY;
Platinum agents are defined as CARB+LPAM, CY+CARB, CARB.
Table 2.
Characteristics of patients who underwent autologous transplant for Wilms Tumor from 1990–2013, as registered to the CIBMTR, by Disease status at transplantation.
| Variable | In remission | Disease present | Missing |
|---|---|---|---|
| Number of patients | 158 | 62 | 33 |
| Region of center | |||
| US | 129 (82) | 49 (79) | 23 (70) |
| Canada | 11 (7) | 6 (10) | 1 (3) |
| International | 18 (11) | 7 (11) | 9 (27) |
| Age at diagnosis, years, median (range) | 5 (<1–21) | 4 (<1–20) | 4 (<1–19) |
| Age at diagnosis | |||
| 0–4 | 90 (57) | 39 (63) | 18 (55) |
| 5–9 | 55 (35) | 17 (27) | 12 (36) |
| 10+ | 13 (8) | 6 (10) | 3 (9) |
| Age at transplant, years, median (range) | 7 (2–23) | 6 (2–21) | 7 (1–21) |
| Age at transplant | |||
| 0–4 | 37 (23) | 24 (39) | 8 (24) |
| 5–9 | 91 (58) | 25 (40) | 20 (61) |
| 10+ | 30 (19) | 13 (21) | 5 (15) |
| Gender | |||
| Male | 66 (42) | 34 (55) | 16 (48) |
| Female | 92 (58) | 27 (44) | 16 (48) |
| Missing | 0 | 1 (2) | 1 (3) |
| Disease status | |||
| CR2 | 85 (54) | 0 | 0 |
| CR3+ | 21 (13) | 0 | 0 |
| CR unknown * | 20 (13) | 0 | 0 |
| REL1 | 0 | 28 (45) | 0 |
| REL2 | 0 | 5 (8) | 0 |
| REL3 | 0 | 3 (5) | 0 |
| REL unknown | 0 | 8 (13) | 0 |
| Progression | 0 | 9 (15) | 0 |
| Stable disease | 0 | 9 (15) | 0 |
| Partial response * | 32 (20) | 0 | 0 |
| Missing | 0 | 0 | 33 |
| Time from diagnosis to transplant, months (continuous) | 18 (5–129) | 17 (5–208) | 17 (6–113) |
| Time from diagnosis to transplant, months (categorical) | |||
| < 12 | 22 (14) | 17 (27) | 12 (36) |
| 12–18 | 51 (32) | 17 (27) | 5 (15) |
| ≥ 18 | 85 (54) | 28 (45) | 16 (48) |
| Graft type | |||
| BM | 23 (15) | 21 (34) | 10 (30) |
| PBSC | 133 (84) | 40 (65) | 22 (67) |
| Missing | 2 (1) | 1 (2) | 1 (3) |
| Regimen groups ** | |||
| Alkylating agents | 89 (56) | 39 (63) | 14 (42) |
| Platinum agents | 55 (35) | 15 (24) | 5 (15) |
| Missing | 14 (9) | 8 (13) | 14 (42) |
| Years of transplant | |||
| 1990–1994 | 10 (6) | 15 (24) | 8 (24) |
| 1995–1999 | 34 (22) | 20 (32) | 13 (39) |
| 2000–2004 | 32 (20) | 13 (21) | 7 (21) |
| 2005–2013 | 82 (52) | 14 (23) | 5 (15) |
| Follow-up of survivors, months, median (range) | 49 (1–241) | 73 (1–239) | 33 (1–152) |
Abbreviations: CR = Complete remission, REL = Relapse, BM = Bone marrow, PBSC = Peripheral Blood Stem Cells, CY = Cyclophosphamide, LPAM = Melphalan, BU = Busulfan, THIO = Thiotepa, CARB = Carboplatinum.
Determined patients not in CR1 or PR1 based on duration of time from diagnosis to transplant.
Alkylating agents are defined as CY+LPAM, BU+CY, BU+LPAM, LPAM, CY+THIO, CY;
Platinum agents are defined as CARB+LPAM, CY+CARB, CARB.
The most common source of autologous stem cells for patients enrolled on this study was PBSC (n=195, 77%). The number of patients transplanted in each study period was: 33 (13%) between 1990–1994, 67 (26%) between 1995–1999, 52 (21%) between 2000–2004 and 101 (40%) between 2005–2013. HCT conditioning included alkylating agents in 142 (56%) of the patients, 75 patients (30%) received platinum agents and 36 (14%) were missing conditioning information. The median follow-up of survivors is 57 months (range 1–241). Fourteen (6%) patients received a second transplant. Nine (64%) of second HCT happened within 2 months of the first transplant and were likely scheduled tandem HCTs, all happening after 2000.
The median follow-up of survivors was greater than 92 months (range 1–241) for patients treated between 1990 and 2004, and 26 months (range 3–94) for those treated between 2005 and 2013.
Outcomes
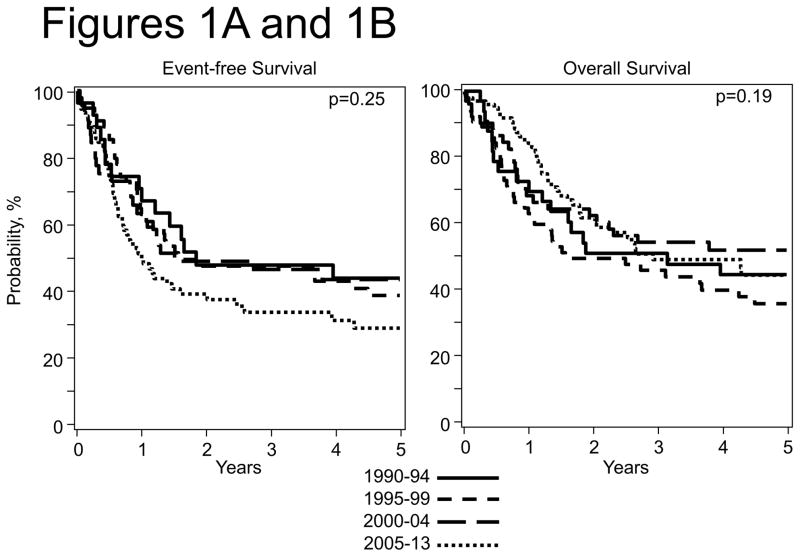
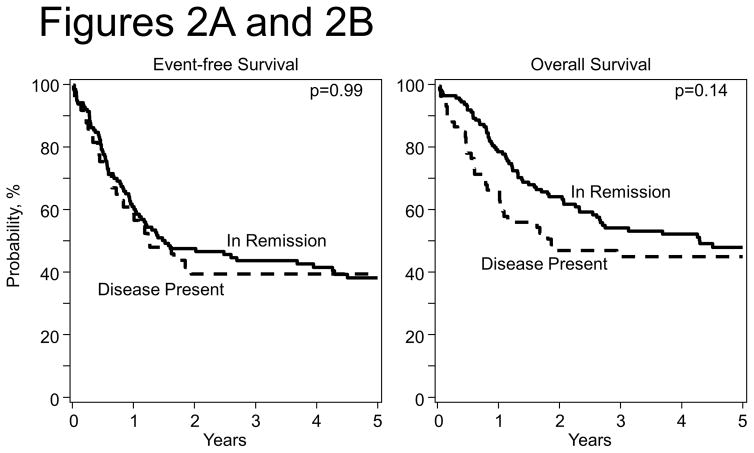
The 5-year EFS and OS rate for all patients were 36% (95% CI; 29 – 43%) and 45% (95% CI; 38 – 51%). Figures 1A and 1B show the probabilities of EFS and OS by transplant period and Figures 2A and 2B show the probabilities of EFS and OS by disease status at transplantation.
Figure 1.
Figure 1A: Event Free Survival after HCT by Year of transplant
Figure 1B: Overall Survival after HCT by Year of transplant
Figure 2.
Figure 2A: Event Free Survival after HCT by Disease status at transplantation
Figure 2B: Overall Survival after HCT by Disease status at transplantation
Relapse of primary disease was the cause of death in 81% of the patients (104 out of 129 evaluable deaths). There were no significant differences in 1, 2, 3 and 5-year EFS OS, relapse, and TRM estimates when outcomes were analyzed by univariate analysis according to disease status at transplant, time from diagnosis to transplant, graft source, conditioning regimen and year of transplant (Table 3). Overall the 1 year TRM incidence was low (4–9%) and did not vary significantly over the length of the study. An analysis of patients who received autologous HCT when not in remission compared with those who were in remission did not show significant differences.
Table 3.
Univariate analysis, by Year of transplant
| Outcomes | 1990–1994 (N = 34) | 1995–1999 (N = 68) | 2000–2004 (N = 54) | 2005–2013 (N = 102) | P-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N Eval | Prob (95% CI) | N Eval | Prob (95% CI) | N Eval | Prob (95% CI) | N Eval | Prob (95% CI) | ||
| Event-Free Survival | 28 | 56 | 48 | 91 | |||||
| 1-year | 68 (50–84)% | 64 (50–76)% | 63 (48–77)% | 47 (36–58)% | 0.11 | ||||
| 2-year | 46 (28–64)% | 48 (34–61)% | 49 (34–63)% | 38 (27–49)% | 0.59 | ||||
| 3-year | 46 (28–64)% | 48 (34–61)% | 49 (34–63)% | 34 (23–45)% | 0.31 | ||||
| 5-year | 42 (24–60)% | 39 (26–53)% | 46 (31–61)% | 29 (18–41)% | 0.34 | ||||
| Overall Survival | 34 | 68 | 54 | 102 | |||||
| 1-year | 74 (58–87)% | 62 (50–74)% | 68 (55–80)% | 84 (76–91)% | 0.007 | ||||
| 2-year | 49 (33–66)% | 49 (36–61)% | 62 (48–74)% | 62 (51–72)% | 0.27 | ||||
| 3-year | 49 (33–66)% | 45 (33–58)% | 54 (41–67)% | 50 (38–61)% | 0.82 | ||||
| 5-year | 43 (27–60)% | 35 (23–48)% | 52 (38–65)% | 45 (33–57)% | 0.35 | ||||
| Relapse | 28 | 56 | 48 | 91 | |||||
| 1-year | 29 (13–46)% | 34 (22–47)% | 28 (15–42)% | 47 (35–57)% | 0.15 | ||||
| 2-year | 47 (27–65)% | 50 (36–63)% | 38 (23–52)% | 56 (43–67)% | 0.30 | ||||
| 3-year | 47 (27–65)% | 50 (36–63)% | 38 (23–52)% | 60 (47–70)% | 0.15 | ||||
| 5-year | 51 (31–68)% | 57 (42–70)% | 41 (25–55)% | 64 (51–75)% | 0.12 | ||||
| TRM | 28 | 56 | 48 | 91 | |||||
| 1-year | 4 (0–16)% | 2 (0–9)% | 9 (3–20)% | 7 (3–13)% | 0.33 | ||||
| 2-year | 7 (1–21)% | 2 (0–9)% | 14 (5–26)% | 7 (3–13)% | 0.12 | ||||
| 3-year | 7 (1–21)% | 2 (0–9)% | 14 (5–26)% | 7 (3–13)% | 0.12 | ||||
| 5-year | 7 (1–21)% | 4 (1–13)% | 14 (5–26)% | 7 (3–13)% | 0.48 | ||||
Conditioning regimen was the only factor found to be statistically significant by multivariate analysis (Table 4). The effect of conditioning regimen on overall mortality changed over time. The use of platinum agents was associated with lower mortality in the first 10 months after HCT but not thereafter. This time-dependent effect explains why in univariate analysis there were no differences in survival by conditioning regimen.
Table 4.
Multivariate analysis results for Overall mortality
| Variable | Hazard Ratio (95% Confidence Interval) | p-value |
|---|---|---|
| Alkylating agent containing regimen | 1 | 0.0008* |
| Platinum agent containing regimens ≤ 10 months after transplant | 0.26 (0.11 – 0.61) | .002 |
| Platinum agent containing regimens > 10 months transplant | 1.42 (0.87 – 2.33) | .16 |
overall p-value
DISCUSSION
Relapsed WT is rare, but outcomes for these patients remain suboptimal. HDT followed by autologous HCT has been used to salvage patients with WT who relapse after chemotherapy. This retrospective study using the CIBMTR database includes one of the largest numbers of patients with recurrent WT treated with high dose chemotherapy followed by autologous HCT reported to date.
The number of HCT per year has not changed significantly since 1995. However, over time, there was a clear trend in change of practice from BM as a stem cell source to PBSC. During the first analysis period, 1990–94, BM was the most common source of stem cells (70%) and it was almost completely replaced by PBSC (93%) in the most recent period, 2005 to 2013.
The conditioning regimens were varied and included alkylating agents for 142 (56%) patients. Platinum-containing regimens became more common during the most recent period (2005–2013). The large number of different conditioning regimens used in the treatment of these patients, highlights one of the biggest challenges for the development of a common approach for these patients. Although our data identified that the use of platinum agents was associated with lower mortality in the first 10 months’ post-transplant, this effect was no longer seen after that.
In a recent manuscript, Ha et al, reviewed the data from 19 relevant publications in an attempt to determine the role of HDT followed by HCT in the treatment of relapsed WT. The authors used pooled data of 1226 patients to compare the overall 3-year EFS and OS between patients treated with HDT followed by HCT with dose treated without HDT (conventional chemotherapy).21 The median 3-year EFS and OS for patients who did not received HDT was 47.6% (1.76–79.83%) and 47.5% (3.7–100%), respectively, compared to 47% (3.7–100%) and 47.5% (12.5–100%) for those treated with HDT followed by HCT.21 The authors concluded that the evidence suggest the value of HDT followed by HCT, particularly for those in the highest risk relapse group based on initial stage, histology, initial treatment received and prior relapse.
A recent international meta-analysis conducted by Presson et al, to provide additional insights in the role of HDT followed by HCT revealed that the patients most likely to benefit from this approach were those initially treated with four or more chemotherapeutic agents and those with multiple relapses or progression on salvage therapy. 22
The biggest limitation of our study is the fact that this study is a retrospective registry-based analysis and that the available data originates from basic level forms, and therefore, did not include the following diagnosis variables: histology, site of metastases, stage of disease, genetic syndrome, tumor spillage, and radiation. The following pre-transplant variables were also not available for the majority of the patients: site, time from diagnosis to relapse. Differently than the 2 studies mentioned above, this lack of detailed data did not allow us to investigate if there is a risk-group of patients that could benefit from the use HDT followed by autologous HCT. Consequently our observation of an absence in EFS and OS by disease status must be interpreted with caution as several pertinent disease and treatment characteristics including histopathological features at diagnosis were not studied.
Unlike than other previous reports,3,5,7,10–16 our results were not influenced by time from diagnosis to transplant, graft source or conditioning regimen. The survival outcomes found in this study are no different than those obtained in previous reports using systemic chemotherapy alone or HDT followed by HCT21–22. Overall, the use of HDT followed by autologous HCT appears to be useful for the full spectrum of disease states. However, the retrospective nature, heterogeneous nature of relapsed Wilms tumor relatively small patient numbers limit this study. The lack of a complete supplemental data set limits the strength of the conclusions and is a reminder of the importance of data collection and submission, particularly in rare diseases.
Our data, including considering its limitations together with those from previous studies suggest that HDT followed by autologous HCT for relapsed WT is well tolerated. The EFS and OS for our study was not different than those reported by Ha et al21 and Presson et al22 for patients with relapsed WT treated with HCT or standard chemotherapy. Since attempts to conduct a randomized trial comparing maintenance chemotherapy with consolidation with high-dose chemotherapy followed by stem cell transplant have failed, one should balance the potential benefits of such approach with the yet unknown long term risks associated with this approach when deciding which patient should be submitted HDT followed by autologous HCT. Since disease recurrence continues to be the most common cause of death for patients who received standard chemotherapy or HDT followed by autologous HCT, future research should focus on the development of consolidation therapies for those patients achieving complete response to therapy.
Acknowledgments
CIBMTR Support List
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics, Inc.; Be the Match Foundation; *Bluebird Bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cellular Dynamics International, Inc.; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; Perkin Elmer, Inc.; Pfizer, Inc; *Sanofi US; *Seattle Genetics; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Fernandez C, Geller JI, Ehrlich PF, Hill DA, Kalapurakal JA, Grundy PE, et al. Renal Tumors. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 6. Lippincott Williams and Wilkins; Philadelphia: 2011. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; Bethesda, MD: [Accessed on December 06, 2015]. based on November 2014 SEER data submission, posted to the SEER web site, April 2015. Available at: http://seer.cancer.gov/csr/1975_2012/results_merged/sect_29_childhood_cancer_iccc.pdf. [Google Scholar]
- 3.Grundy P, Breslow N, Green DM, Sharples K, Evans A, D’Angio GJ. Prognostic factors for children with recurrent Wilms’ tumor: results from the Second and Third National Wilms’ Tumor Study. J Clin Oncol. 1989;7:638–647. doi: 10.1200/JCO.1989.7.5.638. [DOI] [PubMed] [Google Scholar]
- 4.Pinkerton CR, Groot-Loonen JJ, Morris-Jones PH, Pritchard J. Response rates in relapsed Wilms’ tumor. A need for new effective agents. Cancer. 1991;67:567–571. doi: 10.1002/1097-0142(19910201)67:3<567::aid-cncr2820670307>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Pein F, Rey A, de Kraker J. Multivariate analysis of adverse prognostic factors (APF) in children with recurrent (Rec) Wilms’ tumor (WT) after initial treatment according to SIOP-6 or SIOP-9 strategies. Med Pediatr Oncol. 1999;33:170. (11) [Google Scholar]
- 6.Tannous R, Giller R, Holmes E. Intensive therapy for high risk (HR) relapsed Wilms’ tumor (WT). A CCG-4921/POG-9445 Study Report. Proceeding of ASCO; p. 588a. 200. abstract 2315. [Google Scholar]
- 7.Dome JS, Liu T, Krasin M, Lott L, Shearer P, Daw NC, et al. Improved survival for patients with recurrent Wilms tumor: the experience at St. Jude Children’s Research Hospital. J Pediatr Hematol Oncol. 2002;24:192–198. doi: 10.1097/00043426-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Reinhard H, Schmidt A, Furtwangler R, Leuschner I, Rube C, Von Schweinitz D, et al. Outcome of relapses of nephroblastoma in patients registered in the SIOP/GPOH trials and studies. Oncol. 2008;20:463–467. [PubMed] [Google Scholar]
- 9.Hale J, Hobson R, Moroz V, Satori P. Results of UK Children’s Cancer and Leukemia Group (CCLG) protocol for relapsed Wilms tumor (UKWR): Unified relapse strategy improves outcome. 40th meeting of International Society of Paediatric Oncology; 2008. p. 62. [Google Scholar]
- 10.Green DM, Cotton CA, Malogolowkin M, Breslow NE, Perlman E, Miser J, et al. Treatment of Wilms tumor relapsing after initial treatment with vincristine and actinomycin D: a report from the National Wilms Tumor Study Group. Pediatr Blood Cancer. 2007;48:493–499. doi: 10.1002/pbc.20822. [DOI] [PubMed] [Google Scholar]
- 11.Malogolowkin M, Cotton CA, Green DM, Breskiw NE, Perlman E, Miser J, et al. Treatment of Wilms tumor relapsing after initial treatment with vincristine, actinomycin D, and doxorubicin. A report from the National Wilms Tumor Study Group. Pediatr Blood Cancer. 2008;50:236–241. doi: 10.1002/pbc.21267. [DOI] [PubMed] [Google Scholar]
- 12.Garaventa A, Hartmann O, Bernard JL, Zucker JM, Pardo N, Castel V, et al. Autologous bone marrow transplantation for pediatric Wilms’ tumor: the experience of the European Bone Marrow Transplantation Solid Tumor Registry. Med Pediatr Oncol. 1994;22:11–14. doi: 10.1002/mpo.2950220103. [DOI] [PubMed] [Google Scholar]
- 13.Pein F, Michon J, Valteau-Couanet D, Quintana E, Frappaz D, Vannier JP, et al. High-dose melphalan, etoposide, and carboplatin followed by autologous stem-cell rescue in pediatric high-risk recurrent Wilms’ tumor: a French Society of Pediatric Oncology study. J Clin Oncol. 1998;16:3295–3301. doi: 10.1200/JCO.1998.16.10.3295. [DOI] [PubMed] [Google Scholar]
- 14.Kremens B, Gruhn B, Klingebiel T, Hasan C, Laws HJ, Koscielniak E, et al. High-dose chemotherapy with autologous stem cell rescue in children with nephroblastoma. Bone Marrow Transplant. 2002;30:893–898. doi: 10.1038/sj.bmt.1703771. [DOI] [PubMed] [Google Scholar]
- 15.Campbell AD, Cohn SL, Reynolds M, Seshadri R, Morgan E, Geissler G, et al. Treatment of relapsed Wilms’ tumor with high-dose therapy and autologous hematopoietic stem-cell rescue: the experience at Children’s Memorial Hospital. J Clin Oncol. 2008;22:2885–2890. doi: 10.1200/JCO.2004.09.073. [DOI] [PubMed] [Google Scholar]
- 16.Spreafico F, Bisogno G, Collini P, Jankner A, Gandola L, D’Angelo P, et al. Treatment of high-risk relapsed Wilms tumor with dose-intensive chemotherapy, marrow-ablative chemotherapy, and autologous hematopoietic stem cell support: experience by the Italian Association of Pediatric Hematology and Oncology. Pediatr Blood Cancer. 2008;51:23–28. doi: 10.1002/pbc.21524. [DOI] [PubMed] [Google Scholar]
- 17.Dallorso S, Dini G, Faraci M, Spreafico F. SCT for Wilms’ tumour. Bone Marrow Transplant. 2008;41(Suppl 2):S128–30. doi: 10.1038/bmt.2008.70. [DOI] [PubMed] [Google Scholar]
- 18.Klein J. Survival analysis: techniques of censored and truncated data. 2. Springer Verlag; New York, NY: 2003. [Google Scholar]
- 19.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Ha TC, Spreafico F, Graf N, Dallorso S, Dome JS, Malogolowkin M, et al. An international strategy to determine the role of high dose therapy in recurrent Wilms’ tumour. Eur J Cancer. 2013;49:194–210. doi: 10.1016/j.ejca.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Presson A, Moore TB, Kempert P. Efficacy of High-dose Chemotherapy and Autologous Stem Cell transplant for Recurrent Wilms’ Tumor: A Meta-Analysis. J Ped Hematology/Oncology. 2010;32(6):454–461. doi: 10.1097/MPH.0b013e3181e001c2. [DOI] [PubMed] [Google Scholar]