Abstract
Background
Dimethyl fumarate (DMF) is used to treat relapsing multiple sclerosis and causes lymphopenia in a subpopulation of treated individuals. Much remains to be learned about how the drug affects B- and T-lymphocytes.
Objectives
To characterize changes in B- and T-cell phenotype and function induced by DMF and to investigate whether low absolute lymphocyte count (ALC) is associated with unique functional changes.
Methods
Peripheral blood mononuclear cells (PBMCs) were collected from DMF-treated patients, untreated patients and healthy controls. A subset of DMF-treated patients was lymphopenic (ALC <800). Multiparametric flow cytometry was used to evaluate cellular phenotypes. Functional response to non-specific and viral peptide stimulation was assessed.
Results
DMF reduced circulating memory B-cells regardless of ALC. Follicular T-helper cells (CD4+CXCR5+) and mucosal invariant T-cells (CD8+CD161+) were also reduced. DMF reduced T-cell production of pro-inflammatory cytokines in response to polyclonal (PMA/ionomycin) and viral peptide stimulation, regardless of ALC. No differences in activation induced cell death or circulating progenitors were observed between lymphopenic and non-lymphopenic DMF-treated patients.
Conclusions
These data implicate DMF-induced changes in lymphocytes as an important component of the drug’s efficacy and expand our understanding of the functional significance of DMF-induced lymphopenia.
Keywords: Dimethyl fumarate, multiple sclerosis, immunology, disease modifying therapy
Introduction
Dimethyl fumarate (DMF) is a commonly prescribed disease modifying therapy (DMT) for relapsing multiple sclerosis (MS). Clinical trials demonstrated efficacy in reducing relapses and decreasing MRI measures of disease activity as well as in slowing disease progression (1, 2). Despite this, the mechanism of action for DMF remains poorly understood. Early studies suggested that it provided neuroprotection via the Nrf2 pathway (3), yet others demonstrated that much of the therapeutic effect was Nrf2 independent (4). For example, one study found that DMF induced glutathione depletion, which modulated dendritic cells to adopt an anti-inflammatory phenotype (5). DMF also appeared to affect lymphocytes directly, reducing numbers of circulating lymphocytes by 30% or more among patients taking the medication. A significant minority developed critically low absolute lymphocyte counts (ALC) (1, 2, 6). The emergence of DMF-associated opportunistic infections among a few lymphopenic patients heightened safety concerns and underscored the gaps in our knowledge of its mechanism of action (7–10).
Several descriptive studies demonstrated that, in addition to reducing white blood cell numbers, DMF shifted the composition of the circulating lymphocyte population with CD8+ T-cells being disproportionately lost. Moreover, our group and another have observed reductions in circulating memory cells and concurrent expansion of naive cells for both CD4+ and CD8+ T-cells (11, 12). This phenotypic shift was associated with decreased CD4+ T-cell production of several pro-inflammatory cytokines (13). Yet, the scope of DMF-induced changes in T-cell function remains incompletely characterized. Moreover, it has become clear that B-lymphocytes play an important role in MS pathology. Most DMTs for MS affect both B- and T-cells (14). DMF appears to modestly reduce B-cell numbers over time (12, 15), but its effects on B-cell phenotype and function have only recently begun to be examined (16).
We evaluated the phenotype and function of circulating B- and T- cells from DMF-treated MS patients compared with untreated patients. Additionally, we explored possible mechanisms for DMF-induced lymphopenia including whether it is caused by apoptosis or impairment in lymphocyte maturation. A better understanding of DMF effects on peripheral immune cells is needed to help counsel and stratify risks for patients. Moreover, understanding the mechanism of action of DMF in MS will lead to better understanding of the disease.
Methods
Subject selection
This was a cross-sectional, observational study. We included 27 healthy controls, 50 untreated MS patients and 66 patients who had been treated with DMF (Tecfidera, Biogen, Weston, MA; 240mg p.o. bid). Patients with grade 2–3 lymphopenia (ALC of <800 cells/Al) according to the common terminology criteria for adverse events (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf) were specifically recruited (Table 1). Patients who had received steroids within the prior 3 months were excluded. EDSS scores were determined for MS patients based on clinical documentation at the time of enrollment. This study was approved by the Washington University Human Research Protection Office and all subjects provided written informed consent.
Table 1. Cohort Demographics.
ALC: absolute lymphocyte count; EDSS: estimated disability status scale; DMT: disease modifying therapy; DMF: dimethyl fumarate; DMF-N: dimethyl fumarate treated, not lymphopenic; DMF-L: dimethyl fumarate treated, lymphopenic
| Healthy Controls | Untreated MS | DMF - all | DMF-N | DMF-L | ||
|---|---|---|---|---|---|---|
| N | 27 | 50 | 66 | 43 | 23 | |
| Age | mean (SD) | 40.1 (13) | 42.8 (12) | 47.6 (11) | 46 (11) | 49 (11) |
| Female | n (%) | 18 (67) | 40 (80) | 45 (68) | 31 (72) | 13 (57) |
| ALC | (mean, SD) | - | - | 1098 (592) | 1378 (552) | 591 (148) |
| EDSS | (median, range) | - | 2.5 (0–6.5)† | 2.5 (0–7.0) | 2 (0–6.5) | 3 (0–7) |
| Relapsing MS | n (%) | - | 42 (84) | 54 (82)† | 37 (86)† | 18 (78) |
| Years with MS | (mean, SD) | - | 7.8 (8.1)† | 12.1 (9.0)† | 10 (7.1)† | 15.5 (10.4) |
| Number of prior DMTs | - | 2 (0–6) | 2 (0–6) | 2 (0–4) | ||
| median (range) | ||||||
| Time on DMF (months) | - | - | 17 (9) | 15 (9) | 21 (9) | |
| mean (SD) | ||||||
| MS activity on DMF‡ | n (%) | - | - | 11 (16) | 9 (21) | 2 (9) |
Data missing for 1–2 patients
MS activity was defined as: clinical relapse as determined by treating physician ≥3 months after beginning drug, gadolinium enhancing MRI lesion ≥3 months after beginning drug, or new T2 lesion on MRI compared to a baseline MRI taken after initiating DMF.
Leukocyte phenotyping
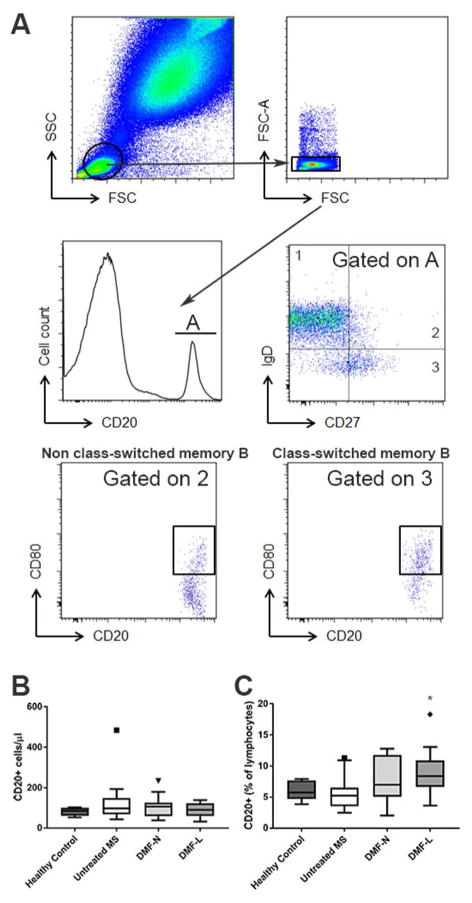
Fresh whole blood was labeled with CD8 (AlexaFluor 488, BD Biosciences), CD185/CXCR5 (PerCP eFluor 710, clone MU5UBEE, eBioscience), CD80 (PE, clone 2D10), CD86 (APC, clone IT2.2), CD27 (AlexaFluor 700, clone MT271), CD20 (APC/Cy7, clone 2H7), CD161 (Brilliant Violet 421, clone Hp-3G10), IgD (Brilliant Violet 510, clone IA6-2), Lineage Cocktail, Lin-1 (APC, CD3, CD14, CD16, CD19, CD20, CD56), CD39 (Brilliant Violet 421, clone A1) and CD34 (PE, clone 561, all from Biolegend), and CD4 (ECD, clone RPA-T4, Beckman Coulter). Fix/Lyse solution (eBioscience) and fluorescent eBeads (eBioscience) were added prior to flow cytometry using a Beckman Coulter Gallios instrument with 10 fluorescent channels and capturing 500,000 – 1,000,000 events per sample. Gating strategy is illustrated in Figure 1. Data were analyzed using Kaluza analysis software.
Figure 1. B-cell flow cytometry gating strategy.
Lymphocytes were gated based on forward/side scatter and doublets were removed (A). CD20+ B-cells were classified as naïve, class-switched or non-class-switched memory cells based on IgD and CD27 expression; CD80 expression was evaluated on memory B-cells (A). Circulating B-cell numbers (B) and proportions (C) were calculated (n=6 healthy control, 22 untreated MS, 17 DMF-N, 11 DMF-L). DMF-N: non-lymphopenic DMF-treated, DMF-L: lymphopenic, DMF-treated. Kruskal Wallis ANOVA with Dunn’s multiple comparison test was used to compare groups. * p<0.05 compared to untreated MS.
Intracellular cytokine analysis
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using Ficoll [GE Healthcare Life Sciences] and cryopreserved at −80°C. For functional analyses, cells were thawed, plated and allowed to rest at 37°C overnight. PBMCs were then stimulated for two hours with 10−7 M PMA and 1 μg/ml ionomycin (both from Sigma Aldrich); monensin was added for an additional four hours. Cells were labeled for CD8 (Krome Orange, clone B9.11), CD4 (Krome Orange, clone 13B8.2, both Beckman Coulter) and Zombie Aqua Fixable Viability Dye (Biolegend) for 20 minutes at 4°C. Cells were then washed, fixed with 2% PFA, permeabilized with 5% saponin buffer and stained for intracellular cytokines with the following antibodies: IL-10 (AlexaFluor 488, clone JES3-9D7), IL-4 (PE, clone 8D4-8), GM-CSF (APC, clone BVD2-21C11), TNF-α (AlexaFluor 700, clone MAb11), IFN-γ (APC-Cy7, clone 4S.B3, all from Biolegend) and IL-17A (eFluor450, clone ebio64DEC17, eBioscience). Flow cytometry was conducted and analyzed as above.
ELISPOT
Assays were performed on Multiscreen Filter Plates (Millipore). PBMCs were plated at 2.5 × 104 cells/well in RPMI 5% AB Human Serum (Sigma Aldrich) and stimulated with 10 μM viral peptides (Mabtech) or 1 μg ml−1 PHA overnight at 37°C. Viral peptides consisted of immunodominant epitopes from the ubiquitous viruses CMV, EBV and HSV. IFN-γ ELISPOT antibody pairs and streptavidin-alkaline phosphatase were from BD Bioscience. Plates were developed with NBT/BCIP substrate (Sigma) and spots were counted on an Immunospot counter (Cellular Technology Ltd.).
Activation induced cell death
PBMCs were thawed, plated and allowed to rest at 37°C overnight, then stimulated with anti-CD3 and anti-CD28 (eBioscience) at 10 μg/mL for 48 h. The cells were then rinsed and stained for CD4 (Alexa Fluor 700, clone RPA-T4, eBioscience) and CD8 (AlexaFluor 488, BD Biosciences) prior to rinsing and labeling with annexin V and propidium iodide (PI; BD Pharmingen) according to the manufacturer’s instructions. Samples were immediately analyzed using flow cytometry (Beckman Culture, Gallios). The annexin V-PE−/PI− population was considered live cells, while the annexin V-PE+/PI− and Annexin V-PE+/PI+ populations were considered early and late apoptotic cells, respectively.
Quantitative immunoglobulins
Immunoglobulins were quantified from patient serum by the Barnes Jewish Hospital clinical laboratory.
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (IBM Corp, Armonk, NY) and GraphPad Prism version 7.0 (La Jolla, CA). Because data were not normally distributed, nonparametric tests were used. Groups were first evaluated using Kruskal Wallis ANOVA and Dunn’s multiple comparison test with non-lymphopenic DMF-treated (DMF-N) and lymphopenic DMF-treated (DMF-L) patients considered as separate groups. For functional assays, we also performed multivariate linear regressions controlling for sex and batch effect; results were comparable to Kruskal-Wallis ANOVAs (Supplementary Table 1). Results were considered significant at p<0.05.
Results
DMF reduces circulating memory B-cells
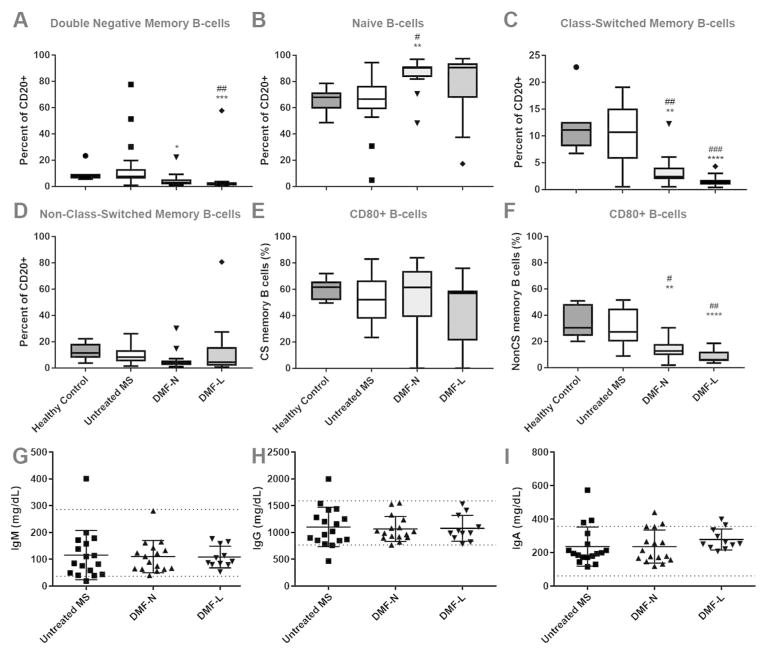
MS reduces IL-10-producing naive B-cells in the circulation (17), and numerous DMTs, including interferons, glatiramer acetate, fingolimod and mitoxantrone expand this population while reducing memory B-cells (14). The effects of DMF on B-cells are not well understood. We evaluated the phenotype of B-cells identified via whole blood staining of DMF-treated MS patients, untreated MS patients and healthy controls. DMF treatment was associated with a marked reduction in circulating class-switched memory B-cells and concurrent expansion of the naive B-cell population. There were smaller reductions in other memory B-cell populations as well (Figure 2A–D; absolute numbers in Supplementary Figure 1). In contrast, there were no differences among circulating B-cell phenotypes when lymphopenic DMF-treated patients were compared to non-lymphopenic patients (Figure 2A–D).
Figure 2. DMF effects on circulating B-cell phenotype and function.
Double negative memory (CD20+ CD27− IgD−), naïve (CD20+ CD27− IgD+), class-switched memory (CD20+ CD27+ IgD−) and non-class-switched memory (CD20+ CD27+ IgD+) B-cells were identified using flow cytometry (A–D). The proportion of activated (CD80+) memory B-cells was also calculated (E–F). n=6–7 healthy control (71% female), 22 untreated MS (95% female), 16–17 DMF-N (71% female) and 11 DMF-L (55% female). Serum concentrations of immunoglobulins were also quantified (H–J); n=17 untreated MS, 16 DMF-N and 11 DMF-L. Kruskal Wallis ANOVA with Dunn’s multiple comparison test was used to compare groups. Boxplots illustrate median/interquartile range; whiskers and outliers are calculated according to Tukey’s method. Dotted lines (H–J) delineate the upper and lower limits of normal. DMF: dimethyl fumarate; CS: class switched; nCS: non-class switched; DMF-N: non-lymphopenic DMF-treated, DMF-L: lymphopenic, DMF-treated. * p<0.05, ** p<0.01, *** p<0.001 compared to untreated MS; #p<0.05, ## p<0.01, ### p<0.001 compared to healthy controls.
DMF effects on markers of B-cell co-stimulation and immunoglobulin levels
B-cells are thought to affect MS mainly via their roles in antigen presentation and cytokine production (18). Antibodies (immunoglobulins) may also play an important role in the disease. We examined how DMF affects several aspects of B-cell function.
CD80 and CD86 are costimulatory molecules that are expressed on B-cells and are upregulated upon B-cell activation. Increased expression of these molecules enhances the ability of B-cells to activate naive T-cells and support T-cell mediated cytokine production. We assessed the expression of CD80 and CD86 on circulating B-cells from DMF-treated MS patients, untreated MS patients and healthy controls. CD80 expression was markedly reduced on circulating non-class switched memory B-cells among DMF treated patients (Figure 2E–F), but CD86 expression was unaffected (data not shown).
Plasma cells are the major source of circulating antibodies, with B cells contributing to a lesser extent. We quantified circulating immunoglobulins among DMF treated and untreated patients and observed no differences in circulating IgG, IgA, or IgM levels (Figure 2G–I).
DMF-reduces pro-inflammatory T-cell cytokine production in response to antigen – independent and antigen-dependent stimulation
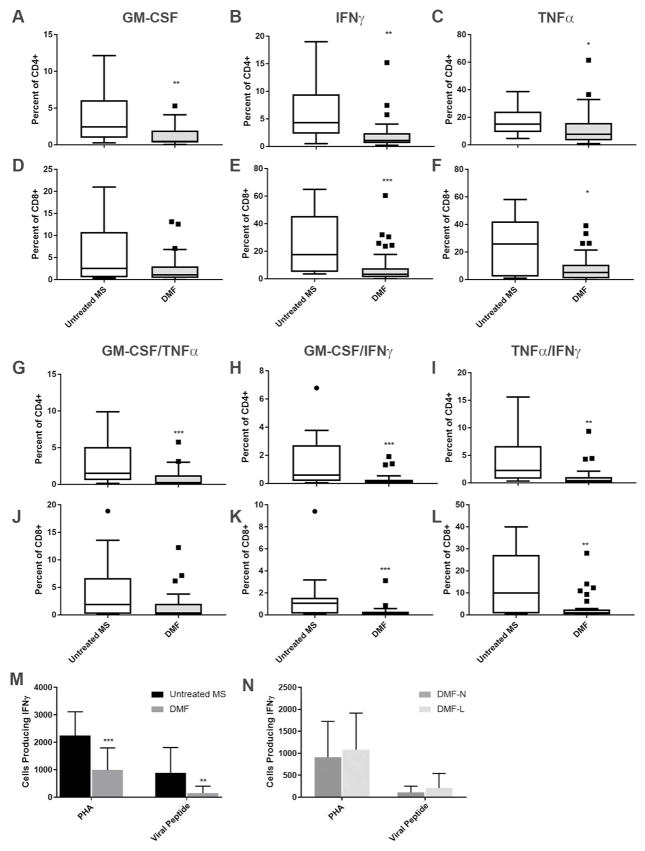
Like B-cells, T-cells contribute to MS pathology in part via secretion of pro-inflammatory cytokines. Previous work demonstrated that CD4+ T-cells from DMF-treated patients produced less IFN-γ, GM-CSF, TNF-α and IL-22 upon ex vivo stimulation (13). After polyclonal stimulation with PMA/ionomycin, we confirmed that CD4+ T-cells from DMF-treated patients produced less GM-CSF, IFN-γ and TNF-α compared to untreated patients (Figure 3A–C). CD4+ lymphocytes producing multiple cytokines (GM-CSF/TNF-α, GM-CSF/IFN-γ, and TNFα/IFN-γ) were also reduced by DMF (Figure 3G–I). No differences in CD4+ T-cell production of IL-4, IL-10, or IL-17 were detected (data not shown). Lymphopenic and non-lymphopenic patients showed no differences in cytokine production (data not shown).
Figure 3. DMF effects on T-cell cytokine production.
The proportion of CD4+ (A–C, G–I) and CD8+ (D–F, J–L) T-cells producing cytokines was evaluated using intracellular flow cytometry after exposure to antigen-independent stimulation (PMA/ionomycin). M–N: Lymphocyte production of cytokines was evaluated by ELISPOT after exposure to antigen independent (PHA) and dependent (viral peptide pool) stimuli. Numbers of cells producing IFN-γ were compared between untreated and DMF treated MS patients (M) and between non-lymphopenic and lymphopenic DMF treated MS patients (N). n=13–16 untreated MS (75% female), 29–33 DMF (14–15 lymphopenic; 70% female) for A–L. n=11 untreated MS, 22 DMF (10 lymphopenic) for M–N. Mann-Whitney U test was used to compare groups. Boxplots illustrate median/interquartile range; whiskers and outliers are calculated according to Tukey’s method. DMF-N: non-lymphopenic DMF-treated, DMF-L: lymphopenic, DMF-treated, PMA: phorbol 12-myristate 13-acetate, PHA: phytohemagglutinin. * p<0.05, ** p<0.01, ***p<0.001.
While DMF treatment disproportionately reduces the numbers of circulating CD8+ T-cells in MS patients (11, 12), the effects of DMF on CD8+ T-cell function have not been reported. Polyclonal stimulation with PMA/ionomycin resulted in less IFN-γ and TNF-α production among CD8+ T-cells from DMF-treated compared to untreated patients (Figure 3E–F). CD8+ cells producing both GM-CSF/IFN-γ and IFN-γ/TNF-α were also reduced (Figure 3J–L). No differences in CD8+ T-cell production of GM-CSF, IL-4, IL-10 or IL-17 were identified (Figure 3D–F; data not shown). Lymphopenic patients were indistinguishable from non-lymphopenic patients (data not shown).
To evaluate DMF effects on the cellular response to a more physiologic stimulus, we stimulated PBMCs with a peptide pool comprised of the immunodominant epitopes of the ubiquitous viruses HSV, EBV and CMV and measured IFN-γ production using ELISPOT. Significantly fewer PBMCs from DMF-treated patients produced IFN-γ in response to viral peptides when compared to those from untreated patients (Figure 3M).
As a primary function of CD8+ T-cells is to mount an immune response to viral infections, this finding may reflect the reduced proportion of CD8+ T cells induced by DMF. However, no significant differences in IFN-γ producing cells between lymphopenic and non-lymphopenic patients were seen (Figure 3N).
Effects of DMF on regulatory and follicular T-cells
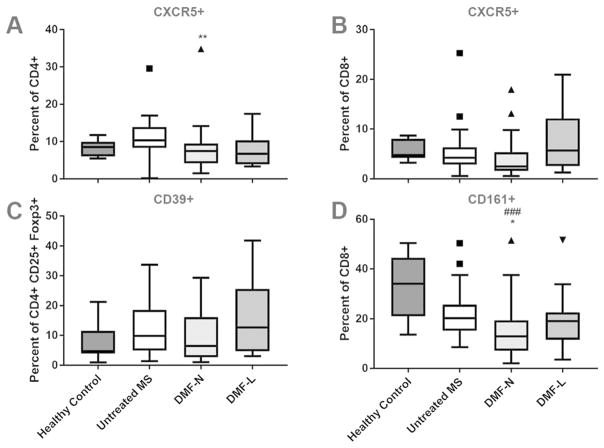
MS affects several functionally distinct T-cell subsets, including follicular T-cells, regulatory T-cells and mucosal invariant T-cells (19–21). CD4+ and CD8+ follicular T-cells express the CXCR5 receptor for CXCL13, and both cell types are reported to be dysregulated in MS (22, 23). DMF induced a modest reduction in the proportion of CD4+ CXCR5+ T-cells relative to untreated MS patients (Figure 4A; absolute numbers in Supplementary Figure 2). The proportion of CD8+ CXCR5+ cells was unchanged (Figure 4B).
Figure 4. DMF effects on functionally distinct T-cell subsets.
Proportions of circulating follicular T-cells (CD4+ CXCR5+ and CD8+ CXCR5+; A–B), CD39+ T-regulatory cells (CD4+ CD25+ Foxp3+, C) and mucosal invariant T-cells (CD8+ CD161+; D) were calculated using flow cytometry. n=9–10 healthy control (70% female), 21 (CD39) or 36 untreated MS (64% or 83% female) 49–50 DMF treated (70% female; 14–16 lymphopenic). Kruskal Wallis ANOVA with Dunn’s multiple comparison test was used to compare groups. Boxplots illustrate median/interquartile range; whiskers and outliers are calculated according to Tukey’s method. DMF-N: non-lymphopenic DMF-treated, DMF-L: lymphopenic, DMF-treated. * p<0.05, ** p<0.01, *** p<0.001.
We previously demonstrated that DMF did not affect the overall proportion of circulating Foxp3+ regulatory T-cells (Tregs) (11). The ectonucleotidase CD39 is expressed by a unique Treg subpopulation which contributes to Th17 suppression and is reported to be dysregulated in MS (24). DMF did not affect the proportion of circulating CD39+ Tregs (Figure 4C). We then evaluated CD8+ CD161+ mucosal invariant T-cells and found that the proportion of circulating cells was reduced in patients taking DMF compared with controls (Figure 4D). No significant differences were observed between T-cell subsets among lymphopenic compared to non-lymphopenic DMF-treated patients (Figure 4A–D).
Effects of DMF on activation induced cell death and circulating precursor lymphocytes
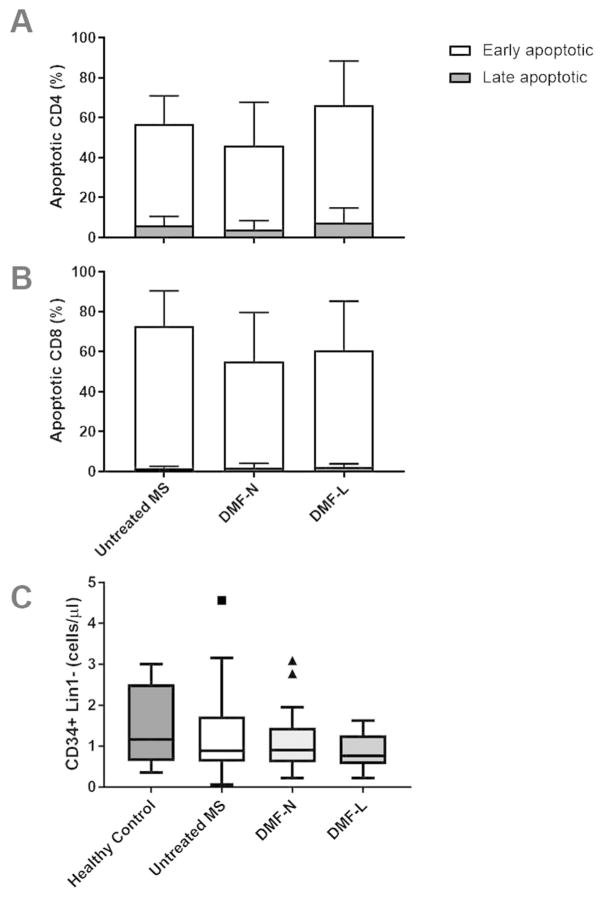
The mechanism by which DMF acts to reduce the numbers of circulating lymphocytes is not known. DMF induces T-cell apoptosis in vitro (16, 25), so to further evaluate its effects on lymphocyte survival, we stimulated PBMCs isolated from untreated and DMF-treated MS patients with anti-CD3 and anti-CD28, then stained for Annexin V and propidium iodide (PI) to quantify activation induced cell death (AICD). No significant differences in AICD were detected between DMF-treated and untreated MS patients. Moreover, AICD was not significantly different between lymphopenic and non-lymphopenic DMF-treated patients (Figure 5A–B).
Figure 5. No significant effects of DMF on activation induced cell death or circulating hematopoietic progenitor cells.
PBMC were stimulated with anti-CD3/anti-CD28 and stained for Annexin V and propidium iodide (PI) to quantify activation induced cell death (A–B). Annexin V-PE+/PI− and Annexin V-PE+/PI+ populations were considered early and late apoptotic cells, respectively. Circulating hematopoietic progenitor cells (CD34+ Lin1−) were identified using flow cytometry (C). DMF-N: non-lymphopenic DMF-treated, DMF-L: lymphopenic, DMF-treated. n=13 untreated MS (77% female), 15 DMF-N (87% female), 14 DMF-L (50% female) for activation induced cell death (A–B). n=12 healthy controls, 20 untreated MS, 26 DMF-N, and 7 DMF-L for circulating progenitors.
Another hypothetical mechanism for DMF-induced lymphopenia is a failure of lymphocyte differentiation. We evaluated circulating CD34+ progenitor cells among DMF-treated and untreated patients. As expected, this population was very small. We observed no differences in either the number or proportion of circulating CD34+ progenitors among treated compared to untreated patients (Figure 5C). Within the DMF-treated group, there were no differences in circulating progenitors between lymphopenic and non-lymphopenic patients.
Discussion
DMF modulates the phenotype and function of both T- and B-cells. Here, we report that DMF induces a phenotypic shift among circulating B-cells, reducing the proportion of memory B-cells and expanding the proportion of naive B-cells. These results corroborate and expand upon the recent report by Li et al. (16). The phenotypic shifts produced by DMF mirror the previously described effects of several other DMTs on B-cells (14). Although each DMT has a unique mechanism of action, the commonalities in their effects on B-cells suggest that this may be an important way by which immunomodulatory drugs benefit MS. Of particular importance may be the reduction of memory B-cells. Anti-CD20 immunotherapies eliminate mature B-cells, sparing progenitors and plasma cells. When immune reconstitution occurs, there is a preferential expansion of immature cells (26, 27). Similarly, after immunoablation with alemtuzumab, immature/naive B-cells reconstitute quickly, but there is prolonged depletion of memory B-cells (28). This phenomenon implicates mature/memory B-cells as permissive for disease, and the selective reduction of this cell population may underlie the beneficial effects of several DMTs.
Markers of B-cell function were also modulated by DMF. Naive T cell activation requires that processed antigen be presented to the T-cell receptor in the presence of a second co-simulatory signal, such as CD80. We observed a reduction in the proportion of CD80-expressing non-class switched memory B-cells in the circulation of DMF-treated patients. That CD80 expression was reduced suggests that B-cell antigen presentation may be regulated by DMF. DMF did not affect circulating immunoglobulin levels, indicating that plasma cell function remained intact during DMF treatment, at least in the short-term.
We previously reported that DMF changes the phenotype of circulating T-cells by expanding the naive compartment while reducing memory cells (11). It is now evident that T-cell function is also affected by DMF. We confirmed that CD4+ T-cells from DMF-treated patients were less able to produce pro-inflammatory cytokines in response to polyclonal stimulation (Figure 3) (13, 16). CD8+ T-cells also had a reduced IFN-γ and TNF-α response to antigen-independent stimulation (Figure 3).
Additionally, we showed for the first time that the cytokine response to common viral peptides is attenuated among circulating blood cells from patients taking DMF. Approximately 20% of T-cells were CD8+ among DMF-treated compared to 25% in untreated MS patients in our dataset (data not shown). This decreased proportion of CD8+ T-cells may contribute to the observed reduction in IFN-y-producing cells after viral peptide stimulation but is unlikely to be fully explanatory. These data suggest that DMF-treated patients may be less able to mount a robust immune response to common viruses. In practice, few serious infections have been associated with DMF although rare cases of progressive multifocal leukoencephalopathy have been reported among lymphopenic patients (7–10). Since CD8+ T-cells are particularly important for CNS immune surveillance (29) and this cell type is disproportionately reduced by DMF (11, 12), quantification of CD8+ T-cells may be a better biomarker for PML risk than ALC. Nevertheless, we observed no functional differences between lymphocytes derived from lymphopenic and non-lymphopenic patients.
Among the functionally distinct T-cell subsets we evaluated, DMF affected follicular T-helper cells (CD4+ CXCR5+). CXCR5 is the receptor for CXCL13, a key chemokine for secondary lymphoid tissue development. It has been hypothesized that the follicular T-helper subpopulation is important for the development of ectopic lymphoid follicles in the CNS (23). Still, little is currently known about how these cells affect MS. Some have observed increased frequency of activated (ICOS+) and memory follicular T-helper cells among progressive MS patients, with partial normalization of these counts after treatment with steroids (22, 23). Thus, the reduction in this cellular population is in line with the overall suppression of the pro-inflammatory milieu that is precipitated by DMF. Circulating CD8+ CD161+ cells were also reduced among DMF-treated patients. These T-cells promote Th1/Th17 cytokine production and a pro-inflammatory environment (19, 30) and are modulated by other DMTs (31). The observed reduction of this population by DMF reiterates that the drug promotes an anti-inflammatory milieu via many mechanisms, all of which likely contribute to its efficacy.
The mechanism for DMF-induced lymphopenia remains uncertain. We uncovered no significant differences in ex vivo AICD between lymphopenic and non-lymphopenic patients, although DMF can induce apoptosis in vitro (16, 25). This may be a function of timing, as the ALC tends to drop within the first months on DMF (6) and remain stably low thereafter. DMF-induced apoptosis may be easiest to detect within the first few months of starting the medication while rapid shifts in the lymphocyte compartment are occurring. Future studies should be designed with this in mind. Additionally, we observed no differences among circulating hematopoietic progenitors when comparing lymphopenic and non-lymphopenic patients. This could relate not only to the length of time that patients had been treated but also to the very small number of hematopoietic progenitors detectable in the bloodstream, since hematopoiesis generally takes place in the bone marrow. To address whether DMF affects lymphocyte maturation, it may be necessary to either study the bone marrow directly, or to significantly expand the circulating progenitor population using a medication such as filgrastim.
This study is the largest to date to examine the effects of DMF on T- and B-cell function. It confirms and expands upon prior work demonstrating that DMF reduced the numbers of both naive and memory B-cells and expanded regulatory B-cells (15, 16). Although our study was limited by a cross-sectional design, the results are in agreement with a smaller, longitudinal study (16). This study also expands our understanding of DMF effects on T-cells by demonstrating that DMF attenuates the T-cell response to viral peptides and documenting that the cytokine response to stimuli is unchanged among lymphopenic compared to non-lymphopenic patients. Addressing the mechanisms by which DMF affects lymphocyte phenotype and function in MS may provide a window into the underlying pathogenesis of the disease.
Supplementary Material
Acknowledgments
We would like to thank Dr. Marina Cella for methodological and study design advice and Mr. Michael Ramsbottom and Mr. Bob Mikesell for technical assistance. We would also like to thank all of the patients, study coordinators and referring physicians who made this study possible.
This work was supported by a pilot grant from the National MS Society (PP-1412-02199). It was also supported in part by NIH training grant UL1 TR000448.
Dr. Longbrake was funded by a Sylvia Lawry Fellowship from the National MS Society.
Dr. Cantoni was supported by a postdoctoral fellowship from the National MS Society (FG-2010A1/2).
Dr. Cross was supported by The Manny & Rosalyn Rosenthal – Dr. John L. Trotter MS Center Chair in Neuroimmunology of the Barnes-Jewish Hospital Foundation.
Dr. Piccio was supported by the Harry Weaver Neuroscience Scholar award of the National MS Society (JF 2144A2/1).
Footnotes
Disclosures:
Dr. Longbrake has received honoraria from consulting/speaking for Sanofi Genzyme, Teva, Genentech, EMD Serono and Biogen.
Dr. Cignarella, Dr. Chahin and Dr. Cantoni have no disclosures.
Dr. Cross has received honoraria for consulting for AbbVie, Bayer, Biogen, EMD Serono, Sanofi Genzyme, Genentech/Roche, Teva and Novartis.
Dr. Piccio has received honoraria for consulting for Biogen and has received research support from Alector.
References
- 1.Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–97. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 2.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098–107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 3.Scannevin RH, Chollate S, Jung MY, Shackett M, Patel H, Bista P, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther. 2012;341(1):274–84. doi: 10.1124/jpet.111.190132. [DOI] [PubMed] [Google Scholar]
- 4.Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, Spencer CM, Shetty A, Sagan SA, et al. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci U S A. 2016;113(17):4777–82. doi: 10.1073/pnas.1603907113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghoreschi K, Brück J, Kellerer C, Deng C, Peng H, Rothfuss O, et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med. 2011;208(11):2291–303. doi: 10.1084/jem.20100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox RJ, Chan A, Gold R, Phillips JT, Selmaj K, Chang I, et al. Characterizing absolute lymphocyte count profiles in dimethyl fumarate-treated patients with MS: Patient management considerations. Neurol Clin Pract. 2016;6(3):220–9. doi: 10.1212/CPJ.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieuwkamp DJ, Murk JL, Cremers CH, Killestein J, Viveen MC, Van Hecke W, et al. PML in a Patient without Severe Lymphocytopenia Receiving Dimethyl Fumarate. N Engl J Med. 2015;372(15):1474–6. doi: 10.1056/NEJMc1413724. [DOI] [PubMed] [Google Scholar]
- 8.Rosenkranz T, Novas M, Terborg C. PML in a Patient with Lymphocytopenia Treated with Dimethyl Fumarate. N Engl J Med. 2015;372(15):1476–8. doi: 10.1056/NEJMc1415408. [DOI] [PubMed] [Google Scholar]
- 9.van Kester MS, Bouwes Bavinck JN, Quint KD. PML in Patients Treated with Dimethyl Fumarate. N Engl J Med. 2015;373(6):583–4. doi: 10.1056/NEJMc1506151. [DOI] [PubMed] [Google Scholar]
- 10.van Oosten BW, Killestein J, Barkhof F, Polman CH, Wattjes MP. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med. 2013;368(17):1658–9. doi: 10.1056/NEJMc1215357. [DOI] [PubMed] [Google Scholar]
- 11.Longbrake EE, Ramsbottom MJ, Cantoni C, Ghezzi L, Cross AH, Piccio L. Dimethyl fumarate selectively reduces memory T cells in multiple sclerosis patients. Mult Scler. 2015 doi: 10.1177/1352458515608961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, Cree BA, Zamvil SS. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e76. doi: 10.1212/NXI.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross CC, Schulte-Mecklenbeck A, Klinsing S, Posevitz-Fejfár A, Wiendl H, Klotz L. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3(1):e183. doi: 10.1212/NXI.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longbrake EE, Cross AH. Effect of Multiple Sclerosis Disease-Modifying Therapies on B Cells and Humoral Immunity. JAMA Neurol. 2016;73(2):219–25. doi: 10.1001/jamaneurol.2015.3977. [DOI] [PubMed] [Google Scholar]
- 15.Lundy SK, Wu Q, Wang Q, Dowling CA, Taitano SH, Mao G, et al. Dimethyl fumarate treatment of relapsing-remitting multiple sclerosis influences B-cell subsets. Neurol Neuroimmunol Neuroinflamm. 2016;3(2):e211. doi: 10.1212/NXI.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Rezk A, Ghadiri M, Luessi F, Zipp F, Li H, et al. Dimethyl Fumarate Treatment Mediates an Anti-Inflammatory Shift in B Cell Subsets of Patients with Multiple Sclerosis. J Immunol. 2016 doi: 10.4049/jimmunol.1601649. [DOI] [PubMed] [Google Scholar]
- 17.Li R, Rezk A, Miyazaki Y, Hilgenberg E, Touil H, Shen P, et al. Proinflammatory GM-CSF–producing B cells in multiple sclerosis and B cell depletion therapy. Science Translational Medicine. 2015;7(310):310ra166–310ra166. doi: 10.1126/scitranslmed.aab4176. [DOI] [PubMed] [Google Scholar]
- 18.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178(10):6092–9. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 19.Annibali V, Ristori G, Angelini DF, Serafini B, Mechelli R, Cannoni S, et al. CD161(high)CD8+T cells bear pathogenetic potential in multiple sclerosis. Brain. 2011;134(Pt 2):542–54. doi: 10.1093/brain/awq354. [DOI] [PubMed] [Google Scholar]
- 20.Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259(1):231–44. doi: 10.1111/imr.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones AP, Kermode AG, Lucas RM, Carroll WM, Nolan D, Hart PH. Circulating immune cells in multiple sclerosis. Clin Exp Immunol. 2016 doi: 10.1111/cei.12878. [DOI] [PMC free article] [PubMed]
- 22.Fan X, Jin T, Zhao S, Liu C, Han J, Jiang X, et al. Circulating CCR7+ICOS+ Memory T Follicular Helper Cells in Patients with Multiple Sclerosis. PLoS One. 2015;10(7):e0134523. doi: 10.1371/journal.pone.0134523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romme Christensen J, Bornsen L, Ratzer R, Piehl F, Khademi M, Olsson T, et al. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS One. 2013;8(3):e57820. doi: 10.1371/journal.pone.0057820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, et al. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183(11):7602–10. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 25.Treumer F, Zhu K, Gläser R, Mrowietz U. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol. 2003;121(6):1383–8. doi: 10.1111/j.1523-1747.2003.12605.x. [DOI] [PubMed] [Google Scholar]
- 26.Anolik JH, Friedberg JW, Zheng B, Barnard J, Owen T, Cushing E, et al. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol. 2007;122(2):139–45. doi: 10.1016/j.clim.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(2):613–20. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 28.Thompson SA, Jones JL, Cox AL, Compston DA, Coles AJ. B-cell reconstitution and BAFF after alemtuzumab (Campath-1H) treatment of multiple sclerosis. J Clin Immunol. 2010;30(1):99–105. doi: 10.1007/s10875-009-9327-3. [DOI] [PubMed] [Google Scholar]
- 29.Loeffler C, Dietz K, Schleich A, Schlaszus H, Stoll M, Meyermann R, et al. Immune surveillance of the normal human CNS takes place in dependence of the locoregional blood-brain barrier configuration and is mainly performed by CD3(+)/CD8(+) lymphocytes. Neuropathology. 2011;31(3):230–8. doi: 10.1111/j.1440-1789.2010.01167.x. [DOI] [PubMed] [Google Scholar]
- 30.Maggi L, Santarlasci V, Capone M, Peired A, Frosali F, Crome SQ, et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol. 2010;40(8):2174–81. doi: 10.1002/eji.200940257. [DOI] [PubMed] [Google Scholar]
- 31.Negrotto L, Canto E, Rio J, Tintore M, Montalban X, Comabella M. Peripheral blood non-MAIT CD8+CD161hi cells are decreased in relapsing-remitting multiple sclerosis patients treated with interferon beta. J Neuroimmunol. 2015;288:98–101. doi: 10.1016/j.jneuroim.2015.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.