Abstract
Objective
Given the increasing use and broadening of indications for antipsychotic medications in the general population, as well as the paucity of information on the safety of this drug class during pregnancy, the study aim was to document patterns of antipsychotic medication use in pregnant women.
Method
Medicaid Analytic eXtract data (2001–2010) from pregnant women who delivered live-born infants was used. Antipsychotic use at both the class and individual drug level was defined based on dispensed outpatient prescriptions. Users’ characteristics, including mental disorder diagnoses, were described. Temporal trends in use, as well as discontinuation patterns and polytherapy with other psychotropic medications during pregnancy were evaluated.
Results
Among 1,522,247 pregnancies, the prevalence of atypical antipsychotic use at any time during pregnancy increased three-fold, from .4% to 1.3%, over the 10-year period while the use of typical antipsychotics remained stable around .1%. The increase in atypical use was largely driven by more frequent use in patients with bipolar disorder. Quetiapine and aripiprazole were the most frequently dispensed drugs, and polytherapy with antidepressants (65.2%), benzodiazepines (24.9%), and/or other mood stabilizers (22.0%) was common among women using antipsychotics during pregnancy. More than 50% of women receiving an antipsychotic in the 3 months prior to pregnancy discontinued during pregnancy.
Conclusions
A growing number of pregnant women in Medicaid are exposed to atypical antipsychotics, frequently in combination with other psychotropic medications. This study highlights the importance of documenting the use and safety of these drugs during pregnancy to inform therapeutic decision making for pregnant women with psychiatric disorders.
INTRODUCTION
Over the past two decades, the use of antipsychotic medications to treat psychiatric disorders has greatly expanded in the United States (US) (1). Schizophrenia and other psychotic disorders have long been treated with both typical and atypical antipsychotics. However, since 2000, a number of atypical antipsychotics have received approvals for broader indications including irritability in autism, mood stabilization in bipolar disorder and adjunct therapy for major depressive disorder (MDD). Increasing off-label use of antipsychotics to treat attention-deficit hyperactivity disorder (ADHD) or behavioral disorders has also been reported in recent years (2–4).
For women, psychiatric disorders that are treated with antipsychotics often present during the childbearing years (5) and the risk-benefit trade-off of treatment during pregnancy is challenging. While continuous treatment may be important to prevent symptomatic episodes or relapse (6), maternal and fetal safety concerns exist related to antipsychotic treatment. Case reports and studies with small samples have reported conflicting findings on the association between typical antipsychotic use and the risk of congenital anomalies (7–9). There are few large, well-controlled studies examining the teratogenicity of atypical antipsychotics (10, 11), but the results from a recent large study with 9,258 women exposed to atypical antipsychotics did not find increased risk of congenital malformation. (12). Atypical antipsychotics are known to cause weight gain and increase the risk of Type 2 diabetes mellitus in the general population (13), which may translate into higher risks for diabetes associated adverse pregnancy outcomes like fetal macrosomia or increased risk of gestational diabetes and its attendant effects.
In light of the rising use in the general population and the broadening of the indications for antipsychotic treatment observed in the last decade (1–3), as well as the limited information on the safety profile of this drug class during pregnancy, it is important to understand the extent and patterns of use of antipsychotics among pregnant women. Describing antipsychotic utilization trends in a publicly insured population is especially important since Medicaid covers the costs for approximately 80% of all antipsychotic prescriptions (14) and close to 50% of all deliveries in the US (15). We used nationwide Medicaid data to investigate the patterns of antipsychotic use among publicly insured women in the US.
METHODS
Data Source and Study Population
We used Medicaid Analytic eXtract (MAX) data from 2001 to 2010 from the Centers for Medicare and Medicaid Services (CMS). MAX contains person-level information on demographics, hospitalizations and outpatient visits, as well as outpatient pharmacy dispensings. We created a linked cohort of pregnant women and their live-born babies based on a process described elsewhere in detail (16). Linkage provided delivery dates that were used to estimate the last menstrual period (LMP) based on a previously validated algorithm (17). Women were required to have continuous coverage from three months before the LMP to one-month after delivery and to not have other private insurance or restricted benefits during pregnancy to ensure complete capture of healthcare use information.
Antipsychotic Medication Use
To define exposure and patient characteristics, the time period was divided into four different windows: 3 months prior to the LMP (baseline), the LMP to gestational day 90 (first trimester), from gestational day 91 to 180 (second trimester), and from gestational day 181 to delivery (third trimester). Antipsychotic exposure was defined as filling at least one prescription during the respective window, based on dispensing dates in the outpatient pharmacy dispensing file (see Table S1 for the full list of antipsychotics considered). Prochlorperazine and promethazine were excluded because they are more commonly used for non-psychiatric conditions (18). We examined antipsychotic use at the class level (atypical or typical) and at the generic level for the six most frequently used drugs (aripiprazole, olanzapine, quetiapine, risperidone, ziprasidone, and haloperidol). Most women (> 99 %) received oral medications, so we did not distinguish injectable forms. When a woman was dispensed more than one type of antipsychotic, each dispensing was counted separately toward each drug exposure category since the main purpose of this study was to describe the extent of antipsychotic use in this population.
Psychiatric Disorder Diagnoses
To document psychiatric disorders, we searched for the presence of International Classification of Diseases, Ninth Revision (ICD-9) codes for anxiety disorder, attention-deficit hyperactivity disorder (ADHD), bipolar disorder, depression, schizophrenia or other psychotic disorder from 90 days prior to LMP to delivery (see Table S1 for diagnostic codes). Following the approach from Crystal et al (1), we created mutually exclusive hierarchical diagnosis categories since multiple diagnoses often occur concurrently (5). Subjects were classified into the highest possible category starting from ADHD only to anxiety with or without ADHD, depression with or without anxiety or ADHD, bipolar disorder with or without depression, anxiety or ADHD, or schizophrenia or other psychotic disorder with or without bipolar disorder, depression, anxiety or ADHD (i.e., the highest category).
Analyses
Trends in Use
Temporal trends of antipsychotic use were examined by delivery year and the p-value for trend was reported. The prevalence of any antipsychotic use was examined stratified by age at time of delivery and race. To evaluate changes in drug use as a function of changes in diagnoses, we examined the yearly prevalence of each diagnosis using the hierarchical definitions, as well as the proportion receiving any antipsychotic medication among those with the diagnosis.
Characteristics of Study Population
We used descriptive statistics to characterize the population in terms of demographics, comorbid diagnoses such as other mental disorders, pain disorders, hypertension, or diabetes, and use of other medications such as anxiolytics, hypnotics, or opioids during the baseline period and the first trimester. Additionally, we investigated concomitant treatment with major psychotropic medications (antidepressants, benzodiazepines, and other mood stabilizers defined in this study as lithium, carbamazepine, lamotrigine, oxcarbazepine, topiramate, and valproate) in women who received antipsychotic medication during pregnancy.
Discontinuation and Switching
To evaluate the potential impact of pregnancy on the decision to continue or to discontinue treatment at different time points during pregnancy, we investigated the patterns of use during pregnancy. ‘Continuation’ was defined as filling a prescription for the same antipsychotic class (class level) or specific drug (generic level) during pregnancy as before pregnancy and ‘initiation’ was defined as filling a prescription during pregnancy among those without a record of use during the 3 months before the start of pregnancy. A ‘Switch’ was defined as filling a prescription for a different antipsychotic (class or generic level) than during the baseline.
RESULTS
Trends in Use
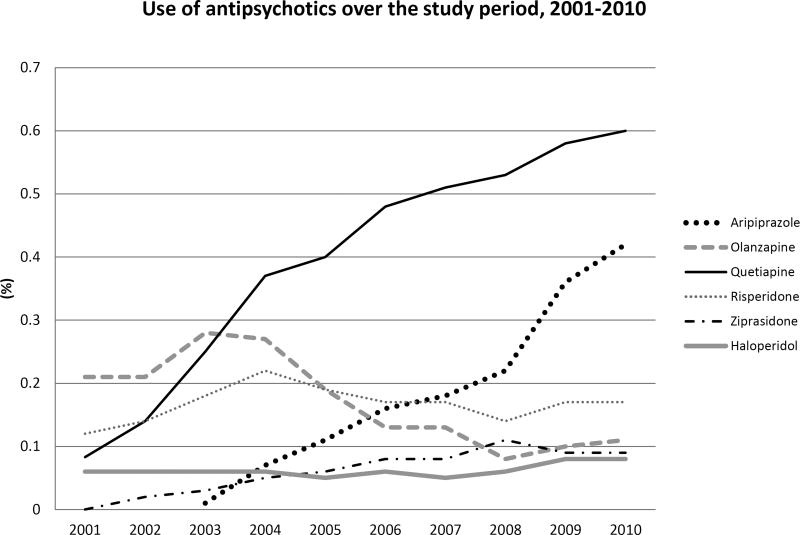
Between 2001 and 2010, we identified 1,522,247 pregnancies meeting our inclusion criteria. Over this 10-year period, the number of women who filled at least one prescription for an atypical antipsychotic during pregnancy increased from .4% (n=376) to 1.3% (n=2,044; p-value for trend < 0.001). The increasing trend was similar across the age and race groups considered. In all years, use was higher among women older than 30 years (Figure S1-A), and remained higher among whites than among Hispanics and blacks (Figure S1-B). The proportion of women who received a typical antipsychotic remained stable at around .1% over the entire study period. At the individual drug level, we observed different trends for each of the 6 antipsychotics considered (Figure 1). The proportion using quetiapine increased substantially from .1% in 2001 to .6% in 2010. Since its introduction to the market in 2002, aripiprazole became the second most frequently used antipsychotic by 2010 with .4% of all women filling a prescription during pregnancy. In contrast, we observed a decreasing trend in the proportion of olanzapine users from .2% to .1%.
Figure 1. Proportion of women who were dispensed an antipsychotic medication during pregnancy in Medicaid, 2001 to 2010.
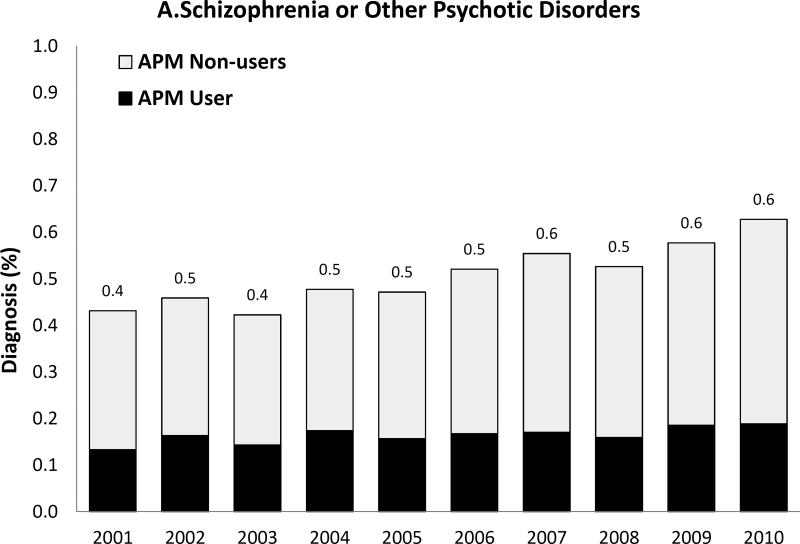
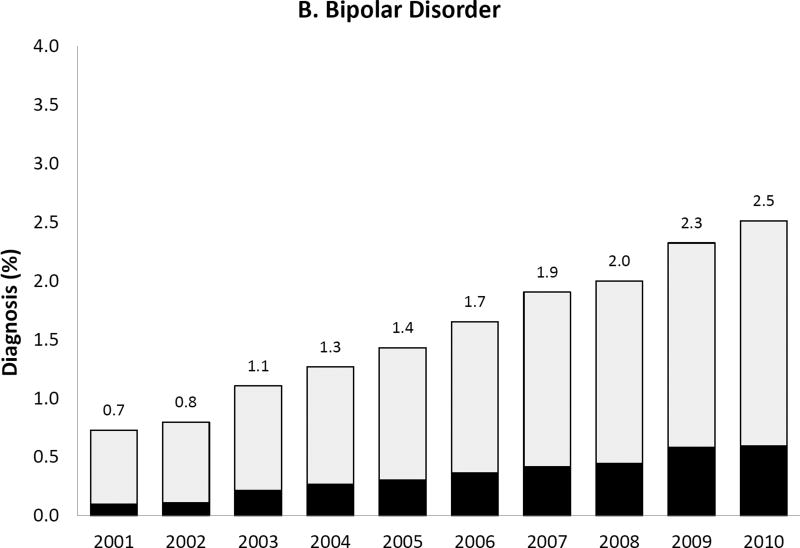
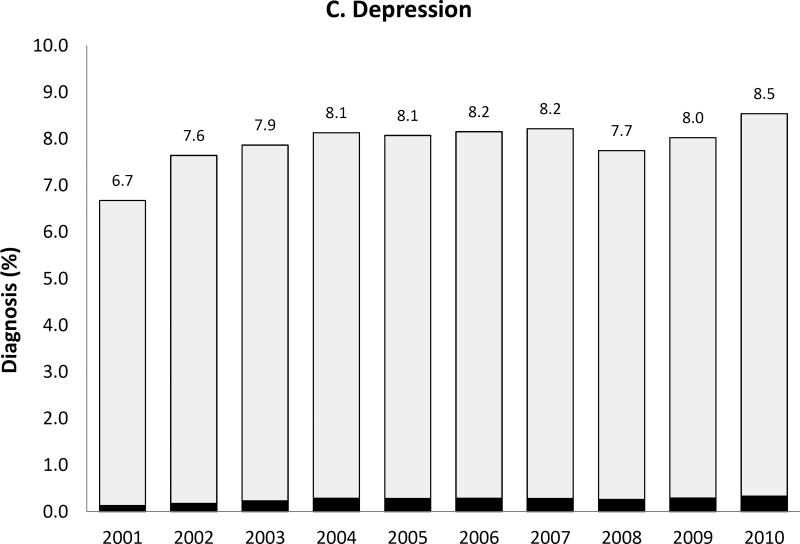
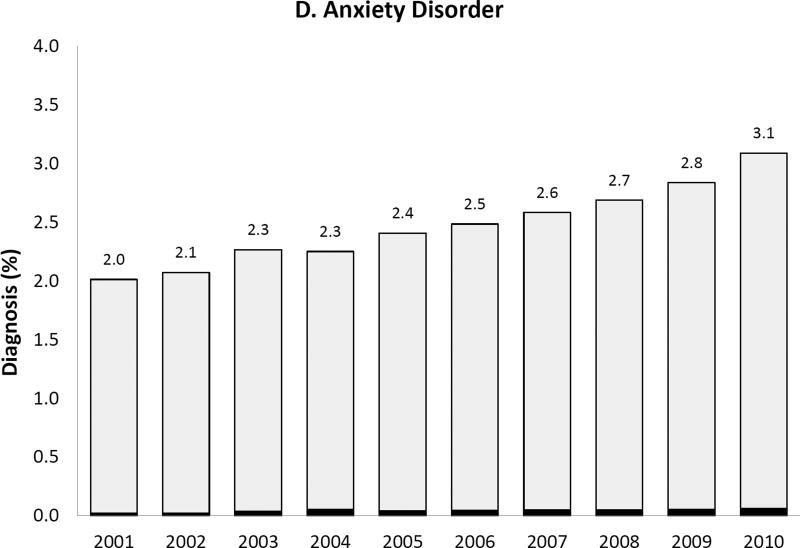
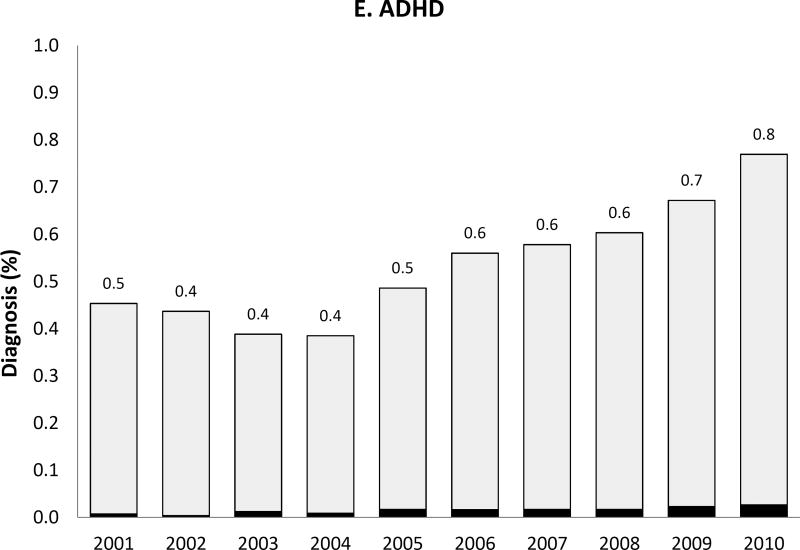
We observed a temporal increase both in the prevalence of the five psychiatric disorder diagnoses of interest and, for some diagnoses, also in the proportion of women with such diagnoses receiving antipsychotic medication during pregnancy (Figure 2). Most strikingly, the prevalence of bipolar disorder diagnosis increased more than 3-fold over 10 years from .7% to 2.5% and the proportion of women with the diagnosis receiving antipsychotics increased from 13.6% in 2001 to 23.6% by 2010. Women with depression, but no schizophrenia, bipolar or other psychotic disorders, represented 6.7% of the study population in 2001 and 8.5% in 2010; the proportion treated with antipsychotics changed from 1.9% to 3.9% representing about a twofold increase. The extent of antipsychotic use in women with apparent off-label indications such as anxiety or ADHD was small but increased over time.
Figure 2. Prevalence of each psychiatric disorder diagnosis (hierarchicala) among pregnant women in Medicaid and proportion of women with the diagnosis who had one or more dispensing of antipsychotic medication, 2000 to 2010.
APM: Antipsychotic medication; ADHD: Attention-deficit hyperactivity disorder
Note that the y-axis scales are different for each diagnosis
aHierarchy of diagnoses: the highest possible category among 1) ADHD only, 2) anxiety with or without ADHD, 3) depression with or without anxiety or ADHD, 4) bipolar disorder with or without depression, anxiety or ADHD, and 5) schizophrenia or other psychotic disorder with or without bipolar disorder, depression, anxiety or ADHD
Characteristics Study Population
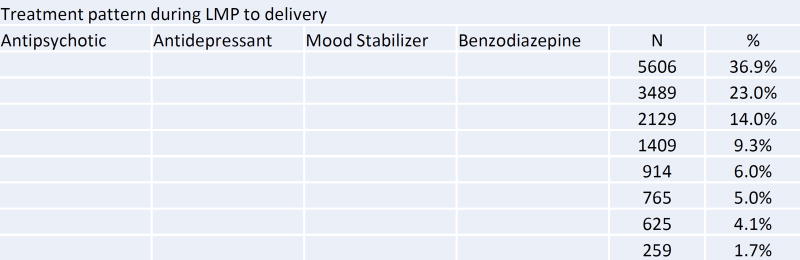
Compared to non-users during pregnancy, antipsychotic users were older, disproportionately white, and had a higher prevalence of non-psychiatric comorbidities and medication use, smoking, alcohol use, and recorded drug abuse or dependence (Table 1). Polytherapy with other psychotropic medications during pregnancy was common (Figure 3). Among the 15,196 women who used antipsychotics at any time during pregnancy, 65.2% also received antidepressants, 24.9% benzodiazepines, and 22.0% mood stabilizers. Five percent (765 women) received at least one prescription for all four drug classes at some point during pregnancy. Opioids were prescribed to more than 40% of women who received antipsychotics during pregnancy.
Table 1.
Characteristics of antipsychotic users vs. non-users among pregnant women in Medicaid, 2001 to 2010
| Users (N=15196) | Non-users (N=1507051) | Standardized Difference* |
|||
|---|---|---|---|---|---|
|
| |||||
| N | (%) | N | (%) | ||
| Demographics | |||||
| Age under 20 | 2888 | 19.0 | 364962 | 24.2 | 13 |
| Age 20 to <30 | 8246 | 54.3 | 880000 | 58.4 | .08 |
| Age 30 to <40 | 3745 | 24.6 | 242338 | 16.1 | .21 |
| Age 40 or older | 317 | 2.1 | 19751 | 1.3 | .06 |
| White | 9144 | 60.2 | 602772 | 40.0 | .41 |
| Black/African American | 3766 | 24.8 | 504884 | 33.5 | .19 |
| Hispanic/Latino | 827 | 5.4 | 225390 | 15.0 | .32 |
| Asian/Other Pacific Islanders | 267 | 1.8 | 53318 | 3.5 | .11 |
| Others | 1192 | 7.8 | 120687 | 8.0 | .01 |
| Mental disorders | |||||
| Schizophrenia or other psychotic disorder | 2526 | 16.6 | 5273 | .3 | .61 |
| Bipolar disorder | 6488 | 42.7 | 20383 | 1.4 | 1.15 |
| Depression | 7910 | 52.1 | 127273 | 8.4 | 1.08 |
| Anxiety disorder | 4470 | 29.4 | 65747 | 4.4 | .71 |
| ADHD | 1374 | 9.0 | 15433 | 1.0 | .37 |
| Other comorbid conditions | |||||
| Personality disorder | 589 | 3.9 | 2724 | .2 | .26 |
| Adjustment disorder | 329 | 2.2 | 8249 | .5 | .14 |
| Sleep disorder | 598 | 3.9 | 9639 | .6 | .22 |
| Epilepsy | 774 | 5.1 | 17378 | 1.2 | .23 |
| Neuropathic pain | 603 | 4.0 | 18462 | 1.2 | .17 |
| Fibromyalgia | 431 | 2.8 | 12541 | .8 | .15 |
| Other pain disorder | 433 | 2.8 | 12984 | .9 | .15 |
| Migraine/headache | 2378 | 15.6 | 105806 | 7.0 | .28 |
| Hypertension | 761 | 5.0 | 32717 | 2.2 | .15 |
| Diabetes | 506 | 3.3 | 23043 | 1.5 | .12 |
| Tobacco use | 1446 | 9.5 | 45623 | 3.0 | .27 |
| Alcohol use | 603 | 4.0 | 7780 | .5 | .24 |
| Other drug abuse or dependence (2001–2006) | 942 | 12.8 | 13418 | 1.5 | .45 |
| N of outpatient visits (median, IQR) | 10 | (6–17) | 4 | (2–8) | .73 |
| Other Medication usea,b | |||||
| Anxiolytics | 644 | 4.2 | 4641 | .3 | .27 |
| Barbiturates | 695 | 4.6 | 24574 | 1.6 | .17 |
| Other hypnoticsc | 3633 | 23.9 | 94835 | 6.3 | .51 |
| Opioids | 6112 | 40.2 | 306000 | 20.3 | .44 |
ADHD: Attention-deficit hyperactivity disorder; IQR: Interquartile range; ED: Emergency department; NSAIDs: Non-steroidal antiinflamatory drugs
Defined based on the information during 90 days prior to LMP to 90 days after LMP.
Use of psychotropic medication (antidepressants, mood stabilizers, and benzodiazepines) is presented in Figure 3.
Hypnotic drugs other than barbiturates, benzodiazepines, antidepressants, or antipsychotics
The standardized difference for each variable between the exposed and unexposed group was calculated as the difference in means expressed in units of the pooled standard deviation (43).
Figure 3. Treatment patterns with other psychotropic medication among women receiving antipsychotic during pregnancy.
LMP: last menstrual period
The shaded cells indicate the use of each medication during LMP to delivery. Each row represents different combinations of psychotropic drug use patterns (does not necessarily indicate concurrent use of drugs).
aMood stabilizers include lithium, carbamazepine, lamotrigine, oxcarbazepine, topiramate, and valproate
Discontinuation and Switching
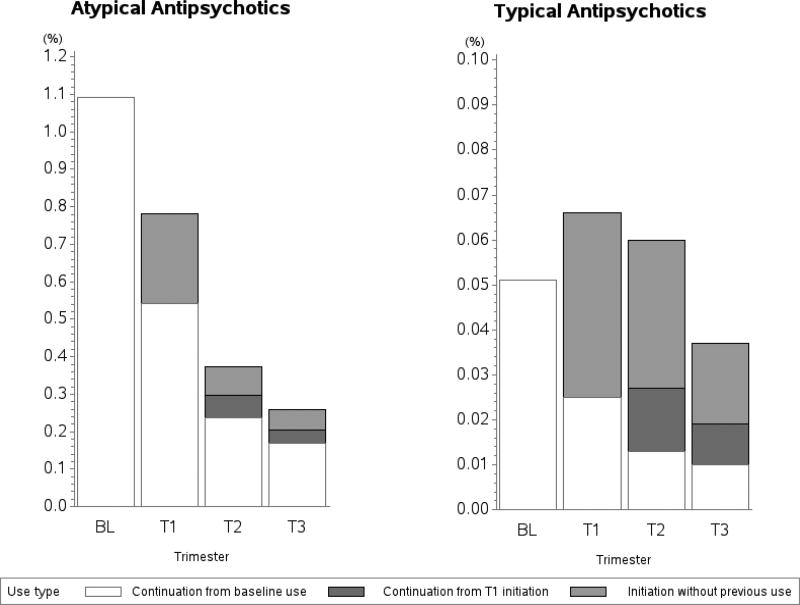
Of the 16,608 women (1.1%) who filled an atypical antipsychotic prescription during the 3 months before the LMP, about half (50.2%) did not fill any additional prescription from LMP until delivery and 15.4% continued throughout pregnancy (Figure 4). Of all women who did not use atypical antipsychotics during the baseline period (n=1,505,639), 5,583 (.4%) filled a prescription during pregnancy: .2% (n=3,641) initiated in the first, .07% (n=1,123) in the second, and .05% (n=819) in the third trimester. For women who used typical antipsychotics prior to the LMP (n=774), the discontinuation rate was similar (51.7%). Regardless of the baseline use, 6,469 (.4%) women filled an atypical antipsychotic and 1,197 (.08%) filled a typical antipsychotic in the second or third trimester, when most women would be aware that they are pregnant.
Figure 4. Discontinuation and initiation of antipsychotic medication during pregnancy, by medication class.
BL: baseline; T1: first trimester; T2: second trimester; T3: third trimester
Note that the y-axis scales are different between the two classes
The observed patterns at the generic level were similar to those at the class level (Figure S2). Depending on the drug 49.6% to 59.6% of women exposed to individual atypical agents discontinued treatment during pregnancy, while 42.8% of women who received haloperidol in the baseline period discontinued. Switching to a different antipsychotic in the first trimester was infrequent among baseline antipsychotic users, and most women who switched did so to quetiapine in the first trimester (Table S2).
DISCUSSION
In a nationwide sample of publicly insured pregnant women in the US, we observed a 3-fold increase in the use of atypical antipsychotics from .4% in 2001 to 1.3% in 2010. The trend was largely driven by an increase in the use of two atypical antipsychotics, aripiprazole and quetiapine. Characteristics of antipsychotic users have changed over time with a notable increase in both diagnosis of and use for bipolar disorders. A large proportion of women are treated concomitantly with other psychotropic medications. More than 50% of women who used atypical antipsychotics during the 3 months before LMP discontinued in the first trimester. Among women who switched, the majority switched to quetiapine.
The prevalence of women on antipsychotic treatment during pregnancy in our study is higher than that found in European cohorts (range .1% to .3%) or privately insured women in the US (.7% for atypical antipsychotics) during partly overlapping periods (18–22). This is not surprising, because the prevalence of mental illness is known to be higher for the Medicaid insured population, due partly to the lower socioeconomic status and higher proportion of minority groups (23). The increase in bipolar disorder diagnoses in our study population is consistent with the increase observed for the general population, including children and adolescents (24, 25). It is not entirely clear why there has been such a rapid increase in bipolar disorder diagnoses, but plausible explanations include improved classification as bipolar spectrum disorders of those previously misdiagnosed as unipolar depression or, perhaps, overdiagnosis of this condition (25, 26). We also observed a small increase in the use of antipsychotics for women with anxiety or ADHD, consistent with previous studies reporting frequent off-label use among non-pregnant populations with either disorder (1, 4). As both disorders often co-occur with other psychiatric disorders it is also possible that we did not capture other diagnoses in the claims for which the antipsychotics were truly used (27).
The utilization trends over time were different for the different antipsychotic agents. The greater increase in the use of aripiprazole and quetiapine may be due in part to their expanded indications for treatment of MDD (2007 for aripiprazole, 2009 for quetiapine). A wide range of off-label uses for quetiapine may partly explain the increase in use observed during the years preceding the expansion of indications, in addition to the perceived relative safety based on prior evidence showing a lower rate of placental passage for quetiapine compared to the other antipsychotics (28, 29). The decrease in the use of olanzapine may be explained by the known risk of metabolic side effects including weight gain (30). Risperidone was the first atypical antipsychotic to be approved, but its potential to cause hyperprolactinemia may be the reason why the use is less common among pregnant women (31).
The discontinuation patterns observed in this study were similar to the results from previous studies in the UK in which close to 50% of women had discontinued atypical antipsychotics by six weeks of pregnancy and 62% to 72.3% by the third trimester (19, 22). Interestingly, a greater number of women filled a prescription for a typical antipsychotic after LMP compared to the baseline period; a similar trend was not seen for atypical antipsychotics. This might be due to the fact that physicians have more experience prescribing typical antipsychotics during pregnancy and therefore feel more comfortable doing so. In particular, concerns about the risk of metabolic side effects may deter physicians from prescribing atypical antipsychotics during pregnancy. A small number of women appear to initiate treatment during pregnancy. Although some might be true initiators, it should be acknowledged that for some of these women it might be a continuation of treatment from the pre-pregnancy period, which was not captured in our 3-month baseline period.
Polytherapy with other psychotropic medications was very common, as antipsychotics are often indicated as an adjunct agent (32). Potential drug interactions between antipsychotics and other psychotropics are largely unknown, particularly in pregnancy; given the high frequency of such use, this is an important priority for future research. It is also notable that opioid use during pregnancy was remarkably high in pregnant women taking antipsychotics, which may be due to a higher prevalence of mental illness among Medicaid recipients and a strong link between mental illness and physical pain (33). There are potential harms such as neonatal abstinence syndrome associated with opioid use in pregnancy (34) and the risk of other negative maternal and fetal outcomes is largely unknown. More attention to appropriate use of opioid in this population is needed.
Strengths and limitations
We used a nationwide sample of more than 1.5 million pregnancies covered by Medicaid. Since Medicaid pays for close to 50% of all deliveries in the US, the results reflect the real-world utilization in a large proportion of the US population. In addition, our study population was racially diverse (non-white race and minority groups representing 58% vs. 39% in general non-elderly US population) and young (mean age of 24.1 vs. 25.4 in the general US population) (35, 36). We were able to study trends by racial groups, which can be a proxy for differential access to mental health care (37).
Our study also has some limitations. The study findings about prevalence of use are not applicable to women with private insurance since the use of psychotropic medication is higher in Medicaid (14), but some of the other trends in use may still be generalizable. Antipsychotic exposure was defined based on filling a prescription, which does not guarantee the actual intake. But claims from the automated dispensing records are considered to be more accurate than other methods to define drug exposures such as patient recall or medical records (38, 39). The date of LMP was estimated, so some misclassification of exposure timing is possible. But the algorithm we used estimated 75% of preterm and 99% of term deliveries within 2 weeks of the clinical gestational age at birth (17). We defined psychiatric morbidity using ICD-9 diagnostic codes that have imperfect sensitivity (40) and as a result we could have underestimated the prevalence of each psychiatric diagnosis in the study. However, since pregnant women have frequent contact with health services and since we required continuous coverage over the pregnancy period, concerns about incomplete capture of diagnoses are reduced. Also a number of women had more than one psychiatric diagnosis recorded, which implies that different diagnoses were recorded over time. We were not able to ascertain the specific diagnosis for which antipsychotics may have been prescribed. The lower than expected prevalence of smoking and alcohol problems in this cohort of women with psychiatric disorders (22, 41) is attributed to the under-recording of lifestyle variables in claims data. Lastly, some of the trends in use might have been affected by changes in each state’s Medicaid program at different points in time such as implementation of prior authorization or coverage expansion (42). We were not able to disentangle the impact of changes in policies versus changes in clinician preference. Nevertheless, this study provides insight into the observed patterns of antipsychotics medication use over time at a national level.
CONCLUSION
A growing number of pregnant women in Medicaid are exposed to antipsychotic medications during pregnancy, reaching 1.3% by the year 2010, but there is still limited evidence regarding the safety of antipsychotic medication during pregnancy. Polytherapy with other psychotropic medications common in this population deserves more attention with regard to fetal safety. High rate of discontinuation observed in this population suggests that clinicians and patients have concerns about the safety of the use of these medications during pregnancy. However, discontinuation of these medications may have implications for maternal mental health. To help clinicians and patients make informed treatment decisions, there is an urgent need for further studies in this area to examine adverse pregnancy outcomes associated with maternal use of antipsychotics in mono- or poly-therapy, as well as studies examining comparative effectiveness specific antipsychotic agents in pregnant population.
Supplementary Material
Acknowledgments
Disclosures: This study was supported by the National Institute of Mental Health R01 MH100216.
Y was supported by Pharmacoepidemiology Program at Harvard TH Chan School of Public Health, partially funded by Pfizer, Takeda, Bayer and Asisa. K was supported by a career development grant K01MH099141 from the National Institute of Mental Health. R is Principal Investigator of a research grant from Merck to the Brigham and Women’s Hospital for unrelated work. L is has received National Pregnancy Registry for Atypical Antipsychotics research support from Alkermes, AstraZeneca, Bristol-Myers Squibb/Otsuka, Forest/Actavis, Ortho-McNeil Janssen, and Sunovion Pharmaceuticals, Inc. and received other research support for unrelated work from Cephalon, Inc., National Institute on Aging, National Institutes of Health, National Institute of Mental Health, and Takeda/Lundbeck. SHD consulted for Boehringer-Ingelheim and UCB, and was the epidemiologist for the North American Antiepileptic Drugs pregnancy registry and advisor for the Antipsychotics Pregnancy Registry, which are funded my multiple companies. Y and B consult for Optum for unrelated projects. K, B, and S are investigators on grants to the Brigham and Women’s Hospital from Lilly and Pfizer and B on grants from Baxalta, unrelated to the topic of this manuscript.
Footnotes
Previous presentation: Some parts of the result of this study were presented at International Conference on Pharmacoepidemiology & Therapeutic Risk Management in Boston, MA in August, 2015.
References
- 1.Crystal S, Olfson M, Huang C, Pincus H, Gerhard T. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health affairs. 2009;28(5):w770–81. doi: 10.1377/hlthaff.28.5.w770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiology and drug safety. 2011;20(2):177–84. doi: 10.1002/pds.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camsari U, Viguera AC, Ralston L, Baldessarini RJ, Cohen LS. Prevalence of atypical antipsychotic use in psychiatric outpatients: comparison of women of childbearing age with men. Archives of women's mental health. 2014;17(6):583–6. doi: 10.1007/s00737-014-0465-0. [DOI] [PubMed] [Google Scholar]
- 4.Olfson M, King M, Schoenbaum M. Treatment of Young People With Antipsychotic Medications in the United States. JAMA psychiatry. 2015;72(9):867–74. doi: 10.1001/jamapsychiatry.2015.0500. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 6.Galbally M, Snellen M, Lewis AJ. A review of the use of psychotropic medication in pregnancy. Curr Opin Obstet Gynecol. 2011;23(6):408–14. doi: 10.1097/GCO.0b013e32834b92f3. [DOI] [PubMed] [Google Scholar]
- 7.Kopelman AE. Limb Malformations Following Maternal Use of Haloperidol. JAMA: The Journal of the American Medical Association. 1975;231(1):62. doi: 10.1001/jama.231.1.62. [DOI] [PubMed] [Google Scholar]
- 8.Hanson JW. Haloperidol and Limb Deformity. JAMA: The Journal of the American Medical Association. 1975;231(1):26. doi: 10.1001/jama.1975.03240130020015. [DOI] [PubMed] [Google Scholar]
- 9.Diav-Citrin O, Shechtman S, Ornoy S, Arnon J, Schaefer C, Garbis H, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. The Journal of clinical psychiatry. 2005;66(3):317–22. doi: 10.4088/jcp.v66n0307. [DOI] [PubMed] [Google Scholar]
- 10.Habermann F, Fritzsche J, Fuhlbruck F, Wacker E, Allignol A, Weber-Schoendorfer C, et al. Atypical antipsychotic drugs and pregnancy outcome: a prospective, cohort study. Journal of clinical psychopharmacology. 2013;33(4):453–62. doi: 10.1097/JCP.0b013e318295fe12. [DOI] [PubMed] [Google Scholar]
- 11.Wichman CL. Atypical antipsychotic use in pregnancy: a retrospective review. Archives of women's mental health. 2009;12(1):53–7. doi: 10.1007/s00737-008-0044-3. [DOI] [PubMed] [Google Scholar]
- 12.Huybrechts KF, Hernandez-Diaz S, Patorno E, Desai RJ, Mogun H, Dejene SZ, et al. Antipsychotic Use in Pregnancy and the Risk for Congenital Malformations. JAMA psychiatry. 2016;73(9):938–46. doi: 10.1001/jamapsychiatry.2016.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophrenia bulletin. 2010;36(3):518–44. doi: 10.1093/schbul/sbn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank RG, Conti RM, Goldman HH. Mental health policy and psychotropic drugs. Milbank Q. 2005;83(2):271–98. doi: 10.1111/j.1468-0009.2005.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandit S. 2012 Maternal and Child Health Update. National Governors Association; 2013. Issue Brief. [Google Scholar]
- 16.Palmsten K, Huybrechts KF, Mogun H, Kowal MK, Williams PL, Michels KB, et al. Harnessing the Medicaid Analytic eXtract (MAX) to Evaluate Medications in Pregnancy: Design Considerations. PloS one. 2013;8(6):e67405. doi: 10.1371/journal.pone.0067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernandez-Diaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiology and drug safety. 2013;22(1):16–24. doi: 10.1002/pds.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toh S, Li Q, Cheetham TC, Cooper WO, Davis RL, Dublin S, et al. Prevalence and trends in the use of antipsychotic medications during pregnancy in the U.S., 2001–2007: a population-based study of 585,615 deliveries. Archives of women's mental health. 2013;16(2):149–57. doi: 10.1007/s00737-013-0330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margulis AV, Kang EM, Hammad TA. Patterns of prescription of antidepressants and antipsychotics across and within pregnancies in a population-based UK cohort. Matern Child Health J. 2014;18(7):1742–52. doi: 10.1007/s10995-013-1419-2. [DOI] [PubMed] [Google Scholar]
- 20.Reis M, Kallen B. Maternal use of antipsychotics in early pregnancy and delivery outcome. Journal of clinical psychopharmacology. 2008;28(3):279–88. doi: 10.1097/JCP.0b013e318172b8d5. [DOI] [PubMed] [Google Scholar]
- 21.Boden R, Lundgren M, Brandt L, Reutfors J, Kieler H. Antipsychotics during pregnancy: relation to fetal and maternal metabolic effects. Archives of general psychiatry. 2012;69(7):715–21. doi: 10.1001/archgenpsychiatry.2011.1870. [DOI] [PubMed] [Google Scholar]
- 22.Petersen I, McCrea RL, Osborn DJ, Evans S, Pinfold V, Cowen PJ, et al. Discontinuation of antipsychotic medication in pregnancy: A cohort study. Schizophrenia research. 2014;159(1):218–25. doi: 10.1016/j.schres.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 23.Frank RG, Goldman HH, Hogan M. Medicaid And Mental Health: Be Careful What You Ask For. Health affairs. 2003;22(1):101–13. doi: 10.1377/hlthaff.22.1.101. [DOI] [PubMed] [Google Scholar]
- 24.Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Archives of general psychiatry. 2007;64(9):1032–9. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- 25.Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996-2004. Biol Psychiatry. 2007;62(2):107–14. doi: 10.1016/j.biopsych.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerman M, Ruggero CJ, Chelminski I, Young D. Is bipolar disorder overdiagnosed? The Journal of clinical psychiatry. 2008;69:935–40. doi: 10.4088/jcp.v69n0608. [DOI] [PubMed] [Google Scholar]
- 27.Kessler R. The Prevalence and Correlates of Adult ADHD in the United States: Results From the National Comorbidity Survey Replication. American Journal of Psychiatry. 2006;163(4):716. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kogut SJ, Yam F, Dufresne R. Prescribing of antipsychotic medication in a medicaid population: use of polytherapy and off-label dosages. J Manag Care Pharm. 2005;11(1):17–24. doi: 10.18553/jmcp.2005.11.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newport DJ, Calamaras MR, DeVane CL, Donovan J, Beach AJ, Winn S, et al. Atypical antipsychotic administration during late pregnancy: placental passage and obstetrical outcomes. The American journal of psychiatry. 2007;164(8):1214–20. doi: 10.1176/appi.ajp.2007.06111886. [DOI] [PubMed] [Google Scholar]
- 30.Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PloS one. 2014;9(4):e94112. doi: 10.1371/journal.pone.0094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peuskens J, Pani L, Detraux J, De Hert M. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421–53. doi: 10.1007/s40263-014-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. [Accessed Jul. 27, 2016];Drugs@FDA. Available at: https://www.accessdata.fda.gov/scripts/cder/drugsatfda.
- 33.Benjamin S, Morris S, McBeth J, Macfarlane GJ, Silman AJ. The association between chronic widespread pain and mental disorder: A population-based study. Arthritis & Rheumatism. 2000;43(3):561. doi: 10.1002/1529-0131(200003)43:3<561::AID-ANR12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.Desai RJ, Huybrechts KF, Hernandez-Diaz S, Mogun H, Patorno E, Kaltenbach K, et al. Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. Bmj. 2015;350:h2102. doi: 10.1136/bmj.h2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Kaiser Family Foundation. [Accessed Feb. 25, 2017];Health Coverage by Race and Ethnicity: The Potential Impact of the Affordable Care Act. 2013 Available at: https://kaiserfamilyfoundation.files.wordpress.com/2014/07/8423-health-coverage-by-race-and-ethnicity.pdf%5Cnhttp://kff.org/disparities-policy/issue-brief/health-coverage-by-race-and-ethnicity-the-potential-impact-of-the-affordable-care-act/
- 36.Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Wilson EC, Mathews TJ. National vital statistics reports. 1. Vol. 61. Hyattsville, MD: National Center for Health Statistics; 2012. Births: Final data for 2010. [PubMed] [Google Scholar]
- 37.Cook TB, Reeves GM, Teufel J, Postolache TT. Persistence of racial disparities in prescription of first-generation antipsychotics in the USA. Pharmacoepidemiology and drug safety. 2015;24(11):1197–206. doi: 10.1002/pds.3819. [DOI] [PubMed] [Google Scholar]
- 38.West SL, Savitz DA, Koch G, Strom BL, Guess HA, Hartzema A. Recall accuracy for prescription medications: self-report compared with database information. American journal of epidemiology. 1995;142(10):1103–12. doi: 10.1093/oxfordjournals.aje.a117563. [DOI] [PubMed] [Google Scholar]
- 39.West SL, Strom BL, Freundlich B, Normand E, Koch G, Savitz DA. Completeness of prescription recording in outpatient medical records from a health maintenance organization. Journal of clinical epidemiology. 1994;47(2):165–71. doi: 10.1016/0895-4356(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 40.Hartung DM, Middleton L, McFarland BH, Haxby DG, McDonagh MS, McConnell KJ. Use of administrative data to identify off-label use of second-generation antipsychotics in a Medicaid population. Psychiatric services. 2013;64(12):1236–42. doi: 10.1176/appi.ps.005482012. [DOI] [PubMed] [Google Scholar]
- 41.Petersen I, McCrea RL, Sammon CJ, Osborn DP, Evans SJ, Cowen PJ, et al. Risks and benefits of psychotropic medication in pregnancy: cohort studies based on UK electronic primary care health records. Health Technol Assess. 2016;20(23):1–176. doi: 10.3310/hta20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Etheredge L. A New Medicaid Program. Health affairs. 2003 doi: 10.1377/hlthaff.w3.426. [DOI] [PubMed] [Google Scholar]
- 43.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.