Abstract
A major challenge in urban ecology is to identify the environmental factors responsible for phenotypic differences between urban and rural individuals. However, the intercorrelation between the factors that characterize urban environments, combined with a lack of experimental manipulations of these factors in both urban and rural areas, hinder efforts to identify which aspects of urban environments are responsible for phenotypic differences. Among the factors modified by urbanization, anthropogenic sound, particularly traffic noise, is especially detrimental to animals. The mechanisms by which anthropogenic sound affects animals are unclear, but one potential mechanism is through changes in glucocorticoid hormone levels. We exposed adult house wrens, Troglodytes aedon, to either traffic noise or pink noise (a non-traffic noise control). We found that urban wrens had higher initial (pre-restraint) corticosterone than rural wrens before treatment, and that traffic noise elevated initial corticosterone of rural, but not urban, wrens. By contrast, restraint stress-induced corticosterone was not affected by noise treatment. Our results indicate that traffic noise specifically contributes to determining the glucocorticoid phenotype, and suggest that glucocorticoids are a mechanism by which anthropogenic sound causes phenotypic differences between urban and rural animals.
Keywords: anthropogenic sound, corticosterone, noise pollution, phenotype, urbanization
1. Introduction
Urban animals differ phenotypically from their rural conspecifics, probably as a result of the modified environment created by urbanization [1,2]. However, the environmental factors modified by urbanization, e.g. light, temperature and noise, are intercorrelated [3]. We are unaware of any experimental manipulations of these factors in both urban and rural areas to evaluate phenotypic responses in birds. Consequently, it is difficult to identify which aspects of the urban environment are responsible for the observed phenotypic differences and which factors determine whether a species can adjust to urban areas.
Anthropogenic sound, which in urban areas comes primarily from motor vehicles, is ubiquitous in urban environments, differs acoustically from most other types of noise, and has spread at an evolutionarily unprecedented rate [4]. It also hinders acoustic communication and predator detection [4,5], and reduces fitness and species diversity [6,7]. The mechanisms by which anthropogenic sound affects animals are unclear, but one potential mechanism is through changes in circulating levels of corticosterone, which is the primary glucocorticoid hormone in birds and a key part of the vertebrate endocrine stress response [8]. Because corticosterone secretion is sensitive to environmental stimuli, its receptors are found in most vertebrate cell types, and binding regulates target cell gene expression, this hormone links environmental change and phenotypic responses [9].
Noisy human activities are generally associated with elevated glucocorticoid levels [10–13], but the results of traffic noise exposure experiments are inconsistent [7,14,15]. Moreover, previous experiments exposed only rural free-ranging animals to noise, and used natural background noise as the control. Therefore, it is unclear, first, whether it is traffic noise per se or elevated noise in general that affects glucocorticoids and, second, whether traffic noise contributes to determining phenotypic differences between urban and rural individuals. To address these issues, we experimentally tested the effects of traffic noise versus pink noise (a non-traffic noise control) on the corticosterone levels of adult house wrens, Troglodytes aedon, in both urban and rural areas. We predicted that exposure to traffic noise or pink noise would elevate initial and stress-induced corticosterone of rural wrens, because they inhabit an environment with little anthropogenic noise. Furthermore, we predicted that traffic noise would have a stronger effect on corticosterone levels of rural wrens than pink noise, potentially because pink noise is less variable than traffic noise. By contrast, because urban wrens inhabit an environment that already has high levels of traffic noise, we predicted that neither traffic noise nor pink noise exposure would affect initial or stress-induced corticosterone levels.
2. Material and methods
We conducted our study from May to July 2016 at one urban location in Reno, NV, USA and one rural location in Sparks, NV, USA (see the electronic supplementary material for details of the study sites and urbanization estimates). We monitored house wren nests to determine the hatch date (day 0). On day 8, we caught the parents at the nest, collected a blood sample (for details of capture and blood collection, see the electronic supplementary material) within 3 min of capture to determine initial corticosterone, then placed each bird in a cloth bag for 30 min to determine stress-induced corticosterone [16]. We also measured body mass (0.1 g) and tarsus length (0.01 mm) to calculate body condition [17]. On day 9, we randomly assigned all nests to a traffic noise (n = 7 rural; 10 urban) or pink noise treatment (n = 7 rural; 5 urban). For details of the traffic and pink noise tracks, see the electronic supplementary material. On day 9, we placed an Arespark AS200 speaker 5 m from each nest-box and broadcast traffic or pink noise from 08.00 to 17.00. On day 10, we caught both parents and collected initial and stress-induced blood samples, as described above. We measured plasma corticosterone using enzyme-linked immunosorbent assays (Enzo Life Sciences; Farmingdale, NY, USA), validated for the house wren (see the electronic supplementary material for details).
(a). Statistical analyses
We conducted statistical analyses using R (v. 3.1.2). All final models met assumptions, and α = 0.05. We used a repeated measures linear mixed model (LMM) with random intercepts to test whether initial and stress-induced corticosterone levels were different within individuals before and after noise treatment, with the interaction of treatment (traffic or pink noise), site (urban or rural) and time (pre- or post noise treatment). If the interaction was significant, we broke down our analyses to LMMs examining whether pre-treatment initial and stress-induced corticosterone levels differed between sites. We included individual and nest as random effects, and sex and body condition as covariates in all models. We also initially included fledgling number as a covariate, but due to lack of variation, we removed it from all models.
3. Results
The three-way interaction between treatment, site and time was significant for initial corticosterone, but not stress-induced corticosterone (table 1). Before noise treatment, rural wrens had lower initial corticosterone than urban wrens (coeff = 9.5, s.e. = 1.8, t = 5.29, p < 0.0001). Traffic noise caused initial corticosterone to increase in rural, but not urban wrens, and pink noise had no effect on initial corticosterone of either urban or rural wrens (figure 1a). Stress-induced corticosterone did not differ between habitats or noise treatment either before or after noise exposure (figure 1b). Sex and body condition were not significant in any model (table 1). The number of fledglings did not differ between habitats (mean ± s.e.m.: urban = 7.1 ± 0.6, rural = 7.3 ± 0.9).
Table 1.
Model estimates of the effects of noise treatment and site on initial corticosterone levels pre- and post noise treatment (time). p-values <0.05 are indicated in bold.
| variable | estimate | s.e. | t-value | p-value |
|---|---|---|---|---|
| intercept | −2.9 | 12.0 | −0.2 | 0.81 |
| treatment × site × time | −12.7 | 5.3 | −2.4 | 0.016 |
| treatment × site | −0.18 | 5.0 | −0.04 | 0.97 |
| treatment × time | 15.9 | 3.7 | 4.3 | <0.0001 |
| site × time | 3.2 | 4.1 | 0.8 | 0.43 |
| treatment | −2.9 | 3.6 | −0.8 | 0.42 |
| site | 9.6 | 3.7 | 2.6 | 0.009 |
| time | −0.7 | 2.6 | −0.3 | 0.78 |
| body condition | 0.9 | 1.2 | 0.7 | 0.48 |
| sex | 0.2 | 1.7 | 0.1 | 0.90 |
Figure 1.
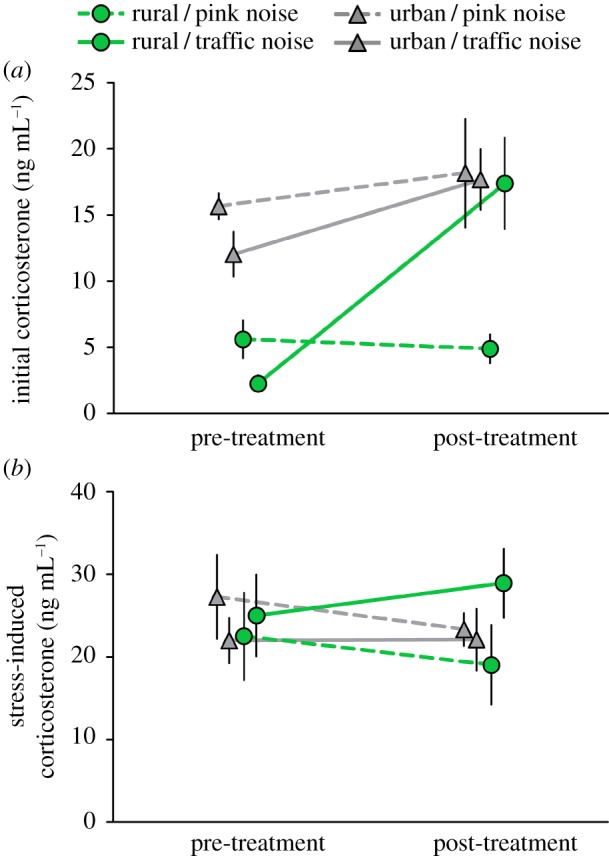
(a) Traffic noise exposure increased initial corticosterone in rural, but not urban, adult house wrens, whereas exposure to pink noise had no effect on initial corticosterone of either rural or urban wrens. (b) Stress-induced corticosterone did not differ between any of the groups. Points depict means (±s.e.m.).
4. Discussion
We found that traffic noise has environmentally dependent effects on glucocorticoid levels of breeding adult house wrens, whereby exposure to traffic noise elevated initial corticosterone of rural, but not urban wrens. By contrast, no corticosterone measure changed in response to pink noise exposure. Our study did not include a ‘no noise’ control, so it is unclear whether the response to pink noise differs from the response to no noise. Nevertheless, our findings indicate that an aspect specific to traffic noise, not noise in general, elevated initial corticosterone levels in rural wrens. The traffic noise-induced increased in initial corticosterone of rural wrens contrasts to the findings of previous studies on free-ranging rural birds [14,15], which found no effect of noise exposure on initial corticosterone. These contrasting findings could be due to differences in the duration of noise exposure. Alternatively, the previous studies investigated nestlings, whereas our study investigated adults, which suggests that nestlings and adults may respond differently to noise exposure.
The specific aspect of traffic noise that caused initial corticosterone to increase in rural, but not urban wrens is unclear. It is possible that, compared to pink noise, the variable nature of traffic noise (i.e., unpredictable changes in frequency and amplitude associated with sirens and other especially loud traffic noises) acted as a stressor for rural wrens. Alternatively or in addition, traffic noise may have been less effective than pink noise at masking other sounds in the environment that elevate corticosterone. Compared to pink noise, traffic noise may have reduced the ability of rural wrens to hear predators and rival neighbours, to communicate acoustically with their mate, and/or to hear the begging calls of their chicks [4,5]. A lack of similar response to experimental noise exposure in urban wrens may be because they have adjusted to the already high levels of traffic noise in urban areas by changing aspects of their anti-predator behaviour and/or vocal communication [5,18]. It is notable that traffic noise exposure elevated initial corticosterone of rural wrens to levels similar to those of urban wrens, which is consistent with the idea that the elevated initial corticosterone levels of urban wrens, relative to rural wrens, is determined, at least partly, by higher levels of traffic noise in urban areas. Unequivocally demonstrating whether higher levels of traffic noise in urban areas elevates initial corticosterone levels would entail a comparison before and after either reducing traffic noise in urban areas or translocating urban individuals to areas lacking traffic noise.
In contrast to initial corticosterone, stress-induced corticosterone of urban and rural wrens was not significantly affected by either noise treatment. This finding suggests that traffic noise specifically affects initial corticosterone levels, which could be related to the different roles of initial versus stress-induced corticosterone. Initial corticosterone is thought to regulate energy availability and daily processes, such as feeding and activity cycles, whereas stress-induced corticosterone is thought to redirect behaviour and energy stores solely towards immediate survival [19].
Our study suggests that traffic noise, in particular, contributes to determining the glucocorticoid phenotype, and is consistent with the proposition that glucocorticoids are a mechanism by which anthropogenic sound causes phenotypic differences between urban and rural animals. As glucocorticoids have wide-ranging effects on physiology and behaviour, this traffic noise-induced difference in glucocorticoids potentially also underlies differences in other phenotypic traits between urban and rural animals. More broadly, our findings demonstrate that phenotypic responses are environmentally dependent, and highlight that evaluating phenotypic responses across environmental gradients, such as urban to rural, can improve our understanding of physiological adaptations to challenging environments.
Supplementary Material
Acknowledgements
We thank the Reno Parks, Recreation and Community Services Department, particularly S. Churchillo, and the UNR Agricultural station for access to field sites; and J. Liou, C. Munguia and D. Staten for assistance in the field.
Ethics
Our methods were approved by the UNR institutional animal care and use committee and conducted under relevant state and federal permits.
Data accessibility
Data from the manuscript are archived in Dryad (http://dx.doi.org/10.5061/dryad.qb08c) [20].
Authors' contributions
J.Q.O. and N.H. conceived of and designed the study, collected and analysed the data and edited the manuscript. S.D. carried out the hormone assay, contributed to data analysis and wrote the manuscript. All authors gave final approval for publication and agree to be accountable for the content therein.
Competing interests
We have no competing interests.
Funding
Funding was provided by the Department of Biology at UNR and an Honours Undergraduate Research Award to N.H.
References
- 1.Deviche P, Davies S. 2014. Reproductive phenology of urban birds: environmental cues and mechanisms. In Avian urban ecology (eds Gil D, Brumm H), pp. 98–115. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Atwell JW, Cardoso GC, Whittaker DJ, Price TD, Ketterson ED. 2014. Hormonal, behavioral, and life-history traits exhibit correlated shifts in relation to population establishment in a novel environment. Am. Nat. 184, E147–E160. ( 10.1086/678398) [DOI] [PubMed] [Google Scholar]
- 3.Pickett STA, Cadenasso ML, Grove JM, Nilon CH, Pouyat RV, Zipperer WC, Costanza R. 2001. Urban ecological sysytems: linking terrestrial ecological, physical, and socioeconomic components of metropolitan areas. Annu. Rev. Ecol. Syst. 32, 127–157. ( 10.1146/annurev.ecolsys.32.081501.114012) [DOI] [Google Scholar]
- 4.Slabbekoorn H, Ripmeester EAP. 2008. Birdsong and anthropogenic noise: implications and applications for conservation. Mol. Ecol. 17, 72–83. ( 10.1111/j.1365-294X.2007.03487.x) [DOI] [PubMed] [Google Scholar]
- 5.Meillère A, Brischoux F, Angelier F. 2015. Impact of chronic noise exposure on antipredator behavior: an experiment in breeding house sparrows. Behav. Ecol. 26, 569–577. ( 10.1093/beheco/aru232) [DOI] [Google Scholar]
- 6.Francis CD, Ortega CP, Cruz A. 2009. Noise pollution changes avian communities and species interactions. Curr. Biol. 19, 1415–1419. ( 10.1016/j.cub.2009.06.052) [DOI] [PubMed] [Google Scholar]
- 7.Hayward LS, Bowles AE, Ha JC, Wasser SK. 2011. Impacts of acute and long-term vehicle exposure on physiology and reproductive success of the northern spotted owl. Ecosphere 2, art65 ( 10.1890/ES10-00199.1) [DOI] [Google Scholar]
- 8.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. 1998. Ecological bases of hormone–behavior interactions: the ‘emergency life history stage’. Am. Zool. 38, 191–206. ( 10.1093/icb/38.1.191) [DOI] [Google Scholar]
- 9.Wingfield JC. 2015. Coping with change: a framework for environmental signals and how neuroendocrine pathways might respond. Front. Neuroendocrinol. 37, 89–96. ( 10.1016/j.yfrne.2014.11.005) [DOI] [PubMed] [Google Scholar]
- 10.Creel S, Fox JE, Hardy A, Sands J, Garrott B, Peterson RO. 2002. Snowmobile activity and glucocorticoid stress responses in wolves and elk. Conserv. Biol. 16, 809–814. ( 10.1046/j.1523-1739.2002.00554.x) [DOI] [Google Scholar]
- 11.Crino OL, Klaassen B, Oorschot V, Johnson EE, Malisch JL, Breuner CW. 2011. Proximity to a high traffic road: glucocorticoid and life history consequences for nestling white-crowned sparrows. Gen. Comp. Endocrinol. 173, 323–332. ( 10.1016/j.ygcen.2011.06.001) [DOI] [PubMed] [Google Scholar]
- 12.Thiel D, Jenni-Eiermann S, Braunisch V, Palme R, Jenni L. 2008. Ski tourism affects habitat use and evokes a physiological stress response in capercaillie Tetrao urogallus: a new methodological approach. J. Appl. Ecol. 45, 845–853. ( 10.1111/j.1365-2664.2008.01465.x) [DOI] [Google Scholar]
- 13.Owen DAS, Carter ET, Holding ML, Islam K, Moore IT. 2014. Roads are associated with a blunted stress response in a North American pit viper. Gen. Comp. Endocrinol. 202, 87–92. ( 10.1016/j.ygcen.2014.04.020) [DOI] [PubMed] [Google Scholar]
- 14.Crino OL, Johnson EE, Blickley JL, Patricelli GL, Breuner CW. 2013. Effects of experimentally elevated traffic noise on nestling white-crowned sparrow stress physiology, immune function and life history. J. Exp. Biol. 216, 2055–2062. ( 10.1242/jeb.081109) [DOI] [PubMed] [Google Scholar]
- 15.Angelier F, Meillère A, Grace J, Trouvé C, Brischoux F. 2016. No evidence for an effect of traffic noise on the development of the corticosterone stress response in an urban exploiter. Gen. Comp. Endocrinol. 232, 43–50. ( 10.1016/j.ygcen.2015.12.007) [DOI] [PubMed] [Google Scholar]
- 16.Ouyang JQ, Sharp PJ, Dawson A, Quetting M, Hau M. 2011. Hormone levels predict individual differences in reproductive success in a passerine bird. Proc. R. Soc. B 278, 2537–2545. ( 10.1098/rspb.2010.2490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891. ( 10.1111/j.1600-0706.2009.17643.x) [DOI] [Google Scholar]
- 18.Slabbekoorn H, Peet M. 2003. Ecology: birds sing at a higher pitch in urban noise. Nature 424, 267 ( 10.1038/424267a) [DOI] [PubMed] [Google Scholar]
- 19.Landys MM, Ramenofsky M, Wingfield JC. 2006. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 148, 132–149. ( 10.1016/j.ygcen.2006.02.013) [DOI] [PubMed] [Google Scholar]
- 20.Davies S, Haddad N, Ouyang JQ. 2017. Data from: Stressful city sounds: glucocorticoid responses to experimental traffic noise are environmentally dependent Dryad Digital Repository. ( 10.5061/dryad.qb08c) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Davies S, Haddad N, Ouyang JQ. 2017. Data from: Stressful city sounds: glucocorticoid responses to experimental traffic noise are environmentally dependent Dryad Digital Repository. ( 10.5061/dryad.qb08c) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data from the manuscript are archived in Dryad (http://dx.doi.org/10.5061/dryad.qb08c) [20].


