Abstract
In wetland ecosystems, birds and fish are important dispersal vectors for plants and invertebrates, but the consequences of their interactions as vectors are unknown. Darwin suggested that piscivorous birds carry out secondary dispersal of seeds and invertebrates via predation on fish. We tested this hypothesis in the great cormorant (Phalacrocorax carbo L.). Cormorants regurgitate pellets daily, which we collected at seven European locations and examined for intact propagules. One-third of pellets contained at least one intact plant seed, with seeds from 16 families covering a broad range of freshwater, marine and terrestrial habitats. Of 21 plant species, only two have an endozoochory dispersal syndrome, compared with five for water and eight for unassisted dispersal syndromes. One-fifth of the pellets contained at least one intact propagule of aquatic invertebrates from seven taxa. Secondary dispersal by piscivorous birds may be vital to maintain connectivity in meta-populations and between river catchments, and in the movement of plants and invertebrates in response to climate change. Secondary dispersal pathways associated with complex food webs must be studied in detail if we are to understand species movements in a changing world.
Keywords: great cormorant Phalacrocorax carbo, fish, piscivory, endozoochory, seed dispersal, wetland
1. Introduction
Dispersal is crucial for the persistence of species inhabiting aquatic habitats, because these are often discontinuous in space and time [1]. Many aquatic species disperse as seeds or diapausing stages by vectors such as water, wind, fish, waterbirds or mammals [2]. Successive transportation by multiple vectors (secondary dispersal) can extend dispersal routes, increasing connectivity for plants and invertebrates [3]. Although waterbirds and fish are both major vectors [4,5], the possibility of secondary dispersal by their interactions has been little explored [6].
After daytime fishing, piscivorous birds such as cormorants, mergansers, pelicans and herons commonly roost close to water at night and regurgitate indigestible prey remains as pellets. The potential of this bird–fish interaction for secondary dispersal previously led Darwin [7] and Mellors [8] to experimentally feed fish containing seeds or invertebrates to piscivorous birds, later retrieving viable propagules in excreta. There are anecdotal observations of endozoochory by piscivorous birds in the field: e.g. one Australian pelican Pelecanus conspicillatus dropping contained seeds and invertebrate eggs, and two great cormorant Phalacrocorax carbo stomachs contained Carex seeds [9,10]. This supports potential dispersal by piscivorous birds, but quantitative evidence is lacking [6].
The aim of this study was to quantify the importance of secondary dispersal of plants and invertebrates by piscivorous birds. Specifically, we considered (i) the taxonomic and ecological diversity of propagules egested by piscivores, (ii) the relationship between ingested fish species and propagules retrieved, (iii) the frequency and generality of this dispersal mechanism across localities. We studied these questions in seven colonies of great cormorants.
2. Methods
(a). Study species
The great cormorant is a widespread colonial waterbird with an expanding population of 120 000 individuals in northwestern Europe and a global population of several million [11]. Great cormorants are piscivorous and forage during daytime in coastal areas, estuaries, lakes and rivers [12]. Important freshwater prey species include Cyprinidae (e.g. common roach Rutilus rutilis, common carp Cyprinus carpio) and Percidae (e.g. European perch Perca fluviatilis) (e.g. [13]). Indigestible prey remains are regurgitated daily in one pellet of 5–10 g dry mass [13].
(b). Field sampling and examination
Pellets were collected below roosting trees or on shores at seven locations in Denmark, Sweden and The Netherlands (electronic supplementary material, figure S1 and table S1). Pellets were individually stored in zip bags at −20°C (n = 61), at 7°C (n = 31) or were lost in the post for several weeks (n = 20). Pellets were weighed and examined in the laboratory for plant diaspores (hereafter ‘seeds’), intact invertebrates (including diapausing stages) and fish remains. To exclude propagules that potentially attached to the exterior of pellets after egestion, we only included propagules completely covered in mucus (electronic supplementary material, figure S1).
Fish remains and propagules were identified and examined for damage under a microscope (keys in the electronic supplementary material, table S2). Fish length was estimated using species-specific regressions for sagittal otolith width [14]. For plant taxa, Ellenberg habitat indicator values for moisture (‘Feuchtigkeit’; F) [15,16] and dispersal syndromes [17] were identified.
We attempted to hatch or germinate propagules from 51 unfrozen pellets. Individual seeds were placed on 1% agar with a 14 h light (22 ± 2°C) to dark (18 ± 2°C) schedule, and monitored daily for two months. Invertebrate propagules were placed at 25°C on tissue culture plates with 1 ml deionized water in the shade (total darkness for sponge gemmules).
(c). Statistical analyses
Non-random co-occurrence patterns among particular fish species and propagules were analysed in a network analysis in R [18]. For every pairwise combination of species in the pellets, we calculated Spearman rank correlations (ρ) to analyse possible associations of their presences. All pairwise combinations formed a co-occurrence matrix for all pellet contents, which we visualized for correlations with ρ > 0.3 and p < 0.05 as edges (connections) between nodes (species) using the plot.network() function in package statnet [19]. Node size is proportional to the number of pellets containing that species, and edge width is proportional to ρ. The R code including more details is available in the electronic supplementary material.
3. Results
Forty-eight of 112 pellets (43%) contained at least one intact plant or invertebrate propagule [20]. Broken propagules were found in a further eight pellets. Thirty-seven pellets (33%) contained one or more intact seed, and 22 pellets (20%) one or more intact invertebrate propagule. Seeds were found at six of seven locations, and invertebrate propagules at two locations (table 1). Mean ± s.d. pellet dry mass was 7.65 ± 6.96 g (range 1.59–49.23 g, n = 83).
Table 1.
Intact plant seeds and invertebrates from cormorant pellets. Ellenberg F classes 4–6 as representing dry-to-moist, 7–9 moist-to-wet and 10–12 wet-or-submerged habitats [16]. Species are sorted by the number of recovered propagules, while indicating the number of pellets, viable propagules that germinated or hatched (per number tested), sampling locations (Ringkøbing Fjord (RK), Roxen Lake (RL), Havsstensfjord Vadholmen (HV), Björningarna (B), North Mittholmarna (NM), South Mittholmarna (SM), Fortmond (F)), and assigned dispersal syndromes [17]. Species indicated in bold are not known to be dispersed by European dabbling ducks [21]. Actinidia deliciosa is alien to Europe, and therefore has no Ellenberg F-value.
| category | species | family | Ellenberg F | dispersal syndrome | # intact propagules | # pellets | # germinated or hatched/ attempted | sampling locations |
|---|---|---|---|---|---|---|---|---|
| plant | unknown | Charophyceae | 15 | 5 | 0/15 | RK, F | ||
| plant | Urtica dioica | Urticaceae | 6 | epizoochory | 11 | 6 | 0/9 | RL, F |
| plant | Schoenoplectus tabernaemontani | Cyperaceae | 10 | barochory | 8 | 6 | 1/7 | RK, RL |
| plant | Betula pendula | Betulaceae | 5 | anemochory | 5 | 5 | — | RL, HV, B |
| plant | Suaeda maritima | Amaranthaceae | 8 | hydrochory | 5 | 5 | 0/4 | B, F |
| plant | Atriplex patula | Amaranthaceae | 5 | epizoochory | 3 | 3 | 1/3 | RK |
| plant | Limosella aquatica | Scrophulariaceae | 8 | barochory | 3 | 2 | 0/3 | F |
| plant | Zannichellia palustris | Potamogetonaceae | 12 | hydrochory | 3 | 1 | 0/3 | F |
| plant | Chenopodium glaucum | Amaranthaceae | 6 | barochory | 4 | 3 | 1/4 | RK |
| plant | Potentilla anserina | Rosaceae | 5 | barochory | 2 | 1 | 0/2 | F |
| plant | Actinidia deliciosa | Actinidiaceae | endozoochory | 1 | 1 | 0/1 | F | |
| plant | Alopecurus pratensis | Poaceae | 5 | barochory | 1 | 1 | — | RL |
| plant | Carex nigra | Cyperaceae | 8 | hydrochory | 1 | 1 | 0/1 | F |
| plant | Cochlearia officinalis | Brassicaceae | 6 | barochory | 1 | 1 | — | SM |
| plant | Eleocharis uniglumis | Cyperaceae | 9 | epizoochory | 1 | 1 | 0/1 | RK |
| plant | Plantago major | Plantaginaceae | 5 | barochory | 1 | 1 | 0/1 | F |
| plant | Rubus fruticosus | Rosaceae | 6 | endozoochory | 1 | 1 | 0/1 | F |
| plant | Ruppia cirrhosa | Ruppiaceae | 12 | hydrochory | 1 | 1 | — | NM, SM |
| plant | Sagina apetala | Caryophyllaceae | 4 | anemochory | 1 | 1 | — | B |
| plant | Salix triandra | Salicaceae | 8 | anemochory | 1 | 1 | 0/1 | F |
| plant | Veronica beccabunga | Plantaginaceae | 10 | barochory | 1 | 1 | 0/1 | F |
| plant | Zostera marina | Zosteraceae | 12 | hydrochory | 1 | 1 | — | B |
| plant | unknown | Apiaceae | 1 | 1 | 0/1 | RK | ||
| plant | unknown | Poaceae | 1 | 1 | — | RL | ||
| invertebrate | Ephydatia fluviatilis | Spongillidae | 186 | 1 | 0/186 | F | ||
| invertebrate | Daphnia pulex agg. (Group) | Daphniidae | 24 | 7 | 0/23 | RL, F | ||
| invertebrate | Cristatella mucedo | Cristatellidae | 19 | 14 | 0/11 | RL, F | ||
| invertebrate | Plumatella repens | Plumatellidae | 12 | 6 | 1/11 | RL, F | ||
| invertebrate | Plumatella fungosa | Plumatellidae | 10 | 4 | 0/10 | F | ||
| invertebrate | Plumatella emarginata | Plumatellidae | 3 | 3 | 0/3 | F | ||
| invertebrate | Plumatella casmiana | Plumatellidae | 1 | 1 | 0/1 | F |
Seventy-three intact diaspores were recovered from 16 families of angiosperms plus Charophyceae. Among intact seeds, we identified 21 taxa to species level and three to family level (table 1). Three plant families (Adoxaceae, Fabaceae and Polygonaceae) and the Potamogeton-genus were represented only by broken seeds. Actinidia deliciosa (Kiwi fruit) is alien to Europe, although common in gardens. Five of the 21 species are characteristic of wet or submerged habitats, five of moist-to-wet habitats and 10 of dry-to-moist habitats. Dispersal syndromes varied, with only two species assigned to endozoochory compared with five for hydrochory and eight for barochory (unassisted, table 1). Three of 54 unfrozen seeds (5.6%) germinated: one Chenopodium glaucum, one Schoenoplectus tabernaemontani and one Atriplex patula.
We found 256 intact invertebrate propagules, including 186 gemmules of the sponge Ephydatia fluviatilis, from one pellet. Seven different invertebrate taxa were found (from four families), a lower diversity than of plants (χ2 = 74.9, d.f. = 1, p < 0.001). One Plumatella casmiana statoblast was found in a Dutch pellet (probably alien for Europe, T. Wood 2017, personal communication) and one Plumatella repens statoblast hatched.
Fish remains were found in 104 pellets, with a mean ± s.d. of 1.5 ± 1.2 fish taxa (range 0–4) and 10.9 ± 12.8 individuals per pellet (range 0–51), of mean length 7.7 ± 3.7 cm (range 3.2–41.3). Common taxa were European perch, Eurasian ruffe Gymnocephalus cernuus and common roach (electronic supplementary material, table S3). Fish lengths varied between species and locations (electronic supplementary material, table S4).
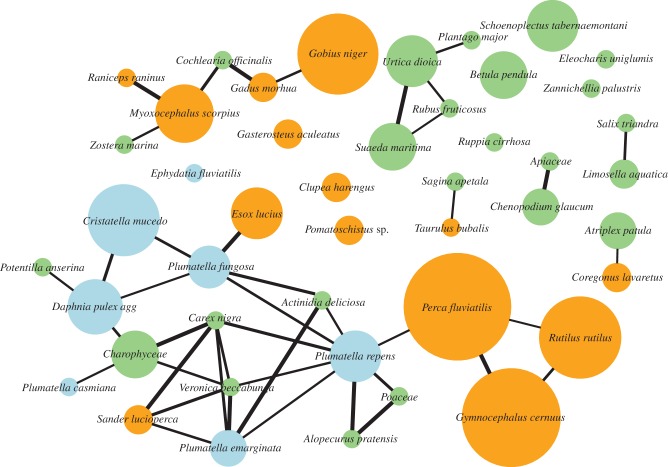
Fish, plant and invertebrate contents of pellets were partly interrelated (electronic supplementary material, table S5). Pellets with more fish held a higher diversity of invertebrates, and pellets with more invertebrate taxa held significantly more plant taxa. Fish species associated with multiple propagule species were Zander Sander lucioperca and bullhead Myoxocephalus scorpius; five additional fish species were associated directly with one propagule species (figure 1; electronic supplementary material, table S6).
Figure 1.
Network visualization of pellet contents depicting fish (orange), plant (green) and invertebrate (blue) species in nodes whose size depicts their abundance on a log-scale. Connecting lines depict correlations among species; line width scales to ρ. Unconnected species have no significant associations.
4. Discussion
This is the first quantitative field study of dispersal of plants and invertebrates by piscivorous birds. Great cormorants regurgitate pellets containing intact propagules previously ingested by fish prey. Pellets contained seeds of terrestrial, freshwater and marine plant species, indicating potential secondary dispersal for species with a range of habitat requirements. Terrestrial seeds are often blown or washed into the water and ingested (like aquatic seeds) by fish, followed by avian secondary dispersal. We confirmed the viability of seeds of three plant species and one bryozoan statoblast, and many of the other taxa we recorded are already known to survive passage through the guts of waterfowl [5]. Our first exploration of species interactions (figure 1) suggests secondary dispersal may connect aquatic and terrestrial environments, e.g. associations of Atlantic cod Gadus morhua with Brassicaceae and longspined bullhead Taurulus bubalis with Caryophyllaceae.
Among prerequisites for effective secondary dispersal are that (i) birds reach a new suitable location before egestion and (ii) propagules can establish in a suitable microhabitat. Both aspects depend on bird behaviour. Many cormorants roost in trees partially overhanging the water and partially above land, providing opportunities for both aquatic and terrestrial plants to reach suitable microhabitats. Cormorants may also provide germinating plants with nutrient-rich guano [22]. Great cormorants often travel up to 45 km between roosting and foraging locations, with occasional movements further than 200 km [23]. Tags inserted in fish have been retrieved further than 39 km from tagging locations [24], and further than 10 km in one of our study locations (Lake Roxen). Dispersal over several tens of kilometres is therefore possible throughout the annual cycle, and perhaps much further during migrations.
Our results raise key questions for future research, including (i) possible overlap of secondary dispersal with primary dispersal by other vectors, e.g. ducks. We found six plants in cormorant pellets not recorded from the diet of European dabbling ducks (table 1), and reported bird-mediated dispersal of freshwater sponges for the first time. Detailed comparisons between primary and secondary dispersal by different avian vectors are needed. (ii) The importance of secondary dispersal relative to other vectors, and how its importance varies with colony size, over seasons and between individual birds. This study found considerable spatial and temporal variability in pellet content, which deserves more detailed investigations. (iii) Germinability of unfrozen seeds was low compared with studies on omnivorous waterbirds, possibly because passing two digestive systems severely impacts viability. Future research should extract propagules quickly from piscivore excreta and study effects of double gut passage on viability. (iv) We found secondary dispersal of alien species (table 1), but further exploration is needed. (v) Associations among particular fish species, among propagule species and between fish and propagule species require more detailed inspections to unravel specific secondary dispersal pathways.
We conclude that piscivorous birds may be major dispersal vectors that require more scientific attention. As most plants dispersed lack a fleshy fruit, they are assumed to rely on mechanisms with less potential for long-distance dispersal than endozoochory (table 1). Secondary dispersal by piscivorous birds may play an important role in maintaining connectivity in meta-populations and between river catchments, and in the movement of plants and invertebrates in response to climate change.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Niels Jepsen, Erik Kleyheeg, Karl Lundström and Anders Nilsson for help collecting pellets. This is publication 6366 of the Netherlands Institute of Ecology.
Ethics
All fieldwork was authorized by landowners.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.fj669. R code for the network analysis is available in the electronic supplementary material.
Authors' contributions
C.H.A.v.L.: Collected pellets, analysed the data and wrote the article. Á.L.-K.: Collected pellets, identified and germinated propagules. M.O.: Collected pellets and identified fish remains. A.J.G.: Conceptualized and designed the study, and co-wrote the article. All authors contributed to writing the manuscript, approved the final version and are accountable for all content.
Competing interests
We have no competing interests.
Funding
This study was supported by Marie S-Curie Actions-H2020 grant no. 750240 of the EU to C.H.A.v.L., by Spanish Ministerio de Economía, Industria y Competitividad project CGL2016-76067-P (AEI/FEDER, EU) to A.J.G. and by ÚNKP-17-3-I-DE-385 New National Excellence Program of the Ministry of Human Capacities to Á.L.-K. Pellet collection was partly financed by LONA (LOcal Nature Conservation, Swedish Environmental Protection Agency).
References
- 1.Howe HF, Smallwood J. 1982. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 13, 201–228. ( 10.1146/annurev.es.13.110182.001221) [DOI] [Google Scholar]
- 2.Bilton DT, Freeland JR, Okamura B. 2001. Dispersal in freshwater invertebrates. Annu. Rev. Ecol. Syst. 32, 159–181. ( 10.1146/annurev.ecolsys.32.081501.114016) [DOI] [Google Scholar]
- 3.Hämäläinen A, Broadley K, Droghini A, Haines JA, Lamb CT, Boutin S, Gilbert S. 2017. The ecological significance of secondary seed dispersal by carnivores. Ecosphere 8, e01685 ( 10.1002/ecs2.1685) [DOI] [Google Scholar]
- 4.Horn MH, et al. 2011. Seed dispersal by fishes in tropical and temperate fresh waters: the growing evidence. Acta Oecol. 37, 561–577. ( 10.1016/j.actao.2011.06.004) [DOI] [Google Scholar]
- 5.van Leeuwen CHA, Van der Velde G, Van Groenendael JM, Klaassen M. 2012. Gut travellers: internal dispersal of aquatic organisms by waterfowl. J. Biogeogr. 39, 2031–2040. ( 10.1111/jbi.12004) [DOI] [Google Scholar]
- 6.Green AJ. 2016. The importance of waterbirds as an overlooked pathway of invasion for alien species. Divers. Distrib. 22, 239–247. ( 10.1111/ddi.12392) [DOI] [Google Scholar]
- 7.Darwin C. 1859. On the origin of species by means of natural selection. London, UK: John Murray. [Google Scholar]
- 8.Mellors WK. 1975. Selective predation of ephippial Daphnia and resistance of ephippial eggs to digestion. Ecology 56, 974–980. ( 10.2307/1936308) [DOI] [Google Scholar]
- 9.Green AJ, Jenkins KM, Bell D, Morris PJ, Kingsford RT. 2008. The potential role of waterbirds in dispersing invertebrates and plants in arid Australia. Freshw. Biol. 53, 380–392. [Google Scholar]
- 10.Sterbertz I. 1992. Alimentation examinations of water birds at Szeged-Fehértó. Móra Ferenc Múzeum Évkönyve (1989–1990) 1, 505–518. [Google Scholar]
- 11.Wetlands-International. 2017. Waterbird Population Estimates. Retrieved from wpe.wetlands.org on Wednesday 22 Mar 2017.
- 12.Magath V, Abraham R, Helbing U, Thiel R. 2016. Link between estuarine fish abundances and prey choice of the great cormorant Phalacrocorax carbo (Aves, Phalacrocoracidae). Hydrobiologia 763, 313–327. ( 10.1007/s10750-015-2384-0) [DOI] [Google Scholar]
- 13.Boström MK, Lunneryd S-G, Ståhlberg H, Karlsson L, Ragnarsson B. 2012. Diet of the Great Cormorant (Phalacrocorax carbo sinensis) at two areas at Lövstabukten, South Bothnian Sea, Sweden, based on otolith size-correction factors. Ornis Fenn. 89, 157. [Google Scholar]
- 14.Leopold MF, van Damme CJG, Phillippart CJM, Winter CJN. 2001. Otoliths of the North Sea: Interactive guide of identification of fish from the SE North Sea, Wadden Sea and adjacent fresh waters by means of otoliths and other hard parts. World Biodiversity Database CD-ROM Series. Amsterdam, The Netherlands: Expert Center for Taxonomic Identification (ETI). [Google Scholar]
- 15.Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulissen D. 1992. Zeigerwerte von Pflanzen in Mitteleuropa. Scr Geobotanica 18, 1–258. [Google Scholar]
- 16.Hill MO, Mountford JO, Roy DB, Bunce RGH. 1999. Ellenberg's indicator values for British plants. ECOFACT volume 2 technical annex (vol. 2). Institute of Terrestrial Ecology. [Google Scholar]
- 17.Julve P. 1998. Baseflor. Index botanique, écologique et chorologique de la flore de France. Lille, France: Institut Catholique de Lille. [Google Scholar]
- 18.R Development Core Team. 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org). [Google Scholar]
- 19.Handcock M, Hunter D, Butts C, Goodreau S, Krivitsky P, Bender-deMoll S, Morris M.2016. Statnet: Software Tools for the Statistical Analysis of Network Data. The Statnet Project http://www.statnet.org .
- 20.van Leeuwen CHA, Lovas-Kiss Á, Ovegård M, Green AJ.2017. Data from: Great cormorants reveal overlooked secondary dispersal of plants and invertebrates by piscivorous waterbirds. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
- 21.Soons MB, Brochet A-L, Kleyheeg E, Green AJ. 2016. Seed dispersal by dabbling ducks: an overlooked dispersal pathway for a broad spectrum of plant species. J. Ecol. 104, 443–455. ( 10.1111/1365-2745.12531) [DOI] [Google Scholar]
- 22.Kolb GS, Jerling L, Hambäck PA. 2010. The impact of cormorants on plant–arthropod food webs on their nesting islands. Ecosystems 13, 353–366. ( 10.1007/s10021-010-9323-8) [DOI] [Google Scholar]
- 23.Wright GA. 2003. Turnover in a wintering cormorant population: implications for management. In Interaction between fish and birds: implications for management (ed. Cowx IG.), pp. 345–353. Oxford, UK: Fishing News Books, Blackwell Science. [Google Scholar]
- 24.Skov C, Jepsen N, Baktoft H, Jansen T, Pedersen S, Koed A. 2014. Cormorant predation on PIT-tagged lake fish. J. Limnol. 73, 177–186. ( 10.4081/jlimnol.2014.715) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- van Leeuwen CHA, Lovas-Kiss Á, Ovegård M, Green AJ.2017. Data from: Great cormorants reveal overlooked secondary dispersal of plants and invertebrates by piscivorous waterbirds. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.fj669. R code for the network analysis is available in the electronic supplementary material.