Abstract
Many ant and termite colonies are defended by soldiers with powerful mandibles or chemical weaponry. Recently, it was reported that several stingless bee species also have soldiers for colony defence. These soldiers are larger than foragers, but otherwise lack obvious morphological adaptations for defence. Thus, how these soldiers improve colony fitness is not well understood. Robbing is common in stingless bees and we hypothesized that increased body size improves the ability to recognize intruders based on chemosensory cues. We studied the Neotropical species Tetragonisca angustula and found that large soldiers were better than small soldiers at recognizing potential intruders. Larger soldiers also had more olfactory pore plates on their antennae, which is likely to increase their chemosensory sensitivity. Our results suggest that improved enemy recognition might select for increased guard size in stingless bees.
Keywords: soldiers, polymorphism, physical castes, Tetragonisca angustula, Meliponini
1. Introduction
Many ant and termite species have a soldier caste for colony defence. Soldiers (or majors) are often equipped with muscular heads and strong mandibles, which is likely to improve the overall defensive performance of soldiers [1]. Despite the benefits of having specialists for colony defence, many social insect species do not have soldiers [1]. It is thought that developmental constraints, individual-level selection or a reduced multifunctionality of soldiers may prevent the evolution of soldiers in many species [1–3]. Moreover, many bee and wasp species might not need soldiers, because workers are equipped with a sting [1].
Stingless bees are the largest group of eusocial bees (greater than 500 species) and several Neotropical species have recently been shown to produce soldiers (i.e. bees responsible for defence that differ in size and/or shape from other workers) for colony defence [3,4]. In these species, entrance guards are larger than their nest-mates, but they otherwise lack obvious defensive adaptations [3,4]. This raises the question how larger entrance guards improve colony defence. Intra- and interspecific robbing of vital resources such as honey, pollen or building material is widespread in stingless bees and often leads to colony death [5,6]. Additionally, workers face a loss of fitness through reproductive parasitism as conspecific virgin queens may try to sneak into colonies [7]. Larger guards might be better at fighting these intruders [4]. The first challenge for guards is to recognize whether incoming bees pose a threat, especially if they are visually less discernible conspecifics [8]. Recognition of conspecific intruders in stingless bees is based on the ability of guards to perceive differences between the cuticular hydrocarbons (CHCs) of an incoming bee (i.e. a label) and the memorized colony odour (i.e. a template) [8]. A large body size could reduce recognition errors because size is an important determinant of sensory perception [9,10]. In bumblebees, larger workers can accommodate more sensory sensilla on the antennae and have increased antennal perception [10]. In weaver ants, aggression towards non-nest-mates increases with the number of antennal sensilla [11].
We hypothesized that larger soldiers are better at recognizing conspecific intruders. This, in turn, could help explain the evolution of larger guards in stingless bees. At the same time, we expected that larger soldiers have more antennal sensilla, which is likely to improve sensory perception. We tested this hypothesis in Tetragonisca angustula and found a positive relationship between soldier size, enemy recognition and olfactory pore plate number. Soldier size did not affect the probability to attack harmless heterospecific intruders, suggesting that larger guards are not more aggressive per se.
2. Material and methods
To investigate the recognition of conspecifics, we used 10 T. angustula colonies kept on the campus of the University of São Paulo in Ribeirão Preto, Brazil. We introduced 54 non-nest-mates and six nest-mates into each colony (total N = 600, six bees from each of the 10 colonies). For details, see the electronic supplementary material, S1, and Jones et al. [12]. All colonies had multiple standing guards (20.8 ± 10.4) and we recorded both the first decision (FD), i.e. the decision of the first guard, and the overall decision (OD), i.e. the cumulative decision of all guards during a test. For example, OD = ’reject’ means that at least one guard attacked the intruder during a maximum of 2 min. At the end of the experiment, we measured the average guard size (head width) of six standing guards per colony using a digital camera. Measurements were done with ImageJ 1.46 [4]. We counted the number of guards before introductions and estimated relative colony size by counting the number of returning foragers per 30 s four times on 1 day with good foraging conditions [13]. Colony size was estimated to explore whether this correlates with the recognition ability of guards. Multiple values were averaged for each colony.
To investigate aggression towards heterospecific intruders, we introduced workers from 10 colonies of the sympatric stingless bee Frieseomelitta varia to 10 different T. angustula test colonies (N = 99, 8–11 introductions per T. angustula colony). Frieseomelitta varia is not known to rob T. angustula colonies (for details, see the electronic supplementary material, S1) [6].
To explore whether there is a link between guard size and sensilla number, we focused on pore plates (sensilla placodea; figure 1) because their role in olfaction has been demonstrated in bees [10,14] and they are easy to identify with a light microscope (figure 1). We captured one standing guard of each of 14 different colonies. Antennae were washed in 1 ml water with a drop of dishwashing liquid for 5 min at 70°C. Water and detergent were then replaced by 1 ml of a 10% NaOH solution for another 5 min at 70°C. Antennae were then mounted on a microscope slide to measure antennal surface and count pore plates using a light microscope. To measure the surface area of antennae, we photographed each pair, measured their length (segments 1–9) and the width of the first and last segment. Average antennal width was determined as the average of segments 1 and 9. Pore plates were counted (1000× magnification) on segments 1–7 (figure 1). Segments 8 and 9 were excluded because they contained very few pore plates.
Figure 1.

Olfactory pore plate measurements. (a) Scanning electron microscope image of a pore plate (sensillum placodea) on a T. angustula antenna. (b) Light microscope image of a segment of a T. angustula antenna with elliptical pore plates. We counted the pore plates (bright areas of elliptical shape) that were completely or partly inside the white circle (50 µm radius) on segments 1–7 on both antennae. (Online version in colour.)
The recognition data were analysed with general and generalized mixed-effect models (LME, GLMM) in R v. 3.0 [15]. To test for an effect of guard size on nest-mate-recognition, we used a binomial response (accept, reject) and we included ‘source colony’ (origin of introduced bee) and ‘discriminator colony’ (colony of focal guards) as random effects. We used Spearman's rank correlation to test for a link between guard size and pore plate number. See the electronic supplementary material, S1, for more details.
3. Results
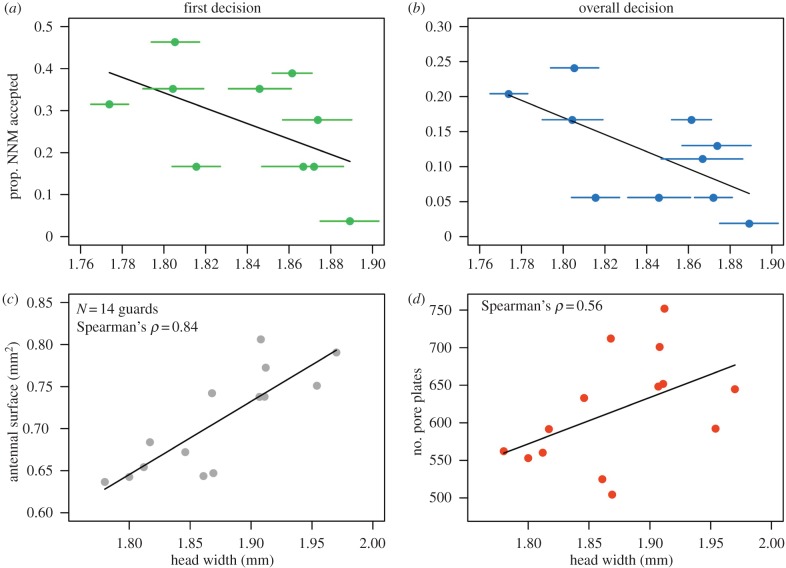
The mean rejection rate for introduced non-nest-mates was 73.1 ± 13.0% (mean ± s.d., N = 10 colonies) for FDs and 88.0 ± 7.4% for ODs. This was approximately 60% higher than for nest-mates (FD: 14.8 ± 14.6%, GLMM: z-value = 7.59, p < 0.0001; OD: 26.7 ± 22.5%, GLMM: z-value = 9.8, p < 0.0001). Guard size and colony size were included as predictors of recognition error probability. Neither guard size nor colony size affected acceptance of nest-mates (FD: guard size: z-value = 0.66, p = 0.51; colony size: z-value = 0.08, p = 0.94; OD: guard size: z-value = 0.8, p = 0.43, colony size: z-value = 0.38, p = 0.71). However, we found that guard size, but not colony size, positively affected both the FD rejection rate of non-nest-mates (FD: guard size: z-value = −2.43, p = 0.015; colony size: z-value = 1.29, p = 0.20; figure 2a) and the OD (guard size: z-value = −2.85, p = 0.004, colony size: z-value = 1.25, p = 0.21; figure 2b).
Figure 2.
Non-nest-mate (NNM) recognition accuracy. (a) Average size of standing guards affects the proportion of accepted non-nest-mates by the first guard that made contact. (b) As in (a) but results show the OD of guards. Data are shown as means and standard error per colony. (c) Relationship between guard head width (mm) and the estimated surface area of antennae (mm2). (d) Relationship between head width (mm) and the estimated number of pore plates on an antenna. (Online version in colour.)
We then tested whether guard size predicts aggression towards a heterospecific intruder. Thirty-one per cent of all introduced F. varia bees were not attacked, probably because they are not a threat to T. angustula. We found that colonies with larger guards were not more likely to attack F. varia intruders (GLMM: z-value = 1.06, p = 0.29).
We found a highly significant positive correlation between head width and estimated antennal surface (ρ = 0.84, N = 14, p = 0.0003; figure 2c). Furthermore, there was a positive relationship between the head width of guards and the estimated number of pore plates (ρ = 0.56, N = 14, p = 0.042; figure 2d), but we found no significant relationship between head width and pore plate density (ρ = −0.11, p = 0.71).
4. Discussion
Our data suggest that larger soldiers make fewer recognition errors when confronted with non-nest-mates (figure 2a,b): the colony with the smallest guards had a 10 times higher error-rate (20.3%) than the colony with the largest guards (1.9%). The effect was found both for the first soldier to contact a conspecific intruder and the collective performance of soldiers. Soldier size, however, did not affect acceptance of nest-mates. Successful intrusions can be harmful because intruders often steal resources [6] or parasitize reproduction [7]. Tetragonisca angustula is the most common stingless bee in the study area and robbing is frequently observed [13]. In such an environment, a low rate of recognition errors might be particularly beneficial. A recent study has found that larger T. angustula soldiers are more often observed guarding than smaller ones [16], which might further help to keep error rates low.
It is possible that guard size is a consequence, rather than the cause of recognition accuracy. For example, colonies with better recognition might build up more reserves, leading to larger colonies that produce larger workers. In addition, differences in colony health could affect both recognition accuracy and colony size, leading to a correlation between guard size and enemy recognition. In both cases, we would expect a positive relationship between colony size and recognition ability, which was not the case in our study. Yet another explanation for our results could be that larger bees are generally more aggressive than smaller bees [17,18]. However, we found no association between guard size and the probability to attack a heterospecific and, thus, easy to recognize bee (F. varia). Therefore, we suggest that soldier size itself affects recognition. Larger soldiers had a greater antennal surface area and a larger number of pore plates (figure 2). In ants, bumblebees and honeybees, sensory sensitivity is determined by the number of sensory sensilla on the antennae [10,14,19], which in turn is linked to the number of olfactory glomeruli in the antennal lobes in Camponotus ants [19]. It is not currently known which type of sensilla enable T. angustula soldiers to detect CHC profiles, but our findings support the view that larger bees can accommodate more antennal sensilla [10]. However, body size does not always improve enemy recognition: in the ant Acromyrmex echinatior majors are more aggressive than medium-sized workers but do not seem to be better at recognizing intruders [17].
Stingless bees were long thought to have a division of labour based mainly on age, but a recent comparative study has found that 10 of 28 species examined in Brazil have entrance guards that are larger than their foraging nest-mates [3], suggesting an important role of body size in task allocation. Given that even moderate size differences among soldiers seem to improve recognition (figure 2), it is unclear why not all species have guards of increased size. It is possible that some species experience less robbing or that developmental constraints prevent the evolution of a distinct soldier caste [3]. In T. angustula, soldiers are not only larger, but also different in shape: foragers have relatively larger heads, while soldiers have larger legs [4]. The reasons for these differences are currently unknown, but it is possible that the cognitive demands of foraging require foragers to have larger brains, whereas larger legs might be beneficial while grappling with an intruder [4]. Future studies on the threats experienced by colonies and the species-specific sensory and chemical ecology [20] will shed light on the circumstances that make the energy investment into soldiers worthwhile.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Ayrton Vollet Neto, Cláudia Inês da Silva and Sidnei Mateus for their help with the experiments.
Ethics
This work did not require an ethical assessment prior to conducting the research. The collection licence number is Ibama #26649-5.
Data accessibility
The raw data are available as the electronic supplementary material.
Authors' contributions
C.G., F.H.I.D.S. and F.S.N. conceived the study; F.H.I.D.S. and F.S.N. contributed to the study design; C.G., F.H.I.D.S., L.L.G.S., B.H. and U. Z. collected the data; C.G. performed the analyses and drafted the manuscript; all authors edited and approved the final version of the manuscript. All authors agree to be held accountable for the content of this study.
Competing interests
We declare we have no competing interests.
Funding
C.G. was funded by a Science without Borders fellowship from the Brazilian CNPq (process-number: 400664/2012-7). B.H. and U.Z. were funded by the UNIBRAL programme of the German Academic Exchange Service (DAAD; project-number: 54433831). L.L.G.S. was funded by a CNPq grant (process-number: 130143/2016-2). F.S.N. was funded by an FAPESP grant (process-number: 2015/25301-9).
References
- 1.Oster GF, Wilson EO. 1978. Caste and ecology in the social insects. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 2.Fjerdingstad EJ, Crozier RH. 2006. The evolution of worker caste diversity in social insects. Am. Nat. 167, 390–400. ( 10.1086/499545) [DOI] [PubMed] [Google Scholar]
- 3.Grüter C, Segers FHID, Menezes C, Vollet-Neto A, Falcon T, von Zuben L, Bitondi MMG, Nascimento FS, Almeida EAB. 2017. Repeated evolution of soldier sub-castes suggests parasitism drives social complexity in stingless bees. Nat. Commun. 8, 4 ( 10.1038/s41467-016-0012-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grüter C, Menezes C, Imperatriz-Fonseca VL, Ratnieks FLW. 2012. A morphologically specialized soldier caste improves colony defence in a Neotropical eusocial bee. Proc. Natl Acad. Sci. USA 109, 1182–1186. ( 10.1073/pnas.1113398109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakagami S, Roubik D, Zucchi R. 1993. Ethology of the robber stingless bee, Lestrimelitta limao (Hymenoptera: Apidae). Sociobiology 21, 237–277. [Google Scholar]
- 6.Grüter C, von Zuben L, Segers F, Cunningham J. 2016. Warfare in stingless bees. Insectes Soc. 63, 223–236. ( 10.1007/s00040-016-0468-0) [DOI] [Google Scholar]
- 7.Van Oystaeyen A, Alves DA, Oliveira RC, Nascimento DL, Nascimento FS, Billen J, Wenseleers T. 2013. Sneaky queens in Melipona bees selectively detect and infiltrate queenless colonies. Anim. Behav. 86, 603–609. ( 10.1016/j.anbehav.2013.07.001) [DOI] [Google Scholar]
- 8.van Zweden JS, D'Ettorre P. 2010. Nestmate recognition in social insects and the role of hydrocarbons. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnères A-G), pp. 222–243. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 9.Chittka L, Niven J. 2009. Are bigger brains better? Curr. Biol. 19, R995–R1008. ( 10.1016/j.cub.2009.08.023) [DOI] [PubMed] [Google Scholar]
- 10.Spaethe J, Brockmann A, Halbig C, Tautz J. 2007. Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Naturwissenschaften 94, 733–739. ( 10.1007/s00114-007-0251-1) [DOI] [PubMed] [Google Scholar]
- 11.Gill KP, Van Wilgenburg E, Macmillan DL, Elgar MA. 2013. Density of antennal sensilla efficacy of communication in a social insect. Am. Nat. 182, 834–840. ( 10.1086/673712) [DOI] [PubMed] [Google Scholar]
- 12.Jones SM, van Zweden JS, Grüter C, Menezes C, Alves D, Nunes-Silva P, Czaczkes TJ, Imperatriz-Fonseca VL, Ratnieks FLW. 2012. The role of wax and resin in the nestmate recognition system of a stingless bee, Tetragonisca angustula. Behav. Ecol. Sociobiol. 66, 1–12. ( 10.1007/s00265-011-1246-7) [DOI] [Google Scholar]
- 13.Segers FHID, Von Zuben LG, Grüter C. 2016. Local differences in parasitism and competition shape defensive investment in a polymorphic eusocial bee. Ecology 97, 417–426. ( 10.1890/15-0793.1) [DOI] [PubMed] [Google Scholar]
- 14.Riveros AJ, Gronenberg W. 2010. Sensory allometry, foraging task specialization and resource exploitation in honeybees. Behav. Ecol. Sociobiol. 64, 955–966. ( 10.1007/s00265-010-0911-6) [DOI] [Google Scholar]
- 15.R Core Team. 2016. R: A language and environment for statistical computing . Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org. [Google Scholar]
- 16.Hammel B, Vollet-Neto A, Menezes C, Nascimento FS, Engels W, Grüter C. 2016. Soldiers in a stingless bee: work rate and task repertoire suggest guards are an elite force. Am. Nat. 187, 120–129. ( 10.1086/684192) [DOI] [PubMed] [Google Scholar]
- 17.Larsen J, Fouks B, Bos N, d'Ettorre P, Nehring V. 2014. Variation in nestmate recognition ability among polymorphic leaf-cutting ant workers. J. Insect Physiol. 70, 59–66. ( 10.1016/j.jinsphys.2014.09.002) [DOI] [PubMed] [Google Scholar]
- 18.Nowbahari E, Fénéron R, Malherbe M-C. 1999. Effect of body size on aggression in the ant, Cataglyphis niger (Hymenoptera; Formicidae). Aggressive Behav. 25, 369–379. () [DOI] [Google Scholar]
- 19.Mysore K, Shyamala BV, Rodrigues V. 2010. Morphological and developmental analysis of peripheral antennal chemosensory sensilla and central olfactory glomeruli in worker castes of Camponotus compressus (Fabricius, 1787). Arthropod Struct. Dev. 39, 310–321. ( 10.1016/j.asd.2010.04.003) [DOI] [PubMed] [Google Scholar]
- 20.Leonhardt SD. 2017. Chemical ecology of stingless bees. J. Chem. Ecol. 43, 385–402. ( 10.1007/s10886-017-0837-9) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data are available as the electronic supplementary material.