Abstract
Peptide and low molecular weight amino acid-based materials that self-assemble in response to environmental triggers are highly desirable candidates in forming functional materials with tunable biophysical properties. In this paper, we explore redox-sensitive self-assembly of cationic phenylalanine derivatives conjugated to naphthalene diimide (NDI). Self-assembly of the cationic Phe-NDI conjugates into nanofibrils was induced in aqueous solvent at high ionic strength. Under reducing conditions, these self-assembled Phe-NDI conjugate fibrils underwent a morphological change to non-fibril aggregates. Upon reoxidation, the initially observed fibrils were reformed. The study herein provides an interesting strategy to effect reversible switching of the structure of supramolecular materials that can be applied to the development of sophisticated stimulus-responsive materials.
Keywords: self-assembly, stimulus-responsive, phenylalanine, naphthalene diimide
1. Background
Amyloid-inspired self-assembly of peptides and amino acids into highly ordered fibril assemblies has been exploited for the creation of functional materials [1–8]. The emergent properties of peptide-derived self-assembled materials depend on the molecular structure of the assembly motifs. Covalent [9] or non-covalent [10–12] incorporation of additional functionality into these self-assembling materials is beneficial in forming self-assembly systems with integrated molecular functions. Stimulus-responsive self-assembly is a highly desired function for supramolecular materials [13–17].
Recently, development of short peptide sequences decorated with organic π-systems has emerged as a powerful method to tune the properties of self-assembly [9]. Electron-deficient, aromatic 1,4,5,8-naphthalene diimides (NDIs) with well-defined redox and spectroscopic properties have been widely used in π-conjugate systems to fabricate supramolecular nanomaterials [18–29]. For example, incorporation of an electron-deficient NDI functional module into a β-sheet forming dipeptide resulted in n-type one-dimensional nanostructures [30] that can be modified to form self-supporting hydrogels [31]. Ulijn and co-workers [32] have shown that NDI–tyrosine conjugates can be enzymatically combined with Phe-NH2 to form NDI-YF-NH2 self-supporting hydrogels. Similarly, Lin et al. [33] recently demonstrated that NDI-Phe-Phe and NDI-Phe-Gly conjugates formed coloured hydrogels under both acidic and physiological conditions. More recently, they demonstrated that equimolar ratios of NDI–lysine and NDI–serine conjugates were able to co-assemble into hydrogels under neutral conditions [34]. Bhosale and co-workers also demonstrated the assembly of several NDI–amino acid and dipeptide systems into distinct materials, including golf ball-like nanostructures derived from an NDI–dipeptide [35] as well as nanobelts formed from co-assembly of NDI motifs containing phosphonic acid groups with l- and d-arginine [36].
NDI-grafted peptides have also been found to provide precise stimulus-responsive control in initiating supramolecular self-assembly processes. Ghadiri and co-workers investigated the redox-triggered self-assembly of cyclic peptides with alternating l- and d-amino acid sequences in which NDI derivatives were appended to Lys side chains [37]. They found that these cyclic peptides assembled into d, l-α-peptide nanotubes upon reduction of the attached NDI side chain groups. This demonstration of reduction-triggered self-assembly highlights the interesting potential of NDI to influence the supramolecular self-assembly behaviour of NDI conjugates. Examples of supramolecular systems that can reversibly assemble/disassemble or switch between different emergent morphologies in response to stimuli are more rare. We reasoned that the redox potential of NDI makes it an attractive moiety to develop materials that can exhibit altered properties under oxidizing or reducing conditions and that these properties may be switchable as a function of repeated alteration of the redox environment.
As such, we sought to demonstrate that NDI conjugates could be used to reversibly alter supramolecular material properties in a stimulus-responsive manner. Fluorenylmethoxycarbonyl-Phe (Fmoc-Phe) and side chain modified derivatives of Fmoc-Phe are privileged supramolecular self-assembly motifs [38–42]. We reasoned that NDI may be an effective proxy of the Fmoc-group, and that NDI-Phe conjugates may undergo effective self-assembly into fibrils in which redox environment may influence the nature of the supramolecular materials. We thus synthesized symmetrical, NDI–phenylalanine (NDI-Phe) conjugates 1a and 1b (figure 1). We have previously shown in the context of Fmoc-Phe self-assembly, that pentafluorophenylalanine (F5-Phe) has enhanced self-assembly propensity relative to Phe [43]. Thus, both Phe (1a) and F5-Phe (1b) NDI conjugates were prepared. The cationic appendages to the Phe-derivative termini were used to enhance the water solubility of these conjugates. It was found that these NDI-Phe conjugates self-assembled into one-dimensional fibrils in aqueous solutions with high ionic strength. Upon reduction, the resulting anionic species affected a conformational rearrangement of the supramolecular fibrils to amorphous aggregates. Upon reoxidation, the fibril morphology was restored. This process was reversible and the cycle could be repeated with the same results each time. Detailed below is a discussion of these findings that elucidate the interesting potential for NDI to be used to alter the emergent properties of supramolecular materials.
Figure 1.
Chemical structures of NDI-Phe conjugates used in these studies.
2. Material and methods
2.1. Synthesis
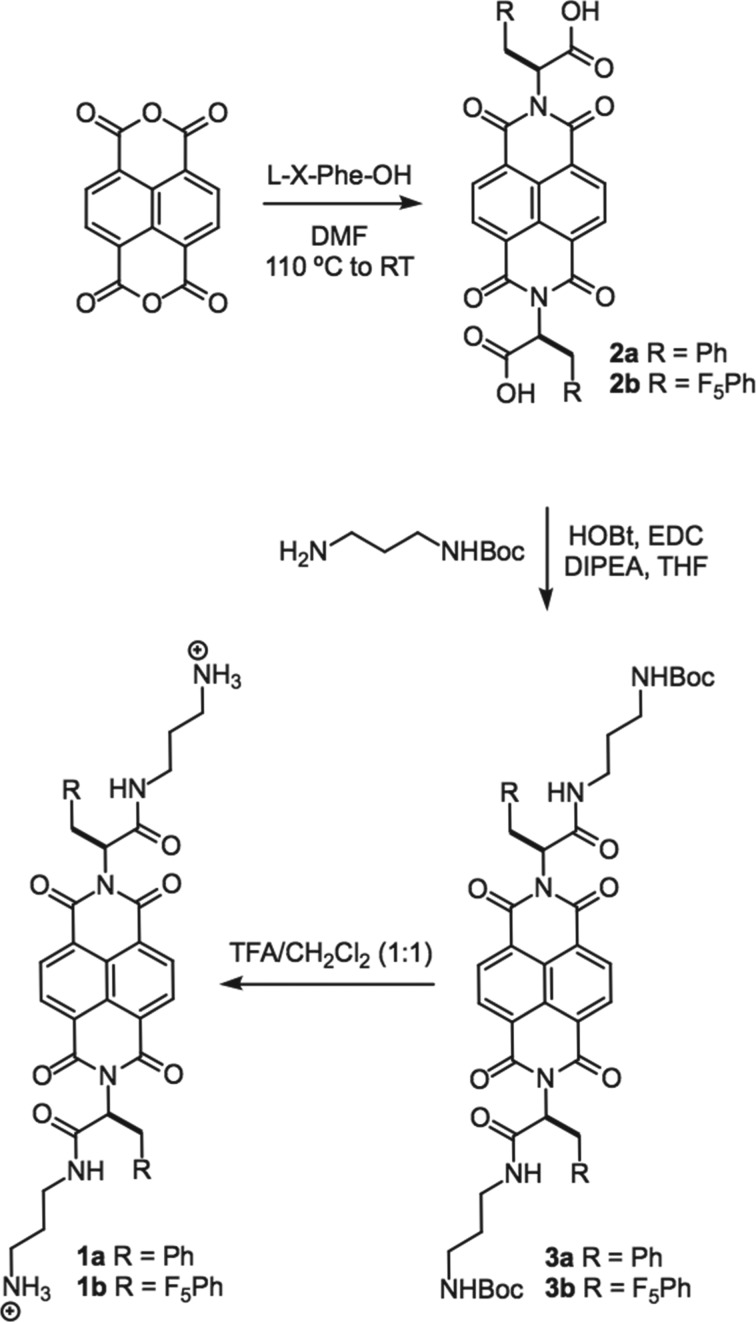
The synthesis of NDI congeners 1a and 1b is shown in scheme 1 and described in detail in the electronic supplementary material. These analogues were prepared by a modification to a published NDI conjugation protocol [44]. Briefly, 1,4,5,8-naphthalenetetracarboxylic dianhydride in dimethylformamide was condensed with Phe derivatives to obtain diimide functionalized compounds 2a and 2b. Tert-butyl-(3-aminopropyl)carbamate moieties were appended at the C-termini of these Phe derivatives to yield 3a and 3b. Trifluoroacetic acid-mediated deprotection of N-Boc residues then afforded 1a and 1b. Characterization data, including NMR and mass spectroscopy analysis can be found in electronic supplementary material, figures S1–S14.
Scheme 1.
Synthesis of NDI-Phe conjugates.
2.2. Self-assembly
Self-assembly was initiated by dissolving 1a or 1b in unbuffered filtered water (Barnstead NANOpure 0.2 µm filter, 18 Ω) to obtain NDI-Phe conjugate stock solutions (10 mM). Stock NDI-Phe conjugate solutions were then diluted to 5 mM in aqueous brine solutions (100–1000 mM NaCl).
2.3. Circular dichroism spectroscopy
Circular dichroism (CD) spectra were recorded on an AVIV 202 CD spectrometer. NDI-Phe conjugate samples (5 mM) were analysed immediately after dilution into 100–1000 mM NaCl. Spectra were obtained from 300 to 190 nm with a 1.0 nm step, 1.0 nm bandwidth and a 6 s collection time per step at 25°C in a 0.1 mm path length quartz cuvette (Hellma). The AVIV software was used for background subtraction, conversion to molar ellipticity and data smoothing with a least-squares fit.
2.4. Transmission electron microscopy
Images were obtained with a Hitachi 7650 transmission electron microscope with an accelerating voltage of 80 kV. Freshly prepared samples (10 µl) were spotted onto 200 mesh carbon-coated copper grids and allowed to stand for 1 min. Excess sample was carefully removed by capillary action using filter paper and then grids were immediately stained with uranyl acetate negative stain (10 µl for 2 min). Excess stain was removed by capillary action and grids were allowed to air-dry for 10–15 min. Dimensions of nanostructures were determined using ImageJ64 software.
2.5. Scanning electron microscopy
Images were taken on a Zeiss Supra 40VP FESEM scanning electron microscope with 10 kV accelerating voltage. Samples were placed on glass plates and excess solvent was removed by capillary action (filter paper). Then the air-dried samples were sputter coated with gold at a 1 Å s−1 rate using low vacuum sputter-coating system under 100 mTorr pressure in 15 mA current.
2.6. UV–visible and fluorescence spectroscopy
UV spectra were collected using a TECAN Infinite plate reader fluorimeter from 230–800 nm and background was subtracted. Spectra of each NDI-Phe conjugate sample were obtained from 200 µM samples in H2O. The reduced NDI-Phe conjugate samples were prepared by diluting NDI-Phe conjugate stock solutions into aqueous 50 mM Na2S2O4.
2.7. Cyclic voltammetry
Cyclic voltammetry (CV) measurements of the NDI-Phe conjugate samples (1 M) were performed with a CHI 680D potentiostat using a glassy-carbon working electrode, a glassy-carbon auxiliary electrode and SCE reference electrode. The CV was recorded for 0.5 mM 1a or 1b in the presence of 0.1 M TBAPF6 in a 1/1 acetonitrile/H2O mixture at room temperature with a mM s−1 scan rate. The instrument was purged with argon 10 min before performing a CV experiment. Under these solvent conditions no assembly was observed, indicating that the observed redox potentials are independent of the assembly properties of the NDI-Phe and NDI-F5-Phe conjugates.
2.8. Electron paramagnetic resonance studies
Electron paramagnetic resonance (EPR) spectra were obtained with a Bruker X-band EPR spectrometer model EMXplus operating at approximately 9.8 GHz microwave frequency with a high 100 kHz magnetic field modulation frequency at 298 K. A standard EPR cavity was used and the microwave power was kept to a minimum to avoid saturating the radical spins. The magnetic fields and g-values were calibrated with a standard solid powder sample of diphenyl picryl hydrazyl (g = 2.0036). The EPR of the blank quartz tube was measured to calibrate EPR baseline for the samples. Quartz AquaX EPR tubes were used. The quartz EPR tubes were washed thoroughly with distilled and deionized water and dried before each use. Samples were prepared at concentrations of 5 mM NDI-Phe with 1 M NaCl and 50 mM Na2S2O3.
3. Results and discussion
3.1. Self-assembly of NDI-Phe conjugates
We initially attempted to study the aqueous self-assembly of the 2a and 2b carboxylic acid derivatives, but the poor water solubility of these molecules was limiting. To circumvent the poor water solubility of 2a and 2b, tert-butyl-(3-aminopropyl) amine was coupled to the C-termini of the Phe derivatives of 2a and 2b to provide amides 3a and 3b. Deprotection of the Boc-amine group gave amines 1a and 1b. Compounds 1a and 1b exhibited dramatically improved water solubility.
While other amino acid–NDI conjugates have been previously shown to self-assemble [34], compounds 1a and 1b failed to self-assemble in simple aqueous solutions. The amine cations of these molecules that impart water solubility also introduce repulsive charge effects that impede intermolecular interactions. Other cationic self-assembling peptide systems have been shown to undergo self-assembly at high ionic strength in solutions with high salt concentrations [16,45]. At high salt concentrations cationic charges can be shielded and repulsive effects are minimized.
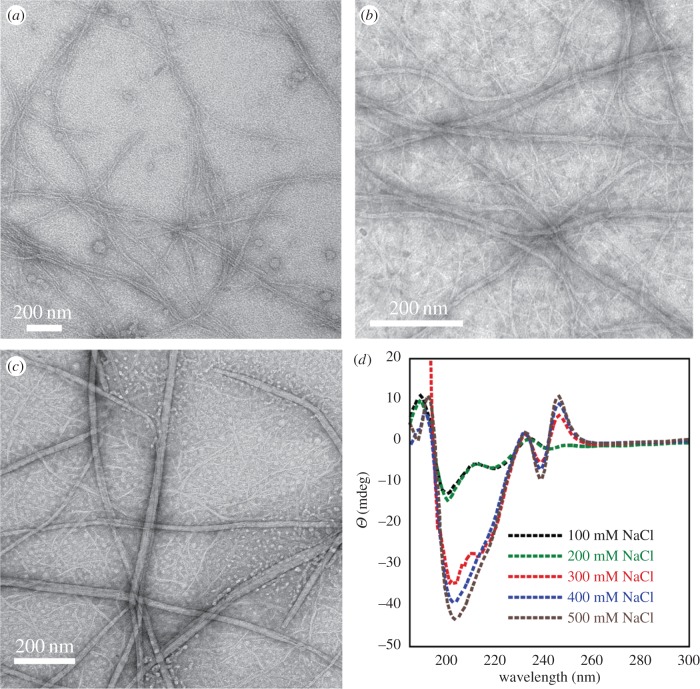
Accordingly, we assessed the self-assembly behaviour of 1a and 1b in the presence of increasing concentrations of NaCl. Compounds 1a and 1b were dissolved in unbuffered water (18 Ω) to obtain homogeneous NDI-Phe conjugate solutions, which were diluted into aqueous brine solutions (5 mM NDI-Phe derivative) with NaCl concentrations ranging from 100 to 1000 mM. The least hydrophobic Phe-NDI conjugate 1a showed no evidence of self-assembly as evidenced by extensive transmission electron microscopy (TEM) analysis even after increasing NaCl concentration to 1000 mM. However, the more hydrophobic F5-Phe-NDI compound 1b effectively self-assembled into rigid fibrils over a range of NaCl concentrations (100–1000 mM; figure 2). The fibril solutions were optically transparent, indicating that the assembled fibrils remained in a solvated state after assembly. At 100 mM NaCl, 1b formed fibrils of 12 ± 2 nm in diameter (figure 2a). As solvent ionic strength increased, wider fibrils ranging from 20 to 30 nm in diameter formed through the apparent bundling of the smaller 12 nm fibrils (figure 2b,c). The influence of ionic strength on the self-assembled structure of 1b was also assessed by CD spectroscopy. The CD spectrum of 1b showed a distinctive minimum at 221 nm, a maximum at 194 nm, with a crossover at 205 nm (figure 2d). The intensity of the CD signal increased as the solvent ionic strength increased, reaching a maximum intensity at approximately 500 mM NaCl. These data are consistent with an increased propensity towards self-assembly as solution ionic strength is increased, similar to observations from self-assembling peptide systems [45]. The failure of compound 1a to self-assemble can be attributed to the much lower hydrophobicity of Phe relative to the F5-Phe groups found in compound 1b.
Figure 2.
TEM images of fibrils of 1b (5 mM) in aqueous solutions with varying concentrations of NaCl: (a) 100 mM NaCl, (b) 500 mM NaCl and (c) 1000 mM NaCl. (d) CD spectra of fibrils of 1b (5 mM) at varying concentrations of NaCl. The decreased signal intensity at 194 nm is an artefact of increased dynode voltage in the spectrometer from high salt concentrations, which obscures signals in the far-UV.
3.2. Influence of redox environment on self-assembly
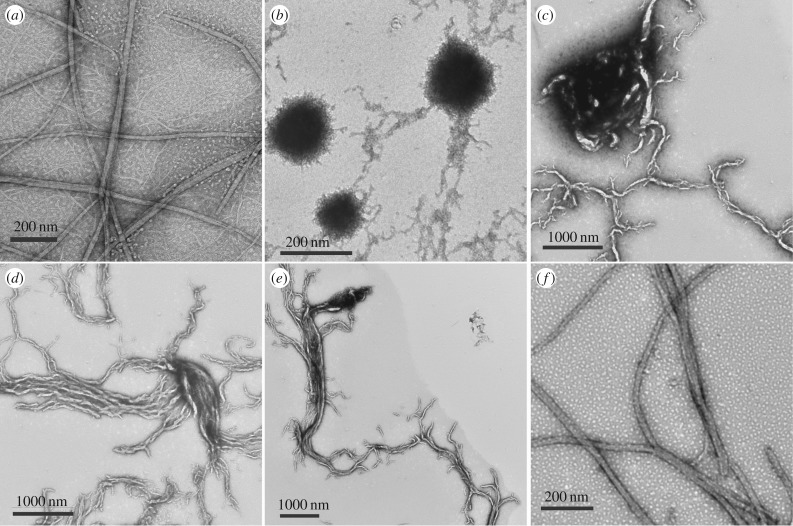
The effect of redox environment on the self-assembled fibrils of 1b was next explored. The addition of aqueous sodium dithionite (Na2S2O4, 50 mM), a mild chemical reductant, to self-assembled 1b fibrils resulted in an immediate conversion from fibril to spherical/amorphous aggregates as evidenced by TEM images (figure 3b). The reduced 1b aggregates were globular in appearance, with diameters of approximately 100–200 nm. Also, the optically transparent solutions of the self-assembled fibrils become turbid upon addition of reductant. Upon air oxidation, the globular aggregates begin to convert into protofibril species that eventually (over 16 h) completely revert back to the originally observed supramolecular fibrils (figure 3b–f). The transient protofibril intermediates observed during the NDI radical quenching process provide interesting insight into the self-assembly process, providing a glimpse of possible early aggregates formed during 1b fibril assembly. It is also significant that repeated addition of sodium dithionite followed by a period of reoxidation in ambient oxygen causes this morphological conversion to occur in a repeatable manner. This is a significant demonstration of reversible switching from one supramolecular aggregate to another, showing the great potential of NDI conjugates to be used in the design of dynamic supramolecular materials.
Figure 3.
TEM images demonstrating the effects of reducing agents on the self-assembled fibril of compound 1b. (a) Fibrils of 1b (5 mM 1b, 1 M NaCl) under ambient oxidizing conditions. (b) Fibrils of 1b immediately after reduction by addition of Na2S2O4 show loss of fibril morphology, replaced by irregular spherical aggregates. (c–f) Reduced 1b aggregates 5 min (c), 30 min (d), 1 h (e) and 16 h (f) after addition of Na2S2O4. Over this time frame, atmospheric oxygen re-establishes an oxidizing environment, facilitating complete reformation of the initially observed fibrils.
Interestingly, our morphological transition from fibrils under oxidizing conditions to globular aggregates under reducing conditions differs from previously observed reduction-triggered assembly of NDI-bearing cyclic peptides. As described in the Introduction, Ghadiri and co-workers [37] previously showed that cyclic d/l-peptides undergo selective assembly under reducing conditions. This was explained based on the assumption that the NDI radical anions that form under reducing conditions enable attractive NDI–NDI interactions possibly due to charge delocalization between stacked NDI residues. This cyclic peptide system is considerably more complex than our simple NDI-Phe conjugates. In addition to putative NDI interactions, the cyclic peptide is also stabilized by formation of an extensive hydrogen bond network. Our NDI-Phe conjugates most likely assemble into structures that are primarily mediated by aromatic effects. Since our NDI conjugate 1b has such a low molecular weight relative to Ghadiri's cyclic peptides, the effect of introducing negative charge to 1b most likely results in a dominating repulsive effect that destabilizes the one-dimensional fibrils in favour of globular aggregates in which repulsive effects are presumably minimized.
We next analysed the nature of the reduced NDI species that exist upon addition of sodium dithionite in order to confirm formation of radical anions. We confirmed the formation of NDI radicals in our system by EPR spectroscopy (electronic supplementary material, figures S15–S17). We analysed both compounds 1a and 1b under conditions in which both were soluble and unassembled in order to simplify the measurements and also to confirm that formation of radical anions alone does not promote assembly of 1b. Under oxidizing conditions, compounds 1a and 1b do not display any EPR signal (diamagnetic). In the presence of chemical reductant, however, both congeners display a characteristic EPR pattern at 298 K that is consistent with radical formation. The isotropic EPR spectra of 1a and 1b in aqueous solutions were obtained using a narrow magnetic field sweep width of 20 G. Both 1a and 1b showed similar EPR spectra (electronic supplementary material, figures S16 and S17, respectively) with 13 lines centred on a free radical g-value of 2.0, indicating free radical formation in both species. Owing to the hyperfine coupling, the main EPR transition of the radical anion is split into two equivalent nitrogen nuclei (14N, I = 1; aN = 1.0 G or 1.0 × 10−4 cm−1), giving a 5-line spectrum. This is further split by four equivalent aromatic protons (1H, I = 1/2; aH = 2.0 G or 2.0 × 10−4 cm−1) giving the resulting 13 lines. Small splitting from the two equivalent side chain CH protons is not resolved in either of the 1a or 1b EPR spectra. The larger line width seen in the 1b EPR spectra may be attributed to further unresolved hyperfine splitting from fluorine nuclei. The g-values and hyperfine couplings are shown in electronic supplementary material, table S1. Taken together, these data indicate the formation of a free radical located on the NDI ring.
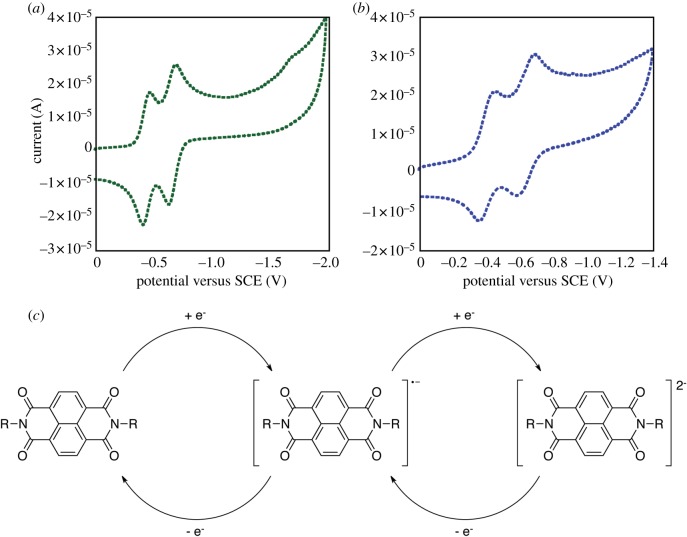
The redox properties of both 1a and 1b were further characterized using electrochemical methods. CV of these congeners was recorded in acetonitrile/water (1 : 1) with tetra-N-butylammonium perchlorate (TBAPC) as the supporting electrolyte (figure 4). Both 1a and 1b exhibited two well-separated reversible one-electron reduction processes, which are characteristic of sequential reduction of NDI to the mono radical anion (NDI•–) and dianion (NDI2–) species. The anion radical was stable in anaerobic environments and compounds 1a and 1b regained their original molecular properties upon exposure to oxidizing conditions. The reduction and oxidation of these species could be repeated over many cycles (electronic supplementary material, figure S18) consistent with our observation that the morphological switching of aggregates of 1b is repeatable.
Figure 4.
Cyclic voltammetry analysis of Phe-NDI conjugates in acetonitrile/H2O (1 : 1). Tetra-N-butylammonium perchlorate is used as the supporting electrolyte; experiments were performed at 20°C with a glassy-carbon working electrode at a scan rate of 200 mV s−1. (a) Cyclic voltammagram of compound 1a, (b) cyclic voltammagram of compound 1b. (c) A schematic of the reduction and reoxidation processes of NDI conjugates.
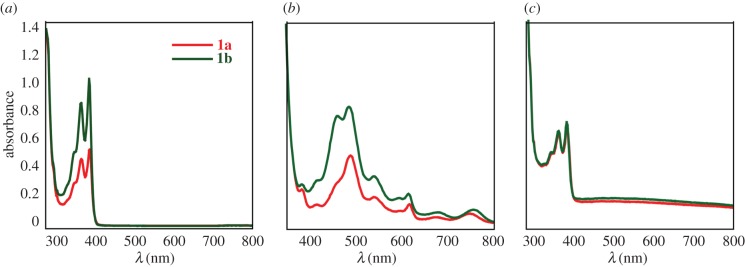
Despite the observed differences in self-assembly propensity, both NDI-Phe congeners 1a and 1b possess similar typical photochemical behaviour under reducing conditions. UV–visible (UV–Vis) spectra of 1a and 1b were recorded in unbuffered H2O (200 µmol), in the presence of chemical reductant (50 mM Na2S2O4), and in the reoxidized state after addition of air/O2 (figure 5). In native state, the spectra of both 1a and 1b exhibit two prominent absorption peaks at approximately 360 nm and 390 nm indicative of NDI π–π* transition polarized along the Z-axis of the NDI chromophore [31]. In the presence of Na2S2O4, chemically reduced 1a and 1b peaks shifted to the visible region (λmax approx. 454 nm and 476 nm, respectively), consistent with formation of radical anions [46]. However, in the presence of air/O2 the peaks shifted back to near UV region (λmax approx. 360 nm, 390 nm), confirming reoxidation of these species over time in ambient air.
Figure 5.
UV–Vis spectra of compounds 1a and 1b (a) in the native oxidized state, (b) in the reduced state after addition of sodium dithionite and (c) in the reoxidized state.
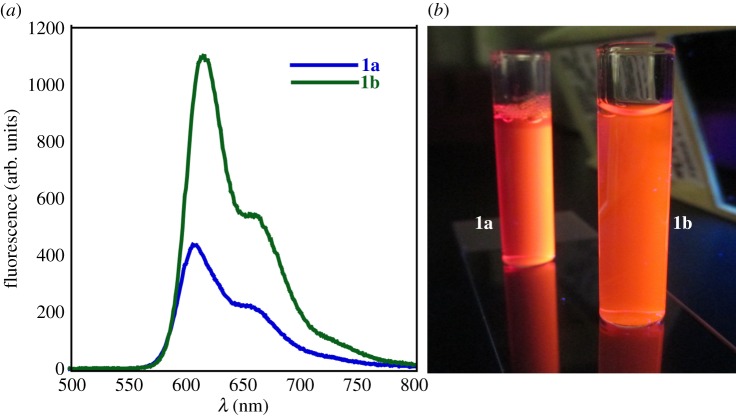
The fluorescence emission spectra of 1a and 1b in the reduced state were analysed (figure 6). Spectra were collected at excitation wavelength of 476 nm. The fluorescence spectra from 1a and 1b in the reduced state show intense emission maxima (λex) at 616 nm with a low energy shoulder at 660 nm (figure 6a). The red-shifted fluorescence emission is indicative of NDI aggregation in the reduced state. The fluorescence quantum yields of 1a and 1b were measured with fluorescein as a standard (Φ1a = 0.001 and Φ1b = 0.023, respectively) [47]. The low quantum yields obtained for these congeners are consistent with typical N-substituted NDI containing dyes [48,49], in which the decrease in fluorescence quantum yield may be due to strong hydrophobic interactions in water. However, in the presence of O2/air, fluorescence intensity gradually decreased upon reoxidation to the native state.
Figure 6.
(a) Fluorescence emission spectra of reduced 1a and 1b (187 µmol) excited at 476 nm (fluorescence was detected at 616 nm); (b) digital images showing the fluorescence of reduced solutions of 1a and 1b.
As a whole, the EPR, CV and UV–Vis data described herein are consistent with previously reported data on similar NDI compounds [37,46,50–52]. These data suggest the formation of a radical anion and dianion species, which results in the degradation of fibrils and formation of large aggregates upon reduction. It is significant that 1a and 1b have similar redox properties, but that only 1b assembles into fibrils. This affirms the importance of hydrophobicity as a critical driving force in supramolecular assembly processes. It is also possible that the electron-deficient fluorinated benzene side chain the F5-Phe conjugates mediates a unique intermolecular π–π effect, accounting for this distinctive assembly behaviour. It also confirms that the self-assembly behaviour of these NDI-Phe conjugates under oxidizing conditions is independent of the redox potential of these molecules.
Precise structural insight into the self-assembled fibrils and globules of 1b is impossible to derive from the data reported herein. Analysis of supramolecular structure of peptide and amino acid-based self-assemblies is often initiated using low resolution spectroscopic techniques such as CD and infrared spectroscopy. While these techniques are useful in providing clues about peptide secondary structure and, in some cases, other intermolecular interactions such as π–π effects, they do not provide data of sufficient resolution to build strong packing models of supramolecular structures. To better understand the driving forces of self-assembly, X-ray crystal diffraction can be used. Unfortunately, materials that assemble into high-aspect ratio fibrils are notoriously difficult to crystallize. We were, however, able to obtain high-resolution crystal analyses of several of our synthetic intermediates (compounds 2a, 3a and 3b; see electronic supplementary material, figures S19–S21 and appendices S1–S3). It is tempting to use these structural data to infer models of the self-assembled fibrils of 1b, but we have opted to avoid this temptation. The crystals of these synthetic intermediates were formed in organic solvents that do not accurately reflect the environment of 1b fibril assembly. In addition, these synthetic intermediates lack charge and, in the case of compounds 3a and 3b, include bulky Boc protecting groups on the amine functionality. As such, we suspect, based on the CD data, that intermolecular Phe side chain-side chain interactions occur. We also suspect that NDI–NDI interactions occur based on the morphological changes observed in the aggregates upon radical ion formation. However, we currently resist the proposal of a fibril packing model in the absence of high-resolution data that pertain directly to compound 1b in water.
While the packing mode of the fibrils of 1b remains unresolved, it is likely that the dramatic change in morphology that occurs upon reduction of the NDI moieties enforces a structural rearrangement that is more tolerant of intermolecular interactions that arise as a function of the negative charge on NDI in the reduced state. While it is premature to assign a distinct structure to the aggregates observed herein, their packing may be related to previously reported NDI–peptide or amino acid aggregates in which side chains stabilize NDI residues and allow for NDI stabilization [9,19,31,37,53]. Without high-resolution information regarding packing architecture in these aggregates, a detailed understanding of the mechanism(s) at play in the morphological transformations that are observed as a function of varying redox conditions is purely speculative. It is possible that NDI–NDI interactions occur in fibrils, which are then disrupted by Coulombic repulsion upon formation of radical anions under reducing conditions. Upon reoxidation, these repulsive effects are eliminated, enabling reorganization in the fibril packing mode. The ability to control the morphology of these NDI-Phe aggregates via a reductive trigger provides a useful template for future developments in supramolecular materials that respond dynamically to the environment.
4. Conclusion
Herein we report the stimulus-responsive morphological switching of supramolecular NDI-Phe aggregates in water. The superior self-assembly propensity of NDI-Phe conjugate 1b can be attributed to the greater hydrophobicity of 1b as well as the unique electron-deficient nature of the F5-Phe side chain, reaffirming the critical nature of hydrophobic and aromatic effects in supramolecular self-assembly of low molecular weight peptides and amino acid derivatives. The observation of repeatable morphological shifting from supramolecular one-dimensional nanofibrils under oxidizing conditions to globular 100–200 nm aggregates with altered photophysical properties under reducing conditions is a dramatic demonstration of a material that responds dynamically to changes in the environment. These observations indicate the great potential of NDI/peptide-based materials for the creation of dynamic and responsive supramolecular architectures.
Supplementary Material
Acknowledgements
We gratefully acknowledge Karen Bentley (University of Rochester Electron Microscopy Core) for her assistance with TEM imaging, Dr Scott Kennedy (University of Rochester) for assistance with CD experiments, Sandip Sur (University of Rochester) for assistance with EPR experiments, and Dr William Brennessel (University of Rochester) for assistance with XRD experiments.
Authors' contributions
W.L. designed and carried out synthesis of materials and performed CD, UV–Vis, CV, EPR, NMR, TEM, SEM and self-assembly experiments. She also helped to draft the manuscript. P.W.R. supplemented W.L.'s synthetic and self-assembly work and assisted in drafting the manuscript. B.L.N. provided materials for experiments, helped with planning and designing of experiments and assisted in drafting of the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the NSF (DMR-1148836).
References
- 1.Hirst AR, Escuder B, Miravet JF, Smith DK. 2008. High-tech applications of self-assembling supramolecular nanostructured gel-phase materials: from regenerative medicine to electronic devices. Angew. Chem. Int. Ed. 47, 8002–8018. ( 10.1002/anie.200800022) [DOI] [PubMed] [Google Scholar]
- 2.Gao Y, Zhao F, Wang Q, Zhang Y, Xu B. 2010. Small peptide nanofibers as the matrices of molecular hydrogels for mimicking enzymes and enhancing the activity of enzymes. Chem. Soc. Rev. 39, 3425–3433. ( 10.1039/B919450A) [DOI] [PubMed] [Google Scholar]
- 3.Zhao F, Ma ML, Xu B. 2009. Molecular hydrogels of therapeutic agents. Chem. Soc. Rev. 38, 883–891. ( 10.1039/B806410P) [DOI] [PubMed] [Google Scholar]
- 4.Xu B. 2009. Gels as functional nanomaterials for biology and medicine. Langmuir 25, 8375–8377. ( 10.1021/la900987r) [DOI] [PubMed] [Google Scholar]
- 5.Du X, Zhou J, Shi J, Xu B. 2015. Supramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterials. Chem. Rev. 115, 13 165–13 307. ( 10.1021/acs.chemrev.5b00299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo Q, Hou C, Bai Y, Wang R, Liu J.. 2016. Protein assembly: versatile approaches to construct highly ordered nanostructures. Chem. Rev. 116, 13 571–13 632. ( 10.1021/acs.chemrev.6b00228) [DOI] [PubMed] [Google Scholar]
- 7.Hauser CAE, Maurer-Stroh S, Martins IC. 2014. Amyloid-based nanosensors and nanodevices. Chem. Soc. Rev. 43, 5326–5345. ( 10.1039/C4CS00082J) [DOI] [PubMed] [Google Scholar]
- 8.Bowerman CJ, Nilsson BL. 2012. Self-assembly of amphipathic β-sheet peptides: insights and applications. Biopolymers 98, 169–184. ( 10.1002/bip.22058) [DOI] [PubMed] [Google Scholar]
- 9.Tovar JD. 2013. Supramolecular construction of optoelectronic biomaterials. Acc. Chem. Res. 46, 1527–1537. ( 10.1021/ar3002969) [DOI] [PubMed] [Google Scholar]
- 10.Hirst AR, Smith DK. 2005. Two-component gel-phase materials—highly tunable self-assembling systems. Chem. Eur. J. 11, 5496–5508. ( 10.1002/chem.200500241) [DOI] [PubMed] [Google Scholar]
- 11.Hirst AR, Miravet JF, Escuder B, Noirez L, Castelletto V, Hamley IW, Smith DK. 2009. Self-assembly of two-component gels: stoichiometric control and component selection. Chem. Eur. J. 15, 372–379. ( 10.1002/chem.200801475) [DOI] [PubMed] [Google Scholar]
- 12.Hanabusa K, Miki T, Taguchi Y, Koyama T, Shirai H. 1993. Two-component, small molecule gelling agents. J. Chem. Soc. Chem. Commun. 1382–1384. ( 10.1039/C39930001382) [DOI] [Google Scholar]
- 13.Zelzer M, Todd SJ, Hirst AR, McDonald TO, Ulijn RV. 2013. Enzyme responsive materials: design strategies and future developments. Biomater. Sci. 1, 11–39. ( 10.1039/C2BM00041E) [DOI] [PubMed] [Google Scholar]
- 14.Li X, Gao Y, Kuang Y, Xu B. 2010. Enzymatic formation of a photoresponsive supramolecular hydrogel. Chem. Commun. 46, 5364–5366. ( 10.1039/C0CC00163E) [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang B, Kuang Y, Gao Y, Shi J, Zhang XX, Xu B. 2013. A redox responsive, fluorescent supramolecular metallohydrogel consists of nanofibers with single-molecule width. J. Am. Chem. Soc. 135, 5008–5011. ( 10.1021/ja402490j) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozbas B, Kretsinger J, Rajagopal K, Schneider JP, Pochan DJ. 2004. Salt-triggered peptide folding and consequent self-assembly into hydrogels with tunable modulus. Macromolecules 37, 7331–7337. ( 10.1021/ma0491762) [DOI] [Google Scholar]
- 17.Schneider JP, Pochan DJ, Ozbas B, Rajagopal K, Pakstis L, Kretsinger J. 2002. Responsive hydrogels from the intramolecular folding and self-assembly of a designed peptide. J. Am. Chem. Soc. 124, 15 030–15 037. ( 10.1021/ja027993g) [DOI] [PubMed] [Google Scholar]
- 18.Bhosale SV, Jani CH, Langford SJ. 2008. Chemistry of naphthalene diimides. Chem. Soc. Rev. 37, 331–342. ( 10.1039/B615857A) [DOI] [PubMed] [Google Scholar]
- 19.Katz HE, Lovinger AJ, Johnson J, Kloc C, Slegrist T, Li W, Lin YY, Dodabalapur A. 2000. A soluble and air-stable organic semiconductor with high electron mobility. Nature 404, 478–481. ( 10.1038/35006603) [DOI] [PubMed] [Google Scholar]
- 20.Kobaisi MA, Bhosale SV, Latham K, Raynor AM, Bhosale SV. 2016. Functional naphthalene diimides: synthesis, properties, and applications. Chem. Rev. 116, 11 685–11 796. ( 10.1021/acs.chemrev.6b00160) [DOI] [PubMed] [Google Scholar]
- 21.Nalluri SKM, Liu Z, Wu Y, Hermann KR, Samanta A, Kim DJ, Krzyaniak MD, Wasielewski MR, Stoddart JF. 2016. Chiral redox-active isosceles triangles. J. Am. Chem. Soc. 138, 5968–5977. ( 10.1021/jacs.6b02086) [DOI] [PubMed] [Google Scholar]
- 22.Schroot R, Schlotthauer T, Schubert US, Jäger M. 2016. Modular assembly of poly(naphthalene diimide) and Ru(II) dyes for an efficient light-induced charge separation in hierarchically controlled polymer architectures. Macromolecules 49, 2112–2123. ( 10.1021/acs.macromol.5b02717) [DOI] [Google Scholar]
- 23.Chen M, Yang C, Xu Z, Tang Y, Jiang J, Liu P, Su Y, Wu D. 2016. A facile self-assembly strategy towards naphthalene diimide/graphene hybrids as high performance organic cathodes for lithium-ion batteries. RSC Adv. 6, 13 666–13 669. ( 10.1039/C5RA26181C) [DOI] [Google Scholar]
- 24.Pandeeswar M, Khare H, Ramakumar S, Govindaraju T. 2015. Crystallographic insight-guided nanoarchitectonics and conductivity modulation of an n-type organic semiconductor through peptide conjugation. Chem. Commun. 51, 8315–8318. ( 10.1039/C5CC01996F) [DOI] [PubMed] [Google Scholar]
- 25.Avinash MB, Govindaraju T. 2011. Engineering molecular organization of naphthalenediimides: large nanosheets with metallic conductivity and attoliter containers. Adv. Funct. Mater. 21, 3875–3882. ( 10.1002/adfm.201101001) [DOI] [Google Scholar]
- 26.Avinash MB, Govindaraju T. 2011. A bio-inspired design strategy: organization of tryptophan-appended naphthalenediimide into well-defined architectures induced by molecular interactions. Nanoscale 3, 2536–2543. ( 10.1039/c0nr00766h) [DOI] [PubMed] [Google Scholar]
- 27.Das A, Ghosh S. 2016. H-bonding directed programmed supramolecular assembly of naphthalene-diimide (NDI) derivatives. Chem. Commun. 52, 6860–6872. ( 10.1039/C6CC01983H) [DOI] [PubMed] [Google Scholar]
- 28.Rananaware A, Samanta M, Bhosale RS, Kobaisi MA, Roy B, Bheemireddy V, Bhosale SV, Bandyopadhyay S, Bhosale SV. 2016. Photomodulation of fluoride ion binding through anion-π interactions using a photoswitchable azobenzene system. Sci. Rep. 6, 22928 ( 10.1038/srep22928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhosale RS, Al Kobaisi M, Bhosale SV, Bhargava S, Bhosale SV. 2015. Flower-like supramolecular self-assembly of phosphonic acid appended naphthalene diimide and melamine. Sci. Rep. 5, 14609 ( 10.1038/srep14609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao H, Nguyen T, Romano NC, Modarelli DA, Parquette JR.. 2009. Self-assembly of 1-Dn-type nanostructures based on naphthalene diimide-appended dipeptides. J. Am. Chem. Soc. 131, 16 374–16 376. ( 10.1021/ja906377q) [DOI] [PubMed] [Google Scholar]
- 31.Shao H, Parquette JR. 2010. A π-conjugated hydrogel based on an Fmoc-dipeptide naphthalene diimide semiconductor. Chem. Commun. 46, 4285–4287. ( 10.1039/C0CC00701C) [DOI] [PubMed] [Google Scholar]
- 32.Nalluri SKM, Berdugo C, Javid N, Frederix PWJM, Ulijn RV. 2014. Biocatalytic self-assembly of supramolecular charge-transfer nanostructures based on n-type semiconductor-appended peptides. Angew. Chem. Int. Ed. 53, 5882–5887. ( 10.1002/anie.201311158) [DOI] [PubMed] [Google Scholar]
- 33.Liu Y-H, Hsu S-M, Wu F-Y, Cheng H, Yeh M-Y, Lin H-C. 2014. Electroactive organic dye incorporating dipeptides in the formation of self-assembled nanofibrous hydrogels. Bioconjugate Chem. 25, 1794–1800. ( 10.1021/bc500299c) [DOI] [PubMed] [Google Scholar]
- 34.Hsu L-H, Hsu S-M, Wu F-Y, Liu Y-H, Nelli SR, Yeh M-Y, Lin H-C. 2015. Nanofibrous hydrogels self-assembled from naphthalene diimide (NDI)/amino acid conjugates. RSC Adv. 5, 20 410–20 413. ( 10.1039/C5RA00172B) [DOI] [Google Scholar]
- 35.Goskulwad SP, La DD, Bhosale RS, Al Kobaisi M, Bhosale SV, Bhosale SV. 2016. Golf ball-like architecture fabricated by supramolecular self-assembly of naphthalene diimide. RSC Adv. 6, 39 392–39 395. ( 10.1039/C6RA06927D) [DOI] [Google Scholar]
- 36.Nandre KP, Bhosale SV, Rama Krishna KVS, Gupta A, Bhosale SV. 2013. A phosphonic acid appended naphthalene diimide motif for self-assembly into tunable nanostructures through molecular recognition with arginine in water. Chem. Commun. 49, 5444–5446. ( 10.1039/C3CC41259H) [DOI] [PubMed] [Google Scholar]
- 37.Ashkenasy N, Horne WS, Ghadiri MR. 2006. Design of self-assembling peptide nanotubes with delocalized electronic states. Small 2, 99–102. ( 10.1002/smll.200500252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao K, Levin A, Adler-Abramovich L, Gazit E. 2016. Fmoc-modified amino acids and short peptides: simple bio-inspired building blocks for the fabrication of functional materials. Chem. Soc. Rev. 45, 3935–3953. ( 10.1039/C5CS00889A) [DOI] [PubMed] [Google Scholar]
- 39.Ryan DM, Nilsson BL. 2012. Self-assembled amino acids and dipeptides as noncovalent hydrogels for tissue engineering. Polym. Chem. 3, 18–33. ( 10.1039/c1py00335f) [DOI] [Google Scholar]
- 40.Ryan DM, Anderson SB, Nilsson BL. 2010. The influence of side-chain halogenation on the self-assembly and hydrogelation of Fmoc-phenylalanine derivatives. Soft Matter 6, 3220–3231. ( 10.1039/c0sm00018c) [DOI] [Google Scholar]
- 41.Draper ER, Morris KL, Little MA, Raeburn J, Colquhoun C, Cross ER, McDonald TO, Serpell LC, Adams DJ. 2015. Hydrogels formed from Fmoc amino acids. CrystEngComm 17, 8047–8057. ( 10.1039/C5CE00801H) [DOI] [Google Scholar]
- 42.Sutton S, Campbell NL, Cooper AI, Kirkland M, Frith WJ, Adams DJ. 2009. Controlled release from modified amino acid hydrogels governed by molecular size or network dynamics. Langmuir 25, 10 285–10 291. ( 10.1021/la9011058) [DOI] [PubMed] [Google Scholar]
- 43.Ryan DM, Anderson SB, Senguen FT, Youngman RE, Nilsson BL. 2010. Self-assembly and hydrogelation promoted by F5-phenylalanine. Soft Matter 6, 475–479. ( 10.1039/b916738b) [DOI] [Google Scholar]
- 44.Andric G, et al. 2004. Spectroscopy of naphthalene diimides and their anion radicals. Aust. J. Chem. 57, 1011–1019. ( 10.1071/CH04130) [DOI] [Google Scholar]
- 45.Bowerman CJ, Liyanage W, Federation AJ, Nilsson BL. 2011. Tuning β-sheet peptide self-assembly and hydrogelation behavior by modification of sequence hydrophobicity and aromaticity. Biomacromolecules 12, 2735–2745. ( 10.1021/bm200510k) [DOI] [PubMed] [Google Scholar]
- 46.Gosztola D, Niemczyk MP, Svec W, Lukas AS, Wasielewski MR. 2000. Excited doublet states of electrochemically generated aromatic imide and diimide radical anions. J. Phys. Chem. A 104, 6545–6551. ( 10.1021/jp000706f) [DOI] [Google Scholar]
- 47.Hapuarachchige S, et al. 2011. Design and synthesis of a new class of membrane-permeable triazaborolopyridinium fluorescent probes. J. Am. Chem. Soc. 133, 6780–6790. ( 10.1021/ja2005175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basak S, Nanda J, Banerjee A. 2013. Assembly of naphthalenediimide conjugated peptides: aggregation induced changes in fluorescence. Chem. Commun. 49, 6891–6893. ( 10.1039/C3CC43538E) [DOI] [PubMed] [Google Scholar]
- 49.Würthner F, Kaiser TE, Saha-Möller CR. 2011. J-aggregates: from serendipitous discovery to supramolecular engineering of functional dye materials. Angew. Chem. Int. Ed. 50, 3376–3410. ( 10.1002/anie.201002307) [DOI] [PubMed] [Google Scholar]
- 50.Ford WE, Hiratsuka H, Kamat PV. 1989. Photochemistry of 3,4,9,10-perylenetetracarboxylic dianhydride dyes. 4. Spectroscopic and redox properties of oxidized and reduced forms of the bis(2,5-di-tert-butylphenyl)imide derivative. J. Phys. Chem. 93, 6692–6696. ( 10.1021/j100355a025) [DOI] [Google Scholar]
- 51.Viehbeck A, Goldberg MJ, Kovac CA. 1990. Electrochemical properties of polyimides and related imide compounds. J. Electrochem. Soc. 137, 1460–1466. ( 10.1149/1.2086690) [DOI] [Google Scholar]
- 52.Lee SK, Zu Y, Herrmann A, Geerts Y, Muellen K, Bard AJ. 1999. Electrochemistry, spectroscopy and electrogenerated chemiluminescence of perylene, terrylene, and quaterrylene diimides in aprotic solution. J. Am. Chem. Soc. 121, 3513–3520. ( 10.1021/JA984188M) [DOI] [Google Scholar]
- 53.Pengo P, Pantoş GD, Otto S, Sanders JKM. 2006. Efficient and mild microwave-assisted stepwise functionalization of naphthalenediimide with α-amino acids. J. Org. Chem. 71, 7063–7066. ( 10.1021/jo061195h) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.