Abstract
Islet amyloid polypeptide, also known as amylin, is the main component of the amyloid deposits present in approximately 90% of people with type 2 diabetes mellitus (T2DM). In this disease, amylin aggregates into multimeric β-pleated sheet structures which cause damage to pancreatic islet β-cells. Inhibitors of early-stage amylin aggregation could therefore provide a disease-modifying treatment for T2DM. In this study, overlapping peptides were designed to target the ‘binding’ region (RLANFLVHSS, residues 11–20) of human amylin, and their effects on amyloid fibril formation were determined by thioflavin-T assay. The first generation peptides showed less than 50% inhibition of aggregation, but a second generation peptide (H2N-RGANFLVHGR-CONH2) showed strong inhibitory effects on amylin aggregation, and this was confirmed by negative stain electron microscopy. Cytotoxicity studies revealed that this peptide protected human pancreatic 1.4E7 (ECACC 10070102) insulin-secreting cells from the toxic effects of human amylin. Unlike the retro-inverso version of this peptide, which stimulated aggregation, two N-methylated peptides (H2N-RGAmNFmLVmHGR-CONH2 and H2N-RGANmFLmVHmR-CONH2) gave very clear dose-dependent inhibition of fibril formation. These two peptides were also stable against a range of different proteolytic enzymes, and in human plasma. These N-methylated peptides could provide a novel treatment for slowing progression of T2DM.
Keywords: diabetes, amylin, islet amyloid polypeptide, IAPP, N-methylated peptide, amyloid
1. Introduction
Amyloid is a generic term for pathological protein deposits that accumulate in many different organs and tissues when protein molecules in a predominantly β-pleated sheet conformation self-associate, mainly by hydrogen bonds, to form long and unbranching 8–10 nm diameter fibrils [1,2]. More than 30 different proteins are known to form these fibrils in a wide variety of diseases in humans, including various forms of systemic and inherited amyloidosis [3–5], Alzheimer's disease (AD) [6] and other neurodegenerative diseases [7], and type 2 diabetes mellitus (T2DM) [8–10]. One of the most prevalent of these diseases (along with AD) is T2DM, where the amyloid deposits are found in the islets of Langerhans of the pancreas, and are composed of islet amyloid polypeptide (IAPP), also known as amylin [10]. Amylin is a 37 amino acid peptide belonging to the calcitonin family, members of which have a disulfide bridge between Cys residues 2 and 7, as well as an amidated carboxyl terminus [10,11]. Amyloid deposits have been reported in around 90% of cases of T2DM [12,13] and amylin aggregation has been strongly linked with the development of islet β-cell failure in this disease [13,14]. Early studies demonstrated the toxicity of human amylin to cultured islet cells, through induction of membrane damage, Ca2+ ion influx, and apoptosis, and suggested that this toxicity resides in the amyloid fibrils themselves [15–18]. However, as is the case with other amyloids, more recent studies have indicated that smaller ‘soluble oligomers’ could be the most toxic form of this molecule [19–21].
Currently, approximately 425 million people globally have diabetes and this figure is expected to rise to 642 million by the year 2040 [22]. Moreover, total deaths from diabetes have been predicted to increase by 50% in the next 10 years [23]. Diabetes leads to a number of secondary complications including blindness, heart disease, kidney failure and stroke. A healthy diet, weight control, and exercise, are all necessary for management of T2DM [24,25]. In addition to these lifestyle changes, a number of drug treatment options are available, including insulin therapy. However, these drugs do not provide a cure for diabetes, or prevent secondary complications. There is, therefore, a great need for more research to develop new and potentially more effective treatment options for diabetes.
Compounds that inhibit the self-assembly of amylin are a potential therapeutic target for limiting damage to pancreatic islet cells in T2DM, and this would be expected to slow progression of this disease (i.e. have a disease-modifying effect). The objective of this study was to develop novel peptide-based inhibitors of amylin aggregation that impede the spontaneous assembly of amylin into oligomers and fibrils in vitro. In general, it has been challenging to find suitable drug-like therapeutic agents that inhibit the aggregation of amyloid proteins. However, small organic molecules, peptides, peptidomimetics and nanoparticles have all been developed for this purpose. In the case of AD, where this type of therapy is most advanced, a number of inhibitors of aggregation of the Aβ peptide found in senile plaques, including small molecules and peptides, have been developed over the years, but none of these compounds have been successful yet in human clinical trials [26,27]. This is partly due to the fact that inhibition of amyloid aggregation involves blocking the interactions between protein monomers, and protein–protein interactions are recognized as difficult therapeutic targets [28,29]. Generally, regions for protein–protein interactions are 1500–3000 Å in size [30,31], while the region for protein-small molecule interactions is only 300–1000 Å [32,33]. Therefore, small molecules are not able to build adequate steric interruptions to inhibit protein aggregation [34]. These challenges make it difficult to develop potent and selective small molecule inhibitors of amyloid aggregation.
An alternative strategy for inhibition of amyloid aggregation is the use of peptide-based inhibitors. Peptide-based inhibitors directed against specific amyloid sub-regions represent the first generation of amyloid-based therapeutics, which can then be developed further into more drug-like molecules, and this could be a promising avenue for development of a new disease-modifying therapy for T2DM. In the case of amylin, previous studies along these lines have focused almost exclusively on the primary ‘amyloidogenic’ region of the peptide (amino acid residues 22–28, with sequence NFGAILS), which is the main region involved in protein misfolding into the toxic β-sheet conformational structure [35,36]. These peptide inhibitors are designed to act as ‘β-sheet breakers’ and are typically compounds that consist of this amyloidogenic motif in combination with a β-sheet breaker element. The latter can be comprised, for example, of methylated amino acids, or prolines [37,38]. However, these ‘β-breaker’ peptides do not completely inhibit fibril formation and their inhibitory effects are often seen only at high concentrations, when the peptides are present in molar excess compared to amylin [39–41]. In contrast, the peptide inhibitors described in this report are designed to interact with amylin at the 11–20 ‘binding’ region (RLANFLVHSS), peptide derivatives from which show maximum binding to full-length human amylin [42]. Many peptides face the challenge of insolubility in aqueous solution and/or susceptibility to proteolytic degradation. To improve the solubility of the peptides described here, and to limit their self-aggregation, arginine–glycine residues (RG…GR) were placed at both N- and C-termini (figure 1). This approach differs from the ‘β-sheet blockers’ presented in other studies [43–45] and this rationale follows previous successful research where a peptide inhibitor (OR2) with the sequence H2N-RGKLVFFGR-CONH2 was found to inhibit Aβ oligomer and fibril formation [46]. A proteolytically stable retro-inverso version of this peptide (RI-OR2), with sequence reversal and substitution of l-amino acids with d-amino acids, was then developed [47]. The next step was to attach a ‘TAT’ transit sequence (trans-activator of transcription from HIV) to RI-OR2 to allow it to penetrate into cells, and cross the blood–brain barrier [48]. In a final iteration, RI-OR2-TAT was covalently bound to the surface of nanoliposomes to produce a highly potent multivalent inhibitor [49,50]. Here, the first steps of a similar strategy are described for inhibition of aggregation of the amylin peptide in T2DM.
Figure 1.
Example of ThT fluorescence curves for human amylin in the presence of different concentrations of an inhibitor (IO4). Data are means for a single experiment carried out in triplicate, with readings taken every 10 min. Amylin alone (at 25 µM) displays a characteristic increase in fluorescence corresponding to the ‘sigmoidal’ and ‘plateau’ phases of amyloid fibril formation, while the addition of the inhibitor, at varying concentrations, has a dose-dependent effect on fibril formation. The buffer control (‘control’) contained neither amylin nor inhibitor.
2. Material and methods
2.1. Peptides
Full length human amylin (1–37 amide) was purchased from American Peptide Company, California, USA. Structures of the new peptides designed for this study are presented in table 1. Seven peptide inhibitors (IO1-IO7) derived from the 11–20 binding region of amylin (RLANFLVHSS), together with IO8 (the combined amino acid sequences of IO4 and IO5), and NFGAILS (H2N-RGNFGAILSGR-CONH2, from the primary amyloidogenic region), were purchased from ChinaPeptide Company, Shanghai, China. RI-IO8 (retro-inverso IO8), and two N-methylated peptides (N1-IO8, N2-IO8), were synthesized by Cambridge Peptides, Birmingham, UK. The effects of two previously published inhibitors were also assessed. The first of these is the hexapeptide H2N-NF(N-Me)GA(N-Me)IL-COOH (abbreviated here to NMeG24 NMeI26) which is a modification of the amylin 22–27 fragment (NFGAIL), with an N-methylation of the amide bonds at G24 and I26 [51], and was purchased from Anaspec EGT Group, California, USA. The second of these peptides, with amino acid sequence H2N-ANFLVH-COOH [52], was synthesized by ChinaPeptide Company. All peptides were analysed for purity (table 1) and had their mass confirmed by high performance liquid chromatography (HPLC)-mass spectrometry (MS) (electronic supplementary material, figure S1).
Table 1.
Design of peptide inhibitors employed for this study. (Online version in colour.)
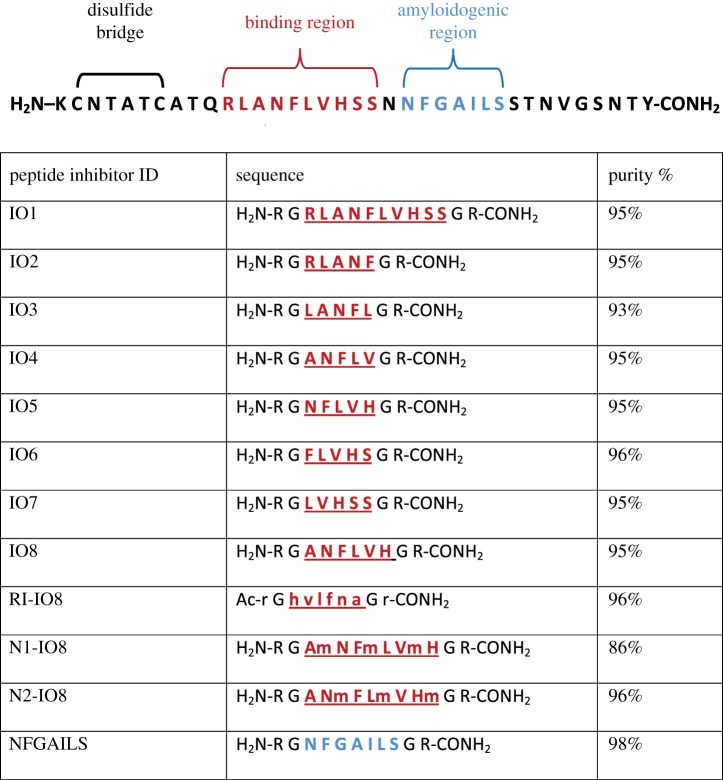 |
The amino acid sequence of human amylin, showing the binding region for amylin self-association [42] and the main amyloidogenic region [35]. All of the short peptide inhibitors are designed to interact with the binding region of full-length amylin, except for the last peptide. The arginine–glycine flanking residues (RG…GR) impede peptide self-aggregation. In the retro-inverted peptide (RI-IO8), d amino acids are in lower case. N-methylated peptide residues are indicated by lower case ‘m’.
2.2. Determination of peptide aggregation by thioflavin-T assay
Amylin was ‘deseeded’ in trifluoroacetic acid (TFA), followed by 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), to remove any preformed aggregates prior to these experiments. ThT assays were carried out in 384-well clear-bottomed microtitre plates (NUNC) by incubating the amylin peptide (25 µM) in the presence of ThT (15 µM) in 10 mM phosphate-buffered saline (PBS), pH 7.4, at 30°C. The inhibitors, when present, were at varying molar ratios relative to amylin, with the total volume of solution in each well set at 60 µl. The plates were shaken every 10 min, and the fluorescence was then read (λex = 442 nm, and λem = 483 nm) in a BioTek Synergy 2 plate reader. Triplicate readings were taken for each condition, with each experiment being repeated three times.
2.3. Cell toxicity experiments
Human pancreatic 1.4E7 (ECACC 10070102) insulin-secreting cells were obtained from Public Health England Culture Collection. 1.4E7 is a hybrid cell line formed by the electrofusion of a primary culture of human pancreatic islets with PANC-1, a human pancreatic ductal carcinoma cell line (ECACC 87092802). These cells were routinely grown in RPMI-1640 medium with l-glutamine (Gibco Life Technologies), supplemented with 10% fetal calf serum and 1% penicillin/streptomycin. Monolayers of cells were grown in 75 cm3 flasks with incubation at 37°C, 5% CO2. Cell viability was assessed using the Promega CellTiter 96 aqueous one solution cell proliferation (MTS) assay. A confluent layer of cells was detached using trypsin, washed, and then suspended and replated, at 250 000 cells/ml, in culture medium. After 24 h, the medium was replaced with fresh medium containing amylin (10 or 20 µM), with the required concentration of peptide inhibitor, with replicates of six wells. After incubation for 24 h, 20 µl of CellTiter 96 aqueous one solution reagent was added to each well and the plate was incubated for a further 3 h. Absorbance at 490 nm was determined using a Wallac Victor2 1420 multilabel counter (PerkinElmer). Each experiment was repeated three times.
2.4. Determination of peptide stability
Reverse-phase (RP) HPLC was used to determine the stability of the peptide inhibitors in plasma, and in the presence of the stated proteolytic enzymes. A C18 column (Phenomenex, 250 × 4 mm) was used for these experiments, with elution by a gradient of acetonitrile, containing 0.01% trifluoro acetic acid (TFA). A sample of human plasma was obtained, with ethical approval (Oldham Ethics Committee), from Prof. David Mann (University of Manchester). Each peptide (5 µl of 100 µM peptide) was added to 95 µl of 50% plasma. To assess the stability of peptides in the presence of proteolytic enzymes, 2 µl of enzyme (1 mg ml−1) was added to 98 µl of peptide (100 µM) in PBS. After incubation, the samples were injected onto the RP-HPLC column and eluted with a linear gradient of 0–60% acetonitrile, with continuous monitoring of absorbance (λ = 220 nm).
2.5. Transmission electron microscopy
Solutions of amylin (25 µM), and amylin in the presence of inhibitors at varying concentrations, were incubated in PBS at room temperature for 48 h, with continuous orbital shaking, and a 5 µl sample was applied to a carbon-coated formvar grid. After 3 min, the liquid was adsorbed by filter paper, then 5 µl of 2% aqueous phosphotungstic acid (PTA) (adjusted to pH 7.3 using 1N NaOH) was applied, and left for 1 min. Excess liquid was removed, and the grid was allowed to dry overnight before observation under a Jeol JEM-1010 electron microscope. Five fields were photographed at random for each sample, after first examining the grids for uniformity.
2.6. Statistical analysis
Data for ThT and cell toxicity assays are expressed as mean ± standard error of mean (s.e.m.), for one representative experiment. Statistical analysis was performed using a two-tailed Student's t test. ANOVA and confidence interval (CI) analysis (p < 0.05 + 95% CI) was used to compare mean values.
3. Results
The aggregation of human amylin at 25 µM in the presence of varying concentrations of peptides IO1-IO7 was monitored by ThT assay. Figure 1 shows typical examples of ThT aggregation curves, demonstrating the effects of one of these peptides (IO4) on fibril formation. Figure 2 presents data for percentage aggregation of amylin when incubated in the presence of different concentrations of each peptide, as determined by ThT fluorescence after 48 h incubation (corresponding to the level of the final plateau phase of fibril formation). Surprisingly, IO1 (H2N-RGRLANFLVHSSGR-CONH2), which spans the entire binding region of amylin, gave no significant inhibition. Lower concentrations (0.6 and 2 µM) of all of the peptides IO1-IO7 appeared to stimulate fibril formation, and no peptide inhibited aggregation to less than 50% of the non-treated control. The most convincing inhibition of amylin aggregation was obtained with IO4 and IO5, and particularly with IO5 (H2N-RGNFLVHGR-CONH2) which inhibited at all concentrations ≥12.5 µM, and so another inhibitor, IO8 (H2N-RGANFLVHGR-CONH2), was designed by combining the sequences of these two peptides. In order to protect IO8 from proteolysis, a retro-inverso version (RI-IO8, Ac-rGhvlfnaGr-CONH2) was also made, by reversing the peptide sequence and replacing the l-amino acids with d-amino acids. IO8 displayed pronounced inhibitory effects on amylin aggregation at all concentrations ≥1 µM (i.e. down to 1 : 25 molar ratio of inhibitor to amylin), with 100 µM IO8 decreasing ThT fluorescence to levels comparable with a buffer only control (figure 3a). In contrast, RI-IO8 showed no inhibitory effects on amylin aggregation, but appeared to enhance fibril formation at all concentrations ≤50 µM (figure 3a). In addition to retro-inversion, another method to improve the physiochemical properties of IO8 is through N-methylation, and so the next step was to carry out ThT assays with two different N-methylated peptides, N1-IO8 and N2-IO8. Both of these peptides displayed a clear and almost identical dose-dependent inhibition of amylin aggregation (figure 3b). Results for IO8 and related peptides from three independent experiments, each carried out in triplicate, are presented in electronic supplementary material, figure S2. Inhibitor IO8 was then compared with peptide ‘NFGAILS’ (H2N-RGNFGAILSGR-CONH2) which was derived from the amyloidogenic region of human amylin. NFGAILS enhanced amylin aggregation at all concentrations ≥25 µM. The effects of NMeG24 NMeI26 [51] and ANFLVH [52], which are inhibitors reported in the literature to reduce amylin fibril formation, were also assessed. ANFLVH did not dissolve in aqueous solution, and NMeG24 NMeI26 showed no inhibitory effects (figure 3d).
Figure 2.
ThT data showing effects of IO1, IO2, IO3, IO4, IO5, IO6 and IO7 peptides on amylin aggregation, after 48 h incubation. All peptides were assayed at 0.6, 2.5, 5, 12.5, 25, 50 and 100 µM concentrations in the presence of 25 µM amylin. Results are means ± s.e.m., n = 3, for a single experiment. Student's t-test was used to establish significance at *p < 0.05, **p < 0.01, or ***p < 0.001, compared to 100% control (amylin alone). (Online version in colour.)
Figure 3.
ThT data showing the effects of IO8 and related peptides, as well NFGAILS and NMeG24 NMeI26, on amylin aggregation, after 48 h incubation. (a) IO8 and RI-IO8. (b) N1-IO8 and N2-IO8. (c) NFGAILS (H2N-RGNFGAILSGR-CONH2). (d) NMeG24 NMeI26. All peptides were assayed at 0.6, 2.5, 5, 12.5, 25, 50 and 100 µM in the presence of 25 µM amylin. Results show means ± s.e.m., n = 3, for a single experiment. See electronic supplementary material, figure S2 for the data from three independent experiments. Note the clear dose-dependent effects of the two N-methylated peptides (N1-IO8 and N2-IO8). (Online version in colour.)
Figure 4 focusses on the early stages of amylin (25 µM) aggregation in the presence of varying concentrations (0.1–100 µM) of N1-IO8 (figure 4a) and N2-IO8 (figure 4b). It can be seen that increasing concentrations of these inhibitors were found to progressively reduce the steepness of the curve during the fibril growth phase, indicating a reduction in the rate of fibril growth. There was also a progressive decrease in the level of the final plateau phase, indicating a reduction in the amount of fibrils formed (it has been demonstrated previously that ThT fluorescence correlates linearly with amyloid fibril concentration [53]). The ThT curves, both in the absence and presence of inhibitors, showed virtually no ‘lag’ phase, and so any effects of the inhibitors on the initial nucleation steps are not clearly defined.
Figure 4.
ThT fluorescence curves for the first 10 h of incubation of amylin in the presence of different concentrations of (a) N1-IO8 and (b) N2-IO8. Data are means for a single experiment carried out in triplicate, with readings taken every 10 min. Amylin alone (at 25 µM) displays a characteristic increase in fluorescence corresponding to the ‘sigmoidal’ and ‘plateau’ phases of amyloid fibril formation (top curve in both cases). In both (a) and (b), the stepwise decrease in the final level of ThT fluorescence in the 10 curves underneath is due to addition of the inhibitors at concentrations of 0.1, 0.3, 0.6, 1, 2.5, 5, 12.5, 25, 50 and 100 µM.
TEM was used to monitor the effects of IO8, RI-IO8, N1-IO8 and N2-IO8 peptides on amylin aggregation, with samples being negatively stained by 2% phosphotungstic acid (PTA). Amylin (at 25 µM) was incubated with 100, 50, 25, 5 and 0 µM (non-inhibited control) of each of these peptides. Figure 5a shows the typical dense meshwork of amyloid fibrils that was observed after 48 h incubation of amylin alone. With addition of 100, 50 or 25 µM of IO8 (i.e. 4 : 1, 2 : 1 and 1 : 1 molar ratios of IO8 to amylin), no fibrils were observed (illustrated for 25 µM IO8 in figure 5b). At 5 µM IO8 (1 : 5 molar ratio of IO8 to amylin) some fibrils were observed, but at a lower density than that seen in the amylin control. On addition of 100, 50, 25 or 5 µM RI-IO8 to 25 µM amylin, very dense fibrillar aggregates of amylin were observed (illustrated for 25 µM RI-IO8 in figure 5c). In the presence of 100, 50 or 25 µM of either N1-IO8 or N2-IO8, no fibrils were seen after 48 h incubation (illustrated for 25 µM N1-IO8 and N2-IO8 in figure 5d,e). At 5 µM of N1-IO8, a few fibrils were observed, but no fibrils were seen with 5 µM of N2-IO8. None of the peptides tested showed any tendency to form oligomers or fibrils when incubated alone (figure 5f–i). These TEM results support the ThT data and confirm that IO8, N1-IO8 and N2-IO8 are effective inhibitors of amylin aggregation, whereas RI-IO8 has no inhibitory effect, and may even stimulate fibril formation.
Figure 5.
Negative stain EM images of amylin incubated in the presence and absence of inhibitors. (a) Sample of amylin (25 µM) incubated for 48 h in PBS at room temperature and stained with phosphotungstic acid (2% w/v). (b) Amylin (25 µM) + IO8 (25 µM); (c) amylin (25 µM) + RI-IO8 (25 µM); (d) amylin (25 µM) + N1-IO8 (25 µM); (e) amylin (25 µM) + N2-IO8 (25 µM); (f) IO8 alone (100 µM); (g) RI-IO8 alone (100 µM); (h) N1-IO8 alone (100 µM); and (i) N1-IO8 alone (100 µM). Scale bar, 100 nm.
The stability of the most promising inhibitory peptides (IO8, N1-IO8 and N2-IO8) towards individual proteolytic enzymes (see electronic supplementary material, figure S3), and in human plasma, was assessed by RP-HPLC. The data are summarized in table 2, with examples of RP-HPLC traces of peptides in plasma presented in figure 6. IO8 was highly susceptible to the effects of trypsin and chymotrypsin, which rapidly degraded this peptide, but it was also degraded by cathepsin G, elastase, thrombin, kallikrein, plasmin, and factor X. Not surprisingly, therefore, IO8 was very unstable in human plasma (figure 6A). In contrast, both N1-IO8 and N2-IO8 were stable for up to 24 h incubation in plasma (figure 6B,C), and, unlike IO8, remained intact after 3 h incubation with each of the individual proteolytic enzymes, although some degradation was noted after 24 h incubation (table 2).
Table 2.
Stability of peptide-inhibitors to proteolysis. √, stable; X, degraded, after 3 h incubation with the enzyme.
| IO8 | RI-IO8 | N1-IO8 | N2-IO8 | |
|---|---|---|---|---|
| blood plasma | X | √ | √ | √ |
| trypsin | X | √ | √a | √a |
| chymotrypsin | X | √ | √ | √ |
| cathepsin G | X | √ | √ | √ |
| elastase | X | √ | √ | √ |
| thrombin | X | √ | √a | √ |
| kallikrein | X | √ | √a | √a |
| plasmin | X | √ | √ | √ |
| factor X | X | √ | √ | √ |
aSome degradation seen after 24 h incubation. HPLC traces for IO8, N1-IO8 and N2-IO8 are given in electronic supplementary material, figure S3.
Figure 6.
Reverse-phase HPLC traces showing stability of peptide inhibitors in human plasma. Column (A) IO8; column (B) N1-IO8; column (C) N2-IO8. For each column, the top trace shows elution of the peptide standard without plasma; the middle trace is for 0 h incubation in plasma; and the lower trace is after 48 h incubation in plasma. Note that IO8 is degraded, whereas N1-IO8 and N2-IO8 are more stable.
The toxic effect of human amylin (20 and 10 µM) on human pancreatic 1.4E7 cells was determined by MTS assay, in the presence and absence of 1 : 1 and 1 : 4 molar ratios (inhibitor:amylin) of the IO8, N1-IO8 and N2-IO8 peptides (for results see figure 7). Amylin at 20 µM was cytotoxic to PANC-1 cells and consistently reduced cell viability to approximately 40% of untreated control cells, whereas at 10 µM amylin the results were more variable and cell viability was reduced to 60–90%. All of these inhibitor peptides, at both molar ratios, rescued the cells from the toxic effect of 20 µM amylin and 10 µM amylin. None of the inhibitors alone, at concentrations of up 20 µM, had any effect on cell viability. Cell toxicity results for IO8 and related peptides from three independent experiments, each carried out with n = 6 replicates, are presented in electronic supplementary material, figure S4.
Figure 7.

Cytotoxic effect of amylin on human pancreatic 1.4E7 insulin-secreting cells in the presence or absence of inhibitor peptides. (a) Cells were incubated for 24 h with 20 µM or 10 µM human amylin in RPMI-1640 medium, with or without IO8 inhibitor, and viability was measured using the MTS assay. (b) Results for N1-IO8. (c) Results for N2-IO8. In all cases, results show mean ± s.e.m., n = 6. ANOVA followed by Student's t-test established significance at *p < 0.05, **p < 0.01, ***p < 0.001. (Online version in colour.)
4. Discussion
T2DM is the most widespread endocrine disorder [54], and is characterized by a reduction in β-cell mass, insulin resistance, and the presence of amyloid deposits in the pancreatic islets, the main component being amylin [55]. The 22–28 (NFGAILS) segment of amylin is regarded as the most highly amyloidogenic region of this peptide, and will itself assemble into amyloid fibrils [39,56]. However, residues 8–20 [57], 14–20 [58], and 30–37 [59] have also been reported to form β-sheet fibrils. Although several amyloidogenic regions of human amylin have been proposed, this study was concerned with developing peptide inhibitors from the ‘binding’ region of human amylin, corresponding to amino acid residues 11–20 (RLANFLVHSS), and on studying their impact on the fibrillogenesis of full-length human amylin. This region is thought to be involved in the initial interactions between two misfolded amylin molecules, after which they begin to aggregate [42]. Thus, preventing this interaction should impede aggregation. This strategy, to target the binding region, has been successfully applied to the development of inhibitors of Aβ aggregation as a potential disease-modifying treatment for AD [47–50].
Seven potential inhibitor peptides were derived from this binding region, and investigated for their ability to influence amylin fibril formation, based on the ThT fluorescence assay [60]. Peptides IO2, IO3, IO4, IO5 and IO7 (table 1) showed some inhibitory effects, but IO4 and IO5 gave the most promising results, and were considered for further investigation. These two amino acid sequences were combined to give IO8 (amino acid sequence: H2N-RGANFLVHGR-CONH2). Retro-inverso peptides often retain bioactivity and are stable to proteolysis [47,61], and so the retro-inverso version of IO8 (RI-IO8: Ac-rGhvlfnaGr-CONH2) was derived by sequence reversal and d-amino acid substitution. The IO8 peptide showed a strong inhibitory effect on amylin aggregation, and, unlike peptides IO1-IO7, did not stimulate amylin aggregation at low concentrations. However, RI-IO8 had no inhibitory effect on amylin aggregation, except at a 4 : 1 molar ratio RI-IO8 to amylin, where the peptide reduced amylin aggregation to only 77% of a non-inhibited control (figure 3). At lower concentrations, RI-IO8 actually stimulated amylin aggregation. This finding was unexpected and suggests that RI-IO8 does not interact in the same way as IO8 to full-length human amylin. Congo red binding experiments have also confirmed the inhibitory effect of IO8, and the stimulatory effect of RI-IO8, on amylin aggregation (data not shown). This finding was further supported by TEM studies, where IO8 abolished and RI-IO8 increased amylin fibril formation (figure 5). This result with RI-IO8 is contrary to a previous study, where the retro-inverso peptide RI-OR2, developed against β-amyloid (Aβ) aggregation, was shown to inhibit amyloid fibril formation, and also rescue cells from the toxic effects of Aβ, as well as being highly resistant to proteolysis [47].
Since RI-IO8 did not inhibit amylin aggregation, N-methylation was considered as an alternative means to improve its stability and pharmacokinetic properties. It is not surprising that IO8 was rapidly degraded in plasma, and in the presence of proteolytic enzymes, because l-peptides are quickly metabolized in this way [62]. IO8 would be cleaved after amino acid 5 (Phe) by high specificity chymotrypsin, and after amino acids 5, 6 and 8 (Phe, Leu, His) by low specificity chymotrypsin, while trypsin will cleave after position 1 (Arg). N-methylation has been shown to improve the pharmacokinetic properties of peptides, by protecting them from proteolysis [63]. Also, N-methylation of alternate amino acid residues gives one face of the peptide molecule that is not available for H-bonding, which impedes amyloid fibril formation [64]. N-methylated derivatives of Aβ(25–35) have been reported to impede the aggregation of fibrils and prevent Aβ cytotoxicity, and N-methylated analogues of amylin do not form fibrils [18,65]. Here, IO8 was stabilized against proteolytic degradation through N-methylation of alternate amino acid residues, to give N1-IO8 (H2N-R-G-Am-N-Fm-L-Vm-H-G-R-CONH2) and N2-IO8 (H2N-R-G-A-Nm-F-Lm-V-Hm-G-R-CONH2). ThT and TEM data showed that both NI-IO8 and N2-IO8 are excellent and highly convincing inhibitors of amylin aggregation (figures 3–5) and are relatively stable against proteolytic degradation (figure 6).
New inhibitors of amylin aggregation are desired, as many of the reported inhibitors only work when present in molar excess over amylin [65–67]. For example, a study on peptide fragments corresponding to human amylin residues 20–25 (SNNFGA) and 24–29 (GAILSS) showed an inhibitory effect on β-sheet transition and amyloid aggregation at 10 : 1 and 20 : 1 molar ratios of peptide to amylin, and the GAILSS peptide had no significant effect on amylin-induced cytotoxicity [41]. The inadequacy of some previously published inhibitors is also highlighted by the comparison of the effects of IO8, ANFLVH [52] and NMeG24 NMeI26 [51] on amylin aggregation in this report. ANFLVH has the same amino acid sequence as IO8, but without the flanking cationic arginine residues together with their glycine spacers. Although ANFLVH was reported to inhibit amylin fibril formation in vitro and to protect against amylin cytotoxicity, the latter effect was only observed at 10-fold and 20-fold molar excess concentrations of the peptide [52]. In contrast, the IO8 peptide inhibits amylin aggregation at much lower concentrations than this, and, given the inability to dissolve ANFLVH in aqueous solution, is clearly much more soluble. Moreover, IO8, N1-IO8 and N2-IO8 were seen to rescue human pancreatic 1.4E7 cells from the toxic effects of amylin at a 1 : 4 molar ratio of these peptides to amylin. In contrast to a previous report [51], no evidence of inhibition of aggregation was observed upon addition of NMeG24 NMeI26, at reasonable concentrations, to amylin. In fact, NMeG24 NMeI26 was seen to promote fibril formation (figure 3). This discrepancy could be due to the fact that NMeG24 NMeI26 was reported to inhibit IAPP aggregation when added before nucleation [68] but the aggregation system reported here lacks any lag-phase (figure 4), and so nucleation may be too rapid for this inhibitor to be effective. Two human amylin-derived peptides, with sequences NFGAIL and SNNFGAILSS, were unable to inhibit fibrillation of human amylin [69]. Another study has indicated that NFGAIL causes an immediate shift of amylin to the β-sheet conformation, suggesting that this peptide promotes fibril formation [41]. These results, together with the lack of effect of the H2N-RGNFGAILSGR-CONH2 peptide in this study (figure 3), justify the decision to focus on the RLANFLVHSS (residues 11–20) amylin binding region.
As noted above, modified full-length amylin with N-methylation at positions 24 and 26 has been shown to impede amylin aggregation and its associated cytotoxicity [65]. In addition, a human amylin derived peptide marketed as pramlintide, with proline substitutions at positions 25, 28 and 29, has undergone clinical trials [70–73] where it was administered, alongside insulin, for management of T2DM. This combination of drugs was able to maintain near-normal glycaemic levels, but pramlintide peptide does not appear to have been assessed as an inhibitor of human amylin aggregation. The short peptides described in this report would be much easier and less expensive to synthesize than these full-length human amylin analogues, and would, potentially, be less immunogenic. N1-IO8 and N2-IO8 in particular appear to be potent and stable aggregation inhibitors that are suitable for further development and testing in human amylin transgenic rodent models as potential disease-modifying agents for T2DM. However, the effects of these inhibitors on oligomer formation are not clear from the data presented here, and will need to be examined in further studies. Also, it is emerging that a significant component of amylin toxicity is mediated by inflammation [74], and so the ability of these inhibitors to attenuate amylin-mediated macrophage activation and associated β-cell dysfunction will also need to be determined.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
D.A. wishes to thank Professor Ian Hamley and the other conference organizers for his invitation to this very interesting and stimulating meeting. The authors also thank the editor and anonymous reviewers for their helpful comments and suggestions, and Dr Fuyuki Kametani (Tokyo, Japan) for peptide HPLC-MS analysis.
Data accessibility
This article has no additional data.
Authors' contributions
D.A. conceived of the study and wrote the manuscript. I.O. carried out all of the experiments, data collection and statistical analyses. M.T. designed some of the peptides and N.J.F. was responsible for the electron microscopy. All authors contributed to design of the study and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was partially supported by a scholarship to I.O. from the Faculty of Health and Medicine, Lancaster University.
References
- 1.Dobson CM. 1999. Protein misfolding, evolution and disease. Trends Biochem. Sci. 24, 329–332. ( 10.1016/S0968-0004(99)01445-0) [DOI] [PubMed] [Google Scholar]
- 2.Rochet JC, Lansbury PT. 2000. Amyloid fibrillogenesis: themes and variations. Curr. Opin. Struct. Biol. 10, 60–68. ( 10.1016/S0959-440X(99)00049-4) [DOI] [PubMed] [Google Scholar]
- 3.Westermark P. et al 2007. A primer of amyloid nomenclature. Amyloid 14, 179–183. ( 10.1080/13506120701460923) [DOI] [PubMed] [Google Scholar]
- 4.Westermark P. 2005. Aspects on human amyloid forms and their fibril polypeptides. FEBS Lett. 272, 5942–5949. ( 10.1111/j.1742-4658.2005.05024.x) [DOI] [PubMed] [Google Scholar]
- 5.Pepys MB. 2006. Amyloidosis. Annu. Rev. Med. 57, 223–241. ( 10.1146/annurev.med.57.121304.131243) [DOI] [PubMed] [Google Scholar]
- 6.Allsop D, Mayes J. 2014. Amyloid-β protein and Alzheimer's disease. Essays Biochem. 56, 99–110. ( 10.1042/bse0560099) [DOI] [PubMed] [Google Scholar]
- 7.Soto C, Estrada LD. 2008. Protein misfolding and neurodegeneration. Arch. Neurol. 65, 184–189. ( 10.1001/archneurol.2007.56) [DOI] [PubMed] [Google Scholar]
- 8.Ahmad E, Ahmad A, Singh S, Arshad M, Khan AH, Khan RH. 2011. A mechanistic approach for islet amyloid polypeptide aggregation to develop anti-amyloidogenic agents for type-2 diabetes. Biochimie 93, 793–805. ( 10.1016/j.biochi.2010.12.012) [DOI] [PubMed] [Google Scholar]
- 9.Kapurniotu A. 2001. Amyloidogenicity and cytotoxicity of islet amyloid polypeptide. Biopolymers 60, 438–459. ( 10.1002/1097-0282(2001)60:6%3C438::AID-BIP10182%3E3.0.CO;2-A) [DOI] [PubMed] [Google Scholar]
- 10.Westermark P. 2011. Amyloid in the islets of Langerhans: thoughts and some historical aspects. Upsala J. Med. Sci. 116, 81–89. ( 10.3109/03009734.2011.573884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wimalawansa SJ. 1997. Amylin, calcitonin gene-related peptide, calcitonin, and adrenomedullin: a peptide superfamily. Crit. Rev. Neurobiol. 11, 167–239. ( 10.1615/CritRevNeurobiol.v11.i2-3.40) [DOI] [PubMed] [Google Scholar]
- 12.Kahn SE. 2003. The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46, 3–19. ( 10.1007/s00125-002-1009-0) [DOI] [PubMed] [Google Scholar]
- 13.Zraika S, et al. 2010. Toxic oligomers and islet β cell death: guilty by association or convicted by circumstantial evidence? Diabetologia 53, 1046–1056. ( 10.1007/s00125-010-1671-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hull RL, Westermark GT, Westermark P, Kahn SE. 2004. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab. 89, 3629–3643. ( 10.1210/jc.2004-0405) [DOI] [PubMed] [Google Scholar]
- 15.Hiddinga HJ, Eberhardt NL. 1999. Intracellular amyloidogenesis by human islet amyloid polypeptide induces apoptosis in COS-1 cells. Am. J. Pathol. 154, 1077–1088. ( 10.1016/S0002-9440(10)65360-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapurniotu A, Bernhagen J, Greenfield N, Al-Abed Y, Teichberg S, Frank RW, Voelter W, Bucala R. 1998. Contribution of advanced glycosylation to the amyloidogenicity of islet and amyloid polypeptide. Eur. J. Biochem. 251, 208–216. ( 10.1046/j.1432-1327.1998.2510208.x) [DOI] [PubMed] [Google Scholar]
- 17.Lorenzo A, Yankner BA. 1996. Amyloid fibril toxicity in Alzheimer's disease and diabetes. Ann. NY Acad. Sci. 777, 89–95. ( 10.1111/j.1749-6632.1996.tb34406.x) [DOI] [PubMed] [Google Scholar]
- 18.Yan LM, Velkova A, Tatarek-Nossol M, Andreetto E, Kapurniotu A. 2007. Amylin mimic blocks Aβ cytotoxic self-assembly: cross-suppression of amyloid toxicity of Aβ and amylin suggests a molecular link between Alzheimer's disease and type II diabetes. Angew. Chem. Int. Ed. 46, 1246–1252. ( 10.1002/anie.200604056) [DOI] [PubMed] [Google Scholar]
- 19.Kodali R, Wetzel R. 2007. Polymorphism in the intermediates and products of amyloid assembly. Curr. Opin. Struct. Biol. 17, 48–57. ( 10.1016/j.sbi.2007.01.007) [DOI] [PubMed] [Google Scholar]
- 20.Aitken JF. et al 2010. Tetracycline treatment retards the onset and slows the progression of diabetes in human amylin/islet amyloid polypeptide transgenic mice. Diabetes 59, 161–171. ( 10.2337/db09-0548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritzel RA, Meier JJ, Lin C-Y, Veldhuis JD, Butler PC. 2007. Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes 56, 65–71. ( 10.2337/db06-0734) [DOI] [PubMed] [Google Scholar]
- 22.Diabetes UK. 2015. Key statistics on diabetes. http://www.diabetes.org.uk (accessed 17 August 2015).
- 23.WHO. 2016. World Health Organisation. Diabetes. http://www.who.int/diabetes/en/ (accessed 12 January 2016).
- 24.Colhoun HM. et al 2004. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364, 685–696. ( 10.1016/S0140-6736(04)16895-5) [DOI] [PubMed] [Google Scholar]
- 25.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. 2001. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 44, S14–S21. ( 10.1007/PL00002934) [DOI] [PubMed] [Google Scholar]
- 26.Castello MA, Jeppson JD, Soriano S. 2014. Moving beyond anti-amyloid therapy for the prevention and treatment of Alzheimer's disease. BMC Neurol. 14, 311 ( 10.1186/s12883-014-0169-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenblum WI. 2014. Why Alzheimer trials fail: removing soluble oligomeric β amyloid is essential, inconsistent, and difficult. Neurobiol. Ageing 35, 969–974. ( 10.1016/j.neurobiolaging.2013.10.085) [DOI] [PubMed] [Google Scholar]
- 28.Whitty A, Kumaravel G. 2006. Between a rock and a hard place. Nat. Chem. Biol. 2, 112–118. ( 10.1038/nchembio0306-112) [DOI] [PubMed] [Google Scholar]
- 29.Hajduk PJ, Burns DJ. 2002. Integration of NMR and high-throughput screening. Comb. Chem. High Throughput Screen. 5, 613–621. ( 10.2174/1386207023329996) [DOI] [PubMed] [Google Scholar]
- 30.Keskin O, Gursoy A, Ma B, Nussinov R. 2008. Principles of protein–protein interactions: what are the preferred ways for proteins to interact? Chem. Rev. 108, 1225–1244. ( 10.1021/cr040409x) [DOI] [PubMed] [Google Scholar]
- 31.Teichmann SA. 2002. Principles of protein–protein interactions. Bioinformatics 18, S249 ( 10.1093/bioinformatics/18.suppl_2.S249) [DOI] [PubMed] [Google Scholar]
- 32.Smith RD, Hu L, Falkner JA, Benson ML, Nerothin JP, Carlson HA. 2006. Exploring protein–ligand recognition with Binding MOAD. J. Mol. Graph. Model. 24, 414–425. ( 10.1016/j.jmgm.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 33.Cheng AC, Coleman RG, Smyth KT, Cao Q, Soulard P, Caffrey DR, Salzberg AC, Huang ES. 2007. Structure-based maximal affinity model predicts small-molecule druggability. Nat. Biotechnol. 25, 71–75. ( 10.1038/nbt1273) [DOI] [PubMed] [Google Scholar]
- 34.Wells JA, McClendon CL. 2007. Reaching for high-hanging fruit in drug discovery at protein–protein interfaces. Nature 450, 1001–1009. ( 10.1038/nature06526) [DOI] [PubMed] [Google Scholar]
- 35.Goldsbury C, Goldie K, Pellaud J, Seelig J, Frey P, Müller SA, Kistler J, Cooper GJS, Aebi U. 2000. Amyloid fibril formation from full-length and fragments of amylin. J. Struct. Biol. 130, 352–362. ( 10.1006/jsbi.2000.4268) [DOI] [PubMed] [Google Scholar]
- 36.Tenidis K. et al 2000. Identification of a penta- and hexapeptide of islet amyloid polypeptide (amylin) and with amyloidogenic and cytotoxic properties. J. Mol. Biol. 295, 1055–1071. ( 10.1006/jmbi.1999.3422) [DOI] [PubMed] [Google Scholar]
- 37.Elgersma RC, Meijneke T, Posthuma G, Rijkers DTS, Liskamp RMJ. 2006. Self-assembly of amylin (20–29) amide-bond derivatives into helical ribbons and peptide nanotubes rather than fibrils. Chemistry 12, 3714–3725. ( 10.1002/chem.200501374) [DOI] [PubMed] [Google Scholar]
- 38.Soto C, Kindy MS, Baumann M, Frangione B. 1996. Inhibition of Alzheimer's amyloidosis by peptides that prevent β-sheet conformation. Biochem. Biophys. Res. Commun. 226, 672–680. ( 10.1006/bbrc.1996.1413) [DOI] [PubMed] [Google Scholar]
- 39.Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C. 1990. Islet amyloid polypeptide—pinpointing amino-acid residues linked to amyloid fibril formation . Proc. Natl Acad. Sci. USA 87, 5036–5040. ( 10.1073/pnas.87.13.5036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abedini A, Meng FL, Raleigh DP. 2007. A single-point mutation converts the highly amyloidogenic human islet amyloid polypeptide into a potent fibrillization inhibitor. J. Am. Chem. Soc. 129, 11 300–11 301. ( 10.1021/ja072157y) [DOI] [PubMed] [Google Scholar]
- 41.Scrocchi LA, Chen Y, Waschuk S, Wang F, Cheung S, Darabie AA, McLaurin J, Fraser PE. 2002. Design of peptide-based Inhibitors of human islet amyloid polypeptide fibrillogenesis. J. Mol. Biol. 318, 697–706. ( 10.1016/S0022-2836(02)00164-X) [DOI] [PubMed] [Google Scholar]
- 42.Mazor Y, Gilead S, Benhar I, Gazit E. 2002. Identification and characterization of a novel molecular-recognition and self-assembly domain within the islet amyloid polypeptide. J. Mol. Biol. 322, 1013–1024. ( 10.1016/S0022-2836(02)00887-2) [DOI] [PubMed] [Google Scholar]
- 43.Nie Q, Du X, Geng M. 2011. Small molecule inhibitors of amyloid β peptide aggregation as a potential therapeutic strategy for Alzheimer's disease. Acta Pharmacol. Sin. 32, 545–551. ( 10.1038/aps.2011.14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aitken JF, Loomes KM, Konarkowska B, Cooper GJ.. 2003. Suppression by polycyclic compounds of the conversion of human amylin into insoluble amyloid. Biochem. J. 374, 779–784. ( 10.1042/bj20030422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. 2003. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer's disease. J. Neurochem. 87, 172–181. ( 10.1046/j.1471-4159.2003.01976.x) [DOI] [PubMed] [Google Scholar]
- 46.Austen BM, Paleologou KE, Ali SAE, Qureshi MM, Allsop D, El-Agnaf OMA. 2008. Designing peptide inhibitors for oligomerization and toxicity of Alzheimer's β-amyloid peptide. Biochemistry 47, 1984–1992. ( 10.1021/bi701415b) [DOI] [PubMed] [Google Scholar]
- 47.Taylor M, Moore S, Mayes J, Parkin E, Beeg M, Canovi M, Gobbi M, Mann DMA, Allsop D. 2010. Development of a proteolytically stable retro-inverso peptide inhibitor of β-amyloid oligomerization as a potential novel treatment for Alzheimer's disease. Biochemistry 49, 3261–3272. ( 10.1021/bi100144m) [DOI] [PubMed] [Google Scholar]
- 48.Parthsarathy V. et al 2013. A novel retro-inverso peptide inhibitor reduces amyloid deposition, oxidation and inflammation and stimulates neurogenesis in the APPSWE/PS1ΔE9 mouse model of Alzheimer's disease. PLoS ONE 8, e54769 ( 10.1371/journal.pone.0054769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gregori M. et al 2016. Retro-inverso peptide inhibitor nanoparticles as potent inhibitors of aggregation of the Alzheimer's Aβ peptide. Nanomedicine 13, 723–732. ( 10.1016/j.nano.2016.10.006) [DOI] [PubMed] [Google Scholar]
- 50.Sherer M, Fullwood NJ, Taylor M, Allsop D.. 2015. A preliminary electron microscopic investigation into the interaction between Aβ1-42 peptide and a novel nanoliposome-coupled retro-inverso peptide inhibitor, developed as a potential treatment for Alzheimer's disease. J. Phys. Conf. Ser. 644, 012040 ( 10.1088/1742-6596/644/1/012040) [DOI] [Google Scholar]
- 51.Sellin D, Yan L, Kapurniotu A, Winter R. 2010. Suppression of amylin fibrillation at anionic lipid membranes via amylin-derived amyloid inhibitors and insulin. Biophys. Chem. 150, 73–79. ( 10.1016/j.bpc.2010.01.006) [DOI] [PubMed] [Google Scholar]
- 52.Potter KJ, Scrocchi LA, Warnock GL, Ao Z, Younker MA, Rosenberg L, Lipsett M, Verchere CB, Fraser PE. 2009. Amyloid inhibitors enhance survival of cultured human islets. Biochim. Biophys. Acta 1790, 566–574. ( 10.1016/j.bbagen.2009.02.013) [DOI] [PubMed] [Google Scholar]
- 53.Xue C, Lin TY, Chang D, Guo Z. 2017. Thioflavin T as an amyloid dye: fibril quantification, optimal concentration and effect on aggregation. R. Soc. open sci. 4 160696 ( 10.1098/rsos.160696 ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hossain P, Kawar B, Nahas ME. 2007. Obesity and diabetes in the developing world—a growing challenge. N. Engl. J. Med. 356, 213–235. ( 10.1056/NEJMp068177) [DOI] [PubMed] [Google Scholar]
- 55.Westermark P, Wernstedt C, Wilander E, Hayden DW, O'Brien TD, Johnson KH. 1987. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc. Natl Acad. Sci. USA 84, 3881–3885. ( 10.1073/pnas.84.11.3881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moriarty DF, Raleigh DP. 1999. Effects of sequential proline substitutions on amyloid formation by human amylin 20–29. Biochemistry 38, 1811–1818. ( 10.1021/bi981658g) [DOI] [PubMed] [Google Scholar]
- 57.Jaikaran ET, Higham CE, Serpell LC, Zurdo J, Gross M, Clark A, Fraser PE. 2001. Identification of a novel human islet amyloid polypeptide β-sheet domain and factors influencing fibrillogenesis. J. Mol. Biol. 308, 515–525. ( 10.1006/jmbi.2001.4593) [DOI] [PubMed] [Google Scholar]
- 58.Sawaya MR. et al 2007. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature 447, 453–457. ( 10.1038/nature05695) [DOI] [PubMed] [Google Scholar]
- 59.Nilsson MR, Raleigh DP. 1999. Analysis of amylin cleavage products provides new insights into the amyloidogenic region of human amylin. J. Mol. Biol. 294, 1375–1385. ( 10.1006/jmbi.1999.3286) [DOI] [PubMed] [Google Scholar]
- 60.Biancalana M, Koide S. 2010. Molecular mechanism of thioflavin-T binding to amyloid fibrils. Biochem. Biophys. Acta 1804, 1405–1412. ( 10.1016/j.bbapap.2010.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X, Fan Z, Chen Y, Fang X, Sha X, Barchi JJ. 2013. Retro-inverso carbohydrate mimetic peptides with annexin1-binding selectivity, are stable in vivo, and target tumor vasculature. PLoS ONE 8, e80390 ( 10.1371/journal.pone.0080390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kellock J, Hopping G, Caughet B, Daggetti V. 2016. Peptides composed of alternating l- and d-amino acids inhibit amyloidogenesis in three distinct amyloid systems independent of sequence. J. Mol. Biol. 428, 2317–2328. ( 10.1016/j.jmb.2016.03.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chatterjee J, Rechenmacher F, Kessler H. 2013. N-methylation of peptides and proteins: an important element for modulating biological functions. Angew. Chem. Int. Ed. 52, 254–269. ( 10.1002/anie.201205674) [DOI] [PubMed] [Google Scholar]
- 64.Hughes E, Burke RM, Doig AJ. 2000. Inhibition of toxicity in the β-amyloid peptide fragment β-(25–35) using N-methylated derivatives: a general strategy to prevent amyloid formation. J. Biol. Chem. 275, 25 109–25 115. ( 10.1074/jbc.M003554200) [DOI] [PubMed] [Google Scholar]
- 65.Yan L, Tatarek-Nossol M, Velkova A, Kazantzis A, Kapurniotu A. 2006. Design of a mimic of nonamyloidogenic and bioactive human islet amyloid polypeptide (amylin) as nanomolar affinity inhibitor of amylin cytotoxic fibrillogenesis. Proc. Natl Acad. Sci. USA 103, 2046–2051. ( 10.1073/pnas.0507471103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meng F, Abedini A, Plesner A, Middleton CT, Potter KJ, Zanni MT, Verchere CB, Raleigh DP. 2010. The sulfated triphenyl methane derivative acid fuchsin is a potent inhibitor of amyloid formation by human islet amyloid polypeptide and protects against the toxic effects of amyloid formation. J. Mol. Biol. 400, 555–566. ( 10.1016/j.jmb.2010.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saraogi I, Hebda JA, Becerril J, Estroff LA, Miranker AD, Hamilton AD. 2010. Synthetic α-helix mimetics as agonists and antagonists of islet amyloid polypeptide aggregation. Angew. Chem. Int. Ed. 49, 736–739. ( 10.1002/anie.200901694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tatarek-Nossol M, Yan L-M, Schmauder A, Tenidis K, Westermark G, Kapurniotu A. 2005. Inhibition of hIAPP amyloid-fibril formation and apoptotic cell death by a designed hIAPP amyloid- core-containing hexapeptide. Chem. Biol. 12, 797–809. ( 10.1016/j.chembiol.2005.05.010) [DOI] [PubMed] [Google Scholar]
- 69.Andreasen M. et al 2012. Modulation of fibrillation of hamylin core fragments by chemical modification of the peptide backbone. Biochim. Biophys. Acta 1824, 274–285. ( 10.1016/j.bbapap.2011.10.014) [DOI] [PubMed] [Google Scholar]
- 70.Pullman J, Darsow T, Frias JP. 2006. Pramlintide in the management of insulin-using patients with type 2 and type 1 diabetes. Vasc. Health Risk Manag. 2, 203–212. ( 10.2147/vhrm.2006.2.3.203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kong MF. et al 1998. The effect of single doses of pramlintide on gastric emptying of two meals in men with IDDM. Diabetologia 41, 577–583. ( 10.1007/s001250050949) [DOI] [PubMed] [Google Scholar]
- 72.Maggs DG. et al 2004. Pramlintide reduces postprandial glucose excursions when added to insulin lispro in subjects with type 2 diabetes: a dose-timing study. Diabetes Metab. Res. Rev. 20, 55–60. ( 10.1002/dmrr.419) [DOI] [PubMed] [Google Scholar]
- 73.Thompson RG, Pearson L, Schoenfeld SL, Kolterman OG. 1998. Pramlintide, a synthetic analog of human amylin, improves the metabolic profile of patients with type 2 diabetes using insulin. The Pramlintide in type 2 Diabetes Group. Diabetes Care 21, 987–993. ( 10.2337/diacare.21.6.987) [DOI] [PubMed] [Google Scholar]
- 74.Westwell-Roper CY, Ehses JA, Verchere CB. 2014. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1β production and β-cell dysfunction. Diabetes 63, 1698–1711. ( 10.2337/db13-0863) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.








