Abstract
Amyloidogenic peptides are well known for their involvement in diseases such as type 2 diabetes and Alzheimer's disease. However, more recently, amyloid fibrils have been shown to provide scaffolding and protection as functional materials in a range of organisms from bacteria to humans. These roles highlight the incredible tensile strength of the cross-β amyloid architecture. Many amino acid sequences are able to self-assemble to form amyloid with a cross-β core. Here we describe our recent advances in understanding how sequence contributes to amyloidogenicity and structure. For example, we describe penta- and hexapeptides that assemble to form different morphologies; a 12mer peptide that forms fibrous crystals; and an eight-residue peptide originating from α-synuclein that has the ability to form nanotubes. This work provides a wide range of peptides that may be exploited as fibrous bionanomaterials. These fibrils provide a scaffold upon which functional groups may be added, or templated assembly may be performed.
Keywords: amyloid fibrils, cross-β structure, phenylalanine, X-ray fibre diffraction, electron microscopy
1. Introduction
Amyloid fibrils are formed by numerous proteins and peptides that have been most commonly described in their pathogenic form. Over 30 proteins are known to abnormally self-assemble to form amyloid fibrils, which are deposited in tissues and are related to diseases such as Alzheimer's disease, type 2 diabetes and familial amyloidotic polyneuropathy [1]. Each of the diseases is characterized by a different protein that self-assembles and a broad range of primary sequences are able to form amyloid [2]. A number of algorithms have been developed to explore how amyloidogenicity can be predicted from primary sequence [2–4]. Collectively these in silico approaches reveal that the composition and position of side chains are important for amyloid formation [5]. Aromatic residues are essential and, in particular, phenylalanine and tyrosine are found to drive assembly within numerous amyloidogenic sequences [6].
Amyloid fibrils are defined as sharing a well-defined three-dimensional, repetitive fibrous structure. They are β-sheet rich and share a cross-β architecture which can be detected using X-ray fibre diffraction [7]. The cross-β structure was first defined for insect silk [8] and consists of β-strands that run perpendicular to the fibre axis and elongate to form β-ribbons. Elongation of the fibrils is predominantly mediated via hydrogen bonding between the main chain, but the association of the β-sheet is stabilized by interactions between the side chains [9,10]. The cross-β structure represents the core of the protofilaments, and several protofilaments associate laterally to form the mature fibril [11–13]. Importantly, small changes in the sequence of amyloidogenic peptides can result in different morphologies arising from different packing arrangements. Furthermore, substitutions can result in changes in the propensity to self-assemble [14–17].
Relatively recently, amyloid fibrils have been discovered that play a functional role in a range of organisms [18–20] including the formation of curli fibrils in bacteria [19], hydrophobins in fungi [21] and PMEL17 in melanosomes [20]. This highlights the potential for uses in applications that require highly stable, self-assembling molecules.
Self-assembling molecules are increasingly used for the production of polymeric materials, fibrous assemblies and hydrogels with applications as diverse as photovoltaic cells [22] to platforms for tissue engineering [23]. Amyloid fibrils are extremely strong and stable structures [24,25], resistant to degradation and can be strengthened even further with intermolecular crosslinking [26,27]. They can be fragmented, regenerated and can be used to make hydrogels and molecular scaffolds for further decoration [28]. Ultimately, understanding the molecular basis of peptide self-assembly could lead to the ability to harness amyloidogenesis for the production of novel peptide-based materials, including nanowires, nanotubes, laterally associated ribbons and hydrogelating networks [28–30]. These structures have the potential for use for a diverse range of applications such as cell culture, cell adhesion, bio-sensing, cell differentiation, long-acting drugs and cartilage regeneration [30–36]. For example, the sequence of the C-terminus of the Alzheimer's related peptide, amyloid-β42 (Aβ42), was varied and protected using 9-fluorenylmethoxycarbonyl to produce a range of hydrogels that showed thixotropic properties (shear thinning) and the ability to support cell growth [31]. The stiffness of the gels could be tuned using subtle changes in peptide concentration and ionic strength with the possibility that these gels could then be used to support differentiation of stem cells. Interestingly, these gels supported viable cell growth in contrast with the expected effects of the Aβ42 peptide which is known to be cytotoxic [37]. Peptides derived from the Parkinson's disease (PD) associated protein, α-synuclein, also form hydrogels that could be further developed to support the growth of mesenchymal stem cells for substantia nigra graft implantation in a PD mouse model [33]. For cellular growth, amyloidogenic peptides can be further decorated with cell-adhesive sequences such as RGD. These functional groups were found to alter the topography of the surface of the fibrils and led to reduced toxicity resulting in the ability to provide an attachment surface for cell growth and spreading [32]. In another study, two different peptides were developed that alone remained soluble, but together formed a β-sheet rich hydrogel after mixing. When an RGD sequence was also added to one of the components, the ability to support 3T3 cell growth was further improved and alteration of the ratios of the two components allowed the gel stiffness to be tuned [30].
2. The contribution of sequence to assembly and structure
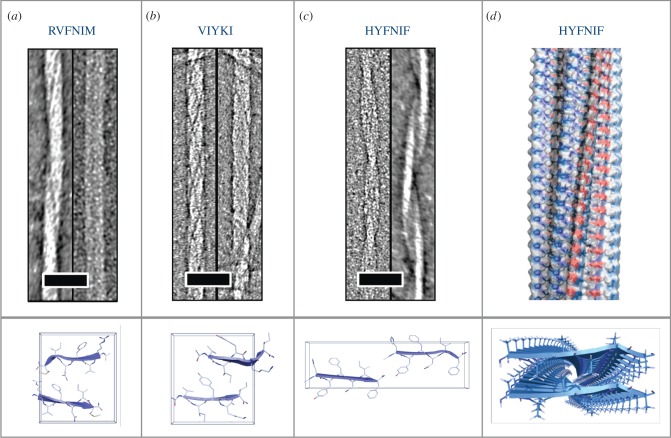
Many different sequences have the ability to self-assemble to form amyloid fibrils. A series of short amyloidogenic peptides have been crystallized to form microcrystals and their assembled structures determined by X-ray crystallography [10]. The first of these was GNNQQNY which is a sequence derived from the yeast prion, Sup35 [38]. It was revealed that this peptide is able to form at least two crystallographic arrangements. The crystals have a hydrogen bonding direction, which is attributed to the fibre axis and the peptides are arranged within the crystallographic structure to form steric zippers in which the side chains associate and interdigitate [10,38]. Further studies using solid-state NMR [39], X-ray fibre diffraction [40] and small angle X-ray scattering [41] revealed that the fibrillar structure differs from the arrangement with the crystal packing. However, many short peptide structures have been solved crystallographically [10,42–44], contributing valuable understanding of how sequence contributes to stability within the amyloid architecture. In order to better understand the role of sequence in amyloidogenicity, a number of groups have designed algorithms that explore the hydrophobicity, β-sheet propensity and residue positional information within the primary sequence [2–4,45,46]. Several of the algorithms have been developed to further explore the structural basis of amyloidogenicity by incorporating residue positional and structural information [2,4]. One of these algorithms, WALTZ, was developed using an experimentally validated group of hexapeptides. In order to contribute further structural information to this algorithm, we explored three peptides with the sequences HYFNIF, RVFNIM and VIYKI [47]. All three of these peptides are strongly predicted to form amyloid fibrils by WALTZ. Using a combined approach of electron microscopy, X-ray fibre diffraction and linear dichroism, we were able to generate model structures that were consistent with the experimental data [47]. It is clear from these three sequences that the aromatic residues (Y and F) play an important role in the amyloidogenic propensity as well as in the final structures. Interestingly, although the sequences appear similar, the morphology of the fibrils by electron microscopy differ significantly, whereby RVFNIM forms twisted ropes and paired ribbons (figure 1a), VIYKI forms fibrils that have a complex network of twisted protofilaments (figure 1b) and HYFNIF forms pairs of twisted protofilaments (figure 1c). The diffraction patterns from each of the fibrils formed by each of the peptides were analysed and revealed the structures organize into different cross-β architectures (figure 1). A model of the structure for a HYFNIF protofilament is shown in figure 1d. These structures highlight the influence of primary sequence on the architecture of the peptides within the protofilament and the packing of protofilaments which gives rise to the macromolecular morphology.
Figure 1.
Characterization of the structure of three amyloidogenic peptides identified by WALTZ [2]. Electron micrographs of fibrils formed by the sequences (a) RVFNIM, (b) VIYKI and (c) HYFNIF, with the structures determined from X-ray fibre diffraction shown in the panels below. (d) The structural model for a protofilament formed by HYFNIF, with the fibre shown above and the view down the fibre axis, highlighting the interdigitation of the side chains, shown below.
2.1. Aromatic and charged residues contribute to the assembly, stability and structure of amyloid
The imperfect repeat peptide sequence KFFEAAAKKFFE assembles to form fibrous nanocrystals. X-ray fibre diffraction and electron microscopy were used to propose a model for the structure of the fibrous architecture. In this arrangement, the phenylalanine side chains play an important role in stabilizing the three-dimensional structure, in both the fibrillar and intersheet directions [9]. To explore the contribution of sequence to the structure, a series of substituted peptides were designed and the resulting fibrillar morphology and molecular structure characterized. Importantly, substitution of any phenylalanine for alanine resulted in assembly incompetent peptides, inferring that the π–π stacking of aromatic residues is important for assembly and stability [9]. Others have shown that phenylalanine can play an important role in amyloidogenicity [6]. Indeed, di- and tri-phenylalanine peptides have been shown to self-assemble to form fibrous, amyloid-like structures [48,49]. To explore the role of charge in the structure of KFFEAAAKKFFE, lysines were substituted for alanine or arginine [50]. The resulting peptides were able to self-assemble, but those with lysine for alanine substitutions formed narrow fibrils rather than the laterally associated nanocrystals that are formed by wild-type, or arginine variants. This result indicates that the lysine residues play an essential role in the further lateral association that takes place between protofilaments to form the crystalline structures for KFFEAAAKKFFE [9,50] and suggests that several of the lysines are exposed on the surface of the fibrils. Many other studies have been conducted and show that the position of particular amino acids in the sequence can influence the organization of the protofilaments [2,47,51–53]. Contribution of basic amino acids to assembly and lateral association have been previously explored using a peptide with sequence (VK)4-VPPT-(KV)4. The proline residues in the centre of the precursor peptide prevented twisting and allowed the formation of flat, laterally associated protofilaments [54]. Hydrophobicity of the sequence has been shown to be essential for the amyloidogenicity [6,55]. MAX3 is a designed sequence containing two repeating VK motifs separated by a threonine flanked d-proline-l-proline region which produces a hairpin [55]. The peptide self-assembles with increasing temperature as the hydrophobic regions self-associate and assembly can be reversed by cooling. The properties of the peptides can be tuned by altering the hydrophobic content of the sequence [55]. The aromatic content of amyloidogenic sequences has been highlighted particularly in reference to the contribution of phenylalanine [6] and tyrosine [16,47,56]. Structures of amyloidogenic peptides have revealed that association of aromatic residues can stabilize the structures [9,10,47,57,58]. Furthermore, the formation of dityrosine, whereby a covalent bond is formed between two close tyrosine residues under oxidative conditions, has been shown to increase the stability of amyloid fibrils [26,59,60].
2.2. Decorating the exterior of amyloid fibrils
The ability to decorate amyloid fibrils formed by self-assembled β-sheet peptides is of considerable interest and has been reported in a number of studies. For example, preformed fibrils assembled from a (Ala)10-(His)6 amphiphilic peptide were decorated with nanogold [61]. Pilkington et al. [62] showed that fibrils could be decorated with an enzyme, glucose oxidase, which remained folded and functional, retaining the ability to inhibit the growth of Escherichia coli. Amyloid fibrils formed by a yeast prion (N-terminal and middle (NM) region of Sup35) in which surface exposed cysteine residues were introduced, were covalently functionalized using gold and silver deposition to produce conducting nanowires [29]. Alternatively, the amyloidogenic region can be conjugated with the functional domain and then assembled. An excellent example is a study using an SH3 domain peptide, which was produced as a cytochrome b562 fusion protein and then allowed to self-assemble to form amyloid fibrils under controlled conditions. The fibrils formed were able to bind metalloporphyrins in 50% of the constituent molecules [63].
Diatom cells are beautifully elaborate silica shells that have nanoscale hooks, holes and gorges that are believed to be formed by a fibrous protein core [64,65]. Indeed, it has been previously shown that fibrous proteins can mediate templating of tetraethyl orthosilicate to form silica [66–68] and that arginine and lysine play an important role in the templating process [67–69]. In recent work, we used the library of variants of the sequence ‘KFFEAAAKKFFE’ to explore whether these fibrils can form silica wires and how the sequence contributes to the morphology of the resulting structures. Yuwono et al. compared the templating of tetraethyl orthosilicate by a series of peptide amphiphiles and revealed that His and Lys were important for calcination, while glutamate was not sufficient to template silica [68].
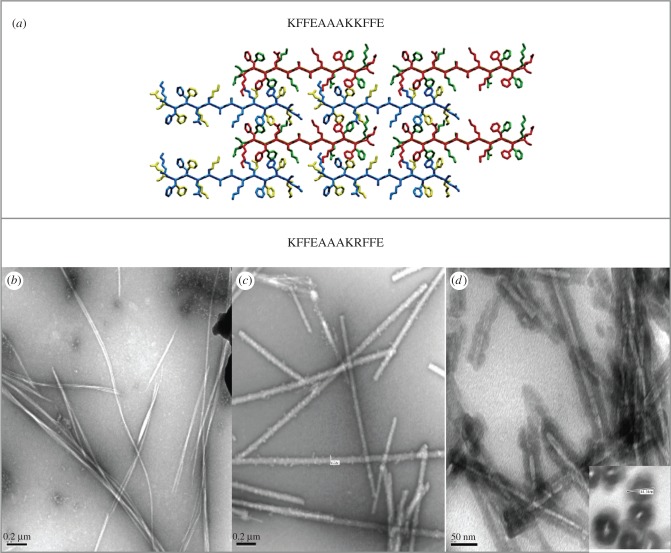
Preformed fibrils composed of KFFEAAAKKFFE variants were incubated with tetraethyl orthosilicate and then washed with ethanol and water. The morphologies of resulting structures were investigated using electron microscopy, which revealed that the alanine mutants were less able to template silica and that lysine at position 1 was particularly important to enable the fibrils to become coated. Arginine variants were efficiently coated with silica and some formed very highly organized silica nanowires with diameters of 70 nm [70] (figure 2). Fourier transform infrared spectroscopy and X-ray fibre diffraction of the silica nanowires revealed that the cross-β core of the nanowires remained after templating. Furthermore, after incubation in a range of harsh conditions, such as low pH, organic solvents and high temperatures, the silica nanowires retained their organized fibrous structure [71] indicating that they could be useful for bionanotechnological processes as narrow wires. In summary, we demonstrated that amyloidogenic arginine–lysine containing peptides represent a viable nucleus for templating silica to produce very stable nanowires.
Figure 2.
Exploiting fibrils formed by the sequence KFFEEAAAKKFFE [9,50]. (a) Structural models elucidated from X-ray fibre diffraction and electron diffraction data [9]. (b) Electron microscopy shows that the variant peptide K9R forms amyloid-like fibrils, (c) which can be coated with silica to form silica nanowires. (d) Silica nanowires following embedding in resin and sectioning to highlight the exterior density from the silica and the electron lucent core [71].
2.3. Cross-β with an alternative morphology
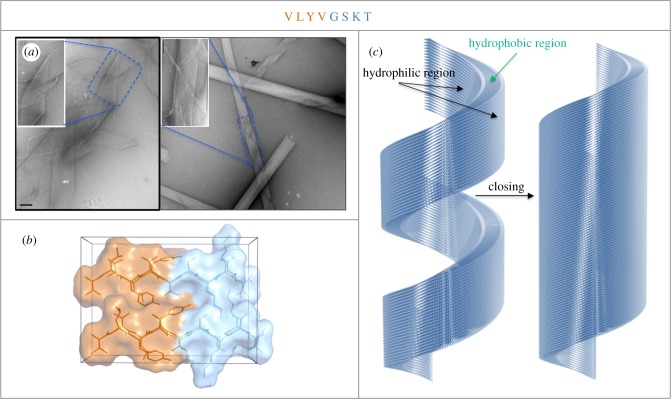
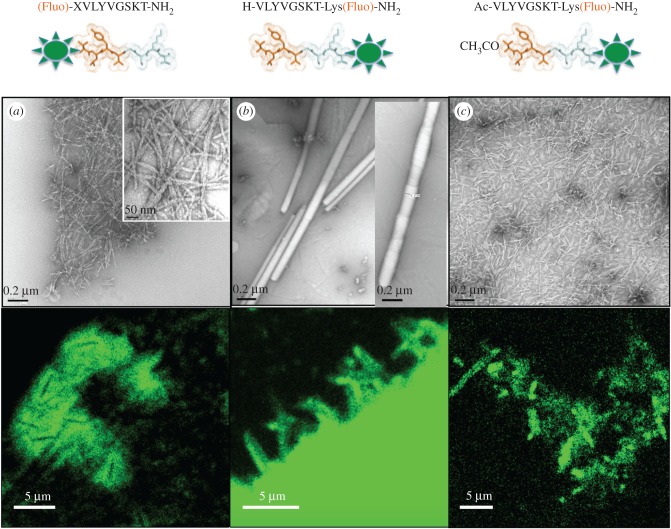
Short amyloidogenic sequences have the possibility of forming amyloid-like fibrils, and also more elaborate structures. A short peptide derived from Aβ16-22 (Ac-KLVFF-NH2) was shown to form conventional amyloid fibrils at neutral pH or to form distinctive, thin-walled nanotubes at low pH [72]. A model of the peptides in the nanotube architecture was built from X-ray and electron diffraction data with β-strands tilted at 23° from the fibre axis [72]. An octapeptide (VLYVGSKT) derived from a predicted β-strand within the Parkinson's related protein, α-synuclein [73], was found to form cylindrical nanotubes following incubation at high concentration in water [74]. These appear to be very lightly stained tubes under the electron microscope, which resemble thin-walled cylinders that occasionally unravelled from a tubular structure to expose helical ribbons (figure 3a). We conducted X-ray fibre diffraction analysis, which revealed a cross-β pattern but with important differences arising from the helical architecture and texture within the diffracting samples [74]. Modelling revealed that the amphipathic peptide formed cross-β-strands, which associated to form a bilayer forming the walls of the cylinder (figure 3b). In the model, the hydrophobic regions of the peptide are shielded from solvent by the inner and outer walls of the cylinder and also when the helical ribbon structure seals to form a tube (figure 3c) [74]. These fibres are excellent candidates for decoration to form fluorescent nanotubes. By conjugating a fluorescein moiety to the N- or C-termini of the peptide, we were able to explore the assembly process and to generate fluorescent tubes. In support of the model structure (figure 3b,c) the addition of a fluorescein tag (X-Fluo) to the hydrophobic N-terminus prevented the formation of the tubes, resulting only in narrow amyloid-like fibrils (figure 4a). Addition of the fluorescein tag (Lys(Fluo)) to the hydrophilic C-terminus resulted in the formation of nanotubes (150 nm) similar to those formed by the wild-type peptide (250 nm). Interestingly, unravelling of the nanotubes into nanoribbons was not observed, unlike those structures formed by the wild-type octamer. The addition of the uncharged lysine (linked to the fluorescein) may have influenced the formation of a more stable helical nanotube (figure 4b). Capping the hydrophobic N-terminus of the C-terminal tagged peptide prevented the formation of nanotubes and resulted in only short nanofibrils (figure 4c). Confocal microscopy images demonstrated the formation of a network of fluorescent fibres from N-terminal tagged peptide and a cluster of narrow fluorescent fibres by the C-terminal tagged peptide. Acetylated C-terminal tagged peptide appeared as small fluorescent particles by confocal microscopy. The results highlight the importance of a free N-terminus to preserve the discrete nanotubular architecture. A unique feature of the wild-type design is its ability to incorporate the fluorescein moiety within the hydrophilic surface and retain its nanotubular structure.
Figure 3.
VLYVGSKT forms helical nanotubes and nanoribbons [74]. (a) Electron micrograph showing the thin-walled cylinders formed by the octapeptide. (b) The structural model built following analysis of X-ray fibre diffraction data. (c) Helical nanotube and nanoribbon model proposed to incorporate the amphipathic peptide into a bilayer which forms the walls of the cylinder.
Figure 4.
Fluorescein tagging of VLYVGSKT at the N- or C-termini (composition is depicted in top panel). Upper panels show transmission electron micrographs showing the fibres formed. Confocal microscopy images showing the fluorescence of the assembled structures are shown in the panels below. (a) N-terminus tagged, (b) C-terminus tagged, (c) N-terminus acetylated and C-terminus tagged. The X in the sequence of the N-terminal tagged peptide is a spacer (6-aminohexanoic acid) to provide a distance between the tag and the peptide sequence.
3. Conclusion
Amyloid fibrils have inherent strength and stability due to their extensive hydrogen bonding along the fibre axis and the contribution of hydrophobic, aromatic and electrostatic interactions between side chains. The diverse amino acid sequences of peptides that form amyloid fibrils result in an extensive range of different morphologies each with unique surface properties which can be used for chemical decoration and functionalization. Here we have discussed three examples of our work to demonstrate the wide range of different amyloid fibrous structure that can be formed and further functionalized with the potential for use in bionanotechnology.
4. Experimental methods
All methodologies for Waltz peptides and KKFFEAAAKFFE peptides are described in [9,47,50,70,71].
Structural study of fluorescent labelled peptides. Peptides were synthesized as C-terminal amides by Fmoc/t-Bu-based solid-phase synthesis, as previously described [75]. The wild-type and C- and N-terminus labelled peptides were dissolved in Milli-Q water (15 mg ml−1) and allowed to self-assemble at room temperature. After 7 days, peptides were placed in a lyophiliser (45°C, 90 000 r.p.m.) for 1 h. Samples (100–120 mg ml−1) from each peptide were then collected and examined using transmission electron microscopy and confocal microscopy. A measure of 4 µl of the fibril samples was incubated on 400 mesh carbon-formvar coated copper grids (Agar Scientific Ltd) for 2 min, washed with Milli-Q, 0.2 µm filtered water for another 2 min and stained using filtered uranyl acetate 2% w/v for 2 min. Grids were examined using a Hitachi 7100 electron microscope operated at 80 kV and images were collected using a Gatan Ultrascan 1000 CCD camera (Gatan, Abingdon, UK). For confocal imaging, 5 µl of sample (50 mg ml−1) was dried onto a clean slide, and a coverslip was placed on top and sealed with nail polish. Images were taken using a Leica TCS SP8 confocal microscope with a 63× 1.2 NA water objective. Samples were excited with a 488 nm laser line and emission collected between 500 and 550 nm.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Dobson CM. 2004. Principles of protein folding, misfolding and aggregation. Semin. Cell Dev. Biol. 15, 3–16. ( 10.1016/j.semcdb.2003.12.008) [DOI] [PubMed] [Google Scholar]
- 2.Maurer-Stroh S, et al. 2010. Exploring the sequence determinants of amyloid structure using position-specific scoring matrices. Nat. Methods 7, 237–242. ( 10.1038/nmeth.1432) [DOI] [PubMed] [Google Scholar]
- 3.Pawar AP, Dubay KF, Zurdo J, Chiti F, Vendruscolo M, Dobson CM. 2005. Prediction of ‘aggregation-prone’ and ‘aggregation-susceptible’ regions in proteins associated with neurodegenerative disease. J. Mol. Biol. 350, 379–392. ( 10.1016/j.jmb.2005.04.016) [DOI] [PubMed] [Google Scholar]
- 4.Thompson MJ, Sievers SA, Karanicolas J, Ivanova MI, Baker D, Eisenberg D. 2006. The 3D profile method for identifying fibril-forming segments of proteins. Proc. Natl Acad. Sci. USA 103, 4074–4078. ( 10.1073/pnas.0511295103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamodrakas SJ. 2011. Protein aggregation and amyloid fibril formation prediction software from primary sequence: towards controlling the formation of bacterial inclusion bodies. FEBS J. 278, 2428–2435. ( 10.1111/j.1742-4658.2011.08164.x) [DOI] [PubMed] [Google Scholar]
- 6.Gazit E. 2002. A possible role for pi-stacking in the self-assembly of amyloid fibrils. FASEB J. 1, 77–83. ( 10.1096/fj.01-0442hyp) [DOI] [PubMed] [Google Scholar]
- 7.Morris KL, Serpell LC. 2012. X-ray fibre diffraction studies of amyloid fibrils. Methods Mol. Biol. 849, 121–135. ( 10.1007/978-1-61779-551-0_9) [DOI] [PubMed] [Google Scholar]
- 8.Geddes AJ, Parker KD, Atkins EDT, Beighton E. 1968. ‘Cross b’ conformation in protein. J. Mol. Biol. 32, 343–358. ( 10.1016/0022-2836(68)90014-4) [DOI] [PubMed] [Google Scholar]
- 9.Makin OS, Atkins E, Sikorski P, Johansson J, Serpell LC. 2005. Molecular basis for amyloid fibril formation and stability. Proc. Natl Acad. Sci. USA 102, 315–320. ( 10.1073/pnas.0406847102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawaya MR, et al. 2007. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 447, 453–457. ( 10.1038/nature05695) [DOI] [PubMed] [Google Scholar]
- 11.Jimenez JL, Tennent G, Pepys MB, Saibil HR. 2001. Structural diversity of ex vivo amyloid fibrils studied by cryo-electron microscopy. J. Mol. Biol. 311, 241–247. ( 10.1006/jmbi.2001.4863) [DOI] [PubMed] [Google Scholar]
- 12.Jimenez JL, Nettleton EJ, Bouchard M, Robinson CV, Dobson CM, Saibil HR. 2002. The protofilament structure of insulin amyloid fibrils. Proc. Natl Acad. Sci. USA 99, 9196–9201. ( 10.1073/pnas.142459399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldsbury C, Aebi U, Frey P. 2001. Visualizing the growth of Alzheimer's A beta amyloid-like fibrils. Trends Mol. Med. 7, 582 ( 10.1016/S1471-4914(01)02180-3) [DOI] [PubMed] [Google Scholar]
- 14.Fraser PE, McLachlan DR, Surewicz WK, Mizzen CA, Snow AD, Nguyen JT, Kirschner DA. 1994. Conformation and fibrillogenesis of Alzheimer Aβ peptides with selected substitution of charged residues. J. Mol. Biol. 244, 64–73. ( 10.1006/jmbi.1994.1704) [DOI] [PubMed] [Google Scholar]
- 15.Hilbich C, Kisters-Woike B, Reed J, Masters CL, Beyreuther K. 1992. Substitutions of hydrophobic amino acids reduce the amyloidogenicity of Alzheimer's disease β A4 peptides. J. Mol. Biol. 228, 460–473. ( 10.1016/0022-2836(92)90835-8) [DOI] [PubMed] [Google Scholar]
- 16.Lopez de la Paz M, Serrano L. 2004. Sequence determinants of amyloid fibril formation. Proc. Natl Acad. Sci. USA 101, 87–92. ( 10.1073/pnas.2634884100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall KE, et al. 2016. A critical role for the self-assembly of Amyloid-β1-42 in neurodegeneration. Sci. Rep. 6, 30182 ( 10.1038/srep30182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen P, Nielsen JL, Dueholm MS, Wetzel R, Otzen D, Nielsen PH. 2007. Amyloid adhesins are abundant in natural biofilms. Environ. Microbiol. 9, 3077–3090. ( 10.1111/j.1462-2920.2007.01418.x) [DOI] [PubMed] [Google Scholar]
- 19.Barnhart MM, Chapman MR. 2006. Curli biogenesis and function. Annu. Rev. Microbiol. 60, 131–147. ( 10.1146/annurev.micro.60.080805.142106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fowler DM, Koulov AV, Balch WE, Kelly JW. 2007. Functional amyloid—from bacteria to humans. Trends Biochem. Sci. 32, 217–224. ( 10.1016/j.tibs.2007.03.003) [DOI] [PubMed] [Google Scholar]
- 21.Sunde M, Kwan AH, Templeton MD, Beever RE, Mackay JP. 2007. Structural analysis of hydrophobins. Micron 39, 773–784 ( 10.1016/j.micron.2007.08.003) [DOI] [PubMed] [Google Scholar]
- 22.Bolisetty S, Adamcik J, Heier J, Mezzenga R. 2012. Amyloid directed synthesis of titanium dioxide nanowires and their applications in hybrid photovoltaic devices. Adv. Funct. Mater. 22, 3424–3428. ( 10.1002/adfm.201103054) [DOI] [Google Scholar]
- 23.Rajagopal K, Schneider JP. 2004. Self-assembling peptides and proteins for nanotechnological applications. Curr. Opin. Struct. Biol. 14, 480–486. ( 10.1016/j.sbi.2004.06.006) [DOI] [PubMed] [Google Scholar]
- 24.Smith JF, Knowles TP, Dobson CM, Macphee CE, Welland ME. 2006. Characterization of the nanoscale properties of individual amyloid fibrils. Proc. Natl Acad. Sci. USA 103, 15 806–15 811. ( 10.1073/pnas.0604035103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostaert AS, Higgins MJ, Fukuma T, Rindi F, Jarvis SP. 2006. Nanoscale mechanical characterisation of amyloid fibrils discovered in a natural adhesive. J. Biol. Phys. 32, 393–401. ( 10.1007/s10867-006-9023-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Hilaly YK, et al. 2013. A central role for dityrosine crosslinking of Amyloid-β in Alzheimer's disease. Acta Neuropathol. Commun. 1, 83 ( 10.1186/2051-5960-1-83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grip S, Johansson J, Hedhammar M. 2009. Engineered disulfides improve mechanical properties of recombinant spider silk. Protein Sci. 18, 1012–1022. ( 10.1002/pro.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mankar S, Anoop A, Sen S, Maji SK. 2011. Nanomaterials: amyloids reflect their brighter side. Nano Rev. 2, 6032 ( 10.3402/nano.v2i0.6032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheibel T, Parthasarathy R, Sawicki G, Lin XM, Jaeger H, Lindquist SL. 2003. Conducting nanowires built by controlled self-assembly of amyloid fibers and selective metal deposition. Proc. Natl Acad. Sci. USA 100, 4527–4532. ( 10.1073/pnas.0431081100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King PJ, Giovanna Lizio M, Booth A, Collins RF, Gough JE, Miller AF, Webb SJ. 2016. A modular self-assembly approach to functionalised β-sheet peptide hydrogel biomaterials. Soft Matter 12, 1915–1923. ( 10.1039/C5SM02039E) [DOI] [PubMed] [Google Scholar]
- 31.Jacob RS, et al. 2015. Self healing hydrogels composed of amyloid nano fibrils for cell culture and stem cell differentiation. Biomaterials 54, 97–105. ( 10.1016/j.biomaterials.2015.03.002) [DOI] [PubMed] [Google Scholar]
- 32.Reynolds NP, Charnley M, Bongiovanni MN, Hartley PG, Gras SL. 2015. Biomimetic topography and chemistry control cell attachment to amyloid fibrils. Biomacromolecules 16, 1556–1565. ( 10.1021/acs.biomac.5b00114) [DOI] [PubMed] [Google Scholar]
- 33.Das S, et al. 2016. Implantable amyloid hydrogels for promoting stem cell differentiation to neurons. NPG Asia Mater. 8, e304 ( 10.1038/am.2016.116) [DOI] [Google Scholar]
- 34.Gras SL, Bongiovanni MN, Scanlon DB. 2011. Functional fibrils derived from the peptide TTR1-cycloRGDfk that target cell adhesion and spreading. Biomaterials 32, 6099–6110. ( 10.1016/j.biomaterials.2011.05.021) [DOI] [PubMed] [Google Scholar]
- 35.Kim S, Kim JH, Lee JS, Park CB. 2015. Beta-sheet-forming, self-assembled peptide nanomaterials towards optical, energy, and healthcare applications. Small 11, 3623–3640. ( 10.1002/smll.201500169) [DOI] [PubMed] [Google Scholar]
- 36.Maji SK, Schubert D, Rivier C, Lee S, Rivier JE, Riek R. 2008. Amyloid as a depot for the formulation of long-acting drugs. PLoS Biol. 6, e17 ( 10.1371/journal.pbio.0060017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh DM, Selkoe DJ. 2007. A beta oligomers—a decade of discovery. J. Neurochem. 101, 1172–1184. ( 10.1111/j.1471-4159.2006.04426.x) [DOI] [PubMed] [Google Scholar]
- 38.Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, Eisenberg D. 2005. Structure of the cross-β spine of amyloid-like fibrils. Nature 435, 773–778. ( 10.1038/nature03680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Wel PC, Lewandowski JR, Griffin RG. 2007. Solid-state NMR study of amyloid nanocrystals and fibrils formed by the peptide GNNQQNY from yeast prion protein Sup35p. J. Am. Chem. Soc. 129, 5117–5130. ( 10.1021/ja068633m) [DOI] [PubMed] [Google Scholar]
- 40.Marshall KE, Hicks MR, Williams TL, Hoffmann SV, Rodger A, Dafforn TR, Serpell LC. 2010. Characterizing the assembly of the Sup35 yeast prion fragment, GNNQQNY: structural changes accompany a fiber-to-crystal switch. Biophys. J. 98, 330–338. ( 10.1016/j.bpj.2009.10.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langkilde AE, Morris KL, Serpell LC, Svergun DI, Vestergaard B. 2015. The architecture of amyloid-like peptide fibrils revealed by X-ray scattering, diffraction and electron microscopy. Acta Crystallogr. D Biol. Crystallogr. 71, 882–895. ( 10.1107/S1399004715001674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colletier JP, et al. 2011. Molecular basis for amyloid-beta polymorphism. Proc. Natl Acad. Sci. USA 108, 16 938–16 943. ( 10.1073/pnas.1112600108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitago Y, Nagae M, Nakata Z, Yagi-Utsumi M, Takagi-Niidome S, Mihara E, Nogi T, Kato K, Takagi J. 2015. Structural basis for amyloidogenic peptide recognition by sorLA. Nat. Struct. Mol. Biol. 22, 199–206. ( 10.1038/nsmb.2954) [DOI] [PubMed] [Google Scholar]
- 44.Spencer RK, Kreutzer AG, Salveson PJ, Li H, Nowick JS. 2015. X-ray crystallographic structures of oligomers of peptides derived from β2-microglobulin. J. Am. Chem. Soc. 137, 6304–6311. ( 10.1021/jacs.5b01673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zibaee S, Makin OS, Goedert M, Serpell LC. 2007. A simple algorithm locates beta-strands in the amyloid fibril core of α-synuclein, Aβ, and tau using the amino acid sequence alone. Protein Sci. 16, 906–918. ( 10.1110/ps.062624507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DuBay KF, Pawar AP, Chiti F, Zurdo J, Dobson CM, Vendruscolo M. 2004. Prediction of the absolute aggregation rates of amyloidogenic polypeptide chains. J. Mol. Biol. 341, 1317–1326. ( 10.1016/j.jmb.2004.06.043) [DOI] [PubMed] [Google Scholar]
- 47.Morris KL, Rodger A, Hicks MR, Debulpaep M, Schymkowitz J, Rousseau F, Serpell LC. 2013. Exploring the sequence–structure relationship for amyloid peptides. Biochem. J. 450, 275–283. ( 10.1042/BJ20121773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamamis P, Adler-Abramovich L, Reches M, Marshall K, Sikorski P, Serpell L, Gazit E, Archontis G. 2009. Self-assembly of phenylalanine oligopeptides: insights from experiments and simulations. Biophys. J. 96, 5020–5029. ( 10.1016/j.bpj.2009.03.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adler-Abramovich L, Reches M, Sedman VL, Allen S, Tendler SJ, Gazit E. 2006. Thermal and chemical stability of diphenylalanine peptide nanotubes: implications for nanotechnological applications. Langmuir 22, 1313–1320. ( 10.1021/la052409d) [DOI] [PubMed] [Google Scholar]
- 50.Marshall KE, Morris KL, Charlton D, O'Reilly N, Lewis L, Walden H, Serpell LC. 2011. Hydrophobic, aromatic, and electrostatic interactions play a central role in amyloid fibril formation and stability. Biochemistry 50, 2061–2071. ( 10.1021/bi101936c) [DOI] [PubMed] [Google Scholar]
- 51.Krysmann MJ, Castelletto V, Kelarakis A, Hamley IW, Hule RA, Pochan DJ. 2008. Self-assembly and hydrogelation of an amyloid peptide fragment. Biochemistry 47, 4597–4605. ( 10.1021/bi8000616) [DOI] [PubMed] [Google Scholar]
- 52.Rajagopal K, Lamm MS, Haines-Butterick LA, Pochan DJ, Schneider JP. 2009. Tuning the pH responsiveness of beta-hairpin peptide folding, self-assembly, and hydrogel material formation. Biomacromolecules 10, 2619–2625. ( 10.1021/bm900544e) [DOI] [PubMed] [Google Scholar]
- 53.Elsawy MA, Smith AM, Hodson N, Squires A, Miller AF, Saiani A. 2016. Modification of β-sheet forming peptide hydrophobic face: effect on self-assembly and gelation. Langmuir 32, 4917–4923. ( 10.1021/acs.langmuir.5b03841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamm MS, Rajagopal K, Schneider JP, Pochan DJ. 2005. Laminated morphology of nontwisting β-sheet fibrils constructed via peptide self-assembly. J. Am. Chem. Soc. 127, 16 692–16 700. ( 10.1021/ja054721f) [DOI] [PubMed] [Google Scholar]
- 55.Pochan DJ, Schneider JP, Kretsinger J, Ozbas B, Rajagopal K, Haines L. 2003. Thermally reversible hydrogels via intramolecular folding and consequent self-assembly of a de novo designed peptide. J. Am. Chem. Soc. 125, 11 802–11 803. ( 10.1021/ja0353154) [DOI] [PubMed] [Google Scholar]
- 56.Bemporad F, Taddei N, Stefani M, Chiti F. 2006. Assessing the role of aromatic residues in the amyloid aggregation of human muscle acyphophatase. Protein Sci. 15, 862–870. ( 10.1110/ps.051915806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diaz-Avalos R, Long C, Fontano E, Balbirnie M, Grothe R, Eisenberg D, Caspar DLD. 2003. Cross-beta order and diversity in nanocrystals of an amyloid-forming peptide. J. Mol. Biol. 330, 1165–1175. ( 10.1016/S0022-2836(03)00659-4) [DOI] [PubMed] [Google Scholar]
- 58.Fitzpatrick AWP, et al. 2017. Cryo-EM structures of tau filaments from Alzheimer's disease. Nature 547, 185–190. ( 10.1038/nature23002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atwood CS, et al. 2004. Copper mediates dityrosine cross-linking of Alzheimer's amyloid-β. Biochemistry 43, 560–568. ( 10.1021/bi0358824) [DOI] [PubMed] [Google Scholar]
- 60.Cukalevski R, Boland B, Frohm B, Thulin E, Walsh D, Linse S. 2012. Role of aromatic side chains in amyloid beta-protein aggregation. ACS Chem. Neurosci. 3, 1008–1016. ( 10.1021/cn300073s) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamley IW, et al. 2014. Self-assembly of a model peptide incorporating a hexa-histidine sequence attached to an oligo-alanine sequence, and binding to gold NTA/nickel nanoparticles. Biomacromolecules 15, 3412–3420. ( 10.1021/bm500950c) [DOI] [PubMed] [Google Scholar]
- 62.Pilkington SM, Roberts SJ, Meade SJ, Gerrard JA. 2010. Amyloid fibrils as a nanoscaffold for enzyme immobilization. Biotechnol. Prog. 26, 93–100. [DOI] [PubMed] [Google Scholar]
- 63.Baldwin AJ, Bader R, Christodoulou J, MacPhee CE, Dobson CM, Barker PD. 2006. Cytochrome display on amyloid fibrils. J. Am. Chem. Soc. 128, 2162–2163. ( 10.1021/ja0565673) [DOI] [PubMed] [Google Scholar]
- 64.Fernandes FM, Coradin T, Aime C. 2014. Self-assembly in biosilicification and biotemplated silica materials. Nanomaterials 4, 792–812. ( 10.3390/nano4030792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Likhoshway YV, Sorokovikova EG, Belykh OI, Kaluzhnaya OV, Belikov SI, Bedoshvili YD, Kaluzhnaya OV, Masyukova JA, Sherbakova TA. 2008. Visualization of the silicon biomineralization in cyanobacteria, sponges and diatoms. In Biosphere origin and evolution (eds Dobretsov N, Kolchanov N, Rozanov A, Zavarzin G), pp. 219–230. Novcsibirsk, Russia: Springer. [Google Scholar]
- 66.Dehsorkhi A, Hamley IW. 2014. Silica templating of a self-assembling peptide amphiphile that forms nanotapes. Soft Matter 10, 1660–1664. ( 10.1039/c3sm52324a) [DOI] [PubMed] [Google Scholar]
- 67.Holmstrom SC, King PJ, Ryadnov MG, Butler MF, Mann S, Woolfson DN. 2008. Templating silica nanostructures on rationally designed self-assembled peptide fibers. Langmuir 24, 11 778–11 783. ( 10.1021/la802009t) [DOI] [PubMed] [Google Scholar]
- 68.Yuwono VM, Hartgerink JD. 2007. Peptide amphiphile nanofibers template and catalyze silica nanotube formation. Langmuir 23, 5033–5038. ( 10.1021/la0629835) [DOI] [PubMed] [Google Scholar]
- 69.Jan JS, Lee SJ, Carr CS, Shantz DF. 2005. Biomimetic synthesis of inorganic nanospheres. Chem. Mater. 17, 4310–4317. ( 10.1021/cm0504440) [DOI] [Google Scholar]
- 70.Al-Garawi ZS, Thorpe JR, Serpell LC. 2015. Silica nanowires templated by amyloid-like fibrils. Angew. Chem. 54, 13 327–13 331. ( 10.1002/anie.201508415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Garawi ZS, Kostakis GE, Serpell LC. 2016. Chemically and thermally stable silica nanowires with a beta-sheet peptide core for bionanotechnology. J. Nanobiotechnol. 14, 79 ( 10.1186/s12951-016-0231-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mehta AK, et al. 2008. Facial symmetry in protein self-assembly. J. Am. Chem. Soc. 130, 9829–9835. ( 10.1021/ja801511n) [DOI] [PubMed] [Google Scholar]
- 73.Goedert M. 2001. Alpha-synuclein and neurodegenerative diseases. Nat. Neurosci. 7, 492–501. ( 10.1038/35081564) [DOI] [PubMed] [Google Scholar]
- 74.Morris KL, Zibaee S, Chen L, Goedert M, Sikorski P, Serpell LC. 2013. The structure of cross-beta tapes and tubes formed by an octapeptide, αSβ1. Angew. Chem. 52, 2279–2283. ( 10.1002/anie.201207699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gross A, Rodel K, Kneidl B, Donhauser N, Mossl M, Lump E, Munch J, Schmidt B, Eichler J. 2015. Enhancement and induction of HIV-1 infection through an assembled peptide derived from the CD4 binding site of gp120. ChemBioChem 16, 446–454. ( 10.1002/cbic.201402545) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.