Abstract
Innovations are generally unexpected, often spectacular changes in phenotypes and ecological functions. The contributions to this theme issue are the latest conceptual, theoretical and experimental developments, addressing how ecology, environment, ontogeny and evolution are central to understanding the complexity of the processes underlying innovations. Here, we set the stage by introducing and defining key terms relating to innovation and discuss their relevance to biological, cultural and technological change. Discovering how the generation and transmission of novel biological information, environmental interactions and selective evolutionary processes contribute to innovation as an ecosystem will shed light on how the dominant features across life come to be, generalize to social, cultural and technological evolution, and have applications in the health sciences and sustainability.
This article is part of the theme issue ‘Process and pattern in innovations from cells to societies’.
Keywords: culture, information, innovation ecosystem, network, niche, novelty
1. Introduction
A central feature of life is change. Change may be rapid, such as a population crash during a disease epidemic or the evolution of antibiotic resistance. Change may also be slow, a prime example being the evolution of novel biochemical pathways leading to altered phenotypic traits, possibly generating new species. Both rapid and slow change can occur at the level of the genotype and the phenotype, as well as in ecological aggregates such as populations and communities. Its magnitude ranges anywhere from imperceptible and difficult to quantify, to highly visible and transformational, impacting not only individuals and their interactions but also their biotic and abiotic environments. Innovations comprise a class of changes that signal an often a priori unexpected departure from a previous state (see glossary for definitions of this and other terms). Many of the conceptual foundations of innovation can be traced at least back to Darwin [1,2], who suggested that novelties could emerge through either natural selection or the co-opting of traits originally used for other functions. His ideas presaged some current concepts of innovation.
Innovations are important to understand because they are distinctive, apparently improbable changes to the phenotype, and yet pervade biology, culture and technology. Innovations are central to the evolution of complexity, to phenotypic diversity, and to the emergence and filling of new niches. Insights into the processes generating innovations are not only central to understanding life, but also for predicting the future of human society and natural ecosystems. The growth of innovation research is evidenced by a keyword search of articles published in ecology or evolution between 1997 and 2017,1 which yielded an approximate fourfold increase in the fraction of articles using the keyword ‘innovation’ (currently about 1%).
The aim of this theme issue is to present our current understanding of innovations and the innovation process across a spectrum of systems and organizational levels, with the ultimate objective of advancing a theory of innovations. Each of the papers of this theme issue articulates with one or more of the steps in the innovation process depicted in figure 1 or the patterns they produce. Although the focus of most of the contributions in this theme issue is biological, given that ecological and evolutionary processes influence human culture, society, economics and technology, the insights emerging could contribute to a theory that encompasses complex behavioural systems, and be transferrable to applications in the biological and social sciences, ranging from conserving biodiversity to managing pathogens, pests and invasive species, to understanding the impacts of medical breakthroughs on human demography and the effects of global change on resource sustainability, and to forecasting the growth of cities, economies and technological breakthroughs. Therefore an important objective of this theme issue and the discussion below is to better understand the commonalities and contrasts of innovation in biology, culture and technology.
Figure 1.
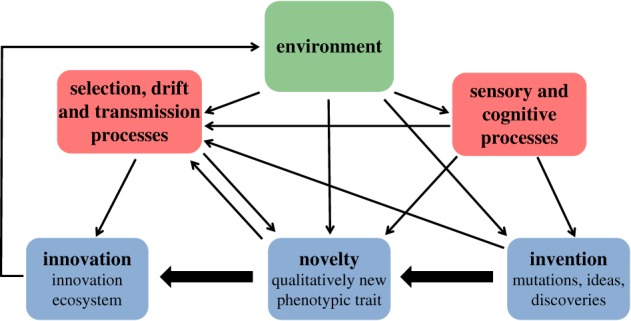
A simplified diagram of processes leading to innovation. An innovation is the result of inventions and their recombination that generates a novelty that spreads and evolves in conjunction with selective and stochastic effects. As emphasized by papers in this theme issue, the environment influences the emergence of innovations, which, in turn, impact the environment. Note that the conventional notion of biological innovation (as opposed to cultural and technological) downplays the role of sensory and cognitive processes in the generation, filtering and horizontal spread of information (and generation of possible novelties) among individuals. See main text for discussion.
2. What is an innovation?
Innovation and the related concepts of invention and novelty have been extensively discussed in the biological [3] and social sciences [4]. Given the many disciplines and systems to which innovations matter, a diversity of definitions is not surprising [5]. Even within disciplines, such as in biology, concepts such as evolutionary novelty are difficult to define rigorously [6,7]. In our opinion, synthesizing this vast literature (for discussion and reviews, see e.g. [8–18]) with the aim of finding a simple, one-size-fits-all definition of innovation may be futile, because the development of innovation as a concept has largely occurred at the discipline level with limited cross-talk, and different aspects of innovations are emphasized in different disciplines. Thus, regardless of how innovation is defined—mathematically, statistically or verbally—it invariably encompasses some degree of discipline- or system-specific subjectivity. Nevertheless, certain basic features common to most innovation concepts are worth highlighting.
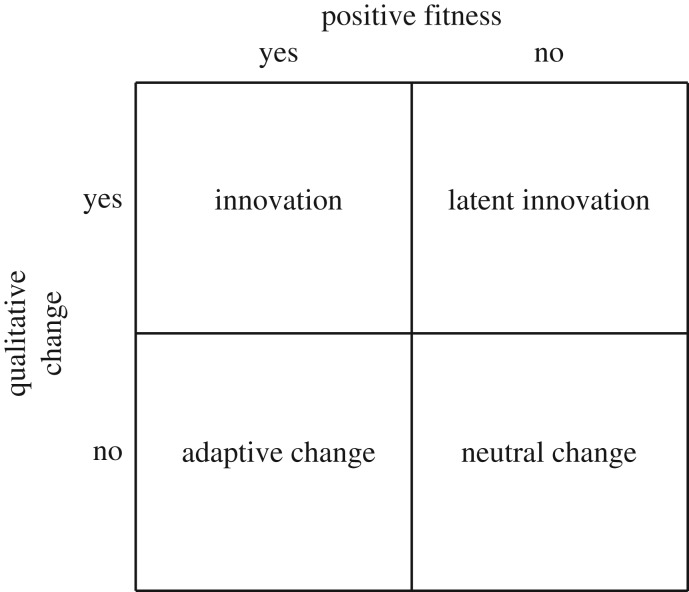
In biology, although all innovations involve adaptive change, not all adaptations are innovations (figure 2). This is because an innovation is a qualitatively new phenotypic trait that is associated with a step departure from an evolutionary trend (figure 3). The terms innovation and novelty are often used interchangeably in the literature, but here we make the distinction that a novelty is an individual-level concept of qualitative phenotypic change, whereas only a subset of novelties become innovations, the latter having population, community, ecosystem and evolutionary consequences. Determining what constitutes a sufficient change in the phenotype to qualify as a novelty or an innovation is a major challenge (box 1), as is quantifying (and possibly statistically evaluating) innovation (box 2).
Figure 2.
A classification of change. Most adaptations are not innovations (lower left), because the necessary conditions for an innovation (upper left) include both a qualitative change in a phenotypic trait (a novelty) and that the novelty has positive fitness; that is, it either spreads through the population (positive relative fitness and competitive elimination of the ancestor) or expands into a new niche (positive absolute fitness). Some qualitative trait changes have nearly neutral or even negative fitness effects, and either persist at low frequencies (latent innovations; upper right), or go extinct and may only return if specific mutation or recombination events occur, or (in the realm of human technology) if an invention is rediscovered and integrated into an existing process or product. See main text for discussion of additional conditions for innovation.
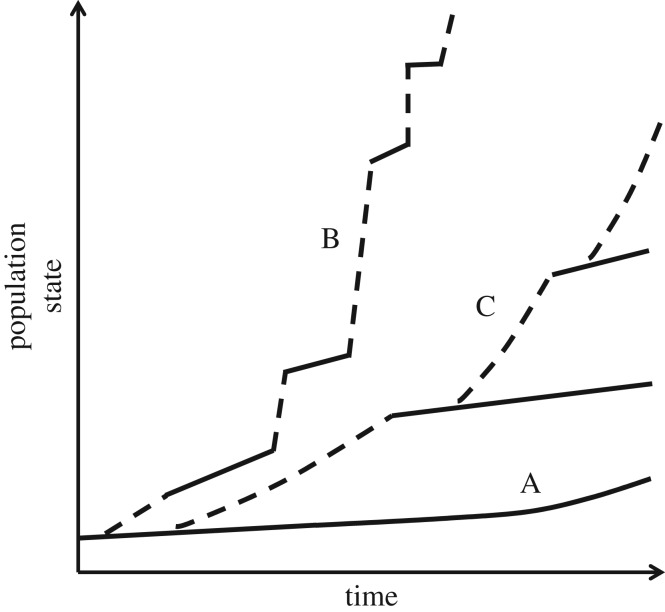
Figure 3.
Illustration of how innovations may impact the state of a population through time. In (A), the population adapts, but does not innovate. It is also possible that no adaptation occurs during certain periods (zero slope) or that the population maladapts (negative slope; not shown). In (B), the population innovates without appreciably altering its ecological niche. Innovations (dotted segments) are infrequent step departures from the prevailing adaptive trend (solid segments). Innovations become increasingly frequent as the population grows in size (due to, for example, more mutations or more recombination events) and as the population accumulates or acquires more information (e.g. trait complexity and variants; cumulative culture). In (C), the population innovates (dotted segments) and in so doing invades and subsequently radiates in a new ecological niche. Four such populations (or species) are illustrated here (ancestor line A, the initial innovating lineage, and two subsequent derived populations/species, each innovation represented by a dashed black line followed by a solid line, the latter representing periods of adaptation). Note that ‘population state’ could be represented as any of a number of possible correlates of evolutionary adaptation, including population size, absolute fitness, relative fitness (selection), phenotypic complexity or cumulative culture. The trajectories in this figure therefore represent a hypothetical index that increases with adaptive (continuous) or innovative (step) change.
Box 1. Sorites paradox.
Much of our understanding of innovations is based on descriptive, conceptual frameworks. An important problem of many conceptual frameworks is encapsulated in the Sorites paradox, where the categorical labelling of a state or function is subjective, yet different grades of the (subjectively) labelled state are quantitative measures themselves (e.g. [19]). For example, the determination of baldness in humans has no objective threshold as measured by the number of hairs on the scalp; however, a male with fewer hairs per unit scalp area could be said to be balder than someone with more hairs. The Sorites paradox highlights why some qualitatively new phenotypic traits are deemed ‘innovations’, and it highlights the role of consensus and subjective judgement in this labelling. It raises the unsolved question of how or whether relative levels or magnitudes of innovation (marginal to transformational) can be scientifically distinguished from one another (see box 2).
Box 2. Statistical detection of innovation?
The very definition of new, novel, never before seen, makes most (if not all) innovations unknowable before they occur, or increasingly knowable (but still probabilistic) as candidate inventions emerge and novelties begin to spread. Relevant notions of feasibility and probability have been conceptualized as the ‘actual and the possible’ [20] and the ‘adjacent possible’ [21].
Recent computational and theoretical modelling work has addressed numerous themes relating to innovation, including patterns generated by the process of technological evolution [22], distinguishing novelty [23], quantifying the magnitude of innovation [24], measuring the time to attain an innovation [25], detecting innovation as revealed by power-law distributions of activities as proxies for inventions [26], and predicting innovations from the adjacent possible [27]. In the context of macroevolution, several interrelated approaches to assessing innovations have been proposed (reviewed in [28]; for adaptive radiations, see reviews in e.g. [14,29]). For example, Wagner [16] has established a rigorous definition of morphological novelty based on mechanisms leading to departures from homologous phenotypic characters (see also [30]). Heard & Hauser [31] proposed a two part test for key innovations, whereby (i) an ecological/functional change in a taxon is associated with increased speciation or decreased extinction rates compared with a sister taxon and (ii) a number of innovative and sister clades are treated as independent observations. Similarly, Bond & Opell [32] proposed three criteria for a key innovation: the trait must (i) be present in two or more organisms and inherited exclusively from their common ancestor, (ii) be functionally advantageous, and (iii) be capable of contributing to adaptive change. Rabosky [28] argues that components of these and other approaches are fraught with conceptual issues and/or methodological problems (see also [11]).
Based on the discussion above and on some of the contributions in this volume, we propose the following sequential criteria to identify innovations. Criterion 1: The new trait is a qualitative departure from the ancestral trait, as revealed by a change in phenotypic information or complexity. The change can in principle be quantified using information theory [33] (see also [34]). This criterion aims to exclude adaptations that result from a loss of traits [10]. Criterion 2: The qualitatively new phenotypic trait is associated with one or more measures of increased performance and/or utilization of a novel niche. Criterion 3: The novel trait confers positive relative fitness (if in same niche) or positive absolute fitness (if entering a new niche). Criterion 4: The growth of the population harbouring the novelty is associated with significant community and ecosystem impacts, and in the longer term (particularly for niche innovations), adaptive radiation of the innovated lineage. In the spirit of figure 3, we suggest that these four criteria can each be assessed statistically as (significant) deviations from prevailing trends. Given difficulties in obtaining sufficiently rich data to evaluate these criteria (e.g. [34]), we expect that their application will be difficult if not impossible for many systems.
A novelty stems from a series of inventions (mutations in biology; discoveries, ideas, or new devices in culture and technology) and/or recombination events to a blueprint, buttressed by facilitating phenotypic traits, such as standards. A novelty may affect structure (e.g. biochemical pathways, tissue architecture, appendages) and/or function, and particularly for the latter be associated, for example, with the ways organisms acquire resources, evade predators, interact with other organisms and modify their environments through niche construction or technologies.
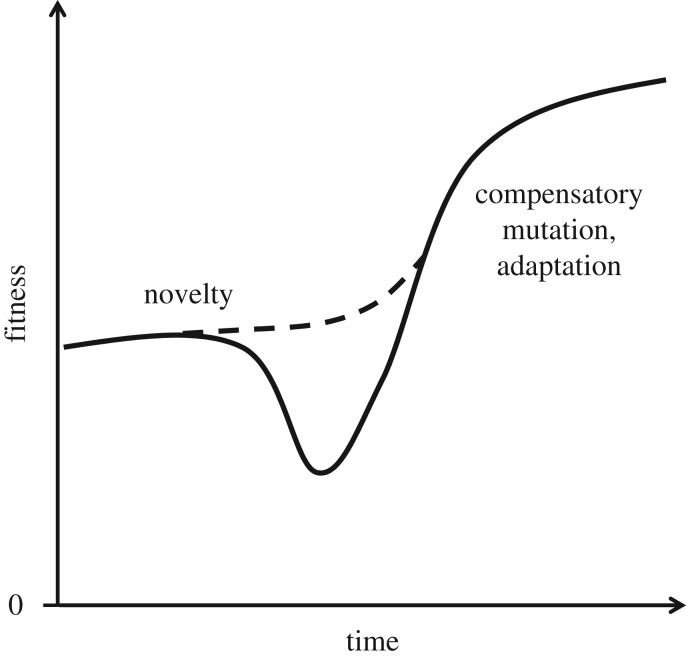
Novelties may derive either from de novo constructs, or from an existing phenotypic trait that is co-opted as an exaptation, that is, from a trait originally associated with other functions or no function at all [35–37]. Although rarely investigated in detail, because a novelty balances benefits to survival and reproduction with costs due to trade-offs and initial maladaptation, the invasion fitness of a novelty may be neutral or even negative (figure 4; e.g. horizontal gene transfer [38]). This means that some inventions never become competitive novelties (a fascinating example in technology being the Einstein–Szilard refrigerators [39]), whereas others do, but only emerge as innovations at some later time (‘latent innovations’; figure 2), either when additional mutations or recombination events occur, or when the environment becomes favourable. By contrast to the hypothesized roles of selection in innovation, the implications of drift are more contentious [40,41] and require further study (see also [42]).
Figure 4.
Simplified representation of the fitness of an innovated lineage through time. Inventions (e.g. mutations) and recombinations lead to a novelty, which may show an increase in fitness of the novel phenotype relative to the ancestral phenotype (dashed line), no change (not shown) or a net cost (solid line). The latter may occur if major rearrangements underlie the novelty, or if environmental conditions are not favourable. To potentially become an innovation, the novelty (or states leading up to it) must spread in the population, or promote population invasion into new niches, and either of these may require some combination of refinements (e.g. DNA mutations that compensate costs; ameliorations that make a technology more efficient or productive), adaptations in other phenotypic traits and conducive environmental conditions.
It is useful to distinguish two basic types of novel traits leading to innovation.
In performance innovations, the novel phenotypic trait is associated with increases in performance, without fundamental changes to the organism's ecological niche (figures 3B, 5B). To become an innovation, such a trait needs to confer a fitness advantage and spread through the population2 (figure 4), and in so doing impact the surrounding community or ecosystem, and possibly create longer-term opportunities for further adaptation and innovation. In the economics and technological spheres, such innovations are often associated with the replacement or ‘creative destruction’ of existing processes or products, akin to some scenarios of competitive exclusion in ecology. The novelty underlying this type of innovation is typically found at lower phenotypic levels, such as, for example, a biochemical pathway permitting more efficient metabolism or tissue repair, or processes enabling the use of low-grade (less expensive) silicon for producing solar cells.
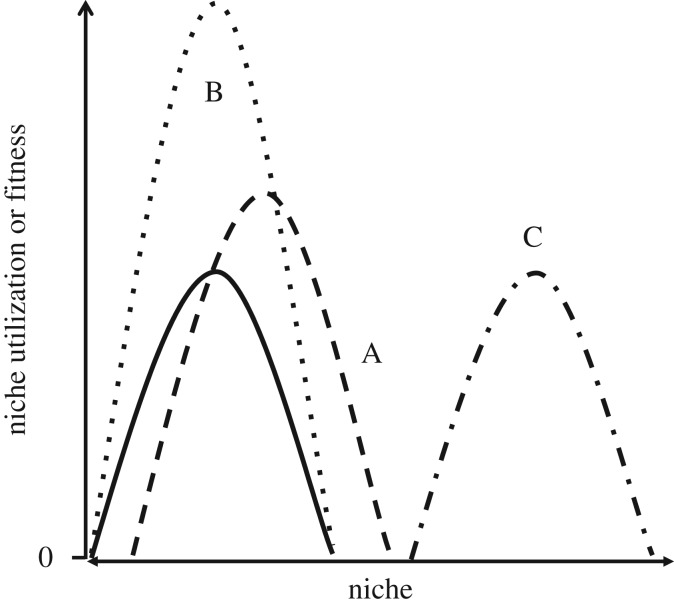
Figure 5.
Effect of innovation on the niche. Niche breadth before a hypothetical innovation is shown by the solid curve. In (A), the innovation is associated with simultaneous expansion (right extreme) and contraction (left extreme) of the niche. In (B), the innovation permits increased effectiveness, efficiency and/or yield, resulting in greater domination of part of the same niche. In (C), the innovation is associated with entering a new niche.
In niche innovations, the novel phenotypic trait is associated with the utilization of a new ecological niche (figures 3C and 5C). These are sometimes referred to in biology as key innovations (e.g. [14,28,31,43]). Entering a novel niche or ecological space may or may not result in higher fitness relative to the source population, because competition with the source population may have little or nothing to do with the success of the novelty in the new ecological niche. Similar to performance innovations, this type of novelty becomes an innovation if invasion fitness into the new niche is positive and the emerging population impacts the surrounding environment (i.e. the community and ecosystem). Niche innovations notably create opportunities for adaptive radiation. Novelties underlying niche innovations typically introduce a new function, such as homeothermy in mammals enabling survival over a wider range of temperature conditions, or radically alter an existing function, for example, the microprocessor enabling personalization of computers and telephones.
Importantly, these two types of novelty/innovation are not mutually exclusive: a novelty may affect both performance and the niche (figure 5A), as opposed to only intensifying domination of an existing niche (figure 5B), or only creating a new niche without changes in performance (figure 5C). Mirroring their effects on niches, innovations also produce phylogenetic pattern. By increasing in frequency and eventually becoming common and refined, innovations can produce some combination of replacing previous phenotypes, or entering a new niche and fostering adaptive radiations.
3. The scope of innovation and the innovation ecosystem
Historically, the study of innovation as a stand-alone concept is often associated with human society, culture and technology. Research topics range from the evolution of important technologies like hafting and fire, to the social transition of hunter–gatherers and the more sedentary agriculturalists who domesticated animals and plants [44], to the growth of cities and states, and the development of an immense array of technologies [45–49]. Indeed, niche construction, the manipulation of the environment resulting in increased prospects for survival and reproduction, is a recurring theme that bridges innovative phenomena in biology and social sciences [50,51]. Whereas most if not all innovations stemming from human activity can be interpreted as forms of niche construction, only a subset of those in biology fit this definition.
Although innovation has been (arguably) a less dominant theme in biology compared with the social sciences, the related concept of novelty was an important topic of discussion during the Modern Synthesis, and both innovation and novelty have garnered increased attention over the past few decades. Innovations in biology are often gauged in terms of their transformative effects on form and function. The most revolutionary include transitions in individuality [52] and fundamental adaptations to the environment [53]. Some innovations are polyphyletic (e.g. the ‘minor–major’ transition of multicellularity [54]), others are monophyletic (e.g. photosynthesis), whereas others—particularly in the human sphere—are more challenging to accurately determine in terms of spatial and temporal origins [55]. Cultural innovations in humans and in other animals include the ability to use (tools) or modify the environment [56], specific (social or asocial) behaviours [57] and adornment that influences mating success [58]. Moreover, several studies have investigated the tendency to innovate itself as a trait (with little supporting evidence, [59,60]), or as a correlate of either life-history traits such as maximum lifespan in birds [61], or cognitive traits such as brain size in birds and primates [62,63].
These and other general phenomena hint at an important feature of innovations: they usually involve multiple levels of organization [16,30,64,65]. Examples include an enzyme's newly acquired ability to catalyse a chemical reaction that allows an organism to survive in a new environment [15], gene expression changes that help generate novel tissues which provide a quantitative advantage for development [66], or the metabolism and musculature supporting the function of a new and advantageous body structure, such as a limb or wing, which may help in locomotion [67]. Moreover, in being a qualitative departure from a previous state, an emerging novelty will have consequences for the functioning and fitness contributions of existing traits, meaning that organisms harbouring such a novelty will be under selection at the loci of other traits to accommodate the novelty as it spreads and becomes an innovation (figure 4).
A useful way to understand these multiple levels of organization and their environmental interactions is as composing an innovation ecosystem. Table 1 presents a highly simplified representation of the biological levels at which inventions, novelties and innovations operate in the innovation ecosystem of winged flight in birds. Winged flight in birds emerged as an innovation in their reptile ancestors, ultimately contributing to taxonomic diversification in the Aves [81,82]. For flying animals such as bats and birds, wings were derived from forelimbs, and thus the innovation is not the de novo emergence of a trait, but rather the radical modification (exaptation) of an existing trait. For wings to be an innovation supporting flight, it is necessary that (i) wings specifically fulfil the proximal function of generating lift for flight, (ii) flying individuals have the scaffolding (e.g. musculature, energy allocation, cognition) required for flight and (iii) the trait complex is maintained by selection. Thus, gliding species such as certain squirrels and snakes do not fit this definition because they do not possess appendages that enable uplift, but neither do kiwi birds, which have vestigial wings. Ostriches are flightless, but can use their wings for balance and direction changes while running. Albatrosses, on the other hand, have built on the innovation of winged flight through adaptations permitting air travel over thousands of kilometres without landing, in great part due to behavioural aerodynamics (using wind updrafts). Determining whether a phenotypic trait is an innovation based on the presence/absence or quantitative criteria alone is difficult, because the trait itself may not be specific to the function being innovated and vice versa [17]. Thus, as alluded to above, birds may use their wings for one or more of several functions in addition to flight, including flap-running, paddle-swimming, thermoregulation, brooding and displays [67].
Table 1.
Winged flight as an innovation ecosystem. This table shows multiple (non-exhaustive) biological levels of organization, how they are associated with invention, novelty and innovation, and how these associations manifest in the example of winged flight in birds, which originated in dinosaurs and persists to this day in their avian descendants with numerous adaptive and novel modifications [68,69], and loss of flight in certain lineages. ‘Winged flight’ is a convenient label for an innovation, but the underlying adaptations and novelties form a trait complex, and together with environmental interactions, compose an innovation ecosystem. All components of this innovation ecosystem (e.g. developmental pathways, tissue architectures, and behaviours) contribute to winged flight, and generic examples given (e.g. in the ‘molecules, cells, tissues’ level) are non-exhaustive. Components may themselves be novelties or innovations, may be singular to wings and flight, and may have been co-opted or modified from other trait complexes (see e.g. [70] for the origins of feathers). Symbol x (?) indicates that the entity does (may) affect the associated biological level.
| biological level | genes | molecules, cells, tissues | organs, systems, appendages | individual | population | community, ecosystem |
|---|---|---|---|---|---|---|
| role in innovation ecosystem | encoding | expression and development | physiology and behaviour | ecological function and performance | selection and evolution | ecological impact |
| invention | x | x | ? | ? | ||
| novelty | x | x | x | x | ? | |
| innovation | x | x | x | x | x | x |
| example of winged flight in birds | genes encoding the development and expression of bird appendages and wings in particular | development and expression (tissue structure and metabolism) produce and maintain wings and their substructures (muscles, feathers, cartilage) | wings enable propelled flight, nervous systems permit diverse flight behaviours | flight enables escape from antagonists, local foraging, long-distance displacement, thereby increasing survival and resource acquisition | enhanced performance increases fitness and representation of heritable novel trait in future generations | ecological success of innovated species and subsequent adaptive radiation may have impacts |
| references | [71] | [71–73] | [67,70] | [74–76] | [75,77,78] | [79,80] |
4. The roles of evolution and ecology in innovation
Whereas the basis of evolution and its importance in biology can be traced back more than 150 years [1], foundations in culture [83,84], economics [85] and technological change [48,86,87] are much more recent. Although there is a discipline-specific, historical component to the integration of evolutionary thought in studying innovations, we claim that our limited understanding of innovation ultimately derives from the complexity of the underlying processes (figure 1).
At a coarse scale, biological evolution (heritability, mutation, recombination, drift and selection) has analogues in culture and technology. But even if operational in broadly similar ways across these disciplines, there are contrasts at finer scales in how different steps of the evolutionary process operate. This system/process-level specificity is associated with substantial scientific challenges, such as, for example, the identification of heritable information [88], understanding the roles of simple and complex cognitive and social processes [18] in cultural and technological systems, and assessing the extent to which biological evolution is in any way ‘goal-oriented’. Regarding the latter, insofar as some sexually reproducing organisms exhibit mate choice, they influence the identity of their mate and thus indirectly, which genetic material is given an opportunity to recombine and help produce their offspring. Although this process may generate novelty in sexually selected characters (e.g. peacock's tails), evidence is limited as to whether it accelerates natural selection in the short term [89], or facilitates phenotypic novelty in the longer term [90].
Evolution is the exploration of the possible, which leads to the to-be possible. This exploration, however, occurs in a space subject to constraints, which affect the emergence of novelties and the speed at which they may become innovations (box 3). Obtaining a novelty may require multiple iterations in the generation of inventions and recombinants (see e.g. [93] for why single mutations are unlikely to produce complex adaptations), whereby intermediates either initially persist without changing appreciably in frequency (e.g. the Dykhuizen–Hartl effect [94]), or are positively selected and increase in frequency until the novel trait is fully formed and spreads through the population [95–97]. For example, Blount et al. [95] experimentally demonstrated how multiple clades of Escherichia coli persisted for over 10 000 generations before a citrate metabolism trait arose in one lineage through the capture of a promoter exapting a previously silent duplication of the citrate transporter. Wallbank et al. [97] showed that introgression between lineages of Heliconius butterflies can produce novel colour patterns based on the recombinatory shuffling of cis-regulatory modules.
Box 3. The limits to innovation.
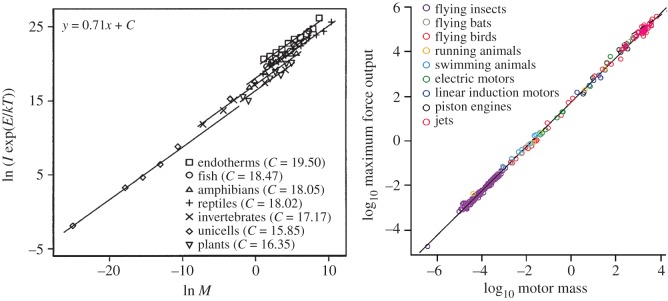
Scaling theory in biology and technology offers several examples of contrasts between different organisms or products that—although apparently substantially different—are nevertheless variations on the same theme. In the accompanying figure, we show two cases. The first involves metabolic rate. Even though a unicellular organism is clearly different than an elephant, both actually satisfy the same statistical scaling law between mass and metabolic rate. Metabolic innovations, however, are strongly suggested by the visible discontinuities (changes in the intercept) that define qualitatively different groups (poikilotherms and homeotherms) (cf. discontinuities portrayed in figure 3). The second example involves motors, both biological and engineered, and their force output. Here, the constraints appear more severe, resulting in that the maximum force output generated by a fly's muscles satisfies the same constraint as the force output of a jet engine. Thus, the ‘space of the possible’ in this relationship is constrained by simple, general rules stemming from the fundamental laws of chemistry, physics and biology.
Ecological interactions and the environment impact survival, reproduction and dispersal, and may play a key role in the transition from novelty to innovation. A good example is the competition among technologies that serve the same purpose, such as transportation. Invented by 1834, electric cabs looked to outcompete horse-drawn cabs at the turn of the twentieth century, but after a period of national and international expansion they failed (and so did the whole electric car industry) and by 1930 were outcompeted by internal combustion engine vehicles. This event was due not only to the quality of the technology itself, but also to insufficient environmental conditions, and in particular to battery limitations and the lack of electric infrastructure in the form of central stations [98]. Interestingly, the same technology re-emerged in California during the 1990s owing to an environmental factor (air pollution), as the Zero Emission Vehicle Mandate required major car companies to make available zero emissions vehicles [99]. But again electric vehicles failed, this time owing to reasons including lack of customer interest, competition with other industries, and insufficient cooperation with key technological developers in the battery sector. More recently, the environmental context has become favourable for electric vehicles, owing to both concerns about climate warming and the longer charge period and lifespan of electric batteries.
5. Overview of the theme issue
The idea for this collection of reviews, perspectives and original research stems from an interdisciplinary workshop entitled ‘Origins of novelty in biological, social and technological systems: towards a general theory of innovation’, held at the Santa Fe Institute in October 2014. It was clear from the workshop that the field is ready for synthesis, and that a reasonable step forward is to centre this synthesis in the biological sciences, with bridges to culture and technology. Box 4 and the discussion below summarize important topics addressed by contributions to this theme issue, and their promise for future research.
Box 4. The importance of innovation.
A deeper understanding of innovative change will be built on a research programme integrating behaviour, ecology, evolution, environment, network theory and information theory. This programme is important for several reasons and generates numerous challenges.
— Understanding innovation contributes to crystallizing notions of the predictability of evolution. How predictable (or inevitable) are innovations in the biological and in the cultural and technological realms? This is important because a major distinction between biology and culture/technology is the latter's (apparent) goal-oriented, rapid generation and filtering of ideas and inventions (the sensory and cognition box in figure 1; see also [100]). Indeed, there is evidence that some innovations in culture/technology stem from serendipitous events [101,102]. In biology, novelties are similarly difficult if not impossible to predict [103,104].
— Innovations and population growth are expected to be mutually reinforcing. Features of a population including its size, social interactions and established standards [102,105,106] influence the probability of innovation. Innovation may foster ecological opportunity, which in turn promotes population expansion and adaptive radiation [14,107]. In cultural evolution where this phenomenon is also reasonably well studied, population growth may promote cultural evolution [108] and demographic change [109]. Therefore, population growth and innovation are expected to be mutually reinforcing [26,110–113].
— Innovation is often associated with solving problems and/or realizing opportunities. Problems or challenges occur within the current niche and include unfavourable abiotic conditions and difficulties in obtaining resources. Realizing opportunities may involve operating in other niches, and include access to more favourable abiotic conditions and the availability of novel resources (for biology and technology, see [114]; for behavioural innovations, see [115,116]). To the extent that unsolved problems severely limit population size (and the associated likelihood of inventions), does this also stymie innovation (e.g. [117])?
— Studying innovation is central to understanding the diversity and complexity of life. Do innovations eventually saturate or become progressively smaller in magnitude? Or rather, is innovation open-ended [41]? Are environmental changes, disturbances or population extinctions necessary to ‘reset the clock’, providing adaptive space for new innovations? Does innovation simplify system structure in some contexts and complexify it in others? Under what conditions does organism complexity [118] or system robustness [119] affect innovation?
— Innovation instructs on associations between adaptation and fitness. Mean population fitness is expected to increase for adaptations to the current ecological niche, but mean fitness may increase, decrease or remain unchanged when adapting to a new habitat (for discussion and examples, see [120]). In producing a (considerable) rewiring of phenotypic traits and being constrained by trade-offs, do niche innovations decrease certain measures of fitness? Headway in understanding the fitness dynamics of innovation has recently been made using an experimental evolution approach [121].
— Innovations influence existing trade-offs and may create new ones. Trade-offs occur when there is competition involving phenotypic traits, gene expression, hormones and signalling molecules, and resource allocation [122]. In rewiring the phenotypic traits of an organism, or influencing levels of selection [123], will the emergence of a novelty also shift traits along existing trade-off surfaces or change the shapes of trade-offs themselves? This can be viewed metaphorically as altering the ruggedness and shifting the fitness peaks on a fitness landscape, or adding new trait dimensions to a multidimensional landscape [124].
— Innovations may have negative externalities and evolve in terms of their function and impact on the environment. The impacts of innovations on the surrounding environment can be complex, a prime example being domestication [125]. Innovations can have net benefits, but also negative externalities, such as, for example, the effects of domestication on farm animal well-being and associated land use changes (see e.g. [126], and theoretical development in [110]). In addition, the same innovation can have different functions or uses, such as the exaptation of fertilizer components for making explosives [127]. Vermeij & Leigh [114] argue that human-driven innovative systems are more prone to negative externalities than are natural systems.
— A general theory of innovation will identify processes that unite biological, cultural and technological spheres. Many authors have compared and contrasted evolutionary frameworks for innovation between biology, culture and technology (e.g. [48,51,106,114]). Is there a level of resolution at which all innovations follow the same basic rules? Or rather, are the apparent differences between innovations in generational (slow evolutionary) systems and intra-generational (fast) systems actually just different points along a continuum?
(a). Searching for and discovering innovation
A central theme linking the contributions to this theme issue is how innovations, which appear a priori unlikely or infeasible, can originate. Wagner [34] aims to address this problem by connecting evolutionary biology to information theory. Classical population genetics focuses on genotypes and the information encoded therein, but more recent work highlights the importance of phenotypes as gatherers and processors of environmental information [128]. Wagner employs information theory to quantify the information content of a phenotype and to investigate the feasibility of acquiring or ‘discovering’ novel and beneficial phenotypes. He develops a metric of the information change associated with a new phenotype (see also box 2), and illustrates its use in the context of DNA duplication (one route to more evolvable genotypes and a mechanism for exaptation), new transcription factor binding sites on DNA (resulting in new gene regulation and new phenotypic traits), and genes encoding biochemical reaction networks that transform environmental nutrients into biomass (e.g. amino acids and nucleotides). A limitation of this framework is that very large numbers of individuals may need to be sampled in order to estimate phenotypic information content and its change associated with novel phenotypes. Wagner proposes to mitigate this problem by using sequence data sampled from evolution experiments to quantify information differences between phenotypes. Although this approach also has limitations, for example, if a novel phenotype is highly information-rich, or requires many mutational steps to be ‘discovered’, Wagner shows that current technology suffices to quantify phenotypic information gain if the number of individuals sequenced from a population is sufficiently large relative to the amount of genetic variation in the population. In addition, Wagner suggests that the information-theoretic framework can address the broad question of what makes a system evolvable, by positing that evolvable phenotypes have low information content. Quantifying evolvability can help answer important questions about the rates at which species diversify (see also [28]), and about the related concept of the open-endedness of evolution in exploring spaces of possible adaptations.
With the exception of a handful of well-studied model systems, most involving microbes (e.g. [129]), and inferences linking major changes in phenotypic traits and phylogenetic structure (reviewed in [14]), we know little about how evolution brings about structural/functional novelty and innovation. The question is whether the evolutionary searches of the adjacent possible described by Wagner [34] typically describe more attainable, but less revolutionary innovations than many of those observed over macroevolutionary time, which could involve families of adaptations and innovations bundled into a single phenotypic feature (e.g. bird wings, table 1). Erwin [103] discusses this important topic, starting with an overview of the intellectual history of ‘spaces’, two of the most intuitive being how the states of different genes or different phenotypic traits in an individual are associated with fitness. But the metaphor of spaces extends to ecological strategies or ‘ecospaces’, and relationships between phenotype and function. Erwin develops the idea that a static predetermined landscape of how evolution could proceed is a considerable oversimplification of how many novelties and innovations occur. He argues that over macroevolutionary time scales, adaptive multidimensional surfaces change in topology and new dimensions are added and (these or others already existing) potentially lost. Moreover, the topology of many spaces is likely to be non-Euclidian, meaning that probabilities of shifting from A to B some topological distance away could be complex, and even change during the macroevolutionary process. Changes to topology are a form of a priori blind ‘construction’, in that contingency in population states and environments provides the fuel for generally unpredictable changes in fitness landscape topology, including trade-offs between phenotypic traits. Erwin summarizes the importance of construction versus search in three points. First, topologies are complex, such that, locally, they are knowable and searchable, but as the space becomes more distant and less defined it is unlikely that search algorithms apply. Second, spaces evolve. Third, novelty and innovation alter spaces and generate new spaces. Erwin wonders whether novelty and innovation are space-topology specific, but notes that the pervasive observation of phenotypic convergence between otherwise independent taxa suggests that topological constraints exist and, as such, innovation is not (entirely) open-ended. More research is needed to investigate the implications of topological construction.
There are considerable challenges in evaluating competing hypotheses to explain macroevolutionary patterns in innovation. Rabosky [28] addresses some of the main issues in statistically evaluating the impact of key innovations on adaptive radiation. Key innovations are important testing grounds for innovations more generally, because in opening a new ecological space, the former are a bellwether for the rates and extent of adaptive radiation (e.g. [29]). The expectation is that ecological opportunity is created when a new adaptive zone is opened, and innovations (e.g. functional novelties) are one possible source of that opportunity [14]. Rabosky evaluates innovations on phylogenetic trees, and begins by briefly reviewing the large literature on phylogenetic inference and the interpretation of innovations. A persistent problem is the circularity of the concept (key innovations occur when traits permitting invasion are obtained) and consequential difficulties in testing it. An alternative is to view the key innovation as an evolutionarily successful trait that results in increased diversification rates. Rabosky argues on the contrary that differential rates of lineage diversification should not be used to evaluate the existence of key innovations. If species richness is largely regulated by diversity-dependent (equilibrial) mechanisms, then there is little theoretical justification for linking innovations to faster diversification. This harkens to the idea that speciation generally entails both reproductive isolation and changes to the ecological niche (e.g. [130]); opening up a new niche (a key innovation) could facilitate the total number of new species, but does not necessarily increase the rates at which the species are formed. Rabosky argues that most tests of the predictions of key innovation have serious limitations, a central one being the low information content of many time-calibrated phylogenetic trees. He concludes that future work should focus on ecological and evolutionary mechanisms in generating macroevolutionary patterns and put less weight on oversimplified hypotheses.
Figure 6.
Scaling in biology and technology. Left panel shows relationship between temperature-corrected metabolic rate, ln (I exp(E/kT)), measured in watts, and body mass, ln M, measured in grams. Variables are M, body size; I, individual metabolic rate; k, Boltzmann's constant; T, absolute temperature (in K). E is the activation energy (after [91]). Right panel shows the scaling of force output for different biological and engineered motors (after [92]).
(b). Challenge and opportunity
The contributions to this theme issue highlight the fundamental importance of scale in innovation. Scale in evolution is evidently spatial and temporal, but more generally it characterizes the information stored and transmitted over networks. Information is in the genotype and in the phenotype, but also, as Wagner [34] relates, in the environment. The concept of information is a calculus. Information is differentiated in microevolutionary time through mutation, recombination and selection, and integrated in macroevolutionary time when living systems change in size and complexity. This raises the intriguing question of how the rate, magnitude and nature of innovations change as a system evolves from inception to maturity, and then possibly to senescence, decline and death [110]. West et al. [131] consider the inception of what is arguably the first major transition in life—heredity—and more specifically how membrane replication in protocells is an important step towards the emergence of nucleic acid blueprints and more complex cellular structures. Most theory regards RNA as the key initiator of heredity in biology. But as West et al. argue, there are some practical difficulties in heredity ‘jump-starting’ with RNA. Rather, innovations associated with the emergence of nucleic acids were possibly preceded by an innovation of membranes themselves as the blueprint of protocellular reproduction and inheritance. Here, the genotype is in many respects also the phenotype. The authors present an evolutionary, computational model based on the biochemistry of some of the earliest cells [132]. Leaky fatty-acid vesicles grow and reproduce based on positive feedbacks emerging from the chemical dynamics of proton gradients across the membrane, in interaction with mineral catalysts (FeS crystals), amino acids and fatty acids in the outer and inner environments. Even this conceptually simple, empirically based model is complex, and although data for parameter value estimates are either rudimentary or lacking, the canonical values used are plausible: when sufficiently high catalytic rates and tight amino acid binding constants are assumed, the protocells grow and reproduce. As the system evolves based on selection for larger quantities of organics inside the protocell, the likelihood of the next novelty—RNA heredity—becomes feasible. Once RNA and polymerase enzymes emerge, selection shifts in favour of a trade-off between replication speed and growth, avoiding parasitic collapse. This study highlights the pervasive observation that more complex, sophisticated levels of individuality are more likely to obtain and (generally) perform better if cheaters are prevented, kept at bay or eliminated.
Arguably, one of the most fundamental forms of innovation is the transition from autonomous individuals to the coordinated cooperating collective and to the new integrated individual [133]. Ratcliff et al. [123] consider how the evolution of collective life cycles could be associated with transitions to higher-level individuality. A major obstacle to transitions to higher-level individuality is the difficulty with which lower-level autonomy is lost, reflected by defection and freeloading. Interestingly, even when a transition is achieved, lower-level autonomy is never completely relinquished, as evidenced for example, in cancers [134]. Key to how life cycles select for higher-level dominance is the extent to which life cycle behaviours create variability within versus between collectives. Greater heritable variation between groups compared with within groups favours cooperation and transitions to higher-level individuals [135]. Life cycles that reproduce through a single-cell bottleneck and subsequently develop clonally are thus the most likely route to multicellularity. Ratcliff et al. review the life cycles of key systems, including Pseudomonas fluorescens biofilms, snowflake yeast, volvocine algae, and choanoflagellates as examples of different stages of transitions in individuality. The authors use mathematical models to compare and contrast the propensity for different life cycles to produce a transition by assessing the likelihood that a beneficial mutation affecting lower- and higher-level fitness would spread in the population. Previous work identified ‘ratcheting mutations’ (beneficial to collectives but deleterious to single cells) as those most likely to transfer command to the higher-level individual; here the authors show that these mutations should spread most quickly when multicellular individuals develop clonally and the life cycle lacks a persistent unicellular phase. Interestingly, the transition in the cases of contingent multicellularity mentioned above each require only a single mutation, suggesting the ease with which the initial novelty can emerge, which would most likely be built-upon by a battery of further genetic changes to achieve the obligate multicellular individual.
The study by Ratcliff et al. shows the complexity of innovations leading to multicellular emergence in the face of cheats. Cheating can take a number of non-mutually exclusive forms including: not executing a cooperative function, not contributing to the public good or abandoning the group. But, in leading to what amounts to competition or parasitism, cheating separates a population into two or more populations, one of which is coordinated and cooperative, and the other(s) that may form a single population of autonomous individuals, or break up into a heterogeneous ensemble of lineages or quasi-species. A key expectation is that both the cooperative ‘host’ and the cheating ‘parasite’ will evolve (and possibly coevolve). This opens the possibility that some adaptations in cooperators and cheaters will entail qualitative novelty and ultimately emerge as innovations. Aktipis & Maley [134] explore the routes to and maintenance of multicellularity, and draw parallels between innovations in cellular systems and those in human society. Although the analogies remain to be investigated in detail, there is appeal in the basic insight that similarities exist between relinquishing autonomy to form complex, cooperative structures such as multicellular organisms, and in complying with norms, morals, rules and laws in human society. Aktipis & Maley discuss how the tension between cooperation and cheating in multicellular organisms leads to innovations in the host, such as programmed cell death and immune systems that protect against disease from within and disease caused by other organisms. Moreover, innovations may emerge in cellular cheaters, such as invasive neoplasms (cancer), examples including evading the immune system, adapting to and changing the microenvironment, and dispersing to colonize new, hospitable tissues and organs [136]. Similar reasoning applies to visitors or residents in the organism, such as beneficial, neutral or pathogenic species and strains in the microbiota [137]. The authors conclude that insights into how cellular societies innovate so as to protect themselves could be translated into medical approaches that improve human health.
Social cheating as a form of parasitism is an example of a more general manifestation of ecological abundance: where there are resources to consume, there will be consumers; where there are living organisms to predate or parasitize, there will be predators or parasites. The natural question is to know how hosts avoid the negative fitness effects of parasites, and how parasites can counter any such adaptations in the host. There is a considerable empirical and theoretical literature on antagonistic coevolution (e.g. [138,139]), but the origins of new adaptive strategies still remain unclear. Fortuna and coworkers [140] investigate this question using self-replicating computer programs (i.e. digital organisms) that interact and evolve in a user-defined computational environment. This approach has proved very useful for understanding evolutionary and coevolutionary processes [141,142]. The authors find that when host resistance traits emerge additionally from non-adaptive origins (i.e. exaptations), coevolution leads to complex and phenotypically diverse networks of interacting hosts and parasites. The resulting coevolutionary outcome not only is predicted to increase population size and thereby facilitate additional adaptations and innovations, but also increases the persistence of entire communities. Interestingly, innovation mediated through exaptations may scale up to generate novel network structures that promote community persistence. These novel structures can be interpreted as forms of niche construction, and in a sense are innovations themselves.
(c). Transmission, selection and construction of novelty
The two most discussed routes to innovation are de novo construction of a qualitatively new phenotypic trait and exaptation of such a trait through gene duplication or the modification of single non-functional or functional genes [15]. There is however another, oft neglected, mechanism in biology, and in culture and technology: the introduction of novelty from another population or another species, and the diffusion of novelty between individuals within a population. The importance of horizontal gene transfer or HGT has been recognized since the 1950s, stemming from its role in the transfer of antibiotic resistance, but it is only recently that an appreciation for its diversity, complexity and importance has emerged. A defining characteristic of many prokaryotes is the fuzziness of the species concept. This is largely due to their ability to transfer and accept genetic material between closely and distantly related individuals, but also between what we would consider to be different species [143]. Accepting genetic material implies that—at least sometimes—there is a fitness benefit that maintains the character in the lineage, but also that other life forms or mobile genetic elements such as phages and plasmids benefit from this permissivity on the part of their hosts. Hall and colleagues [129] explore how the diverse battery of HGT mechanisms contributes to the introduction and diffusion of novelties in bacteria. Hall and coworkers take significant strides towards an evolutionary framework for how HGT has been so successful and effectively conquered many conceivable routes towards expression and carriage in the recipient individual. Unique here is that the would-be innovation is likely to have been of adaptive significance (and possibly an innovation) in the donor species or lineage. The authors make the important point that owing to the multitude of traits under selection in HGT, notions of the boundaries of what is an organism can become complex. In the context of innovation, the question is whether the novelty is shared between the transferred element and the donor, a sort of mutualism, which under certain environmental conditions can turn the relationship into conflict, more resembling parasitism. In either mutualistic or parasitic scenarios, there will be selection on the interacting entities to enhance or restrict subsequent transfer events, opening a world of possibilities of how HGT impacts other life-history traits, creates ecological opportunity resulting in adaptive radiations, and more generally how we view parasitism and mutualism as engines of novelty and innovation.
Horizontal information transfer such as that occurring in HGT is akin to the social transfer of information in multicellular animals. Social species span the gamut from microbes to men, but it is in the latter and in non-human animals where the transmission of novel information is best understood [18,126,144]. Specifically, a defining behaviour in many animal species is their ability to learn from others (social learning) and in so doing, incorporate the novelty (and the diffusion of the innovation through the population). However, the importance of network structure and social learning in the diffusion of innovations is difficult to assess. This is in part because agents may also learn asocially through trial and error [145]. Hoppitt [146] assesses methods for characterizing how an innovation diffuses through a social network of non-human animals when individuals can learn from others or, rather, learn through trial and error. The problem is that most past analyses have assumed basic one-on-one interactions, and have not developed more sophisticated tools for analysing more realistic social networks. Hoppitt evaluates an approach called network based diffusion analysis (NBDA) which can be used to (a) detect the action and strength of social transmission relative to asocial transmission, and (b) determine the typical pathways for diffusion of innovations in populations of animals. Hoppitt assesses what types of network should be used in an NBDA in order to accomplish each of these two goals. One problem encountered in such analyses is that the data may contain biases and noise. Hoppitt investigates different network types, among them observation networks, where social transmission can be inferred if the order of individual observation predicts the order of diffusion, and association networks where the probability of transmission is expected to correlate with the association time. He finds that these two network types are robust to bias and error in parameters. However, numerous other challenges remain, important among them being network-specific interpretations of social learning strength in the diffusion of innovations.
Many cultural variants achieve appreciable population frequencies without any apparent selective effect [147,148]. Examples in present-day humans include fads, fashions, adornment and first names. Defining characteristics of such cultural variants include their rapid emergence, spread and decline, coexistence with alternative variants, and their promotion via the copying or the emulation of prestigious individuals [149]. One hypothesis to explain their near-neutrality, is that the present-day manifestations of these behaviours reflect evolutionary mismatches with what were more important phenomena in our distant past, and that the background behaviours promoting them have persisted. This does not preclude that the mechanisms responsible for their dynamics function the same way today as they did in the distant past. O'Dwyer & Kandler [42] explore the dynamics of what would appear to be a trait with no effect on survival or reproduction—baby's names—with the objective of determining the extent to which transmission might follow neutral or non-neutral processes. Bentley et al. [150] previously showed a power-law distribution of newborn names, with an exponent that depends on invention rate and total population size. O'Dwyer & Kandler derive an analytical approximation for this progeny distribution under neutrality and show that the distribution of names over an interval of intermediate abundances shows an exponent −3/2, but this exponentially declines at and beyond sufficiently abundant names. Maximum-likelihood estimates of the neutral parameter allow direct application of the theory to data. Moreover, the authors show how selective differences between names, in the form of pro- and anti-novelty bias, can be modelled and applied to a data set of newborn names in Australia. They find that the empirical patterns are best described by anti-novelty selection against inventions before they reach appreciable frequencies, but once they do, selection is relaxed and names interact in a neutral manner. These results have potential implications for how marker information (i.e. neither too common, nor too unique) is achieved in the human population. Furthermore, the authors show that analyses based on only the most common cultural variants can lead to misleading inferences about underlying transmission processes.
Two defining characteristics of humans are the complexity of their innovations and the remarkable diversity of their innovation toolbox. Innovation complexity and diversity reflect cumulative culture, that is, building culture over lifetimes and through generations. Whereas models and data indicate the probable routes of cumulative culture [151], less is known about how culture forms within the individual. Building culture during a lifetime requires repeated cycles of individual learning (a form of invention) and copying others (e.g. parents, peers, and prestigious individuals). A critical period in endogenizing is childhood. McGuigan and colleagues [152] review the growing literature on the significance of childhood in cumulative culture, and identify the underlying ‘dual engines’ as invention and copying/transmission. The authors then present a study of children in which the subjects were free to invent, rather than have adult models introduce inventions. The authors focus on the scenario where the origin of certain inventions that contribute to cumulative culture occur during childhood [153]. The experimental design is complex, similar in spirit to an ethological field study. Despite inherent limitations in interpreting these kinds of controlled but complex experiments, the study reveals that social learning can facilitate more cumulative innovation than asocial learning, that higher-level problem solving was more likely following success on a simple problem in a series, and that cumulative success could be driven both by the challenges of ecological loss of existing solutions and by the involvement of occasional superior innovators (older children). The broader picture is that humans, and ostensibly certain other primates, may be adapted to experiment and selectively transmit successful outcomes, providing a basis for the exaptation or amelioration of existing behaviours and the emergence and spread of novelties that become cumulative cultural innovations.
(d). Interacting with, constructing and destructing the environment
The abilities of life forms to generate inventions, refine novel phenotypic traits, and diffuse some of these to become innovations are, sometimes, innovations themselves. Cognition and information processing is a vast space for such innovations. Dukas [154] provides an overview of the foundations of cognition and associated innovations leading to ever-greater complexity, particularly in mammals. An important theme in the development of cognition is the perception of danger (e.g. temperature, predators) and opportunity (e.g. resources), in particular information gathering and transmission in a social environment. A basic form of information gathering found in single cells, from bacteria to vertebrate immune systems, relies on receptors and responses, both expressed via gene networks. These mechanisms, although rapid, are limited in the range of environmental responses they can accommodate. A major innovation in animals was the advent of the nervous system, which promoted more effective environmental sensing and inter-individual communication, and further innovation enabled capacities for information storage and retrieval. The advent of learning and long-term memory required the employment of existing standards, and in particular, biochemical chains and changes in gene expression. Learning and memory, in turn, provided the basis for further behavioural innovations and in particular, social learning. Thus, each successive innovation has either incorporated or complemented previous cognitive innovations. Dukas relates how social learning would have been selected in taxa with repeated interactions between individuals and long life-spans. This highlights a unique aspect of social and individual learning: expertise. Expertise is how experience (through improved skills and increased knowledge) improves the performance of complex tasks. Once social learning was established, there would have been significant selection pressure to adapt it to complex social and asocial environments. Language fills this gap, and produces new opportunities and challenges that result in further adaptations for improved cognitive ability (e.g. [155]) and (its correlate) further innovation [61]. More research is necessary, particularly in understanding how evolution underpins innovation epochs in cognition and expertise, and how these employ automated and plastic responses.
The evolution of ever more sophisticated cognition in response to environmental opportunities and challenges is a prime example of how phenotypic traits diversify and complexify in form and function. Signalling (including language) and social learning would have had major consequences for how well organisms could cope with and exploit changing environments. One of the most fundamental ways in which this is accomplished is through altering the environment: niche construction. Niche construction is a powerful conduit of adaptation and innovation (e.g. [156]). However, niche construction is not limited to the extra-organismal environment—it can involve the organism's phenotype itself, through for example, ‘phenotypic accommodation’ [156,157]. Arguably, the main distinction between the (classic conception of) the organism and its environment is that the former is an autonomous, reproducing entity. Beyond this, there may or may not be a disjunct in how information is stored and transmitted between an organism and its environment [158]. Niche construction is therefore an extension of the phenotype, subjected to selection, and manifests in gene–culture coevolution [159]. Allaby et al. [44] review one of the most fascinating forms of niche construction: domestication. Briefly, one species selects for sought-after traits in another, especially predictability, value and yield, and in so doing the former evolves through the development of management techniques and technologies. Although domestication and agriculture in particular occur across a broad range of taxa (slime moulds, snails, beetles, termites, ants), nowhere are they more diverse and complex than in the human population. Allaby et al. relate how true domestication results from directional selection on major traits, whereas the specific forms of agricultural traits in crop improvement or selection for particular varieties are often idiosyncratic to specific needs or environments. The authors discuss the well documented example of cereals, such as wheat and barley, where archaeobotanical evidence suggests that morphological change occurred over thousands rather than hundreds of years, indicative of generally weak selection, but not incompatible with periods of strong selection due to environmental influences, social conditions, and prevailing technology. Allaby et al. evaluate this hypothesis using a combined model–data analysis of the fossil record of crop domestication to predict periods when human-driven selection in several cereals was particularly strong, and estimate when the earliest selection for domestication began. They find that selection is indeed generally weak, and that periods of stronger selection may occur in parallel with technological innovations such as sickle technologies. Additionally, when many traits are under selection and no two are necessary for crop improvement, selection tends to be diluted on most traits and over most time periods. The authors relate how environmental and cultural contingencies were crucial to domestication rates and particular sought-after traits. Similar to other cultural and technological innovations (e.g. [105,158,160]), it appears that sufficient human populations sizes would have been necessary for cereal domestication and production innovations to obtain.
The potential for biological evolution ultimately derived from mutation. Because many if not most mutations have negative impacts on fitness, genomes have adaptations to control mutation. The stochastic nature of mutation means that their appearance (i.e. nearly neutral or beneficial mutation) depends on population size. Although controversial, this same dependence—large population size—has been hypothesized to favour cumulative cultural evolution [161]. Fogarty & Creanza [111] review the literature, which suggests that this process operates in food producing societies but not in food gathering societies, the latter of which depend more on environmental risk. The authors develop a model to investigate the independent and interactive influences of population size and environment on cultural accumulation in the form of tools and technologies. They assume that innovation is primarily driven by environmental challenge, but that innovations are to some extent environment-specific, meaning that innovations are potentially gained and lost when populations migrate or environments change [55,162,163]. However and importantly, those populations that construct their environments (e.g. building shelters, domesticating plants and animals and associated agriculture and husbandry) buffer themselves from environmental vagaries, and population size will dominate environmental contingency in driving innovative cultural accumulation. Taken together this harkens at predictions of evolutionary theory whereby adaptation to harsh or variable environmental conditions may be achieved by increased mutation rates or phenotypic plasticity, whereas adapting to long-term predictable environmental trends depends on occasional beneficial germ line changes (mutation), which become more probable with population size [164].
How novelty manifests itself in terms of phenotypic traits, functions and fitness depends on where we look in the innovation ecosystem, the time frame in the emergence and development of the innovation, and the prevailing environmental conditions. Innovations may be beneficial for one species and detrimental for other species, as for example, an innovation permitting a host to better resist its parasite, although as shown by Fortuna et al. [140] the precise effects can be complex. More generally, an innovation may be detrimental to individuals, populations or ecosystems in several non-mutually exclusive ways. First, an initially beneficial innovation may be expatiated or co-opted to become a detrimental one, as in the case of dynamite invented for mining but subsequently then used for artillery. Second, an innovation may outcompete existing phenotypes, resulting in their demise or extinction (‘creative destruction’ in economics and technology). Third, the innovation may have negative externalities, such as pollution produced by combustion engines. And fourth, in favouring population growth, the innovation may result in overexploitation of resources and the tragedy of the commons. Weinberger et al. [110] consider innovation in the human population and how it can sustain growth or rather result in population collapse or even extinction. They model how innovation increases the flux of ecosystem services, which boosts population size and results in further innovation and population growth via cumulative cultural evolution. A key point underscored by the authors is that innovations can also have social and environmental costs, which limit population growth [165]. The authors find that continued innovation and population growth is only possible if the positive externalities of the innovations are sufficient, which allows a large stock of technologies. When externalities are sufficiently negative, the population can only persist, albeit at lower numbers, if the stock is reduced to a minimum. Finally, the model shows that population collapses are possible in between these two extremes, and these further depend on the minimum standard of technology humans are willing to accept. This study suggests that the coevolutionary dynamics between population and innovation critically depend on the positive or negative externalities of innovations and on the changing minimum technological requirements of populations.
6. Concluding remarks
Innovation research encompasses a vast spectrum of phenomena, spanning the biological, cultural and technological sciences. This theme issue takes modest steps in surveying and investigating the scope of interesting problems surrounding innovation, and yields a number of insights that will guide future directions. The conceptual frameworks, mathematical and computational models and experiments in this theme issue support the view that only a small number of key mechanisms may be necessary to understand innovations. Nevertheless, robust assessments of putative driving processes are elusive, particularly for innovations manifesting over long time periods. In this regard sufficiently rich data can reveal macroevolutionary patterns through, for example, ancient DNA analysis [166]. At the other extreme, the recent explosion of experimental evolution [104,167,168] and ever-increasing sophistication in behavioural studies [18] have important roles to play in scientifically evaluating competing hypotheses—depending on the system—over time scales amenable to many research programmes. Mathematical and computational models will be central in evaluating candidate processes driving innovation, regardless of time scales.
Despite our suggestion that contributors to this theme issue adhere to a common definition of the term ‘innovation’, there were notable contrasts. These spanned the gamut from single quantitative changes to the phenotype through to qualitative novelties requiring multiple mutations and recombination events. All had in common either some form of phenotypic novelty, or a major step in the performance of a function. This state of affairs reflects both contrasts in perspective of where adaptation stops and innovation begins, and differences in the complexity of the topology of evolutionary spaces on the path to innovation. We view this as an enriching, challenging reality of the current state of the field.
The contributions to this theme issue highlight the intricacy of the innovation ecosystem, which consists of networks of phenotypic traits on different scales, associated changes in behaviour and function, performance and fitness, and current and future impacts on the surrounding community and ecosystem. Clearly, developing a constellation of theories for such multi-layered systems will be a considerable undertaking, and insights into how the complexity of novel traits limits their occurrence and influences their evolvability [169] will be key to theoretical developments. Given current lacunae, it is not surprising that innovation largely remains an a posteriori assessment of change and its impact. Future study should identify the milestones that precede and predict innovations.
In closing, we believe that innovation processes in biology, culture and technology have a small set of key features in common. The main defining contrast appears to be the dynamics and complexity of sensory–cognition–communication interactions (figure 1). In this regard, we claim that these disciplines are not as distinct as they may appear, but rather they form an overlapping continuum. The moon-shot is to understand the central features that drive innovation in all living systems, paving the way for a general theory.
Acknowledgements
We thank the authors for their hard work and the quality of their contributions to this theme issue, and the many external reviewers who provided constructive feedback. We would also like to thank Jennifer Dunne, Doug Erwin, Stuart Kauffman, Mimi Koehl, Hanna Kokko, Manfred Laubichler, Jose Lobo, Doyle McKey, John Miller, Rolando Rebolledo, Deborah Strumsky, Peter Turchin, Jorge Velasco-Hernandez and Andrew Whiten for their support and discussions, Helen Eaton for advice, the contributors to this theme issue for their comments on this introductory paper, and to the Santa Fe Institute for facilitating our discussions over the past several years.
Biographies
Editor profiles
 Michael E. Hochberg is Research Director with the Centre National de la Recherche Scientifique and based at the University of Montpellier, France. He uses mathematical models and experiments to unravel complex ecological interactions and their evolution at scales from cells to societies. His main line of research seeks to understand how environments influence infectious diseases and cancers. Although most of his work is fundamental science, he has a keen interest in applications and has published widely on phage therapy and novel approaches to cancer therapy. Michael has associate positions at the Santa Fe Institute and at the Institute for Advanced Study in Toulouse, and headed a focus group at the Wissenschaftskolleg zu Berlin from which a theme issue in Phil. Trans. on comparative oncology was published in 2015. He is currently completing a book on writing and publishing scientific articles, based on his experience as Editor in Chief of the journal Ecology Letters.
Michael E. Hochberg is Research Director with the Centre National de la Recherche Scientifique and based at the University of Montpellier, France. He uses mathematical models and experiments to unravel complex ecological interactions and their evolution at scales from cells to societies. His main line of research seeks to understand how environments influence infectious diseases and cancers. Although most of his work is fundamental science, he has a keen interest in applications and has published widely on phage therapy and novel approaches to cancer therapy. Michael has associate positions at the Santa Fe Institute and at the Institute for Advanced Study in Toulouse, and headed a focus group at the Wissenschaftskolleg zu Berlin from which a theme issue in Phil. Trans. on comparative oncology was published in 2015. He is currently completing a book on writing and publishing scientific articles, based on his experience as Editor in Chief of the journal Ecology Letters.
 Pablo A. Marquet is interested in postdisciplinary science. He tackles questions in different complex systems, from ecological to social ones, in collaboration with physicists, mathematicians and social scientists. He has devoted most of his research to the search for, and the integration of, the general principles that underlie the structure and dynamics of complex ecological systems. Particular areas of research are the emergence and significance of scaling relationships within ecological systems, the constraints that area imposes on organism size and persistence, the evolution of body size in landmasses, the distribution of abundance within communities, metapopulation theory and macroecology, the emergence of social complexity and the impact of global change upon socio-ecological systems.
Pablo A. Marquet is interested in postdisciplinary science. He tackles questions in different complex systems, from ecological to social ones, in collaboration with physicists, mathematicians and social scientists. He has devoted most of his research to the search for, and the integration of, the general principles that underlie the structure and dynamics of complex ecological systems. Particular areas of research are the emergence and significance of scaling relationships within ecological systems, the constraints that area imposes on organism size and persistence, the evolution of body size in landmasses, the distribution of abundance within communities, metapopulation theory and macroecology, the emergence of social complexity and the impact of global change upon socio-ecological systems.
 Rob Boyd is Origins Professor in the School of Human Evolution and Social Change at Arizona State University. Much of his research focuses on incorporating cultural transmission into the Darwinian theory of evolution, and using the modified theory to understand why humans are such peculiar creatures. Rob has also published on the evolution of social behaviour, especially on the evolution of cooperation in large groups of individuals who are not closely related. He also organized a project on cross-cultural behavioural experimentation using economic games, and implemented some of this work in collaboration with Joe Henrich at a field site in Fiji.
Rob Boyd is Origins Professor in the School of Human Evolution and Social Change at Arizona State University. Much of his research focuses on incorporating cultural transmission into the Darwinian theory of evolution, and using the modified theory to understand why humans are such peculiar creatures. Rob has also published on the evolution of social behaviour, especially on the evolution of cooperation in large groups of individuals who are not closely related. He also organized a project on cross-cultural behavioural experimentation using economic games, and implemented some of this work in collaboration with Joe Henrich at a field site in Fiji.
 Andreas Wagner is professor and chairman of the Institute of Evolutionary Biology and Environmental Studies at the University of Zurich in Switzerland, and External Professor at the Santa Fe Institute. His main research interests are the evolution of biological systems, from genomes to complex molecular networks. Wagner has authored nearly 200 scientific publications in journals that include Nature, Science and PNAS, as well as two scientific monographs: Robustness and evolvability in living systems (Princeton University Press, 2005) and The origins of evolutionary innovations (Oxford University Press, 2011). Wagner received his PhD in 1995 at Yale University, where his research won the J. S. Nicholas prize for best dissertation in his field. He has lectured worldwide, and held research fellowships at several institutions, such as the Institute for Advanced Studies in Berlin, Germany, and the Institut des Hautes Etudes in Bures-sur-Yvette, France. Wagner is an elected member of the European Molecular Biology Organization (EMBO), an elected fellow of the American Association for the Advancement of Sciences (AAAS), and a member of the Faculty of 1000 Biology. An Austrian-born U.S. citizen, he lives with his wife and their son in Zurich, Switzerland.
Andreas Wagner is professor and chairman of the Institute of Evolutionary Biology and Environmental Studies at the University of Zurich in Switzerland, and External Professor at the Santa Fe Institute. His main research interests are the evolution of biological systems, from genomes to complex molecular networks. Wagner has authored nearly 200 scientific publications in journals that include Nature, Science and PNAS, as well as two scientific monographs: Robustness and evolvability in living systems (Princeton University Press, 2005) and The origins of evolutionary innovations (Oxford University Press, 2011). Wagner received his PhD in 1995 at Yale University, where his research won the J. S. Nicholas prize for best dissertation in his field. He has lectured worldwide, and held research fellowships at several institutions, such as the Institute for Advanced Studies in Berlin, Germany, and the Institut des Hautes Etudes in Bures-sur-Yvette, France. Wagner is an elected member of the European Molecular Biology Organization (EMBO), an elected fellow of the American Association for the Advancement of Sciences (AAAS), and a member of the Faculty of 1000 Biology. An Austrian-born U.S. citizen, he lives with his wife and their son in Zurich, Switzerland.
Endnotes
Conducted on 15 August 2017 using the ISI ‘All Databases’. Search term used for each year between 2000 and 2017 was (‘innovation*’ and (‘ecolog*’ or ‘evolut*’))/(‘ecolog*’ or ‘evolut*’). The annual fraction has increased at a slightly greater than linear rate between 1997 and 2017.
‘Spread’ as employed here includes scenarios where phenotypic variants contributing to the full novelty attain appreciable population frequencies prior to the emergence and spread of the full novelty itself.
Authors' contributions
M.E.H. wrote the manuscript. All authors contributed significantly to writing and revisions.
Competing interests
We have no competing interests.
Funding
M.E.H. acknowledges the James S. McDonnell Foundation Studying Complex Systems Research Award no. 220020294, the CNRS PICS Award no. 06313, the Agence National de la Recherche (EvoCan ANR-13-BSV7-0003-01), and the Institut National du Cancer (2014-1-PL-BIO-12-IGR-1), for funding. P.A.M. acknowledges support from Grants Programa de Investigación Asociativa (PIA), Anillo SOC1405, ICM-MINECON P05-001 and PFB-CONICYT P-053. R.B. was supported by grant no. 48952 from the John Templeton Foundation to the Institute of Human Origins at Arizona State University. The opinions expressed in this publication are those of the authors and do not necessarily reflect the views of the John Templeton Foundation. A.W. acknowledges support by ERC Advanced grant no. 739874, by Swiss National Science Foundation grant no. 31003A_146137, by an EpiphysX RTD grant from SystemsX.ch, as well as by the University Priority Research Program in Evolutionary Biology at the University of Zurich.
Glossary
- Adaptation
A trait that helps an organism survive or reproduce in a given environment.
- Blueprint
Stored information that interacts with the environment, resulting in a phenotype. The stored information may be transmitted from parents to offspring via genetic material or epigenetic alterations, or between individuals via horizontal gene transfer or through cultural/behavioural inheritance. Blueprints are subject to alteration (e.g. mutation and recombination) and to evolution due to random drift and selection. Examples of structures containing or embodying blueprints include DNA, brains, patents, instruction manuals and semiconductors.
- Exaptation
The radical modification or co-opting of an existing phenotypic trait, which may or may not have served a function, into a novel trait with a new function.
- Function
A characteristic influence of a phenotypic trait on species ecology. A given trait may be associated with different functions at different biological scales. In the example of bird wings, proximal functions include flying and paddling. Ultimate functions include resource acquisition and predator avoidance.
- Innovation
A qualitatively novel phenotype associated with increased performance (performance innovations) or the establishment of a new ecological niche (niche innovations). Innovations have significant impacts on the environment (i.e. the surrounding community and ecosystem), and notably for niche or ‘key’ innovations, on subsequent lineage diversification.
- Innovation ecosystem
The ensemble of components (DNA, molecular, cellular and tissue architectures, other complex phenotypic traits) forming an innovation, and their interactions with each other and the surrounding environment. An innovation ecosystem has one or more novel phenotypic traits of reference (e.g. feathers, wings and flight behaviour in birds; table 1). A given component may be specific to a particular innovation, whereas other components may be associated with the innovation and to other (possibly unrelated) traits.
- Invention
The appearance of a new phenotypic variant or the modification of an existing variant. The variant potentially contributes to producing a novelty. In biology, certain DNA mutations produce inventions, whereas in culture and technology inventions result from new ideas and discoveries.
- Key innovation
Novel traits that promote a change in the ecological niche and result in lineage diversification.
- Novelty
The radical modification of an existing phenotypic trait or the emergence of a qualitatively new phenotypic trait. A novelty may form the basis of an innovation if the former spreads through a population, or results in population expansion into a new ecological niche.
- Performance
Effectiveness, efficiency or yield associated with the expression of a phenotypic trait or the execution of a function. Performance is expected to correlate with fitness, subject to trade-offs with other phenotypic traits and functions.
- Phenotypic trait
Any (bio)chemical, physiological, morphological or behavioural feature of the individual or its constructed niche, resulting from the interaction between blueprints and environments.
- Standards
Specifications or properties shared by the components of a technology. Commonly applied to human technologies, the notion can also be applied to adaptations and innovations that occur in different organisms.
References
- 1.Darwin C. 1859. On the origin of species. London, UK: John Murray. [Google Scholar]
- 2.Darwin C. 1877. The various contrivances by which orchids are fertilised by insects. London, UK: John Murray. [Google Scholar]
- 3.Erwin DH. 2015. Novelty and innovation in the history of life. Curr. Biol. 25, R930–R940. ( 10.1016/j.cub.2015.08.019) [DOI] [PubMed] [Google Scholar]
- 4.Fagerberg J. 2006. Innovation: a guide to the literature. In The Oxford handbook of innovation (eds Fagerberg J, Mowery DC), pp. 1–31. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Erwin DH, Krakauer DC. 2004. Evolution. Insights into innovation. Science 304, 1117–1119. ( 10.1126/science.1099385) [DOI] [PubMed] [Google Scholar]
- 6.Pigliucci M. 2008. What, if anything, is an evolutionary novelty? Philos. Sci. 75, 887–898. ( 10.1086/594532) [DOI] [Google Scholar]
- 7.Brigandt I, Love AC. 2012. Conceptualizing evolutionary novelty: moving beyond definitional debates. J. Exp. Zool. B Mol. Dev. Evol. 318, 417–427. ( 10.1002/jez.b.22461) [DOI] [PubMed] [Google Scholar]
- 8.Simpson GG. 1944. Tempo and mode in evolution. New York, NY: Columbia University Press. [Google Scholar]
- 9.Mayr E. 1960. The emergence of novelty. In The evolution of life (ed. Tax S.), pp. 349–380. Chicago, IL: University of Chicago Press. [Google Scholar]
- 10.Muller GB, Wagner GP. 1991. Novelty in evolution: restructuring the concept. Annu. Rev. Ecol. Syst. 22, 229–256. ( 10.1146/annurev.es.22.110191.001305) [DOI] [Google Scholar]
- 11.Galis F. 2001. Key innovations and radiations. In The character concept in evolutionary biology (ed. Wagner GP.), pp. 581–605. San Diego, CA: Academic Press. [Google Scholar]
- 12.Reader SM, Laland KN. 2003. Animal innovation: an introduction. Oxford, UK: Oxford University Press. [Google Scholar]
- 13.West-Eberhard MJ. 2008. Toward a modern revival of Darwin's theory of evolutionary novelty. Philos. Sci. 75, 899–908. ( 10.1086/594533) [DOI] [Google Scholar]
- 14.Yoder JB, et al. 2010. Ecological opportunity and the origin of adaptive radiations. J. Evol. Biol. 23, 1581–1596. ( 10.1111/j.1420-9101.2010.02029.x) [DOI] [PubMed] [Google Scholar]
- 15.Wagner A. 2011. Arrival of the fittest: solving evolution’s greatest puzzle. New York, NY: Penguin. [Google Scholar]
- 16.Wagner GP. 2014. Homology, genes, and evolutionary innovation. Princeton, NJ: Princeton University Press. [Google Scholar]
- 17.Wagner GP. 2015. Evolutionary innovations and novelties: let us get down to business! Zool. Anz. J. Comp. Zool. 256, 75–81. ( 10.1016/j.jcz.2015.04.006) [DOI] [Google Scholar]
- 18.Reader SM, Morand-Ferron J, Flynn E. 2016. Animal and human innovation: novel problems and novel solutions. Phil. Trans. R. Soc. B 371, 20150182 ( 10.1098/rstb.2015.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher P. 2000. Sorites paradox and vague geographies. Fuzzy Sets Syst. 113, 7–18. ( 10.1016/S0165-0114(99)00009-3) [DOI] [Google Scholar]
- 20.Jacob F. 1982. The possible and the actual. Seattle, WA: University of Washington Press. [Google Scholar]
- 21.Kauffman SA. 1993. The origins of order: self-organization and selection in evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Axtell RL, Casstevens R, Hendrey M, Kennedy W, Litsch W.2013. Competitive innovation and the emergence of technological epochs. See http://www.css.gmu.edu/~axtell/Rob/Research/Pages/Technology_files/Tech%20Epochs.pdf .
- 23.Nguyen AM, Yosinski J, Clune J. 2016. Understanding innovation engines: automated creativity and improved stochastic optimization via deep learning. Evol. Comput. 24, 545–572. ( 10.1162/EVCO_a_00189) [DOI] [PubMed] [Google Scholar]
- 24.Arbilly M, Laland KN. 2017. The magnitude of innovation and its evolution in social animals. Proc. R. Soc. B 284, 20162385 ( 10.1098/rspb.2016.2385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatterjee K, Pavlogiannis A, Adlam B, Nowak MA. 2014. The time scale of evolutionary innovation. PLoS Comput. Biol. 10, e1003818 ( 10.1371/journal.pcbi.1003818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettencourt LM, Lobo J, Helbing D, Kühnert C, West GB. 2007. Growth, innovation, scaling, and the pace of life in cities. Proc. Natl Acad. Sci. USA 104, 7301–7306. ( 10.1073/pnas.0610172104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loreto V, Servedio VDP, Strogatz SH, Tria F. 2016. Dynamics on expanding spaces: modeling the emergence of novelties. In Creativity and universality in language (eds Degli Esposti M, Altmann EG, Pachet F), pp. 59–83. Cham, Switzerland: Springer. [Google Scholar]
- 28.Rabosky DL. 2017. Phylogenetic tests for evolutionary innovation: the problematic link between key innovations and exceptional diversification. Phil. Trans. R. Soc. B 372, 20160417 ( 10.1098/rstb.2016.0417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Losos JB. 2010. Adaptive radiation, ecological opportunity, and evolutionary determinism. Am. Nat. 175, 623–639. ( 10.1086/652433) [DOI] [PubMed] [Google Scholar]
- 30.Moczek AP. 2008. On the origins of novelty in development and evolution. Bioessays 30, 432–447. ( 10.1002/bies.20754) [DOI] [PubMed] [Google Scholar]
- 31.Heard SB, Hauser DL. 1995. Key evolutionary innovations and their ecological mechanisms. Hist. Biol. 10, 151–173. ( 10.1080/10292389509380518) [DOI] [Google Scholar]
- 32.Bond JE, Opell BD. 1998. Testing adaptive radiation and key innovation hypotheses in spiders. Evolution 52, 403–414. ( 10.1111/j.1558-5646.1998.tb01641.x) [DOI] [PubMed] [Google Scholar]
- 33.Cover TM, Thomas JA. 2006. Elements of information theory, 2nd edn. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 34.Wagner A. 2017. Information theory, evolutionary innovations and evolvability. Phil. Trans. R. Soc. B 372, 20160416 ( 10.1098/rstb.2016.0416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gould SJ, Vrba ES. 1982. Exaptation—a missing term in the science of form. Paleobiology 8, 4–15. ( 10.1017/S0094837300004310) [DOI] [Google Scholar]
- 36.Arnold EN. 1994. Investigating the origins of performance advantage: adaptation, exaptation and lineage effects. In Phylogenetics and ecology (eds Eggleton P, Vane-Wright RI), pp. 123–168. New York, NY: Academic Press. [Google Scholar]
- 37.Armbruster WS. 1997. Exaptations link evolution of plant–herbivore and plant–pollinator interactions: a phylogenetic inquiry. Ecology 78, 1661–1672. ( 10.1890/0012-9658(1997)078%5B1661:ELEOPH%5D2.0.CO;2) [DOI] [Google Scholar]
- 38.Baltrus DA. 2013. Exploring the costs of horizontal gene transfer. Trends Ecol. Evol. 28, 489–495. ( 10.1016/j.tree.2013.04.002) [DOI] [PubMed] [Google Scholar]
- 39.Dannen G. 1997. The Einstein-Szilard refrigerators. Sci. Am. 276, 90–95. ( 10.1038/scientificamerican0197-90) [DOI] [Google Scholar]
- 40.Koonin EV. 2011. The logic of chance: the nature and origin of biological evolution. Upper Saddle River, NJ: FT Press. [Google Scholar]
- 41.de Vladar HP, Santos M, Szathmáry E. 2017. Grand views of evolution. Trends Ecol. Evol. 32, 324–334. ( 10.1016/j.tree.2017.01.008) [DOI] [PubMed] [Google Scholar]
- 42.O'Dwyer JP, Kandler A. 2017. Inferring processes of cultural transmission: the critical role of rare variants in distinguishing neutrality from novelty biases. Phil. Trans. R. Soc. B 372, 20160426 ( 10.1098/rstb.2016.0426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunter JP. 1998. Key innovations and the ecology of macroevolution. Trends Ecol. Evol. 13, 31–36. ( 10.1016/S0169-5347(97)01273-1) [DOI] [PubMed] [Google Scholar]
- 44.Allaby RG, Stevens C, Lucas L, Maeda O, Fuller DQ. 2017. Geographic mosaics and changing rates of cereal domestication. Phil. Trans. R. Soc. B 372, 20160429 ( 10.1098/rstb.2016.0429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diamond J. 2002. Evolution, consequences and future of plant and animal domestication. Nature 418, 700–707. ( 10.1038/nature01019) [DOI] [PubMed] [Google Scholar]
- 46.Boyd R, Richerson PJ, Henrich J. 2013. The cultural evolution of technology: facts and theories. In Cultural evolution, society, technology, science and religion (eds Richerson PJ, Christiansen M), pp. 119–142. Cambridge, MA: MIT Press. [Google Scholar]
- 47.Barham L. 2013. From hand to handle: the first industrial revolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 48.Solé RV, Valverde S, Casals MR, Kauffman SA, Farmer D, Eldredge N. 2013. The evolutionary ecology of technological innovations. Complexity 18, 15–27. ( 10.1002/cplx.21436) [DOI] [Google Scholar]
- 49.Mokyr J. 2016. A culture of growth: the origins of the modern economy. Princeton, NJ: Princeton University Press. [Google Scholar]
- 50.Erwin DH. 2008. Macroevolution of ecosystem engineering, niche construction and diversity. Trends Ecol. Evol. 23, 304–310. ( 10.1016/j.tree.2008.01.013) [DOI] [PubMed] [Google Scholar]
- 51.Wagner A, Rosen W. 2014. Spaces of the possible: universal Darwinism and the wall between technological and biological innovation. J. R. Soc. Interface 11, 20131190 ( 10.1098/rsif.2013.1190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szathmáry E. 2015. Toward major evolutionary transitions theory 2.0. Proc. Natl Acad. Sci. USA 112, 10 104–10 111. ( 10.1073/pnas.1421398112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lane N. 2009. Life ascending: the ten great inventions of evolution. New York, NY: W. W. Norton & Company. [Google Scholar]
- 54.Grosberg RK, Strathmann RR. 2007. The evolution of multicellularity: a minor major transition? Annu. Rev. Ecol. Evol. Syst. 38, 621–654. ( 10.1146/annurev.ecolsys.36.102403.114735) [DOI] [Google Scholar]
- 55.Powell A, Shennan S, Thomas MG. 2009. Late Pleistocene demography and the appearance of modern human behavior. Science 324, 1298–1301. ( 10.1126/science.1170165) [DOI] [PubMed] [Google Scholar]
- 56.Odling-Smee JF, Laland KN, Feldman MW. 2003. Niche construction: the neglected process in evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 57.Aplin LM, Farine DR, Morand-Ferron J, Cockburn A, Thornton A, Sheldon BC. 2015. Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature 518, 538–541. ( 10.1038/nature13998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keagy J, Savard J-F, Borgia G. 2009. Male satin bowerbird problem-solving ability predicts mating success. Anim. Behav. 78, 809–817. ( 10.1016/j.anbehav.2009.07.011) [DOI] [Google Scholar]
- 59.Griffin AS. 2016. Innovativeness as an emergent property: a new alignment of comparative and experimental research on animal innovation. Phil. Trans. R. Soc. B 371, 20150544 ( 10.1098/rstb.2015.0544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quinn JL, Cole EF, Reed TE, Morand-Ferron J. 2016. Environmental and genetic determinants of innovativeness in a natural population of birds. Phil. Trans. R. Soc. B 371, 20150184 ( 10.1098/rstb.2015.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sol D, Sayol F, Ducatez S, Lefebvre L. 2016. The life-history basis of behavioural innovations. Phil. Trans. R. Soc. B 371, 20150187 ( 10.1098/rstb.2015.0187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Overington SE, Morand-Ferron J, Boogert NJ, Lefebvre L. 2009. Technical innovations drive the relationship between innovativeness and residual brain size in birds. Anim. Behav. 78, 1001–1010. ( 10.1016/j.anbehav.2009.06.033) [DOI] [Google Scholar]
- 63.Navarrete AF, Reader SM, Street SE, Whalen A, Laland KN. 2016. The coevolution of innovation and technical intelligence in primates. Phil. Trans. R. Soc. B 371, 20150186 ( 10.1098/rstb.2015.0186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagner GP, Lynch VJ. 2010. Evolutionary novelties. Curr. Biol. 20, R48–R52. ( 10.1016/j.cub.2009.11.010) [DOI] [PubMed] [Google Scholar]
- 65.Wagner A. 2011. The molecular origins of evolutionary innovations. Trends Genet. 27, 397–410. ( 10.1016/j.tig.2011.06.002) [DOI] [PubMed] [Google Scholar]
- 66.Fidler AL, et al. 2014. A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc. Natl Acad. Sci. USA 111, 331–336. ( 10.1073/pnas.1318499111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heers AM. 2016. New perspectives on the ontogeny and evolution of avian locomotion. Integr. Comp. Biol. 56, 428–441. ( 10.1093/icb/icw065) [DOI] [PubMed] [Google Scholar]
- 68.Dyke G, Kaiser G. 2011. Living dinosaurs: the evolutionary history of modern birds. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 69.Brusatte SL, O'Connor JK, Jarvis ED. 2015. The origin and diversification of birds. Curr. Biol. 25, R888–R898. ( 10.1016/j.cub.2015.08.003) [DOI] [PubMed] [Google Scholar]
- 70.Prum RO. 1999. Development and evolutionary origin of feathers. J. Exp. Zool. 285, 291–306. ( 10.1002/(SICI)1097-010X(19991215)285:4%3C291::AID-JEZ1%3E3.0.CO;2-9) [DOI] [PubMed] [Google Scholar]
- 71.Seki R, et al. 2017. Functional roles of Aves class-specific cis-regulatory elements on macroevolution of bird-specific features. Nat. Commun. 8, 14229 ( 10.1038/ncomms14229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vargas AO, Fallon JF. 2005. Birds have dinosaur wings: the molecular evidence. J. Exp. Zool. B Mol. Dev. Evol. 304, 86–90. ( 10.1002/jez.b.21023) [DOI] [PubMed] [Google Scholar]
- 73.Vargas AO, Fallon JF. 2005. The digits of the wing of birds are 1, 2, and 3. A review. J. Exp. Zool. B Mol. Dev. Evol. 304, 206–219. ( 10.1002/jez.b.21051) [DOI] [PubMed] [Google Scholar]
- 74.Hutchinson JR, Allen V. 2008. The evolutionary continuum of limb function from early theropods to birds. Naturwissenschaften 96, 423–448. ( 10.1007/s00114-008-0488-3) [DOI] [PubMed] [Google Scholar]
- 75.Pap PL, Osváth G, Sándor K, Vincze O, Bărbos L, Marton A, Nudds RL, Vágási CI. 2015. Interspecific variation in the structural properties of flight feathers in birds indicates adaptation to flight requirements and habitat. Funct. Ecol. 29, 746–757. ( 10.1111/1365-2435.12419) [DOI] [Google Scholar]
- 76.Tobalske BW. 2016. Evolution of avian flight: muscles and constraints on performance. Phil. Trans. R. Soc. B 371, 20150383 ( 10.1098/rstb.2015.0383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Norberg UM. 1995. How a long tail and changes in mass and wing shape affect the cost for flight in animals. Funct. Ecol. 9, 48–54. ( 10.2307/2390089) [DOI] [Google Scholar]
- 78.Hedenstrom A. 2008. Adaptations to migration in birds: behavioural strategies, morphology and scaling effects. Phil. Trans. R. Soc. B 363, 287–299. ( 10.1098/rstb.2007.2140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morse DH. 1975. Ecological aspects of adaptive radiation in birds. Biol. Rev. Camb. Philos. Soc. 50, 167–214. ( 10.1111/j.1469-185X.1975.tb01056.x) [DOI] [Google Scholar]
- 80.Martin-Albarracin VL, Amico GC, Simberloff D, Nuñez MA. 2015. Impact of non-native birds on native ecosystems: a global analysis. PLoS ONE 10, e0143070 ( 10.1371/journal.pone.0143070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Videler JJ. 2006. Avian flight. Oxford, UK: Oxford University Press. [Google Scholar]
- 82.Hanson T. 2012. Feathers: the evolution of a natural miracle. New York, NY: Basic Books. [Google Scholar]
- 83.Cavalli-Sforza LL, Feldman MW. 1981. Cultural transmission and evolution: a quantitative approach. Monogr. Popul. Biol. 16, 1–388. [PubMed] [Google Scholar]
- 84.Boyd R, Richerson PJ. 1985. Culture and the evolutionary process. Chicago, IL: University of Chicago Press. [Google Scholar]
- 85.Nelson RR, Winter SG. 2002. Evolutionary Theorizing in Economics. J. Econ. Perspect. 16, 23–46. ( 10.1257/0895330027247) [DOI] [Google Scholar]
- 86.Basalla G. 1989. The evolution of technology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 87.Arthur WB. 2009. The nature of technology: what it is and how it evolves. New York, NY: Simon and Schuster, Free Press. [Google Scholar]
- 88.Bonduriansky R. 2012. Rethinking heredity, again. Trends Ecol. Evol. 27, 330–336. ( 10.1016/j.tree.2012.02.003) [DOI] [PubMed] [Google Scholar]
- 89.Nosil P. 2015. Evolution: sex limits adaptation. Curr. Biol. 25, R613–R616. ( 10.1016/j.cub.2015.06.019) [DOI] [PubMed] [Google Scholar]
- 90.Bonduriansky R. 2011. Sexual selection and conflict as engines of ecological diversification. Am. Nat. 178, 729–745. ( 10.1086/662665) [DOI] [PubMed] [Google Scholar]
- 91.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789. ( 10.1890/03-9000) [DOI] [Google Scholar]
- 92.Marden JH. 2005. Scaling of maximum net force output by motors used for locomotion. J. Exp. Biol. 208, 1653–1664. ( 10.1242/jeb.01483) [DOI] [PubMed] [Google Scholar]
- 93.Charlesworth B. 1990. The evolutionary genetics of adaptation. In Evolutionary innovations (ed. Nitecki NH.), pp. 47–70. Chicago, IL: University of Chicago Press. [Google Scholar]
- 94.Dykhuizen D, Hartl DL. 1980. Selective neutrality of 6PGD allozymes in E. coli and the effects of genetic background. Genetics 96, 801–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blount ZD, Barrick JE, Davidson CJ, Lenski RE. 2012. Genomic analysis of a key innovation in an experimental Escherichia coli population. Nature 489, 513–518. ( 10.1038/nature11514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barve A, Wagner A. 2013. A latent capacity for evolutionary innovation through exaptation in metabolic systems. Nature 500, 203–206. ( 10.1038/nature12301) [DOI] [PubMed] [Google Scholar]
- 97.Wallbank RWR, et al. 2016. Evolutionary novelty in a butterfly wing pattern through enhancer shuffling. PLoS Biol. 14, e1002353 ( 10.1371/journal.pbio.1002353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kirsch DA. 1997. The electric car and the burden of history: studies in automotive systems rivalry in America, 1890–1996. Bus. Econ. Hist. 26, 304–310. [Google Scholar]
- 99.Collantes G, Sperling D. 2008. The origin of California's zero emission vehicle mandate. Transp. Res. A: Policy Pract. 42, 1302–1313. ( 10.1016/j.tra.2008.05.007) [DOI] [Google Scholar]
- 100.Johnson S. 2010. Where good ideas come from: the natural history of innovation. London, UK: Penguin. [Google Scholar]
- 101.Kell DB, Lurie-Luke E. 2015. The virtue of innovation: innovation through the lenses of biological evolution. J. R. Soc. Interface 12, 20141183 ( 10.1098/rsif.2014.1183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muthukrishna M, Henrich J. 2016. Innovation in the collective brain. Phil. Trans. R. Soc B 371, 20150192 ( 10.1098/rstb.2015.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Erwin DH. 2017. The topology of evolutionary novelty and innovation in macroevolution. Phil. Trans. R. Soc. B 372, 20160422 ( 10.1098/rstb.2016.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Visser JAGM, Krug J. 2014. Empirical fitness landscapes and the predictability of evolution. Nat. Rev. Genet. 15, 480–490. ( 10.1038/nrg3744) [DOI] [PubMed] [Google Scholar]
- 105.Kline MA, Boyd R. 2010. Population size predicts technological complexity in Oceania. Proc. R. Soc. B 277, 2559–2564. ( 10.1098/rspb.2010.0452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wagner A, Ortman S, Maxfield R. 2016. From the primordial soup to self-driving cars: standards and their role in natural and technological innovation. J. R. Soc. Interface 13, 20151086 ( 10.1098/rsif.2015.1086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Erwin DH. 2011. Novelties that change carrying capacity. J. Exp. Zool. B Mol. Dev. Evol. 318, 460–465. ( 10.1002/jez.b.21429) [DOI] [PubMed] [Google Scholar]
- 108.Kolodny O, Creanza N, Feldman MW. 2016. Game-changing innovations: how culture can change the parameters of its own evolution and induce abrupt cultural shifts. PLoS Comput. Biol. 12, e1005302 ( 10.1371/journal.pcbi.1005302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Richerson PJ, Boyd R, Bettinger RL. 2009. Cultural innovations and demographic change. Hum. Biol. 81, 211–235. ( 10.3378/027.081.0306) [DOI] [PubMed] [Google Scholar]
- 110.Weinberger VP, Quiñinao C, Marquet PA. 2017. Innovation and the growth of human population. Phil. Trans. R. Soc. B 372, 20160415 ( 10.1098/rstb.2016.0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fogarty L, Creanza N. 2017. The niche construction of cultural complexity: interactions between innovations, population size and the environment. Phil. Trans. R. Soc. B 372, 20160428 ( 10.1098/rstb.2016.0428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kremer M. 1993. Population growth and technological change: one million B.C. to 1990. Q. J. Econ. 108, 681–716. ( 10.2307/2118405) [DOI] [Google Scholar]
- 113.Shennan S. 2001. Demography and cultural innovation: a model and its implications for the emergence of modern human culture. Cambr. Archaeol. J. 11, 5–16. ( 10.1017/S0959774301000014) [DOI] [Google Scholar]
- 114.Vermeij GJ, Leigh EG. 2011. Natural and human economies compared. Ecosphere 2, 1–16. ( 10.1890/ES11-00004.1) [DOI] [Google Scholar]
- 115.Griffin AS, Guez D. 2014. Innovation and problem solving: a review of common mechanisms. Behav. Processes 109, 121–134. ( 10.1016/j.beproc.2014.08.027) [DOI] [PubMed] [Google Scholar]
- 116.Tebbich S, Griffin AS, Peschl MF, Sterelny K. 2016. From mechanisms to function: an integrated framework of animal innovation. Phil. Trans. R. Soc. B 371, 20150195 ( 10.1098/rstb.2015.0195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fogarty L, Wakano JY, Feldman MW, Aoki K. 2017. The driving forces of cultural complexity. Hum. Nat. 28, 39–52. ( 10.1007/s12110-016-9275-6) [DOI] [PubMed] [Google Scholar]
- 118.Orr HA. 2000. Adaptation and the cost of complexity. Evolution 54, 13–20. ( 10.1111/j.0014-3820.2000.tb00002.x) [DOI] [PubMed] [Google Scholar]
- 119.Wagner A. 2012. The role of robustness in phenotypic adaptation and innovation. Proc. R. Soc. B 279, 1249–1258. ( 10.1098/rspb.2011.2293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hendry AP. 2016. Eco-evolutionary dynamics. Princeton, NJ: Princeton University Press. [Google Scholar]
- 121.Toll-Riera M, San Millan A, Wagner A, MacLean RC. 2016. The genomic basis of evolutionary innovation in Pseudomonas aeruginosa. PLoS Genet. 12, e1006005 ( 10.1371/journal.pgen.1006005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Flatt T, Heyland A. 2011. Mechanisms of life history evolution. In The genetics and physiology of life history traits and trade-offs (eds Flatt T, Heyland A), pp. 375–379. Oxford, UK: Oxford University Press. [Google Scholar]
- 123.Ratcliff WC, Herron M, Conlin PL, Libby E. 2017. Nascent life cycles and the emergence of higher-level individuality. Phil. Trans. R. Soc. B 372, 20160420 ( 10.1098/rstb.2016.0420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gavrilets S. 2004. Fitness landscapes and the origin of species. Princeton, NJ: Princeton University Press. [Google Scholar]
- 125.Zeder MA. 2015. Core questions in domestication research. Proc. Natl Acad. Sci. USA 112, 3191–3198. ( 10.1073/pnas.1501711112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rogers EM. 2003. Diffusion of innovations, 5th edn New York, NY: Simon & Schuster, Free Press. [Google Scholar]
- 127.Hager T. 2008. The alchemy of air: a Jewish genius, a doomed tycoon, and the scientific discovery that fed the world but fueled the rise of Hitler. New York, NY: Random House. [Google Scholar]
- 128.van Baalen M. 2013. Biological information: why we need a good measure and the challenges ahead. Interface Focus 3, 20130030 ( 10.1098/rsfs.2013.0030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hall JPJ, Brockhurst MA, Harrison E. 2017. Sampling the mobile gene pool: innovation via horizontal gene transfer in bacteria. Phil. Trans. R. Soc. B 372, 20160424 ( 10.1098/rstb.2016.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sobel JM, Chen GF, Watt LR, Schemske DW. 2010. The biology of speciation. Evolution 64, 295–315. ( 10.1111/j.1558-5646.2009.00877.x) [DOI] [PubMed] [Google Scholar]
- 131.West T, Sojo V, Pomiankowski A, Lane N. 2017. The origin of heredity in protocells. Phil. Trans. R. Soc. B 372, 20160419 ( 10.1098/rstb.2016.0419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sojo V, Herschy B, Whicher A, Camprubí E, Lane N. 2016. The origin of life in alkaline hydrothermal vents. Astrobiology 16, 181–197. ( 10.1089/ast.2015.1406) [DOI] [PubMed] [Google Scholar]
- 133.Buss LW. 1987. The evolution of individuality. Princeton, NJ: Princeton University Press. [Google Scholar]
- 134.Aktipis A, Maley CC. 2017. Cooperation and cheating as innovation: insights from cellular societies. Phil. Trans. R. Soc. B 372, 20160421 ( 10.1098/rstb.2016.0421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Frank SA. 1998. Foundations of social evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 136.Aktipis CA, Boddy AM, Jansen G, Hibner U, Hochberg ME, Maley CC, Wilkinson GS. 2015. Cancer across the tree of life: cooperation and cheating in multicellularity. Phil. Trans. R. Soc. B 370, 20140219 ( 10.1098/rstb.2014.0219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wasielewski H, Alcock J, Aktipis A. 2016. Resource conflict and cooperation between human host and gut microbiota: implications for nutrition and health. Ann. NY Acad. Sci. 1372, 20–28. ( 10.1111/nyas.13118) [DOI] [PubMed] [Google Scholar]
- 138.Thompson JN. 2009. The coevolving web of life. Am. Nat. 173, 125–140. ( 10.1086/595752) [DOI] [PubMed] [Google Scholar]
- 139.Brockhurst MA, Chapman T, King KC, Mank JE, Paterson S, Hurst GDD. 2014. Running with the red queen: the role of biotic conflicts in evolution. Proc. R. Soc. B 281, 20141382 ( 10.1098/rspb.2014.1382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fortuna MA, Zaman L, Wagner A, Bascompte J. 2017. Non-adaptive origins of evolutionary innovations increase network complexity in interacting digital organisms. Phil. Trans. R. Soc. B 372, 20160431 ( 10.1098/rstb.2016.0431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lenski RE, Ofria C, Pennock RT, Adami C. 2003. The evolutionary origin of complex features. Nature 8, 139–144. ( 10.1038/nature01568) [DOI] [PubMed] [Google Scholar]
- 142.Zaman L, Meyer JR, Devangam S, Bryson DM, Lenski RE, Ofria C. 2014. Coevolution drives the emergence of complex traits and promotes evolvability. PLoS Biol. 12, e1002023 ( 10.1371/journal.pbio.1002023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cordero OX, Polz MF. 2014. Explaining microbial genomic diversity in light of evolutionary ecology. Nat. Rev. Microbiol. 12, 263–273. ( 10.1038/nrmicro3218) [DOI] [PubMed] [Google Scholar]
- 144.Laland KN, Galef BG. 2009. The question of animal culture. Cambridge, MA: Harvard University Press. [Google Scholar]
- 145.Hobaiter C, Poisot T, Zuberbühler K, Hoppitt W, Gruber T. 2014. Social network analysis shows direct evidence for social transmission of tool use in wild chimpanzees. PLoS Biol. 12, e1001960 ( 10.1371/journal.pbio.1001960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hoppitt W. 2017. The conceptual foundations of network-based diffusion analysis: choosing networks and interpreting results. Phil. Trans. R. Soc. B 372, 20160418 ( 10.1098/rstb.2016.0418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hochberg ME. 2004. A theory of modern cultural shifts and meltdowns. Proc. R. Soc. B 271, S313–S316. ( 10.1098/rsbl.2004.0172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Enquist M, Ghirlanda S, Eriksson K. 2011. Modelling the evolution and diversity of cumulative culture. Phil. Trans. R. Soc. B 366, 412–423. ( 10.1098/rstb.2010.0132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Farrell W. 1998. How hits happen: forecasting predictability in a chaotic marketplace. New York, NY: HarperBusiness. [Google Scholar]
- 150.Bentley RA, Hahn MW, Shennan SJ. 2004. Random drift and culture change. Proc. R. Soc. B 271, 1443–1450. ( 10.1098/rspb.2004.2746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mesoudi A. 2015. Cultural evolution: a review of theory, findings and controversies. Evol. Biol. 43, 481–497. ( 10.1007/s11692-015-9320-0) [DOI] [Google Scholar]
- 152.McGuigan N, Burdett E, Burgess V, Dean L, Lucas A, Vale G, Whiten A. 2017. Innovation and social transmission in experimental micro-societies: exploring the scope of cumulative culture in young children. Phil. Trans. R. Soc. B 372, 20160425 ( 10.1098/rstb.2016.0425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Whiten A, McGuigan N, Marshall-Pescini S, Hopper LM. 2009. Emulation, imitation, over-imitation and the scope of culture for child and chimpanzee. Phil. Trans. R. Soc. B 364, 2417–2428. ( 10.1098/rstb.2009.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dukas R. 2017. Cognitive innovations and the evolutionary biology of expertise. Phil. Trans. R. Soc. B 372, 20160427 ( 10.1098/rstb.2016.0427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lotem A, Halpern JY, Edelman S, Kolodny O. 2017. The evolution of cognitive mechanisms in response to cultural innovations. Proc. Natl Acad. Sci. USA 114, 7915–7922. ( 10.1073/pnas.1620742114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Laland KN, Boogert N, Evans C. 2014. Niche construction, innovation and complexity. Environ. Innov. Societal Transit. 11, 71–86. ( 10.1016/j.eist.2013.08.003) [DOI] [Google Scholar]
- 157.West-Eberhard MJ. 2005. Phenotypic accommodation: adaptive innovation due to developmental plasticity. J. Exp. Zool. B Mol. Dev. Evol. 304, 610–618. ( 10.1002/jez.b.21071) [DOI] [PubMed] [Google Scholar]
- 158.Frank SA. 2012. Natural selection. V. How to read the fundamental equations of evolutionary change in terms of information theory. J. Evol. Biol. 25, 2377–2396. ( 10.1111/jeb.12010) [DOI] [PubMed] [Google Scholar]
- 159.O'Brien MJ, Laland KN. 2012. Genes, culture, and agriculture: an example of human niche construction. Curr. Anthropol. 53, 434–470. ( 10.1086/666585) [DOI] [Google Scholar]
- 160.Marquet PA, Santoro CM, Latorre C, Standen VG, Abades SR, Rivadeneira MM, Arriaza B, Hochberg ME. 2012. From the cover: feature article: emergence of social complexity among coastal hunter-gatherers in the Atacama Desert of northern Chile. Proc. Natl Acad. Sci. USA 109, 14 754–14 760. ( 10.1073/pnas.1116724109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Henrich J, Boyd R, Derex M, Kline MA, Mesoudi A, Muthukrishna M, Powell AT, Shennan SJ, Thomas MG. 2016. Understanding cumulative cultural evolution. Proc. Natl Acad. Sci. USA 113, E6724–E6725. ( 10.1073/pnas.1610005113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Henrich J. 2004. Demography and cultural evolution: how adaptive cultural processes can produce maladaptive losses—the Tasmanian case. Am. Antiq. 69, 197–214. ( 10.2307/4128416) [DOI] [Google Scholar]
- 163.Stiner MC, Kuhn SL. 2006. Changes in the ‘connectedness’ and resilience of Paleolithic societies in Mediterranean ecosystems. Hum. Ecol. 34, 693–712. ( 10.1007/s10745-006-9041-1) [DOI] [Google Scholar]
- 164.Snell-Rood EC, Van Dyken JD, Cruickshank T, Wade MJ, Moczek AP. 2010. Toward a population genetic framework of developmental evolution: the costs, limits, and consequences of phenotypic plasticity. Bioessays 32, 71–81. ( 10.1002/bies.200900132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ehrlich PR, Holdren JP. 1971. Impact of population growth. Science 171, 1212–1217. ( 10.1126/science.171.3977.1212) [DOI] [PubMed] [Google Scholar]
- 166.Cieslak M, Pruvost M, Benecke N, Hofreiter M, Morales A, Reissmann M, Ludwig A. 2010. Origin and history of mitochondrial DNA lineages in domestic horses. PLoS ONE 5, e15311 ( 10.1371/journal.pone.0015311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Blount ZD, Borland CZ, Lenski RE. 2008. Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli. Proc. Natl Acad. Sci. USA 105, 7899–7906. ( 10.1073/pnas.0803151105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Meyer JR, Dobias DT, Weitz JS, Barrick JE, Quick RT, Lenski RE. 2012. Repeatability and contingency in the evolution of a key innovation in phage lambda. Science 335, 428–432. ( 10.1126/science.1214449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wagner GP, Altenberg L. 1996. Complex adaptations and the evolution of evolvability. Evolution 50, 967–976. ( 10.1111/j.1558-5646.1996.tb02339.x) [DOI] [PubMed] [Google Scholar]