Abstract
The capacity to innovate is often considered a defining feature of human societies, but it is not a capacity that is unique to human societies: innovation occurs in cellular societies as well. Cellular societies such as multicellular bodies and microbial communities, including the human microbiome, are capable of innovation in response to novel opportunities and threats. Multicellularity represents a suite of innovations for cellular cooperation, but multicellularity also opened up novel opportunities for cells to cheat, exploiting the infrastructure and resources of the body. Multicellular bodies evolve less quickly than the cells within them, leaving them vulnerable to cellular innovations that can lead to cancer and infections. In order to counter these threats, multicellular bodies deploy additional innovations including the adaptive immune system and the development of partnerships with preferred microbial partners. What can we learn from examining these innovations in cooperation and cheating in cellular societies? First, innovation in social systems involves a constant tension between novel mechanisms that enable greater size and complexity of cooperative entities and novel ways of cheating. Second, cultivating cooperation with partners who can rapidly and effectively innovate (such as microbes) is important for large entities including multicellular bodies. And third, multicellularity enabled cells to manage risk socially, allowing organisms to survive in challenging environments where life would otherwise be impossible. Throughout, we ask how insights from cellular societies might be translated into new innovations in human health and medicine, promoting and protecting the cellular cooperation that makes us viable multicellular organisms.
This article is part of the themed issue ‘Process and pattern in innovations from cells to societies’.
Keywords: multicellularity, cancer, infectious disease, autoimmune disorders, microbiome
1. Introduction
For the first half of the history of life on Earth, all living creatures were individual cells. Then, approximately 2 billion years ago, evolution generated one of the most spectacular innovations in the history of life, multicellularity [1,2]. Complex multicellularity, with tissue differentiation, inter-cellular communication and cells that are not in direct contact with the environment, evolved only 1 billion years ago and has evolved independently since then at least seven times [3]. The evolution of multicellularity required the constraint of evolution and innovations at the cell level which, if left unchecked, can lead to cancer and the death of the organism [2,4–6]. Multicellularity itself is an innovation that spawned further innovations in the organization, specialization and regulation of those cells. The result? ‘… endless forms most beautiful and most wonderful have been, and are being, evolved’ [7, p. 490]. These endless forms arose from the tension between cooperation and cheating in cellular societies. Multicellular bodies require both cooperation and the management of cheating and conflict through implicit rules that are coded into the genomes of each cell [8–10]. These rules include both directions for how to cooperate and restrictions on cellular behaviour that keep cells from cheating and exploiting the organism. These multicellular societies have some parallels to human societies living together and interacting in a community: many multicellular societies promote the common good, prevent exploitation and help coordinate higher level functions like resource transport and division of labour. Thus, every multicellular organism can be considered a society of cells, regulated not by explicit laws, but rather by rules encoded in the genomes.
Multicellularity is essentially a collection of innovations for cooperation and the control of cellular cheating. The tension between cooperation (which benefits the organism/group) and cheating (which benefits the individual) is central to the evolution of multicellularity and the breakdown of multicellularity during cancer [4]. Evolution can simultaneously operate both on cells within organisms and on the organisms themselves. This tension is central to the evolution of both cancer within the body and cancer suppression mechanisms at the organism level [5]. This tension has also driven innovation, both for multicellular societies to become more cooperative and better at suppressing cheating, and for cancer cells to cheat in novel ways that get around the body's defenses. In this paper, we discuss how multicellular bodies and other cellular societies use cooperative innovations and manage the novel forms of cheating that arise as cellular societies become more complex and cooperative. This includes the evolution of multicellularity itself, the control of cellular cheating in the form of cancer, the evolution of the adaptive immune system to counter exploitation from pathogens and also the evolution of cooperative partnerships between multicellular bodies and the microbial species that reside in and on them. Throughout, we ask how these insights might be translated into new innovations in human health and medicine, promoting and protecting the cellular cooperation that makes us viable multicellular organisms.
2. What qualifies as an innovation?
It is difficult to discuss cooperation and cheating as innovations without a definition of innovation. However, the definition of innovation is controversial. In the introduction of this issue, Hochberg et al. say:
In performance innovations, the novel phenotypic trait is associated with increases in performance, without fundamental changes to the organism's ecological niche … To become an innovation, such a trait needs to confer a fitness advantage and spread through the population … and in so doing impact the surrounding community or ecosystem, and possibly create longer-term opportunities for further adaptation and innovation. … In niche innovations, the novel phenotypic trait is associated with the utilization of a new ecological niche … Similar to performance innovations, this type of novelty becomes an innovation if invasion fitness into the new niche is positive and the emerging population impacts the surrounding environment (i.e. the community and ecosystem).
Hochberg et al. [11]
By this definition, all innovations are the results of adaptive selection, but not all the results of adaptive selection are innovations. To be an innovation, the adaptation must rise to the level of a performance improvement or lead to a new function that establishes new ecological space. Parts of this definition are subjective and clearly the degree of novelty exists on a spectrum from minor to major changes. Let us take the example of body size. From one perspective, a change in body size is just a minor quantitative change, not a qualitative innovation. But the evolution of a large body size can dramatically change the ecology of an organism, allowing it to escape predators, invade harsher climates and also provide new ecological niches for parasites and pathogens, thereby establishing new ecological space (another criterion from Hochberg et al. for an innovation [11]). Should we regard larger body size as an innovation? Perhaps, if this larger size enables a qualitative change in terms of how an organism interacts with its environment. But perhaps not if the change in size is merely incremental and does not result in a qualitative change in the organism's modus operandi.
During the evolution of multicellularity, many new cooperative cellular traits arose and were favoured by natural selection. Many of these new cooperative cellular traits qualify as innovations according to Hochberg's definition, but others are perhaps not as clearly innovations, or are minor innovations. For example, during the evolution of multicellularity cells evolved seemingly minor innovations, such as the inhibition of proliferation in response to environmental stresses [12] and controlled cell death [13]. But these more minor innovations set the stage for a much larger qualitative shift in cellular sociality—allowing cells to form proper multicellular bodies that regulate their growth and size rather than just being a collection of cells.
3. Innovations occur in large and diverse societies
Societies innovate [14,15]. New ideas and inventions arise that are adopted and spread through the population, through a process of memetic selection [16–18], sometimes to the benefit of that society and other times to its detriment [15]. But it is not just human societies that innovate, societies of cells—in our bodies and in our environment—innovate as well. Cellular societies such as multicellular bodies and microbial communities, including the human microbiome, are diverse populations of individuals capable of innovation in response to novel opportunities and threats. As with human societies, some of the innovations in cellular societies are innovations for cooperation; others are innovations that allow some individuals to cheat, acquiring benefits at the expense of the higher-level function of the cellular society.
Innovation can be beneficial for the society if it promotes cooperation, but harmful if it opens up avenues for cheating. In human societies, innovations in the form of new ideas and technologies change how we work, how we socialize and even how we reproduce. These innovations allow us to take advantage of new opportunities, but they also can expose us to new risks. For example, the innovation of market capitalism allowed human societies to take advantage of many opportunities arising from cooperation through division of labour, but this same innovation led to new risks and costs such as environmental damage and the introduction of new ways of cheating the systems, e.g. white-collar crime. Some of these examples have direct analogues in cellular societies.
Cellular societies also encounter new opportunities as a result of innovation. For example, multicellular bodies or facultative multicellular communities can innovate through division of labour. Cells in our bodies capitalize on this opportunity through having different cell types doing different jobs. Within our bodies, our liver cells specialize in detoxification, our heart cells specialize in pumping blood through our bodies and our immune cells specialize in protecting us from threats from the outside world. Division of labour also occurs in microbial communities that are composed of different species—even species from different kingdoms. Take, for example, the multi-species (multi-kingdom) biofilm formed during the process of fermenting sweet tea into kombucha. In this system, eukaryotic yeast (mainly Zygosaccharomyces) specialize in cleaving valuable sugars so that they can be metabolized, and bacteria (predominantly Gluconacetobacter and Lactobacillus) specialize in producing a floating glycan layer that may protect the multispecies system from invasion by other potentially contaminating microbes [19]. By dividing labour, cellular societies are able to not just become larger, but also become more complex, deploying multiple innovations simultaneously to reach higher levels of functionality.
Innovation occurs most readily when there is a population of individuals who can produce and test novel solutions to problems. This bears some similarities to the process of evolution by natural selection itself. If a population is diverse (i.e. has diverse solutions), those different solutions have some effect on the individuals or society, and there is some mechanism for those innovations to be passed along to others with reasonable fidelity (e.g. genes or the written word).
Diversity allows a society to explore more potential solutions. In fact, a lack of genetic diversity can be a challenge for organismal multicellularity. Most organismal multicellularity is a result of development from a single-cell bottleneck, where high relatedness enables a highly cooperative organism to evolve. However, like a monoculture of crops, this high relatedness can be a major vulnerability when on organism encounters a threat from a diverse and rapidly evolving population [20], whether it be a diverse population of Staphylococcus in the environment or a highly mutated population of cancer cells within the body.
The size of the population and the pace at which individuals reproduce also influence how rapidly cellular societies can innovate. Innovations are a subset of the changes that are generated by evolution, so the determinants of the rate of evolution are also the determinants of the rate of innovations in biology. Large populations have a greater capacity for innovation than small populations because there are more individuals who can participate in the innovation process [14]. The pace of reproduction is also important: faster reproduction means faster innovation. Evolution within cellular societies can occur rapidly (with generation times as short as 20 min in bacteria, and 24 h in cancer cells) in comparison to evolution at the level of the multicellular organism. The pace of innovation in cellular societies can be much faster than innovations in societies of multicellular organisms simply because the population size and rate of reproduction are usually orders of magnitude greater within cellular societies. Interestingly, some multicellular organisms discovered a way to leverage diversity within themselves to handle unexpected threats using cellular variation, by evolving an adaptive immune system that actually generates diversity in order to respond to novel threats, a topic we will come back to later in this paper.
Sometimes innovations in cellular societies have positive effects on the fitness of the multicellular organism. Other times these innovations have negative effects on the fitness of the multicellular organism. When these innovations have positive effects on the fitness of the organism we refer to them as cooperation innovations, and when they have a negative effect we refer to them as cheating innovations. For the purposes of this paper, we adopt the convention that the relevant point of view is the fitness of the multicellular organism, i.e. innovations that enhance multicellular fitness are referred to as cooperative, those that comprise multicellular fitness are referred to as cheating.
4. Innovations in cellular cooperation spurred the evolution of multicellularity
Natural selection is perhaps the most powerful force shaping biological innovation. Mutation and natural selection have generated a suite of innovations in cellular cooperation including division of labour, resource allocation and inhibition of cellular reproduction that made multicellular life possible (as reviewed in [2]). These complex forms of cellular cooperation gave rise to many opportunities for cellular cheating, which cells then evolved to take advantage of through new innovations for cheating. Both innovations in cellular cooperation and innovations in cellular cheating have shaped multicellularity in important ways, enabling the evolution of large body size, long lifespan [4], the adaptive immune system and even sexual ornamentation [21–24]. But biological innovations have also introduced new challenges, leaving multicellular bodies susceptible to cancer, infectious disease and autoimmune disorders. This vulnerability is inherent to our bodies because cells within us (including both cancer cells and pathogens) can innovate as well, sometimes exploiting those very innovations that enabled us to become multicellular.
Complex multicellularity evolved as a result of many innovations for cellular cooperation being brought together under one umbrella (internal pentagon in figure 1). These include innovations for economic cooperation among cells: division of cellular labour, resource sharing/transportation systems and the creation/maintenance of the extracellular environment. Innovations for demographic cooperation were also critical: control of cellular reproduction and death allowed for complex and well-regulated multicellularity. Together these five innovations for cellular cooperation represent the foundations upon which multicellularity is built [4]. Although there might be debate about the extent to which each of these foundations of multicellular cooperation qualify as a true innovation, it is clear that when these traits are combined they generate a qualitative shift in how organisms can operate. They allow for regulation (of proliferation, cell death, metabolism and more) to happen at the aggregate level, where these processes can be coordinated to create a coherent life-history strategy for the multicellular entity.
Figure 1.
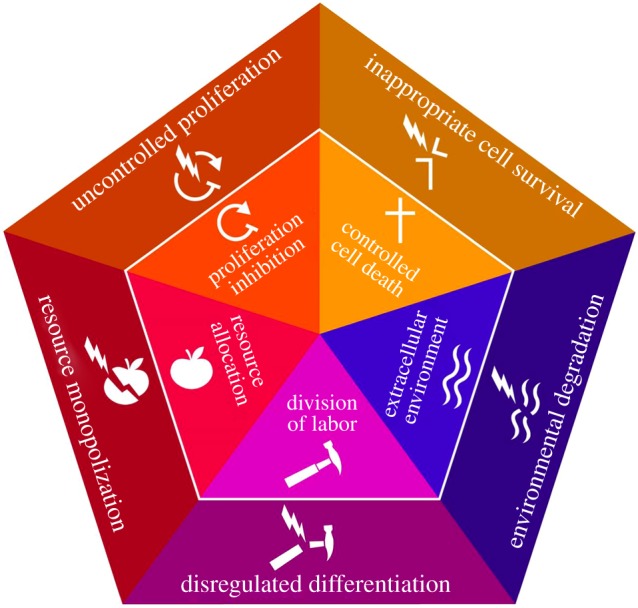
The foundations of multicellular cooperation and cheating. The evolution of multicellularity involved the bringing together of five foundations for cellular cooperation (inside pentagon), in a way that led to a major innovation in this history of life. These five foundations are resource allocation (e.g. circulatory systems), division of labour (e.g. development of specialized organs), the extracellular environment (e.g. skeletons), controlled cell death (e.g. tissues structures such as digits, neural structures) and proliferation inhibition (e.g. cell cycle control). Cheating in each of these foundations (outside pentagon) can disrupt multicellular cooperation, requiring the evolution of further controls in order to maintain effective multicellular function. These forms of cheating can either result from the loss of innovations that make multicellularity viable or from innovations that disrupt and exploit multicellularity through novel functions. Adapted from Aktipis et al. [4].
The emergence of life cycles at this higher level of organization are an important step in the transition from unicellular to multicellular life, allowing selection to act on the phenotype of the collective more strongly than it acts on the individuals making up that collective. This can tip the scales in favour of mechanisms that increase cellular cooperation within the multicellular body and reduce conflict among cells within the body [10]. These multicellular life cycles also open up new ecological space, with cells creating and maintaining an extracellular environment that is both their ecological niche and the substrate through which they express their life-history strategy. Thus, multicellular life cycles, realized through coordination of the foundations of multicellular cooperation, appear to represent a key innovation [11] in the evolution of life.
The five foundations of multicellular cooperation arose every time organismal multicellularity evolved. They represented important innovations, allowing organisms to solve adaptive problems, such as growing larger to avoid predators and managing the risks of environmental uncertainty by storing resources in the body. Each of these forms of cooperation is encoded in genetic networks inside each cell. Also encoded into each cell are systems for detecting and responding to cheating in these implicitly encoded ‘rules’ for cooperation.
The problem of controlling cheating has several solutions, and the best solution depends on the type of cellular society. There are two fundamentally different forms of multicellularity: aggregates of genetically different cells that come together to form a body, and clonal organisms that derive from a single cell (e.g. a zygote). The difficulty of controlling cheating when genetically diverse cells come together to form an organism probably explains why multicellular aggregates, such as Dictyostelium, are generally smaller and less complex than multicellular organisms that develop as a clone from a unicellular bottleneck [22,25]. In fact, one viable strategy for stabilizing cooperation in aggregative multicellularity is for the cooperating cells to ‘walk away’ from aggregates where the burden of cheaters is too high [26,27]. The ability to walk away also applies to cheaters, which may spread from one multicellular aggregate to another [22]. Clonal multicellular organisms avoid this problem by passing through a unicellular life stage (e.g. a zygote). That unicellular bottleneck ensures all the cells in the body have the same genes and follow the same rules of cooperation. Only somatic mutations and pathogens can introduce cheaters into clonal multicellular organisms. Below, unless otherwise specified, when we refer to multicellularity, we mean clonal multicellularity.
5. Cellular cheating can disrupt multicellular innovations
We define cheating here as simply the breakdown of shared rules (including genetically encoded phenotypes or behaviors) that leads to a fitness advantage on the cellular level for the cheater.
Aktipis et al. [4, p. 2]
Innovations can open up new opportunities for cheating through the loss of the innovation. When mutations occur in the genetic networks that promote cellular cooperation and suppress cellular cheating, this can cause disruptions to the function of the cellular society. In multicellular bodies, cheating on the foundations of multicellular cooperation typically gives rise to cancer or cancer-like phenomena [4]. These forms of cheating often lead to radical performance improvements for the cheating cells, and change their microenvironment, which establishes new ecological space for further cellular evolution [28]. Thus, cooperation and cheating in multicellular life can be considered a sort of arms race between cellular societies evolving to promote cooperation and suppress cheating within, while the cells within evolve to ‘break’ the rules of multicellularity in order to maximize cell-level fitness.
The innovation of growth control in the evolution of multicellularity created the opportunity for cells to cheat by not regulating their growth while their neighbours do. And cells that produce their own growth signals cheat by using novel functions. The innovation of using growth signals to regulate the growth of multicellular organisms opened up the possibility that these growth signals could be ‘hacked’ by cells within the body that produce their own growth signals, even when it is detrimental to the fitness of the organism. This innovation can generate radical performance improvements for the somatic cells that produce their own growth signals [28].
This is exactly what happens in cancer: cancer cells evolve novel genes that allow them to generate proliferative signals for themselves [29]. And it does not end there. Cheating can provide the impetus for innovation, selecting for more sophisticated methods of conflict management and cooperation. This is what happened during the evolution of growth regulation systems in multicellular life. Because a growth regulation system based on proliferative signalling can be easily hijacked, selection favoured a complex system of checks and balances on growth that involves many hormones and growth factors whose activity is monitored and controlled by a complex genetic network [30,31].
Cheating can be horizontally transmitted among clonally derived multicellular organisms via pathogens. For example, when human papilloma virus (HPV) causes a chronic infection, it hijacks the host cells, interfering with the p53 and pRb tumour suppressor proteins, and causing their host cells to become cheaters, which may eventually evolve into cancers [32].
Cells in multicellular bodies do not always follow the implicit, genetically encoded rules of multicellularity, sometimes innovating in ways that cheat on these rules. These innovating cells can contribute to neoplasms which sometimes evolve into cancer [33]. Neoplasms have a variety of cellular innovations [34]. We use the technical term neoplasm here, rather than tumour or cancer, because tumours may refer to masses of fibre that are not related to cancers, and innovations occur in neoplasms while they are still benign, not just in cancers. Neoplasms are diverse populations of billions to trillions of mutant cells that can evolve over periods of decades, for tens of thousands of cell generations [34]. New mutations arise and spread through the neoplastic cell population. Some of the mutations that spread are gain-of-function mutations that cause adaptations in the cells, for example, evading the immune system, generating their own growth signals and stimulating the growth of new blood vessels to feed the neoplasm [28]. Other mutations are loss-of-function mutations that destroy a previous innovation of multicellular cooperation, such as loss of programmed cell death, and loss of sensitivity to anti-growth signals [28]. These loss of function mutations, while beneficial to neoplastic cells, are losses of innovations. In other words, some forms of cellular cheating (that can lead to cancer) do not qualify as innovations according to Hochberg et al.'s definition.
But many of the forms of cheating that occur in neoplasms clearly fit this definition of innovation, as they involve the introduction of novel functions and radically improve the performance of the neoplastic cells. Gain-of-function mutations often dramatically increase proliferation rates, and the innovation of using anaerobic metabolism, even in the presence of oxygen, increases biosynthesis efficiency, further increasing neoplastic cell proliferation rates [28]. In fact, some of the innovations in neoplasms actually spur on additional innovations, further disrupting the multicellular body's proper functioning. For example, the genomes of neoplastic cells are often doubled during carcinogenesis, such that the cells have four instead of two copies of all their genes [35]. This then loosens the constraints on further innovations in the copy numbers of individual genes or chromosomal segments. With more copies of each gene, there are also more opportunities for cellular innovations: adding or subtracting copies of these genes, and modifying some copies while keeping at least one pristine copy of genes necessary for cell survival. Some genes become isolated on pieces of DNA that are not connected to any chromosome, which can facilitate further innovations, including massive amplification, with as many as 30 copies in some cells [36].
Innovations in cancer go beyond simply adding genes, or even modifying existing genes by mutating base pairs in their coding regions or regulatory regions. Structural rearrangements, like translocations and inversions, can fuse exons from separate genes so as to form entirely novel genes. These fusion genes are often important drivers of carcinogenesis [37].
Just as multicellularity involves the combining of novel cooperative cell level traits into a new phenotype that represents a true innovation, cancer appears to also involve the combining of several forms of cheating into a new phenotype that represents a true cheating innovation. For example, cheating in both demographic cooperation and economic cooperation may both be required for cancer across species [4]. This same review suggested that cancer-like phenomena such as overproliferation or dedifferentiation can occur without both types of cheating, but that cancer (i.e. invasive or metastatic growth) may require a combination of both. In other words, the cellular ‘innovation’ of cancer may require multi-faceted cheating, just as the innovation of multicellularity may require the coordination of several types of cooperation. Future work should help to clarify whether this pattern applies across all forms of multicellularity and cancer or whether it is limited to the species and types of cancer examined in Aktipis et al. [4]
There is also evidence for innovations in the social behaviour of neoplastic cells. In some cases, neoplastic cells may re-evolve the foundations of multicellular cooperation but within the neoplasm. For example, there is evidence for division of labour in neoplasms. A minority of cells sometimes will produce a public good, like an angiogenic factor that stimulates the growth of new blood vessels [38] or an invasion factor like a protease that allows other non-invasive cells to invade neighbouring tissues [39]. The existence of division of labour in neoplasms helps to resolve the paradox of why many cancers retain cells that terminally differentiate (non-stem cells). Such evolutionary dead-end cells can persist, via kin selection, if they provide a fitness advantage to their closely related stem cells, which they can potentially do through division of labour [40].
Resource allocation systems open up the possibility for cheaters to monopolize resources. The complexity of the insulin system [41] may be due to the need to regulate resource allocation and prevent cells from cheating. Dysregulation of the insulin system, in the form of diabetes, leads to an increased risk of cancer, thought to be due to a loss of growth control [41]. There is evidence that metformin, a diabetes drug, prevents cancer [42]. Similarly, Crespi & Semeniuk have argued that the complexity in the reproductive system is due to the difficulty of managing cooperation and cheating in parent–offspring conflict [43].
Looking at multicellularity, we see that innovations in cooperation led to more opportunities for cheating, and cheating spurs the need for more complex and well-coordinated forms of cooperation and conflict management [9]. Cell cycle controls help regulate multicellular phenotypes but can be disrupted by cells that proliferate too quickly and exploit the body. These cheating opportunities then further spur the evolution of new innovations for cooperation and conflict control among cells [4,9]. Conflict management and policing of cheaters is done in part by tumour suppressor genes [4]. While there was a significant burst of new tumour suppressor genes that evolved at the dawn of multicellularity [44], new tumour suppressor genes continued to evolve afterwards [44]. For example, as elephantids evolved larger bodies, and thus more opportunities for cellular cheaters, they also evolved extra, modified copies of the TP53 tumour suppressor gene [45,46]. TP53 is a cheater detection and elimination gene that responds to DNA damage, viral and other stresses by either halting the cell cycle for DNA repair or eliminating the cell through apoptosis [47]. Researchers are now working on translating those elephant forms of TP53 into human cancer prevention and treatment. In general, we may pursue innovations in cancer prevention through reinforcing cellular cooperation or enhancing multicellular mechanisms of cheater detection and control.
6. The unexpected societal benefits of cheating
Cheating often spurs on further innovation in human societies and technological systems as well. Disruption to systems sometimes facilitates reorganization and restructuring that improves the overall functioning of the system. For example, in computer security, some strain on the system in the form of small breaches can help test for, identify and lead to fixes that ultimately reduce vulnerabilities. In fact, the knowledge acquired from being hacked is so valuable that it is actively promoted in some areas of computer security research, where ‘honeypots’ are created to attract hackers and discover vulnerabilities of the computer systems so that they can be redesigned more effectively [48]. The same may be the case for the evolution of cancer suppression mechanisms, where the vulnerability of organisms to cancer has provided selective pressures for cheater suppression mechanisms. For example, exposure to solar radiation exposes vulnerabilities of organisms to cancer and selects for cancer suppression mechanisms such as the DNA damage response. Without this selective pressure that came from solar radiation during the evolution of life, organisms would probably be much more susceptible to cancer because their defense against cancer would not be as good. Thus, some testing and disruption of system function can sometimes be a good thing for systems that are actively growing and innovating because they may be less likely to retain vulnerabilities in their infrastructure as they get larger and more complex.
More generally, innovations for cheating are not always a bad thing for the systems in which they happen. Sometimes disruptive innovations can actually create novel and even more effective or fit systems through enhancing viability, efficiency or in the case of multicellular bodies, fertility and attractiveness. Phenomena that look like cheating initially can sometimes give rise to important new phenotypes. Major phenotypic effects can result from small mutations in genes that regulate cell division, resource allocation and other genes involved in cellular cooperation [37]. Larger body size and the evolution of ornaments are some of the potential innovations that can happen when mutations occur in these genes. Like large body size, discussed earlier, sexual ornamentation is also arguably an innovation. The dramatic colouration and appendages can radically improve mating success, driven by a positive feedback mechanism of sexual selection [49]. Depending on the environment in which organisms find themselves, innovative traits like these may be a net benefit (e.g. because of enhanced reproductive opportunities) or a net cost (because of greater susceptibility to cancer or other diseases) [24]. This means that some phenomena that may initially look like cheating in the foundations of multicellularity may actually represent important innovations that can contribute to the reproductive success of the organism. This may also be the case in human groups, when phenomena that look like cheating within may end up being beneficial for the society in which they occur. There may be some interesting parallels between innovations in biology and some innovations that occur in scientific and political revolutions [50], when individuals challenge outdated or problematic rule systems and succeed in replacing them with new rules that better serve the society of which they are a part.
7. Pathogens evolve innovations that enhance their resilience at our expense
Pathogens evolve innovations to evade host defenses and therapeutic interventions. Influenza viruses regularly evolve new envelop proteins that evade both our prior immunity and vaccinations, opening up new ecological space for themselves [51]. Pathogens often evolve and release novel enzymes that digest our drugs before they can act on the microbes [52,53]. These therapeutic resistance innovations rapidly spread through microbial populations to the point that entire classes of pharmaceuticals become effectively useless [52].
Microbes cooperate to spread these resistance innovations horizontally through microbial populations [54,55]. The mechanisms underlying the dissemination of these drug resistance innovations are diverse, including horizontal gene transfer through uptake of naked DNA (transformation), bacteriophage-mediated transfer (transduction), pili-mediated plasmid transfer (conjugation) and transfer of phage-like particles known as gene transfer agents (as reviewed in [55,56]). Microbes appear to have a wide range of mechanisms for sharing innovations in drug resistance, including even the production of nanotubes that enable sharing of molecules that confer temporary resistance to drugs without requiring integration and expression of new genes [57]. Many of these mechanisms involve the costly production and transmission of DNA and/or molecules, suggesting that these mechanisms are not simply a result of cells extracting resources from one another. Instead, these mechanisms may themselves be novel innovations for sharing among microbes.
8. Multicellular bodies must innovate to dynamically respond to pathogens
The rapid evolution and innovation of microbes poses a challenge for the more slowly evolving multicellular hosts. All else being equal, we are caught on the losing side of a lopsided evolutionary arms race. A key organismal innovation in the history of that arms race with microbes was the evolution of the adaptive immune system. The adaptive immune system uses cell-level evolution, with recombination and hypermutation, as a mechanism of innovation to generate and refine detectors that can respond to pathogens that an organism has never seen before in its lifetime (or even in its evolutionary history). Furthermore, it does this on the same time scale as the evolution of the pathogens. In this way, multicellular organisms harnessed the power of evolution to innovate by keeping a population of cells (in the adaptive immune systems) actively evolving within it.
The innovation of the adaptive immune system does not come without costs, as anybody with an autoimmune condition can testify. Autoimmune disease and organ transplantation rejection occur when the immune system's innovative capacities accidentally get turned against the host. This means that successful organ transplant requires the suppression or deception of the innovations in the adaptive immune system that have evolved to destroy foreign cells. Autoimmune disease can be understood as false-positive signal processing errors [58]. The adaptive immune system is, in part, a signal processor, evolved to distinguish disease agents from benign cells. This is difficult, because pathogens are constantly innovating ways to evade the immune system and appear to be benign cells. An autoimmune reaction is thus the false identification of normal tissue as disease agents. It is the price we pay for a system that can handle most of the diversity of pathogens in our environments.
9. Multicellular bodies use innovations from microbial partners
Our immune systems are not just designed to prevent pathogen growth, but they also have an ancient and important role in supporting microbes that provide host benefits [59]. From the beginning, multicellular organisms have evolved, covered inside and out, with microbes. Some of the interactions between microbes and host cells involve cooperation, other interactions involve competition, sometimes with the very same microbes depending on their expression of virulence factors [60]. These microbes are involved in diverse ecological interactions with other microbes in and on us as well, cooperating with some and killing others. Some microbes benefit from their host's health, being supplied with food and shelter by their host. In this case, the microbes are under selective pressure to benefit their hosts, which can lead to the microbes providing nutritional benefits and protection from pathogens [60].
Close interactions with microbes have allowed us to use many of their innovations for our benefit. For example, many microbes allow us to extract nutritional value out of foods that we could not otherwise digest, expanding our ecological range. For example, Firmicutes, primarily Lachnospiraceae and Ruminococcaceae, partially digest complex carbohydrates (fibre), making sugars available to us that we could not access [61]. This fibre digestion capacity represents a microbial innovation that our bodies use to our benefit [60]. Microbes provide other nutritional benefits as well, including supplying vitamins and other limiting nutrients to their hosts [62,63].
Not only do we get nutritional benefits through using microbial innovations, we may get important immune benefits as well. Our microbiomes can contribute important pathogen defense functions, acting as a sort of auxiliary immune system. Microbes can prevent the invasion of some pathogens, either indirectly, by filling ecological niches, or directly, by killing pathogens through the release of antimicrobial molecules or viruses [64,65]. Most of our antibiotics have been derived from microbial products that evolved to kill other microbes [66]. Microbes can take up ecological niches in the mucins at the interface of our gut lining, preventing pathogenic microbes from invading our bodies [67,68]. Microbial occupation of ecological niches is an important contributor to infant nutrition and health as well. Breast milk contains oligosaccharides that cannot be digested by the infant without microbial assistance. The microbes that can process these oligosaccharides contribute to infant health by helping to preventing invasion of the intestinal lining by pathogenic microbes [69]. Most of our antibiotics have been derived from microbial products that evolved to kill other microbes [66]. Together, these facts suggest that multicellular organisms may be using the innovations of benign microbes to protect us from pathogenic microbes.
Future innovations in human medicine may be based on harnessing microbial innovation. Stable microbial communities have evolved mechanisms to exclude invaders [70]. We may be able to cultivate more invasion-resistant microbiomes that have the capacity to dynamically respond to new threats from pathogens [71–73]. Such microbial ecosystems have the advantage that they can continually evolve innovations for repelling or destroying pathogens. This may explain why multispecies ecological systems are an important part of many human societies and cultures in the form of fermented foods and beverages. As discussed earlier in this paper, the fermented tea known as kombucha is a symbiotic community of yeast and bacteria that engages in many cooperative interactions including resource sharing and defense against invading microbes [19,74]. Preliminary data collection in our laboratories suggests that actively evolving multi-species ecosystems like kombucha may have the capacity to combat human pathogens [75].
10. Socially managing risk opens new frontiers
In the North, a loner doesn't survive.
Comment from a Koryak man [76].
Some environments are harsher than others. Risks such as an uncertain resource supply, potentially hazardous weather and predators are just some of the challenges that individuals face when they venture into new environments. How has life solved the problem of surviving and thriving in risky, difficult and uncertain environments? One way of dealing with risks like these is by teaming up with others to more effectively deal with the challenges and buffer individuals from risk. Before multicellularity evolved, every cell was a loner. One of the important innovations of multicellularity was enabling cells to better manage risks in the environment. Cells in multicellular bodies invest in an infrastructure that buffers them from the variability of the external environment. This involves a variety of sub-strategies including becoming larger than predators (risk reduction), stockpiling resources in the extracellular environment (risk retention) and creating resource delivery systems to send resources to cells that need them (risk transfer). Multicellularity is just one example of social risk management. Social insects also create structures to store resources and bacteria can produce biofilms that help protect them from toxins and invading species [77].
Like cellular societies, humans also must manage risks and we engage in complex risk management behaviours, many of which are highly social. For example, cooperative building of shelters, collective efforts towards pest elimination and even arrangements for resource sharing in case individuals encounter unexpected negative events occurs across human societies [14,77,78]. Widespread sharing of resources in particularly harsh environments is a pattern seen across many small-scale societies [77], including the Koryak who live in very cold conditions in far-east Russia [76] (quoted at the beginning of this section).
These social risk management strategies are important cooperative innovations, but as with other cooperative innovations they can open up the potential for cheating. Indeed, many societies have implicit and explicit rules that help prevent cheating in these social risk management strategies including reputational mechanisms and strong cultural norms of behaviour that help enforce behaviours that provide risk management benefits to the group [77,78].
There are some interesting parallels between the risk management strategies employed by human societies and those used in cellular societies. For example, human shelter building is similar to multicellular bodies creating an external protective cell layer or microbes producing biofilms. Resource storage and group defense are other strategies used both by human societies and cellular societies that help to buffer risk from environmental volatility and invasion, respectively. In human societies, engineered solutions to our problems produce a form of guided variation. That guided variation and cultural transmission of innovations lead to similar dynamics as natural selection in cellular societies, but with biased exploration of possibilities and biased transmission [79–81].
Humans have novel innovations for transmitting information that allow us to share many things, including strategies for dealing with risky and uncertain environments [82]. Generally speaking, mechanisms of inheritance allow the transmission of new innovations from one individual to another. In evolutionary theory, this includes mechanisms of genetic inheritance in sexual and asexual reproduction as well as mechanisms of horizontal gene transfer, common in bacteria [55]. But in human groups, transmission mechanisms for innovations include oral traditions, the written word/printing press and modern information sharing technologies including the Internet. The ability to share information and technologies—our innovations for sharing innovations—may have played an important role in our ability to colonize environments around the globe, some of which would have been otherwise uninhabitable [15,81].
This capacity to share information may make humans uniquely well suited for responding flexibly to challenges. We have the ability to learn from others who may have previously encountered similar risks, and even to anticipate future risks and innovate new risk-management strategies [82]. This capacity to dynamically manage risks and innovate may have been an important contributor to humans colonizing much of the Earth including many harsh and marginal environments [43,48] and might, therefore, also be important to our capacity to manage future challenges through innovation.
11. Conclusion
Innovations in cooperation and cheating have shaped cellular societies. The evolution of multicellularity is essentially the coming together of five forms of cellular cooperation: inhibition of proliferation, controlled cell death, division of labour, resource allocation and creation/maintenance of the extracellular environment. These forms of cooperation, when brought together, enable major innovation in the forms that life can take: allowing for the diversity of multicellular life forms we see today. Multicellularity is threatened when cells within the body cheat on these foundations of multicellular cooperation, innovating through mutation and other genetic changes that can lead to cancer. Multicellular bodies are also threatened by pathogens that can exploit the cooperative interactions occurring within multicellular bodies. In order to counter this threat, many organisms (us included) have evolved an adaptive immune system that can respond rapidly to microbial innovation. We also leverage relationships with rapidly evolving microbial ecosystems that can provide benefits for us, for example, protecting us from pathogens and helping us extract nutrients from the food that we eat.
Examining how cellular societies such as multicellular bodies and the microbiome have leveraged innovations leads to several insights that may apply to human societies as well. First, working from evolutionary theory, we can see that innovation is more likely to arise and be disseminated in large and diverse communities where new variants can arise and increase in frequency (as with the process of natural selection itself). Second, cultivating cooperation with partners who can rapidly and effectively innovate is important for large entities like multicellular bodies that evolve more slowly than the entities that threaten them (e.g. cancer cells and pathogens). And third, managing risk socially (as multicellular bodies do) can facilitate survival in challenging environments where individuals could not survive on their own, making exploration of frontiers more viable than is possible when individuals strike out on their own. These insights can inform new innovations in human health and medicine, including the translation of cheater detection and control mechanisms from other systems (e.g. translating metformin from diabetes to cancer) or even from other organisms (e.g. elephant TP53 for human cancer prevention). By understanding innovations in cooperation and cheating that occur in cellular societies, we can more effectively promote and protect the cellular cooperation that makes us viable multicellular organisms.
Acknowledgements
We thank the Cancer Evolution working group at the Institute for Advanced Study, Berlin, Wissenschaftskolleg for many interesting discussions that led to the genesis of some ideas that appear in this paper. We thank the Cooperation and Conflict Lab at ASU, The Evolution and Cancer Lab at ASU, the members of the Human Generosity Project and the ASU Cooperation and Cheating Symposium participants, especially Lee Cronk. We thank Cristina Baciu for her assistance with the final preparation of this manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
A.A. and C.C.M conceptualized, wrote and edited the manuscript equally.
Competing interests
We declare we have no competing interests.
Funding
This work was supported in part by NIH grants P01 CA91955, R01 CA149566, R01 CA170595, R01 CA185138 and R01 CA140657 as well as CDMRP Breast Cancer Research Program Award BC132057 and John Templeton Foundation (Generous by Nature: The Human Generosity Project).
References
- 1.Szathmáry E, Smith JM. 1995. The major evolutionary transitions. Nature 374, 227–232. ( 10.1038/374227a0) [DOI] [PubMed] [Google Scholar]
- 2.Schirrmeister BE, Antonelli A, Bagheri HC. 2011. The origin of multicellularity in cyanobacteria. BMC Evol. Biol. 11, 117 ( 10.1186/1471-2148-11-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knoll AH. 2011. The multiple origins of complex multicellularity. Annu. Rev. Earth Planet. Sci. 39, 217–239. ( 10.1146/annurev.earth.031208.100209) [DOI] [Google Scholar]
- 4.Aktipis CA, Boddy AM, Jansen G, Hibner U, Hochberg ME, Maley CC, Wilkinson GS. 2015. Cancer across the tree of life: cooperation and cheating in multicellularity. Phil. Trans. R. Soc. B 370, 20140219 ( 10.1098/rstb.2014.0219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aktipis C, Nesse RM. 2013. Evolutionary foundations for cancer biology. Evol. Appl. 6, 144–159. ( 10.1111/eva.12034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michod RE. 2000. Darwinian dynamics: evolutionary transitions in fitness and individuality. Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Darwin C. 1968. [1859] On the origin of species by means of natural selection. London, UK: John Murray.
- 8.Strassmann JE, Queller DC. 2010. The social organism: congresses, parties, and committees. Evolution 64, 605–616. ( 10.1111/j.1558-5646.2009.00929.x) [DOI] [PubMed] [Google Scholar]
- 9.Michod RE, Roze D. 2001. Cooperation and conflict in the evolution of multicellularity. Heredity 86, 1–7. ( 10.1046/j.1365-2540.2001.00808.x) [DOI] [PubMed] [Google Scholar]
- 10.Ratcliff WC, Herron M, Conlin PL, Libby E. 2017. Nascent life cycles and the emergence of higher-level individuality. Phil. Trans. R. Soc. B 372, 20160420 ( 10.1098/rstb.2016.0420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hochberg ME, Marquet PA, Boyd R, Wagner A. 2017. Innovation: an emerging focus from cells to societies. Phil. Trans. R. Soc. B 372, 20160414 ( 10.1098/rstb.2016.0414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nedelcu AM. 2009. Environmentally induced responses co-opted for reproductive altruism. Biol. Lett. 5, 805–808. ( 10.1098/rsbl.2009.0334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durand PM, Sym S, Michod RE. 2016. Programmed cell death and complexity in microbial systems. Curr. Biol. 26, R587–R593. ( 10.1016/j.cub.2016.05.057) [DOI] [PubMed] [Google Scholar]
- 14.Fogarty L, Creanza N. 2017. The niche construction of cultural complexity: interactions between innovations, population size and the environment. Phil. Trans. R. Soc. B 372, 20160428 ( 10.1098/rstb.2016.0428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberger VP, Quiñinao C, Marquet PA. 2017. Innovation and the growth of human population. Phil. Trans. R. Soc. B 372, 20160415 ( 10.1098/rstb.2016.0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawkins R. 2016. The selfish gene. Oxford, UK: Oxford University Press. [Google Scholar]
- 17.Buchanan M, et al. 2015. The evolution of everything: how new ideas emerge. Nature 526, 36–37. ( 10.1038/526036a) [DOI] [Google Scholar]
- 18.Boyd R, Richerson PJ. 1988. Culture and the evolutionary process. Chicago, IL: University of Chicago Press. [Google Scholar]
- 19.Marsh AJ, O'Sullivan O, Hill C, Ross RP, Cotter PD. 2014. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 38, 171–178. ( 10.1016/j.fm.2013.09.003) [DOI] [PubMed] [Google Scholar]
- 20.King KC, Lively CM. 2012. Does genetic diversity limit disease spread in natural host populations? Heredity 109, 199–203. ( 10.1038/hdy.2012.33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michod RE, Viossat Y, Solari CA, Hurand M, Nedelcu AM. 2006. Life-history evolution and the origin of multicellularity. J. Theor. Biol. 239, 257–272. ( 10.1016/j.jtbi.2005.08.043) [DOI] [PubMed] [Google Scholar]
- 22.Grosberg RK, Strathmann RR. 2007. The evolution of multicellularity: a minor major transition? Annu. Rev. Ecol. Evol. Syst. 38, 621–654. ( 10.1146/annurev.ecolsys.36.102403.114735) [DOI] [Google Scholar]
- 23.Pancer Z, Cooper MD. 2006. The evolution of adaptive immunity. Annu. Rev. Immunol. 24, 497–518. ( 10.1146/annurev.immunol.24.021605.090542) [DOI] [PubMed] [Google Scholar]
- 24.Boddy AM, Kokko H, Breden F, Wilkinson GS, Aktipis CA. 2015. Cancer susceptibility and reproductive trade-offs: a model of the evolution of cancer defences. Phil. Trans. R. Soc. B 370, 20140220 ( 10.1098/rstb.2014.0220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher RM, Cornwallis CK, West SA. 2013. Group formation, relatedness, and the evolution of multicellularity. Curr. Biol. 23, 1120–1125. ( 10.1016/j.cub.2013.05.004) [DOI] [PubMed] [Google Scholar]
- 26.Aktipis CA. 2011. Is cooperation viable in mobile organisms? Simple walk away rule favors the evolution of cooperation in groups. Evol. Hum. Behav. 32, 263–276. ( 10.1016/j.evolhumbehav.2011.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aktipis CA. 2004. Know when to walk away: contingent movement and the evolution of cooperation. J. Theor. Biol. 231, 249–260. ( 10.1016/j.jtbi.2004.06.020) [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 29.Lugo TG, Pendergast A-M, Muller AJ, Witte ON. 1990. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science 247, 1079–1082. ( 10.1126/science.2408149) [DOI] [PubMed] [Google Scholar]
- 30.Bertoli C, Skotheim JM, De Bruin RA. 2013. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 14, 518 ( 10.1038/nrm3629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert J. 2015. Textbook of cell signalling in cancer: an educational approach. Berlin, Germany: Springer. [Google Scholar]
- 32.Werness BA, Levine AJ, Howley PM. 1990. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248, 76–79. ( 10.1126/science.2157286) [DOI] [PubMed] [Google Scholar]
- 33.Greaves M, Maley CC. 2012. Clonal evolution in cancer. Nature 481, 306 ( 10.1038/nature10762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fortunato A, Boddy A, Mallo D, Aktipis A, Maley CC, Pepper JW. 2017. Natural selection in cancer biology: from molecular snowflakes to trait hallmarks. Cold Spring Harb. Perspect. Med. 7, a029652 ( 10.1101/cshperspect.a029652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zack TI, et al. 2013. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 45, 1134–1140. ( 10.1038/ng.2760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner KM, et al. 2017. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature 543, 122–125. ( 10.1038/nature21356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Croce CM. 2008. Oncogenes and cancer. N. Engl. J. Med. 358, 502–511. ( 10.1056/NEJMra072367) [DOI] [PubMed] [Google Scholar]
- 38.Marusyk A, Tabassum DP, Altrock PM, Almendro V, Michor F, Polyak K. 2014. Non-cell autonomous tumor-growth driving supports sub-clonal heterogeneity. Nature 514, 54 ( 10.1038/nature13556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman A, del Ama LF, Ferguson J, Kamarashev J, Wellbrock C, Hurlstone A. 2014. Heterogeneous tumor subpopulations cooperate to drive invasion. Cell Rep. 8, 688–695. ( 10.1016/j.celrep.2014.06.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprouffske K, Aktipis CA, Radich JP, Carroll M, Nedelcu AM, Maley CC. 2013. An evolutionary explanation for the presence of cancer nonstem cells in neoplasms. Evol. Appl. 6, 92–101. ( 10.1111/eva.12030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taniguchi CM, Emanuelli B, Kahn CR. 2006. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96. ( 10.1038/nrm1837) [DOI] [PubMed] [Google Scholar]
- 42.Quinn BJ, Kitagawa H, Memmott RM, Gills JJ, Dennis PA. 2013. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol. Metab. 24, 469–480. ( 10.1016/j.tem.2013.05.004) [DOI] [PubMed] [Google Scholar]
- 43.Crespi B, Semeniuk C. 2004. Parent-offspring conflict in the evolution of vertebrate reproductive mode. Am. Nat. 163, 635–653. ( 10.1086/382734) [DOI] [PubMed] [Google Scholar]
- 44.Domazet-Lošo T, Tautz D. 2010. Phylostratigraphic tracking of cancer genes suggests a link to the emergence of multicellularity in metazoa. BMC Biol. 8, 66 ( 10.1186/1741-7007-8-66) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abegglen LM, et al. 2015. Potential mechanisms for cancer resistance in elephants and comparative cellular response to DNA damage in humans. JAMA 314, 1850–1860. ( 10.1001/jama.2015.13134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sulak M, et al. 2016. Correction: TP53 copy number expansion is associated with the evolution of increased body size and an enhanced DNA damage response in elephants. eLife 5, e24307 ( 10.7554/eLife.24307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farnebo M, Bykov VJ, Wiman KG. 2010. The p53 tumor suppressor: a master regulator of diverse cellular processes and therapeutic target in cancer. Biochem. Biophys. Res. Commun. 396, 85–89. ( 10.1016/j.bbrc.2010.02.152) [DOI] [PubMed] [Google Scholar]
- 48.Spitzner L. 2003. Honeypots: tracking hackers. Reading, MA: Addison-Wesley. [Google Scholar]
- 49.West-Eberhard MJ. 2014. Darwin's forgotten idea: the social essence of sexual selection. Neurosci. Biobehav. Rev. 46, 501–508. ( 10.1016/j.neubiorev.2014.06.015) [DOI] [PubMed] [Google Scholar]
- 50.Kuhn TS, Hawkins D. 1963. The structure of scientific revolutions. Am. J. Phys. 31, 554–555. ( 10.1119/1.1969660) [DOI] [Google Scholar]
- 51.Ferguson NM, Galvani AP, Bush RM. 2003. Ecological and immunological determinants of influenza evolution. Nature 422, 428 ( 10.1038/nature01509) [DOI] [PubMed] [Google Scholar]
- 52.Hyde JE. 2005. Drug-resistant malaria. Trends Parasitol. 21, 494–498. ( 10.1016/j.pt.2005.08.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arias CA, Murray BE. 2009. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N. Engl. J. Med. 360, 439–443. ( 10.1056/NEJMp0804651) [DOI] [PubMed] [Google Scholar]
- 54.Stokes HW, Gillings MR. 2011. Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into Gram-negative pathogens. FEMS Microbiol. Rev. 35, 790–819. ( 10.1111/j.1574-6976.2011.00273.x) [DOI] [PubMed] [Google Scholar]
- 55.Hall JPJ, Brockhurst MA, Harrison E. 2017. Sampling the mobile gene pool: innovation via horizontal gene transfer in bacteria. Phil. Trans. R. Soc. B 372, 20160424 ( 10.1098/rstb.2016.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jansen G, Aktipis CA. 2014. Resistance is mobile: the accelerating evolution of mobile genetic elements encoding resistance. J. Evol. Med. 2, 1–3. ( 10.4303/jem/235873) [DOI] [Google Scholar]
- 57.Dubey GP, Ben-Yehuda S. 2011. Intercellular nanotubes mediate bacterial communication. Cell 144, 590–600. ( 10.1016/j.cell.2011.01.015) [DOI] [PubMed] [Google Scholar]
- 58.Germain RN. 2001. The art of the probable: system control in the adaptive immune system. Science 293, 240–245. ( 10.1126/science.1062946) [DOI] [PubMed] [Google Scholar]
- 59.Dishaw LJ, Cannon JP, Litman GW, Parker W. 2014. Immune-directed support of rich microbial communities in the gut has ancient roots. Dev. Comp. Immunol. 47, 36–51. ( 10.1016/j.dci.2014.06.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wasielewski H, Alcock J, Aktipis A. 2016. Resource conflict and cooperation between human host and gut microbiota: implications for nutrition and health. Ann. NY Acad. Sci. 1372, 20–28. ( 10.1111/nyas.13118) [DOI] [PubMed] [Google Scholar]
- 61.Biddle A, Stewart L, Blanchard J, Leschine S. 2013. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 5, 627–640. ( 10.3390/d5030627) [DOI] [Google Scholar]
- 62.LeBlanc JG, Milani C, de Giori GS, Sesma F, Van Sinderen D, Ventura M. 2013. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 24, 160–168. ( 10.1016/j.copbio.2012.08.005) [DOI] [PubMed] [Google Scholar]
- 63.McNeil NI. 1984. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 39, 338–342. [DOI] [PubMed] [Google Scholar]
- 64.Roossinck MJ. 2011. The good viruses: viral mutualistic symbioses. Nat. Rev. Microbiol. 9, 99 ( 10.1038/nrmicro2491) [DOI] [PubMed] [Google Scholar]
- 65.Breinig F, Sendzik T, Eisfeld K, Schmitt MJ. 2006. Dissecting toxin immunity in virus-infected killer yeast uncovers an intrinsic strategy of self-protection. Proc. Natl Acad. Sci. USA 103, 3810–3815. ( 10.1073/pnas.0510070103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davies J, Davies D. 2010. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 74, 417–433. ( 10.1128/MMBR.00016-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. 2012. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int. J. Food Microbiol. 152, 189–205. ( 10.1016/j.ijfoodmicro.2011.05.025) [DOI] [PubMed] [Google Scholar]
- 68.Sonnenburg JL, Angenent LT, Gordon JI. 2004. Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nat. Immunol. 5, 569 ( 10.1038/ni1079) [DOI] [PubMed] [Google Scholar]
- 69.Hinde K, Lewis ZT. 2015. Mother's littlest helpers. Science 348, 1427–1428. ( 10.1126/science.aac7436) [DOI] [PubMed] [Google Scholar]
- 70.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. 2009. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697. ( 10.1126/science.1177486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dillon RJ, Vennard CT, Buckling A, Charnley AK. 2005. Diversity of locust gut bacteria protects against pathogen invasion. Ecol. Lett. 8, 1291–1298. ( 10.1111/j.1461-0248.2005.00828.x) [DOI] [Google Scholar]
- 72.Guarner F, Malagelada J-R. 2003. Gut flora in health and disease. Lancet 361, 512–519. ( 10.1016/S0140-6736(03)12489-0) [DOI] [PubMed] [Google Scholar]
- 73.Robinson CJ, Bohannan BJ, Young VB. 2010. From structure to function: the ecology of host-associated microbial communities. Microbiol. Mol. Biol. Rev. 74, 453–476. ( 10.1128/MMBR.00014-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.May AN, Medina J, Alcock J, Maley C, Aktipis A. 2017. Kombucha as a model system for multispecies microbial cooperation: theoretical promise, methodological challenges and new solutions ‘in solution’. Presented at the Am. Soc. Microbiol. Conf. on Mechansims of Bacterial Cooperation and Competition, Washington, DC, 26 September. Retrieved from osf.io/qn8dg. [Google Scholar]
- 75.May AN, Alcock J, Maley C, Varsani A, Aktipis A. 2017. Can multispecies ecological systems resist invasion by human pathogens? Developing a model system of multispecies cooperation and group defense. Presented at Dept Defense Microbiome Symp., Colorado Springs, CO, 26 September. Retrieved from osf.io/jaz36. [Google Scholar]
- 76.Gerkey D, et al. 2013. Cooperation in context: public goods games and post-Soviet collectives in Kamchatka, Russia. Curr. Anthropol. 54, 144–176. ( 10.1086/669856) [DOI] [Google Scholar]
- 77.Cronk L, et al. In press. Managing risk through cooperation: need-based transfers and risk pooling among the societies of the Human Generosity Project. In Global perspectives on long-term community resource management (eds LR Lozny and TH McGovern). Berlin, Germany: Springer.
- 78.Wiessner PW. 1977. Hxaro: a regional system of reciprocity for reducing risk among the Kung San. Ann Arbor, MI: University Microfilms International.
- 79.Mesoudi A. 2011. Cultural evolution: how Darwinian theory can explain human culture and synthesize the social sciences. Chicago, IL: University of Chicago Press. [Google Scholar]
- 80.Kronfeldner ME. 2007. Is cultural evolution Lamarckian? Biol. Phil. 22, 493–512. ( 10.1007/s10539-006-9037-7) [DOI] [Google Scholar]
- 81.DeVore I, Tooby J. 1987. The reconstruction of hominid behavioral evolution through strategic modeling. In The Evolution of human behavior: primate models (ed. Kinzey WG.), pp. 183–237. Albany, NY: University of New York Press. [Google Scholar]
- 82.Hoppitt W. 2017. The conceptual foundations of network-based diffusion analysis: choosing networks and interpreting results. Phil. Trans. R. Soc. B 372, 20160418 ( 10.1098/rstb.2016.0418) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.


