Abstract
Animal life can be perceived as the selective use of information for maximizing survival and reproduction. All organisms including bacteria and protists rely on genetic networks to build and modulate sophisticated structures and biochemical mechanisms for perceiving information and responding to environmental changes. Animals, however, have gone through a series of innovations that dramatically increased their capacity to acquire, retain and act upon information. Multicellularity was associated with the evolution of the nervous system, which took over many tasks of internal communication and coordination. This paved the way for the evolution of learning, initially based on individual experience and later also via social interactions. The increased importance of social learning also led to the evolution of language in a single lineage. Individuals' ability to dramatically increase performance via learning may have led to an evolutionary cycle of increased lifespan and greater investment in cognitive abilities, as well as in the time necessary for the development and refinement of expertise. We still know little, however, about the evolutionary biology, genetics and neurobiological mechanisms that underlie such expertise and its development.
This article is part of the themed issue ‘Process and pattern in innovations from cells to societies’.
Keywords: cognition, evolution, expertise, innovation, learning, social learning
1. Introduction: the major cognitive innovations
As they interact with their surroundings, animals rely on their cognitive system to make decisions that ultimately determine their survival and reproductive success. Broadly defined, the cognitive system comprises the structures and processes concerned with the acquisition, retention and use of information [1]. Animal cognition can be divided into a few interrelated components. The first essential stage is perception, which involves capturing information from the external environment and translating it into internal representations retained by neurons. Information acquisition is carried out by receptors specialized to capture distinct cue attributes including visual, auditory, olfactory, flavour and physical contact. Some types of receptors are typically located in dedicated organs positioned and structured to enhance information capture. Newly perceived information may either fade quickly, remain for a short duration necessary to perform a given task or consolidate into long-lasting internal representations that can persist anything between days to decades. The process of adding new representations to the internal storage is termed learning, and the information retained is referred to as memory. The sole utility of information acquisition and retention is to maximize survival and reproduction. To this end, individuals have to constantly determine their subsequent action given the known states of relevant environmental features and their experience. Once individuals make a decision, they have to execute the sequence of behaviours constituting a given action [1–3].
While single-cell organisms such as bacteria and protists already possess remarkable information-processing abilities, a few major innovations have dramatically increased animals’ capacities to selectively use information for maximizing fitness. This review focuses on these innovations, which include the nervous system, learning, social learning and language. I will begin with an analysis of information processing by genetic networks because they occur in all organisms and constitute the most fundamental organic system for the acquisition and use of information. I will then discuss, in turn, the added features enabled by each successive innovation as well as its ecological and evolutionary impacts. I will argue that the major cognitive innovations have fuelled an evolutionary cycle of greater investment per individual, which, in turn, selected for a larger allocation of resources and time for the development of enhanced cognitive abilities. This, in turn, empowered individuals to devote long time periods to acquiring complex skills. The evolutionary trend of increased individual expertise opened up novel niches requiring and selecting for greater animal intelligence.
Most categorizations have an arbitrary element, meaning that one can argue that there is room for including additional innovations or perhaps excluding ones that I have included. Such feasible future modifications are of course appropriate, especially if the focus remains on enhancing our fundamental knowledge of the evolutionary biology of animal cognition. Similarly, one can debate the very meaning of ‘innovation’. Most notably, there is perhaps an expectation of innovation being a single abrupt modification with dramatic consequences. Such expectation, however, is not consistent with our knowledge of the intricate mechanisms enabling gradual evolutionary change [4]. Hence, biological innovation most likely summarizes a long and complex evolutionary process. Similarly, while we often perceive innovation in modern humans as a sudden event typically attributed to a single person, extensive research indicates that it is most often a long process involving many individuals and spanning many years or even decades [5,6]. Finally, space limitation compels me to focus on conceptual essentials while leaving out many details of the enlightening key sources I cite.
2. Information processing by genetic networks
There are two primary means by which genetic networks control cognition. First, during development and throughout life, they assemble and maintain all the structures that handle information. Second, the modulation of gene action can lead to changes in the way organisms perceive, process and respond to information. Through these mechanisms, unicellular organisms such as bacteria continuously monitor the environment and adaptively respond by altering behaviour, physiology, development and virulence. Here, I will briefly review information processing by genetic networks. My goal is to establish a clear baseline that helps us understand the significance of subsequent cognitive innovations.
In the extensively studied bacterium Escherichia coli, genetic networks orchestrate information reception and processing, and subsequent action through production of the complex molecular machinery of the cell membrane, cytoplasm and flagella. Bacteria can gain from moving towards higher food concentration and away from harmful substances. While receptors at the cell surface can detect several relevant molecules, simultaneous comparison of gradients is impossible owing to the small bacterium size. Instead, the bacterium relies on a sequential comparison. For example, when the glucose concentration increases over time, the time-averaged occupancy of the binding site of the glucose receptors increases and they send a signal to the flagella. This causes the flagella to raise their rotational bias in a counterclockwise direction, which results in directional movement towards higher glucose concentration [7–10]. Furthermore, genetic modulation allows E. coli to switch diet. When glucose, E. coli's preferred sugar, is not available but lactose is present, lactose detectors turn on alternative genes that enable lactose metabolism [11,12].
At the other end of organismal complexity, genetic networks orchestrate the adaptive immune system in vertebrates. Two remarkable features of this system are that it can detect novel pathogens and retain a memory of the microbes it has encountered [13–15]. The immunological memory allows an individual's immune system to respond more rapidly and more effectively to a pathogen that it has encountered previously [16].
The above examples illustrate that, through the construction of a complex cellular machinery and alterations in gene expression, genetic networks enable fast and effective responses to a multitude of environmental challenges and changes. The examples, however, implicitly demonstrate what genetic networks may not readily achieve. To respond to a certain nutrient, a bacterium must possess the receptor for that specific nutrient. To adjust to change in the availability of that nutrient, the bacterium has to have the built-in genes and mechanism for their adaptive activation. Even in the exceptional case of vertebrate adaptive immunity, the system can handle a very narrow range of targets and responses. By contrast, the evolution of the nervous system, which is discussed in the next section, has allowed many animals to exploit and respond to limitless resources and environmental features. While very simple nervous systems are primarily determined by genetic networks, more complex ones increasingly rely on experience and thus generate individuals with somewhat unique cognitive abilities and even expertise. I will elaborate on these issues in §§4–6.
3. The first innovation: the nervous system
The evolution of the nervous system is such a significant innovation because it has led to the establishment of the only other major biological information system besides genetic networks. Initially, nervous systems took over the tasks of communication, coordination and modulation already present in unicellular organisms. Further evolution, however, has allowed the basic neuronal architecture to evolve the unique abilities for the acquisition and long-term retention of vast amounts of information (figure 1). These capacities will be discussed in §4. In the subsections below, I will assess the new features facilitated by neurons as a specialized novel system for the transmission, coordination and modulation of information.
Figure 1.
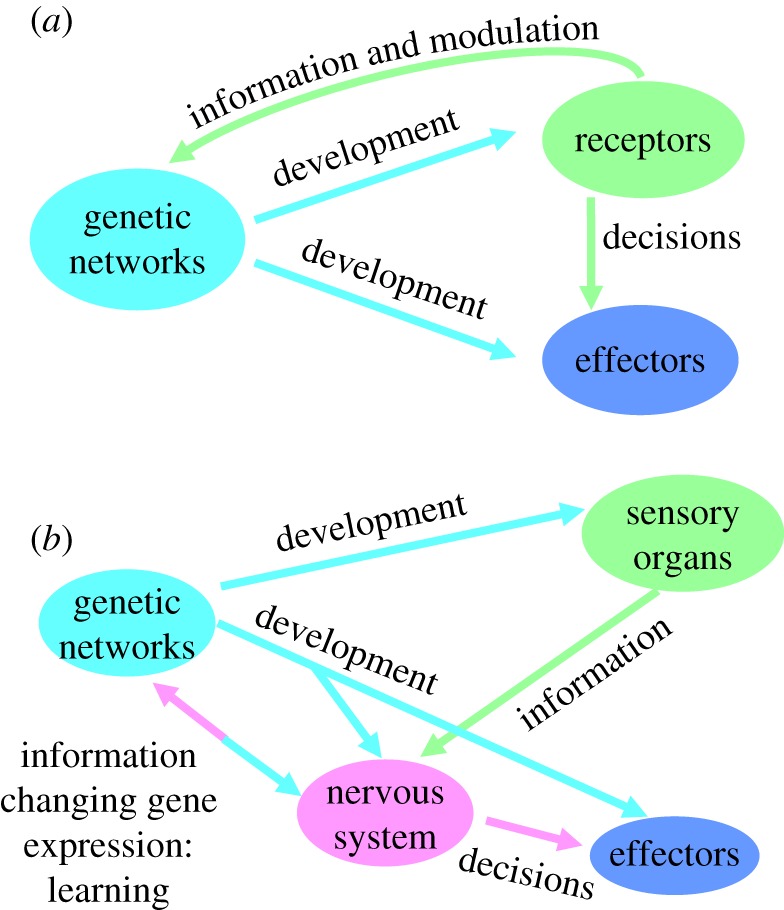
(a) All organisms including bacteria and protists rely on genetic networks to orchestrate reception and processing of information and responses to environmental changes. (b) The first major cognitive innovation involved the evolution of the nervous system. This set the stage for the subsequent major cognitive innovations: learning and memory, social learning and language. In animals possessing more of these innovations, individuals have greater abilities to acquire expertise over long periods of practice. (Online version in colour.)
(a). Communication
The evolution of multicellularity opened up ample opportunities for cell specialization. This allowed the division of the three fundamental cognitive tasks of information acquisition, signal transmission and action among dedicated cells. Neurons, which took over the task of transmitting information from receptors to effectors, co-opted many of the genes and mechanisms used by single-cell organisms for communication [17–19]. There were two major advantages for neurons mediating between sensors and effectors such as cilia and muscles. First, when each cell contains both the sensor and the effector, the system efficiency is low given the 1:1 ratio of sensor to effector. This can limit the number of costly sensor types. When neurons mediate between sensors and effectors, one sensor can communicate with many effectors. The high efficiency of such a system can lead to the evolution of many specialized receptors [20,21]. The other advantage of neuronal communication between sensors and effectors is that each cell type can be positioned optimally without constraining the placement of the other type. That is, each sensor type can be placed optimally in the body region where it is most likely to detect specific cues, while effector location can be optimized based on biomechanical principles.
(b). Coordination
In addition to an increased need for inter-cell communication, the evolution of multicellularity also posed novel challenges of integration and coordination among cells. Given their initial role in communication, neurons were naturally preadapted for coordinating action among cells. Such coordination of complex behaviour can already be seen in cnidarians such as hydras and jellyfish, which possess relatively simple, diffused neural nets. Moving can be risky to an individual hydra because it can be swept away by the water current. A hydra solves this challenge by performing an elaborate somersault, which involves bending its body so that its tentacles can reach and attach to the bottom, releasing its foot, swinging its body in the desired direction, reattaching the foot and then releasing the tentacles to regain its typical erect position (see lower right panel of fig. 2 in [22]). The exact neuronal mechanisms orchestrating this somersaulting are still unknown.
Early in the evolution of animals, nervous systems already showed clear centralization, termed central nervous system or brain [23]. In addition to coordinating behaviour based on the integration of sensory information from the periphery, brains carry out multiple organizational tasks including the control of growth and development, and a variety of physiological features such as circadian activity, digestion, metabolism and excretion [24].
(c). Modulation
The output from neural networks can be modulated via the action of a variety of substances including neurotransmitters, neuropeptides and hormones. Such neuromodulators act through two major mechanisms. First, neuromodulators can modify the type, number or kinetic properties of the ion channels in neurons' membranes. This can lead to changes in the excitability of neurons and their response properties. Second, neuromodulators can alter either the amount of transmitters released from presynaptic terminals or the postsynaptic responsiveness [25,26]. A single neuromodulator can affect multiple neurons and the activity of multiple ion channels. Furthermore, a single neuron can respond to multiple modulators. Consequently, neuromodulators can generate enormously complex dynamics by changing the configuration and output of neural networks [27,28]. For example, foraging animals typically alternate between exploration for and exploitation of resources, characterized by movement through the environment in the former and a relatively stationary state in the latter. In the nematode Caenorhabditis elegans, long-lasting exploration and exploitation states are modulated by two opposing neuromodulators, the neuropeptide pigment-dispersing factor (PDF) and serotonin. PDF promotes roaming through PDF receptors, whereas serotonin induces dwelling states through serotonin-gated chloride channels [29]. Neuromodulation is essential for the retention of information in neural networks, which is discussed next.
4. The second innovation: learning and memory
The evolution of learning and associated long-term memory is probably the most important cognitive innovation throughout the evolution of organismal life because it opened up numerous novel ecological and evolutionary opportunities. In the ecological domain, learning allows animals to exploit abundant environmental features that are unique to certain times and places. For example, an individual can learn the spatial features unique to its shelter location. This means that it can invest more in this shelter because it can return to the shelter after exploring for and exploiting resources such as food, and thus occupy the shelter throughout its life. This also means that spatial learning and memory can improve parental care because parents can invest more time and resources in a nest and keep using it while providing their offspring with shelter and food. Furthermore, provisioners such as bees can learn unique features including the spatial location, odour and colour of their preferred flowers, and learn new motor patterns for optimizing the handling of these flowers. Finally, in many animal species, individual recognition allows one to identify parents, neighbours, competitors, potential mates and offspring [30,31].
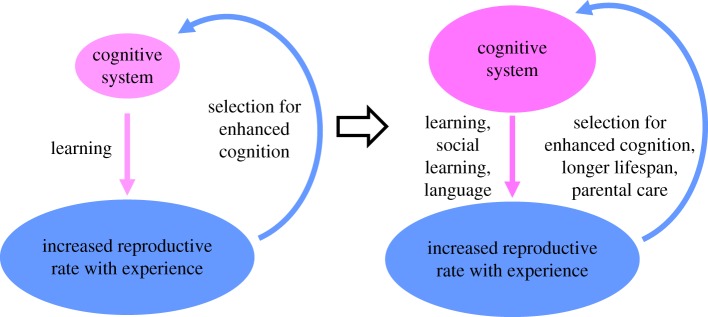
In the evolutionary arena, learning increases the value of the individual. This is because experience can lead to older individuals having much higher reproductive success relative to that of young novices. Strong positive effects of experience on performance or reproductive success have indeed been documented in a broad range of species, including honeybees (Apis mellifera) [32,33], a large variety of birds (e.g. [34,35]), and both human hunter–gatherers [36] and people in developed countries [6,37]. Increased individual value due to experience may have led to an evolutionary cycle of enhanced learning and memory selecting for life histories characterized by longer lifespans. In turn, longer lifespans may have increased the value of further investment in structures that improve learning and memory as well as other cognitive abilities, and so forth. Moreover, the increased value of longer-lived individuals has probably selected for tilting the trade-off between offspring quantity and quality towards the quality end. With greater investment in fewer offspring, parents could allocate more resources and parental care time per offspring. Consequently, young could allocate more physical resources to cognitive structures and more protected time for developing and refining, based on experience, the cognitive mechanisms handling the acquisition, retention and use of information (figure 2). Some of that experience could come via social learning, discussed in §5.
Figure 2.
Learning increases individual value over time because cumulative experience increases the reproductive rate. In some lineages, this may have led to an evolutionary cycle of selection for life histories characterized by longer lifespans, greater resource and time investment in enhanced cognitive abilities, and the evolution of parental care and social learning. (Online version in colour.)
From a mechanistic point, learning and memory rely on the basic organismal tools already well used by bacteria and protists for altering behaviour based on experience. These tools consist of biochemical chains and changes in gene expression. The use of these processes in neurons, however, opened up novel opportunities. Briefly, long-term memory is the culmination of multiple, interactive, dynamic processes that start with the neuronal encoding of new information via the modulation of synaptic properties by neurotransmitters. The initial chemical modulation is followed by a molecular cascade leading to gene expression activated by the cAMP-response element-binding protein (CREB). The products of these genes cause structural and functional changes in selected synapses as well as changes in the intrinsic properties of certain neurons, which affect their subsequent activity [38,39].
Basic learning abilities already exist in animals with simple nervous systems, including the nematode C. elegans, which possesses only about 302 neurons. Caenorhabditis elegans shows associative learning, which allows it to acquire and exploit an association between some novel stimulus and an environmental state affecting its fitness. For example, in an experiment with C. elegans, individuals experienced their favourite food, a suspension of E. coli bacteria with either sodium or chloride ions, and the alternative ions with no food. In the subsequent test, worms showed a strong preference for the ions previously associated with food. Worms in the control groups, which were naive or had experienced either food with no ions or ions with no food, showed no ion preference [40].
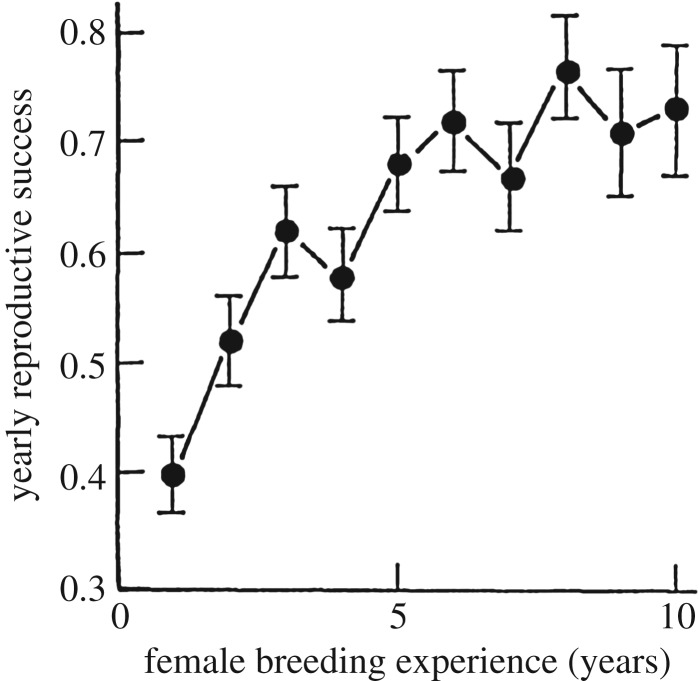
Learning and long-term memory abilities have been documented in most bilateral animals that have been closely examined [41]. The full power of learning, however, is best expressed in long-lived animals. Individuals in such species can acquire complex learned skills over years of practice, ultimately achieving performance dramatically superior to that of inexperienced individuals. Learning improves all stages of information processing within the heavily practised tasks, including selective perceptual improvements, greater abilities to learn new information and retrieve old information from memory, superior decision-making and refined motor skills. While the most detailed data on the development of expertise exist for humans [37,42], it is well known in other species as noted earlier in this section. A general definition of expertise applicable to all species can thus be ‘the characteristics, skills and knowledge allowing individuals with extensive experience to perform significantly better than novices on a given complex task’. For example, in shearwaters (Puffinus tenuirostris), female birds with a single year of breeding experience produce an average of 0.4 young. The birds' reproductive success gradually increases over subsequent breeding seasons, peaking at a yearly average of about 0.7 young after 6 years of experience [34] (figure 3).
Figure 3.
The gradual development of expertise leads to a corresponding increase in performance in a long-lived bird: the average (±s.e.) proportion of eggs that developed into free-flying young as a function of the breeding experience of their short-tailed shearwater mother. Adapted from Wooler et al. [34].
Factors other than expertise can explain some of the increase in the performance with individual age. These factors include higher survival rates of individuals of higher quality, an increase in effort with age, and physical and physiological improvements. Nevertheless, studies that either considered all the variables that can enhance performance with experience or addressed specific features such as foraging performance or anti-predatory behaviour have convincingly shown a dominant role for expertise. For example, the effects of differential survival rates were eliminated in studies that compared the same individuals over time (e.g. [32,43,44]). Effort had a negligible effect on increased performance with age in studies that measured it directly (e.g. [45–47]). And at least one study that carefully measured physiological factors documented peak physiological performance much earlier than the peak in foraging success [48]. Similarly, many studies have documented that young birds require very long periods of time for acquiring complex foraging skills and for reducing mortality rates due to starvation and predation (e.g. [44,49–51]).
Learning can contribute to large improvements in performance even in short-lived species such as honeybees [32,33]. Expertise in short-lived individuals, however, is severely limited by the short time, typically several days, available for the development of cognitive abilities that depend on extensive learning and practice. Intriguingly, two whole-genome duplication events early in the evolution of vertebrates [52] opened up ample opportunities for evolutionary innovations that expanded neuronal complexity and cognitive abilities in that lineage [53]. On the gross anatomical scale, vertebrate innovations include complex brains, neural crest and placodes. Complex brains have specialized forebrain, midbrain and hindbrain regions. The neural crest contributes to a few structures including the sensory ganglia. Sensory placodes contribute to the eye, ear, lateral line and olfactory organs, while neurogenic placodes contribute sensory neurons to cranial ganglia [54].
At the molecular level, the two whole-genome duplications in vertebrates led to gene expansion in many synaptic gene families. Consequently, many fruit fly (Drosophila melanogaster) synaptic genes have up to four orthologues in mice (Mus musculus) [18,55]. At least two innovative experimental studies have attempted to link synaptic gene expansion and its associated increased complexity in synapse signalling mechanisms to cognition and behaviour in mice [56,57]. Given the enormous complexity of the synapse proteome, which consists of over 2000 proteins in mice [18], further experimental work is needed in order to assess the exact contribution of the massive synaptic gene expansion to vertebrate cognition in general and to expertise development in particular.
Another evolutionary consequence of advanced learning and associated cognitive abilities is that they open up opportunities for individuals to adopt novel behaviours. First, learning often involves an early exploratory phase. When numerous individuals of each generation explore, the combined outcome is a massive search, which can lead to the discovery and adoption of novel, fitness-enhancing behaviours. Second, some types of learning lead to each individual changing its behaviour based on its specific experience at a certain place and time. Thus, if a given task (e.g. locating food) has a new optimum in a new environment (e.g. settings with novel foods), an individual may find that optimum via learning. That is, learning is a mechanism that can naturally lead to individuals adopting novel behaviours [58]. If such novel behaviours disappear when individual innovators die, they would probably have negligible evolutionary impact. This is because the novel behaviours are not associated with heritable genetic variation that natural selection can act upon. Novel behaviours, however, can be transmitted between individuals and across generations in species with social learning, which is discussed next.
5. The third innovation: social learning
Social learning, defined as the acquisition of novel information from other individuals [59,60], deserves its place on the cognitive innovations list owing to two unique effects it has had on animal evolution. First, as noted in §4, the evolution of learning probably led to an evolutionary cycle of increased individual value leading to selection on further improvement in learning, memory and other cognitive abilities that contribute to enhanced information capture and utilization. The trouble with individual expertise is that it is lost when that individual dies. Social learning resolves this organic constraint by allowing other individuals and, most importantly, members of subsequent generations, to acquire relevant knowledge from experts. Second, the evolutionary cycle of increased individual value has probably also led to the evolution of parental care. Parental care can merely mean providing food and protection. Such care alone can dramatically increase a young individual's probability of reaching adulthood. With social learning, however, parental care enables young novices to acquire successful skills from their experienced parents. This opens up novel ecological niches that require the use of extensive knowledge and complex abilities that are acquired through prolonged practice. Such niches may even further promote the evolution of cognitive features that enable the development of expertise. Examples include carnivores (e.g. cheetahs, Acinonyx jubatus) and raptors (e.g. sparrowhawks, Accipiter nisus) that feed on highly evasive or large prey [61,62].
We do not know the number of independent evolutionary origins of social learning, but it probably has had multiple beginnings as it is well established in a few classes of vertebrates and at least two insect orders [59,60,63]. There is currently too little information for generalizing about the biological mechanisms that enabled the evolution of social learning (but see [64–67]). It is feasible, however, that reliance on social cues for learning is attainable by many animals with some threshold cognitive machinery. The ecological context for the evolution of social learning was most probably the tendency of individuals in many species to join others. For naive individuals, the presence of conspecifics at a given site might indicate the availability of good resources and safety. Joining others can also enhance feeding and predatory avoidance [68–70]. An individual's tendency to associate with others can readily lead to that individual biasing its resource preference to that chosen by the other group members. A tendency to aggregate opens up further opportunities for taking advantage of the social setting, including the acquisition of relevant information from others.
A basic tendency to socially bias food selection has been documented in fruit flies (D. melanogaster). In addition to their natural attraction to odours of yeast and fermenting fruit [71], which constitute the larval and adult food, fruit flies show long-distance attraction to the pheromone cis-vaccenyl acetate (cVA), which is produced by males and transferred to females during copulation [72]. Consequently, fruit flies typically occur in aggregations in the field and show modest social behaviours [73]. In controlled experiments, Sarin & Dukas [74] found that focal females that experienced novel food together with mated females, who had laid eggs on that food, subsequently showed a stronger preference for laying eggs on that food over another novel food compared with focal females that experienced the food alone. We observed no social learning, however, when observers experienced food with more ambiguous social information provided by the presence of either virgin models or the aggregation pheromone, cVA, alone.
At the other extreme of the social learning spectrum, many vertebrates with extensive parental care provide opportunities for their offspring to learn complex skills. For example, parents in a variety of carnivores and raptors provide wounded prey for young, who can then practise killing the animal [75]. Similarly, adult helpers in meerkats (Suricata suricatta) provide young with live scorpions without their stingers. The young can then safely practise handling that dangerous food [76]. Another type of advanced social learning with a limited taxonomic distribution is imitation, defined as the copying of an action performed by another individual. With a few exceptions, most notably, vocal imitation in birds [77,78], imitation may have had significant effects on behaviour only in great apes (family Hominidae) [79]. By far the most significant case of imitation is language acquisition in human infants.
6. The fourth innovation: language
Language has evolved only once in a single lineage and set its possessors apart from all other animals. Language is acquired via vocal imitation, which is rare among animals in general and absent even in humans' closest extant relatives [80,81]. The evolution of language most probably furthered the evolutionary cycle of enhanced cognitive abilities enabling greater expertise, with the latter selecting for improved cognition. Specifically, improved social learning and memory abilities allowed the evolution of language. In turn, improved language expertise probably selected for better cognitive abilities for better language acquisition and optimal handling of the larger volume of information acquired through language. Most notably, language could dramatically advance human social behaviour and the transmission of cultural information [82,83].
Language acquisition is highly cognitively demanding. Each newly born human infant initiates a long process of acquiring language expertise culminating in a vocabulary of 50 000–100 000 words and complex grammar in adulthood [84]. Infants rely heavily on social learning from their parents, who talk to them in infant-directed speech (motherese) characterized by slower speech with simplified sentence structure, repetition, longer pauses, a higher fundamental frequency and greater pitch variation than in adult-directed speech [85,86]. Language learning involves sophisticated social understanding, detection of statistical regularities, perception across modalities (e.g. connecting face to speech, sight with sound and symbol with object) and extensive memory [87,88].
We cannot reconstruct the evolutionary dynamics among enhanced cognition, cultural changes and language in early hominid evolution [89]. It is commonly agreed, however, that language capacity has existed for at least 100 000 years [90]. It is also widely recognized that language precipitated a range of unique abilities that have allowed humans to successfully spread to all continents except Antarctica. These abilities include enhanced tool technologies such as composite tools with handles, use of novel tool materials including bones and antlers, long-distance exchange of raw materials, hunting of large, dangerous animals, structured use of domestic space, use of pigments and self ornaments, and rituals [91,92]. The strong reliance on rich language for social learning in increasingly larger social groups and the rapid accumulation of culture led to the evolution of human agriculture, writing and, more recently, to the industrial and technological revolutions. A consequence of the large increase in social groups, social organization and rapid accumulation of cultural knowledge in the past few thousands of years has been increased specialization among individual humans. In addition to general expertise in language and social skills shared by all individuals, some humans spend many years, even decades, honing their highly specialized narrow expertise. Well-studied examples include expertise in specific fields of science, medicine and arts, and in competitive games such as chess [37,93,94].
7. The evolutionary biology of expertise: conclusion and prospects
An individual's life can be perceived as acquiring and relying on information to make decisions that maximize fitness. Key innovations throughout animal evolution have led to some lineages expanding resource allocation to cognitive structures handling information and time necessary both for their development and for the acquisition of complex skills, or expertise. The evolution of the nervous system was a natural consequence of the increased complexity associated with multicellularity. Such complexity allows for greater efficiency and regulation of specialized cells, and provides more opportunities for further refinement, improvement and innovation [95,96]. While neurons' initial role was internal communication and coordination, the associated neuromodulation opened up mechanistic opportunities leading to information acquisition and retention. Learning, initially via individual experience and later also through social interactions, probably stimulated an evolutionary cycle of increased investment in and reliance on cognitive skills culminating in the evolution of language and modern human civilization.
With a few exceptions [32,46,97], expertise has been subjected to research only in the field of human psychology [37,42]. While we know little about the genetics and neurobiology of complex skill acquisition, it is likely that humans share similar mechanisms with non-humans. It is possible, however, that a major axis of evolutionary change within vertebrates has been associated with cognitive improvements leading to increased capacities for storing and effectively using vast amounts of knowledge and experience. The two whole-gene duplications early in vertebrate evolution and further events of gene duplication in primates and humans [52,53,98,99] have indeed vastly increased brain complexity and probably enhanced complex skill acquisition. The exact genetic, neurobiological and physiological mechanisms, as well as the evolutionary biology of expertise and expert performance, deserve rigorous examination.
On the mechanistic side, we have to test whether there are unique mechanisms underlying expertise acquisition in certain species. In humans, a remarkable aspect of expertise is that experts can handle more information more rapidly and execute better decisions within their expertise than can novices. Moreover, tasks that are initially very challenging and attention demanding become ‘automated’ with extensive practice, meaning that they are performed easily with little attention. Familiar examples include reading and driving [100]. If there are universal mechanisms that underlie complex skill acquisition, they can most readily be examined in classical animal model systems such as fruit flies and mice. Alternatively, documenting variation among taxa in the mechanisms underlying expertise will open up a search for the genetic and neurobiological mechanisms generating such probable differences.
On the evolutionary side, we ought to quantify genetic variation in the rate of expertise acquisition and peak expert performance, and their association with fitness. We know, for example, that young in a variety of species that rely on complex foraging skills face high mortality rates while in the process of attaining their expertise (e.g. [49,51]). But we know little about the evolutionary biology of such and other cases of slow and costly expertise development, which is likely followed by large benefits to the surviving experts. The functional and mechanistic properties of expertise constitute an exciting central topic for future research.
Acknowledgements
I thank P. Andrew, L. Dukas, M. O'Brien and two anonymous referees for comments on the manuscript, and the numerous colleagues and students who have helped me build my expertise.
Data accessibility
This article has no additional data.
Competing interests
I have no competing interests.
Funding
I thank the Natural Sciences and Engineering Research Council of Canada, Canada Foundation for Innovation and Ontario Ministry of Research and Innovation for supporting my research.
References
- 1.Dukas R. 2004. Evolutionary biology of animal cognition. Annu. Rev. Ecol. Evol. Syst. 35, 347–374. ( 10.1146/annurev.ecolsys.35.112202.130152) [DOI] [Google Scholar]
- 2.Anderson JR. 2015. Cognitive psychology and its implications, 8th edn New York, NY: MacMillan. [Google Scholar]
- 3.Rolls ET. 2014. Emotion and decision-making explained. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Futuyma DJ, Kirkpatrick M. 2017. Evolution, 4th edn Sunderland, MA: Sinauer. [Google Scholar]
- 5.Johnson S. 2010. Where good ideas come from. New York, NY: Penguin. [Google Scholar]
- 6.Bessen J. 2015. Learning by doing. New Haven, CT: Yale University Press. [Google Scholar]
- 7.Koshland D. 1980. Bacterial chemotaxis as a model behavioral system. New York, NY: Raven Press. [Google Scholar]
- 8.Hazelbauer GL, Falke JJ, Parkinson JS. 2008. Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends Biochem. Sci. 33, 9–19. ( 10.1016/j.tibs.2007.09.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segall JE, Block SM, Berg HC. 1986. Temporal comparisons in bacterial chemotaxis. Proc. Natl Acad. Sci. USA 83, 8987–8991. ( 10.1073/pnas.83.23.8987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenbach M, Lengeler JW. 2004. Chemotaxis. London, UK: Imperial College Press. [Google Scholar]
- 11.Beckwith JR, Zipser D. 1970. The lactose operon. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- 12.Jacob F, Monod J. 1961. Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. Biol. 3, 318–356. [DOI] [PubMed] [Google Scholar]
- 13.Rajewsky K. 1996. Clonal selection and learning in the antibody system. Nature 381, 751–758. ( 10.1038/381751a0) [DOI] [PubMed] [Google Scholar]
- 14.Tonegawa S. 1983. Somatic generation of antibody diversity. Nature 302, 575–581. ( 10.1038/302575a0) [DOI] [PubMed] [Google Scholar]
- 15.Abbas AK, Lichtman AH, Pillai S. 2016. Basic immunology: functions and disorders of the immune system, 5th edn St Louis, MI: Elsevier Health Sciences. [Google Scholar]
- 16.Weng N-P, Araki Y, Subedi K. 2012. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat. Rev. Immunol. 12, 306–315. ( 10.1038/nri3173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackie GO. 1970. Neuroid conduction and the evolution of conducting tissues. Q. Rev. Biol. 45, 319–332. ( 10.1086/406645) [DOI] [PubMed] [Google Scholar]
- 18.Ryan TJ, Grant SGN. 2009. The origin and evolution of synapses. Nat. Rev. Neurosci. 10, 701–712. ( 10.1038/nrn2717) [DOI] [PubMed] [Google Scholar]
- 19.Anderson PAV. 1989. Evolution of the first nervous systems. New York, NY: Plenum Press. [Google Scholar]
- 20.Jékely G. 2011. Origin and early evolution of neural circuits for the control of ciliary locomotion. Proc. R. Soc. B 278, 914–922. ( 10.1098/rspb.2010.2027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jekely G, Colombelli J, Hausen H, Guy K, Stelzer E, Nedelec F, Arendt D. 2008. Mechanism of phototaxis in marine zooplankton. Nature 456, 395–399. ( 10.1038/nature07590) [DOI] [PubMed] [Google Scholar]
- 22.Bosch TCG, et al. 2017. Back to the basics: cnidarians start to fire. Trends Neurosci. 33, 435–445. ( 10.1016/j.tins.2016.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arendt D, Denes AS, Jékely G, Tessmar-Raible K. 2008. The evolution of nervous system centralization. Phil. Trans. R. Soc. B 363, 1523–1528. ( 10.1098/rstb.2007.2242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jékely G, Keijzer F, Godfrey-Smith P. 2015. An option space for early neural evolution. Phil. Trans. R. Soc. B 370, 20150181 ( 10.1098/rstb.2015.0181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris-Warrick RM, Marder E. 1991. Modulation of neural networks for behavior. Annu. Rev. Neurosci. 14, 39–57. ( 10.1146/annurev.ne.14.030191.000351) [DOI] [PubMed] [Google Scholar]
- 26.Marder E. 2012. Neuromodulation of neuronal circuits: back to the future. Neuron 76, 1–11. ( 10.1016/j.neuron.2012.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bargmann CI. 2012. Beyond the connectome: how neuromodulators shape neural circuits. BioEssays 34, 458–465. ( 10.1002/bies.201100185) [DOI] [PubMed] [Google Scholar]
- 28.Bargmann CI, Marder E. 2013. From the connectome to brain function. Nat. Methods 10, 483–490. ( 10.1038/nmeth.2451) [DOI] [PubMed] [Google Scholar]
- 29.Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, Bargmann CI. 2013. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell 154, 1023–1035. ( 10.1016/j.cell.2013.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dukas R. 1998. Evolutionary ecology of learning. In Cognitive ecology (ed. Dukas R.), pp. 129–174. Chicago, IL: University of Chicago Press. [Google Scholar]
- 31.Dukas R. 2008. Evolutionary biology of insect learning. Annu. Rev. Entomol. 53, 145–160. ( 10.1146/annurev.ento.53.103106.093343) [DOI] [PubMed] [Google Scholar]
- 32.Dukas R, Visscher PK. 1994. Lifetime learning by foraging honey bees. Anim. Behav. 48, 1007–1012. ( 10.1006/anbe.1994.1333) [DOI] [Google Scholar]
- 33.Dukas R. 2008. Life history of learning: performance curves of honeybees in the wild. Ethology 114, 1195–1200. ( 10.1111/j.1439-0310.2008.01565.x) [DOI] [Google Scholar]
- 34.Wooler RD, Bradley JS, Skira IJ, Serventy DL. 1990. Reproductive success of short-tailed shearwater Puffinus tenuirostris in relation to their age and breeding experience. J. Anim. Ecol. 59, 161–170. ( 10.2307/5165) [DOI] [Google Scholar]
- 35.Leach AG, Sedinger JS. 2016. Male breeding experience, not mate familiarity, affects reproductive output in black brant geese. Behav. Ecol. 27, 1851–1858. ( 10.1093/beheco/arw122) [DOI] [Google Scholar]
- 36.Walker R, Hill K, Kaplan H, McMillan G. 2002. Age-dependency in hunting ability among the Ache of Eastern Paraguay. J. Hum. Evol. 42, 639–657. ( 10.1006/jhev.2001.0541) [DOI] [PubMed] [Google Scholar]
- 37.Ericsson KA, Charness N, Feltovich PJ, Hoffman RR. 2006. The Cambridge handbook of expertise and expert performance. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 38.Benito E, Barco A. 2010. CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 33, 230–240. ( 10.1016/j.tins.2010.02.001) [DOI] [PubMed] [Google Scholar]
- 39.Kandel ER, Dudai Y, Mayford MR. 2014. The molecular and systems biology of memory. Cell 157, 163–186. ( 10.1016/j.cell.2014.03.001) [DOI] [PubMed] [Google Scholar]
- 40.Wen JYM, Kumar N, Morrison G, Rambaldini G, Runciman S, Rousseau J, van der Kooy D. 1997. Mutations that prevent associative learning in C. elegans. Behav. Neurosci. 111, 354–368. ( 10.1037/0735-7044.111.2.354) [DOI] [PubMed] [Google Scholar]
- 41.Dukas R. 2009. Learning: mechanisms, ecology and evolution. In Cognitive ecology II (eds Dukas R, Ratcliffe J), pp. 7–26. Chicago, IL: University of Chicago Press. [Google Scholar]
- 42.Ericsson KA, Lehmann AC. 1996. Expert and exceptional performance: evidence of maximal adaptation to task constraints. Annu. Rev. Psychol. 47, 273–305. ( 10.1146/annurev.psych.47.1.273) [DOI] [PubMed] [Google Scholar]
- 43.Sergio F, Tanferna A, De Stephanis R, Jimenez LL, Blas J, Tavecchia G, Preatoni D, Hiraldo F. 2014. Individual improvements and selective mortality shape lifelong migratory performance. Nature 515, 410–413. ( 10.1038/nature13696) [DOI] [PubMed] [Google Scholar]
- 44.Desrochers A. 1992. Age and foraging success in European blackbirds: variation between and within individuals. Anim. Behav. 43, 885–894. ( 10.1016/S0003-3472(06)80002-3) [DOI] [Google Scholar]
- 45.Reid WV. 1988. Age-specific patterns of reproduction in the glaucous-winged gull: increased effort with age? Ecology 69, 1454–1465. ( 10.2307/1941642) [DOI] [Google Scholar]
- 46.Dukas R. 2008. Life history of learning—short and long term performance curves of honeybees in settings that minimize the role of learning. Anim. Behav. 75, 1125–1130. ( 10.1016/j.anbehav.2007.08.029) [DOI] [Google Scholar]
- 47.Desrochers A. 1992. Age-related differences in reproduction by European blackbirds: restraint or constraint? Ecology 73, 1128–1131. ( 10.2307/1940186) [DOI] [Google Scholar]
- 48.Schippers M-P, Dukas R, Smith RW, Wang J, Smolen K, McClelland GB. 2006. Lifetime performance in foraging honeybees: behaviour and physiology. J. Exp. Biol. 209, 3828–3836. ( 10.1242/jeb.02450) [DOI] [PubMed] [Google Scholar]
- 49.Daunt F, Afanasyev V, Adam A, Croxall JP, Wanless S. 2007. From cradle to early grave: juvenile mortality in European shags Phalacrocorax aristotelis results from inadequate development of foraging proficiency. Biol. Lett. 3, 371–374. ( 10.1098/rsbl.2007.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weathers WW, Sullivan KA. 1989. Juvenile foraging proficiency, parental effort, and avian reproductive success. Ecol. Monogr. 59, 223–246. ( 10.2307/1942600) [DOI] [Google Scholar]
- 51.Sullivan KA. 1989. Predation and starvation: age specific mortality in juvenile juncos (Junco phaeonotus). J. Anim. Ecol. 58, 275–286. ( 10.2307/5000) [DOI] [Google Scholar]
- 52.Putnam NH, et al. 2008. The amphioxus genome and the evolution of the chordate karyotype. Nature 453, 1064–1071. ( 10.1038/nature06967) [DOI] [PubMed] [Google Scholar]
- 53.Van de Peer Y, Maere S, Meyer A. 2009. The evolutionary significance of ancient genome duplications. Nat. Rev. Genet. 10, 725–732. ( 10.1038/nrg2600) [DOI] [PubMed] [Google Scholar]
- 54.Shimeld SM, Holland WH. 2000. Vertebrate innovations. Proc. Natl Acad. Sci. USA 97, 4449–4452. ( 10.1073/pnas.97.9.4449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emes RD, Grant SG.N. 2012. Evolution of synapse complexity and diversity. Annu. Rev. Neurosci. 35, 111–131. ( 10.1146/annurev-neuro-062111-150433) [DOI] [PubMed] [Google Scholar]
- 56.Nithianantharajah J, et al. 2013. Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nat. Neurosci. 16, 16–24. ( 10.1038/nn.3276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryan TJ, et al. 2013. Evolution of GluN2A/B cytoplasmic domains diversified vertebrate synaptic plasticity and behavior. Nat. Neurosci. 16, 25–32. ( 10.1038/nn.3277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dukas R. 2013. Effects of learning on evolution: robustness, innovation and speciation. Anim. Behav. 85, 1023–1030. ( 10.1016/j.anbehav.2012.12.030) [DOI] [Google Scholar]
- 59.Heyes CM, Galef BG. 1996. Social learning in animals. San Diego, CA: Academic Press. [Google Scholar]
- 60.Hoppitt WJE, Laland KN. 2013. Social learning: an introduction to mechanisms, methods and models. Princeton, NJ: Princeton University Press. [Google Scholar]
- 61.Caro TM. 1994. Cheetahs of the Serengeti plains: group living in an asocial species. Chicago, IL: University of Chicago Press. [Google Scholar]
- 62.Newton I. 1986. The sparrowhawk. London, UK: A&C Black. [Google Scholar]
- 63.Dukas R. 2010. Insect social learning. In Encyclopedia of animal behavior (eds Breed M, Moore J), pp. 176–179. Oxford, UK: Academic Press. [Google Scholar]
- 64.Olsson A, Phelps EA. 2007. Social learning of fear. Nat. Neurosci. 10, 1095–1102. ( 10.1038/nn1968) [DOI] [PubMed] [Google Scholar]
- 65.Munger SD, et al. 2010. An olfactory subsystem that detects carbon disulfide and mediates food-related social learning. Curr. Biol. 20, 1438–1444. ( 10.1016/j.cub.2010.06.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kavaliers M, Choleris E. 2013. Neurobiological correlates of sociality, mate choice and learning. Trends Ecol. Evol. 28, 4–5. ( 10.1016/j.tree.2012.08.019) [DOI] [PubMed] [Google Scholar]
- 67.Zeigler HP, Marler P. 2008. Neuroscience of birdsong. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 68.Prokopy RJ, Roitberg BD. 2001. Joining and avoidance behavior in nonsocial insects. Annu. Rev. Entomol. 46, 631–665. ( 10.1146/annurev.ento.46.1.631) [DOI] [PubMed] [Google Scholar]
- 69.Allee WC. 1931. Animal aggregations. A study in general sociology. Chicago, IL: University of Chicago Press. [Google Scholar]
- 70.Danchin E, Wagner RH. 1997. The evolution of coloniality: the emergence of new perspectives. Trends Ecol. Evol. 12, 342–347. ( 10.1016/S0169-5347(97)01124-5) [DOI] [PubMed] [Google Scholar]
- 71.Becher PG, et al. 2012. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct. Ecol. 26, 822–828. ( 10.1111/j.1365-2435.2012.02006.x) [DOI] [Google Scholar]
- 72.Bartelt RJ, Schaner AM, Jackson LL. 1985. cis-Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J. Chem. Ecol. 11, 1747–1756. ( 10.1007/BF01012124) [DOI] [PubMed] [Google Scholar]
- 73.Ramdya P, Schneider J, Levine JD. 2017. The neurogenetics of group behavior in Drosophila melanogaster. J. Exp. Biol. 220, 35 ( 10.1242/jeb.141457) [DOI] [PubMed] [Google Scholar]
- 74.Sarin S, Dukas R. 2009. Social learning about egg laying substrates in fruit flies. Proc. R. Soc. B 276, 4323–4328. ( 10.1098/rspb.2009.1294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caro TM, Hauser MD. 1992. Is there teaching in nonhuman animals? Q. Rev. Biol. 67, 151–174. ( 10.1086/417553) [DOI] [PubMed] [Google Scholar]
- 76.Thornton A, McAuliffe K. 2006. Teaching in wild meerkats. Science 313, 227–229. ( 10.1126/science.1128727) [DOI] [PubMed] [Google Scholar]
- 77.Catchpole CK, Slater PJ.B. 1995. Bird song: biological themes and variation. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 78.Beecher MD, Brenowitz EA. 2005. Functional aspects of song learning in songbirds. Trends Ecol. Evol. 20, 143–149. ( 10.1016/j.tree.2005.01.004) [DOI] [PubMed] [Google Scholar]
- 79.Whiten A. 2011. The scope of culture in chimpanzees, humans and ancestral apes. Phil. Trans. R. Soc. B 366, 997–1007. ( 10.1098/rstb.2010.0334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jarvis ED. 2006. Selection for and against vocal learning in birds and mammals. Ornithol. Sci. 5, 5–14. ( 10.2326/osj.5.5) [DOI] [Google Scholar]
- 81.Tomasello M. 2002. Some facts about primate (including human) communication and social learning. In Simulating the evolution of language (eds Cangelosi A, Parisi D), pp. 327–340. Berlin, Germany: Springer. [Google Scholar]
- 82.Dunbar RIM. 2003. The social brain: mind, language, and society in evolutionary perspective. Annu. Rev. Anthropol. 32, 163–181. ( 10.1146/annurev.anthro.32.061002.093158) [DOI] [Google Scholar]
- 83.Herrmann E, Call J, Hernández-Lloreda MV, Hare B, Tomasello M. 2007. Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science 317, 1360–1366. ( 10.1126/science.1146282) [DOI] [PubMed] [Google Scholar]
- 84.Clark EV. 2009. First language acquisition. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 85.Thiessen ED, Hill EA, Saffran JR. 2005. Infant-directed speech facilitates word segmentation. Infancy 7, 53–71. ( 10.1207/s15327078in0701_5) [DOI] [PubMed] [Google Scholar]
- 86.Ferguson CA. 1964. Baby talk in six languages. Am. Anthropol. 66, 103–114. ( 10.1525/aa.1964.66.suppl_3.02a00060) [DOI] [Google Scholar]
- 87.Kuhl PK. 2004. Early language acquisition: cracking the speech code. Nat. Rev. Neurosci. 5, 831–843. ( 10.1038/nrn1533) [DOI] [PubMed] [Google Scholar]
- 88.Hollich G. 2012. Early language. In Infant development (eds Bremner JG, Wachs TD), pp. 426–449. Chichester, UK: Wiley. [Google Scholar]
- 89.Henrich J. 2016. The secret of our success: how culture is driving human evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 90.Johansson S. 2005. Origins of language: constraints on hypotheses. Amsterdam, The Netherlands: John Benjamins Publishing. [Google Scholar]
- 91.Klein RG. 2009. The human career: human biological and cultural origins. Chicago, IL: University of Chicago Press. [Google Scholar]
- 92.McBrearty S, Brooks AS. 2000. The revolution that wasn't: a new interpretation of the origin of modern human behavior. J. Hum. Evol. 39, 453–563. ( 10.1006/jhev.2000.0435) [DOI] [PubMed] [Google Scholar]
- 93.Stephan PE, Levin SG. 1992. Striking the mother lode in science: the importance of age, place, and time. New York, NY: Oxford University Press. [Google Scholar]
- 94.Chase W, Simon H. 1973. Skill in chess. Am. Sci. 61, 394–403. [Google Scholar]
- 95.Sterling P, Laughlin S. 2015. Principles of neural design. Cambridge, MA: MIT Press. [Google Scholar]
- 96.Maynard SJ, Szathmary E. 1997. The major transitions in evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 97.Helton WS. 2008. Expertise acquisition as sustained learning in humans and other animals: commonalities across species. Anim. Cogn. 11, 99–107. ( 10.1007/s10071-007-0093-4) [DOI] [PubMed] [Google Scholar]
- 98.Dorus S, Vallender EJ, Evans PD, Anderson JR, Gilbert SL, Mahowald M, Wyckoff GJ, Malcom CM, Lahn BT. 2004. Accelerated evolution of nervous system genes in the origin of Homo sapiens. Cell 119, 1027–1040. ( 10.1016/j.cell.2004.11.040) [DOI] [PubMed] [Google Scholar]
- 99.Somel M, Liu X, Khaitovich P. 2013. Human brain evolution: transcripts, metabolites and their regulators. Nat. Rev. Neurosci. 14, 112–127. ( 10.1038/nrn3372) [DOI] [PubMed] [Google Scholar]
- 100.Feltovich PJ, Prietula MJ, Ericsson KA. 2006. Studies of expertise from psychological perspectives. In The Cambridge handbook of expertise and expert performance (eds Ericsson KA, Charness N, Feltovich PJ, Hoffman RR), pp. 41–67. Cambridge, UK: Cambridge University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.