Abstract
Carotenoids are among the most important organic compounds present in Nature and play several essential roles in biology. Their configuration is responsible for their specific photophysical properties, which can be tailored by changes in their molecular structure and in the surrounding environment. In this review, we give a general description of the main electronic and vibrational properties of carotenoids. In the first part, we describe how the electronic and vibrational properties are related to the molecular configuration of carotenoids. We show how modifications to their configuration, as well as the addition of functional groups, can affect the length of the conjugated chain. We describe the concept of effective conjugation length, and its relationship to the S0 → S2 electronic transition, the decay rate of the S1 energetic level and the frequency of the ν1 Raman band. We then consider the dependence of these properties on extrinsic parameters such as the polarizability of their environment, and how this information (S0 → S2 electronic transition, ν1 band position, effective conjugation length and polarizability of the environment) can be represented on a single graph. In the second part of the review, we use a number of specific examples to show that the relationships can be used to disentangle the different mechanisms tuning the functional properties of protein-bound carotenoids.
Keywords: carotenoids, resonance Raman, vibrational properties, electronic properties
1. Introduction
There are more than 700 known carotenoids in Nature, with different chemical structures, which play essential roles in biology [1,2]. Carotenoids display a number of different functions in a large range of different organisms, including bacteria, algae, plants, starfish, salmon, humans, birds, lobsters…, in most cases bound to protein [3]. They are mainly synthesized by photosynthetic organisms and provide vibrant natural colours—often red, orange and yellow; even blue [4]. As a general rule, other organisms only acquire carotenoid molecules (which they may then eventually modify) through their diet, although there are rare cases of animals acquiring carotenoid biosynthetic capabilities through lateral gene transfer [5,6]. Dietary intake of carotenoids by mammals is thought to be associated with reduced risks of several chronic health disorders including heart disease, age-related macular degeneration and certain cancers [7]. It has been postulated that these actions are related to the ability of carotenoids to quench reactive oxygen species [8]. Carotenoid binding to proteins can confer solubility in the aqueous cellular environment (most carotenoid molecules are highly apolar). Additionally, this binding allows tuning of their electronic and vibrational properties via the chemical properties of the binding site. The most common result of such carotenoid–protein interactions is a red-shift of the carotenoid absorption maximum—such as the shift in absorption of the carotenoid astaxanthin from 480 to 630 nm in crustacyanin, the blue carotenoid protein complex in the shell of the lobster Homarus gammarus or Homarus americanus [9–13]. Carotenoids are highly involved in the first steps of the photosynthetic process, where they assume a paradoxical double function: they play a role as light harvesters [14–18], and at the same time they act as photoprotective molecules via a number of different mechanisms, including excitation energy quenching. As light harvesters, carotenoids transfer the absorbed excited state energy to (bacterio)chlorophylls ((B)Chl); this excitation energy is eventually trapped by a reaction centre pigment–protein complex and converted into an electrical potential [19,20]. They also act as protective molecules against the photobleaching of photosynthetic organisms by quenching (B)Chl triplet states [21,22], which prevents the (B)Chl-sensitized formation of singlet state oxygen [23–29], by scavenging singlet oxygen directly [8,30] or by quenching (B)Chl singlet states [31–33]. Carotenoids have also been reported to stabilize protein structures, because many photosynthetic pigment–protein complexes do not fold properly without these molecules [34–36]. However, it is unclear whether this represents a specific function, as removing such large cofactors from a caroteno-protein structure induces the presence of a void, which is expected per se to dramatically influence the process of folding.
2. Molecular configuration and energy levels of carotenoids
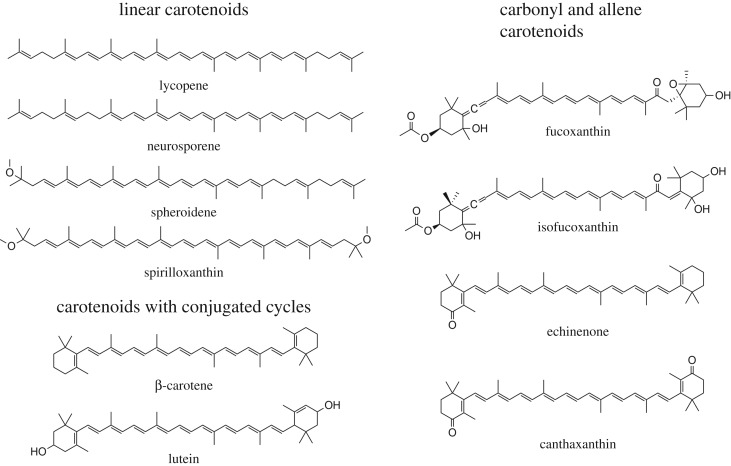
Carotenoids are tetraterpenoid derivatives which are initially formed of eight isoprene molecules [2,37,38]. Carotenoids split into two main classes—carotenes (which are pure hydrocarbons) and xanthophylls (which contain oxygen). Carotenoids present a significant structural diversity because their carbon skeletons may vary from purely linear, including cyclic structures, or contain functional groups such as carbonyls or allenes; in each case, the grouping may be conjugated or not with the isoprenoid chain (figure 1) [1]. The electronic structure of carotenoids has been studied for more than a century, but for many years it was assumed that the lowest energy excited state in all π-electron-conjugated molecules could be reached by one-photon absorption, promoting a single electron from its highest occupied molecular orbital to its lowest unoccupied molecular orbital. Work in the early 1970s by Hudson & Kohler [39] and Schulten & Karplus [40] challenged this molecular orbital theoretical interpretation of the electronic absorption spectra for linear π-electron-conjugated polyenes (which include carotenoids). They proposed that the lowest-lying excited state, 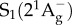 , is absorption silent, displaying the same symmetry as the ground state, and that the strong absorption of carotenoids arises from a transition from the ground to the second excited state,
, is absorption silent, displaying the same symmetry as the ground state, and that the strong absorption of carotenoids arises from a transition from the ground to the second excited state, 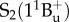 . This excited S2 state decays by internal conversion (less than 200 fs) to the low-lying
. This excited S2 state decays by internal conversion (less than 200 fs) to the low-lying 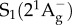 state, which itself decays to the ground state S0 by internal conversion in several picoseconds (fluorescence occurs with extremely low yield) [41]. Other ‘dark’ S* states have been proposed in the vicinity of S1 and S2 to account for the network of relaxation pathways observed in carotenoids [42–44]. A detailed discussion of the energetic levels of carotenoids can be read in [45,46]. Carotenoids have remarkably complex excited-state dynamics, but a system of three electronic states, described in figure 2, with
state, which itself decays to the ground state S0 by internal conversion in several picoseconds (fluorescence occurs with extremely low yield) [41]. Other ‘dark’ S* states have been proposed in the vicinity of S1 and S2 to account for the network of relaxation pathways observed in carotenoids [42–44]. A detailed discussion of the energetic levels of carotenoids can be read in [45,46]. Carotenoids have remarkably complex excited-state dynamics, but a system of three electronic states, described in figure 2, with 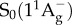 ,
, 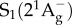 and
and 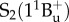 electronic levels can account for most of the observed properties. The S0 → S2 transition of carotenoids usually exhibits a characteristic three-peak structure corresponding to the lowest three vibronic bands of the electronic transition S0 → S2, termed 0–0, 0–1 and 0–2 (figure 2). For simplicity, during the rest of this review, when we address the energy of the S0 → S2 electronic transition, we will refer specifically to the energy of the (0–0) band.
electronic levels can account for most of the observed properties. The S0 → S2 transition of carotenoids usually exhibits a characteristic three-peak structure corresponding to the lowest three vibronic bands of the electronic transition S0 → S2, termed 0–0, 0–1 and 0–2 (figure 2). For simplicity, during the rest of this review, when we address the energy of the S0 → S2 electronic transition, we will refer specifically to the energy of the (0–0) band.
Figure 1.
Structures of several carotenoids grouped as a function of their molecular complexity.
Figure 2.
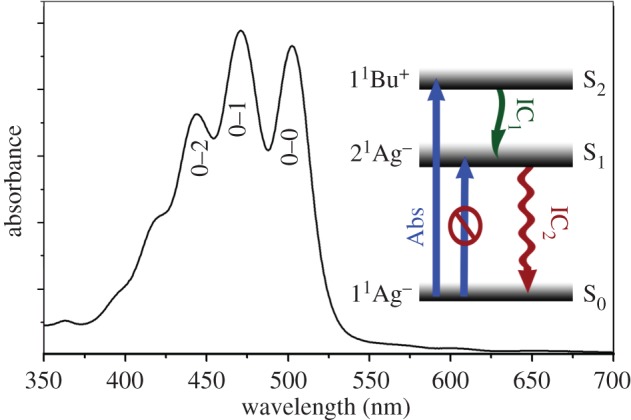
Typical absorption spectrum of carotenoids: lycopene at room temperature in n-hexane. Inset: a simplified energy diagram of carotenoids; the blue arrows represent absorption (Abs), which is forbidden for S0 → S1, green and red arrows represent internal conversion by non-radiative decay for S2 → S1 (IC1) and S1 → S0 (IC2), respectively. There is negligible fluorescence from S2 → S0, and no intersystem crossing to produce carotenoid triplet states.
3. A tailored vibrational technique for carotenoids
Resonance Raman is ideally suited to the study of carotenoids because the resonance coefficient of these molecules, which may reach more than six orders of magnitudes, is the highest among natural biomolecules. As a vibrational technique, resonance Raman yields direct information on the molecular properties of their electronic ground state. The resonance Raman spectra of carotenoids contain four main groups of bands, termed ν1 to ν4, which were observed as early as 1970 [47]. Figure 3 shows the resonance Raman spectrum of the linear carotenoid lycopene with the four major regions labelled. The most intense ν1 band, appearing above 1500 cm−1, arises from stretching vibrations of conjugated C=C double bonds [48]. Its position depends on the length of the π-electron-conjugated chain and on the molecular configuration of the carotenoid [49–53], such that an increase in conjugation length and trans–cis isomerization both result in an increase in ν1 frequency (the more central the cis bond along the chain, the greater the effect) [49,52,53]. Additionally, the ν1 frequency shows a linear dependence according to temperature in the 77–295 K range. This was proposed to arise from changes affecting both the vibronic coupling and the extent of π-electron delocalization in the carotenoid molecule [54]. A shift of approximately 5 cm−1 in the position of the ν1 band is generally observed between 293 and 77 K [55]. The ν2 band is actually constituted by a cluster of contributions around 1160 cm−1, that arise from stretching vibrations of C–C single bonds coupled with C–H in-plane bending modes, and this region is a fingerprint for the assignment of cis-isomers [49,56]. The ν3 band (approx. 1000 cm−1) arises from in-plane rocking vibrations of the methyl groups attached to the conjugated chain, which are coupled with in-plane bending modes of the adjacent C–H's [48], and can be used as a fingerprint for the configuration of conjugated end-cycles [55]. Finally, the ν4 band around 960 cm−1 arises from C–H out-of-plane wagging motions coupled with C=C torsional modes (out-of-plane twists of the carbon backbone) [48]. When the carotenoid conjugated system is planar, these out-of-plane modes will not be coupled with the electronic transition, and so these bands are not resonance enhanced. However, distortions around C–C single bonds increase the coupling of these modes with the electronic transition, resulting in an increase in the structure and intensity of this band. Hence, they can be used as an indicator of such distortions (twisting) of the carotenoid backbone (see [57]). Given the apparent structural simplicity of common carotenoids such as β-carotene and lycopene, it might be supposed that their electronic and vibrational properties should be easily modelled through modern molecular physics. However, it is only recently that the calculation of these properties could be achieved with any reasonable precision, through the application of density functional theory and time-dependent density functional theory [58–62]. A full analysis of the resonance Raman spectra of carotenoids is outside the scope of this work, but it can be found in the review by Robert [63].
Figure 3.
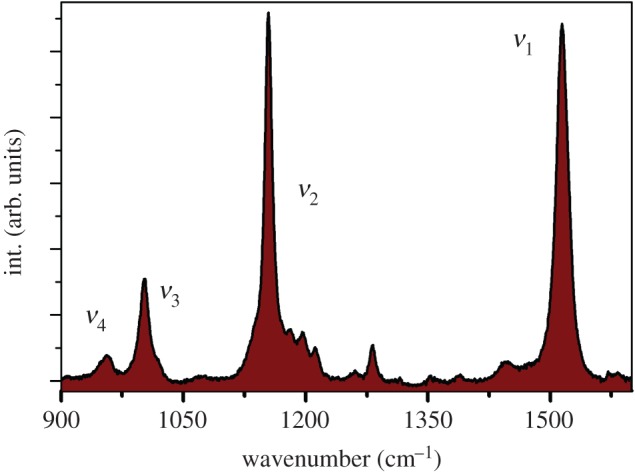
Typical resonance Raman spectrum of a carotenoid molecule (lycopene) in n-hexane. (Online version in colour.)
4. Linear carotenoids and the effect of conjugated end-cycles in solution
4.1. Effect of C=C conjugated length on electronic and vibrational properties
Araki & Murai [64] established in the early 1950s that the number of C=C double bonds in the carotenoid structure (N) is inversely related to the position of the absorption maximum, and this fundamental property has been validated by a large number of experimental studies [65–67]. This effect can be predicted with the simplest theoretical models which describe π–π* transitions [46]. The dependence of excited state energies and lifetimes of linear carotenoids on N is straightforward for linear carotenoids such as neurosporene (N = 9), spheroidene (N = 10) and lycopene (N = 11), where the conjugated backbone consists of a linear chain of π-electron-conjugated C=C bonds. The same linear relationship has also been demonstrated for all five low-lying excited states of linear carotenoids [68], even though the existence of three of these states is still questioned. Empirical linear relationships have been established for several series of polyene and carotenoid homologues having differing N, providing extrapolated values for the energy of their S0 → S2 transition [46,68,69]. Extrapolating the results toward infinite polyenes and carotenoids, the experimental data give an asymptotic limit of 700 nm [70,71]. Linear carotenoids also show a dependence on N−1 for their S1 lifetime. The pioneering work of Wasielewski & Kispert [72] demonstrated a systematic dependence of the measured S1 lifetime on conjugation length for toluene solutions of β-carotene (8.4 ± 0.6 ps), canthaxanthin (5.2 ± 0.6 ps) and β-8′-apocarotenal (25.4 ± 0.2 ps). More recently, studies on spheroidene and linear analogues confirmed this dependence, yielding lifetimes of 400 ps (N = 7), 85 ps (N = 8), 25 ps (N = 9), 8.7 ps (N = 10), 3.9 ps (N = 11), 2.7 ps (N = 12) and 1.1 ps (13) [73]. A similar dependence was found for a β-carotene series (although with slightly different values): 282 ps (N = 7), 96 ps (N = 8), 52 ps (N = 9) and 8.1 ps (N = 11) [68]. Finally, the vibrational properties, and specifically the position of the ν1 Raman band, are also dependent on the conjugation length (N) [69,74,75]. Figure 4 displays the linear correlation between the ν1 position and N−1 for the linear carotenoids neurosporene (N = 9), spheroidene (N = 10), lycopene (N = 11) and spirilloxanthin (N = 13) in n-hexane. As for their S1 decay rates and the energy of their S0 → S2 transition, the measurement of the ν1 Raman band can give accurate values for the conjugation chain length N of these molecules.
Figure 4.
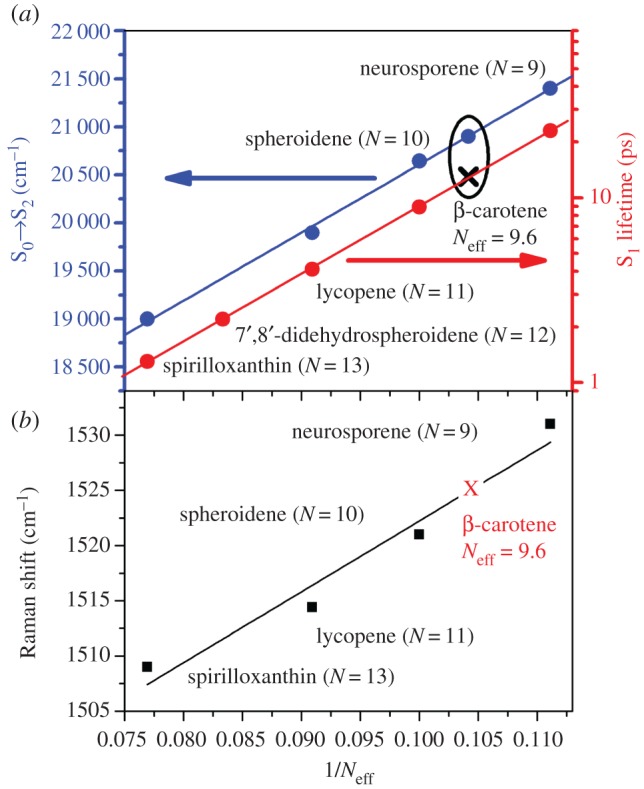
(a) Correlation between the position of the S0 → S2 electronic transition (blue data) [76,77] and the S1 decay rate (red data—y axis is in log scale) [73] with the inverse of the number of conjugated double bonds, N, for linear carotenoids in n-hexane. (b) Correlation between the position of the ν1 Raman band and the inverse of the effective conjugated double bonds, Neff, for linear carotenoids in n-hexane.
This elegant linear relationship between the nominal conjugation length N (that assumed from the chemical structure) and the S0 → S2 electronic transition, S1 decay rate and ν1 Raman band is not always readily followed by carotenoids containing chemical groups, such as conjugated end-cycles, β-rings, ketones and allene groups. In carbonyl carotenoids the presence of a conjugated C=O group extends the conjugated part of the chromophore, resulting in a shift of the absorption transition to longer wavelengths. Aryl-carotenoids and linear carotenoids with conjugated end-cycles (the class which has been the most extensively studied up to now) behave, from the point of view of their absorption, vibrational and photochemical properties, as carotenoids with shorter conjugation length than expected. This was proposed to arise from a decrease in orbital overlap between the π-orbital of the ring double bond and those of the polyene chain, as steric hindrance results in twisting of the conjugated end-cycles out of the conjugated plane [78]. Although the conjugated end-cycle contributes to the conjugation chain length [76,79], it extends it by the equivalent of only 0.3 of a C=C bond. For instance, β-carotene, instead of showing the properties of a carotenoid with 11 C=C bonds (as would be expected from its structure), presents the spectroscopic properties of a carotenoid with only 9.6 C=C bonds [75,76]. This value was termed the effective conjugation length (Neff), as it accounts for the carotenoids' electronic and vibrational properties. Studies on a series of β-carotene derivatives with different chain lengths showed that these follow a similar relationship to linear ones, but shifted due to the partial conjugation of their end-rings [75]. Similar results were also observed for aryl-carotenoids [76]. The Neff value works exceedingly well for predicting the electronic properties of carotenoids—their absorption position, but also their S1 decay rate. The relationship between the S0 → S2 electronic transition and the S1 decay rate with the inverse of N is displayed in figure 4 for linear carotenoids (where the effective and nominal conjugation length is the same) as well as for β-carotene (Neff = 9.6) in n-hexane (blue line). For both relationships, the Neff value calculated for β-carotene indicates that it obeys the same trend as linear carotenoids, once its effective conjugation is taken into account. Similarly, the correlation between the frequency of the ν1 Raman band with the inverse of the effective carotenoid conjugation length (Neff) is also well established in the literature [69,74,75]. Using β-carotene to illustrate this, the measured ν1 frequency of 1525 cm−1 gives the same value of Neff = 9.6 as that obtained using the other methods, demonstrating that they are equivalent. For simplicity, only β-carotene is plotted here, but this concept can be extended to all carotenoids with conjugated end-cycles, as well as to aryl-carotenoid molecules.
4.2. Effect of environment polarizability
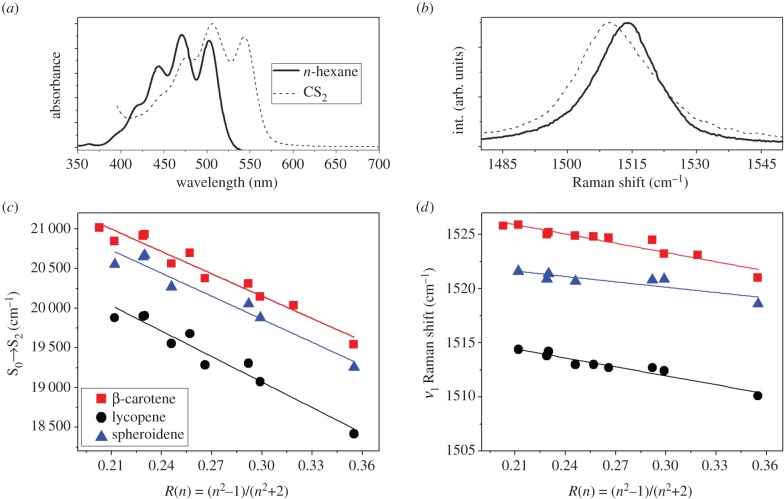
The effect of solvent properties, specifically the refractive index, n, and dielectric constant, ɛ, on the position of the absorption transition of carotenoid molecules has been studied extensively [64,80–87]. The position of the S0 → S2 electronic transition in solution depends on the solvent polarizability defined as R(n) = (n2 − 1)/(n2 + 2), n being the refractive index of the solvent. For linear carotenoids the S0 → S2 transition shifts to a longer wavelength as the refractive index increases [86] due to dispersive interactions between the solvent environment and the large transition dipole moment of the carotenoid [86]. A significant linear correlation was found between the frequency of the ν1 Raman band and the polarizability of the solvent for different linear carotenoids (including those with conjugated end-cycles), proving an influence of the solvent polarizability on the carotenoid ground state [75]. Figure 5a,b represents a practical example of the polarizability effect on the absorption spectra and ν1 Raman band for lycopene in n-hexane and carbon disulfide. Figure 5c plots the correlation between the S0 → S2 electronic transition and the polarizability of the solvent for β-carotene, lycopene and spheroidene. It illustrates a clear linear relationship that can be extended to a great variety of linear carotenoids. Figure 5d plots the correlation between the position of the ν1 Raman band and the polarizability of the solvent for β-carotene, lycopene and spheroidene. Again, this linear relationship extends to a large variety of carotenoids (linear, linear with conjugated end-cycles, aryl-carotenoids and in this case those with allene groups) [75,88]. The ν1 Raman band reflects polarizability-induced changes in the ground state only, while the absorption shift results from the combined effects on both S0 and S2. It is also of note that the dependence on polarizability appears to be similar for all carotenoid molecules and exhibits comparable trends, albeit the slopes are not identical.
Figure 5.
(a) Absorption red-shift of a typical linear carotenoid (lycopene) in solvents with different polarizability. (b) ν1 Raman band shift of lycopene in solvents with different polarizability. (c) Correlation between the S0 → S2 electronic transition and solvent polarizability for β-carotene, lycopene and spheroidene. (d) Correlation between the ν1 band position and solvent polarizability for β-carotene, lycopene and spheroidene [75].
4.3. Combining intrinsic and extrinsic effects: S0 → S2, ν1 Raman band, polarizability of the environment (R), and Neff
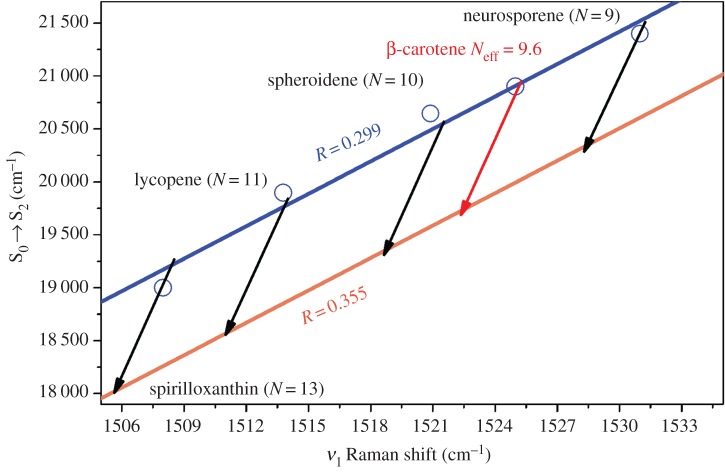
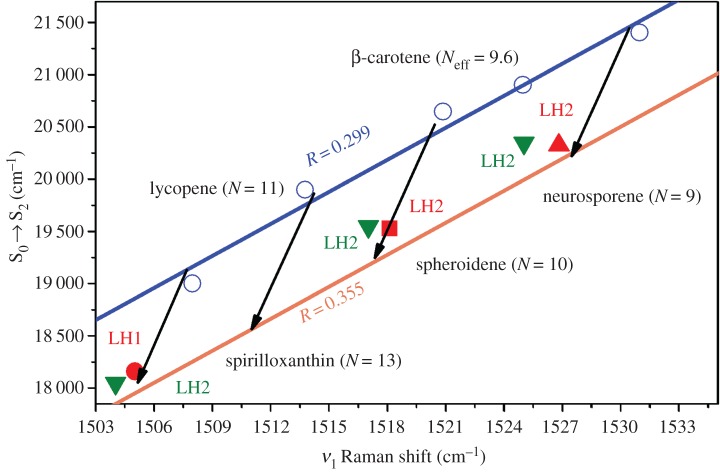
In the previous sections, we have seen that the energy of the S0 → S2 electronic transition and the frequency of the ν1 Raman band are linearly dependent on intrinsic factors, namely 1/Neff, and on environmental factors, namely the polarizability of the environment. As all these properties are linked by linear dependences, it is possible to conceive a graph containing all the information discussed above, plotting the linear relationship between the position of the carotenoid S0 → S2 electronic transition and the frequency of its ν1 Raman band [51,69]. As both of these parameters strictly depend on Neff, they present an excellent correlation for all the carotenoids studied, as shown in figure 6. In addition, the effect of polarizability on the effective conjugation length of these molecules can easily be distinguished, as it results in a shift of this straight line (e.g. between the blue and orange lines in figure 6). For simplicity, we will refer to this type of plot, which relates the position of the electronic transition to the frequency of the ν1 Raman band at room temperature, as the MP graph (from the first author of the original paper in 2013, Mendes-Pinto) [75]. In the MP graph in figure 6, we have removed most of the experimental points obtained for different solvents, showing only those for n-hexane (blue line and circles), a common solvent with low polarizability (0.299), and for carbon disulfide (orange line), a solvent with high polarizability (0.355). Each arrow represents the MP relationship for a single carotenoid species according to the polarizability of the environment, and illustrates the shift from the blue to the orange line. This graph may be used to disentangle the different mechanisms underlying the tuning of the energy of the S0 → S2 transition observed in complex media, and in particular in proteins or in vivo.
Figure 6.
MP graph showing the correlation between the position of the S0 → S2 electronic transition and the ν1 Raman band, as a function of solvent polarizability at room temperature, for spirilloxanthin, lycopene, spheroidene, β-carotene and neurosporene.
5. Carotenoids containing carbonyl and allene groups in solution
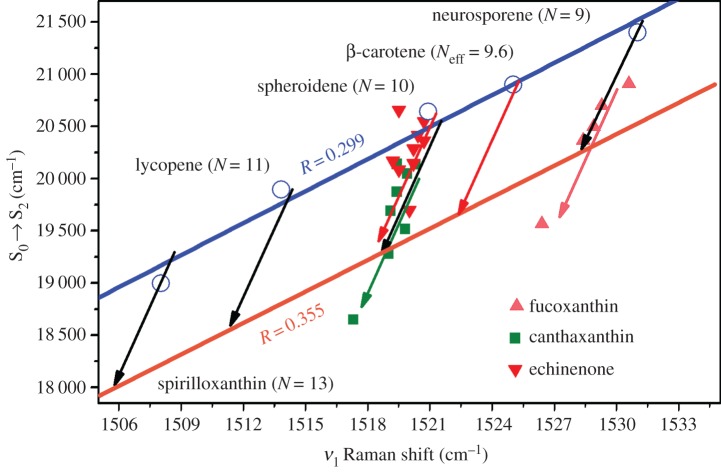
Carotenoids display a vast structural variability, and the presence of additional chemical groups makes analysis of their electronic behaviour increasingly difficult. For example, the presence of carbonyl or allene groups can influence the effective conjugation length or S2 excited state; however, this is in a different way from that observed in linear or linear with conjugated end-cycle carotenoids. Figure 7 illustrates this, as it displays an MP graph where two keto-carotenoid molecules, namely echinenone and canthaxanthin [89], as well as one allene carotenoid, fucoxanthin [88], are represented. Echinenone (downward red triangles) differs from β-carotene by one C=O on one of its rings, and presents an Neff slightly longer than that of β-carotene. However, canthaxanthin, which contains one C=O on each of its two rings, is clearly off the blue line. The introduction of a keto group in the carotenoid thus has a complex effect on its electronic structure. A similar effect is observed with fucoxanthin, a more complicated carotenoid containing both keto groups and an allene group. Fucoxanthin has seven nominal double bonds plus an allene group and a keto group. The representation of the pair (ν1, S0 → S2) for fucoxanthin (upward pink triangles) shows how it falls off the line for Neff, also indicating a perturbation of its S2 excited state.
Figure 7.
Correlation between the position of the S0 → S2 electronic transition and the ν1 band frequency for different Neff and polarizability of the environment for linear carotenoids at room temperature. The values of fucoxanthin (pink triangles), 3-hydroxyechinenone (red triangles) and canthaxanthin (green squares) are plotted for different solvents according to [88,89]. For simplicity, the name of the solvent is not written and the arrows of the corresponding colour mark the tendency with increasing polarizability from n-hexane to carbon disulfide.
6. Carotenoids in photosynthetic protein complexes
The electronic properties of carotenoid molecules underlie their multiple functions throughout Nature. In biological systems, carotenoids are generally present in highly anisotropic environments and most often bound to proteins, and their properties are tuned by these complex binding sites. In this review we restrict ourselves to the scope of carotenoids present in well-defined environments, and it is mainly in photosynthesis that the environment of the different carotenoids is precisely known (due to the existence of three-dimensional structures for a large number of photosynthetic pigment-binding proteins). In light-harvesting (LH) complexes, carotenoids perform both LH and photoprotective roles. The electronic properties of several carotenoids in photosynthetic proteins have been studied extensively, including (i) linear molecules in purple bacteria, spheroidene, neurosporene and spirilloxanthin [33,90,91], (ii) cyclic molecules, β-carotene, lutein and xanthophyll cycle pigments in higher plants [92–94] and cyanobacteria [32], and (iii) carbonyl carotenoids in marine algae, 3-hydroyechinenone [95,96], peridinin [60,97], fucoxanthin [98,99] and fucoxanthin derivatives [88]. In the following sections, we will describe practical cases where the relationships obtained above are useful for conveying new information; we also discuss their current limitations.
6.1. Linear carotenoids in purple bacteria
LH pigment–protein complexes from purple photosynthetic bacteria can bind many different carotenoids, with significant variations observed not only between bacterial species but also for the same species in different habitats [90]. These carotenoids can have different functions depending on their configuration and environment, either as auxiliary LH molecules [100,101] or as photoprotective species quenching Chl exited states [33,90,91]. In native LH pigment–protein complexes, spheroidene and neurosporene bind to LH2 from Rhodobacter sphaeroides, strains 2.4.1 (grown anaerobically) and G1C, respectively, whereas spirilloxanthin is present in LH1 from Rhodospirillum rubrum strain S1 [90,91,102]. The correlation between the ν1 Raman band and the position of the S0 → S2 transition for these carotenoid-bound proteins was compared with the correlation found for in vitro carotenoids (figure 8, red symbols). The position of the pairs of values (ν1, S0 → S2) for LH-bound spheroidene, neurosporene and spirilloxanthin clearly follows the correlation seen when varying the polarizability of the solvent for the corresponding isolated carotenoid. These results suggest that the average polarizability of the protein binding pocket is the dominant factor for tuning the position of the S0 → S2 transition upon binding to their LH protein host. This average polarizability, which is nearly identical for the three proteins, corresponds to a value, R(n), of about 0.334, slightly lower than that found in carbon disulfide (R = 0.355). This value is very high, and can be explained by the fact that LH-bound carotenoids are in close contact with (B)Chl molecules, which may provide them with a highly polarizable environment [103]. Given that the carotenoid in each case occupies an equivalent binding position in these homologous LH proteins, the binding pocket is also expected to exhibit similar electrostatic properties, as observed here [75]. Similar results were found by Dilbeck et al. [33] for six LH2 proteins from genetically modified strains of the purple photosynthetic bacterium Rhodobacter (Rb.) sphaeroides. It was again found that the shift in the ν1 Raman band and the absorption spectrum for the studied LH2-bound carotenoids (neurosporene, spheroidene, lycopene, spirilloxanthin, ketospirilloxanthin or diketospirilloxanthin) could be explained by the polarizability of the environment alone. Figure 8 compares the results obtained for neurosporene, spheroidene and spirilloxanthin in the two studies described here. Both studies describe a similar behaviour of the carotenoids in LH1 and LH2 from purple bacteria, down-shifting their energy levels due to the polarizability of their binding environment. The data obtained by Dilbeck et al. (green symbols) are slightly red-shifted by approximately 1–2 cm−1 from the results obtained by Mendes-Pinto et al. (red symbols), but this should be considered as within experimental error because they were obtained in different set-ups.
Figure 8.
Correlation between the position of the S0 → S2 electronic transition and the ν1 Raman band for spirilloxanthin, spheroidene and neurosporene, in n-hexane (blue circles) and bound to LH proteins (results reproduced from Mendes-Pinto et al., red symbols [75], and Dilbeck et al., green symbols [33]). For comparison, the relationship between carotenoids of different conjugation length in n-hexane (blue line) and CS2 (orange line) is added, as well as the relationship as a function of solvent polarizability (black arrows) [75].
6.2. Cyclic carotenoids in higher plants and cyanobacteria
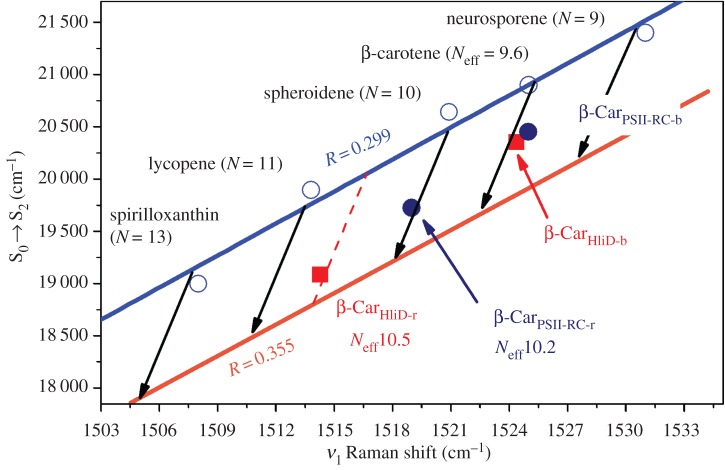
The use of the relationship described here is not only applicable to changes caused by the polarizability of the environment. The LHCII protein, the major LH protein from higher plants, binds two lutein molecules which exhibit electronic transitions at different positions. LHCII is a very complex protein–pigment complex, which assembles into a trimer in the photosynthetic membrane, with each monomer containing two lutein molecules whose binding sites are related by pseudo-symmetry. Whereas in LHCII monomers both luteins absorb at 495 nm, in LHCII trimers one lutein (lut1) absorbs at 495 nm whereas the second one (lut2) is shifted to 510 nm [92,93]. Plotting the lut1 pair of (ν1, S0 → S2) values on an MP plot shows that the position of its electronic transition is mainly governed by the polarizability of its protein binding site (as is the case for both luteins in LHCII monomers). Indeed, this pair strictly obeys the correlation obtained for lutein according to the solvent refractive index. However, the (ν1, S0 → S2) pair for lut2 shows that the energy shifts between the blue- and the red-absorbing lutein molecules are not induced by a variation in polarizability of their binding sites. Instead, the lut2 values suggest that the conjugated chain of the carotenoid is increased by nearly one C=C double bond at constant polarizability. The apparent length of the conjugated chain for lutein in solvent (and for lut1 in LHCII) is 9.3—as discussed above, the ring is only partially conjugated as steric hindrance causes rotation of the ring out of the conjugated plane. On the other hand, Neff for the red-absorbing lut2 in LHCII is approximately 10. This increase in Neff was suggested to be due to rotation of the β-ring back towards a planar conformation, resulting in a gain in conjugation length [55]. A similar effect was observed with the two β-carotene molecules in the photosystem II reaction centre, PSII-RC, which also displays shifted absorption [104–106]. Plotting the (ν1, S0 → S2) pair for each of these molecules (figure 9) shows that, while the blue-absorbing β-carotene fits on the line obtained for β-carotene in different solvents, the values obtained for the red-absorbing β-carotene suggests a sizeable increase in the apparent conjugation length (calculated as approx. 10.2). Analysis of the available three-dimensional structures for both LHCII and PSII-RC revealed the presence, in both cases, of an aromatic sidechain forcing the ring of the red-absorbing carotenoid back into the conjugated plane through steric hindrance [55]. In helix high-light-inducible proteins (Hlips) from cyanobacteria (HliD), the two bound β-carotenes display even more distinct electronic transitions: β-CarHliD-b presents S0 → S2 at 498 nm whereas β-CarHliD-r exhibits S0 → S2 at 525 nm [32,94]. Plotting (ν1, S0 → S2) pairs on an MP graph for these two carotenes again shows that, while the pair corresponding to the blue β-CarHliD-b lies on the line obtained for β-carotene in solvents, the red-absorbing one (β-CarHliD-r) deviates from this line. Again it was concluded that the electronic properties of the blue carotene are tuned by the polarizability of its protein binding site, while the red-absorbing molecule must display a longer effective conjugated length, as well as being present in an environment of relatively high polarizability. It was proposed in this case that the effective length of β-CarHliD-r lies between 10.5 and 10.6 C=C, and the polarizability of its binding site is either in the first case very high, similar to carbon disulfide, or similar to toluene in the second case.
Figure 9.
Correlation between the position of the S0 → S2 electronic transition and the ν1 Raman band for β-carotenes in PSII-RC (dark blue circles) and HliD proteins (red squares) at room temperature. For comparison, the relationship between carotenoids of different conjugation lengths in the same solvent (n-hexane) is added as well as the relationship as a function of solvent polarizability (black arrows). The β-CarPSII-RC-b point corresponds to the blue-absorbing β-carotene in PSII-RC, the β-CarPSII-RC-r point corresponds to the red-absorbing β-carotene in PSII-RC, the β-CarHliD-b point corresponds to the blue-absorbing β-carotene in HliD, and the β-CarHliD-r point corresponds to the red-absorbing β-carotene in HliD.
6.3. Proteins containing carbonyl or allene carotenoids
It is difficult to extract similar information for carbonyl and allene carotenoids in biological environments as they do not follow the same patterns as the simpler carotenoids discussed above. However, comparison with their properties in different solvents can nevertheless be useful in addressing their properties in photosynthetic proteins. Orange carotenoid protein (OCP) is a cyanobacterial photoactive protein, involved in the photoprotection of these photosynthetic organisms against intense illumination [107,108]. The bound carotenoid 3-hydroxyechinenone (spectroscopically indistinguishable from echinenone) spans its N-terminal and C-terminal domains [109]. The orange-coloured OCPo before illumination is converted to red OCPr upon illumination with intense blue-green light, and this is linked to a change in configuration of the 3′-hydroxyechinenone [110]. The pairs (ν1, S0 → S2) were plotted on an MP graph for this carotenoid in several solvents, and compared with the values obtained for OCPo and OCPr. The data for OCPr are consistent with a carotenoid of similar effective conjugation length to isolated 3-hydroxyechinenone, in a highly polarizable environment. Thus the OCPr carotenoid is in a planar, all-trans conformation. The OCPo pair indicates that the effective conjugation length of orange 3-hydroxyechinenone (Neff ≅ 9) is much shorter than isolated echinenone in solvents (Neff ≅ 10), although resonance Raman spectra of this molecule otherwise show it is in an all-trans configuration [89]. These results, together with density functional theory calculations of three isomers of echinenone and canthaxanthin, suggest two possible mechanisms for the OCPo to OCPr transition. An s-cis to s-trans isomerization of the carotenoid end-cycle would increase the relative conjugation of this ring; alternatively, bending both of the echinenone rings would bring them from out of the conjugated C=C plane in the OCPo form and into the C=C plane in the OCPr form [89].
Our last example concerns an allene carotenoid, the isofucoxanthin-like carotenoid (Ifx-l) found in the LH complex of Chromera velia [88]. This antenna protein contains, in addition to chlorophyll a and linear carotenoids, two Ifx-l with different configurations, with absorption bands located at 515 and 548 nm, respectively. The measured (ν1, S0 → S2) values for the two protein-bound Ifx-l molecules were compared on an MP graph with a series of data obtained for isolated Ifx-l in several solvents (n-hexane, cyclohexane, diethyl ether, toluene, acetonitrile and carbon disulfide). Even though allenic carotenoids do not behave exactly as linear carotenoids, it was nevertheless possible from such a comparison to conclude that the electronic absorption of the blue-absorbing Ifx-l is mainly tuned by the polarizability of its environment, while the red-absorbing one largely deviates from the solvent-derived relationship. The electronic transition of this carotenoid is approximately 900 cm−1 below that of the blue Ifx-l, even though they both exhibit the same ν1 Raman frequency. It was concluded that the absorption of the red-absorbing Ifx-l2 presents at best a weak charge transfer character [111]. Nonetheless, these results suggest that the shift in energy of the transition of the red-absorbing Ifx-l arises from a change in the excited state structure only [88].
7. Conclusion
In the first part of this review, we address the different electronic and vibrational properties of carotenoids and discuss the influence of the presence of additional, conjugated groups on these properties. For isolated carotenoids, we introduce the concept of effective conjugation length, and how this parameter is related to their S0 → S2 electronic transitions, the decay rate of the S1 energetic level and the frequency of the vibrational ν1 Raman band. We then describe how these parameters depend not only on intrinsic parameters such as effective conjugation length, but also on extrinsic (environmental) parameters such as the polarizability of their environment. We go on to explain how all this information can be represented on a single (MP) graph. The usefulness of this type of plot is then illustrated in the second part of the review. We give several examples of protein-bound carotenoids, and show how the MP graph can be used to disentangle the various parameters responsible for tuning of their functional properties.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by French Infrastructure for Integrated Structural Biology (FRISBI).
References
- 1.Britton G, Liaaen-Jensen S, Pfander H. 2008. Carotenoids, volume 4: natural functions. Basel, Switzerland: Birkhäuser. [Google Scholar]
- 2.Britton G. 1995. Structure and properties of carotenoids in relation to function. FASEB J. 9, 1551–1558. [PubMed] [Google Scholar]
- 3.Vershinin A. 1999. Biological functions of carotenoids—diversity and evolution. Biofactors 10, 99–104. ( 10.1002/biof.5520100203) [DOI] [PubMed] [Google Scholar]
- 4.Isler O, Gutmann H, Solms U. 1971. Carotenoids. Basel, Switzerland: Birkhäuser. [Google Scholar]
- 5.Altincicek B, Kovacs JL, Gerardo NM. 2012. Horizontally transferred fungal carotenoid genes in the two-spotted spider mite Tetranychus urticae. Biol. Lett. 8, 253–257. ( 10.1098/rsbl.2011.0704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran NA, Jarvik T. 2010. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 328, 624–627. ( 10.1126/science.1187113) [DOI] [PubMed] [Google Scholar]
- 7.Cooper DA, Eldridge AL, Peters JC. 1999. Dietary carotenoids and certain cancers, heart disease, and age-related macular degeneration: a review of recent research. Nutr. Rev. 57, 201–214. ( 10.1111/j.1753-4887.1999.tb06944.x) [DOI] [PubMed] [Google Scholar]
- 8.Krinsky NI. 1971. Function. In Carotenoids (eds Isler O, Gutmann H, Solms U), pp. 669–716. Basel, Switzerland: Birkhauser. [Google Scholar]
- 9.Cianci M, Rizkallah PJ, Olczak A, Raftery J, Chayen NE, Zagalsky PF, Helliwell JR. 2002. The molecular basis of the coloration mechanism in lobster shell: β-crustacyanin at 3.2-Å resolution. Proc. Natl Acad. Sci. USA 99, 9795–9800. ( 10.1073/pnas.152088999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilagan RP, Shima S, Melkozernov A, Lin S, Blankenship RE, Sharples FP, Hiller RG, Birge RR, Frank HA. 2004. Spectroscopic properties of the main-form and high-salt peridinin−chlorophyll a proteins from Amphidinium carterae. Biochemistry 43, 1478–1487. ( 10.1021/bi0357964) [DOI] [PubMed] [Google Scholar]
- 11.Buchwald M, Jencks WP. 1968. Properties of the crustacyanins and the yellow lobster shell pigment. Biochemistry 7, 844–859. ( 10.1021/bi00842a043) [DOI] [PubMed] [Google Scholar]
- 12.Britton G, et al. 1997. Carotenoid blues: structural studies on carotenoproteins. Pure Appl. Chem. 69, 2075 ( 10.1351/pac199769102075) [DOI] [Google Scholar]
- 13.Weesie RJ, Merlin JC, De Groot HJM, Britton G, Lugtenburg J, Jansen FJHM, Cornard JP. 1999. Resonance Raman spectroscopy and quantum chemical modeling studies of protein–astaxanthin interactions in α-crustacyanin (major blue carotenoprotein complex in carapace of lobster, Homarus gammarus). Biospectroscopy 5, 358–370. ( 10.1002/(SICI)1520-6343(1999)5:6%3C358::AID-BSPY5%3E3.0.CO;2-1) [DOI] [PubMed] [Google Scholar]
- 14.Goedheer JC. 1969. Energy transfer from carotenoids to chlorophyll in blue-green, red and green algae and greening bean leaves. Biochim. Biophys. Acta 172, 252–265. ( 10.1016/0005-2728(69)90068-1) [DOI] [PubMed] [Google Scholar]
- 15.Govindjee, Govindjee R. 1975. Bioenergetics of photosynthesis. In Cell biology (ed. Govindjee), pp. 2–50. New York, NY: Academic Press. [Google Scholar]
- 16.Cogdell RJ. 1978. Carotenoids in photosynthesis. Phil. Trans. R. Soc. Lond. B 284, 569–579. ( 10.1098/rstb.1978.0090) [DOI] [Google Scholar]
- 17.Frank HA, Christensen RL. 1995. Singlet energy transfer from carotenoids to bacteriochlorophylls. In Anoxygenic photosynthetic bacteria (eds Blankenship RE, Madigan MT, Bauer CE), pp. 373–384. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 18.Scholes GD, Fleming GR, Olaya-Castro A, van Grondelle R. 2011. Lessons from nature about solar light harvesting. Nat. Chem. 3, 763–774. ( 10.1038/nchem.1145) [DOI] [PubMed] [Google Scholar]
- 19.Kirmaier C, Holten D. 1987. Primary photochemistry of reaction centers from the photosynthetic purple bacteria. Photosynth. Res. 13, 225–260. ( 10.1007/bf00029401) [DOI] [PubMed] [Google Scholar]
- 20.Blankenship RE, Madigan MT, Bauer CE. 1995. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 21.Monger TG, Cogdell RJ, Parson WW. 1976. Triplet states of bacteriochlorophyll and carotenoids in chromatophores of photosynthetic bacteria. Biochim. Biophys. Acta 449, 136–153. ( 10.1016/0005-2728(76)90013-X) [DOI] [PubMed] [Google Scholar]
- 22.Niedzwiedzki DM, Blankenship RE. 2010. Singlet and triplet excited state properties of natural chlorophylls and bacteriochlorophylls. Photosynth. Res. 106, 227–238. ( 10.1007/s11120-010-9598-9) [DOI] [PubMed] [Google Scholar]
- 23.Foote CS. 1968. Mechanisms of photosensitized oxidation. Science 162, 963–970. ( 10.1126/science.162.3857.963). [DOI] [PubMed] [Google Scholar]
- 24.Krinsky NI. 1968. The protective function of carotenoid pigments. In Photophysiology Iii (ed. Giese AC.), pp. 123–195. New York, NY: Academic Press. [Google Scholar]
- 25.Goodwin TW. 1976. Chemistry and biochemistry of plant pigments. New York, NY: Academic Press. [Google Scholar]
- 26.Renger G, Wolff C. 1977. Further evidence for dissipative energy migration via triplet states in photosynthesis. The protective mechanism of carotenoids in Rhodopseudomonas spheroides chromatophores. Biochim. Biophys. Acta 460, 47–57. ( 10.1016/0005-2728(77)90150-5) [DOI] [PubMed] [Google Scholar]
- 27.Boucher F, Van der Rest M, Gingras G. 1977. Structure and function of carotenoids in the photoreaction center from Rhodospirillum rubrum. Biochim. Biophys. Acta 461, 339–357. ( 10.1016/0005-2728(77)90224-9) [DOI] [PubMed] [Google Scholar]
- 28.Cogdell RJ, Frank HA. 1987. How carotenoids function in photosynthetic bacteria. Biochim. Biophys. Acta 895, 63–79. ( 10.1016/S0304-4173(87)80008-3) [DOI] [PubMed] [Google Scholar]
- 29.Frank HA, Cogdell RJ. 1993. The photochemistry and function of carotenoids in photosynthesis. In Carotenoids in photosynthesis (eds Young AJ, Britton G), pp. 252–326. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 30.Foote CS, Chang YC, Denny RW. 1970. Chemistry of singlet oxygen. X. Carotenoid quenching parallels biological protection. J. Am. Chem. Soc. 92, 5216–5218. ( 10.1021/ja00720a036) [DOI] [PubMed] [Google Scholar]
- 31.Ruban AV, et al. 2007. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450, 575–578. ( 10.1038/nature06262) [DOI] [PubMed] [Google Scholar]
- 32.Staleva H, Komenda J, Shukla MK, Šlouf V, Kaňa R, Polívka T, Sobotka R. 2015. Mechanism of photoprotection in the cyanobacterial ancestor of plant antenna proteins. Nat. Chem. Biol. 11, 287–291. ( 10.1038/nchembio.1755) [DOI] [PubMed] [Google Scholar]
- 33.Dilbeck PL, Tang Q, Mothersole DJ, Martin EC, Hunter CN, Bocian DF, Holten D, Niedzwiedzki DM. 2016. Quenching capabilities of long-chain carotenoids in light-harvesting-2 complexes from Rhodobacter sphaeroides with an engineered carotenoid synthesis pathway. J. Phys. Chem. B 120, 5429–5443. ( 10.1021/acs.jpcb.6b03305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang HP, Hunter CN. 1994. The relationship between carotenoid biosynthesis and the assembly of the light-harvesting LH2 complex in Rhodobacter sphaeroides. Biochem. J. 298, 197–205. ( 10.1042/bj2980197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto H, Bassi R. 1996. Carotenoids: localization and function. In Oxygenic photosynthesis: the light reactions (eds Ort DR, Yocum CF), pp. 539–563. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 36.Sandonà D, Croce R, Pagano A, Crimi M, Bassi R. 1998. Higher plants light harvesting proteins. Structure and function as revealed by mutation analysis of either protein or chromophore moieties. Biochim. Biophys. Acta 1365, 207–214. ( 10.1016/S0005-2728(98)00068-1) [DOI] [PubMed] [Google Scholar]
- 37.Maiani G, et al. 2009. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 53, S194–S218. ( 10.1002/mnfr.200800053) [DOI] [PubMed] [Google Scholar]
- 38.Walter MH, Strack D. 2011. Carotenoids and their cleavage products: biosynthesis and functions. Nat. Prod. Rep. 28, 663–692. ( 10.1039/C0NP00036A) [DOI] [PubMed] [Google Scholar]
- 39.Hudson BS, Kohler BE. 1972. A low-lying weak transition in the polyene Α,Ω-diphenyloctatetraene. Chem. Phys. Lett. 14, 299–304. ( 10.1016/0009-2614(72)80119-2) [DOI] [Google Scholar]
- 40.Schulten K, Karplus M. 1972. On the origin of a low-lying forbidden transition in polyenes and related molecules. Chem. Phys. Lett. 14, 305–309. ( 10.1016/0009-2614(72)80120-9) [DOI] [Google Scholar]
- 41.Frank HA, Christensen RL. 2008. Excited electronic states, photochemistry and photophysics of carotenoids. In Carotenoids: volume 4: natural functions (eds Britton G, Liaaen-Jensen S, Pfander H), pp. 167–188. Basel, Switzerland: Birkhäuser. [Google Scholar]
- 42.Polívka T, Sundström V. 2009. Dark excited states of carotenoids: consensus and controversy. Chem. Phys. Lett. 477, 1–11. ( 10.1016/j.cplett.2009.06.011) [DOI] [Google Scholar]
- 43.Buckup T, Motzkus M. 2014. Multidimensional time-resolved spectroscopy of vibrational coherence in biopolyenes. Annu. Rev. Phys. Chem. 65, 39–57. ( 10.1146/annurev-physchem-040513-103619) [DOI] [PubMed] [Google Scholar]
- 44.Balevičius V, Abramavicius D, Polívka T, Galestian Pour A, Hauer J. 2016. A unified picture of S* in carotenoids. J. Phys. Chem. Lett. 7, 3347–3352. ( 10.1021/acs.jpclett.6b01455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robert B. 1999. The electronic structure, stereochemistry and resonance Raman spectroscopy of carotenoids. In The photochemistry of carotenoids (eds Frank HA, Young AJ, Britton G, Cogdell RJ), pp. 189–201. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 46.Christensen RL. 1999. The electronic states of carotenoids. In The photochemistry of carotenoids (eds Frank HA, Young AJ, Britton G, Cogdell RJ), pp. 137–159. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 47.Gill D, Kilponen RG, Rimai L. 1970. Resonance Raman scattering of laser radiation by vibrational modes of carotenoid pigment molecules in intact plant tissues. Nature 227, 743–744. ( 10.1038/227743a0) [DOI] [PubMed] [Google Scholar]
- 48.Saito S, Tasumi M. 1983. Normal-coordinate analysis of retinal isomers and assignments of Raman and infrared bands. J. Raman Spectrosc. 14, 236–245. ( 10.1002/jrs.1250140405) [DOI] [Google Scholar]
- 49.Koyama Y, Takii T, Saiki K, Tsukida K. 1983. Configuration of the carotenoid in the reaction centers of photosynthetic bacteria. 2. Comparison of the resonance Raman lines of the reaction centers with those of the 14 different cis-trans isomers of β-carotene . Photobiochem. Photobiophys. 5, 139–150. [Google Scholar]
- 50.Koyama Y, Fujii R. 1999. Cis-trans carotenoids in photosynthesis: configurations, excited-state properties and physiological functions. In The photochemistry of carotenoids (eds Frank HA, Young AJ, Britton G, Cogdell RJ), pp. 161–188. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 51.Rimai L, Heyde ME, Gill D. 1973. Vibrational spectra of some carotenoids and related linear polyenes. Raman spectroscopic study. J. Am. Chem. Soc. 95, 4493–4501. ( 10.1021/ja00795a005) [DOI] [PubMed] [Google Scholar]
- 52.Koyama Y, Kito M, Takii T, Saiki K, Tsukida K, Yamashita J. 1982. Configuration of the carotenoid in the reaction centers of photosynthetic bacteria. Comparison of the resonance Raman spectrum of the reaction center of Rhodopseudomonas sphaeroides G1C with those of cis-trans isomers of β-carotene. Biochim. Biophys. Acta 680, 109–118. ( 10.1016/0005-2728(82)90001-9) [DOI] [Google Scholar]
- 53.Koyama Y, Takatsuka I, Nakata M, Tasumi M. 1988. Raman and infrared spectra of the all-trans, 7-cis, 9-cis, 13-cis and 15-cis isomers of β-carotene: key bands distinguishing stretched or terminal-bent configurations form central-bent configurations. J. Raman Spectrosc. 19, 37–49. ( 10.1002/jrs.1250190107) [DOI] [Google Scholar]
- 54.Andreeva A, Apostolova I, Velitchkova M. 2011. Temperature dependence of resonance Raman spectra of carotenoids. Spectrochim. Acta A Mol. Biomol. Spectrosc. 78, 1261–1265. ( 10.1016/j.saa.2010.12.071) [DOI] [PubMed] [Google Scholar]
- 55.Mendes-Pinto MM, Galzerano D, Telfer A, Pascal AA, Robert B, Ilioaia C. 2013. Mechanisms underlying carotenoid absorption in oxygenic photosynthetic proteins. J. Biol. Chem. 288, 18 758–18 765. ( 10.1074/jbc.M112.423681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salares VR, Young NM, Carey PR, Bernstein HJ. 1977. Excited state (excitation) interactions in polyene aggregates. Resonance Raman and absorption spectroscopic evidence. J. Raman Spectrosc. 6, 282–288. ( 10.1002/jrs.1250060605) [DOI] [Google Scholar]
- 57.Lutz M, Szponarski W, Berger G, Robert B, Neumann J-M. 1987. The stereoisomerization of bacterial, reaction-center-bound carotenoids revisited: an electronic absorption, resonance Raman and NMR study. Biochem. Biophys. Acta 894, 423–433. ( 10.1016/0005-2728(87)90121-6) [DOI] [Google Scholar]
- 58.Wirtz AC, van Hemert MC, Lugtenburg J, Frank HA, Groenen EJJ. 2007. Two stereoisomers of spheroidene in the Rhodobacter sphaeroides R26 reaction center: a DFT analysis of resonance Raman spectra. Biophys. J. 93, 981–991. ( 10.1529/biophysj.106.103473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dokter AM, van Hemert MC, In't Velt CM, van der Hoef K, Lugtenburg J, Frank HA, Groenen EJJ. 2002. Resonance Raman spectrum of all-trans-spheroidene. DFT analysis and isotope labeling . J. Phys. Chem. A 106, 9463–9469. ( 10.1021/jp026164e) [DOI] [Google Scholar]
- 60.Kish E, Mendes Pinto MM, Bovi D, Basire M, Guidoni L, Vuilleumier R, Robert B, Spezia R, Mezzetti A. 2014. Fermi resonance as a tool for probing peridinin environment. J. Phys. Chem. B 118, 5873–5881. ( 10.1021/jp501667t) [DOI] [PubMed] [Google Scholar]
- 61.Macernis M, Galzerano D, Sulskus J, Kish E, Kim Y-H, Koo S, Valkunas L, Robert B. 2015. Resonance Raman spectra of carotenoid molecules: influence of methyl substitutions. J. Phys. Chem. A 119, 56–66. ( 10.1021/jp510426m) [DOI] [PubMed] [Google Scholar]
- 62.Macernis M, Sulskus J, Malickaja S, Robert B, Valkunas L. 2014. Resonance Raman spectra and electronic transitions in carotenoids: a density functional theory study. J. Phys. Chem. A 118, 1817–1825. ( 10.1021/jp406449c) [DOI] [PubMed] [Google Scholar]
- 63.Robert B. 2009. Resonance Raman spectroscopy. Photosynth. Res. 101, 147–155. ( 10.1007/s11120-009-9440-4) [DOI] [PubMed] [Google Scholar]
- 64.Araki G, Murai T. 1952. Molecular structure and absorption spectra of carotenoids. Prog. Theor. Phys. 8, 639–654. ( 10.1143/PTP.8.639) [DOI] [Google Scholar]
- 65.Dale J. 1954. Empirical relationships of the minor bands in the absorption spectra of polyenes. Acta Chem. Scand. 8, 1235–1256. ( 10.3891/acta.chem.scand.08-1235) [DOI] [Google Scholar]
- 66.Hemley R, Kohler BE. 1977. Electronic structure of polyenes related to the visual chromophore. A simple model for the observed band shapes. Biophys. J. 20, 377–382. ( 10.1016/S0006-3495(77)85556-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christensen RL, Barney EA, Broene RD, Galinato MGI, Frank HA. 2004. Linear polyenes: models for the spectroscopy and photophysics of carotenoids. Arch. Biochem. Biophys. 430, 30–36. ( 10.1016/j.abb.2004.02.026) [DOI] [PubMed] [Google Scholar]
- 68.Polívka T, Sundström V. 2004. Ultrafast dynamics of carotenoid excited states−from solution to natural and artificial systems. Chem. Rev. 104, 2021–2072. ( 10.1021/cr020674n) [DOI] [PubMed] [Google Scholar]
- 69.Merlin JC. 1985. Resonance Raman spectroscopy of carotenoids and carotenoid-containing systems. Pure Appl. Chem. 57, 785–792. ( 10.1351/pac198557050785) [DOI] [Google Scholar]
- 70.Knoll K, Schrock RR. 1989. Preparation of tert-butyl-capped polyenes containing up to 15 double bonds. J. Am. Chem. Soc. 111, 7989–8004. ( 10.1021/ja00202a045) [DOI] [Google Scholar]
- 71.Frank HA, Josue JS, Bautista JA, van der Hoef I, Jansen FJ, Lugtenburg J, Wiederrecht G, Christensen RL. 2002. Spectroscopic and photochemical properties of open-chain carotenoids. J. Phys. Chem. B 106, 2083–2092. ( 10.1021/jp013321l) [DOI] [Google Scholar]
- 72.Wasielewski MR, Kispert LD. 1986. Direct measurement of the lowest excited singlet state lifetime of all-trans-β-carotene and related carotenoids. Chem. Phys. Lett. 128, 238–243. ( 10.1016/0009-2614(86)80332-3) [DOI] [Google Scholar]
- 73.Frank HA, Desamero RZB, Chynwat V, Gebhard R, van der Hoef I, Jansen FJ, Lugtenburg J, Gosztola D, Wasielewski MR. 1997. Spectroscopic properties of spheroidene analogs having different extents of Π-electron conjugation. J. Phys. Chem. A 101, 149–157. ( 10.1021/jp962373l) [DOI] [Google Scholar]
- 74.Withnall R, Chowdhry BZ, Silver J, Edwards HGM, de Oliveira LFC. 2003. Raman spectra of carotenoids in natural products. Spectrochim. Acta A Mol. Biomol. Spectrosc. 59, 2207–2212. ( 10.1016/S1386-1425(03)00064-7) [DOI] [PubMed] [Google Scholar]
- 75.Mendes-Pinto MM, Sansiaume E, Hashimoto H, Pascal AA, Gall A, Robert B. 2013. Electronic absorption and ground state structure of carotenoid molecules. J. Phys. Chem. B 117, 11 015–11 021. ( 10.1021/jp309908r) [DOI] [PubMed] [Google Scholar]
- 76.Fuciman M, Keşan G, LaFountain AM, Frank HA, Polívka T. 2015. Tuning the spectroscopic properties of aryl carotenoids by slight changes in structure. J. Phys. Chem. B 119, 1457–1467. ( 10.1021/jp512354r) [DOI] [PubMed] [Google Scholar]
- 77.Fujii R, Inaba T, Watanabe Y, Koyama Y, Zhang J-P. 2003. Two different pathways of internal conversion in carotenoids depending on the length of the conjugated chain. Chem. Phys. Lett. 369, 165–172. ( 10.1016/S0009-2614(02)01999-1) [DOI] [Google Scholar]
- 78.Christensen RL, Kohler BE. 1973. Low resolution optical spectroscopy of retinyl polyenes: low lying electronic levels and spectral broadness. Photochem. Photobiol. 18, 293–301. ( 10.1111/j.1751-1097.1973.tb06424.x) [DOI] [Google Scholar]
- 79.Fuciman M, Chabera P, Zupcanova A, Hribek P, Arellano JB, Vacha F, Psencik J, Polivka T. 2010. Excited state properties of aryl carotenoids. Phys. Chem. Chem. Phys. 12, 3112–3120. ( 10.1039/B921384H) [DOI] [PubMed] [Google Scholar]
- 80.Suzuki H, Mizuhashi S. 1964. Π-electronic structure and absorption spectra of carotenoids. J. Phys. Soc. Jpn. 19, 724–738. ( 10.1143/JPSJ.19.724) [DOI] [Google Scholar]
- 81.LeRosen AL, Reid CE. 1952. An investigation of certain solvent effect in absorption spectra. J. Chem. Phys. 20, 233–236. ( 10.1063/1.1700384) [DOI] [Google Scholar]
- 82.Hirayama K. 1955. Absorption spectra and chemical structure. II. Solvent effect . J. Am. Chem. Soc. 77, 379–381. ( 10.1021/ja01607a042) [DOI] [Google Scholar]
- 83.Andersson PO, Gillbro T, Ferguson L, Cogdell RJ. 1991. Absorption spectral shifts of carotenoids related to medium polarizability. Photochem. Photobiol. 54, 353–360. ( 10.1111/j.1751-1097.1991.tb02027.x) [DOI] [Google Scholar]
- 84.Kuici M, Nagae H, Cogdell RJ, Shimada K, Koyama Y. 1994. Solvent effect on spheroidene in nonpolar and polar solutions and the environment of spheroidene in the light-harvesting complexes of Rhodobacter sphaeroides 2.4.1 as revealed by the energy of the 1Ag−→1Bu+ absorption and the frequencies of the vibronically coupled C=C stretching Raman lines in the 1Ag− and 1Bu− states. Photochem. Photobiol. 59, 116–124. ( 10.1111/j.1751-1097.1994.tb05009.x) [DOI] [Google Scholar]
- 85.Chen Z, Lee C, Lenzer T, Oum K. 2006. Solvent effects on the S0(1Ag−)→S2(1Bu+) transition of β-carotene, echinenone, canthaxanthin, and astaxanthin in supercritical CO2 and CF3H. J. Phys. Chem. A 110, 11 291–11 297. ( 10.1021/jp0643247) [DOI] [PubMed] [Google Scholar]
- 86.Renge I, Sild E. 2011. Absorption shifts in carotenoids—influence of index of refraction and submolecular electric fields. J. Photochem. Photobiol. A 218, 156–161. ( 10.1016/j.jphotochem.2010.12.015) [DOI] [Google Scholar]
- 87.Frank HA, Bautista JA, Josue J, Pendon Z, Hiller RG, Sharples FP, Gosztola D, Wasielewski MR. 2000. Effect of the solvent environment on the spectroscopic properties and dynamics of the lowest excited states of carotenoids. J. Phys. Chem. B 104, 4569–4577. ( 10.1021/jp000079u) [DOI] [Google Scholar]
- 88.Llansola-Portoles MJ, Uragami C, Pascal AA, Bina D, Litvin R, Robert B. 2016. Pigment structure in the FCP-like light-harvesting complex from Chromera velia. Biochim. Biophys. Acta 1857, 1759–1765. ( 10.1016/j.bbabio.2016.08.006) [DOI] [PubMed] [Google Scholar]
- 89.Kish E, Pinto MMM, Kirilovsky D, Spezia R, Robert B. 2015. Echinenone vibrational properties: from solvents to the orange carotenoid protein. Biochim. Biophys. Acta 1847, 1044–1054. ( 10.1016/j.bbabio.2015.05.010) [DOI] [PubMed] [Google Scholar]
- 90.Takaichi S. 1999. Carotenoids and carotenogenesis in anoxygenic photosynthetic bacteria. In The photochemistry of carotenoids (eds Frank HA, Young AJ, Britton G, Cogdell RJ), pp. 39–69. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 91.Angerhofer A, Bornhäuser F, Gall A, Cogdell RJ. 1995. Optical and optically detected magnetic resonance investigation on purple photosynthetic bacterial antenna complexes. Chem. Phys. 194, 259–274. ( 10.1016/0301-0104(95)00022-G) [DOI] [Google Scholar]
- 92.Ruban AV, Pascal AA, Robert B. 2000. Xanthophylls of the major photosynthetic lightharvesting complex of plants: identification, conformation and dynamics. FEBS Lett. 477, 181–185. ( 10.1016/S0014-5793(00)01799-3) [DOI] [PubMed] [Google Scholar]
- 93.Caffarri S, Croce R, Breton J, Bassi R. 2001. The major antenna complex of photosystem II has a xanthophyll binding site not involved in light harvesting. J. Biol. Chem. 276, 35 924–35 933. ( 10.1074/jbc.M105199200) [DOI] [PubMed] [Google Scholar]
- 94.Llansola-Portoles MJ, Sobotka R, Kish E, Shukla MK, Pascal AA, Polívka T, Robert B. 2017. Twisting a β-carotene, an adaptive trick from nature for dissipating energy during photoprotection. J. Biol. Chem. 292, 1396–1403. ( 10.1074/jbc.M116.753723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Polívka T, Kerfeld CA, Pascher T, Sundström V. 2005. Spectroscopic properties of the carotenoid 3‘-hydroxyechinenone in the orange carotenoid protein from the cyanobacterium Arthrospira maxima. Biochemistry 44, 3994–4003. ( 10.1021/bi047473t) [DOI] [PubMed] [Google Scholar]
- 96.Polívka T, Chábera P, Kerfeld CA. 2013. Carotenoid–protein interaction alters the S1 energy of hydroxyechinenone in the orange carotenoid protein. Biochim. Biophys. Acta 1827, 248–254. ( 10.1016/j.bbabio.2012.10.005) [DOI] [PubMed] [Google Scholar]
- 97.Shima S, Ilagan RP, Gillespie N, Sommer BJ, Hiller RG, Sharples FP, Frank HA, Birge RR. 2003. Two-photon and fluorescence spectroscopy and the effect of environment on the photochemical properties of peridinin in solution and in the peridinin-chlorophyll-protein from Amphidinium carterae. J. Phys. Chem. A 107, 8052–8066. ( 10.1021/jp022648z). [DOI] [Google Scholar]
- 98.Premvardhan L, Bordes L, Beer A, Büchel C, Robert B. 2009. Carotenoid structures and environments in trimeric and oligomeric fucoxanthin chlorophyll a/c2 proteins from resonance Raman spectroscopy. J. Phys. Chem. B 113, 12 565–12 574. ( 10.1021/jp903029g) [DOI] [PubMed] [Google Scholar]
- 99.Büchel C. 2003. Fucoxanthin-chlorophyll proteins in diatoms: 18 and 19 kDa subunits assemble into different oligomeric states. Biochemistry 42, 13 027–13 034. ( 10.1021/bi0349468) [DOI] [PubMed] [Google Scholar]
- 100.Feng J, Tseng C-W, Chen T, Leng X, Yin H, Cheng Y-C, Rohlfing M, Ma Y. 2017. A new energy transfer channel from carotenoids to chlorophylls in purple bacteria. Nat. Commun. 8, 71 ( 10.1038/s41467-017-00120-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Desamero RZB, et al. 1998. Mechanism of energy transfer from carotenoids to bacteriochlorophyll: light-harvesting by carotenoids having different extents of Π-electron conjugation incorporated into the B850 antenna complex from the carotenoidless bacterium Rhodobacter sphaeroides R-26.1. J. Phys. Chem. B 102, 8151–8162. ( 10.1021/jp980911j) [DOI] [Google Scholar]
- 102.Mattioli TA, Hoffmann A, Sockalingum DG, Schrader B, Robert B, Lutz M. 1993. Application of near-IR Fourier transform resonance Raman spectroscopy to the study of photosynthetic proteins. Spectrochim. Acta A Mol. Spectrosc. 49, 785–799. ( 10.1016/0584-8539(93)80103-H) [DOI] [Google Scholar]
- 103.McDermott G, Prince SM, Freer AA, Hawthornthwaite-Lawless AM, Papiz MZ, Cogdell RJ, Isaacs NW. 1995. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature 374, 517–521. ( 10.1038/374517a0) [DOI] [Google Scholar]
- 104.van Dorssen RJ, Breton J, Plijter JJ, Satoh K, van Gorkom HJ, Amesz J. 1987. Spectroscopic properties of the reaction center and of the 47 kDa chlorophyll protein of photosystem II. Biochim. Biophys. Acta 893, 267–274. ( 10.1016/0005-2728(87)90048-X) [DOI] [Google Scholar]
- 105.Kwa SLS, Newell WR, van Grondelle R, Dekker JP. 1992. The reaction center of photosystem II studied with polarized fluorescence spectroscopy. Biochim. Biophys. Acta 1099, 193–202. ( 10.1016/0005-2728(92)90027-Y) [DOI] [Google Scholar]
- 106.Tomo T, Mimuro M, Iwaki M, Kobayashi M, Itoh S, Satoh K. 1997. Topology of pigments in the isolated photosystem II reaction center studied by selective extraction. Biochim. Biophys. Acta 1321, 21–30. ( 10.1016/S0005-2728(97)00037-6) [DOI] [Google Scholar]
- 107.Kirilovsky D, Kerfeld CA. 2013. The orange carotenoid protein: a blue-green light photoactive protein. Photochem. Photobiol. Sci. 12, 1135–1143. ( 10.1039/C3PP25406B) [DOI] [PubMed] [Google Scholar]
- 108.Kirilovsky D, Kerfeld CA.. 2016. Cyanobacterial photoprotection by the orange carotenoid protein. Nat. Plants 2, 16180 ( 10.1038/nplants.2016.180) [DOI] [PubMed] [Google Scholar]
- 109.Wilson A, Kinney JN, Zwart PH, Punginelli C, D'Haene S, Perreau F, Klein MG, Kirilovsky D, Kerfeld CA. 2010. Structural determinants underlying photoprotection in the photoactive orange carotenoid protein of cyanobacteria. J. Biol. Chem. 285, 18 364–18 375. ( 10.1074/jbc.M110.115709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilson A, et al. 2008. A photoactive carotenoid protein acting as light intensity sensor. Proc. Natl Acad. Sci. USA 105, 12 075–12 080. ( 10.1073/pnas.0804636105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Durchan M, et al. 2014. Highly efficient energy transfer from a carbonyl carotenoid to chlorophyll a in the main light harvesting complex of Chromera velia. Biochim. Biophys. Acta 1837, 1748–1755. ( 10.1016/j.bbabio.2014.06.001) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.