Abstract
The lidocaine-prilocaine cream (EMLATM) effectively reduces the pain from debridement of chronic leg ulcers. Studies have demonstrated that when applied to leg ulcers, plasma concentrations of the local anaesthetics are well below the threshold for CNS toxicity. However, there are minimal pharmacokinetic data available from EMLA application to burn wounds. This study evaluated EMLA cream for debridement of burns with regard to plasma concentrations of lidocaine and prilocaine, and reviewed the published literature on safety and efficacy of lidocaine-prilocaine applied epicutaneously to burns. Eight patients aged 22-59 received 5 g of EMLA 5% cream applied to 25 cm2 large 2nd degree burn areas for 30 min. Venous blood samples drawn at set intervals up to 120 min after cream application were analyzed for total plasma concentrations of lidocaine and prilocaine. Pain from debridement was assessed on a 4-point verbal scale and a 100-mm visual analog scale (VAS) with the end points “no pain” and “severe pain”. A literature search on the use of lidocaine-prilocaine cream on burn wounds was performed in PubMed. The results showed that six patients felt no pain and two patients mild pain. The median VAS score was 11 (range 2-59). Peak plasma concentrations of lidocaine (mean 205 ng/ml) and prilocaine (mean 97 ng/ml) were observed after 15-60 min. Two published studies and two case reports of overdose of lidocaine-prilocaine cream applied to burns in paediatric patients were retrieved. Peak plasma concentrations of lidocaine and prilocaine combined after application of 5 g EMLA to burns 25 cm2 large for 30 min in adults are far below those associated with toxicity. Bioavailability estimation suggests 5 to 30% of the prilocaine dose applied to burns is percutaneously absorbed. The analgesic efficacy appears satisfactory for debridement of 2nd degree burns.
Keywords: Patient-rated pain, procedure-related pain, pharmacokinetics, methaemoglobin, topical application, wound debridement
Introduction
Cleansing is an important part of burn care. It is a crucial part of infection prevention and healing. In some patients sharp debridement is required [1]. Patients with thermal injuries suffer pain both due to their injury and due to debridement and wound dressing changes [2-4]. Current pain management regimes in burn care are mostly based on opioid analgesics [4]. Debridement of burn wounds can be extremely painful even when large doses of opioids are used as premedication [5].
The lidocaine-prilocaine cream, EMLATM (Eutectic Mixture of Local Anaesthetics, AstraZeneca), effectively reduces the pain from sharp debridement of chronic leg ulcers after application for 30 minutes [6-8], while the plasma concentrations of lidocaine and prilocaine after application of 10 g EMLA cream before debridement of leg ulcers are well below the threshold for CNS toxicity [6,9-11]. However, there are minimal pharmacokinetic data available from EMLA treatment of burn wounds [12,13], although its use on newly healed skin in a dose of 0.5-1.4 g cream per kg bodyweight to ameliorate post-burn pruritus in children was found to result in plasma concentrations well below the threshold associated with systemic adverse effects [14]. Two cases of overdose of application of EMLA cream to paediatric patients (60 g in a 19-month and 45-90 g in a 3.5-year old child) with burns have been reported [15,16]. Dose recommendations for application of EMLA cream to burns are currently not included in prescription formularies approved by regulatory authorities. This study was performed to determine the maximum plasma concentrations of lidocaine and prilocaine following EMLA application to burns, and to evaluate the analgesic efficacy for wound cleansing and debridement. In a post-hoc analysis the bioavailability of lidocaine and prilocaine was estimated by comparison with previous data from intravenous administration in healthy volunteers. Published data from application of lidocaine-prilocaine cream to burns are reviewed.
Materials and methods
Study design and ethics
This open study was performed at the Department of Anaesthesiology, Haukeland University Hospital, Bergen. The Hospital Ethics Committee approved the study, and the patients were informed about the details of the study and gave their consent to participation in accordance with the Declaration of Helsinki.
Patients
Eight male patients aged 22 to 59 years scheduled for cleansing and debridement of 2nd degree burns during their first week in hospital participated in the study (Table 1). None of the patients were allergic to amide-type local anaesthetics.
Table 1.
Demographic and pharmacokinetic data for lidocaine and prilocaine by patient, after application of 5 g EMLA cream to a 25 cm2 area of 2nd degree burns for 30 min. The bioavailability was estimated by comparison of each patient’s AUC value with the mean AUC from intravenous administration in healthy volunteers in a previous study
| Patient number | Age (years) | Sex | Weight (kg) | Duration of burn (days) | Site of burn | Lidocaine | Prilocaine | Lidocaine | Prilocaine | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Cmax (ng/ml) | tmax (min) | Cmax (ng/ml) | tmax (min) | AUC0-inf (ng h/ml) | AUC% t-inf | F (%) | AUC0-inf (ng h/ml) | AUC% t-inf | F (%) | ||||||
| 1 | 59 | M | 89 | 2 | Anterior chest | 412 | 15 | 206 | 15 | 395.9 | 20 | 14.9 | 115.5 | 0 | 11.6 |
| 2 | 40 | M | 96 | 2 | Right forearm | 139 | 30 | 51 | 15 | 370.2 | 67 | 14.0 | 50.7 | 0 | 5.1 |
| 3 | 39 | M | 76 | 9 | Abdomen | 166 | 60 | 51 | 15 | 300.9 | 28 | 11.3 | 245.7 | 207 | 24.7 |
| 4 | 22 | M | 75 | 3 | Dorsum right foot | 47 | 30 | 40 | 30 | 49.2 | 0 | 1.86 | 218.7 | 239 | 22.0 |
| 5 | 23 | M | 69 | 5 | Dorsum left hand | 230 | 30 | 86 | 15 | 317.2 | 29 | 12.0 | 176.5 | 62.3 | 17.7 |
| 6 | 32 | M | 135 | 3 | Upper thorax | 169 | 60 | 59 | 15 | 432.8 | 93 | 16.3 | 178.0 | 92.7 | 17.9 |
| 7 | 23 | M | * | 4 | Hand | 152 | 30 | 92 | 30 | 194.2 | 21 | 7.3 | 265.3 | 142 | 26.7 |
| 8 | 55 | M | 94 | 3 | Upper thorax | 323 | 30 | 191 | 30 | 427.8 | 27 | 16.1 | 299.7 | 50.1 | 30.1 |
| Mean | 36 | 90 | 3 | 205 | 36 | 97 | 21 | 311.0 | 11.7 | 193.8 | 19.7 | ||||
| Range | (22-59) | (69-135) | (2-9) | (47-412) | (15-60) | (40-206) | (15-30) | (49-433) | (1.9-16.3) | (51-300) | (5.1-30.1) | ||||
*missing data, min: minutes, Cmax: maximum observed plasma concentration, tmax: time of Cmax, AUC0-inf: Area under the plasma concentration curve to last observed time point and extrapolated to infinity, AUC% t-inf: percent extrapolated AUC as compared to observed AUC; F: estimated bioavailability of 30 minutes topical application to burns as compared to intravenous administration in healthy volunteers.
Anaesthetic and debridement procedures
A burn area measuring 25 cm2 was selected for debridement. Seven patients were given opioids for acute burn pain (in one case for physiotherapy), or as part of premedication 20 min to 5 hours prior to debridement (Table 2). The wound dressing was removed to the point when the patient felt pain, so that at least a 25 cm2 burn area was exposed. Then a dose of 5 g EMLA 5% cream (sterile formulation) was applied in a thick layer to the burn, covered with plastic cling film forming an occlusive dressing. After 30 min the cling film was removed and the remaining cream was wiped away using a wooden spatula. The area was inspected and local reactions were recorded. Scissors, pincers and cotton swabs were used for debridement and cleansing of the burn for a period of 5-10 min (median 5 min).
Table 2.
Premedication and patients’ assessment of pain from debridement. The median VAS pain score was 11 (100 indicated “severe pain”)
| Patient | Premedication | Premedication time before procedure | Pain from debridement | |
|---|---|---|---|---|
|
| ||||
| VRS | VAS | |||
| 1 | Morphine 5 mg i.v. | 76 min | None | 2 |
| 2 | Morphine 5 mg i.v. | 300 min | Mild | 59 |
| 3 | Morphine 10 mg i.m. | 20 min | None | 2 |
| 4 | Paracetamol 800 mg codeine 60 mg oral | 69 min | None | 18 |
| 5 | No analgesic | Mild | 27 | |
| 6 | Morphine 20 mg i.m. | 20 min | None | 6 |
| 7 | Diazepam 5 mg i.v. pethidine 35 mg i.m. | 68 min | None | 16 |
| 8 | Morphine 20 mg i.v. | 107 min | None | 2 |
VRS: Verbal Rating Scale, VAS: Visual Analogue Scale.
Clinical efficacy
During the application of the cream the patients were asked about the presence of local sensations, particularly an itching or a burning sensation. The severity of the sensations was graded as none, slight, moderate or severe. After the removal of the dressing and remaining cream, the surgeon assessed the wound area for local skin reactions. Immediately after debridement, the patient rated the pain from the procedure on a verbal scale as none, slight, moderate or severe and on a 100-mm horizontal visual analogue scale (VAS) [17], with the end points “no pain” and “severe pain”. The operating surgeon classified the debridement conditions in each patient as satisfactory or unsatisfactory. The surgeon also subjectively ranked the effectiveness of the procedure (better, as usual, or worse) compared to a standard analgesic regimen, consisting of opioids, sometimes in combination with benzodiazepines. The patient was asked about the presence of post-procedural pain in the wound area at the completion of debridement, and in the case of no pain, was requested to report the time of occurrence of first pain.
Blood sampling
Five ml blood samples were drawn from a forearm vein prior to application of cream and 15, 30, 60, 90, and 120 min after application. The blood samples were drawn into heparinised VenojectTM tubes (Terumo Co., Tokyo, Japan). After centrifugation the plasma was transferred to CryotubesTM (Nunc, Roskilde, Denmark) and stored at -20°C until analysis.
Bioanalytical assay of lidocaine and prilocaine
The total plasma concentrations of lidocaine base and prilocaine base were determined at the Department of Bioanalytical Chemistry, AstraZeneca R&D, Sweden, by capillary gas chromatography using a nitrogen-sensitive detector. The limit of quantification was 9 ng/ml [18].
Pharmacokinetic evaluation
The maximum plasma concentrations (Cmax) of lidocaine and prilocaine and the time to reach Cmax (tmax) were obtained directly from the observed plasma concentration data for each patient. The 95% confidence intervals for the mean plasma concentration of each agent were calculated. In a post-hoc analysis, areas under the plasma concentration curve (AUC) for lidocaine and prilocaine were calculated using “linear up and log down” trapezoidal rule implemented in R package PKNCA (version 0.8.1). AUC up to the last sampling time point (AUC0-t) were calculated with observed plasma concentrations, and in patients where the 120 minute plasma sample was above the limit of quantification, the remaining area up to infinity (AUCt-inf) was extrapolated from the last observed value.
In order to estimate the bioavailability (F) from topical application of 5 g EMLA cream (containing 125 mg lidocaine base and 125 mg prilocaine base) to burns, each patient’s total AUC up to infinity (AUC0-inf) was compared to the mean AUC after IV administration (AUCIV) of a mixture of 10 mg lidocaine hydrochloride and 10 mg prilocaine hydrochloride in a previous AstraZeneca study in 13 healthy volunteers. In volunteers the mean AUCIV values were 183.56 and 68.25 ng h/mL for lidocaine and prilocaine, respectively (Jan Sjövall, AstraZeneca R&D Sweden, Personal communication). The bioavailability in each patient was estimated as: (AUC0-inf/AUCIV) x (Dose base formIV/Dose base formtopical), i.e.: for lidocaine: F= (AUC0-inf/183.56) × (8.65/125) for prilocaine: F= (AUC0-inf/68.25) × (8.58/125) as 1 mg lidocaine HCl corresponds to 0.865 mg lidocaine base, and 1 mg prilocaine HCl corresponds to 0.858 mg prilocaine base.
Literature search
Pubmed was searched for the terms (‘lidocaine’ AND ‘prilocaine’) AND (‘burn’ OR ‘wound’) with no restriction in time. Repeated searches were performed, the most recent search in January 2017.
Results
Clinical efficacy
During cream application three patients experienced a slight or moderate burning sensation and no patient reported itching. No erythema or edema was observed after removal of the cream. Six patients felt no pain and two patients slight pain from debridement. The patients’ median VAS pain rating of the procedure was 11 out of 100 (range 2-59) (Table 2). No patient felt immediate post-procedural pain in the burn wound, while 2 patients reported pain after 2 hours. The surgeon considered the debridement to have been satisfactory in 7 patients (data missing for one patient). In all cases the surgeon considered the analgesic effect to be better than the standard regimen.
Pharmacokinetics
The Cmax values of lidocaine ranged from 47 to 412 ng/ml and those of prilocaine from 40 to 206 ng/ml (Figures 1, 2, Table 1). The plasma concentrations of prilocaine were approximately 40-50% of the lidocaine concentrations throughout the sampling intervals (Figure 3). Tmax for lidocaine occurred at 15-30 min and for prilocaine somewhat later, at 30-60 min. The highest concentrations of lidocaine and prilocaine (combined 618 ng/ml) were observed at 15 mins in the same patient (#1), with a substantial decline after 1 hour. At the time of the last sample, 120 min, the lidocaine concentration was below or close to 100 ng/ml in all patients (Figure 1). For prilocaine, the concentrations in all patients were below or close to 50 ng/ml at 120 min (Figure 2).
Figure 1.
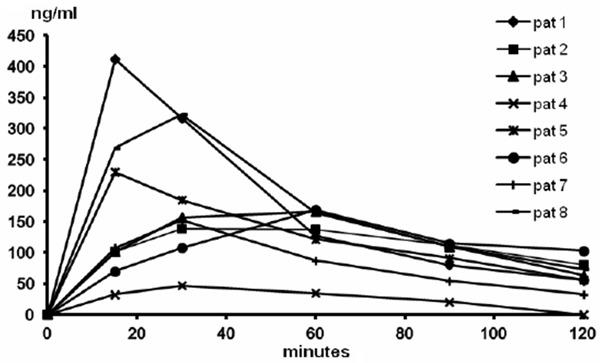
Lidocaine plasma concentration profiles in individual patients after application of 5 g EMLA to a 25 cm2 area of 2nd degree burns for 30 min.
Figure 2.
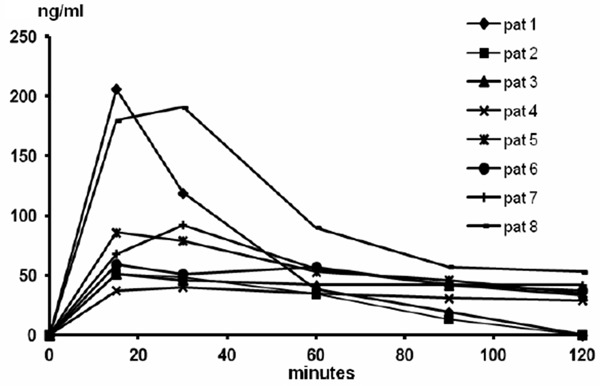
Prilocaine plasma concentration profiles in individual patients after application of 5 g EMLA to a 25 cm2 area of 2nd degree burns for 30 min.
Figure 3.

The 95% confidence intervals for the mean lidocaine and prilocaine plasma concentrations up to 2 hours after application of 5 g EMLA to a 25 cm2 area of 2nd degree burns for 30 min (on the X-axis lidocaine values were moved 2 min to the left to avoid overlap of bars).
The estimation of the AUC to infinity resulted in extrapolation to more than 30% of the observed AUC in two patients for lidocaine, and in six patients for prilocaine (Table 1). The bioavailability was estimated as a mean (range) of 11.7 (1.9-16.3)% and 19.7 (5.1-30.1)% for lidocaine and prilocaine, respectively (Table 1).
Discussion
Efficacy, onset and duration of local anaesthesia for debridement
Procedural pain in burn wound care is considered one of the most difficult forms of acute pain to treat, and adjuvants to opioids such as intravenous lidocaine have been evaluated in a double-blind study with some benefit for patients [4]. The anaesthetic onset time of EMLA for sharp debridement of chronic leg ulcers is 20-30 minutes [6,19], but peak plasma concentrations are observed 60-150 minutes after the start of the application [6]. A comparison with our data suggests that systemic absorption from the cutaneous tissues may be faster when EMLA is applied to burns compared to when it is applied to leg ulcers. This implies that the duration of analgesia may be shorter in patients with burns. However, none of the patients in the current study felt immediate post-procedural pain in the burn wound, although 2 patients reported pain after 2 hours. This compares well with the duration of analgesia in venous leg ulcer patients where EMLA application for 30-45 min significantly decreased the occurrence of post-debridement pain during the first four hours, compared to control patients [20]. In a controlled study in 30 children with painful first or second degree facial burns, incorporation of EMLA cream into the gauze wound dressing, mixed with fucidic acid cream, was reported to reduce the requirement for analgesics (1/15 vs 8/15) in the first 8 hour period after application [13].
Plasma concentrations and relationship with local anaesthetic toxicity
To our knowledge the current study is the first report of complete plasma concentration profiles of lidocaine and prilocaine after application of EMLA to burns. Janezic [12] reported the application of a thick layer of EMLA cream for concomitant split skin grafting and surgical revision of granulation tissue of full-thickness burns covering 3% of the body surface area in an 81-year-old woman, for whom general or spinal anaesthesia was considered to be high risk. A single plasma sample taken 1 hour after removal of the cream had a concentration of 0.73 umol/l lidocaine, corresponding to 171 ng/ml. Kargi and Tekerekoglu [13] collected blood samples at 4 and 8 hours after application of the gauze with a mixture of fucidic acid and EMLA cream to facial burns (1 to 4% body surface area) in 15 children. They did not report actual plasma concentration values, but stated that “lidocaine and prilocaine concentrations were below toxic levels”. It is difficult to evaluate this study as details of the amounts of EMLA used were not given. It should be noted that EMLA cream is produced with a basic pH of 9.4 in order to ensure rapid percutaneous absorption of the local anaesthetic amine base forms. Mixing with fucidic acid cream may have affected the pH and decreased the rate of absorption in the above study.
The magnitude of the peak plasma concentrations of lidocaine and prilocaine are correlated with the occurrence of systemic toxicity. Objective adverse manifestations, such as initial muscular twitching and later convulsions, become increasingly more common with increasing total lidocaine plasma concentrations from 5,000 to 10,000 ng/ml [21]. In our study the local anaesthetic plasma concentrations from application of 5 g EMLA 5% cream (125 mg lidocaine and 125 mg prilocaine) to 2nd degree burns were low, with maximum peak values for lidocaine and prilocaine being 412 and 206 ng/ml, respectively. Assuming that lidocaine and prilocaine toxicity is additive, the combined plasma concentrations in each patient were well below (one-tenth or less) those associated with toxic symptoms.
Throughout the plasma-sampling interval prilocaine concentrations were approximately 40-50% of lidocaine concentrations (Figure 3). Differences in plasma concentrations between prilocaine and lidocaine are also observed after intravenous infusion or neural blockade, the reason being the larger volume of distribution (191 L vs 91 L) and the higher clearance of prilocaine (2.4 L/min vs 0.95 L/min) compared to lidocaine [22]. Cmax values in the majority of patients were reached after 15-30 min, demonstrating a fast absorption.
Considerations of maximum doses in adults and children
Blanke and Hallern [23] used EMLA as a topical anaesthetic for debridement of burns in doses as high as 150 g on burn areas up to 720 cm2 in 44 patients without signs of systemic toxicity. No evaluation of plasma concentration of local anaesthetic was reported. Patients’ ages were not given but it is inferred that the majority were adults. Caution is advised for the use of EMLA on acute burn wounds in children. Serious toxicity was reported after application of high doses of EMLA (estimated to 45-90 g) to a child aged three and a half years with second-degree burns to an extent of 8-10% total body surface area, together with first-degree burns on parts of the face [15]. Fifteen minutes after the application (without occlusive dressing), the patient suffered clonic generalized convulsions and became cyanotic. He was treated successfully with diazepam intravenously, whereupon the convulsions subsided. A similar case in a 19 month old infant with second degree burns on 5% of his body surface (250 cm2) covered with 60 g EMLA, and a methaemoglobin (MetHb) fraction of 16%, was subsequently reported from Germany [16]. The maximum recommended dose of EMLA applied to intact, non-injured skin, in this age group is 10 g to maximum 100 cm2 skin (European Medicines Agency, Summary of Product Characteristics EMLA Cream 2015: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Emla_cream_30/WC500173682.pdf).
Plasma concentrations of lidocaine and prilocaine are related to the likelihood of CNS and cardiovascular toxicity. The extent of percutaneous absorption of prilocaine in relation to an injected dose correlates to the risk of methaemoglobinemia. The metabolism of the prilocaine molecule can lead to oxidation of the ferrous ion (Fe2+) in haemoglobin into the ferric ion (Fe3+), i.e. the formation of methaemoglobin (MetHb) which is incapable of binding to oxygen. MetHb is naturally formed in the body. In normal erythrocytes, MetHb is continuously reduced back to haemoglobin by the enzyme NADH reductase (MetHb reductase or cytochrome b5 reductase) and the circulating fraction remains at normal values of 1-2% [24,25]. It is well known [26] that after treatment with a prilocaine-containing product, MetHb formation is not related to peak plasma concentration, but to the cumulative dose of prilocaine absorbed. At clinically recommended doses of injected prilocaine hydrochloride or epicutaneous application of EMLA this is not a problem in normal patients [26]. However with an overdose the capacity of the Methb reduction processes can be exceeded, causing methaemoglobinemia.
The clinical consequences of methaemoglobinemia are related to the blood fraction of MetHb relative to total haemoglobin: signs of hypoxia, dyspnea, nausea and tachycardia occur at MetHb fractions of 25-30% and higher [25]. Infants are more susceptible [24]. If clinical methaemoglobinemia occurs, it can be rapidly treated by a single intravenous injection of a 1% methylene blue solution, 1 mg/kg body weight, over a 5-minute period. Cyanosis will disappear in about 15 minutes [26]. Methylene blue cannot be given to patients with glucose-6-phosphate dehydrogenase deficiency [25].
Parenteral doses of prilocaine hydrochloride (HCl) exceeding 600 mg, and doses exceeding 8-10 mg/kg, have been associated with methemglobinemia in adults [26-28] These observations resulted in the maximum recommended single parenteral dose of prilocaine hydrochloride was being defined as 500 mg [26] or 600 mg in national formularies [29]. After regional nerve block with prilocaine HCl 300 mg, 600 mg or 900 mg with adrenaline the highest MetHb fraction (max 5%, 14% and 20%, respectively) was usually observed after six hours, but in some subjects at 2, 4 or 8 hours. At 24 hours the MetHb fraction had usually returned to normal values, except in some patients given 900 mg [27]. In infants, recommended epicutaneous doses of EMLA can result in a slight, clinically insignificant increase in metHb fraction from about 3 to 12-13 hours after cream application [24,30], with return to baseline values after 13 hours [30]. This recovery time, as well as the size of the previous dose(s), needs to be considered if repeated applications of EMLA are required.
In 15 children (aged 8-15) with facial burns a gauze dressing containing EMLA was applied for 24 h, and MetHb fraction remained between 1 and 3% in blood samples taken at 4 and 8 hours [13]. In 5 children aged 1-5 yrs, 13 to 20 g EMLA was applied for 1 hour to newly healed skin areas 50-150 cm2 large to ameliorate postburn pruritus. MetHb fractions remained within the normal range of 1-3%, with a slight increase between 2 and 6 hours after application [14]. Doses used in this study by Kopecky et al. corresponded to 0.5-1.4 g EMLA cream per kg bodyweight.
A parenteral dose of 600 mg prilocaine hydrochloride corresponds to 515 mg prilocaine base in EMLA cream, equivalent to a maximum of 20 gram EMLA 5% cream. One gram of EMLA contains 25 mg prilocaine, and thus in our study 125 mg prilocaine was applied to burns. The remaining cream was removed prior to debridement. In patients where EMLA was applied for one hour prior to leg ulcer debridement it has been estimated that approximately 15% of the dose of prilocaine reaches the systemic circulation [31]. Many leg ulcer patients have venous insufficiency and delayed venous return [11]. Our results suggest that systemic absorption from burns may occur faster (Figures 1, 2) than from leg ulcers, and thus the degree of systemic absorption of prilocaine (and lidocaine) should be expected to be higher from burns than from leg ulcers. The mean estimated bioavailability however after a 30-minute application of cream, as compared to IV administration, was 12% for lidocaine and 20% for prilocaine. The variability in prilocaine bioavailability ranged from 5% to 30%, but in the two burn patients with the most reliable AUC estimation (no extrapolation), the bioavailability of prilocaine was lower, 5-12%, than the mean estimate of 20%.
Diagnosis of MetHb
The diagnosis of MetHb should be made using multi-wave length CO-oximetry. This is due to its ability to measure light absorption at 4 wavelengths, rather than a traditional pulse oximeter, which measures only 2 wavelengths [32]. A CO-oximeter measures the absorption spectra of multiple different light wavelengths. The most common correspond to 600 nm (CarboxyHb), 631 nm (MetHb), 660 nm (deoxyHb), and 940 nm (oxyHb). By analysing the peak light absorption spectrum of a blood sample, CO oximetry can be used to diagnose multiple hemoglobinopathies including both carboxyHb and MetHb. Notably the antidote methylene blue absorbs light at 668 nm, which leads to an increase in the measured absorption ratio and appears as arterial desaturation on a traditional pulse oximeter [32].
Use of multi-wave length oximetry allows clinicians to measure abnormal haemoglobin species continuously, noninvasively, and in the presence of hypoxia. With modifications to the device, measurements of methemoglobin up to levels of 14.4% were accurate, even in the presence of hypoxia as low as SpO2 of 74% [32].
Considerations of sterility of the cream formulation
The sterile formulation of EMLA used in our study is no longer commercially available. The current commercial EMLA cream 5% is not sterile, although of good bacteriological quality and with inherent antibacterial properties [33,34]. Lidocaine and prilocaine are antibacterial and antiviral in concentrations above 0.5-2% [35-39]. Solutions of lidocaine 1% to 4% inhibit the growth of pathogens such as Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Lidocaine concentrations of 2% and higher also inhibit the growth of a number of hospital isolates of methicillin-resistant s.aureus and vancomycin-resistant enterococcii [40].
Prolonged local treatment of experimental burns in volunteers with commercial (non-sterile) EMLA cream for 8 h did not show any apparent adverse effect on wound healing [41]. Neither was wound infection observed by Blanke and Hallern [23] in their patients, and all burn wounds in the controlled paediatric study by Kargi and Tekerekoglu [13] had healed at the end of 2 weeks. Currently, use of ordinary tap water for irrigation of burns is recommended by the International Society for Burn Injuries [1].
Limitations of the study
The estimation of bioavailability, comparing AUC values of burn patients with those of healthy volunteers was not part of the original study plan, but was after completion of the study considered to provide valuable information. As the burn patients were aged 22 to 59 with a mean age of 36 years, their clearance of lidocaine and prilocaine may be assumed to be in the normal range and similar to the clearance of healthy volunteers. The plasma sampling schedule was designed to identify the peak, but was relatively short for the estimation of AUC, which resulted in some patients in large proportions of the prilocaine AUC originating from extrapolation of plasma concentrations to infinity. The bioavailability estimate for prilocaine therefore in these patients may be over-estimated. The opioid premedication in our study may have influenced the patients’ evaluation of the analgesic efficacy of EMLA. The doses of morphine used, 5-20 mg, sometimes combined with benzodiazepines, is representative of common analgesic regimens [42,43] employed for the wound care of burns in many centers. Another limitation is that the pain evaluation was not compared to a control group. In our study the median VAS pain score of debridement pain, 11, is low in comparison to the average pain scores of 40 to 54 rated by patients during burn wound dressing changes with opioid analgesia in other studies [42,44], and lower than what burn patients have been reported to consider be an acceptable level of pain (mean VAS score of 59) [42]. The use of EMLA cream facilitated the procedure compared to the ordinary analgesic regimen, as evaluated by the operating surgeon. Evidence in double-blind placebo-controlled trials of the efficacy in reducing sharp debridement pain in leg ulcer patients is supportive [6-9]. It may be argued that the limitation of the debrided wound area to 25 cm2 should limit the interpretation of our results to smaller burns. Minor burns, however, constitute the majority of all burns, and are treated in the out-patient setting, where the administration of opioids is less common, and an alternative effective analgesic would be useful.
In conclusion, our results indicate that a dose of 5 g EMLA cream applied to 2nd degree burns 25 cm2 large in adults for 30 min results in peak plasma concentrations of lidocaine and prilocaine that combined are far below those associated with CNS toxicity. Estimates of bioavailability suggest that 5 to 30% of the prilocaine dose applied to burns is percutaneously absorbed. The analgesic efficacy appears satisfactory for debridement of 2nd degree burns.
Acknowledgements
Funding for this study was provided by AstraZeneca Sweden. We are grateful to Diansong Zhou, Ph.D, Quantitative Clinical Pharmacology, AstraZeneca, Waltham, MA, USA, for the post-hoc estimation of the area under the plasma concentration curves for lidocaine and prilocaine after application of EMLA cream to burns, and for valuable discussions on the interpretability of extrapolated AUC values.
References
- 1.ISBI Practice Guidelines Committee; Steering Subcommittee; Advisory Subcommittee. ISBI practice guidelines for burn care. Burns. 2016;42:953–1021. doi: 10.1016/j.burns.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Atchison NE, Osgood PF, Carr DB, Szyfelbein SK. Pain during burn dressing change in children: relationship to burn area, depth and analgesic regimens. Pain. 1991;47:41–5. doi: 10.1016/0304-3959(91)90009-M. [DOI] [PubMed] [Google Scholar]
- 3.Latarjet J, Choinere M. Pain in burn patients. Burns. 1995;21:344–8. doi: 10.1016/0305-4179(95)00003-8. [DOI] [PubMed] [Google Scholar]
- 4.Wasiak J, Spinks A, Costello V, Ferraro F, Paul E, Konstantatos A, Cleland H. Adjuvant use of intravenous lidocaine for procedural burn pain relief: a randomized double-blind, placebocontrolled, cross-over trial. Burns. 2011;37:951–7. doi: 10.1016/j.burns.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Powers PS, Cruse CW, Daniels S, Stevens BA. Safety and efficacy of debridement under anesthesia in patients with burns. J Burn Care Rehabil. 1993;14:176–80. doi: 10.1097/00004630-199303000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Holm J, Andren B, Grafford K. Pain control in the surgical debridement of leg ulcers by the use of a topical lidocaine-prilocaine cream, EMLA. Acta Derm Venereol. 1990;70:132–6. [PubMed] [Google Scholar]
- 7.Rosenthal D, Murphy F, Gottschalk R, Baxter M, Lycka B, Nevin K. Using a topical anaesthetic cream to reduce pain during sharp debridement of chronic leg ulcers. J Wound Care. 2001;10:503–5. doi: 10.12968/jowc.2001.10.1.26042. [DOI] [PubMed] [Google Scholar]
- 8.Agrifoglio G, Domanin M, Baggio E, Cao P, Alberti AN, Borin F, et al. EMLA anaesthetic cream for sharp debridement of venous leg ulcers. A double-blind, placebo-controlled study. Phlebology. 2000;15:81–3. [Google Scholar]
- 9.Lok C, Paul C, Amblard P, Bessis D, Debure C, Faivre B, Guillot B, Ortonne JP, Huledal G, Kalis B. EMLA cream as a topical anesthetic for the repeated mechanical debridement of venous leg ulcers: a double-blind, placebo-controlled study. J Am Acad Dermatol. 1999;40:208–13. doi: 10.1016/s0190-9622(99)70190-8. [DOI] [PubMed] [Google Scholar]
- 10.Stymne B, Lillieborg S. Plasma concentrations of lignocaine and prilocaine after a 24-h application of analgesic cream (EMLA(R)) to leg ulcers. Br J Dermatol. 2001;145:530–54. doi: 10.1046/j.1365-2133.2001.04408.x. [DOI] [PubMed] [Google Scholar]
- 11.Effendy I, Gelber A, Lehmann P, Huledal G, Lillieborg S. Plasma concentrations and analgesic efficacy of lidocaine and prilocaine in leg ulcer-related pain during daily application of lidocaine-prilocaine cream (EMLA) for 10 days. Br J Dermatol. 2015;173:259–61. doi: 10.1111/bjd.13605. [DOI] [PubMed] [Google Scholar]
- 12.Janezic TF. Skin grafting of full thickness burns under local anaesthesia with EMLA(R) cream. Burns. 1998;24:259–63. doi: 10.1016/s0305-4179(97)00118-6. [DOI] [PubMed] [Google Scholar]
- 13.Kargi E, Tekerekoglu B. Usage of lidocaine-prilocaine cream in the treatment of postburn pain in pediatric patients. Ulus Travma Acil Cerrahi Derg. 2010;16:229–32. [PubMed] [Google Scholar]
- 14.Kopecky EA, Jacobson S, Bch MB, Hubley P, Palozzi L, Clarke HM, Koren G. Safety and pharmacokinetics of EMLA in the treatment of postburn pruritus in pediatric patients: a pilot study. J Burn Care Rehabil. 2001;22:235–42. doi: 10.1097/00004630-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Anonymous. Avoid Emla-cream in burn injuries: Undvik Emla-kräm vid brännskada. Lakartidningen (Swedish Med J) 1995;92:2503. [Google Scholar]
- 16.Book A, Fehlandt C, Krija M, Radke M, Pappert D. [Methemoglobin intoxication by prilocaine in EMLA. Accidental intoxication of an infant with scald injuries] . Anaesthesist. 2009;58:370–4. doi: 10.1007/s00101-009-1512-5. [DOI] [PubMed] [Google Scholar]
- 17.Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2:175–84. [PubMed] [Google Scholar]
- 18.Björk M, Petterson KJ, Österlöf G. Capillary gas chromatographic method for the simultaneous determination of local anaesthetics in plasma samples. J Chromatogr. 1990;533:229–34. doi: 10.1016/s0378-4347(00)82207-0. [DOI] [PubMed] [Google Scholar]
- 19.Holst RG, Kristofferson A. Lidocaine-prilocaine cream (EMLA cream) as a topical anaesthetic for the cleansing of leg ulcers: the effect of length of application time. Eur J Dermatol. 1998;8:245–7. [PubMed] [Google Scholar]
- 20.Hansson C, Holm J, Lillieborg S, Syrén A. Repeated treatment with EMLA cream 5% as a topical analgesic for the cleansing of venous leg ulcers. A controlled study. Acta Derm Venereol. 1993;73:231–3. doi: 10.2340/000155555573231233. [DOI] [PubMed] [Google Scholar]
- 21.Covino BG, Wildsmith JAW. Clinical Pharmacology of Local Anesthetic Agents. In: Cousins MJ, Bridenbaugh PO, editors. Neural Blockade in Clinical Anesthesia and Management of Pain. Philadelphia: Lippincott-Raven Publishers; 1998. pp. 97–128. [Google Scholar]
- 22.Tucker GT. Pharmacokinetics of local anaesthetics. Br J Anaesth. 1986;58:717–31. doi: 10.1093/bja/58.7.717. [DOI] [PubMed] [Google Scholar]
- 23.Blanke W, Hallern BV. Sharp wound debridement in local anaesthesia using EMLA cream: 6 years’ experience in 1084 patients. Eur J Emerg Med. 2003;10:229–31. doi: 10.1097/00063110-200309000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson A, Engberg G, Henneberg S, Danielson K, De Verdier CH. Inverse relationship between age-dependent erythrocyte activity of methaemoglobin reductase and prilocaine-induced methaemoglobinaemia during infancy. Br J Anaesth. 1990;64:72–6. doi: 10.1093/bja/64.1.72. [DOI] [PubMed] [Google Scholar]
- 25.Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf. 1996;14:394–405. doi: 10.2165/00002018-199614060-00005. [DOI] [PubMed] [Google Scholar]
- 26.Wildsmith JAW. Prilocaine: an underutilized local anesthetic. Reg Anesth. 1985;10:155–9. [Google Scholar]
- 27.Hjelm M, Holmdahl MH. Biochemical effects of aromatic amines. II. Cyanosis, methaemoglobinaemia and Heinz-body formation induced by a local anaesthetic agent (prilocaine) Acta Anaesth Scand. 1965;9:99–120. doi: 10.1111/j.1399-6576.1965.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 28.Tucker GT, Mather LE. Properties, absorption, and disposition of local anesthetic agents. In: Cousins MJ, Bridenbaugh PO, editors. Neural blockade in clinical anesthesia and management of pain. Philadelphia: Lippincott-Raven Publishers; 1998. pp. 55–95. [Google Scholar]
- 29.Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med. 2004;29:564–75. doi: 10.1016/j.rapm.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Brisman M, Ljung BM, Otterbom I, Larsson LE, Andréasson SE. Methaemoglobin formation after the use of EMLA cream in term neonates. Acta Paediatr. 1998;87:1191–4. doi: 10.1080/080352598750031202. [DOI] [PubMed] [Google Scholar]
- 31.Enander , Malmros , Nilsen T, Lillieborg S. Plasma concentrations and analgesic effect of EMLA (Lidocaine/Prilocaine) cream for the cleansing of leg ulcers. Acta Derm Venereol (Stockh) 1990;70:227–30. [PubMed] [Google Scholar]
- 32.Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth. 2014;28:1043–7. doi: 10.1053/j.jvca.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Berg JO, Mossner BK, Skov MN, Lauridsen J, Gottrup F, Kolmos HJ. Antibacterial properties of EMLA and lidocaine in wound tissue biopsies for culturing. Wound Repair Regen. 2006;14:581–5. doi: 10.1111/j.1743-6109.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 34.Batai I, Bogar L, Juhasz V, Batai R, Kerenyi M. A comparison of the antimicrobial property of lidocaine/prilocaine cream (EMLA) and an alcohol-based disinfectant on intact human skin flora. Anesth Analg. 2009;108:666–8. doi: 10.1213/ane.0b013e31818f887e. [DOI] [PubMed] [Google Scholar]
- 35.Zaidi S, Healy TE. A comparison of the antibacterial properties of six local analgesic agents. Anaesthesia. 1977;32:69–70. doi: 10.1111/j.1365-2044.1977.tb11562.x. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt RM, Rosenkranz HS. Antimicrobial activity of local anesthetics: lidocaine and procaine. J Infect Dis. 1970;121:597–607. doi: 10.1093/infdis/121.6.597. [DOI] [PubMed] [Google Scholar]
- 37.Wimberley N, Willey S, Sullivan N, Bartlett JG. Antibacterial properties of lidocaine. Chest. 1979;76:37–40. doi: 10.1378/chest.76.1.37. [DOI] [PubMed] [Google Scholar]
- 38.Miller DK, Lenard J. Antihistaminics, local anesthetics, and other amines as antiviral agents. Proc Natl Acad Sci U S A. 1981;78:3605–9. doi: 10.1073/pnas.78.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakuragi T, Ishino H, Dan K. Bactericidal activity of clinically used local anesthetics on staphylococcus aureus. Reg Anesth. 1996;21:239–42. [PubMed] [Google Scholar]
- 40.Parr AM, Zoutman DE, Davidson JS. Antimicrobial activity of lidocaine against bacteria associated with nosocomial wound infection. Ann Plast Surg. 1999;43:239–45. doi: 10.1097/00000637-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen JL, Callesen T, Moiniche S, Kehlet H. Analgesic and anti-inflammatory effects of lignocaine-prilocaine (EMLA) cream in human burn injury. Br J Anaesth. 1996;76:806–10. doi: 10.1093/bja/76.6.806. [DOI] [PubMed] [Google Scholar]
- 42.Byers JF, Bridges S, Kijek J, Laborde P. Burn patients’ pain and anxiety experiences. J Burn Care Rehabil. 2001;22:144–9. doi: 10.1097/00004630-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Thompson EM, Andrews DD, Christ-Libertin C. Efficacy and safety of procedural sedation and analgesia for burn wound care. J Burn Care Res. 2012;33:504–9. doi: 10.1097/BCR.0b013e318236fe4f. [DOI] [PubMed] [Google Scholar]
- 44.Van der Does AJ. Patients’ and nurses’ ratings of pain and anxiety during burn wound care. Pain. 1989;39:95–101. doi: 10.1016/0304-3959(89)90179-6. [DOI] [PubMed] [Google Scholar]


