Abstract
Burn injuries are one of the most devastating injuries in the world. A uniform burn wound is essential for burn research. The objective of this study was to describe a new model for inducing deep partial-thickness burns in rats. Burn wounds were performed on the dorsal part of Sprague-Dawley rats using a constructed heating device in our laboratory. Digital images of each animal were captured every day for macroscopic evaluation and for assessment of the wound contraction rate. Six animals were sacrificed on days 1, 3, 7, 11, 14, and 21 after onset of burn and their skin tissues were harvested for histological analysis. Uniform deep partial-thickness burns could be achieved in Sprague-Dawley rats under the condition of a contact temperature of 70°C, with the weight of heating devices of 300 g, and a duration of 10 s. Macroscopic evaluation recorded the general appearance of the deep partial-thickness burns. Evaluation of the wound contraction rate showed that the deep partial-thickness wound area was reduced by 90.39% of the original wound area by day 21 after burn. Microscopic evaluation by hematoxylin-eosin staining revealed the histological changes during the wound healing process. This is a standardized and reproducible model for inducing deep partial-thickness burns in Sprague-Dawley rats.
Keywords: Animal model, deep partial-thickness burn, macroscopic evaluation, microscopic evaluation
Introduction
Burn injuries are one of the most destructive injuries in the world associated with inflammation, tissue damage, infection and apparent mortality and disability [1]. According to the depth of injury, burns are categorized into superficial burn, partial-thickness burn and full-thickness burn [2]. Burns that affect only the epidermis are known as superficial or first-degree burns. It is congested, dry and painful. If burns are kept clean, there is usually no scar healing in five days [3]. When the lesion penetrates into the dermis, it is denoted as partial-thickness or second-degree burn and further classified as superficial or deep partial-thickness burn [4]. The superficial partial-thickness burn involves the epidermis and half of the dermis. The blister will be present with the pain; however, the hair is still intact. Usually the damage will heal within one to three weeks (no surgery). The deep partial-thickness burn can cause deep dermis damage. Burns appear pale than red. The skin is drier and the sensation of the skin tends to weaken and the hair is easy to fall off. Deep partial-thickness burns heal slowly and are accompanied by scar formation and potential functional loss [4]. In the full-thickness or third-degree burns, the damage extends to all dermis. The skin is dry and leathery and may be white or charred, usually requiring skin grafts [5].
A searching for an immaculate animal experimental model is important for burn research. Animal models can replace direct testing in human beings especially in the case where the test material’s toxicity is unknown. New Zealand rabbits [6], Sprague-Dawley rats [7], albino Wistar rats [8], BALB/c mice [9] and pigs [10] have been used as an animal model in burn research; rats are the most commonly used in all of these animals.
Temperature, duration, and contact pressure are the three primary variables required to achieve a uniform burn [11]; however, in practice, these three factors vary greatly for the induction of deep partial-thickness burn, which indicates a lack of standardization and uniformity for inducing burn wound.
In this study, a new apparatus was designed for studying deep partial-thickness burns in rats. In the model, the contact temperature during the burning process can be controlled precisely, and the exposure time and pressure exerted on the skin are kept constant, all of which renders it as an ideal model for creating a uniform burn wound.
The objective of this study was to describe a new model for inducing deep partial-thickness burns in rats and to record the macroscopic and microscopic changes during the burn wound healing process.
Materials and methods
Animals
Thirty six male Sprague-Dawley rats weighing 250 ± 50 g were used in this study. All rats were kept in a temperature-controlled (25 ± 1°C) environment with a 12-h light/dark cycle and kept in individual cages. They were fed with standard laboratory chow and given water ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee, University Putra Malaysia, Malaysia (AUP Number: R095/2014).
Burn injury
The rats were acclimatized to laboratory conditions for one week prior to the experiment. Then, the animals were weighed and anesthetized by intramuscular injection of 75 mg/kg ketamine (Troy Laboratories, Glendenning, Australia) and 15 mg/kg xylazine (Troy Laboratories, Glendenning, Australia). The dorsum of the sedated animals was shaved with electric clippers and depilated with a commercial depilatory cream (VEETTM; Reckitt Benckiser, NSW, Australia) to remove the remaining stubble that would have hindered the creation of a uniform burn. Then, the rats were injected subcutaneously with tramadol (12.5 mg/kg body weight) for pain control. Burn injuries to the dorsum were created using the 20-mm wide aluminum head in our in-house created heating device (described in greater detail in the Results section) for 10 s. The pressure exerted on the skin was 300 g. Wounds were created at 5-min intervals to allow the aluminum head to recover to the required temperature.
Macroscopic evaluation
Images of the burnt area of each animal were captured by digital camera (Canon SX240 HS) on day 1, 3, 7, 11, 14 and 21 after burn. A ruler was placed on the side of wound to act as a known scale between pixels, which were useful for the calculation of wound area by Image J 1.49v software. Macroscopic change of the wound including the general appearance, the size of the wound, and the time for crust formation and detachment were recorded.
Wound contraction rate
After digitalization, the wound area was analyzed by Image J 1.49v software (National Institutes of Health, Bethesda, MD, USA). The wound contraction rate was expressed as the percentage change in the original wound area using the following formula:
Wound contraction rate = (original wound area - specific day wound area)/Original wound area × 100%
The original wound area was the area of aluminum head and the specific day wound area was the area of wound measured on that particular day.
Histological analysis
After animals were sacrificed, half of the damaged skin tissue was cut off and fixed in 10% formaldehyde. The samples were then embedded in paraffin, cut into 5 µm thick sections and dried at room temperature for at least one day. Prior to staining, the slides were placed in an oven at 60°C for 1 hour to melt the paraffin. The detailed procedure of H&E staining was shown as follows: the slides were washed in xylene twice for 10 minutes each, then rehydrated in a series of ethanol solutions with continuously decreased concentration twice for 5 minutes each (100%→95%→70%). After that, they were washed briefly in distilled water and stained in hematoxylin for 8 minutes, followed by washed in running tap water for 5 minutes. The next step was to immerse the slides in 1% acid alcohol for 30 seconds and rinse in running tap water for 1 minute. The slides were then immersed in 0.2% ammonia water for 30 seconds to 1 minute and rinsed in running tap water for 5 minutes, in 95% alcohol for 10 seconds. The slides were next counterstained in eosin solution for 30 seconds to 1 minute and dehydrated by a series of alcohol solutions with continuously increased concentration (95%→100%) twice for 5 minutes each. Finally they were washed in xylene twice for 5 minutes each. Eventually, the slides were mounted with xylene based mounting medium. Histological changes in stained sections were observed under an optical microscope.
Statistical analysis
In each experiment, the mean value of the repetitions was calculated and the obtained value was used in the statistical analysis. All data are presented as means ± SD. Data were analyzed by repeated measure ANOVA with Bonferroni’s multiple compairisions test using SPSS V22 software. P < 0.05 was considered statistically significant.
Results
Burn apparatus
Burn wounds were introduced on the dorsal part of rats using a newly designed device (Figure 1). The first part of the burn apparatus was a single-channel WD 1000 power unit with 80 watts (Weller, Besigheim, Germany) (Figure 1A). It provided a stable and controlled current to the soldering pencil (Figure 1B). The temperature of the soldering pencil was adjusted by clicking the arrow buttons on the right side of the power unit. The head of the soldering iron was replaced with a 20 mm wide aluminum head (Figure 1C). An additional insulated ring (Figure 1D) was added to the exterior of the aluminum head to create a total weight of 300 g, which was the final weight exerted on the rat dorsum. A small hole was drilled at 2 mm above the contact surface of the aluminum head and inserted by a Type K thermometer probe (Figure 1E) to monitor the actual contact temperature.
Figure 1.
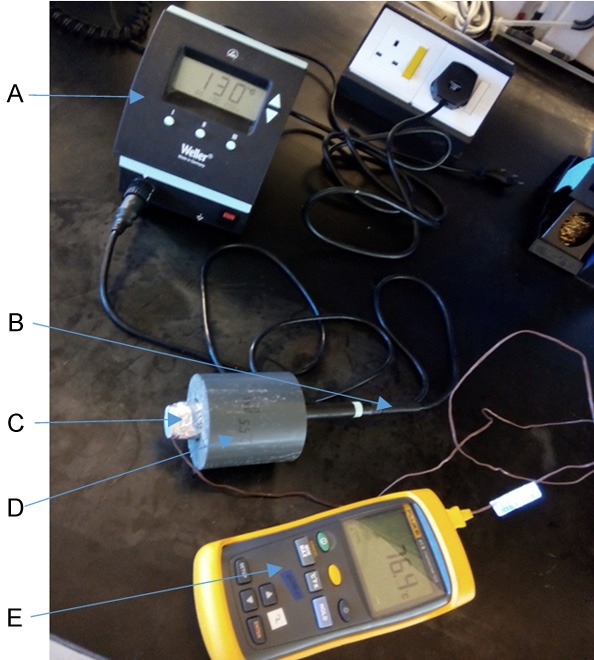
Burn wound creation apparatus. A. A single-channel WD 1000 power unit; B. A soldering pencil; C. An aluminum head with a diameter of 20 mm; D. An insulated ring; E. Type K thermometer.
Optimization of inducing deep partial-thickness burn
The contact temperature of 60°C, 65°C or 70°C was tested to the animals respectively. H&E staining of the burned area on day 3 after burn was used to confirm burn depth. Due to the fact that burn injury is a dynamic process and the depth of burn wound evolves over time; hence the necrosis will stabilize on the third day after burn, therefore day 3 post-burn was used to determine type of burn. The results of H&E staining on day 3 post-burn was shown in Figure 2. Animals subjected to 60°C contact temperature developed superficial burns (Figure 2A). Obviously, only the epidermis was affected. The thickness of the epidermis was thinner and the appendage structures in the dermis remained the same. Exposure to 65°C contact temperature of the animals resulted in superficial partial-thickness burns (Figure 2B), in which the denudation of epidermis was observed and the superficial layer of the dermis was also destroyed, but there were still a presence of skin appendages such as hair follicles. Animals exposed to 70°C contact temperature created deep partial-thickness burns (Figure 2C), where not only the epidermis but also the deep layer of the dermis were affected. The dermis was occupied by many adipose tissues. Collagen fibers were arranged abnormally and hair follicles were degenerated; however, the hypodermis was not affected. In general, the deep partial-thickness burn can be achieved at a contact temperature of 70°C, a weight of the heating devices of 300 g, and duration of 10 s. Therefore this setting has been used for subsequent research.
Figure 2.
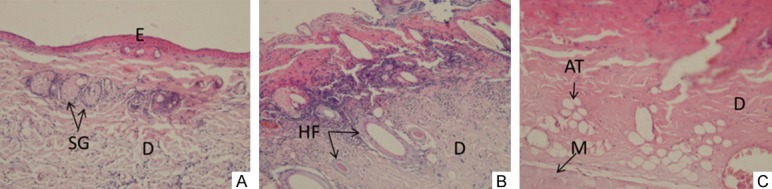
Microscopic assessment of burn depth at different temperatures on day 3 post-burn. Manifestation: ×20 E: epidermis D: dermis SG: sweat gland HF: Hair follicles AT: Adipose tissue M: muscle. A. 60°C burn, showing superficial burn, where the epidermis was affected (became thinner) and the dermis was normal; B. 65°C burn, showing superficial partial-thickness burn, where all epidermal layers could not be observed and the hair follicles still existed; C. 70°C burn, showing deep partial-thickness burn, where all epidermis were destroyed, and the dermis was occupied by adipose tissue.
Macroscopic evaluation
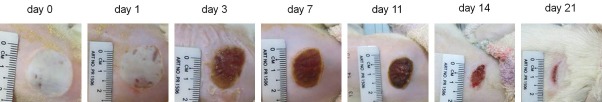
The images of deep partial-thickness burn wounds on day 0, 1, 3, 7, 11, 14, and 21 days after burn were demonstrated in Figure 3. After burns, the burn wound was round, which was due to the burner’s aluminum head. A severe edema was observed, resulting in uniform white wounds. No blister was observed, and the edges between the wound area and the normal skin were clear. On day 1 after burn, edema remained and the smallest crust formation was observed in the area around the wound. Until the third day, the entire area of the burned skin was covered with a solid layer of crust. On the 7th day after burn, the crust became dark and smaller in size. By the 11th day, the crust turned drier and smaller and the edges of the crust were separating from the wound. On the 14th day, the crust was completely detached from the skin, forming a second discreet crust. Reaching day 21, the wound area was small but the complete epithelialization had not yet achieved.
Figure 3.
Macroscopic changes of deep partial-thickness burn wound over time in Sprague-Dawley rats. Immediately after burn, he wound was round in shape and white in color, edema was obvious. From day 1 to day 3, edema gradually disappeared and the wound was covered by a firm crust. From day 7 to day 14, the crust on the wound gradually turned drier and smaller and finally fell off from the wound, and then a second discreet crust was formed. By day 21 after burn, the discreet crust was still present but much less. The wound area was very small but complete epithelialization had not been achieved.
Wound contraction rate
The wound area was captured and the healing state was assessed by the wound area reduction rate, as shown in Figure 4. It was obvious that the percentage of wound contraction rate increased in a time-dependent manner. The wound contraction rate was significantly increased on day 11 after burn (42.79 ± 6.98%) compared with day 3 (15.29 ± 2.48%). The wound contraction rate then continued to rise to 72.20 ± 1.92% on day 14, and the maximum wound contraction was observed on day 21, with a percentage of 90.39 ± 10.48%. In general, the wound area was gradually reduced in the healing process.
Figure 4.
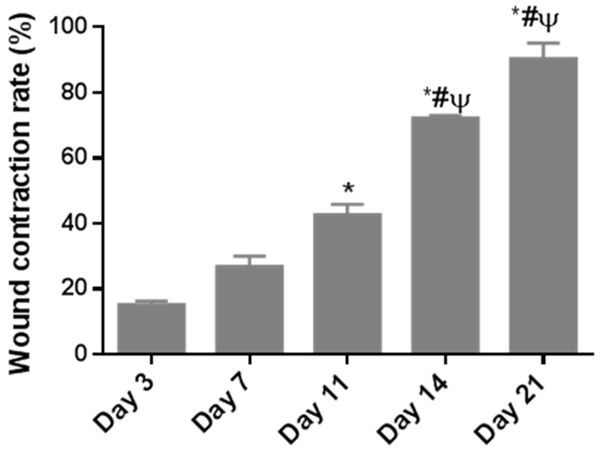
Wound contraction rates of rats with deep partial-thickness burns over time. n = 6. Data are expressed as means ± S.D. *P < 0.05 versus day 3; #P < 0.05 versus day 7; ψP < 0.05 versus day 11.
Histological aspect of deep partial-thickness burn
Microscopic pictures of the deep partial-thickness burn wounds on day 1, 3, 7, 11, 14, and 21 after burn was demonstrated in Figure 5. On day 1 after burn (Figure 5A, 5G), the epidermis in the wound area was complete, but when observed in detail, the rete ridge that protruded into the dermis in normal skin tissue disappeared and the thickness of the dermis became thinner. However, structures inside dermis were normal. The arrangement of collagen was regular, and skin appendeges such as sweat glands were distributed in the dermis. On day 3 after burn (Figure 5B, 5H), the epidermis was completely destroyed and invisible. Deep layer of the dermis were destroyed. The arrangement of collagen fibers became irregular and the structures in the dermis were degraded. The dermis was occupied by much adipose substitution. All of these confirmed the deep partial-thickness burn. On day 7 after burn (Figure 5C, 5I), the crust was attached tightly to the dermis. Adipose tissue was still present in the dermis, inflammatory cells were recruited to the dermis and fibroblasts were present, indicating the granulation tissue was forming. On day 11 after burn (Figure 5D, 5G), the crust was still attached to the dermis, but mild epithelialiazation was observed on both side of the wound. There was a significant reduction in adipose cells and an increase in inflammatory cells. Collagen deposition was present, indicating mild fibrosis. At day 14 after burn (Figure 5E, 5K), the crust detached from the wound, leaving a gap in the wound area. Less adipose tissues, more fibroblasts and more blood vessels were observed in the dermis, showing moderate fibrosis. By day 21 (Figure 5F, 5L), a thin layer of epidermis was formed at the area that eschar fell. The dermis was filled with plenty of fibroblasts and blood vessels, showing well-developed granulation tissue.
Figure 5.
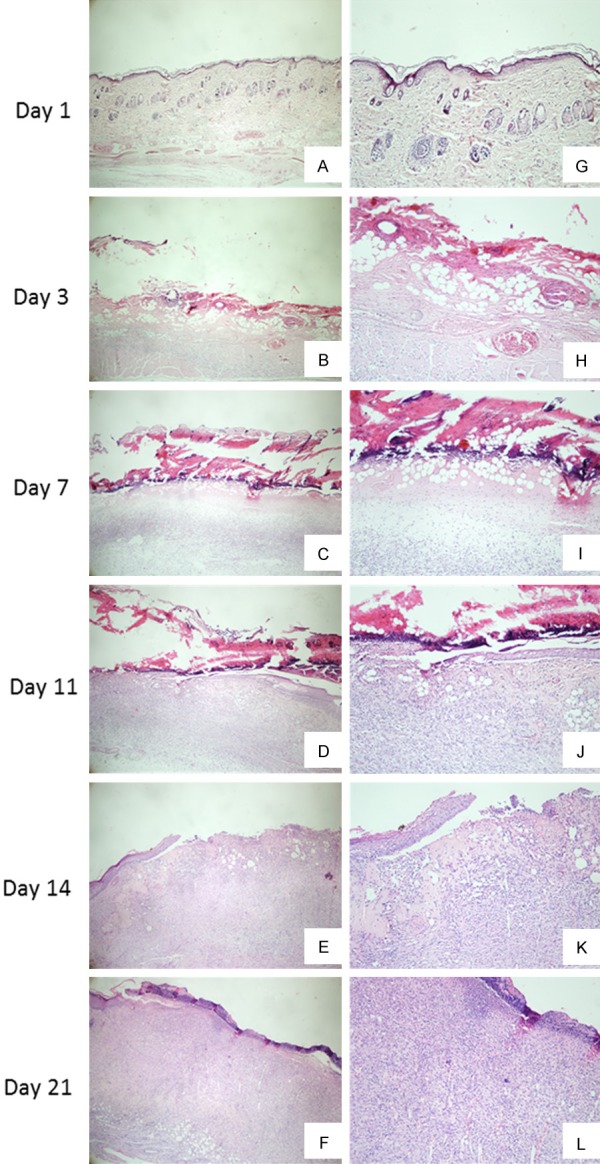
Microscopic images of deep partial-thickness burn wound in Sprague-Dawley rats over time after burn. ×4 (A-F); ×10 (G-L). (A, G) Injured tissue on day 1 after burn, showing thinner epidermis and normal structure of dermis; (B, H) Injured tissue on day 3 after burn, showing complete destruction of epidermis and significant adipose tissue substitution in the dermis; (C, I) Injured tissue on day 7 after burn, showing tight attachment of eschar, moderate adipose tissue substitution and increasing number of inflammatory cells; (D, J) Injured tissue on day 11 after burn, showing moderate epidermal migration, mild adipose tissue substitution and mild fibrosis; (E, K) Injured tissue on day 14 after burn, showing falling of crust and moderate fibrosis; (F, L) Injured tissue on day 21 after burn, showing well-developed granulation tissue and incomplete tissue epithelialization.
Discussion
In the study of burns, the importance of creating consistent and reproducible burn wounds is self-evident. At present, two common models of burn research are the scald model and contact burn model [12,13]. In the scald model, a portion of the shaved animal skin is immersed in hot water (100°C or 70°C) for a few seconds to create burns, during which the skin may be subject to additional damage, such as steam burns, and the operator may also face potential risks [14]. So this is obviously an insecure way of creating burns. In the contact burn model, the heating device, which may be a cylinder, metal rod, branding iron, brass probe, copper plate or aluminum bar, is heated in water (100°C, 98°C or 80°C) to reach thermal equilibrium and then placed on the shaved skin for several seconds to produce burns [15]. Due to the lack of continuous heat sources and heat exchange with the environment, the actual burn temperature is not controlled. In addition, many of the current models can create partial-thickness burn, but the deep partial-thickness burn wound is required in this study. Therefore, a new model was designed to induce uniform and reproducible deep partial-thickness burns.
The burn apparatus consists of five parts, including a single-channel power unit, a soldering pencil, an insulating ring, an aluminum head, and a thermometer. In this model, a stable and controlled current was provided to the solder pencil by the power unit and a fixed pressure of 300 g was achieved by adding an insulating ring external to the solder pencil. The circular wound was introduced from an aluminum head with a diameter of 20 mm and the precise contact temperature was monitored by a thermometer inserted into the aluminum head of the device. Furthermore, the operating of this device was rather simple in creating burns. Two persons were required to operate; one was needed to hold the experimental animals and another one slightly holding the insulation ring of the apparatus. Therefore, the device is a safe and accurate technique to induce burn wounds.
The burn wound area chosen should be large enough for multiple analysis including histological examination, protein purification and RNA extraction yet small enough to avoid any systemic response in the experimental animals. In the present model, a 20-mm wide burn wound was created, which can meet both of these requirements. Similar size of burn wound was also reported by Adam and co-workers [14]. 2.5 cm by 2.5 cm surface area burn wound was produced on pigs for second-degree burn. While the burn wound induced by Neil and colleagues to standardize in experimental burn wound on Wistar rats was 23 mm in diameter [11].
Burns are categorized into three different degrees, which are first-degree, partial-thickness and full-thickness burns. Using our model, first-degree, superficial partial-thickness, and deep partial-thickness burns can be systematically achieved. Although we only test the temperature for these two kinds of burns, it is also possible to create full-thickness burns by changing the contact temperature. In addition, the definition of superficial partial-thickness and deep partial-thickness burns are vague, which renders researchers hard to categorise them based on histology of the dermis. In our research, it is crystal clear that one characteristic that differ between the different burn wound categories lies in the degradation of skin appendages. In superficial partial-thickness burn, skin appendages were still present by day 3 after burn, while they were degraded in deep partial-thickness burn.
The macroscopic evaluation of the deep partial-thickness wound was recorded in the study, including the general appearance and wound contraction rate during healing. Immediately after the burn, the burned skin was round and white. The appearance resembled deep partial-thickness burns created in other studies [16]. In addition, it has been reported that local tissue edema is associated with burns. After injury, the permeability of endothelial cells changes, excess fluid penetrates into the surrounding tissue, leading to the swelling of tissue [17]. Similar phenomena were also present in current study, where edema was observed within three days after burn. Over time, the edema was gradually disappeared and a firm crust (a dead skin tissue caused by coagulation of blood vessels) was formed on the surface of the wound [18]. The wound was always contracted throughout the healing process; however, significant increased contraction rate was only observed 11 days after burn. It was reported that the fibroblasts migrating at the wound margins can produce sufficient force to initiate wound contraction. As contraction proceeded, migrating fibroblasts differentiated into myofibroblast and the actin cytoskeleton was responsible for further contraction of the wound [19]. In addition, more fibroblasts were generally increased at the late stage of healing. Therefore, the evident wound contraction was also observed in the late phase of healing. In our model, the epithelialization had not been achieved until day 21. However, a deep dermal partial thickness burn is normally healed within or more than 3 weeks post burn [16]. Another study showed that it took 35 days for the wound re-epithelialization of second degree burn [11].
Tissue repair is a synchronized and magnificently coordinated interplay of several cellular and biochemical components including homeostasis, inflammation, proliferation and tissue remodeling [20]. To explore the underlying mechanism of burn wound healing, histopathological change of the burn wound healing process was also evaluated. Based on the observations in the present study, the healing processes of the epidermis and dermis were different. Compared to the dermis, the recovery process of the epidermis was relatively simple. After burns, the epidermis was completely destroyed. The epidermal cells then migrated from both sides of the wound and finally covered the wound. It was reported that the migrating epidermal cells were mainly produced from the existing basal layer [21]. The epidermis then continued to increase the thickness and eventually projected into the dermis, forming the rete ridge. In contrast, the healing process of dermis was much more complicated. It required the coordinated completion of a variety of cells and events. Adipose cells were the most abundant cells observed in the wound on the 3rd day after burn. With the healing of the wound, the number of adipose cells gradually decreased and totally disappeared. The role of adipose cells in wound healing was still ambiguous. Inflammatory cells were the first cells that entered into the wound. They appeared in the wound area to scavenge bacteria and other foreign particles to avoid wound infection. Upon completion the function, their numbers were declined [22]. Fibroblasts usually entered in the wound on the 4th day after burn. Once within the wound, fibroblasts proliferated and produced new extracellular proteins such as fibronectin, hyaluronan, collagen and proteoglycans [23]. In addition, fibroblasts underwent phenotypic changes and differentiated into myofibroblast to form very tight adhesions to the surrounding granulation and provided the force for wound contraction [19]. Collagen deposition often occurred at the late stage of wound healing. The collagen fibers were initially evident on both sides of the wound, then expanded to the central area, and finally arranged regularly in the entire dermis. In addition, the recovery of skin appendages took a longer time. Even on day 21 day after burn, only the initial stage of follicles formation was observed in this study. In short, the coordinated efforts of various cells led to the dermal healing.
In conclusion, we described a new apparatus for inducing deep partial-thickness burns in Sprague-Dawley rats. Using a fixed pressure, time, and contact temperature enables the reliable creation of a uniform deep partial-thickness burn. This apparatus can be used to evaluate the efficiency of topical medications in the healing process of deep partial-thickness burns.
Acknowledgements
This work was supported financially by the eScience Fund, Ministry of Science, Malaysia.
Disclosure of conflict of interest
None.
References
- 1.Forjuoh SN. Burns in low- and middle-income countries: a review of available literature on descriptive epidemiology, risk factors, treatment, and prevention. Burns. 2006;32:529–537. doi: 10.1016/j.burns.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Lin YH, Huang CC, Wang SH. Quantitative assessments of burn degree by high-frequency ultrasonic backscattering and statistical model. Phys Med Biol. 2011;56:757–773. doi: 10.1088/0031-9155/56/3/014. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd EC, Rodgers BC, Michener M, Williams MS. Outpatient burns: prevention and care. Am Fam Physician. 2012;85:25–32. [PubMed] [Google Scholar]
- 4.Toussaint J, Singer AJ. The evaluation and management of thermal injuries: 2014 update. Clin Exp Emerg Med. 2014;1:8–18. doi: 10.15441/ceem.14.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nessler M, Puchala J, Wood FM, Wallace HJ, Fear MW, Nessler K, Drukala J. Changes in the plasma cytokine and growth factor profile are associated with impaired healing in pediatric patients treated with INTEGRA (R) for reconstructive procedures. Burns. 2013;39:667–673. doi: 10.1016/j.burns.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Jurjus A, Atiyeh BS, Abdallah IM, Jurjus RA, Hayek SN, Jaoude MA, Gerges A, Tohme RA. Pharmacological modulation of wound healing in experimental burns. Burns. 2007;33:892–907. doi: 10.1016/j.burns.2006.10.406. [DOI] [PubMed] [Google Scholar]
- 7.Alemdaroglu C, Degim Z, Celebi N, Zor F, Ozturk S, Erdogan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns. 2006;32:319–327. doi: 10.1016/j.burns.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 8.de Almeida EB, Cordeiro Cardoso J, Karla de Lima A, de Oliveira NL, de Pontes-Filho NT, Oliveira Lima S, Leal Souza IC, de Albuquerque-Junior RL. The incorporation of Brazilian propolis into collagen-based dressing films improves dermal burn healing. J Ethnopharmacol. 2013;147:419–425. doi: 10.1016/j.jep.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 9.Kubo H, Hayashi T, Ago K, Ago M, Kanekura T, Ogata M. Temporal expression of wound healing-related genes in skin burn injury. Leg Med (Tokyo) 2014;16:8–13. doi: 10.1016/j.legalmed.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Wang XQ, Kravchuk O, Kimble RM. A retrospective review of burn dressings on a porcine burn model. Burns. 2010;36:680–687. doi: 10.1016/j.burns.2009.06.200. [DOI] [PubMed] [Google Scholar]
- 11.Venter NG, Monte-Alto-Costa A, Marques RG. A new model for the standardization of experimental burn wounds. Burns. 2015;41:542–547. doi: 10.1016/j.burns.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Silva MA, Trevisan G, Klafke JZ, Rossato MF, Walker CI, Oliveira SM, Silva CR, Boligon AA, Flores FC, de Bona Silva C, Athayde ML, Ferreira J. Antinociceptive and anti-inflammatory effects of Aloe saponaria haw on thermal injury in rats. J Ethnopharmacol. 2013;146:393–401. doi: 10.1016/j.jep.2012.12.055. [DOI] [PubMed] [Google Scholar]
- 13.Dokumcu Z, Ergun O, Celik HA, Aydemir S, Sezak M, Ozok G, Celik A. Clostridial collagenase aggravates the systemic inflammatory response in rats with partial-thickness burns. Burns. 2008;34:935–941. doi: 10.1016/j.burns.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Singer AJ, Berruti L, Thode HC Jr, McClain SA. Standardized burn model using a multiparametric histologic analysis of burn depth. Acad Emerg Med. 2000;7:1–6. doi: 10.1111/j.1553-2712.2000.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 15.Gokakin AK, Deveci K, Kurt A, Karakus BC, Duger C, Tuzcu M, Topcu O. The protective effects of sildenafil in acute lung injury in a rat model of severe scald burn: a biochemical and histopathological study. Burns. 2013;39:1193–1199. doi: 10.1016/j.burns.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Cuttle L, Kempf M, Phillips GE, Mill J, Hayes MT, Fraser JF, Wang XQ, Kimble RM. A porcine deep dermal partial thickness burn model with hypertrophic scarring. Burns. 2006;32:806–820. doi: 10.1016/j.burns.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Infanger M, Schmidt O, Kossmehl P, Grad S, Ertel W, Grimm D. Vascular endothelial growth factor serum level is strongly enhanced after burn injury and correlated with local and general tissue edema. Burns. 2004;30:305–311. doi: 10.1016/j.burns.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Shakespeare P. Burn wound healing and skin substitutes. Burns. 2001;27:517–522. [PubMed] [Google Scholar]
- 19.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harper DY, McNaught CE. The physiology of wound healing. Surgery (Oxford) 2014;32:445–450. [Google Scholar]
- 21.Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304:274–286. doi: 10.1016/j.yexcr.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Enoch SL. Basic science of wound healing. Surgery (Oxford) 2008;26:31–37. [Google Scholar]
- 23.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]