Abstract
MicroRNAs (miRNAs) are important gene regulators that play key roles in tumor genesis. In this study, we investigate the role of miR-148a in the development of papillary thyroid cancer (PTC). Data from the cancer genome atlas (TCGA) indicate that miR-148a is downregulated in PTC tissues; we also find that miR-148a is downregulated in tissue samples from PTC patients and PTC cell lines. Overexpression of miR-148a significantly suppresses PTC cell proliferation, migration and invasiveness in vitro, and inhibits tumor growth in vivo as well. We have identified the cyclin-dependent kinase 8 (CDK8) gene as a direct target of miR-148a using the online software packages TargetScan and miRanda. Overexpression of miR-148a significantly represses CDK8 expression by directly targeting the 3’-untranslated region (3’-UTR) of the CDK8 gene in PTC tissues and cell lines; overexpression of CDK8 reverses the inhibitory effects of miR-148a on PTC cell growth, migration and invasiveness. Taken together, our results indicate that miR-148a functions as a tumor suppressor in PTC by repressing CDK8 expression.
Keywords: miR-148a, papillary thyroid carcinoma (PTC), cyclin-dependent kinase 8 (CDK8), proliferation, metastasis
Introduction
Papillary thyroid carcinoma (PTC) is the most common type of thyroid malignancy, accounting for more than 90% of all thyroid cancers [1]. In general, the prognosis for patients with PTC is believed to be relatively good. However, some PTCs exhibit invasive behaviors, which are associated with low resection rates and poor prognoses [2,3]. The discovery of specific biomarkers characterizing PTCs could help in assessing the invasive potential of tumors to guide surgical parameters or provide new targets for therapy [4,5].
Recently, researchers have begun to focus on examining the associations between cancer development and miRNAs functioning as oncogenes or tumor suppressors [6,7]. Several studies have reported that PTCs with invasive properties exhibit abnormal expression profiles of certain miRNAs and associate these expression profiles with clinicopathologic features [8,9]. The miRNA miR-146b-5p functions as a positive regulator of migration and invasiveness in normal thyroid follicular cells, as well as in tumor cells by regulating the actin cytoskeleton [10]. Another miRNA, miRNA-203, was reported to be a potential biomarker for relapse and poor prognosis in PTCs [9]. The expression of another miRNA, miR-148a, was reported to be downregulated in nasopharyngeal carcinomas [11] and non-small cell lung cancers [12,13]. We also found that the expression levels of miR-148a were lower than normal in PTC tissues.
Cyclin-dependent kinases (CDKs) play fundamental roles in regulating cell proliferation and differentiation in eukaryotes [14,15]. Dysregulation of CDKs and their regulatory partners, known as ‘cyclins’, can disrupt normal cell proliferation, differentiation and apoptosis, thereby leading to abnormal development and diseases such as cancer [16]. CDK8 was found to be upregulated and associated with tumor progression and metastasis in breast cancer [17,18], pancreatic cancer [19], colorectal cancer [20], and prostate cancer [21].
In this study, we find evidence of miR-148a downregulation in PTC cell lines and tissues. We also find that miR-148a overexpression significantly inhibited cell proliferation, migration, and invasiveness by targeting CDK8 gene expression in vitro, and that its overexpression in vivo suppressed tumor growth. Our study demonstrates that miRNA-148a negatively regulates CDK8, and that this miRNA is involved in proliferation and invasion-related processes in PTCs.
Materials and methods
Patients and tissue samples
Clinical samples were obtained from 81 PTC patients who underwent surgery at the Zhejiang Cancer Hospital from 2007 to 2015. Adjacent non-tumor thyroid tissues from these patients served as normal controls. No patient underwent radiation therapy and/or chemotherapy prior to surgery. The medical ethics committee of Zhejiang Cancer Hospital approved this study. Due to the retrospective nature of this study, the medical ethics committee waived the need for written informed consents from patients.
Cell culture
Human PTC cell lines (PTC-1, BCPAP, K1) and the human thyroid epithelial cell line Nthy-ori 3-1 were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 100 units/ml penicillin (Sigma-Aldrich, St. Louis, MO, USA), 0.1 mg/ml streptomycin (Enpromise, Hangzhou, China), and 10% fetal bovine serum. All cell lines were maintained in a humidified incubator at 37°C and 5% CO2.
Quantitative real-time PCR (qRT-PCR)
Tissues were immersed in RNAlaterTM Stabilization Solution (Invitrogen, Waltham, MA, USA) and stored at -20°C until RNA extraction could be carried out. Total RNA, including miRNA, was extracted using the mirVanaTM miRNA isolation kit (Invitrogen) according to the manufacturer’s protocol. Total RNA was extracted from cells using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. Reverse transcription was performed using RevertAidTM H Minus First Strand cDNA Synthesis kit (ThermoFisher Scientific, Waltham, MA, USA). Quantitative real-time PCR (qRT-PCR) was performed using a SYBR Green Premix Ex Taq (Takara) on a Lightcycler® 480 (Roche, Switzerland). The CDK8-specific primer sequences used were: 5’-GGTCGAGGACCTGTTTGAAT-3’ (forward) and 5’-GCCGACATAGAGATCCCAGT-3’ (reverse). The GAPDH gene was used as an internal control, and GAPDH-specific primer sequences used were: 5’-CTCCATCCTGGCCTCGCTGT-3’ (forward) and 5’-GCTGTCACCTTCACCGTTCC-3’ (reverse). For the miRNA expression assays, miRNAs were isolated using a the miRNeasy Mini kit. Following this, the TaqMan MicroRNA Assays kit was used to determine the miRNA expression on a 7500 Fast Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA). The universal small nuclear RNA, U6, was used as an internal control. Each experiment for each sample was independently repeated three times. The relative expression levels of mRNA and miRNA were analyzed with the 2-ΔΔCt method.
Cell proliferation assays
Cell proliferation assays were performed with MTT (Sigma-Aldrich) according to the manufacturer’s instructions. Briefly, 1 × 104 cells/well were plated into 96-well plates which were incubated for 24 h in a humidified chamber at 37°C and 5% CO2. Following this, 50 μl MTT (5 mg/ml) in phosphate buffered saline (PBS) was added to each well and incubated for 4 h in a humidified chamber at 37°C and 5% CO2. In the next step, 150 μl of DMSO was added to each well after emptying it of supernatant. A microplate reader (Bio-Rad) was used to determine absorbance at 490 nm. Each assay was performed three times to obtain triplicate readings.
Xenograft model experiments
All animal experiments were performed according to institutional and international animal care and use regulations. All animal-dependent experimental protocols were approved by the institutional animal care and use committee of Zhejiang Cancer Hospital. Four-week-old male SCID (severe combined immunodeficiency) mice were purchased from Beijing HFK Bioscience Co. Ltd. (Beijing, China). TPC-1 cells transfected with lentivirus miArrestTM miR-103 inhibitor (Genecopeia, Rockville, MD, USA) were injected into the mice either subcutaneously (1 × 106 cells per mouse) or through the tail vein (2 × 106 cells per mouse). Tumor growth in the mice was monitored every 4 days. All mice were euthanized after 40 days, following which, the tumor nodules in the mice were removed. Tumor sizes were measured using a caliper, and tumor volume was calculated using the following equation: tumor volume (mm3) = length (mm) × width (mm)2/2.
Cell transfections
An miR-148a mimic and a corresponding negative control (miR-C) were purchased from GenePharma Co. Ltd. (Shanghai, China) along with the CDK8 overexpression plasmid. The miR-148a mimic, negative control (miR-C) and CDK8 overexpression plasmid were transfected into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Twenty-four hours post-transfection, cells were harvested and subjected to gene expression analyses and cell proliferation, cell cycle, cell migration, and cell invasion assays.
Cell migration and invasion assays
Cell migration and cell invasion assays were carried out in transwell chambers with 8 μm pore filters (Corning, New York, NY, USA). Cells were grown to about 50% confluency and transfected with the desired siRNA; 24 h after transfection, the cells were incubated in serum-free medium for another 24 h. Following this, cells were trypsinized before 5 × 104 cells suspended in serum-free medium were added to the upper chambers of transwell plates. Medium containing 10% FBS was added to the lower chamber. Cells were incubated for 24 h at 37°C, following which, non-migrating cells were completely removed. Cells that had migrated across the membrane to the bottom chamber were fixed in 4% paraformaldehyde and stained with 0.5% crystal violet. The stained cells were visualized under a microscope and quantified by calculating an average of counts from five random fields. For cell invasion assays, the upper chambers of transwell plates were precoated with 1 mg/ml Matrigel (BD Biosciences), following which, the protocol followed was identical to the cell migration assay.
Bioinformatics analyses and dual-luciferase assays
To find genes targeted by miR-148a, we used the online software programs TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org). From the list of target genes obtained, we extracted all genes that could contribute to PTC progression. Since the 3’-UTR of the CDK8 gene was predicted to have miR-148a binding sites, this region was amplified from human cDNA and cloned into a dual-luciferase expression vector (Promega, Madison, WI, USA). The mutant form of the 3’-UTR of the CDK8 gene was obtained by overlap-extension PCR. K1 and PTC-1 cells were co-transfected with CDK8-3’UTR-WT/MU and miR-148a with Lipofectamine 2000. Luciferase activity was measured with a dual-luciferase reporter assay system (Promega) 48 h after transfection. The results were presented as the ratio of Renilla luciferase activity to firefly luciferase activity.
Western blots
Cells were lysed in 200 μl lysis buffer (0.5 M Tris-HCl at pH 6.8, 2 mM EDTA, 10% glycerol, 2% SDS, and 5% β-mercaptoethanol). Protein extracts (20 μg) were electrophoresed on 10% SDS polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat dried milk in Tris-buffered saline and incubated for 2 h at 20°C with the appropriate primary antibodies-CDK8 (Abcam, Cambridge, MA, USA), and GAPDH (Cell Signaling) -following which, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody. The immunocomplexes were visualized using the chemiluminescence phototopehorseradish-peroxidase kit. Actin was used as an internal standard to ensure equivalent protein loading.
Immunohistochemical staining analyses
Tissue samples were fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin. Following this, sections were deparaffinized and rehydrated. Hydrogen peroxide was applied to the sections to block endogenous peroxidase activity for 10 min. After antigen retrieval using a microwave, the sections were treated with 1% bovine serum albumin to block non-specific binding. The sections were then incubated with rabbit anti-CDK8 (Abgent, San Diego, CA) in a humidified chamber overnight at 4°C. After washing three washes lasting 5 min each with PBS, tissue sections were treated with biotinylated anti-rabbit secondary antibody (Zymed, San Francisco, CA), followed by further incubation with streptavidin-horseradish peroxidase complex. After rinsing, diaminobenzidine (DAB; Abcam) was used as a chromogen, and sections were counterstained with hematoxylin. Samples incubated with PBS instead of the primary antibody served as negative controls.
Statistical analyses
All data are presented as mean ± standard deviation (SD). All statistical analyses were performed using the Prism 5.01 software (GraphPad Software, San Diego, CA, USA). Differences between treatment groups were determined using analysis of variance (ANOVA) tests and Student’s t-tests.
Results
miR-148a expression is downregulated in PTC tissues and cell lines
Although miR-148a was found to be underexpressed in various types of cancer, its expression in PTCs remained uninvestigated until now. The expression of miR-148a was examined in PTC tissues and cell lines using qRT-PCR. Data from TCGA indicated that the majority of PTC tissues showed lower miR-148a levels than the corresponding tumor-adjacent normal tissues that acted as controls (Figure 1A, P < 0.01), indicating that miR-148a may act as a tumor suppressor in PTC. When we examined the expression of miR-148a in PTC tissues and normal tissue by qRT-PCR, we found that the expression of miR-148a in PTC tissue samples was significantly lower than that in the corresponding normal tissue samples (Figure 1B, P < 0.01). This result was further supported by our finding that miR-148a expression in PTC cell lines was significantly lower than that in the thyroid epithelial cell line Nthy-ori3-1 (Figure 1C, P < 0.01).
Figure 1.
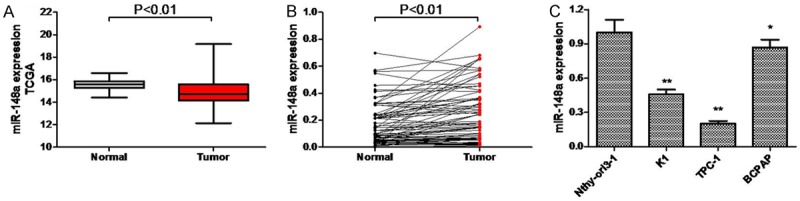
miR-148a is downregulated in PTC tissue samples from patients and PTC cell lines. A. miR-148a expression in PTC tissue samples and adjacent non-tumor thyroid tissues based on TCGA database. B. miR-148a expression levels as detected by qRT-PCR in 81 pairs of PTC tissue samples and adjacent non-tumor thyroid tissues. C. miR-148a expression levels as detected by qRT-PCR in PTC cell lines and normal thyroid cells. Each sample was tested three times. *P < 0.05, **P < 0.01.
miR-148a suppresses cell proliferation, migration, and invasion
To explore the possible anticancer functions of miR-148a in PTC cells, we transfected TPC-1 and K1 cell lines with miR-148a mimics or negative controls. MTT assays demonstrated that overexpression of miR-148a mimics significantly inhibited PTC cell proliferation as compared to that of negative control cells in both TPC-1 and K1 cell lines (Figure 2A). To investigate the effect of miR-148a on cellular motility, we used transwell assays to measure the migration and invasion ability of K1 and TPC-1 cells after modification of miR-148a expression. Cell migration through the transwell membrane was reduced dramatically in miR-148a-transfected K1 and PTC-1 cells as compared to cell migration in negative control cells (Figure 2C). Similarly, the rates of invasive cell movement in K1 and PTC-1 cells transfected with miR-148a mimics were decreased significantly as compared to those of negative control cells (Figure 2D).
Figure 2.
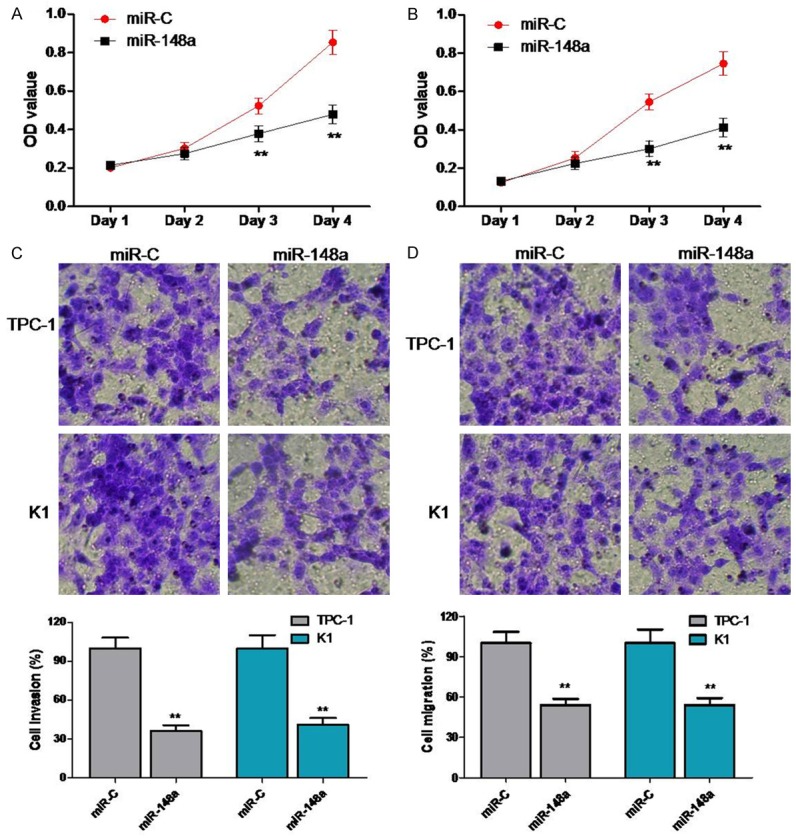
miR-148a suppresses proliferation, migration, and invasiveness of TPC-1 and K1 cells. Cell viability was determined by MTT assays on (A) TPC-1 and (B) K1 cells transfected with either miR-148a mimics or negative control. (C and D) Transwell migration and Matrigel invasion assays for TPC-1 and K1 cells either transfected with miR-148a mimics or negative controls. **P < 0.01.
miR-148a inhibited tumor growth in vivo
In this experiment, we investigated the effects of ectopic miR-148a expression on repressing tumor growth in vivo. PTC-1 cells transduced with miR-148a mimics or a negative control were subcutaneously injected into SCID mice. After 40 days, mice were euthanized and miR-148a expression levels in their tissues were measured. The expression of miR-148a was found to be higher in the group treated with miR-148a mimics than in the miR-C group (Figure 3A). Tumors overexpressing miR-148a grew more slowly and were smaller in size than control tumors (Figure 3B-D). Tumor weights were approximately 2.3-fold lower in miR-148a-overexpressing tumors as compared to those of negative controls (Figure 3B). These results suggest that miR-148a can also inhibit PTC cell growth in vivo.
Figure 3.
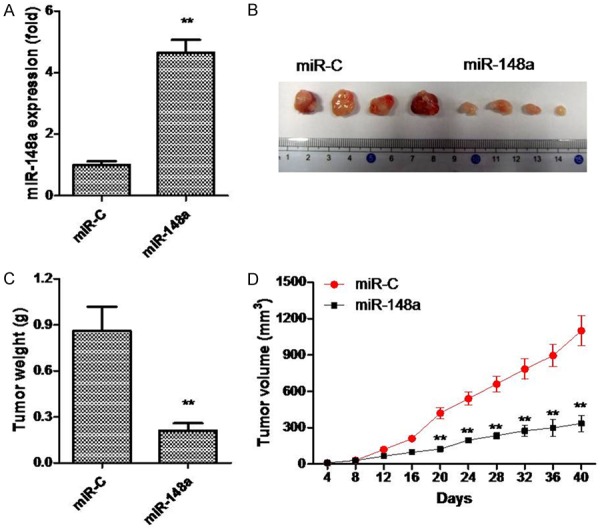
miR-148a inhibits PTC cell growth in vivo. TPC-1 cells transfected with Lv-miR-148a were injected subcutaneously into SCID mice. A. Expression of miR-148a in xenograft tumors as determined by qRT-PCR. B. Representative photograph of xenograft tumors. C. Tumor weights. D. Growth curve for tumor volumes. **P < 0.01.
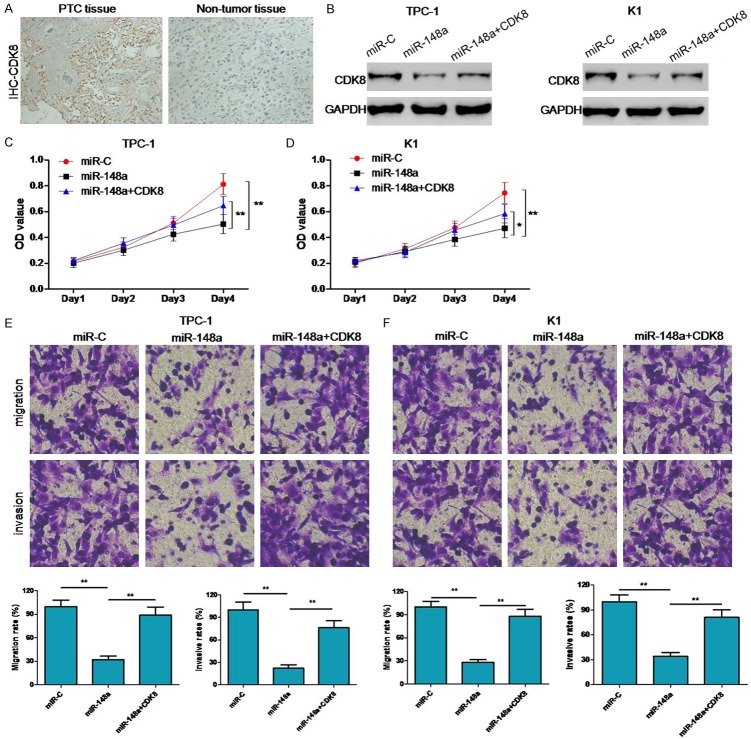
CDK8 is a molecular target of miR-148a regulation
To investigate the molecular mechanism by which miR-148a suppresses PTC cell growth and invasion, we searched for potential mRNA targets of miR-148a using the online software programs TargetScan and miRanda. One of the candidate genes predicted to be targeted by miR-148a was the CDK8 gene since this gene is associated with having positive effects on multiple cancer-related processes (Figure 4A). To determine whether CDK8 was negatively regulated by miR-148a, 3’-UTRs of the CDK8 gene with either wild-type (WT) or mutant (MU) miR-148a target sequences were cloned into firefly luciferase vectors. Following co-transfection with miR-148a mimics, the luciferase activity of the WT 3’-UTR reporter gene was found to be significantly decreased, whereas, the luciferase activity of the mutant reporter gene remained unaffected. These data suggest that miR-148a binds to the 3’-UTR of the CDK8 gene to regulate gene expression (Figure 4B). Our experiments also reveal that CDK8 expression was downregulated in PTC-1 and K1 cells transfected with miR-148a mimics as compared to PTC-1 and K1 cells transfected with miR-C (Figure 4C).
Figure 4.
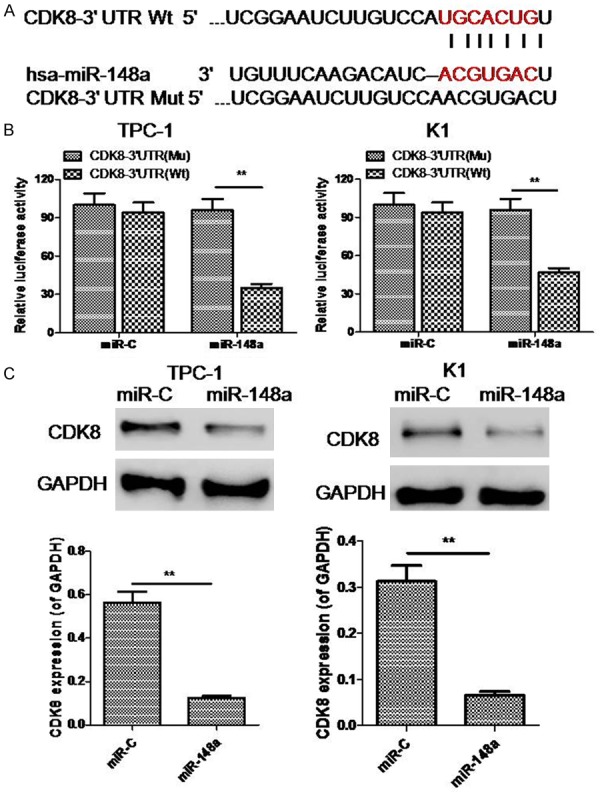
miR-148a targets CDK8 expression. A. Putative binding site of miR-148a in the CDK8 3’-UTR as predicted by TargetScan. B. Dual-luciferase activity in WT and mutant CDK8 3’-UTR reporter constructs, relative to miR-C, in the presence of miR-148a. C. Expression of CDK8 as detected through western blots in TPC-1 and K1 cells transfected with either miR-148a mimics or miR-C. **P < 0.05.
Inhibition of CDK8 expression had opposite effects to miR-148a downregulation in PTC cells
CDK8 expression in PTC tissues was evaluated by immunohistochemistry, and was found to be significantly higher in PTC tissues as compared to corresponding normal adjacent tissues (Figure 5A). To investigate if the suppressive effects of miR-148a on the proliferation, migration, and invasiveness of PTC cells were mediated by CDK8 repression, we rescued CDK8 underexpression in miR-148a-overexpressing K1 and TPC-1 cells (Figure 5B). Restoration of CDK8 expression levels not only increased the proliferation of miR-148a-transfected cells, but also partially rescued their migration and invasion capacities (Figure 5C-F). These results suggest that miR-148a suppresses PTC growth and metastasis by targeting and suppressing CDK8 expression.
Figure 5.
Effects of CDK8 inhibition in PTC cells with upregulated miR-148a expression. A. Detection of CDK8 expression levels through immunohistochemistry. B. Detection of CDK8 expression levels through western blotting for each group of transfected TPC-1 and K1 cells. C-F. Cell proliferation measurements by MTT assays, migration capacity measurements by colony formation assays, and invasion capacity measurements by transwell assays. *P < 0.05, **P < 0.05.
Discussion
MicroRNAs (miRNAs), which are one of the most common non-coding RNA types, are not only engaged in regulating normal physiological processes, but are also involved in a variety of pathological process. The abnormal expression of miRNAs can play important roles in the process of tumor development. Several miRNAs are reported to be abnormally expressed in PTCs and are thought to be involved in PTC tumor initiation and progression. Qiu et al. have reported that miR-613 inhibited cell growth, migration and invasiveness of PTC cells by regulating SPHK2 function [22]. The miRNA miR-363-3p also functions as a tumor suppressor in PTCs by repressing PIK3CA expression [23]. It is also reported that the miRNA miR-146b-5p positively regulates migration and invasion processes in PTC cells through the actin cytoskeleton [10]. The miRNA miRNA-148a has been shown to significantly suppress the migratory and invasive abilities of A549 and H1299 lung cancer cells, and that enforced expression of miRNA-148a in these cells results in a significant reduction in the expression of the DNMT1 gene [12]. Cell migration and invasion are also inhibited by miRNA-148a through its effects on WNT1 in lung cancer [13]. Besides this, the ectopic expression of miR-148a inhibits cell migration in neural progenitor cells (NPC) cells through the suppression of integrin-mediated signaling by targeting VAV2, WASL and ROCK1 [11].
In this paper, we report that the expression of miR-148a is lower in PTC tissues as compared to that in normal adjacent tissues based on information from the TCGA database. To further investigated this, we checked the expression of miR-148a in PTC tissues from patients and PTC cell lines. Interestingly, our results indicate that the expression of miR-148a was lower in PTC tissues as compared to that in adjacent non-tumor tissues, and in PTC cell lines as compared to that in thyroid epithelial cell lines. Functional analyses showed that overexpression of miR-148a suppressed cell proliferation, migration and invasiveness in vitro and inhibited tumor growth in vivo. The CDK8 gene was found to be targeted by miR-148a. CDK8 expression was inversely correlated with miR-148a expression and rescuing CDK8 expression reversed the suppressive effects of miR-148a on the proliferation and invasiveness of PTC cells. Taken together, our results suggest that miR-148a exerts its suppressive effect on the growth and metastasis of PTC by targeting CDK8. CDK8 is an important proto-oncogene that inhibits apoptosis, and abnormal expression of CDK8 is closely associated with the occurrence and development of many tumors [24]. CDK8 expression is upregulated in breast cancer [17,18], pancreatic cancer [19], colorectal cancer [20], and prostate cancer [21], and is associated with tumor progression and metastasis.
To further investigate the mechanisms underlying the inhibitory effect of miR-148a on cell growth, migration, and invasiveness in PTC, we identified genes with miR-148a binding sites using the online software programs TargetScan and miRanda. We found that the 3’-UTR of the CDK8 gene contained miR-148a binding sites, which made it a potential target for regulation by miR-148a. Through dual-luciferase assays, CDK8 was found to be regulated by miR-148a and that restoration of CDK8 expression abolished the inhibitory effect of miR-148a on cell proliferation and invasiveness. Overexpression of miR-148a significantly suppressed cell proliferation, migration, and invasiveness, while upregulation of CDK8 expression in cells transfected with miR-148a mimics dramatically promoted cell proliferation, migration and invasiveness. Our study provides a new mechanism for CDK8 regulation in PTCs.
In conclusion, miR-148a negatively regulates PTC cell proliferation, migration, invasiveness, and tumor growth by downregulating CDK8 expression. Our findings may help to further elucidate the molecular mechanisms underlying PTC progression and provide candidate targets for the prevention and treatment of PTC.
Acknowledgements
This study was supported by grants from the Public Welfare and Social Development Project, Zhejiang Province Science and Technology Department (No. 2015C33211), and from the Zhejiang Province Medical and Health Sciences Project (No. 2015KYB065).
Disclosure of conflict of interest
None.
References
- 1.Das S, Chaudhary N, Ang LC, Megyesi JS. Papillary thyroid carcinoma metastasizing to anaplastic meningioma: an unusual case of tumor-to-tumor metastasis. Brain Tumor Pathol. 2017;34:130–134. doi: 10.1007/s10014-017-0289-5. [DOI] [PubMed] [Google Scholar]
- 2.Melo M, Da RA, Vinagre J, Sobrinho-Simoes M, Soares P. Coexistence of TERT promoter and BRAF mutations in papillary thyroid carcinoma: added value in patient prognosis? J. Clin. Oncol. 2015;33:667–668. doi: 10.1200/JCO.2014.59.4614. [DOI] [PubMed] [Google Scholar]
- 3.Kwak HY, Chae BJ, Eom YH, Hong YR, Seo JB, Lee SH, Song BJ, Jung SS, Bae JS. Does papillary thyroid carcinoma have a better prognosis with or without Hashimoto thyroiditis? Int J Clin Oncol. 2015;20:463–473. doi: 10.1007/s10147-014-0754-7. [DOI] [PubMed] [Google Scholar]
- 4.Selmansberger M, Feuchtinger A, Zurnadzhy L, Michna A, Kaiser JC, Abend M, Brenner A, Bogdanova T, Walch A, Unger K, Zitzelsberger H, Hess J. CLIP2 as radiation biomarker in papillary thyroid carcinoma. Oncogene. 2015;34:3917–3925. doi: 10.1038/onc.2014.311. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Cai J, Yu Y, Fang H, Si Y, Jankee JJ, Shen M. Tumor abnormal protein as a novel biomarker in papillary thyroid carcinoma. Clin Lab. 2017;63:479–485. doi: 10.7754/Clin.Lab.2016.160903. [DOI] [PubMed] [Google Scholar]
- 6.Pallasch CP, Patz M, Park YJ, Hagist S, Eggle D, Claus R, Debey-Pascher S, Schulz A, Frenzel LP, Claasen J, Kutsch N, Krause G, Mayr C, Rosenwald A, Plass C, Schultze JL, Hallek M, Wendtner CM. miRNA deregulation by epigenetic silencing disrupts suppression of the oncogene PLAG1 in chronic lymphocytic leukemia. Blood. 2009;114:3255–3264. doi: 10.1182/blood-2009-06-229898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You Y, Tan JX, Dai HS, Chen HW, Xu XJ, Yang AG, Zhang YJ, Bai LH, Bie P. MiRNA-22 inhibits oncogene galectin-1 in hepatocellular carcinoma. Oncotarget. 2016;7:57099–57116. doi: 10.18632/oncotarget.10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheu SY, Grabellus F, Schwertheim S, Worm K, Broecker-Preuss M, Schmid KW. Differential miRNA expression profiles in variants of papillary thyroid carcinoma and encapsulated follicular thyroid tumours. Br J Cancer. 2010;102:376–382. doi: 10.1038/sj.bjc.6605493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng J, Li J. WITHDRAWN: serum miRNA-203 expression, a potential biomarker for recurrence and prognosis in papillary thyroid carcinoma. Cancer Biomark. 2016 doi: 10.3233/CBM-160653. [DOI] [PubMed] [Google Scholar]
- 10.Lima CR, Geraldo MV, Fuziwara CS, Kimura ET, Santos MF. MiRNA-146b-5p upregulates migration and invasion of different papillary thyroid carcinoma cells. BMC Cancer. 2016;16:108. doi: 10.1186/s12885-016-2146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li HP, Huang HY, Lai YR, Huang JX, Chang KP, Hsueh C, Chang YS. Silencing of miRNA-148a by hypermethylation activates the integrin-mediated signaling pathway in nasopharyngeal carcinoma. Oncotarget. 2014;5:7610–7624. doi: 10.18632/oncotarget.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Min L, Zhang X, Hu S, Wang B, Liu W, Wang R, Gu X, Shen W, Lv H, Zou J, Chen Y, Xu X, Chen L. Decreased miRNA-148a is associated with lymph node metastasis and poor clinical outcomes and functions as a suppressor of tumor metastasis in non-small cell lung cancer. Oncol Rep. 2013;30:1832–1840. doi: 10.3892/or.2013.2611. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Min L, Ren C, Xu X, Yang J, Sun X, Wang T, Wang F, Sun C, Zhang X. miRNA-148a serves as a prognostic factor and suppresses migration and invasion through Wnt1 in nonsmall cell lung cancer. PLoS One. 2017;12:e171751. doi: 10.1371/journal.pone.0171751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Wang G, Ma H, Khan MF. Enhanced expression of cyclins and cyclin-dependent kinases in aniline-induced cell proliferation in rat spleen. Toxicol Appl Pharmacol. 2011;250:213–220. doi: 10.1016/j.taap.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu TY, Pang JH, Wu KP, Lin LP, Tseng WC, Tsai WC. Platelet-rich plasma increases proliferation of tendon cells by modulating Stat3 and p27 to up-regulate expression of cyclins and cyclin-dependent kinases. Cell Prolif. 2015;48:413–420. doi: 10.1111/cpr.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumura K, Nakata S, Taniguchi K, Ii H, Ashihara E, Kageyama S, Kawauchi A, Yoshiki T. Depletion of gamma-glutamylcyclotransferase inhibits breast cancer cell growth via cellular senescence induction mediated by CDK inhibitor upregulation. BMC Cancer. 2016;16:748. doi: 10.1186/s12885-016-2779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crown J. CDK8: a new breast cancer target. Oncotarget. 2017;8:14269–14270. doi: 10.18632/oncotarget.15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott MS, Chumanevich AA, Lim CU, Liang J, Chen M, Altilia S, Oliver D, Rae JM, Shtutman M, Kiaris H, Gyorffy B, Roninson IB, Broude EV. Inhibition of CDK8 mediator kinase suppresses estrogen dependent transcription and the growth of estrogen receptor positive breast cancer. Oncotarget. 2017;8:12558–12575. doi: 10.18632/oncotarget.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu W, Wang Z, Zhang W, Qian K, Li H, Kong D, Li Y, Tang Y. Mutated K-ras activates CDK8 to stimulate the epithelial-to-mesenchymal transition in pancreatic cancer in part via the Wnt/beta-catenin signaling pathway. Cancer Lett. 2015;356:613–627. doi: 10.1016/j.canlet.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Ohtsuka M, Ling H, Ivan C, Pichler M, Matsushita D, Goblirsch M, Stiegelbauer V, Shigeyasu K, Zhang X, Chen M, Vidhu F, Bartholomeusz GA, Toiyama Y, Kusunoki M, Doki Y, Mori M, Song S, Gunther JR, Krishnan S, Slaby O, Goel A, Ajani JA, Radovich M, Calin GA. H19 noncoding RNA, an independent prognostic factor, regulates essential Rb-E2F and CDK8-betacatenin signaling in colorectal cancer. EBioMedicine. 2016;13:113–124. doi: 10.1016/j.ebiom.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bragelmann J, Klumper N, Offermann A, von Massenhausen A, Bohm D, Deng M, Queisser A, Sanders C, Syring I, Merseburger AS, Vogel W, Sievers E, Vlasic I, Carlsson J, Andren O, Brossart P, Duensing S, Svensson MA, Shaikhibrahim Z, Kirfel J, Perner S. Pan-cancer analysis of the mediator complex transcriptome identifies CDK19 and CDK8 as therapeutic targets in advanced prostate cancer. Clin Cancer Res. 2017;23:1829–1840. doi: 10.1158/1078-0432.CCR-16-0094. [DOI] [PubMed] [Google Scholar]
- 22.Qiu W, Yang Z, Fan Y, Zheng Q. MicroRNA-613 inhibits cell growth, migration and invasion of papillary thyroid carcinoma by regulating SphK2. Oncotarget. 2016;7:39907–39915. doi: 10.18632/oncotarget.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Li Q, Li R, Ren P, Dong S. MicroRNA-363-3p inhibits papillary thyroid carcinoma progression by targeting PIK3CA. Am J Cancer Res. 2017;7:148–158. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Xu D, Li CF, Zhang X, Gong Z, Chan CH, Lee SW, Jin G, Rezaeian AH, Han F, Wang J, Yang WL, Feng ZZ, Chen W, Wu CY, Wang YJ, Chow LP, Zhu XF, Zeng YX, Lin HK. Skp2-macroH2A1-CDK8 axis orchestrates G2/M transition and tumorigenesis. Nat Commun. 2015;6:6641. doi: 10.1038/ncomms7641. [DOI] [PMC free article] [PubMed] [Google Scholar]